Abstract

Enhancing catalytic activity through synergic effects is a current challenge in homogeneous catalysis. In addition to the well-established metal–metal and metal–ligand cooperation, we showcase here an example of self-activation by the substrate in controlling the catalytic activity of the two-coordinate iron complex [Fe(2,6-Xyl2C6H3)2] (1, Xyl = 2,6-Me2C6H3). This behavior was observed for aryl acetylenes in their regioselective cyclotrimerization to 1,2,4-(aryl)-benzenes. Two kinetically distinct regimes are observed dependent upon the substrate-to-catalyst ratio ([RC≡CH]0/[1]0), referred to as the low ([RC≡CH]0/[1]0 < 40) and high ([RC≡CH]0/[1]0 > 40) regimes. Both showed sigmoidal kinetic response, with positive Hill indices of 1.85 and 3.62, respectively, and nonlinear Lineweaver–Burk replots with an upward curvature, which supports positive substrate cooperativity. Moreover, two alkyne molecules participate in the low regime, whereas up to four are involved in the high regime. The second-order rate dependence on 1 indicates that binuclear complexes are the catalytically competent species in both regimes, with that in the high one being 6 times faster than that involved in the low one. Moreover, Eyring plot analyses revealed two different catalytic cycles, with a rate-determining step more endergonic in the low regime than in the high one, but with a more ordered transition state in the high regime than in the low one.
Keywords: cooperativity, iron, alkynes, cyclotrimerization, kinetic studies
Introduction
Cooperativity and synergistic effects have tremendous utility in the activation of small molecules and in homogeneous catalysis.1 Current interest focuses on the role of cooperating ligands in the synergistic activation of a substrate and in their behaviors as electron/proton reservoirs, which arises from reversible redox activity or easy aromatization/dearomatization processes, respectively.2 Besides this metal–ligand cooperation, polymetallic systems can benefit from metal–metal cooperation to activate one or two different substrates in a synergistic way, which is unattainable for the corresponding mononuclear species.3 Moreover, polymetallic catalysts showing redox cooperativity generally perform better at multielectron redox reactions.4 Recently, a combination of both metal–metal and metal–ligand cooperation has been observed in binuclear iron complexes.5
Another option involves the substrates behaving as the main cooperative actors.6 This approach is particularly well suited to many enzymes, such as diverse cytochrome P450 isoforms and phosphatases, and is also observed in organocatalysis.7 In this scenario, the binding of the first molecule of substrate increases the binding affinity of a second such that the catalytic activity is controlled by the concentration of the substrate in the reaction media. This self-activation by the substrate, also termed homotropic cooperativity, is characterized by kinetic patterns strongly deviated from the typical hyperbolic Michaelis–Menten curve and can be identified from kinetic studies.6
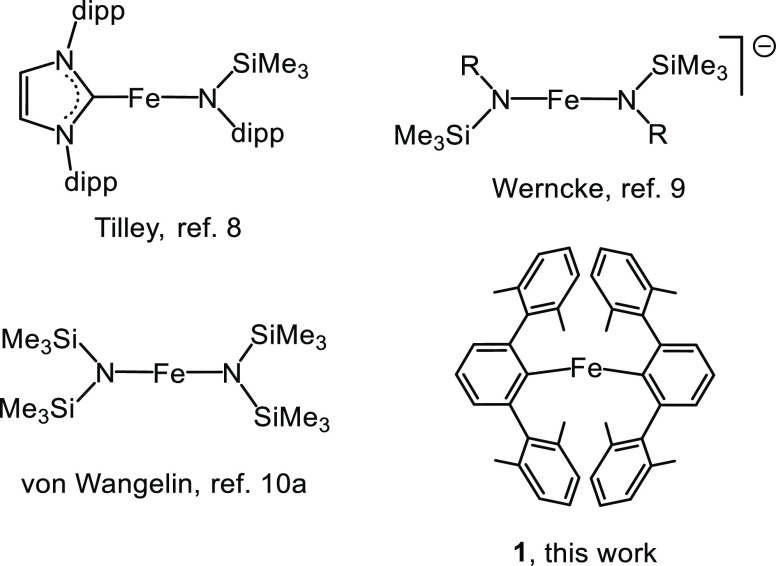
Herein, we showcase an unprecedented case of homotropic cooperativity in the regioselective [2+2+2]-cycloaddition of acetylenes to 1,2,4-(aryl)benzenes catalyzed by a two-coordinate iron complex (1, Figure 1). The potential of such complexes in this field is poorly explored, with complexes [Fe(IPr){N(SiMe3)(dipp)}],8 [Fe{N(SiMe3)(R)}2]−,9 and [Fe{N(SiMe3)2}2],10 as the sole previously reported examples (Figure 1).
Figure 1.
Two-coordinate iron complexes as catalysts for alkyne cyclotrimerization, R = Me3Si, dipp; dipp = 2,6-iPr2C6H3.
Related iron catalysts for this reaction11 include isolated low-valent iron(0) complexes such as tri-/tetra-coordinated complexes with diphosphines,12N-heterocyclic carbenes,13 diolefins,14 and pyrimidinediimine ligands.15 This last example exhibits an unusual 1,3,5-regioselectivity; typically the major product is the unsymmetrical 1,2,4-arene. In other instances, hydride iron complexes formed in situ such as [FeI(pincer)(H)],16 and [FeII(salen)(H)]17 have been proposed as the active species. Additionally, different combinations of iron salts and reductants18 or activators,19 as well as a three-component system (FeCl2/photoredox catalyst/reducing agent)20 also result in catalytically competent low-valent iron species.
Results and Discussion
An initial assessment for the catalytic activity of [Fe(2,6-Xyl2C6H3)2] (1, Xyl = 2,6-Me2C6H3, Figure 1)21 was performed with phenylacetylene (5 mol % 1) in C6D6 at 60 °C (entry 1, Figure 2). The reaction was monitored by 1H NMR spectroscopy requiring around 13 h to achieve a good conversion (87%). The major product from the reaction was found to be the unsymmetrical 1,2,4-tri(phenyl)benzene (2) along with the symmetrical 1,3,5-tri(phenyl)benzene (3) and a small amount of unidentified oligomers with a ratio 2:3:others of 76:11:13 (Scheme 1; Figure 2, entry 1).
