Abstract
A human immunodeficiency virus (HIV) vaccine that will be useful in diverse geographic regions will need to induce a broad immune response characterized by cross-clade immunity. To test whether a clade B-based HIV candidate vaccine could induce interclade humoral responses, including neutralizing activity against primary HIV-1 isolates, sera were tested from recipients of a vaccine consisting of recombinant canarypox virus vCP205 and recombinant gp120SF2. Serum antibodies exhibited strong immunochemical cross-reactivity with V3 peptides from clades B, C, and F, with weaker activity for several V3 peptides from clades A, D, G, and H; essentially no reactivity could be demonstrated with V3 peptides from clades E and O. Extensive cross-clade reactivity was also documented by enzyme-linked immunosorbent assay with all nine recombinant HIV envelope glycoproteins tested from clades B, D, and E. In addition, vaccinees' sera displayed significant neutralizing activity against 5 of 14 primary isolates tested, including one X4 virus and two dualtropic viruses (from clade B) and two R5 viruses (from clades B and C). This is the first demonstration of the induction by a candidate HIV vaccine constructed from clade B laboratory strains of HIV of neutralizing activity against R5 and clade C primary isolates. The data suggest that, by virtue of their ability to induce cross-clade immune responses, appropriately formulated HIV vaccines based on a finite number of HIV isolates may ultimately be able to protect against the wide range of HIV isolates affecting the populations of many geographic regions.
Progress in the development of an effective vaccine for human immunodeficiency virus type I (HIV-1) has been gauged in large part by the ability to elicit measurable virus-specific CD8+ cytotoxic T lymphocytes (CTLs) and neutralizing antibodies (Abs) as critical correlates of protective immune responses (8, 29, 36). The major targets for neutralizing Abs are gp120 and, to a lesser extent, the transmembrane gp41 envelope glycoproteins of the virus (8). The first HIV vaccines advanced to clinical trials were based on recombinant envelope (Env) subunits derived from T-cell line-adapted (TCLA) strains of the virus. While these vaccines generated neutralizing Abs with variable and sometimes potent activity against the homologous TCLA HIV-1 vaccine strain, CTL activity was generally poor against heterologous TCLA strains (5, 25, 27, 41, 62) and the sera from vaccinated volunteers failed to neutralize most primary isolates (28, 41, 42).
Since serum-neutralizing Abs are considered critical to protection against most viral infections (58) and have been shown to protect against HIV and simian immunodeficiency virus (SIV) infection in several animal models (2, 6, 7, 11, 20, 38, 40, 60, 63, 68, 76), the ability to induce neutralizing Abs is thought to be an important characteristic of candidate HIV vaccines. To be protective against the many circulating subtypes of HIV, a vaccine will need to induce broad neutralizing anti-HIV Abs against primary isolates, not only TCLA clade B strains (1, 44, 56).
The current challenge for HIV vaccine design is to develop optimized vaccines able to elicit both stronger cellular immune responses and broader neutralizing responses against genetically diverse viral species. One of the current strategies developed to induce both types of immune responses is called the prime-boost strategy, using a live poxvirus vector expressing the env gene of HIV-1 to prime the immune system and a recombinant subunit HIV-1 envelope protein to boost the immune response (13, 25, 26, 55, 73). Such candidate vaccines have already been shown to induce both cellular and humoral responses in animals (66, 67, 76), and a clade B-based canarypox vaccine was shown to elicit cross-clade CTLs in HIV-uninfected adults (19). However, the repertoire of neutralizing Abs induced by these prime-boost protocols in most volunteers was directed against the homologous TCLA strains from which the vaccine was made, a limited number of heterologous TCLA HIV strains, and a limited number of X4-tropic primary clade B viruses (4, 12, 16, 17, 67, 74, 77). These initial results suggested that this vaccine regimen induced a quite restricted humoral immune response. To test this assumption, the Abs induced by such a prime-boost regimen were tested for their ability to cross-react with V3 peptides and recombinant gp160 proteins derived from viruses of different clades and to neutralize viruses of different tropism from several clades.
MATERIALS AND METHODS
Subjects and specimens tested.
Twenty human sera were obtained from the Division of AIDS (DAIDS), National Institutes of Health, from participants in trials conducted by the AIDS Vaccine Evaluation Group and sponsored by the National Institute of Allergy and Infectious Diseases. Sera were obtained from HIV-uninfected volunteers, 18 to 60 years of age, of both sexes, who were enrolled in AIDS Vaccine Evaluation Group protocol 029. This protocol consisted of an accelerated prime-boost immunization schedule using recombinant canarypox virus (vCP205 [Pasteur Mérieux/Connaught Laboratories] expressing gp120MN, the transmembrane anchoring region of gp41LAI, GagLAI, and a portion of PolLAI) and rgp120SF2 in MF59 adjuvant (Chiron Corp, Emeryville, Calif.). HIV-uninfected subjects were immunized intramuscularly at 0, 1, 2, and 3 weeks with vCP205, boosted at 4 and 12 weeks with rgp120SF2, and bled 2 weeks after the last boost. All reagents were administrated intramuscularly. All sera were heat inactivated at 56°C for 30 min prior to use. The panel of sera received from DAIDS included one HIV-positive serum (from the repository) and three HIV-negative sera (one from a volunteer in the study who received placebo only and two from vaccinees bled prior to immunization). All sera were shipped coded and tested blind. Two additional known HIV-negative sera were used as negative controls, and sera from HIV-positive patients from the Veterans Affairs Medical Center (New York, N.Y.) (designated SX3, SX5, SX7, SX14, and SX16) were used as known positive controls.
Peptides and recombinant proteins.
Fifty-three 19- to 30-mer peptides which span the tip of the V3 loops were used; these were derived from the sequences of seven clade A viruses, eight clade B viruses, eight clade C viruses, eight clade D viruses, two clade E viruses, six clade F viruses, six clade G viruses, three clade H viruses, and five clade O viruses. The peptides were synthesized and purified by standard procedures as described previously (75) and purchased from Intracel, Inc. (Cambridge, Mass.), Genemed Biotechnologies, Inc. (South San Francisco, Calif.), or Princeton Biomolecules Corp. (Columbus, Ohio) or provided by A. Conley (Merck Research Institute), C. Fiol (Colorado State University), or T. VanCott or L. Loomis-Price (H. M. Jackson Foundation). None of the N- or C-terminal amino acids were derivatized, and none of the peptides were cyclic.
