Abstract
Introduction:
Due to low pediatric HIV prevalence, more tests are needed to find one HIV-positive child compared to adults. In Uganda, the number needed to test (NNT) to find one new HIV-positive child was 64 in outpatient departments and 31 through index testing. We aimed to develop and validate a pediatric (1.5 – 14 years) screening tool to optimize testing approaches.
Methods:
Phase 1 evaluated the performance of 10 screening questions in 14 OPDs using a variable selection algorithm to evaluate combinations of screening questions. Using logistic regression, we identified the number of screening questions with the best predictive accuracy using the receiver operation characteristic curve. Phase 2 validated the proposed tool in 15 OPDs and 7 orphan and vulnerable children programs. We estimated sensitivity, specificity, and NNT accounting for inter-cluster correlations.
Results:
3,482 children were enrolled. The optimal model included reported HIV-positive maternal status, or 2/5 symptoms (sickly in past 3 months, recurring skin problems, weight loss, not growing well, history of TB). The proposed tool had a sensitivity of 83.6% (95% CI: 68.1 – 92.4) and specificity of 62.5% (55.0 – 69.4). The tool was validated in a sample of 11,342 children; sensitivity 87.8% (80.9 – 92.5) and specificity 62.6% (54.8 – 69.7) across OPD and community sites. In OPD, sensitivity was 88.1% (80.8 – 92.8), specificity 69.0% (61.9 – 75.3). The NNT across settings was 43 (28 – 67), and 28 (20 – 38) for OPD.
Conclusions:
This HIV screening tool has a high sensitivity and reasonable specificity, increasing testing efficiency and yield for children and adolescents.
Keywords: Pediatric HIV, HIV testing, case finding, mother-to-child transmission, Uganda, screening tool
Introduction
There are an estimated 1.7 million children (<15 years) living with HIV (CLHIV) globally, with 160,000 new child infections each year [1]. According to recent estimates, only 54% of all HIV-positive children have been initiated on antiretroviral treatment [2]. Orphans and vulnerable children (OVC) in resource-limited settings are an important population that requires special support to ensure they access timely testing and treatment services [3].
Provider-initiated testing and counseling (PITC) has been an important strategy for HIV testing. Uganda adopted World Health Organization (WHO) recommendations for provider-initiated testing in 2005 and universal antiretroviral treatment (ART) for all children <15 years in 2013 [4]. However, implementation of these policies has not been consistent across facilities in the public health system. From October 2018 – September 2019, the number needed to test (NNT) to find one new HIV-positive child was 64 in outpatient departments (OPD) and 31 through index testing in Uganda (i.e. OPDs had to test 64 children to identify one new CLHIV, and index testing services had to test 31 children to identify one new CLHIV, using NNT as a measure of HIV testing efficiency). According to the 2019 Uganda Population-based HIV Impact Assessment (UPHIA), only 56.3% of children living with HIV were diagnosed [5]. More than half of the CLHIV (54.3%) were not receiving ART. Among children on ART, pediatric viral suppression (39.3%) was much lower than for adults (59.6%). Factors associated with failure of early identification of children and adolescents living with HIV include individual factors (children living without parents, limited financial resources, lack of awareness of HIV symptoms, limited perinatal care for HIV-positive women and HIV-exposed infants, stigma and fear) and health system factors (limited targeted testing in vulnerable populations such as children in orphanages and in immunization clinics) [6]. HIV risk screening tools have been proposed as one strategy in optimizing PITC coverage in facility settings to improve pediatric case identification and linkages to HIV treatment services [7].
Few studies have evaluated risk screening tools for pediatric and adolescent HIV case identification in facility or community settings. In Zimbabwe, an algorithm for identifying HIV-positive adolescents (10-18 years) in populations at high-risk of infection acquired through mother-to-child transmission was developed using factors highly correlated with HIV status [8]. An optimal five-question tool was developed using logistic regression models and a p-value cutoff to identify questions most predictive of HIV status and resulted in a sensitivity of 74.0%. Similarly, Bandason and others used factors associated with HIV (hospital admission, recurring skin problems, parent death, poor health in last three months) to develop a four-question screening tool [9]. When field-tested in facility settings in Zimbabwe among children/adolescents aged 6-15 years, this tool demonstrated a sensitivity of 80.4%. The four-item tool was then validated in a community setting in Zimbabwe among children and adolescents aged 8-17 years with a sensitivity of 56.3% [10]. Moucheraud and colleagues validated a six-item tool among children/adolescents aged 1-15 years in pediatric inpatient wards in Malawi [11]. Using a cut off score of one (i.e. one positive response to any of the six items), sensitivity was 84.4%. These validation studies provide some evidence for further exploration of the use of screening tools for pediatric HIV diagnosis in resource-limited settings. The aim of this study was to develop and validate a pediatric HIV risk screening tool for use in high-volume entry points across varying levels of health facilities and in community settings in Uganda.
Methods
This cross-sectional study was conducted in two phases – Phase 1 (July – November 2018) and Phase 2 (August – December 2019) to develop and validate a screening tool to improve HIV case identification of children/adolescents (1.5 – 14 years). Research assistants administered screening questions and facility staff tested all study participants for HIV according to the Uganda national HIV testing algorithm with the following serologic tests (initial test Determine™, confirmatory test STAT-PAK®, tie-breaker SD BIOLINE). Written informed consent from caregivers and written assent from children age ≥8 years was obtained before enrollment in the study. Children/adolescents were excluded from the study if they reported being HIV-positive, were non-emancipated minors age <17 years unaccompanied by a caregiver (unaccompanied adolescents 12 years and older, the age of consent for HIV testing in Uganda, that requested testing were referred for programmatic services, i.e. the standard of care), or had a medical condition requiring emergency attention.
