Abstract
Purpose
Our clinical observations showed clot formations in different regions of the left ventricle of the heart in some COVID-19 patients with normal myocardial motion and coronary artery. The aim of this study was to examine the changes caused by COVID-19 disease on blood flow inside the heart as a possible etiology of intracardiac clot formation.
Methods
In a synergic convergence of mathematics, computer science, and cardio-vascular medicine, we evaluated patients hospitalized due to COVID-19 without cardiac symptoms who underwent two-dimensional echocardiography. Patients with normal myocardial motions on echocardiography, normal coronary findings on noninvasive cardio-vascular diagnostic tests, and normal cardiac biochemical examinations but who presented with a clot in their left ventricle were included. To display the velocity vectors of the blood in the left ventricle, motion and deformation echocardiographic data were imported into MATLAB software.
Results
Analysis and output of the MATLAB program indicted anomalous blood flow vortices inside the left ventricular cavity, indicating irregular flow and turbulence of the blood inside the left ventricle in COVID-19 patients.
Conclusion
Our results suggest that in some COVID-19 patients, cardiac wall motion is not satisfactorily able to circulate the blood fluid in normal directions and that, despite normal myocardium, changes in the directions of blood flow inside the left ventricle might lead to clots in different zones. This phenomenon may be related to changes in blood properties, such as viscosity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12574-023-00603-1.
Keywords: Echocardiography, MATLAB, COVID-19, Apical clot , Interventricular septum clot, Vorticity
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a global crisis that spreads quickly throughout the world. This disease is caused by the novel severe acute respiratory syndrome coronavirus 2, which, in addition to the symptoms of the disease, is also associated with several complications [1]. One of the most common complications among different regions is thromboembolism, which led to different theories about the origin of these blood clots [2]. A significant proportion of patients who present with COVID-19 infection requiring hospitalization exhibits biomarker evidence of myocardial injury, which has been shown to be associated with increased risk of in-hospital morbidity and mortality [3, 4]. The pathogenesis of myocardial injury in patients affected by COVID-19 remains unclear. Proposed mechanisms include cytokine-mediated damage, imbalance in oxygen supply demand, ischemic injury due to microvascular thrombi formation, and direct viral invasion of the myocardium [5]. In addition, prior reports showed that the risk of coronary thrombotic events arising from the rupture of atherosclerotic plaque is increased during viral infections [6] Conversely, during routine cardiac examinations of COVID-19 patients, we detected blood clots in different parts of the left ventricle in patients who demonstrate normal myocardial motion on echocardiography and normal coronary findings on noninvasive cardio-vascular diagnostic tests. These clots were often asymptomatic or could be the source of a subsequent stroke or infarction.
Previously published series defined myocardial injury based on elevations in myocardial necrosis biomarkers, but most of these did not use imaging to characterize the structural and functional cardiac abnormalities [7]. On the other hand, performing an extensive cardiac workup in patients with COVID-19 is challenging because of their clinical status and the need to limit the exposure of health care personnel. Therefore, the underlying cardiac abnormalities in patients with COVID-19 infection remain unknown.
The aim of this work is to identify the changes in the blood flow inside the heart resulting from COVID-19 disease and to determine the cause of these clots.
Hypothesis
We hypothesized the heart in some COVID-19 patients cannot pump blood normally even if the heart is not directly affected, resulting in clot formation within the heart's cavities. Demonstrating the direction of blood flow inside the cardiac chambers can provide valuable information on heart disease. Vorticity, defined as the tendency of fluid elements to spin, is the fundamental quantity used to describe fluid motion. Vorticity is calculated using the curl formula [8]. In this formula, if ) is a velocity vector corresponding to a cardiac segment such that and are velocity components along the x-axis and y-axis, respectively, we get
If we correspond the velocity vectors to the selected points in the left ventricular (LV) cavity of the heart, the curl formula shows the amount of the rotations of those velocity vectors. Because the curl is a quantitative vector, it demonstrates both numerical value and direction.
Its direction is observed using the right-hand rule [9] around the axis perpendicular to the x–y plane. Its direction rotates both clockwise or counterclockwise, or the selective velocity vector moves straightforward and demonstrates no rotation.
For convenience, we coded the curl formula as a normalized vector in this article [10]:
In this formula, the denominator is the absolute value of the curl vector. Namely, without loss of generality, we considered numerically only the values of zero and nonzero for the scalar part of the curl vector. This indicates in which areas the selected velocity vectors are circling or non-circling. Whenever a velocity vector is rotating, the curl value is nonzero. In other words, if the curl of the velocity vectors is zero, the selected vectors of the interested region exhibit straightforward or non-rotational directions. The curl formula is usually coded as a computer command in software. In the output file of the software, the curl of each velocity vector shows the amount and direction of the rotations of the velocity vectors in the desired area.
More precisely, vorticity can show the turbulency of the fluid elements and the formation of circulatory areas. Also, quantitative parameters of the intraventricular vortex are extracted based on the vorticity [11].