Recombinant gp160 (rgp160) proteins were provided by M.-P. Kieny (Transgene, France) and were derived from env genes from a clade B strain (rgp1601286), two clade D strains (rgp160ELI and rgp1604020), and one clade E strain (rgp160CM243). The cleavage site of these recombinant glycoproteins was altered, and the hydrophobic env transmembrane domain was removed (34, 54). rgp160IIIB is oligomeric, uncleaved, and truncated at the C terminus of the molecule, has a length of 813 amino acids, and was purchased from Advanced Bioscience Laboratories (Kensington, Md.) (33, 70). Although designated gp160, these rgp160 glycoproteins are actually gp140. rgp120IIIB was purchased from Intracel, rgp120Bal was purchased from SmithKline Beecham (30), and rgp41MN was provided by Jian Zheng (Ortho-Clinical Diagnostics, Raritan, N.J.).
Virus isolates.
A total of 14 primary isolates from different clades were used, all being passaged exclusively in peripheral blood mononuclear cells. These included isolates HIV-1SF2 and HIV-1SF33 (both dualtropic [R5X4]), obtained from J. Levy (University of California at San Francisco), and isolate HIV-1MNp (X4), recently isolated by John Sullivan (University of Massachusetts Medical School, Worcester) from frozen spleen tissue from the patient from whom isolate HIV-1MN had been obtained. HIV-1MNp has never been passaged in cell lines. Isolates HIV-1JR-FL (R5), HIV-1SM993 (R5), HIV-192BR025 (R5), HIV-193BR029 (R5), HIV-193BR020 (R5X4), and HIV-193IN904 (R5) were supplied by the National Institutes of Health AIDS Research and Reference Reagent Program; isolates HIV-1CA4 (R5) and HIV-1CA20 (R5) were obtained from G. van der Groen (Institute of Tropical Medicine, Antwerp, Belgium); HIV-1BX08 (R5) was obtained from H. J. A. Fleury (Université de Bordeaux II, Bordeaux, France); and isolates HIV-1748 (X4) and HIV-12036 (R5X4) were obtained from D. Katzenstein (Stanford University, Stanford, Calif.).
ELISA.
A standard peptide enzyme-linked immunosorbent assay (ELISA) was used (22, 23). Briefly, V3 peptides were coated onto plastic Immulon 2HB plates at 1 μg/ml. Plates were blocked for 2.5 h at 37°C with 7.5% fetal calf serum and 2.5% bovine serum albumin in phosphate-buffered saline (PBS) and then washed four times with PBS containing 0.05% Tween 20 (pH 7.4). Subsequently, 50 μl of each serum, at a dilution of 1:100, was added to each well for 1.5 h at 37°C. After washing, the plates were incubated with alkaline phosphatase-conjugated goat anti-human immunoglobulin G (γ-chain specific), color was developed with p-nitrophenyl phosphate, and plates were read at 410 nm. Negative controls consisted of V3-coated wells reacted with known HIV-negative sera. Sera from five known HIV-positive subjects from New York were introduced into this assay as positive controls for clade B infection. For ELISAs with recombinant proteins, the plates were coated with 0.5 μg of recombinant protein/ml following the same protocol.
Neutralization assay.
The GHOST cell neutralization assay was used (9). GHOST-X4 cells were used as target cells in assays with X4 and dualtropic viruses. GHOST-R5 cells were used with the R5 viruses. The GHOST cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1% glutamine, 2% penicillin and streptomycin, plus 500 μg of geneticin, 50 μg of hygromycin, and 1 μg of puromycin per ml. Cell monolayers, when confluent, were resuspended using 0.25% trypsin. The cells were carried for 15 passages and then replaced with fresh cells from stocks frozen at the second or third passage.
For the GHOST cell neutralization assay, 6 × 104 GHOST cells/well per 0.5 ml were seeded into wells of 24-well tissue culture plates and allowed to grow for 24 h. Each virus stock was diluted to a predetermined concentration which had been found to result in ∼1,000 infected cells per 15,000 total cells measured cytofluorometrically at the end of the assay. Equal volumes of appropriately diluted virus and heat-inactivated serum at a 1:10 dilution were mixed and incubated at 37°C for 1 h before being applied to the GHOST cells in the presence of DEAE-dextran at 8 μg/ml. After overnight adsorption, the virus- and Ab-containing medium was removed, the cell monolayers were washed, and the cells were incubated for 3 to 4 days. For harvest, cells were resuspended using 1 mM EDTA, fixed in 2% formaldehyde, and then analyzed using a FACScan flow cytometer (Becton-Dickinson). The percent neutralization was calculated using the number of infected cells observed in the absence of human serum as the denominator. Two known HIV-negative human sera were used as negative controls in each experiment.
RESULTS
Reactivity of anti-V3 loop antibodies.
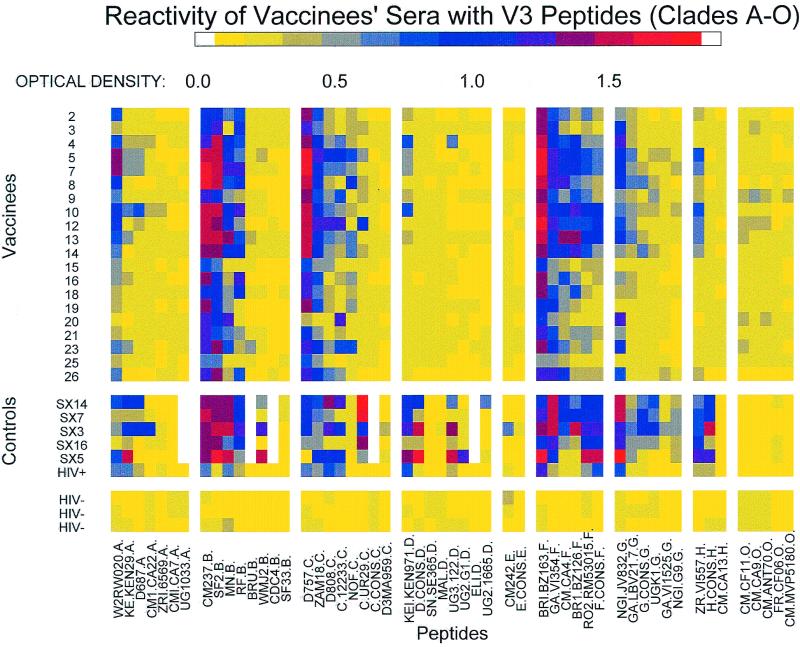
The coded panel of sera and the known HIV-positive sera were tested for cross-reactivity to V3 peptides derived from the sequences of viruses of group M (clades A through H) and group O. Results from each serum-peptide combination are shown in Fig. 1. For clarity, the optical density (OD) values have been represented as a spectrum of colors corresponding to the degree of reactivity observed.