Study facilities that identified ≥ 20 HIV-positive children/adolescents were selected purposively to maximize the targeted sample size [12]. They included a mix of hospitals, Health Center (HC) IV facilities (i.e. small hospitals serving multiple counties within a district able to admit adults and children, and provide surgical and lab services), and HC III facilities (i.e. sub-county level health facilities with outpatient clinic, maternity ward, and lab services). Community intervention points (CIPs) delivering OVC services were utilized to recruit eligible children and adolescents for study enrollment (Phase 2 only) to evaluate tool performance in a low-prevalence setting.
The Advarra Institutional Review Board in the U.S. and Makerere University School of Public Health (MUSPH) Higher Degrees, Research and Ethics Committee in Uganda approved this study. The study was registered with the Uganda National Council for Science and Technology (UNCST). The protocol was reviewed in accordance with the U.S. Centers for Disease Control and Prevention (CDC) human research protection procedures and determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
Phase 1: Screening Tool Development
All children/adolescents (1.5 – 19 years) receiving care at 14 selected facility OPD sites across eight sub-regions in Uganda were eligible for recruitment. Ten screening questions (Appendix 1) based on symptoms or characteristics associated with HIV were identified by the study team, in consultation with HIV testing experts, and administered to caregivers and children/adolescents. Emancipated minors and some adolescents who requested being screened without their caregiver present were administered screening tool questions directly.
We used a computer intensive variable selection algorithm, in which all possible combinations of screening questions, minus the mother’s HIV status, were compared using the log likelihood, Akaike Information Criteria (AIC) and Bayes Information Criteria (BIC), to select the best subset of questions [13]. Maternal HIV-positive status was excluded from the variable selection method since it was an established policy to test all children/adolescents < 15 years born to known HIV-positive mothers. We identified the best subset of screening questions for use if the reported maternal HIV status was unknown or negative based on the minimum AIC/BIC. From the selected best subset of questions, we created a score for each child based on the total number of questions with positive responses. Using logistic regression of the child’s HIV test result on the score, we identified the minimum number of screening questions k from the selected subset with the best predictive accuracy using the receiver operating characteristic (ROC) curve. We defined a two-step screening tool based on testing the child for HIV if the mother was known to be HIV-positive, or if the child had at least k positive responses to the screening questions.
Phase 2: Screening Tool Validation
The six-question tool (Appendix 2) was validated in a different sample of children/adolescents (1.5 – 14 years) receiving care in 15 high HIV prevalence OPD facilities in Central and Midwestern sub-regions and OVC services across 7 districts in Southwestern and Midwestern sub-regions of Uganda, and all children/adolescents receiving care were eligible for study recruitment.
Statistical Analysis
For descriptive purposes, we categorized study participants into three age groups: 1.5 - 4 years, 5 - 9 years and 10 - 14 years, and estimated the median age within the age group. Data on adolescents aged 15 – 19 years will be presented in a separate analysis on the performance of risk screening questions among younger adolescents (10-14 years old) versus older adolescents (15-19 years old) where the majority are vertically-infected versus horizontally-infected, respectively. We summarized baseline characteristics of the study participants using frequencies and proportions by age group. We calculated the frequencies of positive responses to the screening questions (Phase 1). For each screening tool model (Phase 1) and the validated tool (Phase 2), we estimated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). We estimated the number needed to test (NNT) in order to identify serologically one HIV-positive child when the screening tool is used, and in absence of the screening tool, as the inverse of the PPV, or yield. We estimated the confidence intervals (CIs) of the diagnostic measures accounting for potential health facility/community intra-cluster correlations using robust standard errors. All analyses were performed using programs diagt and gvselect in STATA version 16 (StatCorp, Texas, USA).
Results
Phase 1
A total of 5,607 participants were assessed for study eligibility, 5,327 were enrolled, and 3,482 were included in the final analysis (Figure 1). Table 1 summarizes the characteristics of the 3,482 children (1.5 – 14 years). The median age was five years with nearly half of the children, 1,631 (46.8%), under five years, and 52.5% were girls. About half (50.7%) the children were sick in the last three months, 30.0% had recurring skin problems and 20.9% had lost weight in the last three months. Maternal HIV status was reported as positive for 305 (8.7%) children, unknown for 1,289 (37.1%), and HIV-negative for 1,888 (54.2%) children. A total of 55 (1.6%) children tested HIV-positive. Table 2 shows the sensitivity, specificity, PPV and NPV for each screening question. The screening questions with the highest sensitivity were “sick in the last three months” (76.4%), “HIV positive maternal status” (56.4%), “recurring skin problems” (54.5%), and “weight loss in the last three months” (52.7%). The questions with the highest specificity were “not growing well” (93.8%), history of tuberculosis (TB) (93.5%), and “HIV positive maternal status” (92.0%).
Figure 1. CONSORT diagram of participant enrollment (Phase 1).
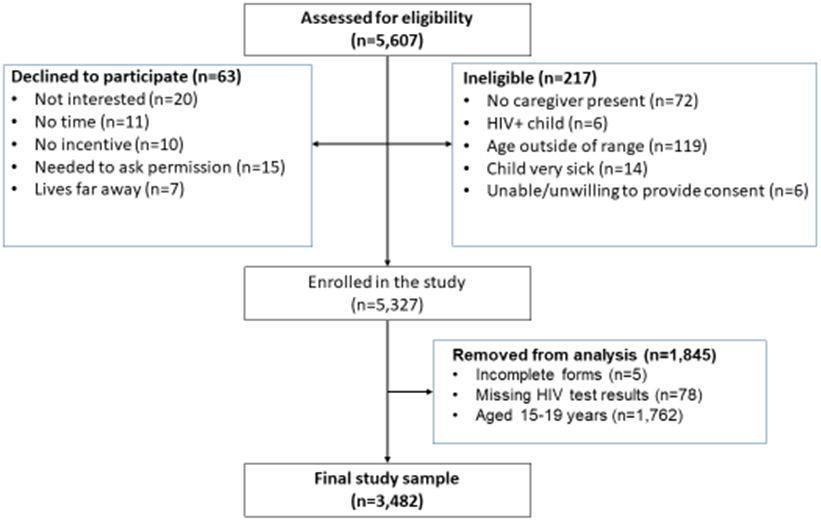
Table 1.
