Abstract
Skeletal muscle is composed of long multinucleated cells, termed myofibers, that are formed through the activation and differentiation of resident muscle stem cells, called satellite cells. In healthy individuals, skeletal muscle enables voluntary locomotion, while also playing a role in energy metabolism and thermoregulation. As skeletal muscle is integral to everyday processes, perturbations to skeletal muscle function can have devastating consequences. Here we describe an integral tool in biomedical research of skeletal muscle regeneration and disease, the immunofluorescence staining of myogenic cells. We highlight useful techniques for immunostaining myogenic cells, and we list validated antibodies for the staining of muscle proteins across different species and multiple developmental time points. This includes methods for unmasking antigens following formaldehyde fixation (using Myosin Heavy Chain staining as an example), and practices for preserving endogenous fluorescent proteins by cardiac perfusion fixation.
Keywords: Skeletal Muscle, Satellite Cell, Muscle Stem Cell, Immunohistochemistry, Immunofluorescence, Regeneration, Differentiation, Myogenesis, Antibodies, Myosin Heavy Chain, Pax7, Heat Induced Antigen Retrieval
1. Introduction
Skeletal muscle is the most abundant tissue in the human body, accounting for approximately 40% of total body mass. It is critical for many bodily functions, such as voluntary locomotion, force generation and energy metabolism. It also exhibits remarkable plasticity and regenerative potential [1]. Unsurprisingly, diseases that affect skeletal muscle function and regeneration can profoundly impact health and well-being, and are common focuses of biomedical research [2].
The study of skeletal muscle frequently relies on the visualization of myogenic cells by immunofluorescent staining. Markers of myogenic cells that are expressed during development and regeneration are also evolutionarily conserved. In mammals, somite-derived embryonic muscle stem cells (eMuSCs) expressing Pax3 and Pax7 give rise to all skeletal muscles of the trunk and limbs. Pax7 expression is maintained in a population of eMuSCs that give rise to adult muscle stem cells, while Pax3 is only maintained in a small subpopulation of MuSCs [3]. Following injury or in diseased conditions, quiescent MuSCs become activated and enter the cell cycle and differentiate in a process reminiscent of muscle development. During differentiation, progenitor cells transiently amplify as myoblasts, differentiate into mononuclear myocytes and either fuse to damaged myofibers or form new myofibers to facilitate muscle development or regeneration. Both processes are regulated by the hierarchical expression of the muscle regulatory factors (MRFs) Myf5, MyoD, Myogenin and Mrf4, while terminally differentiated myofibers are identified by the expression of contractile proteins such as Myosin Heavy Chain (MyHC) and Actin [3].
The immunofluorescence labelling of eMuSCs, MuSCs and derivative cells is an important tool for studying myogenesis in the context of regeneration and disease. However, probing new antigens and testing new antibodies is time consuming and expensive. Moreover, many commercially available antibodies do not robustly react with their target antigen, or do not recognize the target antigen following formaldehyde tissue fixation. This is problematic when formaldehyde-fixation is required, such as for paraffin embedding or to preserve the location of endogenous proteins in transgenic animals and MuSC transplantation assays [4].
Antigens however can be routinely recovered using heat induced antigen retrieval (HIAR). Interestingly, while heating is absolutely necessary for non-enzymatic antigen retrieval, the pH and composition of the retrieval solution can profoundly impact the success of different antibody stains [5]. For example, commonly used antibodies raised against different Myosin Heavy Chain (MyHC) isotypes are highly specific when staining fresh or methanol fixed mouse tissue, but do not recognize their antigens following cardiac perfusion fixation. HIAR with conventional citrate buffer pH6 retrieves the MyHCI antigen recognized by the monoclonal antibody BAF8 but causes nonspecific tissue binding of the antibody clones SC-71 and BF-F3 which normally recognize MyHCIIa and MyHCIIb respectively. However, when the citrate buffer is adjusted to pH 7, all three monoclonal antibodies are highly specific for their target antigens (Fig. 4). The current chapter describes techniques for the immunofluorescence labelling of myogenic cells; methods for preserving endogenously expressed fluorophores in transgenic mouse tissue; and lists useful reagents and validated antibodies for the study of mouse, dog, and human skeletal muscle tissue.
