Abstract
Individuals with internalizing psychopathologies (IPs) demonstrate a negativity bias in emotion and self-related processing that contributes to negative interpretation of neutral information. However, most neuroimaging studies of emotional experience in IPs do not specifically investigate reactivity to neutral stimuli. Thus, little is known about the neural processes underlying emotional experience for neutral stimuli and how those processes may differ between groups and during neutral versus negative stimuli. To address this gap, we asked: (1) does neural reactivity to neutral and negative stimuli differ between IPs and control groups in brain regions associated with emotional and self-referential processing, and (2) does neural activity during neutral condition relate to clinical symptoms? Adults with IPs (n=103) and healthy volunteers (HVs; n=40) completed a well-validated fMRI task probing neural responses to neutral and negative images. A flexible factorial model revealed a significant group-by-condition interaction, such that individuals with IPs had less precuneus activation during the neutral condition relative to HVs. In IPs, precuneus activation during the neutral condition was negatively correlated with depression symptom severity. Individuals with IPs demonstrate abnormal precuneus reactivity to neutral stimuli that is associated with depression symptoms. This may reflect altered default mode network activity and/or self-referential processing in IPs.
Keywords: self-referential processing, default mode network, emotion processing
1. Introduction
Internalizing psychopathologies (IPs), which include anxiety and depressive disorders, are prevalent illnesses characterized by abnormalities in emotional processing and emotion regulation1,2. This includes a negative information processing bias whereby affected individuals, relative to unaffected controls, tend to pay more attention to and have greater memory for negative stimuli relative to positive stimuli3,4. Known as the negativity bias5, this abnormal processing of emotional information is a risk factor for development of internalizing psychopathology6 and a target of psychosocial interventions for IPs (i.e., cognitive-behavioral therapy7). In addition to driving increased reactivity to negative information, research suggests that the negativity bias also contributes to abnormal processing of neutral stimuli. For example, individuals with IPs tend to interpret neutral facial expressions as sad8 and demonstrate abnormal pattens of brain activation when viewing neutral stimuli9. Other evidence further suggests that brain reactivity to neutral stimuli may not be significantly different than reactivity to negative stimuli in IPs10. Of note, negativity bias for neutral stimuli may be an underlying risk factor for development of emotional disorders11.
Individuals with IPs also show additional evidence of negativity bias via abnormalities in the processing of self-referential information. Broadly, self-referential processing describes reactivity to stimuli that one experiences as strongly related to their own person12 at the phenomenal level of experience (i.e., not requiring reflection or objective reasoning13) that is thus presumed to represent a foundational, automatic function14. Individuals with IPs demonstrate a bias toward negative self-referential processing15 characterized by excessive self-focused attention that is persistent and self-critical16,17, particularly in the context of greater emotional intensity18. Critically, higher levels of negative self-referential processing is associated with higher generalized anxiety symptoms and stress-reactivity19 and is predictive of both current and future recurrence of major depressive episodes20,21.
A substantial number of studies have investigated the neural correlates and mechanisms of negativity bias in emotion and self-referential processing in IPs using targeted functional magnetic resonance imaging (fMRI) tasks. Collectively, research suggests that the observed biases toward negative stimuli and aberrant emotion processing in IPs are driven by a frontolimbic neural circuit.22 The amygdala and the insula, which are involved in subjective emotional experience23,24 and threat-related processing25, are key nodes of this circuit that show greater reactivity to negatively valenced stimuli26–28 and are strongly implicated in the pathophysiology of IPs28. Notably, existing data suggest that activation in these regions while viewing aversive stimuli is associated with increased symptoms of anxiety in individuals with IPs29. Further, a large body of literature has demonstrated that the default mode network (DMN), which is comprised of distributed network of brain regions (e.g., medial prefrontal cortex, posterior cingulate cortex/precuneus, and bilateral inferior parietal lobules)30 that are more active during rest31, has a central role in self-referential processing30. Activation of the DMN is thought to indicate self-reflective and stimulus-independent thought that occurs spontaneously when the brain is not engaged in a specific or attention-demanding task32. DMN activity is altered in IPs33,34 – including during the viewing of neutral stimuli35 – and activity in DMN brain regions contributes to disordered self-referential thinking36 and higher levels of negative self-attribution37,38 in IPs. Collectively, existing studies suggest that a negativity bias for both emotion and self-related information in IPs has identifiable neural underpinnings.
