Abstract
Human cytomegalovirus (HCMV) infection is well controlled mainly by cytotoxic CD8+ T lymphocytes (CTL) directed against the matrix protein pp65 despite the numerous immune escape mechanisms developed by the virus. Dendritic cells (DCs) are key antigen-presenting cells for the generation of an immune response which have the capacity to acquire antigens via endocytosis of apoptotic cells and thus present peptides to major histocompatibility complex class I-restricted T cells. We examined whether this mechanism could contribute to the activation of anti-pp65 CTL. In this study, we show that infection by HCMV AD169 induced sensitization of MRC5 fibroblasts to tumor necrosis factor alpha-mediated apoptosis very early after virus inoculation and that pp65 contained in apoptotic cells came from the delivery of the matrix protein into the cell. We observed that immature DCs derived from peripheral monocytes were not permissive to HCMV AD169 infection but were able to internalize pp65-positive apoptotic infected MRC5 cells. We then demonstrated that following exposure to these apoptotic bodies, DCs could activate HLA-A2- or HLA-B35-restricted anti-pp65 CTL, suggesting that they acquired and processed properly fibroblast-derived pp65. Together, our data suggest that cross-presentation of incoming pp65 contained in apoptotic cells may provide a quick and efficient way to prime anti-HCMV CD8+ T cells.
CD8+ T-cell response is a major effector mechanism to contain viral infections. The importance of dendritic cells (DCs) for the initiation of CD8+ T-cell responses against viruses has been recently emphasized by the description of molecular mechanisms involved in naive T-cell stimulation. It has been suggested that CD8+ T-cell expansion could first require CD4+ T-cell help through CD40 ligation on DCs and that this step can be bypassed by direct infection of DCs by a virus (17). Nevertheless, this finding did not fully explain how antiviral CD8+ T cells could be generated in cases where DCs were not susceptible to infection. DCs function as sentinels of the immune system and are characterized by their ability to activate T cells through unusual pathways of antigen capture (2). Since DCs can deliver exogenous antigens in either the soluble or particulate form into the major histocompatibility complex (MHC) class I pathway, it is conceivable that antiviral cytotoxic T-lymphocyte (CTL) priming might be achieved in this way in cases of infection by cytopathic viruses. Moreover, DCs are able to acquire antigens through phagocytosis of infected apoptotic cells and process them into the MHC class I pathway for presentation to CD8+ T cells (1). Although there is evidence that DCs are susceptible to infection by viruses such as influenza virus, human immunodeficiency virus, and measles virus (1, 12), the extent to which each antigen capture mechanism contributes to the activation of virus-specific immune response is not fully known. Nevertheless, one may suggest that the cross-presentation mechanism has direct implications in expansion of CD8+ T cells targeted against noncytopathic viruses which do not infect DCs. We then asked how this could take part in activation of CD8+ T cells against human cytomegalovirus (HCMV).
Infection by HCMV, a latent betaherpesvirus, is well controlled by T cells mainly of the CD8+ subset, whose major target is the matrix protein pp65 (for reviews, see references 3 and 15). Indeed, very high frequencies of anti-pp65 CD8+ T-cell precursors have been found in healthy blood donors compared to other viral proteins (24). Whereas it has been shown that HCMV is latent in DC progenitors (11), it seems that susceptibility of either immature or mature DCs to infection is not well established and may vary according to both cell subset and HCMV strain (18, 21). It was recently demonstrated that HCMV infection could either induce or inhibit apoptosis mediated by Fas, tumor necrosis factor alpha (TNF-α) receptor 1 (TNF-R1), and TRAIL (TNF-related apoptosis-inducing ligand) receptor in vitro (4, 9, 20), and that apoptosis of infected cells in Fas and TNF-R1 knockout mice played a pivotal role in the clearance of murine CMV in vivo (7). In this study, we examined whether cross-presentation of apoptotic cells by DCs could be involved in activation of anti-HCMV-specific CD8+ T cells. We showed that TNF-α could induce apoptosis of HCMV AD169-infected MRC5 fibroblasts very early after infection, providing a suitable source of antigen for phagocytosis by immature DCs. We then derived immature DCs from peripheral blood mononuclear cells (PBMC) and demonstrated that they did not synthesize HCMV immediate-early (IE) antigens even after prolonged incubation with the virus. Finally, we showed that HLA-A2- and HLA-B35-restricted anti-pp65 CD8+ CTL were stimulated following exposure of DCs to HCMV-infected apoptotic fibroblasts, suggesting that DCs acquired and processed properly the matrix protein pp65 through phagocytosis of apoptotic bodies. Thus, seeing that pp65 was internalized by fibroblasts immediately after viral input without de novo synthesis, processing of this incoming antigen by DCs may provide a quick and efficient way to prime anti-HCMV CTL before viral replication.
MATERIALS AND METHODS
Virus and cells.
HCMV AD169 was propagated in MRC5 human fibroblasts (BioMérieux, Marcy l'Etoile, France). Virus was collected when cytopathic effects were >90%. Supernatants were clarified by centrifugation at 1,500 × g for 10 min at 4°C and were stored at −80°C until use. Virus titer was determined by PFU titration in human foreskin fibroblasts (American Type Culture Collection) according to standard procedures. U373MG (A0) astrocytoma cells were a gift from S. Michelson (Institut Pasteur, Paris, France). Both MRC5 and U373MG cells were phenotyped HLA-A2 by the Laboratoire Central d'Immunologie-Rangueil, Pr. Ohayon, Toulouse, France). Infections with HCMV were performed at a multiplicity of infection (MOI) of 3 unless otherwise stated.
Generation of DCs.