Fig 4.
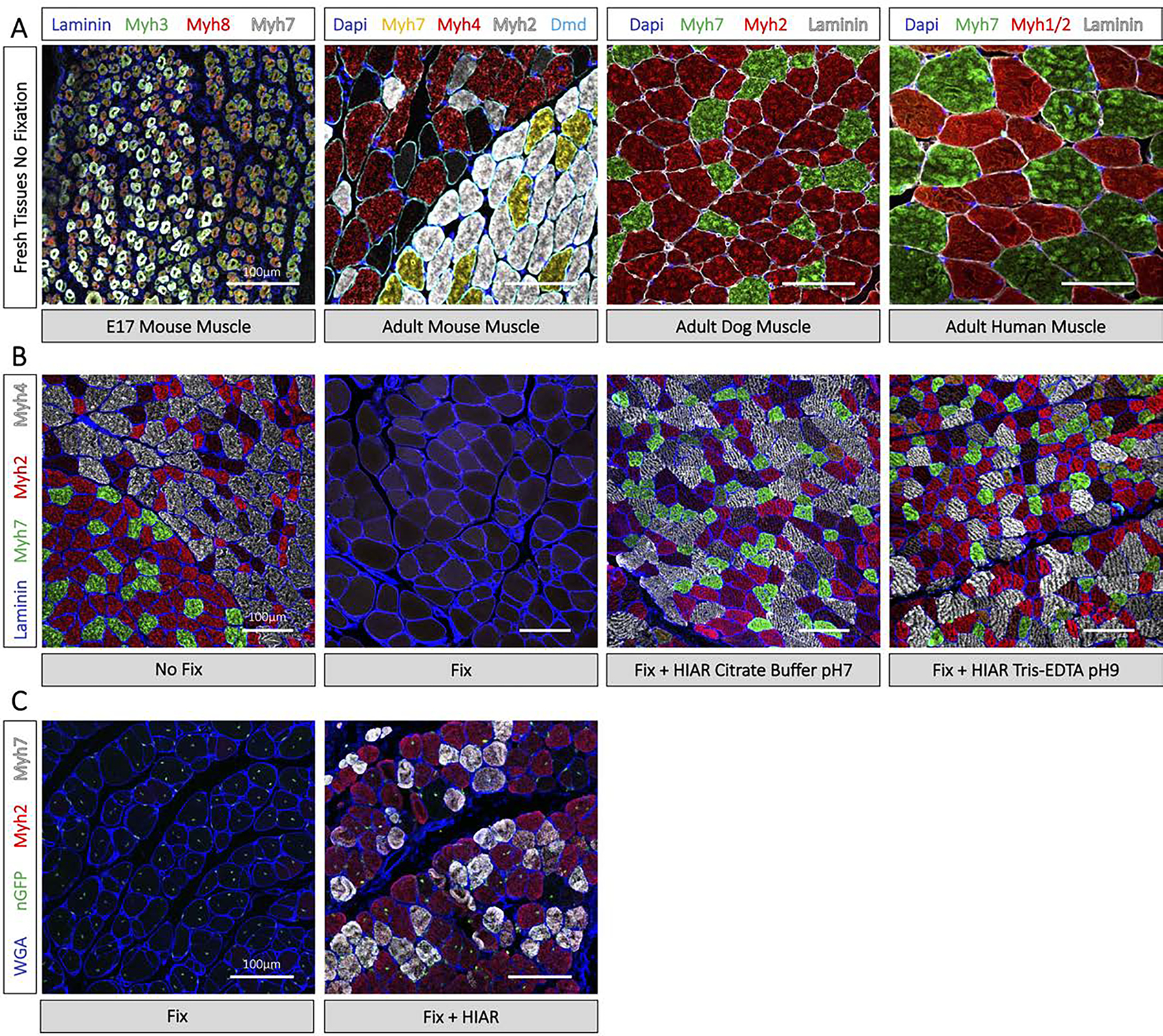
Visualization of muscle fiber type by immunofluorescence histochemistry. (A) Immunostaining of Myosin Heavy Chain isotypes on E17 and adult mouse, adult dog, and adult human muscle with no formaldehyde fixation. Corresponds to Start Point 1, Fig 1. (B) Myosin Heavy Chain immunostaining of adult mouse tissue without formaldehyde fixation, after cardiac perfusion fixation, and after cardiac perfusion fixation with heat induced antigen retrieval (HIAR) in Citrate buffer pH7 and Tris-EDTA pH9 for 10 min. Corresponds to Start Point 2, Fig 1. (C) Myosin Heavy Chain immunostaining of recipient mouse tissue 28 days after muscle stem cell transplantation. Cardiac perfusion fixation preserves donor cell nuclear GFP expression but masks the Myosin Heavy chain epitope (left). Cardiac perfusion fixation followed by HIAR in Tris-EDTA pH9 and immunostaining with a GFP antibody highlights transplanted myofibers and muscle fiber type (right). Corresponds to Start Point 2, Fig 1. Images taken with comparable laser intensity and master gain by confocal microscopy on the LSM900. All scale bars represent 100μm.
2. Materials
2.1. Cardiac Perfusion Fixation
Paraformaldehyde (PFA) 4%: add 100mL of 10X PBS and 800mL of distilled deionized H2O (ddH2O) into a glass beaker. While stirring, add 40g of paraformaldehyde powder and 10 drops of 2N NaOH. Heat the solution to 60°C while stirring to dissolve the powder but do not let the solution heat above 70°C. Adjust the pH to 7.4 using HCl and adjust the volume to 1L using ddH2O. Filter the solution through a 0.2μM filter and store at 4°C for up to one month or freeze aliquots (see Note 1).
PBS 1X pH 7.4 stored at 4°C.
Anesthesia/euthanasia agent: Euthanyl (sodium pentobarbital) at >120mg/kg for mouse administered by intraperitoneal injection to euthanize mice (see Note 2).
Ethanol 70%.
15% sucrose solutions in PBS 1X stored at 4°C.
30% sucrose solutions in PBS 1X stored at 4°C.
Needles (25 gauge for mouse).
Surgical instruments (hemostat, sharp dissection scissors, Vannas spring scissors, forceps).
Peristaltic pump and silicon tubing (we use MCP3000 series 13-310-661; Fisher Scientific) (see Note 3).
Chemical fume hood.
Masking tape.
Large weighing dish (Fisher Scientific, Cat #087-32-115).
15mL Falcon tubes.
2.2. Immersion Fixation
Paraformaldehyde (PFA) 4%: refer to section 2.1 (see Note 1).
PBS 1X pH 7.4 stored at 4°C.
Ethanol 70%.
Dissection instruments (sharp dissection scissors, Vannas spring scissors, forceps, fine forceps).
15mL Falcon tubes.
2.2. Tissue Cryopreservation, Embedding and Sectioning
Tissue-Tek OCT compound.
Tinfoil cryomold (see Note 4).
Liquid nitrogen.
2-methylbutane.
Dry ice.
Superfrost Microscope Slides.
Cryostat.
Slide box.
2.3. Deparaffinization of Paraffin Sections (see Note 5) (see Note 6)
Xylene.
Ethanol 100%.
Ethanol 95%.
Ethanol 70%.
Ethanol 50%.
ddH2O.
Staining jars.
2.4. Antigen Retrieval
Citrate Buffer (10mM sodium citrate, 0.05% Tween 20, pH 6 or pH 7) (see Note 7): dissolve 2.94g of Tri-sodium citrate (dihydrate) in 900mL of ddH2O by mixing. Adjust to pH 6 or 7 using 2M HCl. Add 0.5mL of Tween 20 and adjust to 1L total volume using ddH2O. Store at room temperature for short term use or at 4°C for longer than one month.
Tris-EDTA Buffer (10mM Tris Base, 1mM EDTA, 0.05% Tween 20, pH 9) (see Note 8): dissolve 1.21g Tris base and 0.37g of EDTA in 900mL of ddH2O by mixing. Adjust to pH 9 using 2M NaOH. Add 0.5mL of Tween 20 and adjust volume to 1L. Store at room temperature or at 4°C for longer than one month.
Autoclavable staining jar.
Pressure cooker (and rack) that reaches 12psi (see Note 9).
2.5. Immunofluorescence Staining
Paraformaldehyde (PFA) 4%: refer to section 2.1 (see Note 10).
PBS 1X (see Note 11).
Hydrophobic PAP pen (see Note 12).
Mouse on Mouse (M.O.M.) blocking reagent (Vector laboratories, MKB-2213) (see Note 13).
TrueBlack Lipofuscin Autofluorescence Quencher (Biotium, Cat# 23007) (see Note 14).
Blocking solution: 5% goat serum, 2% bovine serum albumin (BSA) in 1X PBS (0.22μm syringe filtered) (see Note 15) (see Note 16).
Permeabilization solution: 0.1 M Glycine, 0.1% Triton X-100, in PBS 1X (0.22μm syringe filter) (see Note 13).
Cross absorbed fluorescence conjugated secondary antibodies (Alexa Fluor Dyes) (see Note 18).
DAPI (4’,6-diaminidino-phenylindole dihydrochloride).
PermaFluor Aqueous Mounting Medium (Fisher Scientific, TA-030-FM) (see Note 19).
Glass coverslip (1.5 thickness).
Clear nail varnish.
Epifluorescence microscope.
Table 2.