Study participants’ demographics, question responses and HIV status (Phase 1)
| Characteristics | Response | 1.5 – 4 yrs. | 5 – 9 yrs. | 10 – 14 yrs. | 1.5 – 14 yrs. |
|---|---|---|---|---|---|
| N (%) | 1631 (46.8) | 1205 (34.6) | 646 (18.6) | 3482 | |
| Median age (min-max years) | 2 (1.5 – 5) | 7 (5 – 9) | 12 (10 – 14) | 5 (1.5 – 14) | |
| Gender | |||||
| Male | 832 (51.0%) | 561 (46.6%) | 261 (40.4%) | 1654 (47.5%) | |
| Female | 799 (49.0%) | 644 (53.4%) | 385 (59.6%) | 1828 (52.5%) | |
| Screening Questions | |||||
| Has the child (have you) been admitted to a hospital in last 3 months | Yes | 280 (17.2%) | 173 (14.4%) | 81 (12.5%) | 534 (15.3%) |
| Has the child (have you) been sick in last 3 months | Yes | 828 (50.9%) | 622 (51.8%) | 309 (47.8%) | 1759 (50.7%) |
| Has the child (have you) had recurring skin problems | Yes | 510 (31.4%) | 372 (31.0%) | 157 (24.4%) | 1039 (30.0%) |
| Has one or both of the child’s (your) biological parents died | Yes | 105 (6.4%) | 139 (11.6%) | 117 (18.1%) | 361 (10.4%) |
| Has your child (have you) experienced difficulty in performing daily activities | Yes | 288 (17.7%) | 259 (21.5%) | 155 (24.0%) | 702 (20.2%) |
| Is the child (are you) growing well | No | 108 (6.6%) | 80 (6.6%) | 45 (7.0%) | 233 (6.7%) |
| Has the child (have you) lost weight in last 3 months | Yes | 335 (20.6%) | 260 (21.6%) | 133 (20.6%) | 728 (20.9%) |
| Has the child (have you) ever had tuberculosis | Yes | 90 (5.5%) | 99 (8.2%) | 53 (8.2%) | 242 (7.0%) |
| Has the child (have you) had discharge or sores in private parts | Yes | 172 (10.6%) | 152 (12.6%) | 105 (16.3%) | 429 (12.3%) |
| Is the mother of this child HIV positive | Yes | 178 (10.9%) | 80 (6.6%) | 47 (7.4%) | 305 (8.7%) |
| Unknown | 555 (34.0%) | 478 (39.7%) | 256 (40.2%) | 1289 (37.1%) | |
| HIV status (child) | |||||
| Positive | 27 (1.7%) | 18 (1.5%) | 10 (1.5%) | 55 (1.6%) | |
| Negative | 1604 (98.3%) | 1187 (98.5%) | 636 (98.5%) | 3427 (98.4%) |
Table 2.
Sensitivity, specificity, PPV and NPV of individual screening questions and proposed screening tool among children 1.5 - 14 years (Phase 1)
| Screening Questions |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|
| Has the child (have you) been admitted to a hospital in last 3 months | 32.7 (20.7 – 46.7) | 84.9 (83.7 – 86.1) | 3.4 (2.0 – 5.3) | 98.7 (98.3 – 99.1) |
| Has the child (have you) been sick in last 3 months | 76.4 (63.0 – 86.8) | 49.8 (48.1 – 51.4) | 2.4 (1.7 – 3.2) | 99.2 (98.7 – 99.6) |
| Has the child (have you) had recurring skin problems | 54.5 (40.6 – 68.0) | 70.4 (68.9 – 72.0) | 2.9 (2.0 – 4.1) | 99.0 (98.5 – 99.3) |
| Has one or both of the child’s (your) biological parent died | 20.0 (10.4 – 33.0) | 89.8 (88.7 – 90.8) | 3.1 (1.5 – 5.4) | 98.6 (98.1 – 99.0) |
| Has your child (have you) experienced difficulty in performing daily activities | 43.6 (30.3 – 57.7) | 80.2 (78.8 – 81.5) | 3.4 (2.2 – 5.0) | 98.9 (98.4 – 99.2) |
| Is the child (are you) growing well | 37.0 (24.3 – 51.3) | 93.8 (92.9 – 94.6) | 8.6 (5.3 – 12.9) | 99.0 (98.5 – 99.3) |
| Has the child (have you) lost weight in last 3 months | 52.7 (38.8 – 66.3) | 79.6 (78.2 – 80.9) | 4.0 (2.7 – 5.7) | 99.1 (98.6 – 99.4) |
| Has the child (have you) ever had tuberculosis | 33.3 (21.1 – 47.5) | 93.5 (92.6 – 94.3) | 7.4 (4.5 – 11.5) | 98.9 (98.5 – 99.2) |
| Has the child (have you) had discharge or sores in private parts | 21.8 (11.8 – 35.0) | 87.8 (86.7 – 88.9) | 2.8 (1.5 – 4.8) | 98.6 (98.1 – 99.0) |
| Is the mother of this child HIV positive | 56.4 (42.3 – 69.7) | 92.0 (91.0 – 92.9) | 10.2 (7.0 – 14.1) | 99.2 (98.9 – 99.5) |
| Screening tool | 83.6 (68.1 – 92.4) | 62.5 (55.0 – 69.4) | 3.4 (2.0 – 6.0) | 99.6 (99.2 – 99.8) |
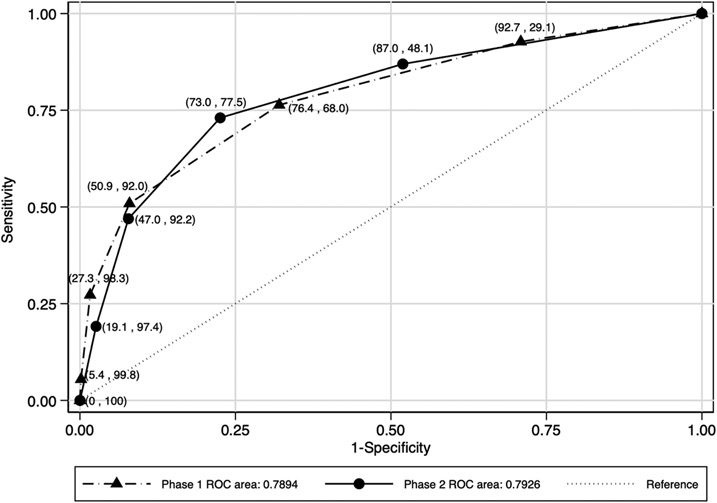