DCs were prepared from PBMC using a VacCell processor as described by Goxe et al. (10). Briefly, PBMC obtained from leukapheresis were cultured for 7 days in hydrophobic bags (Stedim, Marseille, France) in AIMV serum-free medium (Life Technologies, Cergy Pontoise, France) supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; 500 U/ml; provided by Novartis, Ruel Malmaison, France) and interleukin-13 (IL-13; 50 ng/ml; provided by Sanofi-Synthelabo, Labege, France). Fresh IL-13 was added again after 4 days of culture. DCs were isolated on day 7 by elutriation. DC purity was around 90%, and viability was >95%.
Immunophenotypic analysis of DCs.
Flow cytometry analysis was performed using a FACSCalibur (Becton Dickinson, San Jose, Calif.) by gating the cell population of interest on forward scatter/side scatter. Antibodies used were fluorescein isothiocyanate (FITC)-conjugated anti-HLA-ABC (B9.12.1), anti-HLA-DR (B.8.12), anti-CD14 (MoP9), anti-CD64 (22), anti-CD80 (MAB104), and anti-CD83 (HB15A), and phycoerythrin-conjugated anti-CD40 (MAB89), anti-CD86 (HA5-B2B7), anti-CD1a (BL6), and anti-CD11c (B-ly6). All antibodies were purchased from Coulter-Immunotech (Marseille, France) except for CD14 and CD11c, which were provided by Becton Dickinson and Pharmingen, respectively. For staining, cells were washed and resuspended in phosphate-buffered saline (PBS) with 1% fetal calf serum (FCS) containing conjugated antibody. After 30 min on ice, cells were washed and resuspended in PBS–1% FCS. TOPRO-3 (Molecular Probes, Eugene, Oreg.) was added to exclude dead cells from analysis.
Assays for detection of apoptosis.
Cells were cultured in either 24- or 6-well culture plates in RPMI 1640 (RPMI medium; Gibco, Cergy Pontoise, France) containing 10% FCS and supplemented with Glutamax-I, sodium pyruvate, and antibiotics including antimycoplasma OFLOCET (Roussel, Paris, France) unless otherwise stated. MRC5 cells were either mock infected or infected with HCMV AD169 for 6 h. Recombinant TNF-α (1,000 U/ml; R&D Systems, Abington, United Kingdom) was added to the cells and left for 8 h in either the presence or absence of cycloheximide (CHX; 25 μg/ml; Sigma, St. Quentin Fallavier, France). After incubation at 37°C in the conditions specified above, cells were washed with PBS and detached with trypsin. The presence of apoptotic cells was detected by multiparameter flow cytometry (Coulter Epics) using propidium iodide (PI) and FITC-conjugated annexin V (AV; Coulter-Immunotech) according to the manufacturer's instructions.
For Hoechst assay, cells were treated as described above except that adherent cells were grown for 24 h on slides in RPMI medium–10% FCS, labeled with Hoechst 33342 (10 μg/ml in PBS; Sigma) for 30 min at 37°C, and then fixed with 1% paraformaldehyde. Hoechst-stained condensed nuclei were counted, and results were expressed according to the following formula: % specific apoptosis = (% of condensed nuclei from treated cells − % of condensed nuclei from untreated cells/100 − % of untreated cells) × 100. The data shown are means of four independent experiments.
Western blotting.
Cells were pelleted, washed with PBS, and sonicated in water. Total proteins were quantified using a MicroBCA assay (Bio-Rad), and samples were boiled for 5 min in 5% β-mercaptoethanol reducing Laemmli sample buffer. Aliquots were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% denaturing polyacrylamide gels and transferred to nitrocellulose membranes (Hybon C; Amersham). Blots were stained with Ponceau red to visualize total proteins contained in each slot and then probed with a mouse monoclonal antibody (MAb) directed against HCMV IE proteins (IE1 [UL122] and IE2 [UL123], hybridoma supernatant provided by M. Mazeron, Paris, France) at 1:10 dilution. Antibody fixation was revealed using peroxidase-conjugated anti-mouse antibodies at 1:1,000 dilution (ECL detection kit; Amersham).
Metabolic labeling and immunoprecipitation.
Cells were infected with HCMV in 25-cm2 culture flasks and treated or not with the TNF-α–CHX mix. At different times postinfection (p.i.), cells were washed in PBS, incubated in methionine- and cysteine-free medium (Gibco) for 1 h, and then labeled with [35S]methionine-[35S]cysteine (500 μCi/ml; NEN) for 2 h. Cells were treated with trypsin (Gibco), washed, and either stored at −20°C or immediately lysed by incubation for 45 min on ice in lysis buffer (5 mM EDTA, 150 mM NaCl, 1 mM MgCl2, 0.05 mM phenylmethylsulfonyl fluoride, 50 mM Tris [pH 7.6]) containing 2% NP-40. After an overnight preclearing with protein G-conjugated Sepharose beads (Pharmacia), antibody was added to the lysate and the mixture was incubated for 2 h at 4°C. Mouse anti-pp65 antibodies (gift from G. Gerna, Milan, Italy) were used. Protein G-conjugated beads were then added, and incubation continued for an additional 2 h at 4°C. After washing, the beads were pelleted and boiled for 5 min in reducing Laemmli sample buffer containing 5% β-mercaptoethanol. Samples were separated by SDS-PAGE (10% gel). Gels were fixed, incubated in Amplify (Amersham), vacuum dried, and exposed to Hyperfilm-MP (Amersham).
Immunofluorescent intracellular labeling of pp65.