Antibodies for the Study of Myogenic Cells
| Antigen | Clone | Host Species | Isotype | Dilution (IHC-fix frozen) | Product code | Company | AR required (frozen) | Species Reactivity (Rudnicki lab validated) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ms | Dg | Hu | ||||||||
| Pax7 | Monoclonal | Ms | IgG1 | undiluted* | AB_528428 | DSHB | No | ✓ | ✓ | ✓ |
| Pax3 | C2 | Ms | IgG2a | undiluted* | AB_528426 | DSHB | Cit, pH 6 | ✓ | NV | NV |
| Myf5 | Polyclonal | Rb | IgG | 1:500 | sc-302 | Santa Cruz | Cit, pH 6 | ✓ | NV | NV |
| MyoD | G-1 | Ms | IgG2b | 1:30 | sc-377460 | Santa Cruz | No | ✓ | ✓ | NV |
| MyoD | 5.8A | Ms | IgG1 | 1:20 | M351201–2 | Dako | No | ✓ | NV | NV |
| MyoD | EPR6653 | Rb | IgG | 1:500 | ab133627 | Abcam | No | ✓ | × | NV |
| sMyogenin | 671037 | Ms | IgG2a | 1:200 | MAB66861 | R&D | No | ✓ | × | ✓ |
| Myogenin | EPR4789 | Rb | IgG | 1:500 | ab124800 | Abcam | No | ✓ | × | NV |
| Myogenin | F5D | Ms | IgG1 | 1:250 | sc-12732 | Santa Cruz | No | ✓ | ✓ | NV |
| ki67 | Polyclonal | Rb | IgG | 1:500 | ab15580 | Abcam | No | ✓ | ✓ | NV |
| ki67 | Monoclonal | Ms | IgG1 | 1:500 | 550609 | BD Biosciences | No | ✓ | ✓ | NV |
| α-actin | Monoclonal | Ms | IgG1 | 1:2000 | A-7811 | Sigma | No | ✓ | ✓ | NV |
| α-actin | Monoclonal | Ms | IgM | 1:2000 | A-2172 | Sigma | No | ✓ | ✓ | ✓ |
| α-tubulin | Monoclonal | Ms | IgG1 | 1:3000 | T5168 | Sigma | No | ✓ | ✓ | ✓ |
| γ-tubulin | GTU-88 | Ms | IgG1 | 1:2000 | T6557 | Sigma | No | ✓ | ✓ | NV |
| γ-tubulin | Polyclonal | Rb | IgG | 1:1500 | ab11317 | Abcam | No | ✓ | ✓ | NV |
| PH3 (Ser10) | 6G3 | Ms | IgG1 | 1:1000 | 9706 | Cell Signalling | No | ✓ | NV | NV |
| PH3 (Ser10) | Polyclonal | Rb | IgG | 1:1000 | 9701 | Cell Signalling | No | ✓ | NV | NV |
| Laminin | Polyclonal | Rb | IgG | 1:1000 | L9393 | Sigma | No | ✓ | ✓ | ✓ |
| Desmin | Polyclonal | Rb | IgG | 1:500 | ab8592 | Abcam | No | ✓ | ✓ | NV |
| MyHCII | MY-32 | Ms | IgG1 | 1:500 | M4276 | Sigma | No | ✓ | NV | ✓ |
| MyHC | MF20 | Ms | IgG2b | 1:50* | AB_2147781 | DSHB | No | ✓ | ✓ | ✓ |
| MyHCI (Myh7) | NOQ7.5.4D | Ms | IgG1 | 1:3000 | M8421 | Sigma | No | ✓ | ✓ | ✓ |
| MyHCI (Myh7) | A4.480 | Ms | IgM | 1:2* | AB_528384 | DSHB | Tris pH9 | ✓ | ✓ | ✓ |
| MyHCI (Myh7) | BA-F8 | Ms | IgG2b | 1:2* | AB_10572253 | DSHB | Cit pH 6,7; Tris pH9 | ✓ | ✓ | NS |
| MyHCI (Myh7) | BA-D5 | Ms | IgG2b | 1:2* | AB_2235587 | DSHB | Cit pH 6,7; Tris pH9 | ✓ | ✓ | ✓ |
| MyHC IIa (Myh2) | SC-71 | Ms | IgG1 | 1:2* | AB_2147165 | DSHB | Cit pH 7; Tris pH9 | ✓ | ✓ | ✓ |
| MyHC IIb (Myh4) | BF-F3 | Ms | IgM | 1:2* | AB_2266724 | DSHB | Cit pH 7; Tris pH9 | ✓ | ✓ | NV |
| MyHC-emb (Myh3) | F1.652 | Ms | IgG1 | 1:50* | AB_528358 | DSHB | Cit pH 6,7; Tris pH9 | ✓ | ✓ | NV |
| MyHC-peri (Myh8) | N3.36 | Ms | IgM | 1:10* | AB_528380 | DSHB | No | ✓ | ✓ | NV |
| Collagen I | 5D8-G9 | Ms | IgG1 | 1:100* | ab23446 | Abcam | No | ✓ | ✓ | NV |
| Collagen III | 1E7-D7 | Ms | IgG1 | 1:100* | ab23445 | Abcam | No | ✓ | ✓ | NV |
| Dystrophin (CT) | DY8/6C5 | Ms | IgG1 | 1:100* | NCL-Dys2 | Leica | No | ✓ | ✓ | NV |
| Dystrophin (R8–10) | Dy4/6D3 | Ms | IgG2a | 1:100* | Dys1 | Leica | No | ✓ | ✓ | NV |
| Dystrophin (R17) | 5B2 | Ms | IgG1 | 1:100* | AB_2618176 | DSHB; conc. | No | ✓ | ✓ | NV |
| Dystrophin (R8–9) | 1B12 | Ms | IgG2b | 1:100* | AB_2618166 | DSHB; conc. | No | ✓ | ✓ | NV |
| Dystrophin (NT) | 4C7 | Ms | IgG2b | 1:100* | AB_2618175 | DSHB; conc. | No | ✓ | ✓ | NV |
| Dystrophin (NT-H1) | 6F11 | Ms | IgG1 | 1:100* | AB_2753246 | DSHB; conc. | No | ✓ | ✓ | NV |
| Dystrophin (CT) | Polyclonal | Rb | IgG | 1:500 | ab15277 | Abcam | No | ✓ | ✓ | NV |
| GFP (incl. YFP) | Polyclonal | Ckn | IgY | 1:1000 | ab13970 | Abcam | No | SI | SI | SI |
| RFP (incl. tdT) | Polyclonal | Rb | IgG | 1:500 | 600–401-379 | Rockland | No | SI | SI | SI |
| mCherry (incl. tdT) | Polyclonal | Rb | IgG | 1:500 | ab167453 | Abcam | No | SI | SI | SI |
Ms: mouse; Dg: dog; Hu: human; ✓: works; ×: does not work; NS: not specific; NV: not validated; SI: species independent; Cit: Citrate Buffer; Tris: Tris-EDTA Buffer.
