Abstract
Aims/Introduction
Previous studies have reported that the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) delays gastric emptying, and gastric emptying was assessed by the 13C breath test or paracetamol absorption technique. However, neither of them is clinically familiar in real‐world clinical practice. The purpose of the present study was to investigate the association between GLP‐1RA treatment and gastric residue in an esophagogastroduodenoscopy.
Materials and Methods
This study was a matched pair case–control study. The study population consisted of 1,128 individuals with diabetes who had esophagogastroduodenoscopy at our clinic between July 2020 and June 2022. To account for differences in characteristics, such as age, sex, insulin treatment and glycated hemoglobin, we carried out a one‐to‐one nearest neighbor propensity score matching analysis between diabetes patients with and without GLP‐1RA treatment. After matching, we compared the presence of gastric residue in an esophagogastroduodenoscopy by the McNemar test between patients with and without GLP‐1RA treatment.
Results
After the propensity score matching, we selected 205 pairs. In the propensity score‐matched comparison, the proportion of gastric residue was statistically significantly higher in the GLP‐1RA treatment group (0.49% vs 5.4%, P = 0.004). The details of GLP‐1RA prescribed for the 11 patients with gastric residue were liraglutide once daily 1.8 mg (n = 2), dulaglutide once weekly 0.75 mg (n = 5), semaglutide once weekly 0.5 mg (n = 2) and semaglutide once weekly 1.0 mg (n = 2).
Conclusion
GLP‐1RA treatment is associated with gastric residue in an esophagogastroduodenoscopy in patients with diabetes.
Keywords: Esophagogastroduodenoscopy, Gastric residue, Glucagon‐like peptide‐1 receptor agonist treatment
The purpose of the present study was to investigate whether glucagon‐like peptide‐1 receptor agonist treatment is associated with gastric residue in an esophagogastroduodenoscopy. To account for differences in characteristics, such as age, sex, insulin treatment and glycated hemoglobin, we carried out a one‐to‐one nearest neighbor propensity score matching analysis between diabetes patients with and without glucagon‐like peptide‐1 receptor agonist treatment. This matched pair case–control study shows for the first time that taking glucagon‐like peptide‐1 receptor agonist treatment is associated with gastric residue in an esophagogastroduodenoscopy in patients with diabetes.

INTRODUCTION
Glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) has been attracting attention not only for its glucose‐lowering effect, but also effects on bodyweight, lower risk of hypoglycemia, and cardiovascular and renal benefits 1 , 2 .
Previous studies have reported that GLP‐1RAs delay gastric emptying, and in most of these studies, gastric emptying was assessed by the 13C breath test 3 , 4 , 5 or paracetamol absorption technique 6 , 7 , 8 . However, neither of these are familiar in real‐world clinical practice. From this respect, a previous retrospective cohort study with matched cases examined the impact on gastric residue during esophagogastroduodenoscopy 9 . This previous study reported that GLP‐1RA did not significantly increase the odds of gastric residue (6.8% vs 1.7%, odds ratio 4.22, 95% confidence interval 0.87–20.34) 9 . However, a small sample size (59 patients prescribed with GLP‐1RA and 118 matched controls) was one of the limitations.
We have previously reported that insulin treatment is associated with gastric residue in an esophagogastroduodenoscopy independent of age, sex and diabetes or glycated hemoglobin (HbA1c) in a retrospective cohort study conducted at the Institute of Medical Science, Asahi Life Foundation 10 . However, we were unable to confirm the association of GLP‐1RA treatment with gastric residue, because there was only one participant with gastric residue receiving the GLP‐1RA treatment. In our previous study, the study population consisted of individuals who had an esophagogastroduodenoscopy at our clinic (Tokyo, Japan) between January 2003 and December 2019, thus there were not many individuals treated with GLP‐1RA at their first esophagogastroduodenoscopy during the period.
In recent years, the number of diabetes patients treated with GLP‐1RA at our clinic has increased. Furthermore, there is no obvious report on the association between GLP‐1RA and gastric residue in an esophagogastroduodenoscopy in Japanese patients with diabetes. Therefore, the purpose of the present study was to investigate whether GLP‐1RA treatment is associated with gastric residue in an esophagogastroduodenoscopy.