Finally, to justify the abnormal blood flow inside the heart, we must be familiar with viscosity. Changes in viscosity might be one of the reasons for the inability of normal myocardium to pump blood. The flowing of a fluid, such as blood, can be defined as an infinite number of parallel layers sliding against each other. Friction between these layers leads to viscosity. Mathematically, a fluid's shear rate is the velocity difference between any two layers divided by the distance between them. Viscosity is the ratio of shear stress, defined as the force applied to a fluid divided by its contact area and shear rate (viscosity = shear stress/shear rate). In a normal heart, changes in blood viscosity can explain changes in blood flow vectors.
Methods
We conducted this study from March 2020 to September 2020 at Taleghani Hospital (Tehran, Iran), a major medical center caring for COVID-19 patients. This study is a synergic convergence of mathematics, computer science, and cardio-vascular medicine.
We collected patient data from the electronic hospital information system and included patient demographic information, presenting vital signs and symptoms, comorbidities, home medications, chest radiography findings, electrocardiogram findings, laboratory values, echocardiographic findings, inpatient treatments received, and in-hospital outcomes. Then, patients were categorized according to the presence or absence of myocardial injury, defined as a serum cardiac troponin level greater than the upper reference limit for the assay. Then, we conducted echocardiography and noninvasive cardio-vascular diagnostic tests to assess myocardial motion and rule out coronary diseases, respectively. Echocardiographic data included LV ejection fraction, LV diastolic function, LV volumes, presence of regional wall motion abnormalities or global LV dysfunction, right ventricular (RV) size and function, and the presence of pericardial effusions [13]. For echocardiography, we used an ACUSON SC2000 PRIME (Siemens).
We included patients aged 18–65 years who were hospitalized due to COVID-19, as confirmed by polymerase chain reaction assay of nasal or pharyngeal swab specimens or serologic testing. Other inclusion criteria were the absence of cardiac symptoms, no myocardial injury proven by normal serum cardiac troponin, normal coronary findings proven by noninvasive tests such as stress echocardiography or myocardial perfusion imaging, normal myocardial motion in two-dimensional (2D) echocardiography, presence of a clot in the left ventricle on 2D echocardiography, and healing of the clot with anticoagulants during hospitalization (as proven by follow-up 2D echocardiography). Patients who met all inclusion criteria were included in the study. We excluded patients who developed heart or brain complications during hospitalization or who died from the disease. Other exclusion criteria included heart diseases including coronary, valvular, and congenital heart diseases history of open-heart surgery or cardiac interventions; history of deep vein thrombosis or peripheral thrombotic embolism; and known previous hypercoagulable state.
To calculate the curl values and to examine our hypothesis regarding the change in blood flow pattern inside the left ventricle as an etiology of clot creation in COVID-19 patients, we used MATLAB software (MathWorks, Natick, MA; R2015a [8.5.0.197613], 64-bit [win64] February 2015, license No. 161052), interfaced on the echocardiography system. MATLAB is a high-level technical computing language and an interactive environment for algorithm development, data visualization, analysis, and numeric computation. In other words, MATLAB software is a virtual laboratory environment in which ideas, mathematical equations, and physical laws can be entered in the form of computer codes and commands. MATLAB can be used in various applications, including signal/image processing, communications, control design, test and measurement, financial modeling and analysis, and computational biology [14].
In this study, the input data were 2D echocardiography video files of the patients, and the output data were new video files displaying the tracking of anatomical points of the left ventricle. We extracted the retrieved video files and saved them with the encoded command to convert the video to frames.
Each frame consists of several pixels, and each anatomical point of the heart corresponds to one and only one pixel on the Cartesian 2D coordinate plane. The relative position of two anatomical points is equivalent to their corresponding pixels in the Cartesian coordinate plane in each frame. Thus, by changing each frame, the movement and deformation of the anatomical points of the heart were mathematically modeled. The output represents the movement of the selected anatomical points of the heart. By adding together the sequential steps of the multiple fragmented sectors of a fiber, the dynamic orientation contraction (through the cardiac cycle) of every individual myocardial path could be created.
Through the detection of movement of the traced cardiac segments in the new output file, we can observe the altered blood flow pattern inside the left ventricle of the heart, which can be considered a possible etiology of clot formation.
According to data distribution, continuous variables were expressed as mean (± SD) or median (interquartile range). We compared the markers of LV and RV function between baseline and follow-up echocardiograms using paired t tests and calculated the mean of differences (Δ).
Informed consent was obtained from all subjects and the ethical committee of Shahid Beheshti University of Medical Sciences approved this study. All methods were carried out in accordance with relevant guidelines and regulations.
Results
A total of 22 patients met our inclusion and exclusion criteria and entered the study. Table 1 shows the demographics and clinical and laboratory characteristics of the patients. Table 2 reports the baseline medications. Table 3 shows the echocardiographic data of patients enrolled in the study. The median age of the patients was 49 years, and 63.5% were men. None of the patients presented with cardiac symptoms at the time of admission. The median duration of hospital stay was 14 days (interquartile range: 7–23 days).
Table 1.