Despite known biases in the processing of neutral information in IPs, the vast majority of neuroimaging studies of emotion processing do not examine reactivity to neutral stimuli as a unique condition. Instead, neutral stimuli are typically used as a baseline condition that is subsequently contrasted with another experimental condition (i.e., valenced emotional stimuli to create an index of positive or negative emotional brain reactivity)39. Thus, little is known about the neural processes underlying emotional experience for neutral stimuli and how these processes may differ between clinical and non-clinical groups or during neutral versus negative stimuli. As existing research suggests that individuals with IPs are prone to have negative reactions and negative interpretations of neutral information and that these individuals further tend to preferentially engage in self-referential processing for information to which they react negatively, examination of reactivity to neutral stimuli as a unique condition is crucial in clarifying potential group differences in neural reactivity.
Thus, our primary objective in the present study was to examine whether patterns of neural reactivity to neutral and negative stimuli differ between IP and control groups in brain areas relevant to emotional and self-referential processing. We hypothesized that brain activation in these areas would be significantly different between healthy controls and individuals with IPs for both neutral and negative stimuli (i.e., a significant interaction between group and condition). Given strong evidence of a negativity bias in the processing of emotional and neutral information in IPs, we hypothesized that, relative to controls, 1) individuals with IPs would show greater frontolimbic reactivity (i.e., insula and amygdala activation) for both neutral and negative stimuli, and 2) individuals with IPs would show greater DMN activation for negative stimuli. Further, as it remains unclear whether neural reactivity to neutral stimuli is related to anxious and/or affective symptoms among individuals with IPs, we examined correlations between brain activation and clinically-validated symptom measures to address this gap.
2. Methods
2.1. Participants.
The current study included 143 participants in two groups. The study was designed to be consistent with and funded by the NIMH Research Domain Criteria (RDoC) Initiative. Exclusion criteria for all participants included: (i), major active medical or neurological problem, (ii) lifetime history of bipolar disorder or psychosis, (iii) current cognitive or intellectual dysfunction (e.g., pervasive developmental disorder, traumatic brain injury, (iv) substance dependence within the past 6 months (other than nicotine dependence), and (v) any psychiatric treatment including medications within the past 4 weeks. Enrolled participants with available fMRI data were included in the current study. All participants provided written informed consent. Procedures were approved by the University of Illinois Chicago Institutional Review Board.
2.1.1. Internalizing Psychopathologies (IPs) Group.
Individuals in the IPs group (n=103) were treatment-seeking individuals recruited for a clinical trial examining negative valence brain targets and predictors of anxiety and depression treatment40. Individuals included in this study met criteria for at least one DSM-5 depressive or anxiety disorder confirmed by SCID-5 interview,1 had a self-reported score ≥23 on the Depression, Anxiety, and Stress Scale41, had a Global Assessment of Functioning score ≤ 60, and were between the ages of 18 and 65. Enrolled participants with pre-treatment fMRI data were included in the current study.
2.1.2. Healthy Volunteer Group.
Health Volunteers (HVs; n=40) participated as control subjects. HVs were recruited from the local community and had no history of psychiatric diagnoses as determined by SCID-51 interview.
2.2. Procedure and Instruments
2.2.1. Emotion Regulation Task (ERT).
The ERT is a well-validated task to probe neural responses during sustained negative emotional experience and its volitional regulation using cognitive reappraisal27. In a block-design, participants viewed negative and neutral images from International Affective Picture System42 (IAPS) under three conditions: (1) “Look Neutral” by naturally viewing neutral images without attempting to change one’s emotional response; (2) “Look Negative” by naturally viewing negative images without attempting to change one’s emotional response; and (3) “Reappraise Negative” (negative images) by interpreting the depicted scenario in a less negative way to reduce negative affect evoked by the aversive images. At the end of each of the four task blocks, participants rated how negative they felt on a 5-point scale (1 = not at all, 5 = extremely) using a button response. Each condition is presented twice per run, with 2 runs total. In the present study, we were interested in examining reactivity to neutral stimuli as a unique condition and to investigate how reactivity to neutral stimuli differed from reactivity to negative stimuli among individuals with IPs. Thus, we utilized two of the task conditions: (1) “Look” (Neutral), and (2) “Maintain” (Negative). Each run of these conditions contained an equal number of social (i.e., featuring people) and non-social images.