MATERIALS AND METHODS
Study design and participants
The present study was a matched pair case–control study. The protocol was approved by the Committee of Ethics in the Institute of Medical Science, Asahi Life Foundation (approval number 14201). Informed consent was obtained in the form of opt‐out on our website. Investigations were carried out in accordance with the principals of the Declaration of Helsinki.
Those who had an esophagogastroduodenoscopy at our clinic between 1 July 2020 and 30 June 2022 were selected. Among the total number of 3,814 esophagogastroduodenoscopy patients, we excluded 506 patients who did not test HbA1c at our clinic within 3 months before the esophagogastroduodenoscopy, two cases who were unable to keep fasting. As for individuals who has an esophagogastroduodenoscopy more than once, we used the data at the latest esophagogastroduodenoscopy with gastric residue when individuals had gastric residue at least once during the period, and we used the data at the latest esophagogastroduodenoscopy when individuals had no gastric residue during the period. After deduplication, it is found that 2,301 individuals had an esophagogastroduodenoscopy during the period. Among them, we excluded 31 individuals with a history of esophageal or gastric operation and 1,142 individuals without diabetes. Finally, the study comprised of the remaining 1,128 individuals with diabetes (Figure 1).
Figure 1.
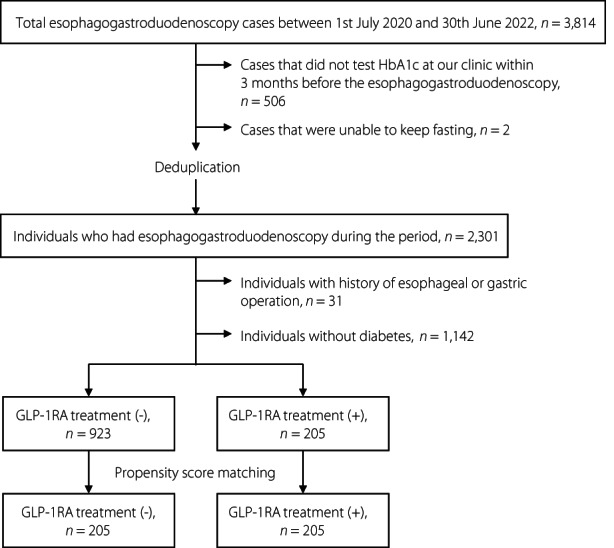
Patient selection flow for propensity score matching. GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin.
Measurements
All individuals started fasting before 09.00 hours on the previous day of the esophagogastroduodenoscopy, and took nothing except for water afterwards. All esophagogastroduodenoscopies were carried out between 09.00 and 11.30 hours. Therefore, the duration of fasting for all individuals was ≥12 h, which should be enough to empty the stomach 11 . All esophagogastroduodenoscopy findings were reported by trained gastroenterologists who carried out the esophagogastroduodenoscopy. We defined the presence of gastric residue as having any solids in the stomach in an esophagogastroduodenoscopy.
We defined having diabetes as either receiving medical treatment for diabetes, fasting blood glucose level ≥126 mg/dL, casual blood glucose level ≥200 mg/dL, HbA1c ≥6.5% or self‐report in a questionnaire.
We used the latest HbA1c and the diabetes medication within 3 months before the esophagogastroduodenoscopy, fasting blood glucose level in the morning on the day of the esophagogastroduodenoscopy, and the latest estimated glomerular filtration rate, urinary protein, urinary albumin creatinine ratio and state of diabetic retinopathy within a year before/after the esophagogastroduodenoscopy for this analysis. Diabetic retinopathy was evaluated by trained ophthalmologist based on the Davis classification: no diabetic retinopathy; simple diabetic retinopathy; pre‐proliferative diabetic retinopathy; and proliferative diabetic retinopathy 12 . Other data collected were sex and age at the esophagogastroduodenoscopy.