The concentration of hybridoma supernatants grown in house or ordered from the Developmental Studies Hybridoma Bank (DSHB) can differ between batches. Therefore, working dilutions may need adjusting.
3. Methods
Extensive formaldehyde fixation of tissue prevents some antibodies from recognizing their target antigens, making immunofluorescence staining of fresh frozen tissue sections the simplest method for examining myogenic cells. In this workflow, tissue sections are briefly fixed prior to immunostaining, or fixation is omitted (Fig. 1A) (see Note 10). However, when formaldehyde fixation by cardiac perfusion fixation (Fig.1B) or by immersion fixation (Fig. 1C) is necessary – either to preserve the location of endogenous fluorescent proteins or when embedding tissue in paraffin – heat induced antigen retrieval (HIAR) can effectively retrieve antigens while preserving tissue morphology. Table 1 summarizes the tissue processing and embedding strategies that best suit most myogenic samples. Table 2 lists validated antibodies for the study of myogenic antigens and the appropriate HIAR method when required. Finally, the workflow from tissue processing to immunostaining of myogenic cells is schematically represented in Figure 1. Sample images are provided to illustrate immunofluorescence staining of common myogenic markers (Fig. 3) and the effectiveness of HIAR on retrieving the Myosin Heavy Chain epitopes from formaldehyde-fixed tissues (Fig. 4). While this chapter covers the processing and staining of paraffin tissue sections, the embedding and sectioning steps are not described.
Fig 1.
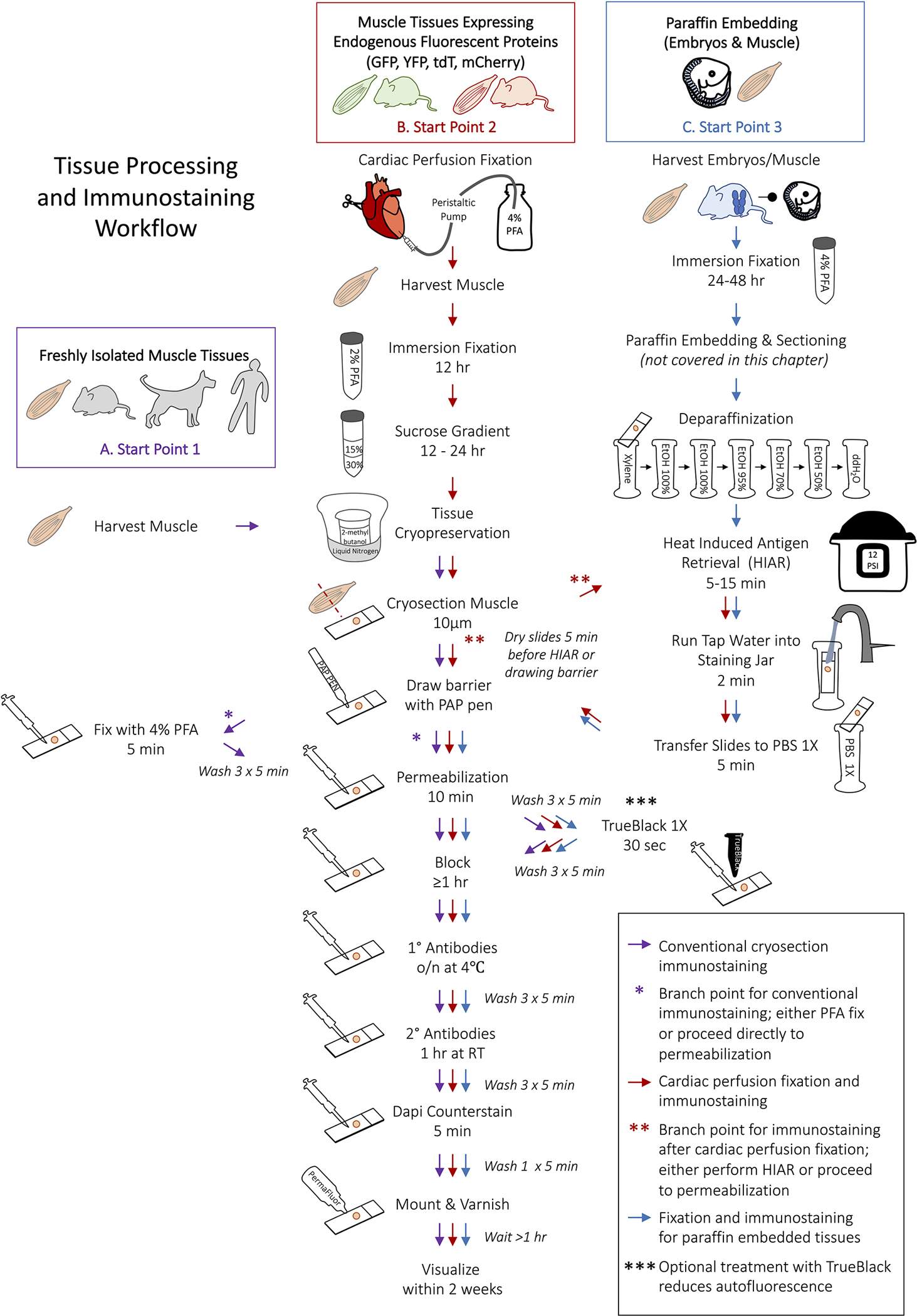
Schematic representation of the workflow for myogenic tissue processing and immunofluorescence staining. (A) Begin from Start Point 1 to process fresh tissue for immunostaining (purple arrows). After surrounding tissues with the hydrophobic barrier, PFA-fixation can be omitted for antigens that are highly sensitive to formaldehyde (purple asterisk). (B) The processing and immunostaining of muscle that requires cardiac perfusion fixation begins at Start Point 2 (red arrows). Heat Induced Antigen Retrieval (HIAR) is not always required for perfusion fixed tissues but is necessary for some primary antibodies (red asterisks). (C) Select Start Point 3 for fixing tissues prior to paraffin embedding and for the subsequent immunostaining of paraffin embedded tissue sections. HIAR is almost always recommended when immunostaining paraffin embedding tissues. Treating tissues with TrueBlack is optional but particularly useful for quenching autofluorescence in the green channel (triple asterisks). Start points A-C correspond to Table 1.
Table 1.