2.2.2. Psychiatric Symptoms.
IPs participants completed self-report symptoms measures of depression (Beck Depression Inventory43 [BDI-II] and anxiety (Beck Anxiety Inventory44 [BAI]). These clinically-validated measures assess the existence of severity of symptoms, with higher scores indicating greater symptom severity. The total score for the BDI-II and BAI were utilized in the current study.
2.3. fMRI data acquisition, preprocessing, and analyses
MR data were acquired on a 3T GE Discovery System MR scanner (General Electric; Waukesha, WI) at the University of Illinois at Chicago (UIC) in Chicago, IL. Gradient echo-planar imaging (EPI) with the following parameters were used to acquire functional data: 44 axial slices, repetition time (TR)=2 s, flip angle 90°, echo time (TE)=25 ms, field of view (FOV) 220 × 220 mm, acquisition matrix 64 × 64, 3 mm slice thickness with no gap, voxel size=3.4 × 3.4 × 3 mm. Each ERT run consisted of 150 T2*-weighted functional volumes. High-resolution, T1-weighted volumetric anatomical scans were acquired for anatomical localization.
Data were analyzed using a general linear model implemented in Statistical Parametric Mapping (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK). Functional images were corrected for errors in slice-timing, realigned, co-registered to structural images, spatially normalized to MNI space, and smoothed with an 8-mm full width at half maximum (FWHM) Gaussian kernel. Translational movement in millimeters (x, y, z) and rotational motion in degrees (pitch, roll, yaw) were calculated based on SPM12 parameters for motion correction of the functional images in each participant. Participants with greater than 3 mm of movement or 3 degrees of rotation during ERT were excluded.
A general linear model identifying the Look Neutral, Look Negative, and Reappraise Negative blocks in the ERT convolved with the canonical hemodynamic function was used to model the hemodynamic signal. A 128-s high-pass filter was applied, and six variables of no interest for motion were included.
For the present study, we created two contrasts: Look Neutral vs. Fixation to capture neutral reactivity, and Look Negative vs. Fixation to capture negative reactivity. Given our hypotheses related to the DMN, insula, and amygdala, we restricted our brain search to these regions of interest (ROIs). A brain mask combining our regions of interest was configured in SPM using the AAL atlas45 to include the amygdala, insula, and the 7 network parcellation from Yeo and colleagues46 for DMN regions.
2.4. Statistical Analyses
We utilized a 2 (group) x 2 (contrast) repeated measures ANOVA using the flexible factorial model in SPM12 to examine differences in reactivity to neutral and negative stimuli. BOLD activation was considered significant only if it exceeded adjustment for multiple comparisons across voxels in the mask. To determine the corrected cluster threshold AFNI’s47,48 3dFWHMx and 3dClustSim programs were used (version AFNI_19.3.16). The ACF method49 was utilized with 3dClustSim. Significance at a voxel level p<0.005 and cluster level α<0.05, required a minimum cluster size of 188 voxels (volume = 1,504 mm3). For any significant effects, the activation (beta weights) was extracted using MarsBar as an 8-mm diameter spherical ROI centered at the peak voxel and then subjected to post-hoc analyses in SPSS. Any effects found to be significant were followed up with independent sample t-test and/or paired t-test as needed. Follow-up bivariate correlations (Pearson’s r) were used to examine the associations between significant brain activations during the neutral condition and clinical symptoms of anxiety and depression.
3. Results
IPs and HVs groups were compared on demographics variables (Table 1). Analyses revealed significant group differences on sex and no significant differences with regard to age, race, or mean years of education (Table 1).