Clinical, demographic, and laboratory characteristics of patients
| Demographics | |
| Total number | 22 |
| Age, years | 49 (18–65) |
| Male (%) | 14 (63.5) |
| Body mass index, kg/m2 | 26.5 (24.3–31.2) |
| Past medical history | n (%) |
| Hypertension | 10 (45.4) |
| Diabetes mellitus | 6 (29.0) |
| Prior myocardial infarction | 0 (0) |
| Prior percutaneous coronary intervention | 0 (0) |
| Prior coronary artery bypass graft surgery | 0 (0) |
| Prior stroke | 1 (4.5) |
| Chronic kidney disease | 2 (9.0) |
| Anemia | 5 (22.7) |
| Chronic obstructive pulmonary disease | 2 (9.0) |
| Asthma | 3 (13.6) |
| History of atrial fibrillation | 0 (0.0) |
| History of heart failure | 0 (0.0) |
| Vital signs at presentation | Mean (range) |
| Temperature, °C | 37.9 (36.5–39.6) |
| Systolic blood pressure, mm Hg | 131 (120–152) |
| Diastolic blood pressure, mm Hg | 77 (69–84) |
| Mean arterial pressure, mm Hg | 95 (85–105) |
| Heart rate, beats/min | 92 (78–106) |
| Oxygen saturation, % | 88 (70–98) |
| Presenting symptoms days from symptoms onset | 5 (3–10) |
| Shortness of breath | 12 (54.5) |
| Cough | 13 (59.0) |
| Fever | 12 (55.0) |
| Chest pain (non-cardiac) | 3 (13.6) |
| Myalgia | 9 (40.9) |
| Dizziness | 1 (4.5) |
| Nausea or vomiting | 3 (13.6) |
| Diarrhea | 3 (13.6) |
| Chest radiography clear | 3 (13.6) |
| Unilateral opacities | 4 (18.1) |
| Bilateral opacities | 15 (68.1) |
| Laboratory characteristics | Mean (range) |
| Cardiac troponin I, ng/ml | |
| Baseline | 0.0 (0.0–0.0) |
| Peak | 0.01 (0.0–0.02) |
| Cardiac troponin T, ng/ml | |
| Baseline | 0.0 (0.0–0.0) |
| Peak | 0.0 (0.0–0.0) |
| High-sensitivity cardiac troponin T, ng/l | |
| Baseline | 6.2 (3.0–9.4) |
| Peak | 9.4 (5.6–14.8) |
| CK-MB, ng/ml | |
| Baseline | 1 (0.7–2.2) |
| Peak | 1.1 (0.6–2.9) |
| Creatinine, mg/dl | |
| Baseline | 0.9 (0.7–1.1) |
| Peak | 1.0 (0.8–1.2) |
| Hemoglobin, g/dl | 13.3 (11.6–14.5) |
| White blood cell count, 103/μl | 10.5 (5.9–13.4) |
| Neutrophil count, 103/μl | 6.2 (4.0–8.8) |
| Lymphocyte count nadir, 103/μl | 0.6 (0.3–1.3) |
| Platelet count, 103/μl | 240 (181–327) |
| Lactate, mmol/l | |
| Baseline | 1.5 (1.0–2.3) |
| Peak | 2 (1.4–3.1) |
| Albumin, g/dl | 3.4 (2.9–3.8) |
| C-reactive protein (peak), mg/l | 230 (54–289) |
| Erythrocyte sedimentation rate (peak), mm/h | 55 (24–89) |
| Interleukin-6 (peak), pg/ml | 90 (25–147) |
| Lactate dehydrogenase (peak), U/l | 445 (306–750) |
| Ferritin (peak), ng/ml | 1100 (219–1848) |
| d-Dimer, μg/ml | |
| Baseline | 0.9 (0.3–1.3) |
| Peak | 3.5 (0.6–5) |
| Procalcitonin (peak), ng/ml | 0.2 (0.1–1.0) |
| Alanine aminotransferase, U/l | 47 (25–114) |
| Aspartate aminotransferase, U/l | 65 (29–116) |
Table 2.
Baseline medications in patients
| (n = 22) | ||
|---|---|---|
| Angiotensin converting enzymes inhibitor | 3 (13.6%) | |
| Angiotensin receptor blocker | 2 (9.1%) | |
| Beta blocker | 6 (27.2%) | |
| Calcium channel blocker | 4 (18.1%) | |
| Diuretics | 2 (9.1%) | |
| Insulin | 3 (13.6%) | |
| Lipid-lowering agent | 6 (27.2%) | |
| Oral diabetes medications | 5 (22.7%) | |
| Anticoagulant | 0 (0.0%) | |
| Direct oral anticoagulant | 0 (0.0%) | |
| Aspirin | 7 (31.8%) | |
| Anti-arrhythmic | 0 (0.0) | |
| Immunosuppressant | 1 (4.5%) | |
Table 3.