One day before the labeling experiment, MRC5 cells were seeded on Labtek chamber slides (Nunc, Naperville, Ill.) at 1.5 × 105 cells per well. DCs were cytocentrifuged (2 × 105 cells/spot) at 900 rpm for 3 min (Cytospin; Shandon, Pittsburg, United Kingdom). Cells were fixed for 10 min in PBS containing 2% saccharose and 5% formaldehyde and then permeabilized with 10% saccharose, 0.5% NP-40, and 1% SVF in PBS for 5 min. After washing with PBS–1% FCS, cells were incubated (30 min, 37°C) with a mouse MAb directed against pp65 (clone 58/2; gift from S. Michelson, Paris, France) at 1/100 dilution. Cells were then washed three times in PBS and incubated with a 1:100 dilution of FITC-labeled goat anti-mouse immunoglobulin (GAM-FITC; Sigma) for 30 min. Slides were washed three more times, mounted, and examined on a Leitz fluorescence microscope. For flow cytometry analysis, the cell pellet was sequentially incubated with 1% formaldehyde (Sigma) for 15 min at 4°C, washed once in PBS, resuspended in cold 80% methanol, and kept at −20°C overnight. Cells were washed and incubated for 45 min at 37°C with a mouse MAb directed against pp65 (clone 9530; gift from S. Michelson). GAM-FITC (Sigma) was used as a second step reagent before flow cytometry analysis. Labeling with GAM-FITC alone served as a control in both cytometry and microscopy. All incubation and washing steps were done in PBS supplemented with 20% human AB serum.
Purification of apoptotic cells.
MRC5 cells were either infected or not with AD169 and treated overnight with a mixture of recombinant TNF-α (1,000 U/ml) and CHX (25 μg/ml). In these conditions, we showed that most (consistently >90%) of the cells underwent apoptosis using detection assays as described above. Then cells were magnetically labeled with AV microbeads (Miltenyi Biotech) for 15 min at room temperature and passed through a MACS column placed in the magnetic field of a MACS separator according to the manufacturer's instructions. Apoptotic cells were eluted in RPMI medium and then labeled with AV and PI as previously described, and their phenotypes were determined by flow cytometry. Samples were then used in coculture experiments as described below.
Phagocytosis assay.
MRC5 cells were dyed red with PKH26 (Sigma) as specified by the manufacturer, infected with HCMV, and then induced to undergo apoptosis as described above. Apoptotic red cells were then cocultured with immature DCs (one DC for five apoptotic cells) in RPMI medium supplemented with GM-CSF and IL-4 for 10 h at either 4 or 37°C. DCs were labeled with a mouse anti HLA-DR antibody (clone L243; American Type Culture Collection) and then with GAM-FITC (Sigma). Labeling with GAM-FITC alone served as a control. Qualitative and quantitative phagocytosis of apoptotic cells by DCs was determined by flow cytometry and fluorescence microscopy.
Expansion of anti-pp65 CD8+ T cells from HCMV-seropositive donor PBMC.
HLA typing was performed by the Laboratoire Central d'Immunologie. PBMC (2 × 106 cells/ml) from HCMV-seropositive healthy donors with the haplotypes HLA-A2 (donors P and V) and HLA-B35 (donor M) were cultured in 24-well plates in RPMI medium containing 10% human AB serum, 1% minimal essential medium with no essential amino acids (Gibco), and 10 mM HEPES (Gibco). pp65-derived peptides corresponding to known CTL epitopes (8, 23) that are recognized in the context of HLA-A2 (NLVPMVATV, N9V) and HLA-B35 (IPSINVHHY, I9Y) were obtained from Neosystem (Strasbourg, France). The restimulation procedure was based on the incubation of PBMC with a mixture containing the appropriate peptide and recombinant fusion protein IE1-pp65 to provide CD4+ T-cell help (unpublished data). Briefly, at day 1 cells were stimulated with a mixture containing either N9V or I9Y (5 μg/ml) and IE1-pp65 (5 μg/ml) associated with a nanoparticulate carrier (SMBV; a gift from Biovector Therapeutics, Labege, France). At days 3 and 7, recombinant human IL-7 (a gift from Sanofi-Synthelabo) was added at a final concentration of 25 ng/ml. Alternatively, after 9 to 15 days of culture, cell lines were incubated with beads coated with a mouse anti-human CD8 antibody (Dynabeads M450 CD8; Dynal) for 50 min at 4°C. Rosetted cells were isolated magnetically and cultured for 16 to 20 h at 37°C. Purity (>90%) of the isolated CD8+ cell subset was determined by dual staining with anti-CD4 and anti-CD8 antibodies (Coulter-Immunotech) and flow cytometry analyses. Activation of anti-pp65 CTL was assessed through quantitation of secreted gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) as described below.
Assay for cross-presentation of apoptotic cells.
Immature DCs (5 × 105 cells/well) obtained from either HLA-A2 or HLA-B35 donors were cocultured in six-well plates for 24 h in medium supplemented with GM-CSF (100 ng/ml; Novartis) and IL-4 (50 ng/ml; gift from J.-P. Marolleau, Paris, France) in the presence of purified apoptotic cells (1:1 ratio) raised from either HCMV-infected or uninfected MRC5 cells. Cells were washed 24 h later, plated in duplicate in a 96-well plate (at 104 cells/well), and incubated for 24 h in the presence of an anti-pp65 T-cell line at different effector-to-DC ratios in a final volume of 200 μl. Alternatively, DCs were used either unlabeled or pulsed overnight with 1 μM N9V or I9Y peptide in the presence of TNF-α (50 ng/ml) or infected with AD169 (MOI of 3) for 24 h.
51Cr release assay and ELISA for IFN-γ secretion.