Table 1:
Group Demographics and Clinical Characteristics
| Patients | Healthy Volunteers | |
|---|---|---|
| Group Difference Statistics | ||
| N | 103 | 40 |
| Mean Age (SD) | 26.6(7.8) | 25.6(9.9) |
| t(141) = .545, p = .59 | ||
| Sex (% Female) | 71.8% | 52.5% |
| χ2 = 4.84, df = 1, p = .03* | ||
| Race (%) | ||
| χ2 = 8.82, df = 5, p = .12 | ||
| Caucasian | 61(59.2%) | 16(40%) |
| Black | 17(16.5%) | 7(17.5%) |
| Asian | 13(12.6%) | 13(32.5%) |
| American Indian or Alaskan Native | 4(3.9%) | 1(2.5%) |
| More Than One Race or Unknown | 8(7.8%) | 3(7.5%) |
| Mean Years of | 15.8 (2.9) | 15.7(3.1) |
| t(141) = .129, p = .89 | ||
| Education (SD) | ||
| Primary Diagnosis | ||
| Generalized Anxiety Disorder | 38.8% | |
| Social Anxiety Disorder | 24.3% | |
| Major Depressive Disorder | 25.2% | |
| Persistent Depressive Disorder | 1.0% | |
| Panic Disorder | 5.8% | |
| Post-traumatic Stress Disorder | 4.9% | |
| Beck Depression | 25.5(9.3) | |
| Inventory Score (SD) | ||
| Beck Anxiety Inventory Score (SD) | 19.1(9.6) | |
p < .05
Examination of standard ERT contrasts confirmed activation of expected brain areas, which have been reported elsewhere40. No significant main effects of group or condition were observed in our restricted brain search.
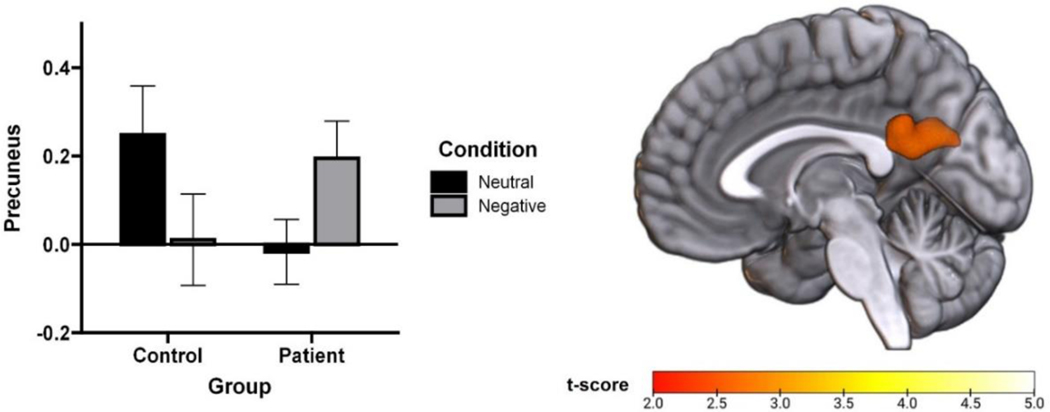
A restricted brain flexible factorial model with group as a between-subjects factor and condition (neutral or negative) as a within-subjects factor revealed a significant group-by-condition interaction in the precuneus (Z=3.18, α<.05, corrected; Figure 1). More specifically, this cross-over interaction indicated that the pattern of precuneus function in the neutral and negative conditions varied as a function of group (i.e., IPs or HVs demonstrated the opposite pattern of results). Follow-up paired t-test showed that individuals with IPs had less precuneus activation during the neutral condition than the negative condition (t(102)=−2.705, p=0.008); meanwhile, HVs demonstrated no significant difference in precuneus activation between conditions (p=0.122). Moreover, follow-up t-test showed that precuneus activation did not differ between individuals with IPs and HVs during the negative condition (p=0.220); however, precuneus activation was marginally higher among HVs than individuals with IPs (t(141)=1.942, p=0.054) during the neutral condition. Significant effects from the restricted-search flexible factorial model are summarized in Table 2.
Figure 1.
Group differences in precuneus response during neutral and negative task conditions. T-map (p < .005) rendered on a canonical brain image and bar graph of extracted BOLD response showing a significant group by condition interaction in the precuneus.