In-hospital outcomes of patients
| N = 22 | |
|---|---|
| Death | 2 (9.0) |
| Intensive care unit admission | 7 (31.8) |
| Mechanical ventilation | 5 (22.7) |
| ARDS | 6 (27.2) |
| Acute kidney injury | 4 (18.1) |
| Shock | 3 (13.6) |
| Ventricular arrhythmia | 1 (4.5) |
| Myocardial perfusion imaging | 8 (36.3) |
| Stress echocardiography | 14 (63.6) |
| Diagnostic catheterization | 0 (0.0) |
| Obstructive coronary artery disease | – |
| Left anterior descending artery disease | – |
| Left circumflex artery disease | – |
| Right coronary artery disease | – |
| Normal coronaries | – |
| Thrombotic occlusion | |
| Percutaneous coronary intervention | – |
Results reported as n (%) or median (interquartile range) as appropriate
Patients demonstrated a high prevalence of hypertension, diabetes mellitus, and chronic kidney disease. They also exhibited high levels of inflammatory biomarkers (e.g., interleukin-6, C-reactive protein, ferritin), serum creatinine, coagulation biomarkers (e.g., d-dimer), and serum lactate (Table 1).
All 22 patients were treated with anticoagulants, and the masses disappeared on echocardiography (Video 1) after 4–15 days (mean 7 days). This finding increases the likelihood that the masses were blood clots. Table 4 reports the in-hospital outcomes of the patients.
Table 4.
Echocardiographic data of patients enrolled in the study
| Patient number | LVEFa (%) (by Simpson’s method) | LVDFb | LV volumes: EDVc-ESVd (cc) | Regional wall motion abnormality | Global dysfunction | RVe size (cm) |
|---|---|---|---|---|---|---|
| 1 | 54 | Dys7 grade1 | 68–36.7 | Negative | Negative | 2.8 |
| 2 | 55 | Normal | 118–53.1 | Negative | Negative | 3 |
| 3 | 56 | Normal | 120–52 | Negative | Negative | 2.71 |
| 4 | 55 | Normal | 132–59 | Negative | Negative | 2.73 |
| 5 | 55 | Normal | 80–36 | Negative | Negative | 2.78 |
| 6 | 57 | Dys7 grade1 | 70–30 | Negative | Negative | 2.54 |
| 7 | 54.5 | Normal | 68–31 | Negative | Negative | 3.2 |
| 8 | 55 | Normal | 95–42.7 | Negative | Negative | 3.1 |
| 9 | 55 | Normal | 102–46 | Negative | Negative | 2.6 |
| 10 | 58 | Normal | 121–51 | Negative | Negative | 2.56 |
| 11 | 60 | Normal | 65–26 | Negative | Negative | 2.43 |
| 12 | 54 | Dys7 grade 1 | 74–34 | Negative | Negative | 2.38 |
| 13 | 54 | Normal | 140–64.5 | Negative | Negative | 2.3 |
| 14 | 54 | Normal | 142–62.3 | Negative | Negative | 3.18 |
| 15 | 56 | Normal | 133–58.5 | Negative | Negative | 2.9 |
| 16 | 55 | Normal | 138–63 | Negative | Negative | 2.97 |
| 17 | 58 | Normal | 125–52.5 | Negative | Negative | 2.48 |
| 18 | 55 | Normal | 128–57.6 | Negative | Negative | 2.43 |
| 19 | 56 | Normal | 66–29 | Negative | Negative | 3 |
| 20 | 57.5 | Normal | 130–55.2 | Negative | Negative | 3.01 |
| 21 | 58.5 | Normal | 132–54.8 | Negative | Negative | 3.15 |
| 22 | 59 | Normal | 100–41 | Negative | Negative | 3 |
aLeft ventricle ejection fraction
bLeft ventricle diastolic function
cEnd diastolic volume
dEnd systolic volume
eRight ventricle
We imported the 2D echocardiography data of the patients into MATLAB software to analyze the vector directions of the regional wall velocity per every cardiac cycle (Video 2) and the blood flow rotations and turbulence inside the left ventricle. Next, different myocardial segments were mapped out during the analysis, and colored vectors represent the velocity vectors. Results of the analysis showed changes in the blood flow path per unit area.
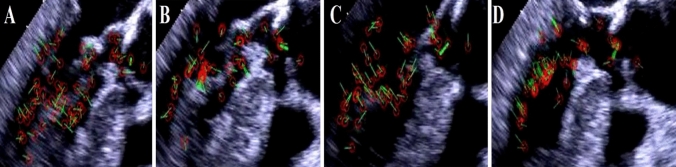
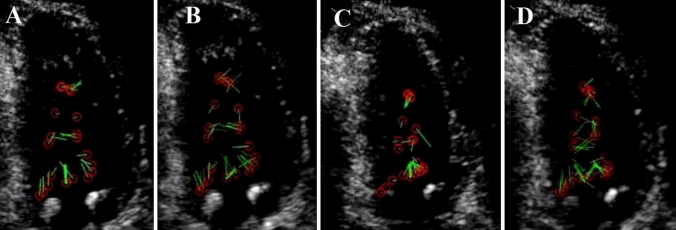
First, a delay occurred in blood circulation as compared with normal heart phases. Second, changes were noted in the amount of divergence of a bundle of selected velocity vectors for each unit area (Figs. 2B, 4B). These changes were observed with different amounts in all the studied patients. Moreover, we observed major variations in the curl of the velocity vectors within every unit area and time in different phases of the cardiac contraction cycle (Figs. 2, 4). Our observations showed that the curl value was nonzero in the rapid filling phase in all cases, which means that the blood flow was rotational (Figs. 2A, 4A), and it was zero in the slow-filling phase, which means that the blood flow was irrotational (Figs. 2B, 4B). In addition, the normalized value of the curl was nonzero in the late atrial contraction phase (Figs. 2C, 4C), during iso-volumic contraction (IVC) period, and in the ejection phase, which means that the blood flow in these phases was rotational (Figs. 2D, 4D).
