Abstract
Our lives are governed by three clocks: the social clock that organizes our lives with others (local time), the biological clock that controls our physiology (circadian time) and the sun clock that defines natural light and darkness. The more misaligned these clocks are, the higher our odds of developing certain diseases. ‘Social jetlag’ quantifies the difference between local and circadian time.
Subject terms: Epidemiology, Type 2 diabetes
Refers to Bouman, E. J., et al. Social jet lag and (changes in) glycemic and metabolic control in people with type 2 diabetes. Obesity 31, 945–954 (2023).
The term ‘social jetlag’ was inspired by a study showing that people who slept at different times during the working week than the weekends had increased tobacco, caffeine and alcohol consumption compared with people who had similar sleeping patterns throughout the week1. Social jetlag quantifies the difference in mid-sleep times on nights before work (or school) days and those before work-free days. It assumes that people live more according to the social clock (local time) during the working week and more according to their biological clock (circadian clock) on work-free days.
The circadian clock has a genetic basis, which contributes to inter-individual differences; that is, different ‘chronotypes’, from early ‘larks’ to late ‘owls’. Owls (who sleep and wake later on work-free days than on workdays) would be expected to have a greater degree of social jetlag than larks (who sleep and wake at approximately the same times on both day types). Being a late chronotype is a strong predictor for having more social jetlag, and as a person’s chronotype changes with age2, so does the amount of social jetlag they experience (Fig. 1). Coining the term ‘social jetlag’ apparently gave a name to a state that many people had experienced: within a month after the paper1 appeared, the term went from zero to 100,000 counts in search engines.
Fig. 1. Average social jetlag across a lifetime.
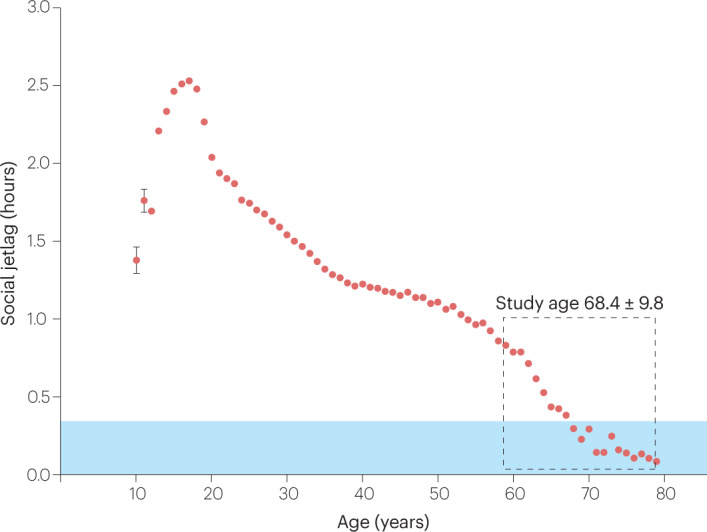
Social jetlag, which can be calculated using the Munich ChronoType Questionnaire (MCTQ)3, is defined as the difference between the mid-point of sleep on workdays and on work-free days. Data are taken from the MCTQ database (n = 198,370) and were first published in Roenneberg et al. (2012)8. Except for the age groups of 10 and 11 years, standard errors of the mean are smaller than the symbols. The blue area indicates where social jetlag is smaller than 20 min, which appears to be the tolerable limit for people6. The average age ± standard deviation of the study population in Bouman et al.4 is indicated by the dashed grey box.
The phenomenon social jetlag is as young as industrialisation, which has drastically changed our exposure to light and darkness, the predominant synchronising signals for circadian clocks. By working inside, we decrease daytime light exposure up to a thousand-fold, and by using artificial light, we eliminate dark exposure (except for when we sleep). Under these conditions, most circadian clocks, except for those of people with extremely early chronotypes, had to become later to ensure stable synchronisation to the 24-h Earth rotation. Because the demands of the social clock (for example, work and/or school times) are early relative to the ‘industrialised’ circadian clocks, over 80% of the population (represented in the MCTQ (Munich ChronotType Questionnaire) database; see legend to Fig. 1) use an alarm clock and accumulate a sleep debt over the course of the working week. Weekend sleep is characterized by both sleeping at more appropriate times for the individual biological clock and by catching up on lost sleep.
Many epidemiological studies have shown strong associations between social jetlag and the prevalence of diverse pathologies, ranging from depression and cardiovascular risks to metabolic dysfunction (reviewed in ref. 3). The most recent epidemiological study, by Bouman and colleagues4, reports apparently contradicting results that offer explanations on how social jetlag and health might be connected. The authors assessed social jetlag and metabolic and glycaemic outcomes in approximately 1,000 people over the age of 60 with type 2 diabetes mellitus (T2DM), using both a cross-sectional and longitudinal design (re-testing after 2 years). A covariate of the study was whether participants were currently employed or retired. While the cross-sectional results in employed people corroborate previous associations between increased social jetlag and increased metabolic dysfunction5, the relationship seemed to be the reverse in retired people. The longitudinal assessment results do not reach statistical significance. The authors postulate that this finding could be because the study population included people with well-controlled T2DM. However, these apparent contradictions might be clarified when put into a larger context of social jetlag.