FIG. 1.
Reactivity of HIV-positive, HIV-negative, and vaccinees' sera with 53 V3 peptides from groups M (clades A through H) and O. The OD values are color coded as shown by the spectrum at the top of the figure. Each row represents data generated with serum from a single individual in the study. The columns show reactions of the sera with each different peptide; the peptides are grouped according to clade in decreasing order of reactivity within that clade. Also shown are results with HIV-positive sera SX3, SX5, SX7, SX14, and SX16 and an HIV-positive serum included with the panel of sera received from DAIDS and HIV-negative sera (one from a vaccine volunteer who received placebo only, and two from HIV-negative participants in the vaccine trials bled prior to their immunization).
No significant reactivity was detected against V3 peptides with any HIV-negative sera. In contrast, cross-clade V3 Abs were detected in sera from each of the 20 recipients of the prime-boost regimen. Positive reactions were defined as greater than the mean +3 standard deviations of the 159 combinations of HIV-negative sera and peptides. Vaccinees' sera reacted with three of seven clade A peptides, four of eight clade B peptides, six of eight clade C peptides, one of eight clade D peptides, six of six clade F peptides, three of six clade G peptides, and one of three clade H peptides. Within this pattern of broad cross-clade reactivity, additional patterns were noted. Strong reactivity (>0.7 OD units) was observed with most of the V3 peptides from clades B, C, and F, while the strength of reactivity to V3 peptides from clades A, D, G, and H was lower and restricted to only a few vaccinees' sera. No reactivity was detected to V3 peptides from clade E or O. These results show that anti-V3 Ab responses induced by MN and SF2 envelope-based vaccines are broadly reactive and clearly not type or clade specific.
To compare the clade B vaccine-induced humoral response to that of natural infection with clade B primary isolates, results of experiments performed with sera from HIV-positive subjects using the same conditions as mentioned previously were analyzed. The reactivity of HIV-positive sera was similar to that of vaccinees' sera, showing broad and strong reactivity to clades B, C, and F. Reactivity to V3 peptides from clades A, D, G, and H was somewhat stronger and broader than that displayed by vaccinees sera, but again no reactivity with clade E and O V3 loops was detected. Thus, the sera from vaccinees show a high degree of cross-reactivity to V3 regions derived from different genetic subtypes and were very similar to sera from clade B-infected subjects with respect to the magnitude and pattern of anti-V3 Ab cross-reactivity.
Reactivity with recombinant HIV envelope proteins.
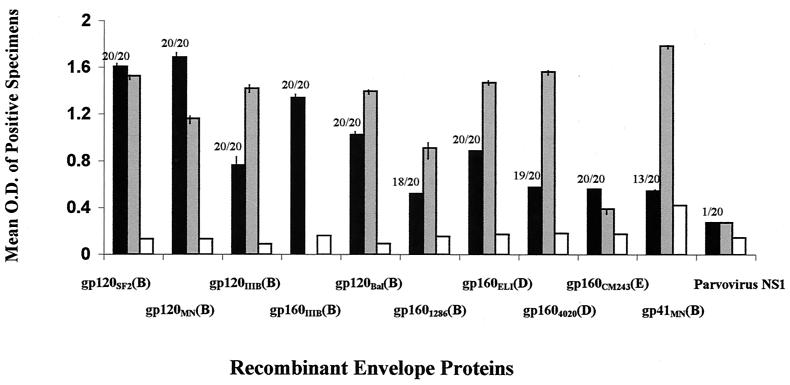
Cross-clade reactivity to the entire envelope was then tested. The availability of such molecules in recombinant form was limited. All that were obtained (six from clade B strains, two from clade D strains, and one from a clade E strain) were tested. All vaccinees' sera reacted strongly with rgp120SF2 and rgp120MN, which were both homologous to the immunizing antigens (Fig. 2). Strong reactivity to rgp160IIIB was also detected. Vaccinees' sera also showed significant reactivity with the heterologous rgp120 and rgp160 molecules from primary isolates of other clade B, D, and E strains. This cross-reactivity was not due to anti-gp41 antibodies, as shown by the weak reactivity of vaccinees' sera with gp41 (Fig. 2). Indeed, it has previously been shown that without a gp120 boost, priming with recombinant virions produces little antibody activity (76), and this would account for the paucity of anti-gp41 Abs in the vaccinees' sera tested here. While vaccinees' sera reacted with all of the recombinant envelope molecules tested, the levels of Abs detectable in the HIV-positive sera against six of eight rgp120 and rgp160 molecules derived from clades B, D, and E were comparable to or higher than those detected in the vaccinees' sera (Fig. 2). Interestingly, reactions were detected with rgp160 of clade E even though no reactivity was noted against any V3 peptides from clade E.
FIG. 2.
Average reactivity of vaccinees' sera with recombinant envelope proteins. The average OD values detected in vaccinees' sera (solid bars) with the designated recombinant proteins are shown on the y axis. Only the results with vaccinees' sera giving reactions above the cut-off were used to calculate the average values. The number of vaccinees' sera (out of 20) giving a positive reaction with a designated protein is shown at the top of each bar. The average OD detected in the three HIV-1-negative control sera (one from a vaccine volunteer who received placebo only, two from HIV-negative participants in the vaccine trials bled prior to their immunization, and two from HIV-negative uninfected unimmunized individuals) with each recombinant protein is also shown (open bars). The average OD detected with all three HIV-positive sera (SX5, SX7, and SX16) (gray bars) is shown. The reactivity with a recombinant protein, NS1, from human parvovirus B19 was used as a negative control. The vertical bars show standard deviations.
Neutralization of primary isolates by vaccinees' sera.