The variable selection method identified “history of child being sick in the last three months,” “recurring skin problems,” “not growing well,” “lost weight in the last three months,” and “ever had tuberculosis” as the best predictive subset of symptoms for HIV positivity. A screening tool based on the best subset of symptoms had an area under the ROC curve of 0.7894 (Figure 2). Having a score of ≥2 questions from the subset had the best predictive accuracy/performance with a sensitivity of 76.4% and specificity of 68.0%. To enhance the performance of the tool, we assumed a two-step screening process whereby children are first screened for “HIV-positive maternal status,” and if the mother is HIV-positive, the child is tested for HIV. If the maternal status is unknown or negative, and the child has a score of ≥2 from the subset of the five screening questions, then the child is tested for HIV. The proposed two-step HIV risk screening process improved the performance of the tool for a combined sensitivity of 83.6%, specificity of 62.5%, PPV of 3.4%, and NPV of 99.6% (Table 2). This tool missed 16.4% of HIV-positive children.
Figure 2. Performance of different number of positive responses to five screening questions*.
* Note: The symbols correspond to increasing numbers of affirmative responses to questions proceeding from the upper right to the bottom left
Phase 2
A total of 12,248 children were screened for eligibility, 11,459 were enrolled into the validation sample, and 11,342 were included in the final analysis (Figure 3). Table 3 summarizes the characteristics of the validation sample. Of the children enrolled, 8,243 (72.7%) were from OPD and 3,099 (27.3%) from OVC CIPs. The median age was six years, and 53.0% were females. Participants exhibited the characteristics noted in the screening questions in the following frequency: 36.4% were “sick in the last three months,” 20.5% “had recurring skin problems,” and 20.5% “lost weight in the last three months.” Maternal HIV status was reported as positive for 2,294 (20.2%) children, unknown for 1,804 (16.0%), and HIV-negative for 7,244 (63.8%) children. A child met the “positive” screening criteria if either the mother was reported to be HIV-positive or if the child reported any two of the five symptoms (i.e. following the 2-step process described in Phase 1). One hundred and fifteen (1.0%) children tested HIV-positive; six of the HIV-positive children were diagnosed in the community sites.
Figure 3: CONSORT diagram of participant (Phase 2).
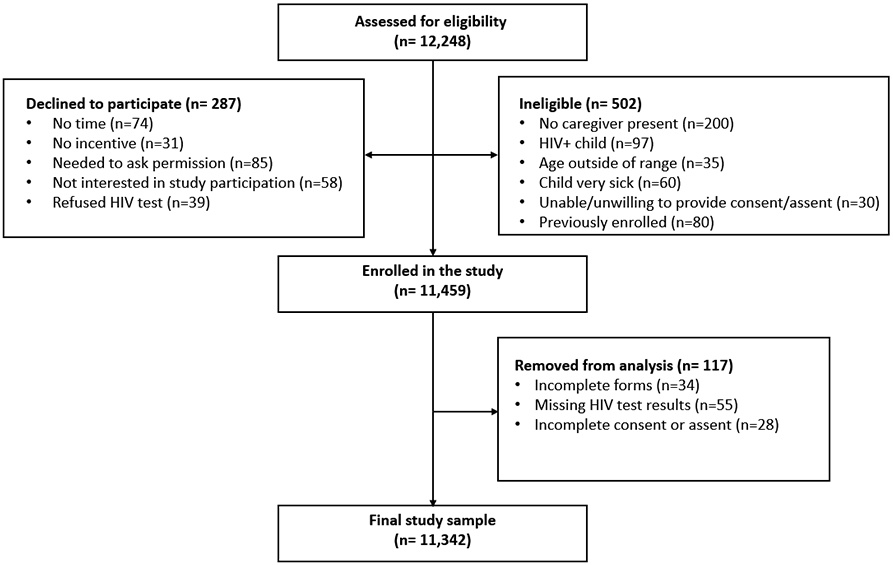
Table 3:
Study participants’ demographics, tool item responses and HIV status (Phase 2)
| Characteristics | Response | 1.5 – 4 yrs. | 5 – 9 yrs. | 10 – 14 yrs. | 1.5 – 14 yrs. |
|---|---|---|---|---|---|
| N (%) | 4252 (37.5) | 4217 (37.2) | 2873 (25.3) | 11342 (100.0) | |
| Study Sites: | |||||
| OVC | 734 (17.3%) | 1165 (27.6%) | 1200 (41.8%) | 3099 (27.3%) | |
| OPD | 3518 (82.7%) | 3052 72.4%) | 1673 (58.2%) | 8243 (72.7%) | |
| Median age (min-max years) | 3 (1.5 – 4) | 7 (5 – 9) | 12 (10 – 14) | 6 (1.5 – 14) | |
| Gender: | |||||
| Male | 2055 (48.3%) | 1976 (46.9%) | 1298 (45.2%) | 5329 (47.0%) | |
| Female | 2197 (51.7%) | 2241 (53.1%) | 1575 (54.8%) | 6013 (53.0%) | |
| HIV status: | |||||
| Positive | 40 (0.94%) | 40 (0.95%) | 35 (1.22%) | 115 (1.00%) | |
| Negative | 4211 (99.0%) | 4176 (99.0%) | 2837 (98.7%) | 11224 (99.0%) | |
| Indeterminate | 1 (0.02%) | 1 (0.02%) | 1 (0.03%) | 3 (0.00%) | |
| Has the child been sick in the last 3 months | Yes | 1652 (38.8%) | 1540 (36.5%) | 933 (32.5%) | 4125 (36.4%) |
| Has the child had recurring skin problems | Yes | 991 (23.3%) | 906 (21.5%) | 428 (14.9%) | 2325 (20.5%) |
| Is the child growing well | No | 350 (8.2%) | 377 (8.9%) | 226 (7.9%) | 953 (8.4%) |
| Has the child lost weight in last 3 months | Yes | 991 (23.3%) | 875 (20.8) | 457 (15.9%) | 2323 (20.5%) |
| Has the child ever had TB | Yes | 22 (0.5%) | 26 (0.6%) | 14 (0.5%) | 62 (0.6%) |