HLA-A2-positive U373MG (A0) cells were either infected with HCMV (MOI of 1) or mock infected for 6 h and divided into two parts. One half was left unlabeled for IFN-γ secretion assay, and the other was labeled with [51Cr]Na2CrO4 (313 mCi/mg; ICN) for 51Cr release assay as follows. Cells were labeled at 100 μCi per 2 × 106 for 2 h and washed three times in RPMI medium-FCS. The effector cells were incubated with 5 × 103 target cells at various effector-to-target ratios in duplicate using 96-well U-bottom microtiter plates and then incubated for further 5 h. Percent specific 51Cr release was calculated as follows: [(cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release)] × 100. Spontaneous release was always less than 25% of the maximal value. ELISA for IFN-γ secretion by activated CTL was performed as follows. Ninety-six-well plates (Nunc) were coated overnight at 4°C with primary anti-IFN-γ MAb (Biosources), washed, and blocked for 2 h at 37°C with provided buffers (Medgenix screening line). Diluted (1:10) supernatants from CTL expansion experiments were added to precoated wells in duplicate and supplemented with secondary antibody (biotin-conjugated anti-IFN-γ MAb; Biosources) for 2 h. After washing with PBS–1% FCS, streptavidin-bound peroxidase was added (Coulter-Immunotech) and left for 30 min at room temperature. Plates were then washed and incubated for 5 min in chromogen buffer (Biosources). Optical density was counted on an ELISA apparatus (Dynatech Laboratories).
RESULTS
HCMV infection sensitizes fibroblasts to TNF-α-mediated apoptosis very early after infection.
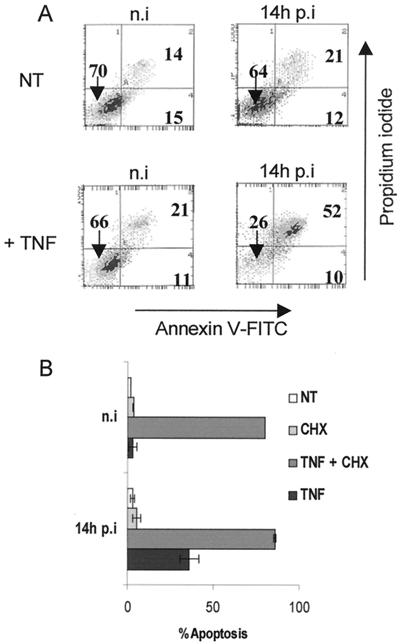
TNF-α has been shown to exert its antiviral activity through induction of apoptosis in HCMV-infected human foreskin fibroblasts (20). We investigated the sensitivity of MRC5 cells to TNF-α-mediated apoptosis using two different approaches: AV-PI double labeling coupled with flow cytometry analysis, and microscopic analysis of Hoechst-stained nuclei. As shown in Fig. 1A, treatment of MRC5 cells with TNF-α did not significantly modify the percentage of both apoptotic (AV+ PI−) and necrotic or late apoptotic (AV+ PI+) cells. However, infection with HCMV resulted in sensitization of cells to apoptosis, since after 6 h of infection followed by an 8-h treatment with TNF-α, cell viability (AV− PI− cells) decreased to 26%, compared to 66% in uninfected cells. Infection with HCMV did not significantly modify the amount of double-positive cells (21% versus 14%). Histograms of condensed nuclei observed in four independent experiments are shown in Fig. 1B. Infection with HCMV for 6 h prior to TNF-α treatment induced apoptosis in about 40% of MRC5 cells. Sensitization of MRC5 cells in the absence of infection was observed when cells were incubated with TNF-α in the presence of CHX, an inhibitor of protein synthesis known to suppress the antiapoptotic effect of NF-κB (5). In these conditions, 90% of nuclei were apoptotic in both uninfected and HCMV-infected samples. These data demonstrate that infection with HCMV initiated the apoptotic process in MRC5 cells through TNF-R1 signaling at a very early stage after infection.
FIG. 1.
HCMV sensitizes MRC5 fibroblasts to TNF-α-mediated apoptosis very early after infection. MRC5 cells were either mock infected (n.i [not infected]) or infected for 6 h with HCMV AD169 at an MOI of 3. Then cells were not treated (NT) or treated with CHX, with TNF-α alone (TNF), or with TNF-α supplemented with CHX (TNF + CHX) for an additional 8 h. The indicated times correspond to the total time p.i. including TNF-α and/or CHX treatment. The presence of apoptotic cells was detected by flow cytometry analysis using PI and FITC-conjugated AV (A) or by fluorescence microscopy using Hoechst 33342 staining to reveal condensed nuclei (B). Histograms show mean values (±standard deviation) of the percentages of condensed nuclei from four independent experiments.
Immature DCs are not permissive to HCMV but internalize HCMV-infected apoptotic fibroblasts.