Binding of Abs to recombinant proteins and peptides of HIV has previously been shown not to be predictive of primary isolate neutralization (69, 71), and this appears to be substantiated by the data presented below. Thus, the capacity of the vaccinees' sera to neutralize 14 HIV-1 primary isolates was tested using the GHOST cell neutralization assay. Ten primary isolates from clade B were previously tested in this assay (9). Neutralization curves showed that for the primary isolate HIV-1SF2, the maximum level of neutralizing activity (70%) of several HIV-1-positive sera with broad reactivity was maintained through a dilution of 1:40 and gradually decreased to 50% neutralization at a dilution of 1:500 and to 25% neutralization at a dilution of 1:1,000 (9). Furthermore, the various primary isolates differed in their capacity to be neutralized by HIV-positive sera, displaying 50% neutralizing titers ranging from 1:20 to 1:5,000. Therefore, for our study, HIV-1-positive sera as well as vaccinees' sera were tested at a dilution of 1:20. Significant neutralization was defined on the basis of the 95% confidence limit of the percentage of neutralization by HIV-negative sera (shown as the shaded area in Fig. 3). This was established on the basis of 54 assays using five HIV-negative sera (at a final serum dilution of 1:20) and the 14 primary isolates. Thus, significant neutralization is depicted when the percent neutralization is above the shaded area in Fig. 3 (>23%). Two HIV-1-positive sera (SX5 and SX16; see Fig. 1) were used as positive controls. The sera from these two HIV-positive individuals had previously been found to neutralize a large majority of primary isolates from clades B and C in the same assay (data not shown); the percent neutralization obtained with these HIV-positive sera is indicated in red symbols in Fig. 3.
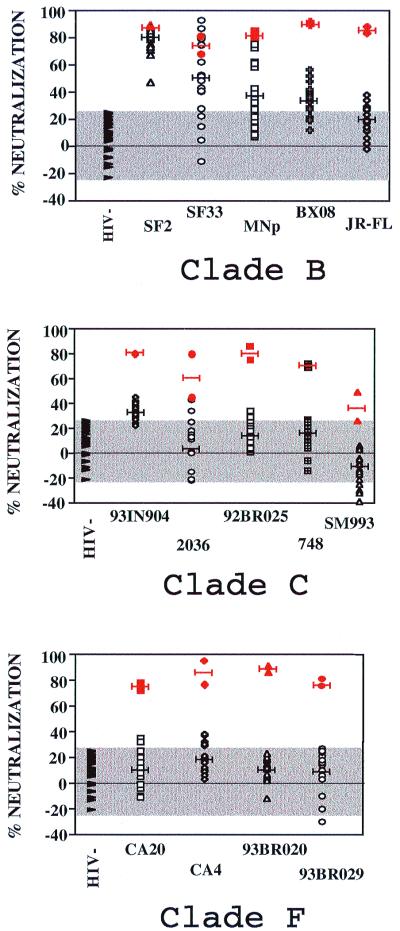
FIG. 3.
Neutralization of HIV primary isolates from several clades by sera from vaccinees and by HIV-positive and HIV-negative control sera. The percent neutralization shown on the y axis was determined for five primary isolates from clade B (top panel), five from clade C (middle panel), and four from clade F (bottom panel) with vaccinees' sera (open symbols), HIV-positive sera (SX5 and SX16) (red symbols), and HIV-negative sera (▸). The mean percent neutralization for each virus strain is indicated by a black line (vaccinees' sera) or by a red line (HIV-positive sera). The GHOST cell neutralization assay was used (9), with sera used at a final dilution of 1:20.
We tested the capacity of the vaccinees' sera to neutralize 14 HIV-1 primary isolates. We selected five clade B primary isolates, five clade C primary isolates, and four clade F primary isolates. Previous studies have shown the neutralizing activity of sera from recipients of a similar prime-boost immunization protocol against 13 clade B HIV-1 primary isolates (77). Among the clade B HIV-1 primary isolates, we chose HIV-1SF2, HIV-1SF33, and HIV-1MNp because they were syncytium-inducing viruses that were homologous to or contemporaneous with the virus from which rgp120SF2 was constructed. HIV-1BX08 and HIV-1JR-FL were chosen because they are two non-syncytium-inducing viruses that have been well characterized in terms of neutralization (9, 14, 45, 46). Clade C and F primary isolates were selected arbitrarily. Sera from all vaccinees (at a final dilution of 1:20) were able to significantly neutralize HIV-1SF2, homologous to the strain of the boosting immunogen (Fig. 3). The majority of vaccinees' sera also displayed significant neutralizing activity against other clade B primary isolates, including HIV-1MNp (homologous to env in the priming immunogen), HIV-1SF33, and HIV-1BX08. Some vaccinees' sera neutralized HIV-1JR-FL, but the majority did not show any activity against this virus. The relative lack of neutralization of this virus has been reported by other authors (9, 14). The neutralization of the heterologous clade B primary isolates is noteworthy: HIV-1SF33, a dualtropic strain, is contemporaneous with HIV-1MN and HIV-1SF2, while HIV-1BX08 is an R5-tropic strain and was isolated much later (46). This is the first demonstration that a vaccine derived from X4-tropic TCLA strains can induce Abs that neutralize an R5-tropic primary isolate.
The magnitude of the neutralizing Ab response against each virus is also reflected in Fig. 3. The mean percent neutralization is indicated as a black line for the vaccinees' sera and as a red line for the broadly cross-reactive clade B HIV-positive sera (Fig. 3). The levels of neutralizing activity detectable in the vaccinees' sera against clade B strains were comparable to those achieved by the HIV-positive serum when HIV-1SF2 was tested. Furthermore, some vaccinees' sera were able to neutralize HIV-1SF33 and HIV-1MNp to approximately the same extent as the HIV-positive sera. However, the mean percent neutralization of vaccinees' sera against the clade B strains was generally lower than that of the broadly cross-reactive clade B HIV-positive sera when HIV-1BX08, HIV-1SF33, and HIV-1MNp neutralization was measured.