| Is the mother of this child HIV-positive | Yes | 782 (18.4) | 870 (20.6%) | 642 (22.4%) | 2294 (20.2%) |
| Unknown | 422 (9.9%) | 666 (15.8) | 716 (24.9%) | 1804 (16.0%) |
Across facility and community settings, the tool’s sensitivity was 87.8% (95% CI: 80.9 – 92.5), specificity 62.6% (95% CI: 54.8 – 69.7), PPV 2.4% (95% CI: 1.5 – 3.6) and NPV 99.8% (95% CI: 99.6 – 99.9) with an estimated NNT of 43 (95% CI: 28 – 67) (Table 4). Among children in facility OPDs, the sensitivity was 88.1% compared to 83.3% among OVCs in the community. Specificity among children in OPD was significantly higher compared to OVC in the community (69.0% vs. 45.6%). Similarly, PPV, or HIV testing yield, was significantly higher among children in the OPD (3.7%) compared to OVC in the community (0.3%). The NNT to identify one HIV-positive child was 28 among children in the OPD compared to 338 among OVC in the community, although there were only six HIV-positive children identified in community settings. The tool missed 12.2% of HIV-positive children across OPD and OVC settings, ranging from 11.9% in OPD to 16.7% in OVC.
Table 4:
Screening tool sensitivity, specificity, PPV, NPV and NNT (Phase 2)
| Validation sample |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
NNT (95% CI) |
|---|---|---|---|---|---|
| OPD and OVC | 87.8 (80.9 – 92.5) | 62.6 (54.8 – 69.7) | 2.4 (1.5 – 3.6) | 99.8 (99.6 – 99.9) | 43 (28 – 67) |
| OPD only | 88.1 (80.8 – 92.8) | 69.0 (61.9 – 75.3) | 3.7 (2.7 – 5.0) | 99.8 (99.6 – 99.9) | 28 (20 - 38) |
| OVC only | 83.3 (5.7 – 99.8) | 45.6 (32.6 – 59.2) | 0.30 (0.1 – 0.8) | 99.9 (99.2 – 100) | 338 (128 – 897) |
In OPD sites, while 2,083 (25.3%) children reported a maternal HIV status that was positive or unknown, 100 (91.7%) of the HIV-positive children were in these two subgroups (Table 5). HIV positivity was highest (7.2%) among children with a reported HIV-positive maternal status followed by 3.0% among children with a reported unknown maternal HIV status, compared to 0.2% among children with a reported HIV-negative maternal status. The tool indicated HIV testing for 2,617 children (31.7%) in OPD sites, decreasing HIV testing by 5,626 tests (68.3%). The screening tool identified 100% of CLHIV with a reported HIV-positive maternal status, 71.4% with a reported unknown maternal status, and 66.7% of CLHIV with a reported HIV-negative maternal status. The NNT to identify one new CLHIV where the maternal HIV status is reported as positive or unknown was 14-15.
Table 5:
Characteristics and tool performance by reported maternal HIV status (Phase 2, OPD sites)
| Characteristics | HIV-positive maternal status |
Unknown maternal HIV status |
HIV-negative maternal status |
OPD only study sample |
|---|---|---|---|---|
| N (%) | 907 (11.0) | 1176 (14.3) | 6160 (74.7) | 8243 |
| Child HIV-status: | ||||
| Positive | 65 (7.2) | 35 (3.0) | 9 (0.2) | 109 (1.3) |
| 1.5-4 years | 20 (2.2) | 17 (1.4) | 3 (0.1) | 40 (0.5) |
| 5-9 years | 27 (3.0) | 8 (0.7) | 3 (0.1) | 38 (0.5) |
| 10-14 years | 18 (2.0) | 10 (0.9) | 3 (0.1) | 31 (0.4) |
| Negative | 841 (92.8) | 1139 (97.0) | 6151 (99.8) | 8131 (98.7) |
| Indeterminate | 1 | 2 | 0 | 3 |
| Tests indicated by PATEST Tool | 907 (100.0) | 366 (31.1) | 1345 (21.8) | 2617 (31.7) |
| HIV-positive children identified by PATEST Tool | 65 (100.0) | 25 (71.4) | 6 (66.7) | 96 (88.1) |
| NNT (95% CI) | 14 (10 - 21) | 15 (8 – 28) | 225 (67 – 761) | 28 (20 - 37) |
Discussion
This two-step HIV risk screening algorithm is a robust tool with high sensitivity and specificity and high pediatric testing yield of 2.4% across OPD and OVC settings, and a yield of 3.7% considering OPD alone. In the context of a national pediatric HIV prevalence of 0.5%, the screening tool has a yield that is nearly seven times the national prevalence. The aggregate NNT to identify one HIV positive child is 43 across facility and community settings.