Figure 2.
Conversion (%) vs time (min) plots (entries 1–4, left) and time-adjusted profiles (entries 1–3, right) for the cyclotrimerization of PhC≡CH (S) catalyzed by 1. Experimental conditions and colors correspond to that shown in the figure. Dioxane (5 μL, 0.058 mmol) was used as internal standard. Dashed lines are for visual aid.
Scheme 1. Phenylacetylene Cyclotrimerization Catalyzed by Complex 1.
A noticeable decrease in the reaction time was observed with a larger substrate loading and identical initial concentration of the precatalyst 1 (Figure 2, entry 2). Under these conditions, 90% of conversion takes place in around 6 h with slightly better yield and selectivity (ratio 2:3:others of 80:13:7).
A particular feature of these two catalytic runs is their kinetic profiles, which were initially analyzed by using the general equation for an nth-order reaction for the substrate.22 The best fit for run 2 was obtained when plotting 1/[PhC≡CH] versus time, suggestive of a second-order reaction for the alkyne (Figure S3). Noticeably, an apparent fractional order (n) of 1.5 was obtained for run 1. A straight line required plotting 1/([PhC≡CH])0.5versus time (Figure S3).
This change in the apparent reaction order in PhC≡CH can be attributed to either the initial concentration of phenylacetylene or to the [PhC≡CH]0/[1]0 ratio. In order to discriminate between these, additional experiments at similar [PhC≡CH]0 but with [PhC≡CH]0/[1]0 ratios of 36 and 100 were carried out (Figure 2, entries 3 and 4, respectively). Run 3 showed again a good averaged fit to n = 1.5. However, the best fit for run 4 corresponds to an unusually high value of n = 2.9 (see below), which clearly indicates that the [PhC≡CH]0/[1]0 ratio plays a more important role than [PhC≡CH]0 (Figure S3).
Additional information was gathered from the time-adjusted profiles23 for runs 1–3 (with identical [1]0 and temperature), which were obtained by shifting the time scale of runs 3 and 1 to the point in which run 2 (with the highest substrate concentration) reaches [PhC≡CH] = 0.479 and 0.266 M, respectively. As shown in Figure 2 (right), once run 2 (in red) reaches a [PhC≡CH] = 0.479 M, it follows the same profile as run 3 (in orange). The same applies to runs 2 and 3 when reaching a [PhC≡CH] = 0.266 M that overlap with run 1. This excellent superposition clearly indicates that product inhibition and catalyst deactivation processes are negligible. In other words, evolution of the catalysis depends on the concentration of phenylacetylene in the reaction media (regardless of their initial concentration) and confirms that only the intrinsic kinetics contributes to the observed reaction rate.
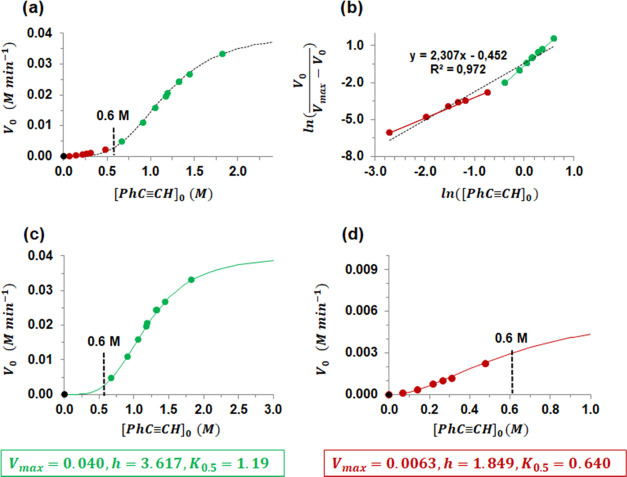
A second snapshot of this unusual behavior was attained by evaluating the dependence of the initial rate (V0) with the initial concentration of phenylacetylene at the same catalyst concentration ([1]0) (Table S1). Saturation kinetics with an increase of the initial concentration of phenylacetylene was observed, but, to our surprise, the curve displayed significant deviation from the hyperbolic Michaelis–Menten behavior, showing a sigmoidal shape. This feature implies that the overall catalytic process is cooperative in nature (Figure 3a).
Figure 3.
(a) Sigmoidal shape of the plot V0vs [PhC≡CH]0 and the simulated best fit (black line) for all of the experimental data: Vmax = 0.040 ± 0.0016 M min–1, K0.5 = 1.17 ± 0.031 M, h = 3.62 ± 0.20 M. (b) Linear plot of the Hill equation clearly showing two straight lines for Vmax = 0.040 M min–1. Experimental (circles) and simulated (lines) V0vs [PhC≡CH]0 plots for the high (c) and low (d) regimes, respectively. Parameters for the simulations of (c) and (d) are included in the green/red squares, respectively. Experimental conditions: [1]0 = 0.0133 M, T = 60 °C.
The data were then analyzed using eq 1, where Vmax refers to the maximum reaction rate, K0.5 is the substrate concentration that results in a reaction rate of 0.5(Vmax), and h (unitless), also known as the Hill index, reflects the level of cooperativity. Values of h > 1 indicate positive cooperativity, whereas h < 1 indicates negative cooperativity. Noncooperative processes correspond to h = 1, and eq 1 reduces to the Michaelis–Menten equation (eq 2).
| 1 |
| 2 |
The major difference between the Michaelis–Menten behavior (eq 2) and those following eq 1 concerns the number of preequilibria previous to the rate-determining step that takes place, which is reduced to one in the latter case (cat + S ⇌ ′cat-S′ → Product).
The best fit24 for all of the data produced the following parameters: Vmax = 0.040 ± 0.0016 M min–1, K0.5 = 1.17 ± 0.031 M, h = 3.62 ± 0.20, although it is not sufficient for [PhC≡CH] below 0.6 M (Figure 3a). Indeed, the linear plot of eq 1 (whose slope is h) for Vmax = 0.040 gives an average value of h = 2.3 (Figure 3b, black line), quite far from the above-estimated value of 3.6. Interestingly, this linear plot revealed two well-defined regimes, namely, low (in red) and high (in green) (Figure 3b).