Tissue Embedding Methods
| Tissue | Fixation method | Embedding material |
|---|---|---|
| Skeletal muscle | No fixation | Cryopreservation in OCT |
| Skeletal muscle with endogenous fluorophore proteins | Cardiac perfusion fixation with 4% PFA followed by immersion in 2% PFA overnight | Sucrose gradient followed by cryopreservation in OCT |
| Whole murine embryos (E9.5 to E18.5) | Immersion fixation in 4% PFA for 12–48 hour (age dependant) | Paraffin embed or sucrose gradient followed by cryopreservation in OCT (cryopreservation provides lower resolution images of somite stage embryos) |
| Murine embryo limbs (E14.5 to E18.5) | No fixation | Cryopreservation in OCT |
| Murine embryo with endogenous fluorophore | Immersion fixation in 4% PFA (see Note 22) | Sucrose gradient and OCT cryopreservation |
Fig 3.
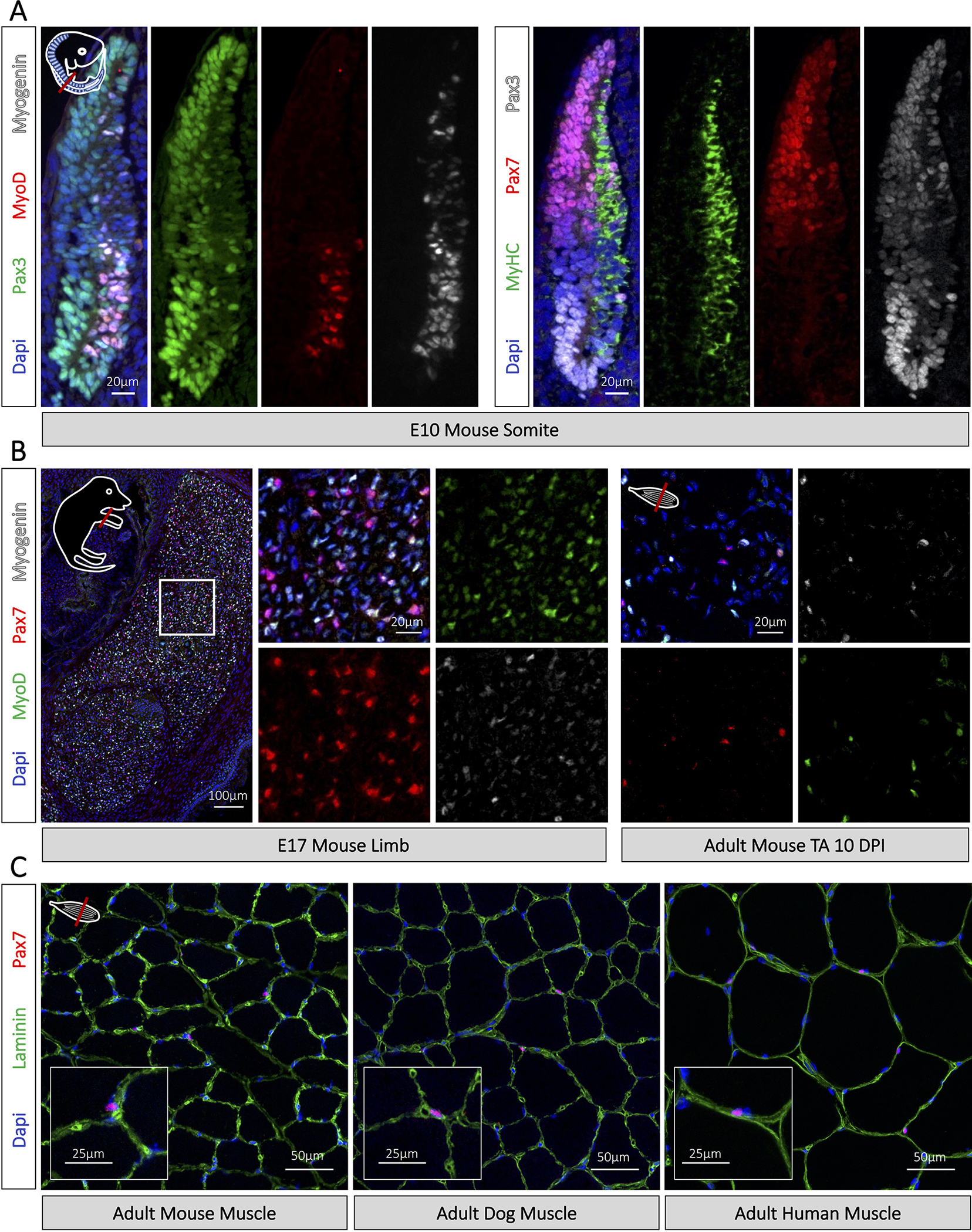
Immunofluorescence staining of myogenic cells. (A) Visualization of myogenic cells in E10.5 murine embryos following deparaffinization and antigen retrieval in citrate buffer pH6 for 10 min. Corresponds to Start Point 3, Fig 1. Images taken with widefield Zeiss D1 microscope (B) Immunostaining of myogenic cells on fresh frozen E17.5 mouse embryo limbs and adult muscle following 4% PFA fixation and blocking with M.O.M. blocking reagent. Corresponds to Start Point 1, Fig 1. (C) Immunostaining of Pax7 muscle stem cells on fresh frozen mouse, dog, and human tissues following tissue fixation in 4% PFA. Corresponds to Start Point 1, Fig 1. Days post injury (DPI). Images acquired on an LSM900 confocal microscope.
3.1. Cryopreservation of Muscle Tissue
3.1.1. Harvesting and Cryopreservation of Fresh Tissue in OCT (Fig 1. A)
Perform all animal experiments in accordance with your institution’s animal care and usage protocols and guidelines. We use CO2 or isoflurane followed by cervical dislocation for mouse euthanization.
Wet the animal’s fur by spraying 70% ethanol.
Harvest muscles, embed in Tissue-Tek OCT compound, and freeze in 2-methylbutane cooled in liquid nitrogen (see Note 20). To prevent cracking, remove the tissue block from the 2-methylbutane before the OCT completely freezes and place it on dry ice. Store embedded tissues at −80°C.
Using a cryostat, section the OCT-embedded tissue (perpendicular to the direction in which the myofibers traverse) at 10μm thickness, and then carefully transfer the section onto a SuperFrost microscope slide. Proceed directly to immunostaining or store the slides at −20°C.
3.1.2. Cardiac Perfusion Fixation and Cryopreservation of Mouse Tissue in OCT (Fig 1. B)
Set up peristaltic pump in a chemical fume hood with a 25-gauge needle affixed to silicon tubing. Run ddH2O through the pump tubing to clear it out, then switch to collecting tube to ice cold PBS. Set the machine to 48.5 RPMs (~4mL/min) and pause the PBS flow.
Anesthetize or euthanize the animal according to your institution SOP and confirm by loss of pedal withdraw. We administer euthanyl (sodium pentobarbital) at >120mg/kg by intraperitoneal injection. Place the animal on a large weighing dish in a supine position and tape down the limbs (Fig. 2A). This allows for safe collection of the fixative.