“While enforced social jetlag disrupts health, voluntary sleep extension on weekends might protect it”
The study population in Bouman et al.4 (shown in the dashed grey box in Fig. 1) has lower social jetlag than younger people (that is, those <60 years old). This known age trajectory is even present within the age range of this population: participants with the greatest social jetlag are the youngest (62 years) and those with the lowest are the oldest (72 years). Above the age of 60, social jetlag drops sharply, most probably due to retirement, and quickly approaches 20 min: when people can reduce social jetlag, as occurred during the COVID-19 lockdowns, they do so only if the pre-lockdown social jetlag was >20 min6 (shown in the blue area in Fig. 1). Individuals who are late chronotypes at the age of 60 years most probably were even later chronotypes for most of their lives. The health effect of social jetlag probably has both acute and chronic components. The immediate consequences of interrupted and insufficient sleep would contribute to the acute effects, while the strain on metabolism by being active and eating at the wrong biological times would accumulate as the chronic effects of social jetlag. The acute social jetlag status (the effects that people report at the time of the study) would then be a marker for the social jetlag load they accumulated over decades, which in turn is a predictor for pathology, especially in older populations. This distinction would also explain why cross-sectional associations between social jetlag and metabolic dysfunctions are statistically significant while the prospective associations after 1 and 2 years are not: at ages >60 years, most of the social jetlag load has already occurred.
The question remains, why does social jetlag in this >60 years age group have a detrimental association with health in those who still work but become protective after retirement. Social jetlag is defined as a difference in sleep timing between workdays and work-free days. It can persist after retirement for three reasons: first, people continue to get up with alarm clocks during the working week but not on weekends; second, people sleep in during the working week and get up earlier on weekends (producing a negative social jetlag); or third, people don’t use alarm clocks throughout the week but allow themselves extra sleep on weekends (beyond their usual biological waking time). While Bouman et al. address the first two possibilities in suggesting that social jetlag in retirement “could be an indication of maintaining an active social life”, the third possibility simply points to the protective role of sleep. A study has shown that we usually underestimate our sleep need7: when people were instructed to stay in bed in the dark for many hours a day, all participants slept longer than their habitual sleep duration, even if they claimed to get enough sleep in their everyday life. Thus, social jetlag occurs both when we are forced to live against our body clock and when we allow ourselves to sleep in on weekends. While enforced social jetlag disrupts health, voluntary sleep extension on weekends might protect it. We know that longer sleep is protective against metabolic dysfunction even on free days8 and that life-expectancy is reduced by not sleeping in on weekends9.
“A reduction of enforced social jetlag should therefore be central to strategies to prevent disease”
The many epidemiological studies investigating social jetlag suggest that the higher its accumulation, the higher the prevalence and the earlier the onset of clinical symptoms for many different pathologies beyond metabolic dysfunction. This effect is similar to the accumulating effects of sleep loss on health10. A reduction of enforced social jetlag should therefore be central to strategies to prevent disease. Candidates for such a prevention could be more flexible work schedules, later school start times for adolescents or eliminating daylight saving time.
Competing interests
The author declares no competing interests.
References
- 1.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol. Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 2.Roenneberg T, et al. A marker for the end of adolescence. Curr. Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and social jetlag: a (self-) critical review. Biology. 2019;8:54. doi: 10.3390/biology8030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouman EJ, et al. Social jet lag and (changes in) glycemic and metabolic control in people with type 2 diabetes. Obesity. 2023;31:945–954. doi: 10.1002/oby.23730. [DOI] [PubMed] [Google Scholar]
- 5.Parsons MJ, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int. J. Obes. 2015;39:842–848. doi: 10.1038/ijo.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korman M, et al. COVID-19-mandated social restrictions unveil the impact of social time pressure on sleep and body clock. Sci. Rep. 2020;10:22225. doi: 10.1038/s41598-020-79299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klerman EB, Dijk D-J. Age-related reduction in the maximal capacity for sleep–implications for insomnia. Curr. Biol. 2008;18:1118–1123. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr. Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Åkerstedt T, et al. Sleep duration and mortality-does weekend sleep matter? J. Sleep Res. 2019;28:e12712. doi: 10.1111/jsr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferini-Strambi L. Sleep disorders and increased risk of dementia. Eur. J. Neurol. 2022;29:3484–3485. doi: 10.1111/ene.15562. [DOI] [PMC free article] [PubMed] [Google Scholar]