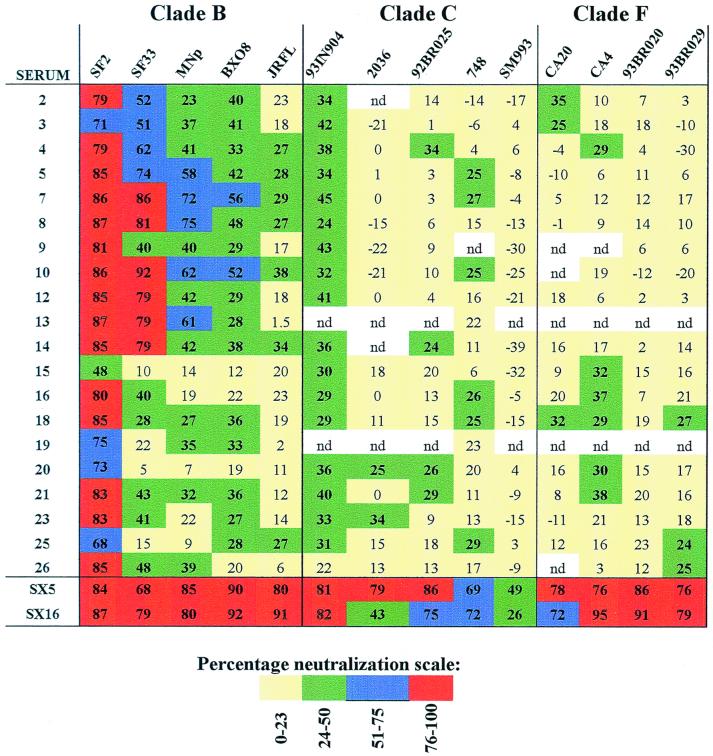
Given these neutralizing responses to heterologous clade B primary isolates and the previously demonstrated cross-clade immunochemical activity in the vaccinees' sera, the sera were further tested for neutralizing activity against five clade C and four clade F primary isolates, clades to which strong cross-reactivities were detected in ELISA experiments (Fig. 1). Significant neutralization was observed with 18 of 20 vaccinees' sera against the clade C, R5-tropic primary isolate HIV-1931N904. Neutralizing Ab levels of vaccinees' sera directed against this virus were lower than those of the broadly cross-reactive clade B HIV-positive serum but comparable to those when HIV-1BX08 and HIV-1MNp were tested. The C2-V3 (6500 to 7300) region of the HIV-1931N904 envelope was sequenced from the virus preparation used here, and its identity was confirmed by comparison with the C2-V3 sequence of this virus in the HIV sequence database (http://hiv-web.lanl.gov/) (data not shown). The neutralizing activity of vaccinees' sera against the other eight clade C and F primary isolates was sporadic and weak (Fig. 3 and 4). As shown in Fig. 4, strong and broad neutralizing activity against clade B viruses was achieved with several vaccinees' sera, but this does not correlate with broad interclade neutralizing activity. Thus, for example, sera 7 and 10 showed good neutralizing activity against four of five clade B viruses but were able to significantly neutralize only two of nine clade C and F viruses. In contrast, vaccinee serum 20 was able to neutralize only one of five clade B viruses, but significantly neutralized four of nine clade C and F primary isolates. Thus, while the cross-clade neutralizing activity cannot be defined as broad, the detection of neutralizing activity against HIV-1931N904 demonstrates for the first time that significant cross-clade neutralizing activity can be induced by a TCLA clade B-derived prime-boost vaccine regimen.
FIG. 4.
Levels of neutralization of each serum (tested at a 1:20 dilution) against each of the 14 primary isolates. The percent neutralization by each serum-virus combination is shown. For ease of interpretation, the levels of neutralization are color coded: yellow represents nonsignificant neutralization (<23%), green represents weak neutralization (24 to 50%), blue represents moderate neutralization (51 to 75%), red represents strong neutralization (76 to 100%), and white depicts serum-virus combinations that were not done (nd).
DISCUSSION
It is currently assumed that both a vigorous HIV-specific CTL response and serum neutralizing activity against primary HIV isolates will be important immune responses induced by a preventive HIV vaccine. To date, the prime-boost regimen, using recombinant canarypox virus vaccine constructs and recombinant envelope subunits, has most successfully induced both of these HIV-specific immune responses. This regimen was able to elicit cross-clade CTL reactivities (19) but was thought to have induced only a very restricted humoral response. In fact, these prime-boost protocols were shown to induce a variety of immunochemically reactive Abs, including Abs mediating Ab-dependent cell-mediated cytotoxicity and Abs that neutralize autologous TCLA strains but relatively few primary isolates of clade B (12, 74, 77). However, while cross-clade CTL activity was studied and demonstrated, as mentioned above, no study of cross-clade primary isolate neutralizing activity has been reported. This led to a tacit assumption, in the absence of data, that cross-clade neutralizing activity has not been and cannot be induced by TCLA clade B-based vaccines.
The experiments presented here investigated the nature of the Ab repertoire in vaccinees' sera following immunization of healthy seronegative volunteers with a prime-boost vaccine regimen derived from TCLA clade B HIV-1MN and HIV-1SF2 isolates. The data demonstrate that vaccine-induced Abs are not strain or even clade B specific, but rather are broadly cross-clade reactive. The sera showed the strongest and most extensive immunochemical reactivity with V3 peptides from clades B, C, and F. In contrast, none of the vaccinees' sera bound to V3 peptides from clade E and group O. These results extend previous studies describing (i) the extensive cross-clade reactivity of human anti-V3 serum and monoclonal Abs (3, 10, 21, 23, 47, 50, 57, 75), (ii) the similarity of clade A and C V3 loops and of clade B and F V3 loops, and (iii) the divergence of the clade D V3 loop, based on serologic and sequence data (3, 35, 57). The lack of reactivity of vaccinees' sera with the clade E and group O V3 peptides is also consistent with previous serological and functional analyses of Ab activity (39, 43, 49). Furthermore, vaccinees' sera were able to bind to gp160 glycoproteins of primary isolates of HIV-1 subtypes B, D, and E. These results are consistent with recent data showing that Abs induced by MN and IIIB recombinant gp120 HIV-1 vaccines were able to bind to oligomeric native HIV-1 envelope glycoproteins of primary isolates of HIV-1 from clades A, D, and E measured by a flow cytometric indirect immunofluorescence assay (24).
Studies of the neutralization of X4, R5, and dualtropic viruses and of viruses from clades B, C, and F also demonstrated broader reactivity of vaccinees' sera than had previously been documented or anticipated. While the breadth of neutralizing activity induced by immunization with a clade B, TCLA-based vaccine is narrower than that induced by infection with clade B strains, vaccinees' sera displayed neutralizing activity against X4, R5, and dualtropic primary isolates, and significant neutralizing activity was found in 18 of 20 vaccinees' sera against an R5 isolate of clade C. Whether this activity is protective cannot be ascertained without phase III efficacy studies; however, it is noteworthy that passive immunization studies conducted in chimpanzees, macaques, and SCID-hu mice suggest that Ab alone can be protective against HIV and SIV challenge (15, 53, 61) and that, in the case of other viral infections, such as polio and hepatitis B, vaccine-induced Ab titers as low as 1:4 are sufficient to confer protection (18, 31, 52, 64, 65).