Fig. 2.
Blood flow circulation inside the left ventricular cavity of a COVID-19 patient with an apical clot (patient number 8): A green velocity vectors show the blood flow during the rapid filling phase and are rotational. B Blood fluid moves in a straight direction at the diastasis phase. C In the late filling phase, the blood flow has a rotational flow again. D In the early iso-volumic contraction (IVC) period, the blood flow has a clockwise rotation toward the LV-free wall
Fig. 4.
Blood flow circulation inside the left ventricular cavity of a COVID-19 patient with a clot in the base of the interventricular septum (patient number 10): A in these transesophageal echocardiography views, green velocity vectors located under the mitral valve during the rapid filling phase have a rotational flow. B A bundle of green velocity vectors moves non-rotationally at the diastasis phase. C In the late atrial contraction time, the blood fluid had a rotational flow again and inclined toward the left ventricle's free wall. D In the early iso-volumic contraction (IVC) period, the blood flow has a clockwise rotation toward the LV-free wall
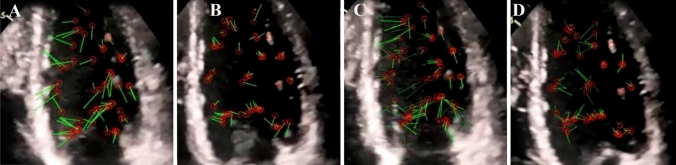
Through these modifications in the selective velocity vectors and their tracking in a cardiac cycle, we observed that the directions of the blood flow vectors were conversely different from those of normal stations. In particular, we tracked abnormal blood flow turbulence inside the LV at different diastolic and systolic phases. In all cases, the directions of blood flow inside the LV were abnormal at the end of the systolic phase, and the blood velocity vectors demonstrated a tendency to the LV-free wall at the middle and apical parts of the LV (where the clots were present) (Figs. 2D, 4D). After the clot resolved with anticoagulation therapy, blood flow direction was the same as when the clot was present, with the difference that the delay in the direction of the blood flow was reduced (Fig. 3).
Fig. 3.
Blood flow direction inside the left ventricular cavity of the same COVID-19 patient (patient number 8) after the clot resolves with anticoagulation therapy is the same as when the clot is present, with the difference that the delay in the direction of the blood flow is reduced. A Green velocity vectors show the blood flow during the rapid filling phase and are rotational. B Blood fluid moves in a straight direction at the diastasis phase. C In the late filling phase, the blood flow has a rotational flow again. D In the early iso-volumic contraction (IVC) period, the blood flow has a clockwise rotation toward the LV-free wall
In other words, in our cases, whenever the blood fluid entered the left ventricle, it exhibited a rotational flow during the rapid filling phase and then moved in a straight direction at the diastasis phase. In addition, a rotational flow was observed again in the late filling phase, followed by the early IVC period, when the blood flow traveled rotationally in a clockwise direction toward the LV-free wall (Figs. 2, 4, Videos 3 and 4). Also, we observed that the blood velocity vectors were relocated to the free wall of the LV during the beginning of mitral valve opening.
Overall, MATLAB software calculations showed anomalous blood vortices inside the LV cavity of COVID-19 patients with normal myocardium and clot in the left ventricle.
Discussion
Mathematics can assist us in understanding the mechanism of the cardiac motion and blood flow directions inside the heart [15–18]. In this regard, MATLAB software is very helpful for calculating and showing the velocity vectors of the LV myocardial samples using motion and deformation echocardiographic data.
In this study, patients hospitalized due to COVID-19 underwent 2D echocardiography examinations.
COVID-19 infection causes a complex interplay of inflammation-related factors such as von Willebrand factor and coagulation factor VIII release, complement activation, and fibrinogen increase, as well as cytokine storm, along with reduced endothelial anti-thrombogenicity due to vascular injury. These factors cause a decrease in blood flow in arteries, veins, and capillaries and lead to thrombus formation. On the other hand, there is a fact that without conditions such as hyper-eosinophilic syndrome (Loffler) or a severe decrease in ejection fraction or wall motion abnormality due to myocardial infarction or primary blood disorders, the formation of clots in different origins of the heart is sporadic. So we went to explore other causes for this phenomenon.