This tool directs HIV tests to children and adolescents more likely to be living with HIV, significantly improving the NNT to identify one HIV positive child from 64 under current testing algorithms to 43 across facility and community settings. In OPD, this tool reduced the NNT to 28. The HIV testing yield using the screening tool in OPD settings (3.7%) is higher than the yield obtained with pediatric index testing based on fiscal year 2019 PEPFAR program data in Uganda for different age groups: 1-4 years (3.5%), 5-9 years (3.4%), and 10-14 years (2.7%) [14]. With 36 fewer tests required to diagnose one child living with HIV, targeted screening in high-volume entry points could result in significant cost savings.
In reducing the number of children needing to be tested, a screening tool could enhance the PITC approach for children with an undocumented HIV status. Typically, PITC programs have focused on replicating HIV testing strategies used for adults rather than designing interventions appropriate to the specific needs of children and adolescents [15]. Innovative strategies are emerging such as combination interventions using screening tools in lower level health facilities to increase equitable access to HIV testing for children, adolescents and previously unreached groups, and accelerate the rate of HIV case identification [16]. In 2019, 66% of HIV tests conducted in children (<15) and 44% of CLHIV identified across 16 PEPFAR-supported African countries were through PITC modalities (NNT 103), including OPDs, well-child under-five clinics and voluntary medical male circumcision settings [17]. Screening tools facilitate the identification of CLHIV already presenting to the OPD and use fewer tests. Future studies could investigate the effect of screening tools on the rate of HIV case identification and the number of children and adolescents initiated on HIV treatment.
This tool could supplement existing testing guidelines for providing an HIV test for all children with HIV-positive mothers by including additional symptom-related indicators for children with a reported HIV maternal status that is negative or unknown. Using a tiered approach by first assessing for HIV-positive maternal status, which will identify 56% of CLHIV, and by screening children when the maternal status is unknown or negative, we can identify more HIV positive children while reducing unnecessary testing by 68%. With the global pivot toward prioritizing funding for index testing, the performance of this screening tool demonstrates the need to optimize targeted HIV testing in OPD settings [18]. The number of children with reported unknown maternal HIV status, 1,804, of which 35 were HIV-positive, translates to an HIV prevalence (1.9%) four times higher than the general population, and indicates an acute need for HIV screening in this population.
While this HIV risk screening tool has the potential to contribute to more efficient HIV case finding strategies among pediatric populations, even the highest performing tools will fail to identify all HIV-positive children, including those who do not yet present with symptoms. With a sensitivity of 88%, this screening tool will miss 12% of children living with HIV. Missing 1 in 10 HIV-positive children is significant. While missed children may still be healthy, not yet presenting with symptoms of HIV infection, the consequence of missing these children is delayed treatment initiation. It is difficult to estimate the current rate of undertesting in outpatient settings; however, an estimated 34% of children living with HIV in Uganda are not receiving ART, and universal testing is not practiced in OPD settings [19, 20]. A combination of strategies is warranted (i.e. scaling-up coverage of index testing and HIV testing in for all children presenting to inpatient, malnutrition, TB and sexually transmitted infection (STI) entry points) to help ensure no CLHIV are missed and to expedite the rate of pediatric HIV case identification through routine HIV testing eligibility screening in OPD settings. Country programs adopting targeted screening could combine this approach with additional strategies to ensure children are not missed and UNAIDS 95-95-95 targets are achieved by 2030 [21]. For example, promising strategies for identifying children in the 2-14 year age band, particularly those who age out of under-five focused care, include those that have adolescent siblings receiving antiretroviral drugs and deceased clients with known HIV infection as the index clients, hybrid community/home-based testing, and integrated testing strategies [22, 23].
A strength of the study is the large sample sizes used for developing and validating the tool, giving smaller confidence intervals and more precise estimates of sensitivity and specificity, PPV, NPV and NNT. In this study, the optimal tool required positive responses to ≥ 2 HIV-related symptoms, providing higher sensitivity (88.1%) and specificity (69.0%) than other facility-based screening tools [24]. Compared to the seven screening algorithms used to identify children for HIV testing from a systematic review [24], this tool was more sensitive than four of seven tools and all three tools with a higher sensitivity had a specificity below 40.0%. A four-question tool, including the same or similar four questions tested in Phase 1, was validated in facility settings in Zimbabwe. The cut off was ≥ 1 positive response to any HIV-related symptom, and it had a sensitivity of 80.4% and 66.3% specificity [9]. When the same four questions were tested in a community setting, using one positive response as a cut off, the tool was less reliable with a sensitivity of 56.3% and 75.1% specificity [10]. Two of the Bandason questions, “sick in the last three months” and “recurring skin problems” performed well and were included in the final Uganda HIV risk screening tool. In addition, this tool was validated in community and facility settings and across a range of facility levels from hospitals down to outpatient health centers at the sub-county level and community intervention points delivering OVC services, extending the use of the tool by health care workers across different settings in the health system.