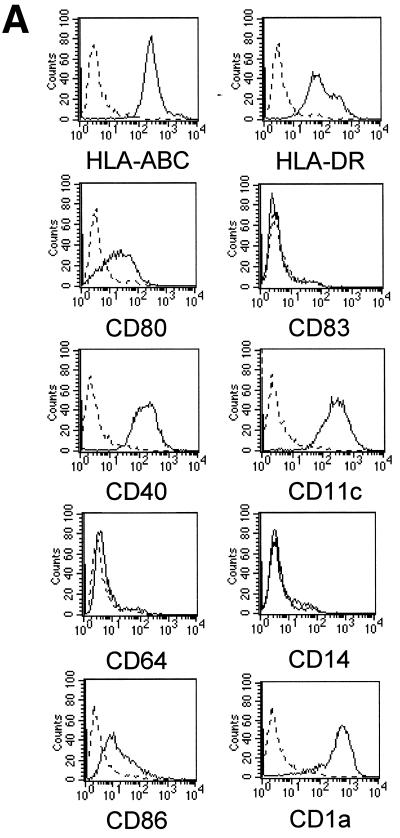
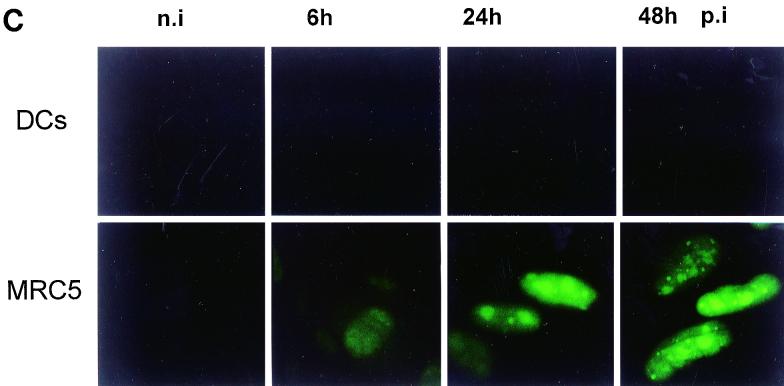
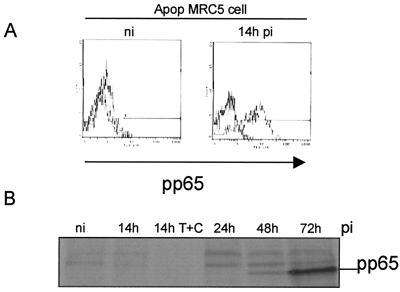
Infection of DCs by viruses may have opposite functional consequences: it could be a prerequisite for the induction of primary antiviral responses, but it could also contribute to viral pathogenesis (12). We first derived DCs from peripheral blood monocytes in vitro as described in Materials and Methods. Immunofluorescent staining and flow cytometry analysis revealed that the nonadherent population consisted mainly of immature DCs characterized by the phenotype HLA-ABC+ HLA-DR+ CD40+ CD80+ CD86+ CD1a+ CD11c+ CD64− CD83− CD14− (Fig. 2A). We then examined whether DCs could acquire viral proteins through direct infection with HCMV. To this end, DCs were infected for 48 h in the same conditions as MRC5 cells (MOI of 3) and checked for the expression of HCMV IE proteins. Western blotting experiments showed that in contrast to infected MRC5 cells, IE proteins were not detected in immature DCs even at 48 h p.i. (Fig. 2B). Since it was previously established that in permissive fibroblasts the matrix protein pp65 from the viral inoculum could be delivered into the cytosol (14), we examined whether this could occur in DCs. In contrast to MRC5 cells exhibiting a typical nuclear staining with anti-pp65 antibodies as soon as 6 h p.i., DCs exhibited no fluorescent labeling even after 48 h of incubation with the virus (Fig. 2C). Since it had been shown that immature DCs could acquire antigens through an efficient phagocytosis of apoptotic cells (1), we investigated whether this could occur with HCMV antigens contained in apoptotic MRC5 cells. We therefore tested whether immature DCs could engulf apoptotic HCMV-infected MRC5 cells. To this end, MRC5 cells were infected with HCMV for 6 h and then treated for 8 h with TNF-α, in the presence of CHX to recover a large amount of apoptotic cells. To further characterize the content of these apoptotic cells, we checked for the expression of pp65 using intracellular staining. Flow cytometry analysis of purified apoptotic cells showed that most (75%) of the cells expressed the matrix protein pp65 at 14 h p.i. (Fig. 3A). We then assessed whether pp65 contained in apoptotic MRC5 cells was synthesized de novo or derived from viral input as previously found (14). To this end, lysates from [35S]Methionine-[35S]cysteine-labeled MRC5 cells were immunoprecipitated with anti-pp65 antibody, submitted to SDS-PAGE, and exposed to autoradiography. Figure 3B shows that 14 h p.i. no pp65 synthesis occurred, since immunoprecipitate clearly appeared only from 48 h p.i. Taken together, these data demonstrate that 14 h p.i., pp65 stemmed from the viral inoculum and that apoptosis occurred prior to de novo pp65 synthesis.
FIG. 2.
Immature DCs are not permissive to HCMV AD169. DCs were derived from peripheral blood monocytes after 7 days of differentiation in AIMV medium supplemented with GM-CSF and IL-13. Filled histograms represent labeling with specific antibody against the indicated surface marker; dotted lines represent staining with the control isotype antibody (A). MRC5 cells and immature DCs were not infected (n.i) or infected with HCMV AD169 (MOI of 3) for the indicated times p.i. Viral IE protein expression was detected by Western blotting using a mouse anti-IE MAb (B), and the matrix protein pp65 was visualized by intracellular staining with anti-pp65 antibody under a Leitz fluorescence microscope (C). Results are representative of two experiments.
FIG. 3.