Table 2:
Significant Effects from Restricted Search Brain Analyses
| Region Name | L/R | MNI Coordinates | Voxels | Z-score | p-value | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Group x Condition Interaction | Precuneus | L | −6 | −52 | 20 | 321 | 3.17 | .005 |
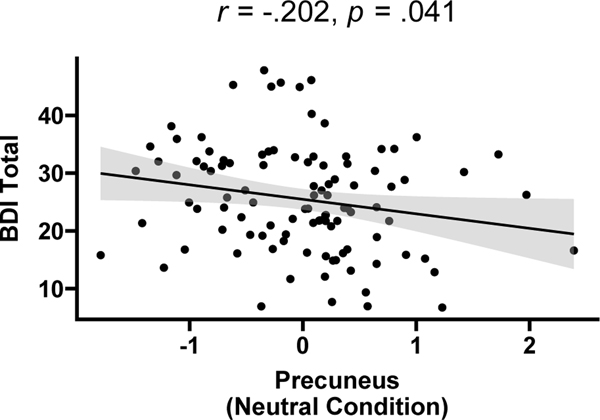
Follow-up correlation analyses revealed that among IPs individuals, precuneus activation during the neutral condition was negatively correlated with depressive symptom severity (r=−.20, p=.04; Figure 2) but not with anxious symptom severity (r=.14, p=.17).
Figure 2.
Scatterplot showing significant correlation between precuneus activation during the neutral condition and depressive symptom severity among Individuals with Internalizing Psychopathology.
Due to sex differences between groups, t tests were used to examine differences in precuneus activation between male and female participants during both the negative and neutral conditions. There were no significant differences in precuneus activation during the neutral or negative tasks between men and women in either the IPs or HVs groups.
4. Discussion
In the present study, the overall pattern of precuneus activation to neutral versus negative stimuli varied as a function of group (i.e., IPs or HVs). More specifically, IPs participants had less activation in the precuneus during the neutral condition and more precuneus activation during the negative condition. Among individuals with IPs, precuneus activation during the neutral condition was negatively correlated with depressive symptom severity.
Our results suggest that the precuneus is implicated in the processing of neutral and emotional stimuli among individuals with IPs. The precuneus is a key hub of the DMN, which is involved in self-referential processing and self-related brain activity. This pattern of results thus suggests a possible role of abnormal self-referential processing whereby IP individuals and HVs exhibit different patterns of neural reactivity to neutral versus negative stimuli in a key node of the DMN. This inference is consistent with existing research noting increased self-referential processing for negative information in IPs15–17 and abnormal neural response to neutral emotional faces among depressed individuals,50 yet to the best of our knowledge this is the first study to demonstrate this effect in the brain when neutral stimuli are considered as a separate condition (i.e., contrasted against fixation and not another experimental condition).
Notably, other studies using different methodology have noted the relevance of the precuneus in IPs. For example, greater activation of the precuneus during self-referential processing among individuals with major depression predicts nonremission 6 months later,51 and greater resting-state functional connectivity stemming from the left precuneus is associated with more severe suicidal thoughts and behaviors in depressed adolescents52. Precuneus activity is also associated with greater levels of rigid, negative beliefs about how negative thoughts can influence real-world outcomes (i.e., thought-action fusion)53. Further evidence suggests that altered precuneus function is evident before IPs onset among youth at elevated risk for developing a mood disorder54, with converters (i.e., those that go on to develop a psychiatric disorder) showing greater activation of the precuneus during the processing of aversive stimuli than non-converters55. Taken together, our current results and other existing literature suggest that the precuneus may play an important role in the phenomenology and underlying pathophysiology of emotion processing in IPs. It is possible that our observed pattern of results may in part reflect altered mind-wandering among individuals with IPs. More specifically, greater levels of precuneus activity during the neutral condition among HVs may reflect normative mind wandering that is down-regulated during the negative condition when other regulatory brain regions become active – and that this process somehow goes awry in IPs, potentially contributing to emotional dysfunction. Though we did not explicitly assess this explanation in the present study, it can be tested in future research.
It is important to acknowledge that aspects of our predictions that were not supported in the present study. First, contrary to our hypotheses brain activation in the amygdala and insula during the neutral and negative conditions did not differ as a function of group. Though an abundant literature demonstrates increased insula and amygdala reactivity to negative stimuli among individuals with IPs22, we did not observe this pattern for either neutral or negative stimuli in the present study. Notably, our study utilized a transdiagnostic sample that may not generalize to other work done in more homogenous clinical groups. Second, precuneus activation during the neutral condition was not significantly correlated with anxiety symptom severity. Though we utilized a transdiagnostic sample of individuals with IPs in the current study, it is possible that our observations of altered precuneus function in response to neutral stimuli is most important in understanding the processes that underlie mood disturbance. More specifically, it is possible that precuneus activity may reflect processes related to distorted or maladaptive self-reflection that aligns with rumination, which is more prominent in mood disorders than anxiety disorders56.