Spray the mouse with 70% ethanol.
Lift the skin at the sternum using blunt forceps and create a lateral incision below the rib cage using sharp dissection scissors (Fig. 2A). Hold the sternum with forceps and cut laterally to expose the diaphragm (Fig. 2B). If the liver adheres to the body wall, gently push it downwards with blunt forceps and avoid piercing it. Cut into the rib cage, but do not cut the lungs.
Cut back the diaphragm using Vannus spring scissors and expose the plural cavity and heart (Fig. 2C). Clamp the sternum using a hemostat and rest it over the animal’s head. Gently remove connective tissue from around the heart.
Locate the left ventricle and right atrium. The right site of the heart will be a deeper red (Fig. 2C). Confirm there are no bubbles in the perfusion tubing and that the machine is paused. Gently insert the needle into the bottom of the left ventricle pointing towards the left atrium. The needle should only be inserted to directly above the bevel to prevent it from puncturing the left atrium or right ventricle, which will detrimentally impact fixation. Affixing tape directly above the bevel can prevent the needle from being inserted too deeply.
Using Vannas spring scissors, create a small incision in the right atrium. Deep red blood should immediately drain into the chest cavity (Fig. 2D).
Turn on the perfusion machine and flush the animal with ice cold PBS until the buffer leaving the animal is no longer red and the liver lightens from deep red to pink (approximately 20–30mL of PBS per mouse) (Fig. 2E). Turn off the machine and switch tubing to ice cold 4% PFA and turn it back on. Perfuse the mouse for 10–15 minutes (approximately 50mL of 4% PFA). The liver and tail should become stiff.
Harvest the hind limb muscle and immerse them in 2% PFA overnight at 4°C (see Note 21).
Wash muscle in PBS three times for 5 min.
Prepare sucrose gradient by placing 30% sucrose in a falcon tube. Break the end of a serological pipet and gently place 15% sucrose above the 30% sucrose in the same tube. Two separate layers should be easily observed.
Gently place the fixed muscle at the top of the sucrose gradient and wait until the muscle sinks to the bottom of the tube before embedding. The muscles can be left in sucrose overnight, but no longer than 24 hours, as this can detrimentally impact the tissue.
Remove the muscle from sucrose and gently tap dry with Kim wipe. Embed the muscle in OCT and let it rest for a minute.
Freeze the muscle in 2-methylbutane cooled in liquid nitrogen (see to tissue fixation to help with penetration of the PFA Note 16). Remove the tissue block from the 2-methylbutane before the OCT completely freezes to prevent cracking. Place the tissue block on dry ice. Store the embedded tissue at −80°C.
Using a cryostat, section the OCT-embedded muscle (perpendicular to the direction of myofibers) at 10μm thickness and carefully transfer the sections onto a SuperFrost microscope slide. Proceed directly to immunostaining or store the slides at −20°C.
Fig 2.
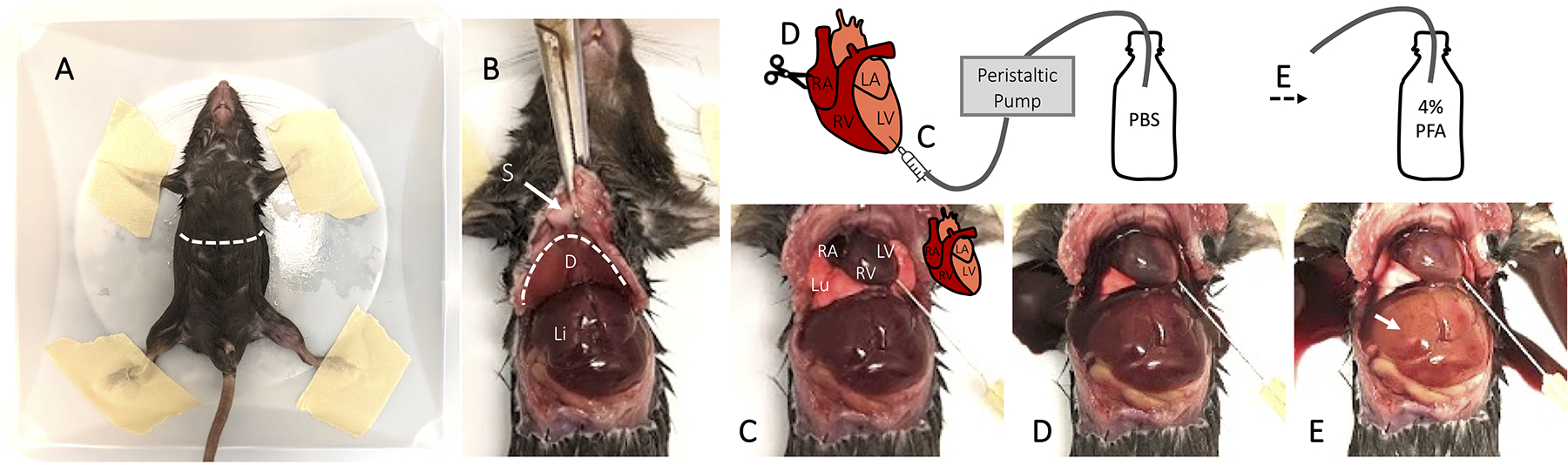
Visual guide for cardiac perfusion fixation of mice. (A) Following euthanasia, affix the mouse to a weighing dish using masking tape. (B) Make a lateral incision below the sternum and rib cage to expose the diaphragm and liver. Cut back the diaphragm using Vannus spring scissors and expose the plural cavity and heart. (C) Identify the left ventricle and insert the needle bevel. (D) Create a small incision in the right atrium and turn on the peristaltic pump to flush the circulatory system with PBS. (E) Once the blood has cleared from the circulatory system and the liver has lightened, switch the tubing to collect 4% PFA. Sternum (S), diaphragm (D), liver (Li), lung (Lu).
3.1.3. Immersion Fixation for Paraffin Embedding
A detailed description of paraffin embedding and sectioning is beyond the scope of this chapter. However, there are numerous readily available resources online for more information, and many institutions provide histology core services that routinely perform paraffin embedding for a nominal fee.
Euthanize the animal according to your institution’s animal care and usage protocols and guidelines. We use CO2 or isoflurane followed by cervical dislocation for mouse euthanasia.
Sterilize and wet the animal’s fur by spraying 70% ethanol.
Harvest muscle or embryos. To isolate embryos, remove the uterine lining, the yolk sac, and the amniotic sac. If necessary, save the yolk sac or placenta for genotyping, as both are derived from embryo and not maternal DNA.
Wash tissues 3 times for 5 min in PBS.
Fix muscles or E9.5-E11.5 embryos for 24 to 48 hours in 4% PFA depending on tissue size. For embryos older than E11.5, remove the head prior to tissue fixation to help with penetration of the PFA (see Note 22).