Statistical analysis
To account for differences in characteristics between diabetes patients with and without GLP‐1RA treatment, we carried out a one‐to‐one propensity score matching analysis. Logistic regression analysis was used to calculate propensity scores for patients with or without GLP‐1RA treatment, using HbA1c and insulin treatment. To calculate propensity scores for patients with weekly injectable GLP‐1RA treatment or without any GLP‐1RA treatment, and propensity scores for GLP‐1RA‐untreated patients with or without dipeptidyl peptidase‐4 (DPP‐4) inhibitor treatment, we used HbA1c, age, sex and insulin treatment. To calculate propensity scores for patients with or without GLP‐1RA treatment among those who had fasting blood glucose level measured in the morning on the day of the esophagogastroduodenoscopy, we used HbA1c, fasting blood glucose level, age, sex and insulin treatment. Each patient with GLP‐1RA treatment was matched with a patient without GLP‐1RA treatment in the analyses of association with GLP‐1RA and gastric residue (Figure 1, Figure S1), and each patient with DPP‐4 inhibitor treatment was matched with a patient without DPP‐4 inhibitor treatment in the analyses of association with DPP‐4 inhibitor and gastric residue (Figure S2), with the closest estimated propensity score on the logit scale with no replacement. The width of the caliper was set at 20% of the standard deviation of the propensity scores on the logit scale. Balances in baseline variables using standardized differences were estimated. Absolute values <0.25 were considered balanced 13 , 14 .
After the propensity score matching, we compared the presence of gastric residue in esophagogastroduodenoscopy by the McNemar test.
In the comparison of clinical characteristics between patients with and without gastric residue among those who were treated with GLP‐1RA, we assessed the normality of continuous variables by histogram. The Wilcoxon rank sum test was used to compare non‐parametric continuous variables. Pearson's χ2‐test or Fisher's exact test was used to compare categorical variables as appropriate.
The threshold of statistical significance was two‐tailed P < 0.05. Statistical analyses were carried out using JMP version 16.2.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The characteristics of the 1,128 study participants are shown in Table 1. Of 1,128 patients, 17 had gastric residue in an esophagogastroduodenoscopy, and details of their clinical characteristics are shown in Table S1. Before matching, patients with GLP‐1RA treatment had higher HbA1c and a higher proportion of insulin treatment. As we previously reported that taking insulin treatment is a risk factor for gastric residue in an esophagogastroduodenoscopy independent of age, sex and diabetes or HbA1c 10 , we carried out propensity score matching analysis to account for differences in characteristics between patients with and without GLP‐1RA treatment. After one‐to‐one nearest neighbor propensity score matching, we selected 205 pairs (Figure 1). After propensity score matching, patients' characteristics were balanced between the two groups (Table 1). In the propensity score‐matched comparison, the proportion of gastric residue was statistically significantly higher in the GLP‐1RA treatment group (Table 2). The comparison of clinical characteristics between patients with and without gastric residue among those who were treated with GLP‐1RA is shown in Table S2. Among patients treated with GLP‐1RA, patients with gastric residue were statistically significantly younger than patients without gastric residue (Table S2). The details of GLP‐1RA prescribed for the 11 patients with gastric residue were liraglutide once daily 1.8 mg (2/19 patients treated with liraglutide once daily 1.8 mg, 10.5%), dulaglutide once weekly 0.75 mg (5/90 patients treated with dulaglutide once weekly 0.75 mg, 5.6%), semaglutide once weekly 0.5 mg (2/17 patients treated with semaglutide once weekly 0.5 mg, 11.8%) and semaglutide once weekly 1.0 mg (2/9 patients treated with semaglutide once weekly 1.0 mg, 22.2%; Tables S1 and S2).
Table 1.
Characteristics before and after propensity score matching
| Variables | Before matching | After matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GLP‐1RA (−) | GLP‐1RA (+) | Standardized difference | GLP‐1RA (−) | GLP‐1RA (+) | Standardized difference | |||||
| (n = 923) | (n = 205) | (n = 205) | (n = 205) | |||||||
| Age (years) | 72 | (63, 77) | 70 | (62, 76) | 0.088 | 72 | (64, 77) | 70 | (62, 76) | 0.099 |
| Sex (male) | 728 | (78.9) | 163 | (79.5) | 0.016 | 154 | (75.1) | 163 | (79.5) | 0.105 |
| HbA1c (%) | 7.0 | (6.6, 7.5) | 7.3 | (6.8, 7.8) | 0.258 | 7.3 | (6.8, 7.8) | 7.3 | (6.8, 7.8) | 0.022 |
| Insulin treatment | 289 | (31.3) | 102 | (49.8) | 0.383 | 101 | (49.3) | 102 | (49.8) | 0.010 |
Data are median (interquartile range) or n (%).GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin.
Table 2.