Our findings comport with the concerns raised by other researchers about the use of neutral stimuli as a “baseline” contrast in fMRI research. In particular, our findings challenge the assumption that neural reactivity to neutral stimuli in IPs is similar to neural reactivity to neutral stimuli in healthy controls. Building on previous findings that the difference scores that are created when two fMRI experimental conditions are contrasted against one another may be problematic with regard to reliability and use as indicators of individual differences39, our results suggest that additional consideration is needed when utilizing neutral stimuli as a baseline for neuroimaging contrast analyses among individuals with internalizing psychopathology or individuals otherwise prone to disease-related biases in the processing of “neutral” information. Further, our results suggest that brain differences in the processing of neutral information are clinically meaningful as they relate to clinical symptoms and thus even greater caution may be warranted.
This study also has several limitations to consider. First, though our analytical approach was guided by a-priori hypotheses, brain search was restricted and thus limited in examining other potential areas of reactivity. Second, the cross-sectional nature of our analyses does not permit inferences about how precuneus function may change or predict changes in other clinical variables over time. Third, though our use of a highly comorbid, transdiagnostic IPs sample likely increases external validity it also limits our ability to make determinations about the impact of specific diagnoses on the current pattern of results. Fourth, we did not have a specific probe of self-related processing in the current study. Finally, we had an unequal number of participants in the IPs and HVs groups. Though ANOVA is robust despite uneven sample size,57 future studies may investigate whether our pattern of results is replicated when group sample sizes are more even.
The current study provides a preliminary characterization of how precuneus function is altered in internalizing psychopathology, and how these alterations relate to clinical symptoms of depression. Future studies may examine how precuneus reactivity may change with treatment and/or predict treatment response in IPs. As psychosocial interventions for IPs target cognitive errors and maladaptive responses to emotions7,58, these treatments are likely to augment self-referential processing and biases in emotion processing. Notably, increased down-regulation of precuneus activation during emotion regulation is associated with better treatment outcomes among individuals receiving CBT for depression59. It is also possible that precuneus function at baseline may influence response to treatments for IPs. Though several studies have examined brain-based predictors for treatment response in IPs40,59, additional studies that investigate reactivity to neutral stimuli as a unique condition may provide additional insights. Finally, though we did not observe sex differences in precuneus function in the present study, other research has demonstrated that sex and mood disorder diagnoses have interactive effects on the neural mechanisms of emotion processing50 and thus potential contributions of sex should be considered in future studies.
Highlights:
Individuals with internalizing psychopathology (IPs) have negative biases in how they process both emotional and neutral information
The majority of neuroimaging studies investigating emotional responding use neutral conditions as a baseline that is contrasted with other emotional valence conditions (i.e., positive or negative) despite possible differences in neutral processing
People with IPs show altered precuneus reactivity to neutral stimuli, and this brain activity is associated with depression symptom severity
Neuroimaging researchers may consider how group differences in brain reactivity to neutral stimuli may relate to disease-related process when creating contrasts for analyses
Acknowledgements.
This study was supported by the National Institute of Mental Health (NIMH) Award R01MH101497 (PI: KLP), the National Center for Advancing Translational Sciences (NCATS) Award KL2TR002734 (to AMM), and a Research Innovation Career Development Award from the Ohio State University College of Medicine (to AMM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest.