If we fit the data to the Hill equation for the high and low regimes independently, the plots in Figure 3c,d, respectively, are obtained. The value of Vmax = 0.040 ± 0.0016 M min–1 for the high regime was directly obtained from the data. However, Vmax for the low regime showed a large error (0.0078 ± 0.0039 M min–1) because of the lack of data at a high concentration of phenylacetylene in this regime. A more precise value of Vmax for this regime of 0.0063 ± 0.00019 M min–1 was independently estimated by alternative methods (see below and Figure S1).
The positive Hill index (h) in both regimes indicates positive cooperativity such that coordination of one molecule of phenylacetylene to the catalyst facilitates the binding of the next, thus enhancing the catalytic activity. Moreover, analysis of the data using the Lineweaver–Burk replot showed the characteristic nonlinear upward curve for both regimes, again indicating positive cooperativity (Figure S2). The Hill index value of 1.85 in the low regime implies that cooperation is restricted to two molecules of phenylacetylene, which increases to four molecules at high [PhC≡CH]0/[1]0 ratio (high regime, h = 3.62).
For a catalytic system (cat) capable of interacting reversibly with up to two molecules of substrate (S) (eq 3), the Hill index reflects the relative values of the equilibrium constants K1 and K2
| 3 |
such that positive cooperativy (h > 1) means that K2 > K1. If the system were “infinitely” cooperative (K2 ≫> K1) such that only species cat and cat-S2 are present in the reaction, a value of h = 2 is expected. Thus, the observed value of 1.85 in the low regime indicates a high level of cooperativity.
The same applies if four equilibria to cat-S4 are involved, where a value of h = 4 would reflect “infinitely” cooperative behavior involving four molecules of substrate and only the species cat and cat-S4 in the reaction media. Accordingly, the value of 3.62 estimated for the high regime is again indicative of a high level of cooperativity.
At this point, it is interesting to comment that, for kinetics following eq 1, apparent partial reaction orders in the substrate from zero to h can be observed depending on the relative values of K0.5 and [S]
It is worth noting that the Hill indexes calculated represent the maximum partial reaction order in the substrate that could be observed in each regime. The observed rate order will be dependent upon the concentration of the substrate. For experiments in the low regime ([PhC≡CH]0/[1]0 < 40) and [1]0 ≈ 0.013 M, the data is represented closer to Scenario A, where the reaction is operating close to the maximum partial reaction order. Consequently, the observed partial reaction order in the alkyne of 1.50 (Figure 2, entries 1 and 3) matches well with the estimated value of h = 1.85.
For experiments in the high regime ([PhC≡CH]0/[1]0 = 100), two different apparent partial reaction orders in the alkyne were observed: 2 and 2.9 (entries 2 and 4, Figures 2 and S3, respectively). The former, which contains a [PhC≡CH]0 ≈ 1.33 M, is in the region between both Scenario A and B where (K0.5)h is comparable to [S]h. This leads to a discrepancy of the partial reaction order of PhC≡CH to the maximum value of h (2 vs 3.82).
Lowering the [PhC≡CH]0 to 0.47 M (at the same [PhC≡CH]0/[1]0 ratio of 100), the experimental conditions approach Scenario A (the term [S]h becomes smaller) and consequently, the apparent partial reaction order in the alkyne should increase (approaching to the maximum value of 3.62). Therefore, the value of 2.9 (entry 4, Figure 2) agrees with that expected for a system that follows eq 1.
It should be emphasized that noninteger (1.5) or excessively high (2.9) values, when fitting the data to the general power law V = k[S]n, are not directly related to the molecularity of any individual elementary step in the mechanism.
Further insight came from the determination of the reaction order for precatalyst 1. For these studies, the standard ‘normalized time scale method‘25 cannot be used since it requires varying [1]0 at a fixed [PhC≡CH]0. This methodology is associated with a change in the [PhC≡CH]0/[1]0 ratio, which in our case is associated with a change in the kinetics. Consequently, experiments were performed varying [1]0 at fixed [PhC≡CH]0/[1]0 ratios of 20 and 100 (with an additional experiment at a ratio = 36 to ensure the goodness of the results) and analyzed by the “adjusted normalized time scale method.”25 To this end, the x-axis of the standard [substrate] vs t[cat]n plots was shifted the standard time adjustment (τ) multiplied by 0.0133 (the initial concentration of 1 in the experiment that contains the highest initial concentration of phenylacetylene) to the power of the order in the catalyst. A good overlap of the substrate profiles was observed for a partial reaction order of 2 for the catalyst (Figure 4).
Figure 4.
Adjusted normalized time scale plots showing the overlapping of the profiles for a partial order in the catalyst of 2. The experimental conditions can be found in Table S2: green circle (run 1), red diamond (run 2), orange diamond (run 3), blue diamond (run 4), pink diamond (run 5), light blue circle (run 6), and yellow circle (run 7). [1]0 = 0.0133 M corresponds to the reference trace (run 2).
In addition, a plot of ln(kobs) vs ln([1]0) for the catalytic runs in the low regime (Table S2), which all displayed the same apparent order in [PhC≡CH] (n = 1.5), also indicated a partial reaction order in the catalyst of 2. A straight line with a slope of 2.08 ± 0.00011 was obtained (Figure S4). Moreover, the y-intercept gives a value for kcat (low) = 35.5, which leads to Vmax = kcat ([1]0)2 = 0.0063 ± 0.00019 M min–1 for a [1]0 = 0.0133 M. This value produces a more precise sigmoidal curve for the low regime (Figure 3d). Conversely, the value of Vmax = 0.04 M min–1 (Figure 3c) corresponds to a value for kcat (high) = 226.1. These results allow us to conclude that the catalytically active species in the high regime reacts around 6 times faster than those in the low regime. Moreover, the homotropic cooperativity, involving up to four molecules of alkyne, accounts for the considerable 54% decrease of the reaction time (from 13 to 6 h) on increasing [PhC≡CH]0 from 0.26 to 1.33 M (Figure 2).