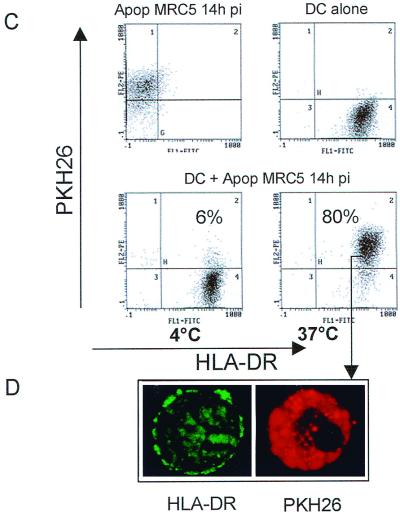
Apoptotic cells containing incoming HCMV pp65 protein are internalized by immature DCs. MRC5 cells either infected (14h pi) or not infected (n.i) were induced to undergo apoptosis (Apop) by treatment with TNF-α plus CHX (T+C). The expression of pp65 in purified apoptotic cells was detected by intracellular staining with mouse anti-pp65 MAb and flow cytometry analysis (A). Lysates from [35S]methionine-[35S]cysteine-labeled MRC5 cells that had been infected with HCMV as indicated were immunoprecipitated with anti-pp65 antibody, submitted to SDS-PAGE, and exposed to autoradiography (B). PKH26-dyed red apoptotic infected MRC5 cells (Apop MRC5 14h pi) were cocultured with immature DCs for 10 h at 4 or 37°C at a DC/apoptotic cell ratio of 1:5. Uptake of apoptotic MRC5 by HLA-DR+ DCs was analyzed by flow cytometry where dot plots were gated on FL1 high positive cells (DCs), thus excluding the uncleared apoptotic material (C). Internalization of PKH26-labeled apoptotic cells was visually confirmed by fluorescence microscopy using flow cytometry samples as starting material (D). Similar results were obtained in three independent experiments.
To quantify the uptake of apoptotic cells by DCs, MRC5 cells were sequentially labeled with PKH26, infected with HCMV, killed by incubation with TNF-α in the presence of CHX, washed, and then cocultured with DCs at a DC/apoptotic cell ratio of 1:5. After incubation at 37°C for 10 h, quantification of phagocytosis was deduced from the amount of HLA-DR+ PKH26+ cells by flow cytometry analysis. Figure 3C shows that 80% of DCs were PKH26+, suggesting that apoptotic MRC5 cells had been engulfed by DCs through an active process. No PKH26+ cells were observed at 4°C. To further confirm that flow cytometry data reflected internalization of apoptotic cells by DCs, samples were analyzed by fluorescence microscopy. Figure 3D shows that HLA-DR labeling appeared mainly on the DC surface, whereas PKH26 staining was localized in the cells except in the nucleus area (dark zone), suggesting that apoptotic cells had been internalized by DCs. Thus, the above results demonstrated that contrary to MRC5 cells, which expressed the inoculum-derived pp65, DCs did not contain detectable levels of pp65 even after prolonged infection but internalized apoptotic HCMV-infected MRC5 cells.
Immature DCs pulsed with HCMV-infected apoptotic fibroblasts induce activation of anti-pp65 CTL.
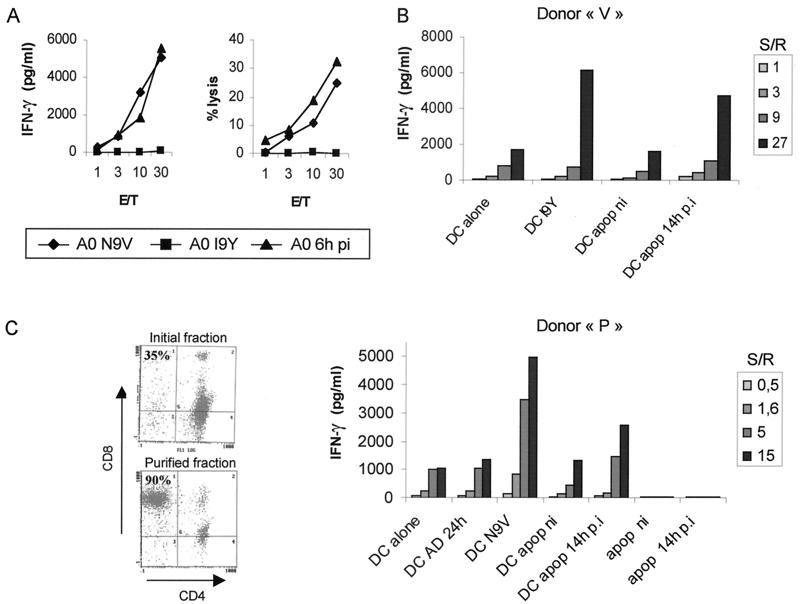
It has been shown that antigen acquired from apoptotic cells could be the target for CD8+ CTL (1). Since the above results suggest that DCs acquire pp65 from apoptotic HCMV-infected MRC5 cells, we assessed presentation of pp65-derived peptides to anti-pp65 CD8+ CTL. Antipeptide CTL from HCMV-seropositive HLA-A2 and HLA-B35 donors were expanded from PBMC. Both 51Cr release and IFN-γ secretion assays showed that these CTL were stimulated by U373MG cells pulsed with N9V peptide or infected with HCMV at an MOI of 1 for 6 h (Fig. 4A). pp65 presentation by DCs was monitored by quantification of IFN-γ secretion using ELISA. Immature HLA-B35 DCs were pulsed at a 1:1 cell ratio for 24 h with apoptotic cells raised from HCMV-infected MRC5 cells and then incubated with HLA-B35-restricted CTL lines at different stimulator-to-responder ratios. Alternatively, DCs were used either alone or pulsed with the relevant peptide I9Y. Figure 4B shows that DCs pulsed with apoptotic infected MRC5 cells specifically induced the activation of antipeptide CTL from donor V at a level close to that observed with I9Y-pulsed DCs. DCs pulsed with apoptotic bodies from mock-infected MRC5 cells were completely inactive. To exclude the possibility that IFN-γ was secreted by anti-IE1-pp65 CD4+ T cells which could have been expanded from PBMC, we prepared DCs from an MHC class II-mismatched donor (donor M). This was confirmed by experiments using HLA-A2 DCs incubated with purified HLA-A2-restricted anti-N9V CD8+ T cells from donor P (Fig. 4C). To further exclude the possibility of direct stimulation by HLA-A2-positive apoptotic MRC5 cells which have not been internalized by DCs, we tested the activation of T cells by apoptotic cells alone (Fig. 4C). Furthermore, that DCs which had been preincubated with HCMV did not stimulate anti-pp65 CTL corroborates the absence of pp65 staining on micrographs as shown above (Fig. 2C). Both N9V-pulsed DCs and DCs incubated with pp65-positive apoptotic bodies stimulated HLA-A2-restricted anti-pp65 CTL. Taken together, these data show that apoptotic cell-derived pp65 was processed by DCs and that peptides were presented by HLA-A2 or HLA-B35 to allow activation of CD8+ T cells.