Clemens et al. identified seven tools implemented across outpatient and inpatient settings [24]. The questions from the validated screening tool in Uganda were included on other algorithms in the following frequency: “reported HIV-positive maternal status” was included in zero of seven tools, “history of child being sick in the last three months” in three of seven, “recurring skin problems” in three of seven, “not growing well” in two of seven, “lost weight in the last three months” in two of seven, and “ever had tuberculosis” in one of seven. While reported HIV-positive maternal status had the second highest sensitivity and specificity in this study, clients may not always feel comfortable disclosing HIV status depending levels of stigma, among other factors. Implementation of similar tools has been a challenge in other countries. For example, in South Africa, an HIV algorithm including six HIV-related symptom questions, used as part of Integrated Management of Childhood Illness (IMCI) program was evaluated in pediatric clinics. The tool correctly identified 90.8% of HIV-positive children as HIV-exposed or suspected HIV, but IMCI providers did not identify any HIV-suspected or HIV-exposed cases using the tool in 66.2% of cases. The limited number of diagnoses were attributed to health workers not systematically implementing the tool for multiple reasons including limited supportive supervision of IMCI trained health workers [25]. More research is needed to understand factors that improve the implementation and utilization of screening tools by frontline healthcare workers.
This study has limitations, especially related to the use of the tool in community settings. A limited number of HIV-positive children (six) were identified in community sites and there is large uncertainty surrounding the performance of this tool in community settings serving OVCs. The HIV yield in the OVC community study participants was 0.2%, and thus, screening items designed for a community setting need to be identified to further advance the performance of screening tools in these settings. Additional testing and validation of this screening tool for community-based settings is warranted. All study data were collected August – December, and thus, screening items were not tested for any differences in performance due to seasonal variation.
Conclusion
A multi-strategy approach where countries continue to invest resources in rapid test kits for OPD settings, while scaling up index testing for eligible children/adolescents could improve pediatric case identification and linkages to care. Screening tools are an important way to target OPD testing in high-volume entry points and increase the HIV testing yield for children and adolescents living with HIV. Maternal HIV status is a key first step to determining the need for HIV testing, and all children presenting to OPDs should be screened for HIV-positive maternal status and tested accordingly. Optimized targeted screening in OPDs with HIV risk screening tools has the potential to generate the same yield as index case testing in children (1.5 – 14 years). Use of such a screening tool has the potential to expedite the rate of HIV case identification in OPD, and should be balanced with the use and coverage of other HIV testing strategies to increase the possibility of diagnosis for all children living with HIV.
Supplementary Material
Appendix 1. PATEST eligibility screening tool (Phase 1)
Appendix 2. PATEST eligibility screening tool (Phase 2)
Acknowledgements
We wish to acknowledge the contribution of the study participants in facility and community settings in all districts where we carried out this study. We would also like to thank the study team, data collectors, and data entry staff for their hard work and dedication, and for the support of the district health offices, implementing partners and the communities they serve, and the Ministry of Health and Ministry of Gender, Labor and Social Development teams. We would like to acknowledge Rashida Ferrand, London School of Hygiene and Tropical Medicine, and Madina Apolot, CDC Uganda, for their technical guidance and support with study design, and Steven Gutreuter and Megumi Itoh, U.S. Centers for Disease Control and Prevention, Division of Global HIV and TB, for his biostatistical and analytic support with the methods and her technical guidance and study supervision, respectively. The Faith-Based Action for Scaling Up Testing and Treatment for Epidemic Response (FASTER) initiative supported dissemination of this study. FASTER aims to mobilize government, civil society and faith-based organizations to achieve targets for finding, linking and retaining children and adolescents in HIV care. Through funding from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention, Catholic Relief Services with Clinton Health Access Initiative is catalyzing the HIV response for children and youth in Nigeria, Tanzania, Uganda, Zambia and Zimbabwe, including supporting the national roll out of this risk screening tool in collaboration with the Uganda MOH.
Funding
This study was supported by PEPFAR through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement #NU2GGH000985 with the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF). This submission was supported by the by PEPFAR through the CDC under the terms of Cooperative Agreement # NU2GGH001463 with Catholic Relief Services.
List of abbreviations
- ART
Antiretroviral Treatment
- CDC
U.S. Centers for Disease Control and Prevention
- CI
Confidence Interval
- CIP
Community Intervention Point
- CLHIV
Children Living with HIV
- DHIS
District Health Information System
- EGPAF
Elizabeth Glaser Pediatric AIDS Foundation
- HC
Health Center
- HIV
Human Immunodeficiency Virus
- IMCI
Integrated Management of Childhood Illnesses
- MUSPH
Makerere School of Public Health
- NNT
Number Needed to Test to Identify One HIV-positive Person
- NPV
Negative Predictive Value
- OPD
Outpatient Department
- OVC
Orphans and Vulnerable Children
- PEPFAR
U.S. President’s Emergency Plan for AIDS Relief
- PITC
Provider-initiated Testing and Counseling
- PMTCT
Prevention of Mother-to-Child Transmission
- PPV
Positive Predictive Value
- ROC
Receiver Operation Characteristics
- STI
Sexually Transmitted Infection
- TB
Tuberculosis
- UNAIDS
Joint United Nations Programme on HIV and AIDS
- UNCST
Uganda National Council for Science and Technology
- UPHIA
Uganda Population-based HIV Impact Assessment
- WHO
World Health Organization
Footnotes
Competing interests
The authors declare that there are no competing interests.
Disclaimer
The findings and conclusions are those of the author(s) and do not necessarily represent the official position of the funding agencies.
References
- 1.Joint United Nations Program on HIV and AIDS (UNAIDS). Fact Sheet for World AIDS Day 2019. Geneva, Switzerland; 2019. Available from: https://www.unaids.org/en/resources/fact-sheet. Accessed March 20, 2020. [Google Scholar]
- 2.UNAIDS. Start Free Stay Free AIDS Free: 2019 report [Internet]. Geneva; UNAIDS; 2019. [cited 2020 Feb 5]. Available from https://www.unaids.org/en/resources/documents/2019/20190722_UNAIDS_SFSFAF_2019. Accessed March 20, 2020. [Google Scholar]
- 3.Thurman TR, Luckett B, Taylor T, Carnay M. Promoting uptake of child HIV testing: an evaluation of the role of a home visiting program for orphans and vulnerable children in South Africa. AIDS Care. 2016;28(2):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Republic of Uganda, Ministry of Health, National HIV Testing Services Policy and Implementation Guidelines, October 2016.