Further support for the binuclear nature of the active species was established by an additional experiment using a substoichiometric amount (0.2 mol-equiv relative to 1) of PMe3 as a catalyst poison. The reaction is slower in the presence of PMe3 with a kobs (PMe3)/kobs (none) ratio (6.073 × 10–3/1.91 × 10–2) of 0.32 (Figure 5, left). This value fits with that expected for a second-order reaction in 1 and PMe3 sequestering a binuclear entity. In this scenario, PMe3 causes a reduction of 0.4 mol-equiv in the catalyst, leading to a ratio [1-PMe3]0/[1-none]0 of 0.6. Since kobs is a function of ([1]0)2, a value for kobs (PMe3)/kobs (none) of 0.36 (0.62) is expected.26
Figure 5.
Left: Plot of 1/[PhC≡CH] (M–1) vs time (min) in the absence of additives (black diamonds) and with 0.2 mol-equiv of PMe3 relative to 1 (blue circles). Right: Eyring plots for the high (green) and low (red) regimes over the temperature range of 40–100 °C.
The effect of temperature was analyzed by performing the catalysis at 40, 80, and 100 °C for both regimes. At room temperature (≈25 °C), the catalysis was found to be very slow achieving only a 32% conversion after 16.5 h (high regime). At all temperatures studied, an apparent reaction order in phenylacetylene of 2 was observed for a ratio of [PhC≡CH]0/[1]0 = 100, but of 1.5 with a ratio of [PhC≡CH]0/[1]0 = 20 (Figure S5). Consequently, temperature does not affect the apparent reaction order in PhC≡CH, which is mainly controlled by the [PhC≡CH]0/[1]0 ratio.
From these data, Eyring plots of ln(kobs/T) vs 1/T over the temperature range of 40–100 °C give the activation parameters ΔH≠ = 8.31 kcal mol–1, ΔS≠ = −41.50 cal mol–1 K–1 and ΔH≠ = 13.98 kcal mol–1, ΔS≠ = −27.40 cal mol–1 K–1 for the high and low regimes, respectively, confirming the presence of two different catalytic cycles (Figure 5, right). The large and negative activation entropy in the high regime can be seen as diagnostic of a highly ordered transition state in the rate-determining step, which is less ordered in the low regime cycle. Conversely, this step is more endergonic in the low regime than in the high regime.
Additionally, the progress of the catalysis in the presence of equimolar amounts of PhC≡CH and PhC≡CD (ratio 1:PhC≡CH:PhC≡CD = 1:50:50 and 1:10:10, for the high and low regimes, respectively) intermolecular competition27 was monitored to determine the isotope effect. This method was chosen mainly because cyclotrimerization of PhC≡CH and PhC≡CD occurs under exactly the same conditions, avoiding experimental errors, and thus the ratio of the products (PH/PD) can be measured by NMR spectroscopy with good precision. Small values of PH/PD of 1.07 and 1.09, for the high and low regimes, respectively, were observed. Therefore, neither the rate-determining step nor the preequilibria detected by the sigmoidal behavior (Figure 2) involve the cleavage of the C–H bond.
To help identify the species formed prior to the rate-determining step, stoichiometric reactions with two and four equivalents of PhC≡CH (relative to 1) were followed by 1H NMR spectroscopy. In both cases, the slow transformation of PhC≡CH into the corresponding trimers takes place, whereas complex 1 was the only observed organometallic species, a clear indication that K1 in our case is small.
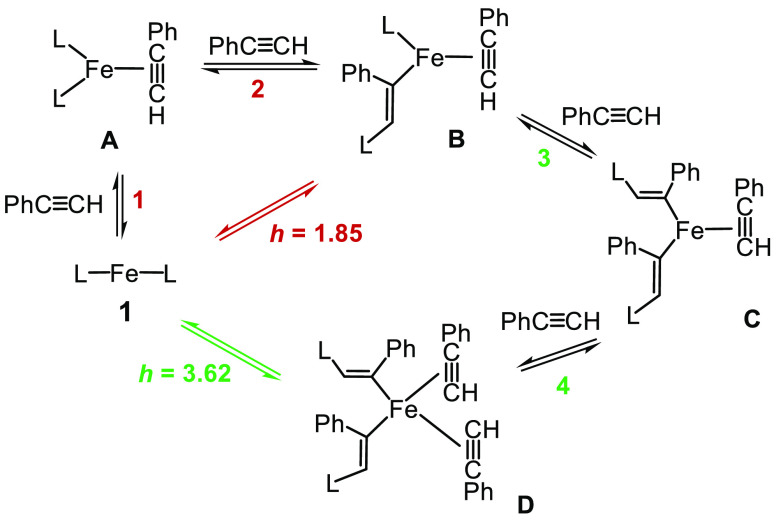
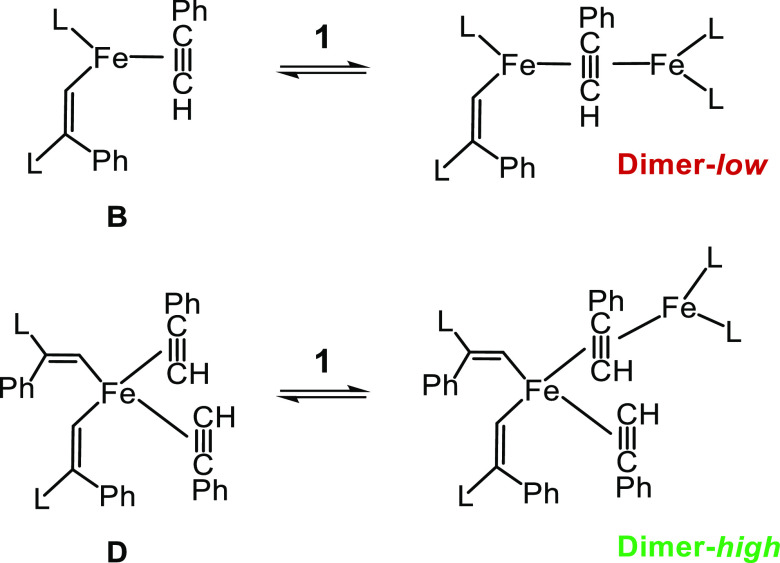
With the information gathered from the Eyring analysis, KIE measurements, and kinetic and stoichiometric studies, a precise proposal on what is happening in the reaction media is, unfortunately, unavailable. Nonetheless, a reasonable picture is shown in Schemes 2 and 3.
Scheme 2. Proposed Preequilibria Undergone by Complex 1 and PhC≡CH; L = 2,6-Xyl2C6H3.