We included patients with normal noninvasive cardio-vascular diagnostic tests and normal myocardial motion in echocardiography and biochemical examinations but with a mass in their left ventricle, for example, in the apex of the heart or in the base of the intraventricular septum area in the study. All included patients were treated with anticoagulant agents during hospitalization, and the masses disappeared within days, which thus supports that the masses were most likely clots. The motion and deformation echocardiographic data were imported into MATLAB software to display the velocity vectors of the blood inside the left ventricle.
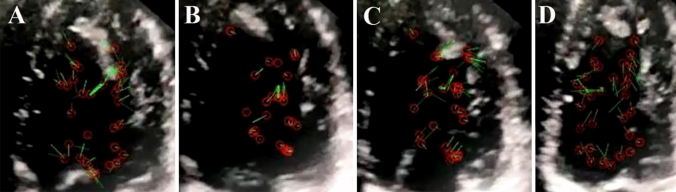
In normal cases, blood flows inside the left ventricle through the mitral valve, hits the lateral LV-free wall, experiences clockwise turbulences at the apex, hits the septal wall, and flows toward the aortic valve (Fig. 1A–D) [19–21]. Analyzing these data using the MATLAB program, we determined that in the rapid filling phase of a normal heart, blood flow inside the left ventricle is irrotational. Conversely, blood moves rotationally in a clockwise direction at the diastasis phase and irrotationally in the late filling phase. This is followed by the early IVC period, during which the blood flow travels rotationally in a counterclockwise direction. In the same way, the vortex located in the proximity of the anterior mitral leaflet in the LV outflow tract (LVOT) region moves in a counterclockwise direction. During the late IVC period, the vortex remains in the LVOT region, and its direction is toward the aortic valves (Fig. 1A–D and Video 5) [22].
Fig. 1.
Blood circulation in a normal heart left ventricle: A the rapid filling phase has an irrational flow shown by green velocity vectors. B Blood as a fluid moves rotationally in a clockwise direction at the diastasis phase. C In the late filling phase, blood has an irrational flow (a non-rotational bunch velocity vectors) obscuring the vortex. D In the early iso-volumic contraction (IVC) period, the blood flow has a counterclockwise rotation. During the late IVC phase and ejection time, the vortex remains in the LVOT region, and the blood flows directly toward aortic valves
Based on previous studies, it seems that the direction of blood flow in healthy people and normal cases follows a common pattern [22].
However, in our cases, we observed that whenever the blood fluid entered the left ventricle, it demonstrated a rotational flow during the rapid filling phase and then moved in a straight direction during the diastasis phase. This reversal of flow direction might be a main reason for clot formation at the apex of the heart. However, the rotational flow was observed again in the late filling phase, followed by the early IVC period, where the blood flow traveled rotationally in a clockwise direction toward the LV-free wall (Figs. 2, 3, 4, Videos 3 and 4). We also observed a delay, which means a slower blood flow inside the left ventricle compared to the normal heart phase. These abnormal flow directions could be a potential etiology for clot formation in the intraventricular septum area.
The anomalous blood trajectories inside the LV cavity suggest that cardiac wall motion in COVID-19 patients is not satisfactorily able to circulate the blood fluid in normal directions. However, regardless of the normal myocardial motion in our patients, what causes this abnormal blood flow?
COVID-19 is an acute infectious disease, and according to Sloop et al. [12], both acute and chronic inflammation exhibit the potential to increase blood viscosity. Because blood is a non-Newtonian fluid (as its viscosity varies with its shear rate), the increase in blood viscosity (caused by inflammation) in low-shear conditions can increase the risk of thrombosis on its own. Conversely, an increase in blood viscosity can lead to an increase in weight and pressure per unit area inside the heart, a decrease in kinetic energy, and an increase in inertial energy. These changes might cause the inability of the normal myocardium to lead the blood in its correct direction during a cardiac cycle and thus change the blood flow vectors inside the heart cavities (Figs. 2A–D, 3A–D and Videos 3 and 4) [23–25]. Based on these findings, the behavior of the blood inside the LV of COVID-19 patients is more like an abnormal Newtonian fluid (Figs. 2, 3, 4). The delay (slower blood flow inside the left ventricle) can be due to the abnormal blood flow direction and/or the increased viscosity of blood, which creates a force in the opposite direction of the blood flow. This viscous force is something like friction that slows down the movement of blood fluid. The above-mentioned conditions can be considered as possible etiologies for the clots inside the heart cavities of COVID-19 patients with normal myocardial motion [26].
Since the blood inside the left ventricular cavity is in interaction with the myocardial muscle as a fluid, according to Newton's laws and Euler Lagrange's laws, changes in blood specification may cause changes in the myocardium, such as the myocardial wall thickening or abnormal changes in the movement of the ventricular myocardial wall in the long term. Proving this hypothesis requires a separate study on COVID-19 patients over an extended period.