FIG. 4.
Immature DCs cross-present incoming pp65 contained in apoptotic cells. Anti-pp65 CTL lines were raised as described in Materials and Methods. Specific stimulation was assayed through either 51Cr release or IFN-γ secretion in the presence of HLA-A2+ U373MG (A0) cells either pulsed with HLA-A2 binding peptide (N9V) or irrelevant peptide (I9Y) or infected with HCMV at an MOI of 1 for 6 h (A0 6h pi) (A). E/T, effector-to-target ratio. MRC5 (HLA-A2) cells infected (14h p.i) or not infected (ni) with HCMV were induced to undergo apoptosis by TNF-α–CHX treatment. Apoptotic (apop) purified cells were fed to immature DCs at a ratio of 1:1. After 24 h of incubation, DCs were collected and cocultured with an HLA-B35-restricted anti-pp65 CTL line from donor V (B) and HLA-A2-restricted purified CD8+ CTL from donor P (C) at different stimulator-to-responder ratios (S/R). Alternatively, HCMV-pulsed DCs (DC AD 24h, MOI of 3), and purified apoptotic MRC5 cells either uninfected (apop ni) or infected (apop 14h p.i) were used as stimulator cells. Additional controls consisted of immature DCs which either had or had not been pulsed with the HLA-A2 (N9V) and HLA-B35 (I9Y) peptides at 1 μM. Stimulation of CTL was assayed by secretion of IFN-γ using ELISA. Results shown are representative of two to four independent experiments.
DISCUSSION
In this study we showed that although immature DCs did not express HCMV antigen after incubation with infectious virus, they could internalize the matrix protein pp65 contained in infected apoptotic fibroblasts. Apoptosis of fibroblasts was induced by TNF-α very early after infection, providing starting material for cross-presentation to anti-pp65 CD8+ CTL. This pathway of pp65 delivery into DCs allowed processing of relevant peptides and presentation to HLA-A2- and HLA-B35-restricted anti-HCMV cytotoxic CD8+ T cells.
Apoptosis is a critical mechanism in many physiological processes, including eradication of cells infected with viruses. It has been suggested that viruses may interfere with apoptosis for their own survival through both inhibition to allow viral replication to continue and induction in the later stages of infection, allowing viral progeny to spread to neighboring cells. In contrast, apoptosis triggered by ligands of the TNF-α family may contribute significantly to the clearance of an acute infection (22). Recent findings showing that DCs may acquire antigen through phagocytosis of apoptotic bodies, and thus play a critical role in priming of antigen-specific immune responses, raised the idea that it could be an efficient way to prime an antiviral response against viruses which do not replicate in DCs. Our data showing stimulation of established anti-pp65 CTL in the presence of apoptotic cells indicate that this could be the case for the generation of an anti-HCMV immune response. The fact that infected fibroblasts were sensitized to TNF-α apoptosis very early after infection, before viral replication, emphasized the importance of cross-presentation for efficient and rapid induction of CTL. It had been demonstrated that TRAIL was inducible after infection of fibroblasts with HCMV AD169 and then allowed sensitization of infected cells to apoptosis (20). This finding suggests that apoptosis may occur through an autocrine mechanism providing starting material for phagocytosis by DCs at local sites of infection. Importantly, our data showing that pp65 was derived from the immediate delivery of the viral matrix into fibroblasts suggest that any cell able to interact with the virus and sensitive to apoptosis could serve as a pp65 source of antigen for DCs. At this stage, one may ask why this process was inefficient in DCs since semipermissive U373MG cells stimulated anti-pp65 CTL after 6 h of infection. We cannot exclude that pp65 from the viral inoculum was delivered into DCs but in amounts much lower than those required for CTL stimulation. We have to consider recent data showing that permissivity of immature DCs may depend on laboratory strains used in in vitro experiments and that with the AD169 strain, pp65 visualization required an MOI of 50 (18). The in vivo relevance of these observations remains to be determined, and the in vitro use of clinical strains may provide additional information. Nevertheless, the failure of DCs to replicate HCMV could be considered a protective effect against the blockade of antigen presentation through expression of the viral US proteins (16).