Scheme 3. Proposed Catalytically Active Binuclear Species in the Low and High Regimes; L = 2,6-Xyl2C6H3.
From the literature, reversible acetylene insertion has been shown to be possible using various metals (Nb,28 Rh,29 and Ru).30 These reactions serve as a basis for the reactivity observed as there can be no cleavage of the acetylene C–H bond during the reaction. Inserting the terphenyl into the acetylene can open up the coordination sphere, allowing more acetylene to bind. Thus, starting from complex 1, coordination of the first molecule of PhC≡CH would give the tricoordinated species A.31 At this point, molecular models revealed that there is not enough space in A to accommodate a second molecule of phenylacetylene. Consequently, insertion of the acetylene into Fe–C bond is proposed to provide access to the second molecule of PhC≡CH. This insertion reaction is associated with a small change in the C–H bond (change of hybridization of the carbon from sp to sp2) and therefore a small KIE is expected as observed. Through this second equilibrium, species B would be formed. Since B is less crowded than A, equilibrium 2 is expected to be shifted to the right (K2 ≫ K1).
At a high concentration of phenylacetylene, a third molecule of PhC≡CH could reproduce the reaction to give intermediate C (less crowded than B), allowing then the easy entry of the fourth acetylene to yield D. The values of the Hill indexes of 1.85 and 3.62 agree with this proposal, with mainly complex 1 and intermediate B in the low regime, but 1 and D in the high regime. In this scenario, a low value for K1 is required to account for complex 1 as the only observable species even with a ratio [PhC≡CH]0/[1]0 = 150, the highest experimentally feasible.
The next point concerns the catalytically active species in the low and high catalytic cycles, which are both binuclear according to the second partial reaction order in complex 1. Among the wide range of possibilities to form binuclear complexes from B and D, a reasonable proposal involves further interaction of both with complex 1, which could give the dimer-low and dimer-high depicted in Scheme 3. The more crowded structure of the latter would account for the higher negative value of entropy estimated at the high regime.
In general terms, mononuclear catalysts have been mainly reported for alkyne trimerizations,32 a picture also found in mononuclear iron complexes and iron(0) nanoparticles. However, a novel binuclear alternative as proposed herein has been recently reported for iron cyclotrimerization,8a as well as in related metal complexes.33
The scope of the reaction was extended to other terminal aryl acetylenes. In all cases, the catalysis is regioselective to the corresponding unsymmetrical 1,2,4-cyclotrimers (85–97%) (Table S3). In addition, Figure 6 (left) evidences the strong influence of the electronic nature of the substituent in the phenyl group on the catalysis. Thus, reducing the electronic density on the arene ring (4-CF3C6H4C≡CH and 4-BrC6H4C≡CH) increases the reaction rate, whereas the opposite trend was observed with the electronically richer alkynes 4-MeC6H4C≡CH and 4-tBuC6H4C≡CH. In the latter case (in pink), the catalysis slows down after 45% conversion, most likely due to an unknown impurity that deactivates the catalyst.
Figure 6.
Left: Conversion (%) vs time (min) plot for acetylene cyclotrimerization catalyzed by 1. Experimental conditions: [1]0 = 0.0130 M, [alkyne]0 = 1.33 M, T = 60 °C, solvent = C6D6. Dashed lines are for visual aid. Right: Plot of V0 obtained for the different alkynes vs their corresponding Hammett constants (σp).
Moreover, plotting a graph of the initial rates (V0) vs Hammett constants (σp)34 reveals a linear correlation (Figure 6, right), indicating a direct influence of the electronic density on the arene ring on the initial rates.
The reaction with 4-MeC6H4C≡CH (in red) is noted to be slightly faster than with the more sterically hindered isomer, 2-MeC6H4C≡CH (in green, Figure 6). This indicates that steric factors play a role in the rate of catalysis, albeit a minor one in this case.
In addition, the highly electron-deficient methyl propiolate (MeO2CC≡CH) immediately reacts with 1 at room temperature to afford a gummy precipitate, insoluble in common organic solvents, which is presumably polymeric. Internal alkynes such as PhC≡CPh did not undergo cyclotrimerization at 60 °C, probably due to steric effects.
As described above for phenylacetylene, the alkynes shown in Figure 6 also displayed homotropic cooperativity. This is evidenced by the apparent reaction order for the alkyne of 1.5 in catalytic mixtures with an [alkyne]0/[1]0 ratio of 20, but an apparent reaction order of 2 for an [alkyne]0/[1]0 ratio of 100 ([1]0 = 0.0133 M; Figures S6–S11).
In summary, this work demonstrates an unprecedented case of homotropic cooperativity of alkynes in regioselective [2+2+2]-cycloadditions to 1,2,4-(aryl)-benzenes. The kinetic behavior is strongly dependent on the [RC≡CH]0/[1]0 ratio in such a way that two alkyne molecules cooperate at ratios <40, whereas up to four molecules cooperate at ratios >40. Values for the Hill index of 1.85 and 3.62 strongly support a noteworthy high level of positive substrate cooperation in both scenarios. Moreover, active species in the high regime were found to react ca. 6 times faster than those involved in the low regime. Accordingly, two sets of parameters for the high and low regimes, respectively, confirming the presence of two different catalytic cycles, were obtained from Eyring analyses. The rate-determining step in the low regime was found to be more endergonic than in the high one, with a more ordered transition state in the high region than in the low one. We believe that our findings reveal unique features of the kinetic behavior of alkynes, providing key insights into detecting and analyzing these atypical phenomena in reactions catalyzed by organometallic complexes.
Acknowledgments
The generous financial support from MCIN/AEI/10.13039/501100011033 (PID2020-119512GB-I00) and Gobierno de Aragón/FEDER, UE (GA/FEDER, Inorganic molecular architecture and applications Group, E50_23R) is gratefully acknowledged. A.M.G. thanks grant IJC2018-035231-I funded by MCIN/AEI/10.13039/501100011033. D.L.K. thanks The Leverhulme Trust (RF-2021-102 and RPG-2021-183) for funding. The authors also thank Dr. Laurence J. Taylor for the gift of a sample of complex 1.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c00764.