Significant neutralizing activity in vaccinees' sera was demonstrated in 45% of the 257 virus-serum combinations tested; this compares favorably with studies of sera from HIV-positive subjects in which 65% of 224 combinations of virus and serum (51), 56% of 107 combinations (72), and 52% of 441 combinations (48) were found to have neutralizing activity. The overall neutralizing activity was, however, weaker in the vaccinees' sera than in the HIV-positive sera and cannot be defined as broad. Nevertheless, these data constitute the first proof of the principle that a clade B, TCLA (X4)-based candidate HIV vaccine can induce detectable neutralizing activity against viruses from a heterologous, R5 phenotype and against a heterologous clade. These data also suggest that the potential protective capacity of vaccines may not be restricted to a single clade. However, it also appears that protection may not be conferred against all viruses belonging to the clade from which a single immunogen is derived. The spectrum of protection may turn out to be more closely related to immunologically defined groups (immunotypes) than to genotypically defined groups (clades), a concept that is supported by several other published studies (50, 72, 75).
Our data suggest that an HIV vaccine, in order to confer truly broad protection, will most probably need to include a mixture of immunogens representative of the different immunologic groups of HIV. Quite possibly not all immunologic groups of HIV will be required as components of such a polyvalent vaccine, since components from a single virus or immunotype are able to induce cross-reactivity to heterologous viruses. This is the case with the monovalent influenza virus vaccine, for example, which is able to induce cross-reactive Abs (32, 37). Similarly, vaccines against bacterial pathogens require a limited number of serotypes to protect against a wide number of immunologic variants: in the case of pneumococcal vaccines, components from approximately 23 common serotypes confer immunity to more than 80 serotypes of Streptococcus pneumoniae (59). Thus, for HIV, the challenges presented to vaccine development against a virus family with extreme genetic variation may be addressed successfully by the ability of the immune system to recognize related, conserved structures and conformations defining antigenic variability which might be less extreme than the well-documented variation in the sequences of HIV.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institutes of Health (R01-AI 32424, R01-AI 36085, R01-HL 59725, and P01-AI 27742 [which supports the Immunology and Flow Cytometry Cores of the NYU Center for AIDS Research]) and from the Department of Veterans Affairs (Research Center for AIDS and HIV Infection and Merit Review funding).
REFERENCES
- 1.Albert J, Abrahamsson B, Nagy K, Aurelius E, Gaines H, Nystrom G, Fenyo E M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990;4:107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- 3.Barin F, Lahbabi Y, Buzelay I, LeJeune B, Baillou-Beaufils A, Denis F, Mathiot C, M'Boup S, Vithayasai V, Dietrich U, Goudeau A. Diversity of antibody binding to V3 peptides representing consensus sequences of HIV type 1 genotypes A to E: An approach for HIV type 1 serological subtyping. AIDS Res Hum Retroviruses. 1996;12:1279–1289. doi: 10.1089/aid.1996.12.1279. [DOI] [PubMed] [Google Scholar]
- 4.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Belshe R B, Graham B S, Keefer M C, Gorse G J, Wright P, Dolin R, Matthews T, Weinhold K, Bolognesi D P, Sposto R, et al. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. NIAID AIDS Vaccine Clinical Trials Network. JAMA. 1994;272:475–480. doi: 10.1001/jama.272.6.475. [DOI] [PubMed] [Google Scholar]
- 6.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van Opstal O, Culp J, Rosenberg M, De Wilde M, Heidt P, et al. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 7.Buge S L, Murty L, Arora K, Kalyanaraman V S, Markham P D, Richardson E S, Aldrich K, Patterson L J, Miller C J, Cheng S M, Robert-Guroff M. Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251. J Virol. 1999;73:7430–7440. doi: 10.1128/jvi.73.9.7430-7440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11:S87–S98. [PubMed] [Google Scholar]
- 9.Cecilia D, KewalRamani V N, O'Leary J, Volsky B, Nyambi P, Burda S, Xu S, Littman D R, Zolla-Pazner S. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheingsong-Popov R, Lister S, Callow D, Kaleebu P, Beddows S, Weber J the WHO Network for HIV Isolation Characterization. Serotyping HIV type 1 by antibody binding to the V3 loop: relationship to viral genotype. AIDS Res Hum Retroviruses. 1994;10:1379–1386. doi: 10.1089/aid.1994.10.1379. [DOI] [PubMed] [Google Scholar]
- 11.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, et al. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J L, Tartaglia J, Paoletti E, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 13.Cooney E L, McElrath M J, Corey L, Hu S L, Collier A C, Arditti D, Hoffman M, Coombs R W, Smith G E, Greenberg P D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreyer K, Kallas E G, Planelles V, Montefiori D, McDermott M P, Hasan M S, Evans T G. Primary isolate neutralization by HIV type 1-infected patient sera in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 1999;15:1563–1571. doi: 10.1089/088922299309856. [DOI] [PubMed] [Google Scholar]
- 15.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 16.Evans T G, Keefer M C, Weinhold K J, Wolff M, Montefiori D, Gorse G J, Graham B S, McElrath M J, Clements-Mann M L, Mulligan M J, Fast P, Walker M C, Excler J L, Duliege A M, Tartaglia J. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J Infect Dis. 1999;180:290–298. doi: 10.1086/314895. [DOI] [PubMed] [Google Scholar]
- 17.Excler J L, Plotkin S. The prime-boost concept applied to HIV preventive vaccines. AIDS. 1997;11:S127–S137. [PubMed] [Google Scholar]
- 18.Farisano G, Trivello R, Moschen M E, Bonello C, Baldo V, Moretti G, Majori S, Marin F, Piron L, Renzulli G. Poliovirus neutralizing antibody persistence after vaccination with the Sabin vaccine: a follow-up study. Ann Clin Lab Sci. 1995;25:200–206. [PubMed] [Google Scholar]