Regarding interference of HCMV with apoptotic processes, it was reported that ectopic expression of IE1 and IE2 proteins in HeLa cells suppressed TNF-α-mediated apoptosis (25) and that UL37-encoded protein functioned as a mitochondrion-localized inhibitor of apoptosis involving Fas and TNF-R1 (9). These processes may act respectively in the IE and early-late phases of HCMV infection, thus providing an escape mechanism allowing viral replication and spread. This emphasizes the importance of both the very immediate delivery of pp65 in infected cells and sensitization to apoptosis before synthesis of these HCMV inhibitors. Altogether, these observations argue in favor of cross-presentation of the incoming pp65 as a major mechanism for priming of CTL directed against pp65 and could explain why CTL are mainly directed against this major matrix protein (24). It was recently shown that optimal cross-presentation of antigen from dead tumor cells required a maturation signal in DCs provided by the uptake of necrotic cells (19). In our experiments, apoptosis was induced through interaction of TNF-α with its receptor on fibroblasts. Such an interaction is usually considered a starting event toward apoptosis, which further moves to secondary necrosis in long-term culture. Such necrotic cells are likely contained in the AV+ PI+ cell population shown in Fig. 1. In cross-presentation experiments, infected fibroblasts were treated with TNF-α in the presence of CHX to provide a large amount of dead cells. Microscopic examination showed that cells were completely fragmented, arguing in favor of a secondary necrotic phenotype. Thus, in these conditions, we suggest that cross-presentation of pp65 occurred through internalization of secondary necrotic cells according to the data obtained by Sauter et al. (19). Moreover, since late after infection fibroblasts die through the cytopathic effect of HCMV even in the absence of proapoptotic ligand, we speculate that derived cell fragments could serve as an antigen source for cross-presentation by DCs. We are currently investigating whether this late cross-presentation of pp65 may be relevant. Nevertheless, we have no clues as to how necrotic cells are able to provide antigen to DCs only for anti-pp65 CTL activation or to induce DC maturation as reported recently (19). In conclusion, our data show that very early after infection, incoming pp65 can be cross-presented to CTL through uptake by DCs of infected fibroblasts which have been previously made apoptotic following TNF-α-mediated signaling but do not exclude that other mechanisms may participate in cross-presentation by DCs. Whether this could take place in vivo for priming of anti-HCMV CTL is not known, but in vitro CTL priming experiments from seronegative donors PBMC may provide more relevance to this hypothesis. Moreover, one may speculate that targeting of DCs with apoptotic cells containing HCMV antigens could allow expansion of CD4+ T cells, which are known to play a significant role in providing help for the expansion of CD8+ T cells (13) and in the direct control of viral replication (6). Finally, cross-presentation of HCMV antigens contained in apoptotic cells might be useful in design of anti-HCMV cellular immunotherapy based on adoptive transfer of CD4+ and CD8+ T cells (23).
ACKNOWLEDGMENTS
This work was supported by institutional grants from INSERM and a grant from ARC (Association de Recherche contre le Cancer). Géraldine Arrode was supported by a MNERT fellowship.
The technical assistance of G. Cassar for flow cytometry analyses is fully acknowledged. We thank Sanofi-Synthelabo for supplying us with IL-2, IL-7, and IL-13.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Bell D, Young J, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- 3.Britt W J, Alford C. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 4.Chaudhuri A R, St. Jeor S, Maciejewski J P. Apoptosis induced by human cytomegalovirus infection can be enhanced by cytokines to limit the spread of virus. Exp Hematol. 1999;27:1194–1203. doi: 10.1016/s0301-472x(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 5.Chu Z L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davignon J L, Castanie P, Yorke J A, Gautier N, Clement D, Davrinche C. Anti-human cytomegalovirus activity of cytokines produced by CD4+ T-cell clones specifically activated by IE1 peptides in vitro. J Virol. 1996;70:2162–2169. doi: 10.1128/jvi.70.4.2162-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleck M, Kern E R, Zhou T, Podlech J, Wintersberger W, Edwards III C K, Mountz J D. Apoptosis mediated by Fas but not tumor necrosis factor receptor 1 prevents chronic disease in mice infected with murine cytomegalovirus. J Clin Investig. 1998;102:1431–1443. doi: 10.1172/JCI3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin M A, Gilbert M J, Riddell S R, Greenberg P D, Bevan M J. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971–3980. [PubMed] [Google Scholar]
- 9.Goldmacher V S, Bartle L M, Skaletskaya A, Dionne C A, Kedersha N L, Vater C A, Han J, Lutz R J, Watanabe S, McFarland E D, Kieff E D, Mocarski E S, Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goxe B, Latour N, Bartholeyns J, Romet-Lemonne J L, Chokri M. Monocyte-derived dendritic cells: development of a cellular processor for clinical applications. Res Immunol. 1998;149:643–646. doi: 10.1016/s0923-2494(99)80031-5. [DOI] [PubMed] [Google Scholar]
- 11.Hahn G, Jores R, Mocarski E S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klagge I M, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–833. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 13.Livingstone A M, Kuhn M. Dendritic cells need T cell help to prime cytotoxic T cell responses to strong antigens. Eur J Immunol. 1999;29:2826–2834. doi: 10.1002/(SICI)1521-4141(199909)29:09<2826::AID-IMMU2826>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin-Taylor E, Pande H, Forman S J, Tanamachi B, Li C R, Zaia J, Greenberg P D, Riddell S R. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 15.Mocarski E E. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 16.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 17.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 18.Riegler S, Hebart H, Einsele H, Brossart P, Jahn G, Sinzger C. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol. 2000;81:393–399. doi: 10.1099/0022-1317-81-2-393. [DOI] [PubMed] [Google Scholar]
- 19.Sauter B, Albert M L, Francisco L, Larsson M, Smersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–433. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedger L M, Shows D M, Blanton R A, Peschon J J, Goodwin R G, Cosman D, Wiley S R. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- 21.Soderberg-Naucler C, Fish K N, Nelson J A. Growth of human cytomegalovirus in primary macrophages. Methods. 1998;16:126–138. doi: 10.1006/meth.1998.0650. [DOI] [PubMed] [Google Scholar]
- 22.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 24.Wills M, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons P J G. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu H, Shen Y, Shenk T. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol. 1995;69:7960–7970. doi: 10.1128/jvi.69.12.7960-7970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]