General information, experimental procedures, kinetic studies, and NMR spectra of isolated tri-(aryl)benzenes (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript
MCIN/AEI/10.13039/501100011033 (PID2020-119512GB-I00, CT), Gobierno de Aragón/FEDER, UE (GA/FEDER, CT), and the Leverhulme Trust (RF-2021-102, RPG-2021-183, DK).
The authors declare no competing financial interest.
Supplementary Material
References
- Stevens M. A.; Colebatch A. L. Cooperative approaches in catalytic hydrogenation and dehydrogenation. Chem. Soc. Rev. 2022, 51, 1881–1898. 10.1039/D1CS01171E. [DOI] [PubMed] [Google Scholar]
- a Kar S.; Milstein D. Sustainable catalysis with fluxional acridine-based PNP pincer complexes. Chem. Commun. 2022, 58, 3731–3746. 10.1039/D2CC00247G. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mondal R.; Guin A. K.; Chakraborty G.; Paul N. D. Metal–ligand cooperative approaches in homogeneous catalysis using transition metal complex catalysts of redox noninnocent ligands. Org. Biomol. Chem. 2022, 20, 296–328. 10.1039/D1OB01153G. [DOI] [PubMed] [Google Scholar]; c Whited M. T. Pincer-supported metal/main-group bonds as platforms for cooperative transformations. Dalton Trans. 2021, 50, 16443–16450. 10.1039/D1DT02739E. [DOI] [PubMed] [Google Scholar]; d Gonçalves T. P.; Dutta I.; Huang K.-W. Aromaticity in catalysis: metal ligand cooperation via ligand dearomatization and rearomatization. Chem. Commun. 2021, 57, 3070–3082. 10.1039/D1CC00528F. [DOI] [PubMed] [Google Scholar]; e Vogt M.; Langer R. The Pincer Platform Beyond Classical Coordination Patterns. Eur. J. Inorg. Chem. 2020, 2020, 3885–3898. 10.1002/ejic.202000513. [DOI] [Google Scholar]; f Elsby M. R.; Baker R. T. Strategies and mechanisms of metal–ligand cooperativity in first-row transition metal complex catalysts. Chem. Soc. Rev. 2020, 49, 8933–8987. 10.1039/D0CS00509F. [DOI] [PubMed] [Google Scholar]; g Alig L.; Fritz M.; Schneider S. First-Row Transition Metal (De)Hydrogenation Catalysis Based On Functional Pincer Ligands. Chem. Rev. 2019, 119, 2681–2751. 10.1021/acs.chemrev.8b00555. [DOI] [PubMed] [Google Scholar]; h Dub P. A.; Gordon J. C. The role of the metal- bound N–H functionality in Noyori- type molecular catalysts. Nat. Rev. Chem. 2018, 2, 396–408. 10.1038/s41570-018-0049-z. [DOI] [Google Scholar]; i Kuijpers P. F.; van der Vlugt J. I.; Schneider S.; de Bruin B. Nitrene Radical Intermediates in Catalytic Synthesis. Chem. – Eur. J. 2017, 23, 13819–13829. 10.1002/chem.201702537. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Berben L. A.; de Bruin B.; Heyduk A. F. Non-innocent ligands. Chem. Commun. 2015, 51, 1553–1554. 10.1039/C4CC90480J. [DOI] [PubMed] [Google Scholar]; k Lyaskovskyy V.; de Bruin B. Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2, 270–279. 10.1021/cs200660v. [DOI] [Google Scholar]
- a Sciortino G.; Maseras F. Computational Study of Homogeneous Multimetallic Cooperative Catalysis. Top. Catal. 2022, 65, 105–117. 10.1007/s11244-021-01493-2. [DOI] [Google Scholar]; b Maity R.; Birenheide B. S.; Breher F.; Sarkar B. Cooperative Effects in Multimetallic Complexes Applied in Catalysis. ChemCatChem 2021, 13, 2337–2370. 10.1002/cctc.202001951. [DOI] [Google Scholar]; c Ghosh A. C.; Duboc C.; Gennari M. Synergy between metals for small molecule activation: Enzymes and bio-inspired complexes. Coord. Chem. Rev. 2021, 428, 213606 10.1016/j.ccr.2020.213606. [DOI] [Google Scholar]; d Wang Q.; Brooks S. H.; Liu T.; Tomson N. C. Tuning metal–metal interactions for cooperative small molecule activation. Chem. Commun. 2021, 57, 2839–2853. 10.1039/D0CC07721F. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Campos J. Bimetallic cooperation across the periodic table. Nat. Chem. Rev. 2020, 4, 696–702. 10.1038/s41570-020-00226-5. [DOI] [PubMed] [Google Scholar]; f Xiong N.; Zhang G.; Sun X.; Zeng R. Metal-Metal Cooperation in Dinucleating Complexes Involving Late Transition Metals Directed towards Organic Catalysis. Chin. J. Chem. 2020, 38, 185–201. 10.1002/cjoc.201900371. [DOI] [Google Scholar]; g Xu W.; Li M.; Qiao L.; Xie J. Recent advances of dinuclear nickel- and palladium-complexes in homogeneous catalysis. Chem. Commun. 2020, 56, 8524–8536. 10.1039/D0CC02542A. [DOI] [PubMed] [Google Scholar]; h Buchwalter P.; Rosé J.; Braunstein P. Multimetallic Catalysis Based on Heterometallic Complexes and Clusters. Chem. Rev. 2015, 115, 28–126. 10.1021/cr500208k. [DOI] [PubMed] [Google Scholar]
- Powers D. C.; Ritter T. Bimetallic Redox Synergy in Oxidative Palladium Catalysis. Acc. Chem. Res. 2012, 45, 840–850. 10.1021/ar2001974. [DOI] [PubMed] [Google Scholar]