Wash tissues 3 times for 5 min in PBS.
Consult your institution’s histology core services for paraffin embedding and sectioning.
3.2. Heat Induced Antigen Retrieval
Refer to Table 2 for the appropriate antigen retrieval solution based on the selected antibody panel (see Note 7) (see Note 23). If the antibody is not listed in Table 2 and does not recognize its target with conventional immunostaining, different pH and buffer combinations can be tested with HIAR.
Insert the metal rack into the pressure cooker pot and fill with enough ddH2O to reach the rack. Fill an autoclavable staining jar with the appropriate antigen retrieval solution. With a loosely fitted lid, place the jar atop the pressure cooker rack and preheat the ddH2O and antigen retrieval solution (see Note 9).
- Perform the appropriate slide preparatory step listed below.
- Paraffin sections: deparaffinize slides by sequential immersion in jars containing the following chemicals (Fig. 1C) (see Note 6).
- 10 min Xylene.
- 10 min 100% EtOH.
- 10 min 100% EtOH.
- 5 min 95% EtOH.
- 5 min 70% EtOH.
- 5 min 50% EtOH.
- 1 min ddH2O.
- Place slides in the jar containing preheated antigen retrieval solution.
With the staining jar atop the rack, set the pressure cooker to 12 psi (or max) for 5–15 min. The time can be adjusted, but 10 min is routinely sufficient (see Note 25).
When the pressure cooker is sufficiently cooled to handle the staining jar safely, remove the jar from the pressure cooker. Gently run tap water into the jar for 2 min. Place the slides in PBS for 5 min at room temperature.
Remove the slides, one at a time, from the water and gently tap them. It is important that the tissues do not dry out (residual PBS should still surround the tissue sections). Use a Kim wipe to remove any moisture where the hydrophobic barrier will be applied. Using a PAP pen, surround the tissues and cover the slide in PBS. If the glass slide is wet, the hydrophobic barrier will not adhere properly.
Proceed directly to permeabilization in the immunofluorescence staining section (steps 5–15). Do not fix the tissues again, and do not let the tissues dry out.
3.3. Immunofluorescence Staining
Immunofluorescence staining is effective for examining multiple antigens simultaneously (see Note 27) (see Note 23). Consult Table 2 for primary antibody concentrations and the corresponding immunostaining method. If Table 2 indicates an antibody does not work following formaldehyde fixation and HIAR is not performed, skip tissue fixation (step 3–4) and proceed with permeabilization. Select secondary antibodies that are compatible with the epifluorescence or confocal microscope available at your institution (see Note 17). All steps are performed at room temperature unless otherwise indicated.
Remove slides from the freezer and let them air dry for 5 min. Ensure there is no residual moisture before beginning immunostaining.
Surround tissue sections with a hydrophobic barrier using a PAP pen.
Cover tissues with 4% PFA and incubate for 5 min (see Note 10).
Aspirate PFA and wash three times with PBS for 5 min.
Incubate slides in permeabilization buffer for 10 min (see Note 26).
Wash three times with PBS for 5 min if using TrueBlack, otherwise proceed directly to blocking (see Note 14).
(Optional) Treat slides with TrueBlack autofluorescence quencher. Dilute 20X Trueblack in 70% ethanol and apply generously to the slide for 30 sec and ensure the tissues do not dry out. It is okay if residual buffer is left on the slide when applying TrueBlack. Wash slides three times with PBS for 5 min, ensuring no black remains in PBS when done. Do not use reagents containing detergents (i.e. Triton X-100 or Tween 20) for the remainder of the protocol.
Cover the slides in blocking solution and incubate for at least 1 hour at room temperature. Include M.O.M. blocking reagent 1:40 when staining mouse tissue with antibodies raised in mouse (see Note 13) (see Note 27).
Dilute primary antibodies in blocking solution (see Table 2 for antibody dilutions). It is acceptable to dilute antibodies in hybridoma supernatant when targeting multiple antigens.
Aspirate the blocking buffer and cover slide with the primary antibody solution without washing. Incubate the slides overnight at 4°C (see Note 28).
Wash three times with PBS for 5 min.
Cover slides with secondary antibodies diluted in blocking buffer for 1 hour at room temperature in the dark (see Note 17). Keep slides in the dark for the remainder of protocol.
Wash slides three times with PBS for 5 min.
Incubate with 1 μg/ml DAPI diluted in PBS for 5 min.
Wash once in PBS for 5 min.
Aspirate PBS and place 1–2 drops of PermaFluor mounting media (see Note 19). Carefully place a coverslip on the slide. Take care to avoid introducing air bubbles. Let the slides dry in the dark for a 1–2 hours before sealing the slides with clear nail polish. Store the slides at 4°C and visualize within 2 weeks.
4. Notes
Personal protective equipment and a chemical fume hood are required to safely handle paraformaldehyde. Make sure to consult the available MSDS. Paraformaldehyde 8% or 16% can also be purchased and diluted to 4% with 1X PBS (for example, 10mL 8% PFA, 2mL 10X PBS, 8 mL ddH2O).
All animal protocols must be approved by your institute’s animal care committee. We use Euthanyl (sodium pentobarbital) at >120mg/kg administered by intraperitoneal injection to euthanize mice. Euthanyl is a controlled substance and must be used according to your institution’s standard operating procedures and approved animal protocols. Ketamine/xylazine mixtures can also be used for anesthesia.
Syringes connected to silicone pump tubing can be used for cardiac perfusion fixation as an alternative to the automated peristaltic pump.
For muscles such as the tibialis anterior or the gastrocnemius, we create cryomolds using tin foil. These tin foil molds fit perfectly in the wells of a 48-well tissue culture dish which is ideal for organization and storage. For embryos or smaller muscles, small plastic molds can be purchased.
For the purposes of this methods chapter, paraffin embedding and sectioning are not covered. However, resources are readily available, and many institutions have core facilities that perform paraffin embedding.
Deparaffinization solutions can be used multiple times but should be replaced when volumes change, as ethanol and xylene do evaporate.
Citrate buffer pH6 is a standard retrieval buffer that works for many antigens; however, if an antibody does not recognize its target with conventional immunostaining, different pH and buffer combinations can be tested. When citrate buffer at pH6 creates excessive background, try increasing to pH7 or switching to Tris-EDTA pH9. Reducing the pH of citrate buffer to 3 or 4 can also improve retrieval of antigens but often creates significant background signal. Generally, citrate buffer pH6 is superior for staining of nuclear antigens [5].
Heat induced antigen retrieval using Tris-EDTA pH9 buffer is effective at retrieving antigens that are not retrieved using an acidic or neutral retrieval solution. However, Tris-EDTA can negatively impact tissue morphology, especially on frozen sections, and thus may not be suited for examining small structures. For perfusion fixed tissues, morphology is improved when the sections are fixed after thawing and before performing antigen retrieval.