- 5.Ministry of Health Uganda Population-based HIV Impact Assessment (UPHIA) 2016-2017: Final Report. Kampala: Ministry of Health; July 2019. [Google Scholar]
- 6.Boender TS, Sigaloff KDE, Kayiwa J, Mussime V, Calis JCJ, Hamers RL, et al. Barriers to initiation of pediatric HIV treatment in UGdna: a mixed methods study. AIDS Res Treat. 2012;2012(817506). doi: 10.1155/2012/817506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medley A, Hrapcak S, Golin RA, Dziuban EJ, Watts H, Siberry GK, et al. Strategies for identifying and linking HIV-infected infants, children and adolescents to HIV treatment services in resource limited settings. J Acquir Immun Defic Syndr. 2018;78:S98–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrand RA, Weiss HA, Nathoo K, Ndhlovu CE, Mungofa S, Munyati S, et al. A primary care level algorithm for identifying HIV-infected adolescents in populations at high risk through mother-to-child transmission. Trop Med Int Health. 2011;16(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandason T, McHugh G, Dauya E, Mungofa S, Munyati SM, Weiss HA, et al. Validation of a screening tool to identify older children living with HIV in primary care facilities in high HIV prevalence settings. AIDS. 2016;30(5):779–85. doi: 10.1097/QAD.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandason T, Dauya E, Dakshina S, McHugh G, Chonzi P, Munyati S, et al. Screening tool to identify adolescents living with HIV in a community setting in Zimbabwe: A validation study. PLoS One. 2018;13(10):e0204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moucheraud C, Chasweka D, Nyirenda M, Schooley A, Dovel K, Hoffman RM. Simple screening tool to help identify high-risk children for targeted HIV testing in Malawian inpatient wards. J Acquir Immune Defic Syndr. 2018;79(3):352–57. [DOI] [PubMed] [Google Scholar]
- 12.PEPFAR FY 2014/15; Monitoring, Evaluation, and Reporting (MER) Indicators. HCT yield results. Unpublished data April 2015. [Google Scholar]
- 13.Hosmer DW, Jovanovic B, Lemeshow S. Best subsets logistic regression. Biometrics. 1989;45: 1265–70. [Google Scholar]
- 14.Wolf HT, Battey KA, Patel M, Mujawar M, Bhatkoti R, Rivadeneira ED, et al. Improving pediatric index testing: data from 12 PEPFAR supported countries in sub-Saharan Africa. International AIDS Society Conference, AIDS 2020, Oral abstract OAB0703. [Google Scholar]
- 15.Wong VJ, Murray KR, Phelps BR, Vermund SH, McCarraher DR. Adolescents, young people, and the 90–90–90 goals: a call to improve HIV testing and linkage to treatment. AIDS. 2017;31 (Suppl 3):S191–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kose J, Tiam A, Ochuka B, Okoth E, Sunguti J, Waweru M, et al. Impact of a comprehensive adolescent-focused case finding intervention on uptake of HIV testing and linkage to care among adolescents in western Kenya. Implementation Science. 2018;79(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross J, Patel M, Battey K, Srivastava M, Grillo M, Wolf H, et al. Considerations to improve pediatric HIV case finding and close the treatment gap in 16 African countries. Association of Nurses in AIDS Care (ANAC) Conference, 2020: poster. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. President’s Emergency Plan for AIDS Relief. PEPFAR 2020 Country Operational Plan Guidance for all PEPFAR Countries. Washington, DC: PEPFAR, 2020. Available from: https://www.state.gov/wp-content/uploads/2020/01/COP20-Guidance.pdf [Google Scholar]
- 19.Joint United Nations Program on HIV and AIDS (UNAIDS). Estimates 2019. Available from: http://aidsinfo.unaids.org/. Accessed March 26, 2020.
- 20.World Health Organization. Consolidated Guidelines on HIV Testing Services for a Changing Epidemic. Geneva: WHO. 2019. Available from: https://www.who.int/publications-detail/consolidated-guidelines-on-hiv-testing-services-for-a-changing-epidemic. Accessed March 10, 2020. [Google Scholar]
- 21.Joint United Nations Program on HIV and AIDS (UNAIDS). Fast-Track. Ending the AIDS Epidemic by 2030. Geneva: UNAIDS; 2014. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed March 20, 2020. [Google Scholar]
- 22.Ayieko J, Chamie G, Balzer L, Kwarisiima D, Kabami J, Sang N, et al. Mobile, population-wide, hybrid HIV testing strategy increases number of children tested in rural Kenya and Uganda. Pediatr Infect Dis J. 2018;37(12):1279–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolters T. Innovative pilots to improve diagnosis of HIV-infected children and adolescents aged 5-14 years. Presentation at the International AIDS Society Conference; Amsterdam, 2018. [Google Scholar]
- 24.Clemens S, Macneal K, Alons C and Cohn J. Screening algorithms to reduce burden of pediatric HIV testing: A systematic review and meta-analysis. Pediatr Infect Dis J. 2020; 39(10): e303–e309. doi: 10.1097/INF.0000000000002715. [DOI] [PubMed] [Google Scholar]
- 25.Horwood C, Vermaak K, Rollins N, Haskins L, Nkosi P, and Qazi S. Pediatric HJIV management at primary care level: an evaluaton of the integrated management of childhood illnesses (IMCI) guidelines for HIV. BMC Pediatrics. 2009;9(59). doi: 10.1186/1471-2431-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. PATEST eligibility screening tool (Phase 1)
Appendix 2. PATEST eligibility screening tool (Phase 2)