- van Beek C. B.; van Leest N. P.; Lutz M.; de Vos S. D.; Gebbink R. J. M. K.; de Bruin B.; Broere D. L. J. Combining metal–metal cooperativity, metal–ligand cooperativity and chemical non-innocence in diiron carbonyl complexes. Chem. Sci. 2022, 13, 2094–2104. 10.1039/D1SC05473B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan B. Explicit Treatment of Non-Michaelis-Menten and Atypical Kinetics in Early Drug Discovery. ChemMedChem 2021, 16, 899–918. 10.1002/cmdc.202000791. [DOI] [PubMed] [Google Scholar]
- See for example; a Mak C. A.; Weis K.; Henao T.; Kuchtova A.; Chen T.; Sharma S.; Meekins D. A.; Thalmann M.; Vander Kooi C. W.; Raththagala M. Cooperative Kinetics of the Glucan Phosphatase Starch Excess4. Biochemistry 2021, 60, 2425–2435. 10.1021/acs.biochem.1c00307. [DOI] [PubMed] [Google Scholar]; b Ikeda H.; Nishikawa S.; Yamamoto Y.; Ueno A. Homotropic cooperativity of cyclodextrin dimer as an artificial hydrolase. J. Mol. Catal. A: Chem. 2010, 328, 1–7. 10.1016/j.molcata.2010.06.005. [DOI] [Google Scholar]; c Sohl C. D.; Isin E. M.; Eoff R. L.; Marsch G. A.; Stec D. F.; Guengerich F. P. Cooperativity in Oxidation Reactions Catalyzed by Cytochrome P450 1A2. J. Biol. Chem. 2008, 283, 7293–7308. 10.1074/jbc.M709783200. [DOI] [PubMed] [Google Scholar]; d Hlavica P.; Lewis D. F. V. Allosteric phenomena in cytochrome P450-catalyzed Monooxygenations. Eur. J. Biochem. 2001, 268, 4817–4832. 10.1046/j.1432-1327.2001.02412.x. [DOI] [PubMed] [Google Scholar]
- a Witzke R. J.; Hait D.; Chakarawet K.; Head-Gordon M.; Tilley T. D. Bimetallic Mechanism for Alkyne Cyclotrimerization with a Two-Coordinate Fe Precatalyst. ACS Catal. 2020, 10, 7800–7807. 10.1021/acscatal.0c01828. [DOI] [Google Scholar]; b Lipschutz M. I.; Chantarojsiri T.; Dong Y.; Tilley T. D. Synthesis, Characterization, and Alkyne Trimerization Catalysis of a Heteroleptic Two-Coordinate FeI Complex. J. Am. Chem. Soc. 2015, 137, 6366–6372. 10.1021/jacs.5b02504. [DOI] [PubMed] [Google Scholar]
- Weller R.; Müller I.; Werncke C. G. Catalytic 1,3-H Atom Shift of a Terminal Benzylic Alkyne by Iron and Alkali Metal Silylamides – Switching between Allene and Internal Alkyne. Eur. J. Inorg. Chem. 2022, 2022, e202100955 10.1002/ejic.202100955. [DOI] [Google Scholar]
- a Brenna D.; Villa M.; Gieshoff T. N.; Fischer F.; Hapke M.; von Wangelin J. Iron-Catalyzed Cyclotrimerization of Terminal Alkynes by Dual Catalyst Activation in the Absence of Reductants. Angew. Chem., Int. Ed. 2017, 56, 8451–8454. 10.1002/anie.201705087. [DOI] [PubMed] [Google Scholar]; b Frazier B. A.; Williams V. A.; Wolczanski P. T.; Bart S. C.; Meyer K.; Cundari T. R.; Lobkovsky E. B.. C–C Bond Formation and Related Reactions at the CNC Backbone in (smif)FeX (smif = 1,3-Di-(2-pyridyl)-2-azaallyl): Dimerizations, 3 + 2 Cyclization, and Nucleophilic Attack; Transfer Hydrogenations and Alkyne Trimerization (X = N(TMS)2, dpma = (Di-(2-pyridyl-methyl)-amide)). Inorg. Chem. 2013, 52, 3295–3312. [DOI] [PubMed] [Google Scholar]; The related tricoordinated adduct [Fe{N(SiMe3)2}2(thf)] catalyzes the cyclotrimerization of 2-butyne
- a Matton P.; Huvelle S.; Haddad M.; Phansavath P.; Ratovelomanana-Vidal V. Recent Progress in Metal-Catalyzed [2+2+2] Cycloaddition Reactions. Synthesis 2022, 54, 4–32. 10.1055/s-0040-1719831. [DOI] [Google Scholar]; b Bauer I.; Knölker H.-J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. 10.1021/cr500425u. [DOI] [PubMed] [Google Scholar]; c Bauer E. B. Iron Catalysis: Historic Overview and Current Trends. Top. Organomet. Chem. 2015, 50, 1–18. [Google Scholar]
- a Burcher B.; Sanders K. J.; Benda L.; Pintacuda G.; Jeanneau E.; Danopoulos A. A.; Braunstein P.; Olivier-Bourbigou H.; Breuil P.-A. R. Straightforward Access to Stable, 16-Valence-Electron Phosphine-Stabilized Fe0 Olefin Complexes and Their Reactivity. Organometallics 2017, 36, 605–613. 10.1021/acs.organomet.6b00803. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Casitas A.; Krause H.; Goddard R.; Fürstner A. Elementary Steps of Iron Catalysis: Exploring the Links between Iron Alkyl and Iron Olefin Complexes for their Relevance in C–H Activation and C–C Bond Formation. Angew. Chem., Int. Ed. 2015, 54, 1521–1526. 10.1002/anie.201410069. [DOI] [PubMed] [Google Scholar]
- a Wang L.; Cheng J.; Ma Y.; Chen Q.; Leng X.; Deng L. Three-coordinate Bis(N-heterocyclic carbene)iron(0) complexes with alkene and alkyne ligation: Synthesis and characterization. Polyhedron 2021, 197, 115054 10.1016/j.poly.2021.115054. [DOI] [Google Scholar]; b Cheng J.; Chen Q.; Leng X.; Ye S.; Deng L. Three-Coordinate Iron(0) Complexes with N-Heterocyclic Carbene and Vinyltrimethylsilane Ligation: Synthesis, Characterization, and Ligand Substitution Reactions. Inorg. Chem. 2019, 58, 13129–13141. 10.1021/acs.inorgchem.9b02009. [DOI] [PubMed] [Google Scholar]
- Breschi C.; Piparo L.; Pertici P.; Caporusso A. M.; Vitulli G. (η6-Cyclohepta-1,3,5-triene)(η4-cycloocta-1,5-diene)iron(0) complex as attractive precursor in catalysis. J. Organomet. Chem. 2000, 607, 57–63. 10.1016/S0022-328X(00)00211-4. [DOI] [Google Scholar]