- 19.Ferrari G, Humphrey W, McElrath M J, Excler J L, Duliege A M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard M, Meignier B, Barre-Sinoussi F, Kieny M P, Matthews T, Muchmore E, Nara P L, Wei Q, Rimsky L, Weinhold K, et al. Vaccine-induced protection of chimpanzees against infection by a heterologous human immunodeficiency virus type 1. J Virol. 1995;69:6239–6248. doi: 10.1128/jvi.69.10.6239-6248.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny M K, Xu J-Y, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150:635–643. [PubMed] [Google Scholar]
- 22.Gorny M K, Gianakakos V, Sharpe S, Zolla-Pazner S. Generation of human monoclonal antibodies to HIV. Proc Natl Acad Sci USA. 1989;86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorny M K, VanCott T C, Hioe C, Israel Z R, Michael N L, Conley A J, Williams C, Kessler II J A, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and inter-clade cross-reactivity. J Immunol. 1997;159:5114–5122. [PubMed] [Google Scholar]
- 24.Gorse G J, Patel G B, Mandava M, Berman P W, Belshe R B. MN and IIIB recombinant glycoprotein 120 vaccine-induced binding antibodies to native envelope glycoprotein of human immunodeficiency virus type 1 primary isolates. National Institute of Allergy and Infectious Disease Aids Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1999;15:921–930. doi: 10.1089/088922299310638. [DOI] [PubMed] [Google Scholar]
- 25.Graham B S. Serological responses to candidate AIDS vaccines. AIDS Res Hum Retroviruses. 1994;10:S145–S148. [PubMed] [Google Scholar]
- 26.Graham B S, Gorse G J, Schwartz D H, Keefer M C, McElrath M J, Matthews T J, Wright P F, Belshe R B, Clements M L, Dolin R, et al. Determinants of antibody response after recombinant gp160 boosting in vaccinia-naive volunteers primed with gp160-recombinant vaccinia virus. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Clinical Trials Network. J Infect Dis. 1994;170:782–786. doi: 10.1093/infdis/170.4.782. [DOI] [PubMed] [Google Scholar]
- 27.Graham B S, Matthews T J, Belshe R B, Clements M L, Dolin R, Wright P F, Gorse G J, Schwartz D H, Keefer M C, Bolognesi D P, et al. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. The NIAID AIDS Vaccine Clinical Trials Network. J Infect Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 28.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 29.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 30.Ivey-Hoyle M, Culp J S, Chaikin M A, Hellmig B D, Matthews T J, Sweet R W, Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack A D, Hall A J, Maine N, Mendy M, Whittle H C. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 32.Johansson B E, Matthews J T, Kilbourne E D. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine. 1998;16:1009–1015. doi: 10.1016/s0264-410x(97)00279-x. [DOI] [PubMed] [Google Scholar]
- 33.Kalyanaraman V S, Rodriguez V, Veronese F, Rahman R, Lusso P, DeVico A L, Copeland T, Oroszlan S, Gallo R C, Sangadharan M G. Characterization of the secreted, native gp120 and gp160 of the human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1990;6:371–380. doi: 10.1089/aid.1990.6.371. [DOI] [PubMed] [Google Scholar]
- 34.Kieny M P, Lathe R, Riviere Y, Dott K, Schmitt D, Girard M, Montagnier L, Lecocq J. Improved antigenicity of the HIV env protein by cleavage site removal. Protein Eng. 1988;2:219–225. doi: 10.1093/protein/2.3.219. [DOI] [PubMed] [Google Scholar]
- 35.Korber B T M, MacInnes K, Smith R F, Myers G. Mutational trends in V3 loop protein sequences observed in different genetic lineages of human immunodeficiency virus type 1. J Virol. 1994;68:6730–6744. doi: 10.1128/jvi.68.10.6730-6744.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 37.Levandowski R A, Gross P A, Weksler M, Staton E, Williams M S, Bonelli J. Cross-reactive antibodies induced by a monovalent influenza B virus vaccine. J Clin Microbiol. 1991;29:1530–1532. doi: 10.1128/jcm.29.7.1530-1532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubeck M D, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy S C, Chanda P K, Nigida S M, Jr, Markham P D, Zolla-Pazner S, Steimer K, Wade M, Reitz M S, Jr, Arthur L O, Mizutani S, Davis A, Hung P P, Gallo R C, Eichberg J, Robert-Guroff M. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 39.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of HIV-1: viral genotype predicts neutralization immunotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 40.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 43.Mauclere P, Damond F, Apetrel C, Loussert-Ajaka I, Souquiere S, Buzelay L, Dalbon P, Jolivet M, Lobe M M, Brun-Vezinet F, Simon F, Barin F. Synthetic peptide ELISAs for detection of and discrimination between group M and group O HIV type 1 infection. AIDS Res Hum Retroviruses. 1997;13:987–993. doi: 10.1089/aid.1997.13.987. [DOI] [PubMed] [Google Scholar]
- 44.McKnight A, Clapham P R, Goudsmit J, Cheingsong-Popov R, Weber J N, Weiss R A. Development of HIV-1 group-specific neutralizing antibodies after seroconversion. AIDS. 1992;6:799–802. doi: 10.1097/00002030-199208000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Moog C, Fleury H J, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moog C, Spenlehauer C, Fleury H, Heshmati F, Saragosti S, Letourneur F, Kirn A, Aubertin A M. Neutralization of primary human immunodeficiency virus type 1 isolates: a study of parameters implicated in neutralization in vitro. AIDS Res Hum Retroviruses. 1997;13:19–27. doi: 10.1089/aid.1997.13.19. [DOI] [PubMed] [Google Scholar]
- 47.Moore J P, Trkola A, Korber B, Boots L J, Kessler II J A, McCutchan F E, Mascola J, Ho D D, Robinson J, Conley A J. A human monoclonal antibody to a complex epitope in the V3 region of gp120 of human immunodeficiency virus type 1 has broad reactivity within and outside clade B. J Virol. 1995;69:122–130. doi: 10.1128/jvi.69.1.122-130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers G, Korber B, Foley B, Smith R F, Jeang K-T, Mellors J W, Wain-Hobson A. Human retroviruses and AIDS: theoretical biology and biophysics. Los Alamos, N.Mex: Los Alamos National Laboratories; 1996. [Google Scholar]
- 50.Nyambi P N, Gorny M K, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact HIV-1 virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol. 1998;72:9384–9391. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyambi P N, Nkengasong J, Lewi P, Andries K, Janssens W, Fransen K, Heyndrickx L, Piot P, van der Groen G. Multivariate analysis of human immunodeficiency virus type 1 neutralization data. J Virol. 1996;70:6235–6243. doi: 10.1128/jvi.70.9.6235-6243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogata N, Cote P J, Zanetti A R, Miller R H, Shapiro M, Gerin J, Purcell R H. Licensed recombinant hepatitis B vaccines protect chimpanzees against infection with the prototype surface gene mutant of hepatitis B virus. Hepatology. 1999;30:779–786. doi: 10.1002/hep.510300309. [DOI] [PubMed] [Google Scholar]