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.First MB, Williams JBW, Karg RS, Spitzer RL. Structured clinical interview for DSM-5, clinician version (SCID-5-CV). Arlington, VA: American Psychiatric Association. Published online 2015. [Google Scholar]
- 2.Kotov R, Krueger RF, Watson D, et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126(4):454. [DOI] [PubMed] [Google Scholar]
- 3.Gollan JK, Hoxha D, Hunnicutt-Ferguson K, et al. Twice the negativity bias and half the positivity offset: Evaluative responses to emotional information in depression. J Behav Ther Exp Psychiatry. 2016;52:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everaert J, Koster EHW, Derakshan N. The combined cognitive bias hypothesis in depression. Clin Psychol Rev. 2012;32(5):413–424. [DOI] [PubMed] [Google Scholar]
- 5.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37(1):117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shook NJ, Fazio RH, Vasey MW. Negativity bias in attitude learning: A possible indicator of vulnerability to emotional disorders? J Behav Ther Exp Psychiatry. 2007;38(2). doi: 10.1016/j.jbtep.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 7.Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behav Ther. 2004;35(2). doi: 10.1016/S0005-7894(04)80036-4 [DOI] [PubMed] [Google Scholar]
- 8.Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry Res. 2008;159(1–2):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira L, Ladouceur CD, Phillips ML, Brammer M, Mourao-Miranda J. What does brain response to neutral faces tell us about major depression? Evidence from machine learning and fMRI. PLoS One. 2013;8(4):e60121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunetti M, Sepede G, Mingoia G, et al. Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: neural correlates of generalized emotional response. Neuroscience. 2010;168(3):670–679. [DOI] [PubMed] [Google Scholar]
- 11.Marusak HA, Zundel CG, Brown S, Rabinak CA, Thomason ME. Convergent behavioral and corticolimbic connectivity evidence of a negativity bias in children and adolescents. Soc Cogn Affect Neurosci. 2017;12(4):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. [DOI] [PubMed] [Google Scholar]
- 13.Legrand D. Pre-reflective self-as-subject from experiential and empirical perspectives. Conscious Cogn. 2007;16(3):583–599. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, Callahan CP, Moser JS. A mind full of self: Self-referential processing as a mechanism underlying the therapeutic effects of mindfulness training on internalizing disorders. Neurosci Biobehav Rev. 2018;92:172–186. [DOI] [PubMed] [Google Scholar]
- 15.Northoff G. Psychopathology and pathophysiology of the self in depression—neuropsychiatric hypothesis. J Affect Disord. 2007;104(1–3):1–14. [DOI] [PubMed] [Google Scholar]
- 16.Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;(OCT). doi: 10.3389/fnhum.2013.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon HJ, Seo EH, Kim JJ, Choo ILH. Neural correlates of self-referential processing and their clinical implications in social anxiety disorder. Clinical Psychopharmacology and Neuroscience. 2019;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mennin DS, Fresco DM. What, me worry and ruminate about DSM-5 and RDoC? The importance of targeting negative self-referential processing. Published online 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracy A, Jopling E, LeMoult J. The effect of self-referential processing on anxiety in response to naturalistic and laboratory stressors. Cogn Emot. 2021;35(7):1320–1333. [DOI] [PubMed] [Google Scholar]
- 20.LeMoult J, Kircanski K, Prasad G, Gotlib IH. Negative self-referential processing predicts the recurrence of major depressive episodes. Clinical Psychological Science. 2017;5(1):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips WJ, Hine DW, Thorsteinsson EB. Implicit cognition and depression: A meta-analysis. Clin Psychol Rev. 2010;30(6):691–709. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169(7):693–703. [DOI] [PubMed] [Google Scholar]
- 23.Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68(5). doi: 10.1016/j.biopsych.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 24.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional Neuroimaging Studies of Human Emotions. CNS Spectr. 2004;9(4). doi: 10.1017/S1092852900009196 [DOI] [PubMed] [Google Scholar]
- 25.Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: An fMRI study. Neuroreport. 2014;25(8). doi: 10.1097/WNR.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37(2):152–163. [DOI] [PubMed] [Google Scholar]
- 27.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral cortex. 2014;24(11):2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10). doi: 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, Phan KL. Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depress Anxiety. 2017;34(7). doi: 10.1002/da.22631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8. [DOI] [PubMed] [Google Scholar]
- 31.Raichle ME. The brain’s default mode network Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38(Jul). [DOI] [PubMed] [Google Scholar]