- Doll J. S.; Eichelmann R.; Hertwig L. E.; Bender T.; Kohler V. J.; Bill E.; Wadepohl H.; Roşca D.-A. Iron-Catalyzed Trimerization of Terminal Alkynes Enabled by Pyrimidinediimine Ligands: A Regioselective Method for the Synthesis of 1,3,5-Substituted Arenes. ACS Catal. 2021, 11, 5593–5600. 10.1021/acscatal.1c00978. [DOI] [Google Scholar]
- Gawali S. S.; Gunanathan C. Iron-catalyzed regioselective cyclotrimerization of alkynes to benzenes. J. Organomet. Chem. 2019, 881, 139–149. 10.1016/j.jorganchem.2018.12.007. [DOI] [Google Scholar]
- Provis-Evans C. B.; Lau S.; Krewald V.; Webster R. L. Regioselective Alkyne Cyclotrimerization with an In Situ-Generated [Fe(II)H(salen)]·Bpin Catalyst. ACS Catal. 2020, 10, 10157–10168. 10.1021/acscatal.0c03068. [DOI] [Google Scholar]
- a Minakawa M.; Ishikawa T.; Namioka J.; Hirooka S.; Zhou B.; Kawatsura M. Iron-catalyzed [2+2+2] cycloaddition of trifluoromethyl group substituted unsymmetrical internal alkynes. RSC Adv. 2014, 4, 41353–41356. 10.1039/C4RA06973K. [DOI] [Google Scholar]; b Liu Y.; Yan X.; Yang N.; Xi C. Highly regioselective cyclotrimerization of terminal alkynes catalyzed by Fe(II) complexes bearing 2-(benzimidazolyl)-6-(1-(arylimino)ethyl)pyridines. Catal. Commun. 2011, 12, 489–492. 10.1016/j.catcom.2010.11.013. [DOI] [Google Scholar]
- Karpiniec S. S.; McGuinness D. S.; Britovsek G. J.; Patel J. Acetylene Cyclotrimerization with an Iron(II) Bis(imino)pyridine Catalyst. Organometallics 2012, 31, 3439–3442. 10.1021/om300084s. [DOI] [Google Scholar]
- Neumeier M.; Chakraborty U.; Schaarschmidt D.; de la Pena O_Shea V.; Perez-Ruiz R.; von Wangelin A. J. Combined Photoredox and Iron Catalysis for the Cyclotrimerization of Alkynes. Angew. Chem., Int. Ed. 2020, 59, 13473–13478. 10.1002/anie.202000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe H. R.; Geer A. M.; Taylor L. J.; Gridley B. M.; Blundell T. J.; Blake A. J.; Davies E. S.; Lewis W.; McMaster J.; Robinson D.; Kays D. L. Selective reduction and homologation of carbon monoxide by organometallic iron complexes. Nat. Commun. 2018, 9, 3757 10.1038/s41467-018-06242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- General equation
for a nth-order
reaction for the substrate:

- Baxter R. D.; Sale D.; Engle K. M.; Yu J.-Q.; Blackmond D. G. Mechanistic Rationalization of Unusual Kinetics in Pd-Catalyzed C–H Olefination. J. Am. Chem. Soc. 2012, 134, 4600–4606. 10.1021/ja207634t. [DOI] [PubMed] [Google Scholar]
- Lmfit integrated into Python software.
- Burés J.A Simple Graphical Method to Determine the Order in Catalyst. Angew. Chem., Int. Ed. 2016, 55, 2028–2031 10.1002/anie.201508983(for ′normalized time scale method′). The ′adjusted normalised time scale method′ used here is described in the Supporting Information section of this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Other combinations, including PMe3 sequestering mononuclear 1, do not match with the observed value
- Simmons E. M.; Hartwig J. F. On the Interpretation of Deuterium Kinetic Isotope Effects in C–H Bond Functionalizations by Transition-Metal Complexes. Angew. Chem., Int. Ed. 2012, 51, 3066–3072. 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- Etienne M.; Mathieu R.; Donnadieu B. Reversible Migratory Insertion/β-Alkyl Elimination in α-Agostic Alkylniobium Alkyne Complexes. J. Am. Chem. Soc. 1997, 119, 3218–3228. 10.1021/ja963697q. [DOI] [Google Scholar]
- Iwamoto T.; Shibuya K.; Takakuwa T.; Kuwabara T.; Ishii Y. Experimental Observation of β-Carbon Elimination from Alkenylrhodium Complexes through Exchange Reactions of the Alkenyl Unit. Organometallics 2022, 41, 182–186. 10.1021/acs.organomet.1c00695. [DOI] [Google Scholar]
- Ikeda Y.; Mutoh Y.; Imai K.; Tsuchida N.; Takano K.; Ishii Y. Reactivities of Indenylruthenium Complex toward Internal Alkynes: Formation of Disubstituted Vinylidene Complexes and Indenyl–Alkyne Coupling. Organometallics 2013, 32, 4353–4358. 10.1021/om4004913. [DOI] [Google Scholar]
- Scheme 2 shows species B, C, and D featuring a “Fe–C(Ph) = C(H)–L” moiety. However, those containing the “Fe–C(H) = C(Ph)–L” moiety or even both of them in C and D could be also possible
- Roglans A.; Pla-Quintana A.; Solà M. Mechanistic Studies of Transition-Metal-Catalyzed [2+2+2] Cycloaddition Reactions. Chem. Rev. 2021, 121, 1894–1979. 10.1021/acs.chemrev.0c00062. [DOI] [PubMed] [Google Scholar]
- Yamamoto K.; Nagae H.; Tsurugi H.; Mashima K. Mechanistic understanding of alkyne cyclotrimerization on mononuclear and dinuclear scaffolds: [4+2] cycloaddition of the third alkyne onto metallacyclopentadienes and dimetallacyclopentadienes. Dalton Trans. 2016, 45, 17072–17081. 10.1039/C6DT03389J. [DOI] [PubMed] [Google Scholar]
- Hansch C.; Leo A.; Taft R. W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. 10.1021/cr00002a004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.