Conclusion
In patients with COVID-19, 2D echocardiography images and their mathematical analysis using MATLAB software revealed an abnormal turbulence of blood flow vortices inside the normal left ventricle, which could be considered one of the etiologies of clot formation in the left ventricle of these patients. This phenomenon can be explained by viscosity changes in the blood or its contents that upset the equilibrium between the myocardium and blood fluid which needs blood viscosity measurement in these patients. Ultimately, these clots might result in embolization, myocardial infarction, or cerebrovascular accident in COVID-19 patients with a normal heart.
Limitations
Our study’s limitations include a relatively small number of patients with paired follow-up echocardiograms. In addition, because of technical problems, we were unable to measure the blood viscosity in patients, which, if done, could be a great help in proving our hypothesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1: 2D echocardiography of a case with an apical clot (patient number 8) after anticoagulation therapy: The mass vanished, and the left ventricular myocardial motion is still normal. Fifteen echocardiographic segments were selected and tracked per cardiac cycle (MP4 491 KB)
Video 2: Imported original 2D echocardiography video file of a patient with an apical clot (patient number 3) into the MATLAB software: 13 echocardiographic segments were selected and tracked per a cardiac cycle before running the MATLAB. Green vectors represent normal velocity vectors and show the normal direction of the chosen point movements during the cardiac cycle (MP4 467 KB)
Video 3: 2D rendering of the abnormal blood velocity vectors inside the LV of a patient with an apical clot (patient number 3) by the MATLAB software: 60 blood velocity vectors were tracked. During the rapid filling phase, the blood fluid has a rotational flow and moves in a straightforward direction at the diastasis phase. In the late filling phase, the blood fluid has a rotational flow again. This was followed by the early iso-volumic contraction (IVC) period, where the blood flow travels rotationally in a clockwise direction toward the LV-free wall (MP4 1356 KB)
Video 4: 2D rendering of the abnormal blood velocity vectors inside the LV of a patient with a clot in the base of the interventricular septum (patient number 18) by MATLAB software: The abnormal blood flow turbulence inside the left ventricle of the heart is observed in different cardiac phases. Sixty blood velocity vectors were tracked. The abnormal blood flow directions inside the LV at the end-systolic phase show that detected blood velocity vectors tend to the LV-free wall at the middle part of the LV (MP4 1637 KB)
Video 5: A 2D rendering of normal blood velocity vectors inside the LV by MATLAB software: We tracked 60 selected green vectors. Blood fluid circulations in the rapid filling phase have irrational flow. In the diastasis phase, the blood fluid moves rotationally in a clockwise direction, and in the late filling phase, the circulation is characterized by an irrational flow obscuring the vortex. After that, in the early iso-volumic contraction (IVC) period, the blood flow travels rotationally counterclockwise. The vortex was relocated in the proximity of the anterior mitral leaflet in the LVOT region and had a counterclockwise movement. During the late IVC period, the vortex of the left ventricular outflow tract region shows flow direction toward the aortic valve (MP4 590 KB)
Author contributions
MK and LPB participated in the study design, data gathering, interpretation, writing, and manuscript revision. AMT participated in the study design, validation of data, writing the manuscript, and manuscript revision. SR also contributed to the mathematics calculation, computer and computational work, statistical analysis, and writing and correction of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mersedeh Karvandi, Email: mr.karvandi@sbmu.ac.ir.
Arash Mohammadi Tofigh, Email: arash_mtofigh@yahoo.com.
Saeed Ranjbar, Email: sranjbar28@gmail.com.
Luigi P. Badano, Email: ihhsurgery@gmail.com
References
- 1.Wenzhong L, Hualan L. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. 2020. 10.26434/chemrxiv.11938173.v7(published online April 27).
- 2.Yue H, Bai X, Wang J, Yu Q, Liu W, Pu J, et al. Gansu Provincial Medical Treatment Expert Group of COVID-19. Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann Palliat Med. 2020;9(4):1404–1412. doi: 10.21037/apm-20-887. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of Myocardial Injury in Patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Special article—acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis. 2020;63(5):682–689. doi: 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schey HMD. Grad, curl, and all that: an informal text on vector calculus. New York: Norton; 1997. [Google Scholar]
- 9.Stewart J. Calculus. Section 13.4 “The Cross Product”, 6th ed. Thomson; 2008.
- 10.Weinreich G. Geometrical vectors. 1. Chicago: University of Chicago Press; 2020. [Google Scholar]
- 11.Green S. Fluid vortices. Berlin: Springer; 2012. [Google Scholar]
- 12.Sloop GD, De Mast Q, Pop G, et al. The role of blood viscosity in infectious diseases. Cureus. 2020;12(2):e7090. doi: 10.7759/cureus.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong WF, Ryan T. Feigenbaum’s echocardiography. 8. Philadelphia: Lippincott Williams & Wilkins; 2019. [Google Scholar]
- 14.Chapman SJ. MATLAB programming for engineers. Boston: Cengage Learning; 2019. [Google Scholar]
- 15.Arnol’d VI. Mathematical methods of classical mechanics. New York: Springer; 2019. [Google Scholar]
- 16.Truesdell CA. A critical summary of developments in nonlinear elasticity. J Ration Mech. 1952;1:125–300. [Google Scholar]
- 17.Ranjbar S, Karvandi M, Hassantash SA, Foroughi M. How to construct a 3D mathematical/computer model of the left ventricle? Arch Cardiovasc Imaging. 2014;2:e20628. doi: 10.48550/arXiv.1406.6150. [DOI] [Google Scholar]
- 18.Ranjbar S. Karvandi M. Ajzachi M. System and method modeling left ventricle of heart. US Patent 2013; published online April 9; patent number US 8,414,490 B2. https://patents.google.com/patent/US8414490B2/en?oq=+patent+number+US+8%2c414%2c490+B2.