- 32.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 33.Gentili C, Ricciardi E, Gobbini MI, et al. Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79(6):409–413. [DOI] [PubMed] [Google Scholar]
- 34.Zhao XH, Wang PJ, Li CB, et al. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63(3):373–378. [DOI] [PubMed] [Google Scholar]
- 35.Petrowski K, Wintermann G, Smolka MN, Huebner T, Donix M. The neural representation of emotionally neutral faces and places in patients with panic disorder with agoraphobia. J Affect Disord. 2014;152:454–461. [DOI] [PubMed] [Google Scholar]
- 36.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences. 2009;106(6):1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimm S, Boesiger P, Beck J, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4). doi: 10.1038/npp.2008.81 [DOI] [PubMed] [Google Scholar]
- 38.Kross E, Davidson M, Weber J, Ochsner K. Coping with Emotions Past: The Neural Bases of Regulating Affect Associated with Negative Autobiographical Memories. Biol Psychiatry. 2009;65(5). doi: 10.1016/j.biopsych.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Infantolino ZP, Luking KR, Sauder CL, Curtin JJ, Hajcak G. Robust is not necessarily reliable: From within-subjects fMRI contrasts to between-subjects comparisons. Neuroimage. 2018;173. doi: 10.1016/j.neuroimage.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorka SM, Young CB, Klumpp H, et al. Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: a randomized trial. Neuropsychopharmacology. 2019;44(9):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basha E, Kaya M. Depression, Anxiety and Stress Scale (DASS): The Study of Validity and Reliability. Universal Journal of Educational Research. 2016;4(12). doi: 10.13189/ujer.2016.041202 [DOI] [Google Scholar]
- 42.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. 1997;1(39–58):3. [Google Scholar]
- 43.Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. Published online 1996. [Google Scholar]
- 44.Beck AT, Epstein N, Brown G, Steer R. Beck anxiety inventory. J Consult Clin Psychol. Published online 1993. [DOI] [PubMed] [Google Scholar]
- 45.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1). doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 46.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. Published online 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor PA, Chen G, Cox RW, Saad ZS. Open Environment for Multimodal Interactive Connectivity Visualization and Analysis. Brain Connect. 2016;6(2). doi: 10.1089/brain.2015.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3). doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 49.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017;7(3). doi: 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins LM, Kendall AD, Kassel MT, et al. Considering sex differences clarifies the effects of depression on facial emotion processing during fMRI. J Affect Disord. 2018;225. doi: 10.1016/j.jad.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delaveau P, Jabourian M, Lemogne C, et al. Antidepressant short-term and long-term brain effects during self-referential processing in major depression. Psychiatry Res Neuroimaging. 2016;247:17–24. [DOI] [PubMed] [Google Scholar]
- 52.Schreiner MW, Klimes-Dougan B, Cullen KR. Neural Correlates of Suicidality in Adolescents with Major Depression: Resting-State Functional Connectivity of the Precuneus and Posterior Cingulate Cortex. Suicide Life Threat Behav. 2019;49(3). doi: 10.1111/sltb.12471 [DOI] [PubMed] [Google Scholar]
- 53.Jones R, Bhattacharya J. A role for the precuneus in thought-action fusion: Evidence from participants with significant obsessive-compulsive symptoms. Neuroimage Clin. 2014;4. doi: 10.1016/j.nicl.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiggins JL, Brotman MA, Adleman NE, et al. Neural Markers in Pediatric Bipolar Disorder and Familial Risk for Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(1). doi: 10.1016/j.jaac.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nimarko AF, Garrett AS, Carlson GA, Singh MK. Neural correlates of emotion processing predict resilience in youth at familial risk for mood disorders. Dev Psychopathol. 2019;31(3). doi: 10.1017/S0954579419000579 [DOI] [PubMed] [Google Scholar]
- 56.Olatunji BO, Naragon-Gainey K, Wolitzky-Taylor KB. Specificity of rumination in anxiety and depression: A multimodal meta-analysis. Clinical Psychology: Science and Practice. 2013;20(3):225. [Google Scholar]
- 57.Kikvidze Z, Moya-Laraño J. Unexpected failures of recommended tests in basic statistical analyses of ecological data. Web Ecol. 2008;8. doi: 10.5194/we-8-67-2008 [DOI] [Google Scholar]
- 58.Barlow DH. Clinical handbook of psychological disorders: A step-by-step treatment manual. Published online 2021. [Google Scholar]
- 59.Rubin-Falcone H, Weber J, Kishon R, et al. Neural predictors and effects of cognitive behavioral therapy for depression: the role of emotional reactivity and regulation. Psychol Med. 2020;50(1):146–160. [DOI] [PubMed] [Google Scholar]