A microwave can be used in lieu of a pressure cooker. However, we highly recommend the use of a pressure cooker if antigen retrieval is performed routinely. Most commercially available pressure cookers will work for this step.
Ice cold 100% methanol or acetone is an effective fixative for cryosections, and more suited to some antigens. Acetone is less harsh than methanol, and they are both precipitating fixatives. For conventional immunostaining, 4% PFA, 100% methanol or 100% acetone are applied following cryosectioning.
TBS or PBS can be used as wash buffers. Further, when high background is observed after immunostaining, 0.1 % Tween-20 can be added to PBS or TBS wash buffers to reduce nonspecific binding of antibodies. However, detergents can negate the effectiveness of some hydrophobic barriers, thus caution should be used to ensure slides do not dry out due to barrier failure.
The barrier created by the hydrophobic PAP pen (RPI, 195505) will be disrupted if reagents containing detergents like Tween-20 or Tx-100 come into direct contact. The ImmEdge hydrophobic pen is less susceptible to solutions containing Tween 20 or Triton X100 when applied and dried appropriately.
When staining mouse tissue with primary antibodies raised in mouse, add a 1:40 dilution of M.O.M. to the blocking solution. Omit M.O.M. reagent when staining tissues from other species or when using antibodies raised in species other than mouse.
TrueBlack Lipofuscin Autofluorescence Quencher (Biotium, Cat# 23007) is a new iteration of Sudan Black that provides less background autofluoresence in the far-red channel. While it is a lipofuscin autofluorescence quencher, it is also a good general background blocking agent. TrueBlack is particularly useful for reducing background fluorescence in the green channel when immunofluorescence staining paraffin sections. Ensure that slides do not dry out when using this reagent, as this will detrimentally impact the immunostain, and it is important not to use any reagents containing detergents (i.e. Triton X-100 or Tween 20) for the remainder of the protocol.
The animal serum should match species in which secondary antibodies are raised.
When blocking or permeabilization solutions are stored longer than one week, either add 0.02% sodium azide or freeze aliquots at −20 °C.
When planning the primary antibody panel, ensure the primary antibodies are raised in different species or possess different IgG isotypes. For example, it is okay to combine a Pax7 mouse IgG1 primary antibody with a Myogenin mouse IgG2a.
Fluorophore selections should match the microscope configuration at your institution. For wide field epifluorescence microscopes, this depends on the light source and filter sets, while the laser and detector combinations must be considered for confocal microscopy. Many tools are available to help identify the best combination of fluorophore-conjugated secondary antibodies. FBbase is an open-source tool that lists most fluorophores and their emission and absorption spectra (https://www.fpbase.org/spectra/). Generally, we use Alexa Fluor dyes, as most are bright and photostable, and the Fisher Scientific website provides information on stability in buffer and fluorophore brightness to dye recommendations based on your filter set and more. Alternatively, the CF dyes by Biotium offer antibody isotypes conjugated to less common fluorphores. For example, CF405 is available in goat anti-mouse IgG1, while the comparable Alexa Fluor dye is only available in goat anti-Mouse IgG. The most common panel our lab uses is DAPI, Alexa Fluor 488/546/647.
PermaFluor is our preferred mounting media, but other mounting media can be substituted. It is a good idea to note the resolution and the refractive index of your microscope objective when choosing an appropriate mounting media.
Ensure the 2-methylbutane begins solidifying prior to freezing the muscle tissues. If the 2-methylbutanol is not sufficiently cooled, the muscles will not freeze fast enough. This causes ice crystal to form in the tissues and can detrimentally impact tissue morphology and immunostaining.
When performing cardiac perfusion, an improperly placed needle can detrimentally impact fixation (i.e., if the needle perforates the right ventricle). When muscles are insufficiently fixed, immersion fix the muscle for 48 hours at 4°C prior to the sucrose gradient.
To improve penetration of PFA, 0.05% NP-40 can be added to the fixative overnight.
If tissues containing fluorescence reporters require antigen retrieval, antibodies raised against the fluorescent proteins must be included in the primary antibody panel, as heat induced antigen retrieval quenches endogenous fluorophores (see Table 2).
Heat induced antigen retrieval using Tris-EDTA pH9 buffer is effective at retrieving antigens that are not retrieved using an acidic or neutral retrieval solution. However, Tris-EDTA can negatively impact tissue morphology, especially on frozen sections, and thus may not be suited for examining small structures. For cardiac perfusion fixed tissues, morphology is improved when the sections are fixed again in 4% PFA after thawing and before antigen retrieval.
Fixed frozen sections can be more sensitive than paraffin sections. If sections detach or morphology is impacted, consider reducing the time in the pressure cooker. Properly dried samples rarely detach from superfrost slides.
When using permeabilization buffer, take care to keep the solution away from the hydrophobic barrier, otherwise it negates its hydrophobicity. If this happens, wash the slide well with PBS.
Controls, including secondary only and antibody isotype in lieu of primary antibodies should be included when possible. Positive and negative controls for the antigen of interest are also important when testing new antibodies or tissues.
We do not generally recommend incubating slides in primary antibodies for only 2–3 hours at room temperature, except for unfixed muscle tissue with MyHC antibodies.
Acknowledgments
We thank Dr. Dongsheng Duan at University of Missouri-Columbia for providing us with dog tissue. M.A.R. holds a Canada Research Chair in Molecular Genetics. These studies were carried out with the support of grants from the Canadian Institutes of Health Research [FDN-148387], the US National Institutes for Health [R01AR044031], E-Rare-3 (Canadian Institutes of Health Research/Muscular Dystrophy Canada), the Foundation for Gene & Cell Therapy, and the Stem Cell Network.
References
- 1.Dumont NA, Bentzinger CF, Sincennes M-C, Rudnicki MA (2015) Satellite Cells and Skeletal Muscle Regeneration. In: Comprehensive Physiology. American Cancer Society, pp 1027–1059 [DOI] [PubMed] [Google Scholar]
- 2.Mercuri E, Bönnemann CG, Muntoni F (2019) Muscular dystrophies. The Lancet 394:2025–2038. 10.1016/S0140-6736(19)32910-1 [DOI] [PubMed] [Google Scholar]
- 3.Bentzinger CF, Wang YX, Rudnicki MA (2012) Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harbor Perspectives in Biology 4:a008342–a008342. 10.1101/cshperspect.a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feige P, Rudnicki MA (2020) Isolation of satellite cells and transplantation into mice for lineage tracing in muscle. Nat Protoc 15:1082–1097. 10.1038/s41596-019-0278-8 [DOI] [PubMed] [Google Scholar]
- 5.Shi SR, Imam SA, Young L, et al. (1995) Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem 43:193–201. 10.1177/43.2.7822775 [DOI] [PubMed] [Google Scholar]