- 19.Ranjbar S, Karvandi M, Ajzachi M. Solution Navier-stocks equations of the blood as a non-Newtonian fluid in the left ventricle. US Patent 2014; published online Aug 12; patent number US 8,805,663 B2. https://patents.google.com/patent/US8805663B2/en?oq=+patent+number+US+8%2c805%2c663+B2.
- 20.Hendabadi S, Bermejo J, Benito Y, Yotti R, Fernández-Avilés F, del Álamo JC, et al. Topology of blood transport in the human left ventricle by novel processing of Doppler echocardiography. Ann Biomed Eng. 2013;41(12):2603–2616. doi: 10.1007/s10439-013-0853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranjbar S, Karvandi M, Hassantash SA. Dynamic features creating (which cause) the blood direction inside the left ventricle. IJMI. 2012;2:14–18. doi: 10.11648/j.ijmi.20140202.12. [DOI] [Google Scholar]
- 22.Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK, et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging. 2008;1(6):705–717. doi: 10.1016/j.jcmg.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranjbar S, Sardari Nia P, Karvandi M, Maessen J. Computational fluid dynamics in aortic arch pathophysiology. Eur J Cardiothorac Surg. 2017;51(2):398. doi: 10.1093/ejcts/ezw286. [DOI] [PubMed] [Google Scholar]
- 24.Belohlavek M. Vortex formation time: an emerging echocardiographic index of left ventricular filling efficiency? Eur Heart J Cardiovasc Imaging. 2012;13(5):367–369. doi: 10.1093/ejechocard/jer311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faludi R, Szulik M, D’hooge J, Herijgers P, Rademakers F, Pedrizzetti G, et al. Left ventricular flow patterns in healthy subjects and patients with prosthetic mitral valves: an in vivo study using echocardiographic particle image velocimetry. J Thorac Cardiovasc Surg. 2010;139(6):1501–1510. doi: 10.1016/j.jtcvs.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 26.Tofigh AM, Karvandi M, Coscas R. Current incidence of peripheral arterial embolism and role of echocardiography. Asian Cardiovasc Thorac Ann. 2008;16(6):439–443. doi: 10.1177/021849230801600602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: 2D echocardiography of a case with an apical clot (patient number 8) after anticoagulation therapy: The mass vanished, and the left ventricular myocardial motion is still normal. Fifteen echocardiographic segments were selected and tracked per cardiac cycle (MP4 491 KB)
Video 2: Imported original 2D echocardiography video file of a patient with an apical clot (patient number 3) into the MATLAB software: 13 echocardiographic segments were selected and tracked per a cardiac cycle before running the MATLAB. Green vectors represent normal velocity vectors and show the normal direction of the chosen point movements during the cardiac cycle (MP4 467 KB)
Video 3: 2D rendering of the abnormal blood velocity vectors inside the LV of a patient with an apical clot (patient number 3) by the MATLAB software: 60 blood velocity vectors were tracked. During the rapid filling phase, the blood fluid has a rotational flow and moves in a straightforward direction at the diastasis phase. In the late filling phase, the blood fluid has a rotational flow again. This was followed by the early iso-volumic contraction (IVC) period, where the blood flow travels rotationally in a clockwise direction toward the LV-free wall (MP4 1356 KB)
Video 4: 2D rendering of the abnormal blood velocity vectors inside the LV of a patient with a clot in the base of the interventricular septum (patient number 18) by MATLAB software: The abnormal blood flow turbulence inside the left ventricle of the heart is observed in different cardiac phases. Sixty blood velocity vectors were tracked. The abnormal blood flow directions inside the LV at the end-systolic phase show that detected blood velocity vectors tend to the LV-free wall at the middle part of the LV (MP4 1637 KB)
Video 5: A 2D rendering of normal blood velocity vectors inside the LV by MATLAB software: We tracked 60 selected green vectors. Blood fluid circulations in the rapid filling phase have irrational flow. In the diastasis phase, the blood fluid moves rotationally in a clockwise direction, and in the late filling phase, the circulation is characterized by an irrational flow obscuring the vortex. After that, in the early iso-volumic contraction (IVC) period, the blood flow travels rotationally counterclockwise. The vortex was relocated in the proximity of the anterior mitral leaflet in the LVOT region and had a counterclockwise movement. During the late IVC period, the vortex of the left ventricular outflow tract region shows flow direction toward the aortic valve (MP4 590 KB)
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.