- 53.Parren P W, Ditzel H J, Gulizia R J, Binley J M, Barbas C F, 3rd, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Pasquali J L, Kieny M P, Kolbe H, Christmann D, Knapp A M. Immunogenicity and epitope mapping of a recombinant soluble gp160 of the human immunodeficiency virus type 1 envelope glycoprotein. AIDS Res Hum Retroviruses. 1990;6:1107–1113. doi: 10.1089/aid.1990.6.1107. [DOI] [PubMed] [Google Scholar]
- 55.Picard O, Achour A, Bernard J, Halbreich A, Bizzini B, Boyer V, Desgranges C, Bertho J M, Lachgar A, Polliotti B, et al. A 2-year follow-up of an anti-HIV immune reaction in HIV-1 gp160-immunized healthy seronegative humans: evidence for persistent cell-mediated immunity. J Acquir Immune Defic Syndr. 1992;5:539–546. [PubMed] [Google Scholar]
- 56.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 57.Plantier J-C, Le Pogam S, Poisson F, Buzelay L, Lejeune B, Barin F. Extent of antigenic diversity in the V3 region of the surface glycoprotein gp120 of human immunodeficiency virus type 1 group M and consequences for serotyping. J Virol. 1998;72:677–683. doi: 10.1128/jvi.72.1.677-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbins J B, Schneerson R, Szu S C. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 59.Robbins J B, Austrian R, Lee C J, Rastogi S C, Schiffman G, Henrichsen J, Makela P H, Broome C V, Facklam R R, Tiesjema R H, et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 60.Robert-Guroff M, Kaur H, Patterson L J, Leno M, Conley A J, McKenna P M, Markham P D, Richardson E, Aldrich K, Arora K, Murty L, Carter L, Zolla-Pazner S, Sinangil F. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J Virol. 1998;72:10275–10280. doi: 10.1128/jvi.72.12.10275-10280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Safrit J T, Fung M S, Andrews C A, Braun D G, Sun W N, Chang T W, Koup R A. hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS. 1993;7:15–21. doi: 10.1097/00002030-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz D H, Gorse G, Clements M L, Belshe R, Izu A, Duliege A M, Berman P, Twaddell T, Stablein D, Sposto R, et al. Induction of HIV-1-neutralising and syncytium-inhibiting antibodies in uninfected recipients of HIV-1IIIB rgp120 subunit vaccine. Lancet. 1993;342:69–73. doi: 10.1016/0140-6736(93)91283-r. [DOI] [PubMed] [Google Scholar]
- 63.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 64.Szmuness W, Stevens C E, Harley E J, Zang E A, Alter H J, Taylor P E, DeVera A, Chen G T, Kellner A. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982;307:1481–1486. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- 65.Taffs R E, Chernokhvostova Y V, Dragunsky E M, Nomura T, Hioki K, Beuvery E C, Fitzgerald E A, Levenbook I S, Asher D M. Inactivated poliovirus vaccine protects transgenic poliovirus receptor mice against type 3 poliovirus challenge. J Infect Dis. 1997;175:441–444. doi: 10.1093/infdis/175.2.441. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi H, Nakagawa Y, Pendleton C D, Houghten R A, Yokomuro K, Germain R N, Berzofsky J A. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science. 1992;255:333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- 67.Tartaglia J, Excler J L, El Habib R, Limbach K, Meignier B, Plotkin S, Klein M. Canarypox virus-based vaccines: prime-boost strategies to induce cell-mediated and humoral immunity against HIV. AIDS Res Hum Retroviruses. 1998;14:S291–S298. [PubMed] [Google Scholar]
- 68.Ui M, Kuwata T, Igarashi T, Ibuki K, Miyazaki Y, Kozyrev I L, Enose Y, Shimada T, Uesaka H, Yamamoto H, Miura T, Hayami M. Protection of macaques against a SHIV with a homologous HIV-1 env and a pathogenic SHIV-89.6P with a heterologous env by vaccination with multiple gene-deleted SHIVs. Virology. 1999;265:252–263. doi: 10.1006/viro.1999.0049. [DOI] [PubMed] [Google Scholar]
- 69.VanCott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 70.VanCott T C, Kalyanaraman V, Earl P, Veit S C D, Burke D S, Redfield R R, Birx D L. Characterization of a soluble oligomeric HIV-1 gp160/gp41 protein as a candidate subunit vaccine. J Immunol Methods. 1995;183:103–117. doi: 10.1016/0022-1759(95)00038-c. [DOI] [PubMed] [Google Scholar]
- 71.VanCott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 72.Weber J, Fenyo E-M, Beddows S, Kaleebu P, Bjorndal A the WHO Network for HIV Isolation Characterization. Neutralization serotypes of HIV-1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zagury D, Bernard J, Cheynier R, Desportes I, Leonard R, Fouchard M, Reveil B, Ittele D, Lurhuma Z, Mbayo K, et al. A group specific anamnestic immune reaction against HIV-1 induced by a candidate vaccine against AIDS. Nature. 1988;332:728–731. doi: 10.1038/332728a0. [DOI] [PubMed] [Google Scholar]
- 74.Zolla-Pazner S, Alving C, Belshe R, Berman P, Burda S, Chigurupati P, Clements M L, Duliege A-M, Excler J-L, Kahn J, McElrath M J, Sharpe S, Sinangil F, Steimer K, Walker M C, Wassef N, Xu S. Neutralization of a clade B primary isolate by sera from HIV-uninfected recipients of candidate AIDS vaccines. J Infect Dis. 1997;175:764–774. doi: 10.1086/513969. [DOI] [PubMed] [Google Scholar]
- 75.Zolla-Pazner S, Gorny M K, Nyambi P N, VanCott T C, Nadas A. Immunotyping of human immunodeficiency virus type 1 (HIV): an approach to immunologic classification of HIV. J Virol. 1999;73:4042–4051. doi: 10.1128/jvi.73.5.4042-4051.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zolla-Pazner S, Lubeck M, Xu S, Burda S, Natuk R J, Sinangil F, Steimer K, Gallo R C, Eichberg J W, Matthews T, Robert-Guroff M. Induction of neutralizing antibodies in T-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime-boost vaccine regimen in chimpanzees. J Virol. 1998;72:1052–1059. doi: 10.1128/jvi.72.2.1052-1059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zolla-Pazner S, Xu S, Burda S, Duliege A-M, Excler J-L, Clements-Mann M L. Neutralization of syncytium-inducing primary isolates by sera from HIV-uninfected recipients of candidate HIV vaccines. J Infect Dis. 1998;178:1502–1506. doi: 10.1086/314452. [DOI] [PubMed] [Google Scholar]