Outcome in the unmatched and propensity score‐matched groups
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| GLP‐1RA (−) | GLP‐1RA (+) | P‐value | GLP‐1RA (−) | GLP‐1RA (+) | P‐value | |
| (n = 923) | (n = 205) | (n = 205) | (n = 205) | |||
| Gastric residue (+) | 6 (0.65) | 11 (5.4) | <0.001 | 1 (0.49) | 11 (5.4) | 0.004 |
Data are n (%). GLP‐1RA, glucagon‐like peptide‐1 receptor agonist.
Considering the blood half‐life of each daily injectable GLP‐1RA and the previous studies 3 , 4 , 5 , there remains a possibility that the influence of daily injectable GLP‐1RAs on gastric emptying does not last to the next morning. Thus, we next carried out propensity score matching analysis between diabetes patients with weekly injectable GLP‐1RA treatment and patients without any GLP‐1RA treatment. After one‐to‐one nearest neighbor propensity score matching, we selected 123 pairs (Table S3). The proportion of gastric residue was significantly higher in the weekly injectable GLP‐1RA groups (0% vs 7.3%, GLP‐1RA‐untreated group vs weekly injectable GLP‐1RA treatment group, respectively). Although we were unable to carry out the McNemar test, because there was no patient with gastric residue in the GLP‐1RA‐untreated group after the propensity score‐matched comparison (Table S4), the weekly injectable GLP‐1RA treatment group and the GLP‐1RA‐untreated group were already balanced before matching (Table S3), and the proportion of gastric residue was statistically significantly higher in the weekly GLP‐1RA treatment group before matching (Table S4).
It is reported that blood glucose level, such as hyperglycemia, hypoglycemia and acute changes in blood glucose, affects gastric emptying. 15 , 16 However, our above‐mentioned propensity score analyses did not include data of glucose levels. Therefore, we next carried out propensity score matching analysis between diabetes patients with and without GLP‐1RA treatment among only those who had fasting blood glucose level measured in the morning on the day of the esophagogastroduodenoscopy. After one‐to‐one nearest neighbor propensity score matching, we selected 134 pairs (Figure S1). After propensity score matching, patients' characteristics were balanced between the two groups (Table S5). In the propensity score‐matched comparison, the proportion of gastric residue was statistically significantly higher in the GLP‐1RA treatment group (Table S6) in this analysis, too.
Among antidiabetic drugs, some drugs have an intestinal adverse effect. Although metformin and alpha‐glucosidase inhibitor have intestinal adverse effects, they do not suppress gastric emptying. In contrast, DPP‐4 inhibitor could delay gastric emptying as well as GLP‐1RA. Therefore, we next investigated the association with DPP‐4 inhibitor and gastric residue. Among 923 patients without GLP‐1RA treatment, we carried out propensity score matching analysis between patients with or without DPP‐4 inhibitor treatment. After one‐to‐one nearest neighbor propensity score matching, we selected 313 pairs (Figure S2). After propensity score matching, patients' characteristics were balanced between the two groups (Table S7). In the propensity score‐matched comparison, there was no significant difference in the proportion of gastric residue between groups with or without DPP‐4 inhibitor treatment (Table S8).
DISCUSSION
The present matched pair case–control study showed that GLP‐1RA treatment was associated with gastric residue in an esophagogastroduodenoscopy.
Although it is well known that GLP‐1RAs delay gastric emptying, in most of the studies, gastric emptying was assessed by the 13C breath test 3 , 4 , 5 or paracetamol absorption technique 6 , 7 , 8 . These are both appropriate to evaluate gastric emptying quantitatively, and gastric emptying was assessed until 4–6 h after ingestion in these tests 3 , 4 , 5 , 6 , 7 , 8 . Therefore, the results from these evaluations are useful information when we manage postprandial glucose level. In contrast, the duration of fasting in esophagogastroduodenoscopy is ≥12 h. Thus, gastric emptying assessed by gastric residue in an esophagogastroduodenoscopy might be different from that assessed by 13C breath test or paracetamol absorption technique. Furthermore, in real‐world clinical practice, whether there is any gastric residue or not might be more important than accurate gastric emptying rate, when we evaluate the image of esophagogastroduodenoscopy. Therefore, the present study is novel in that we set the gastric residue in an esophagogastroduodenoscopy as the outcome according to an interest in real‐world clinical practice in testing of esophagogastroduodenoscopy among Japanese diabetes patients.
GLP‐1RAs are classified into short‐acting GLP‐1RAs and long‐acting GLP‐1RAs 17 , and they have different characteristics. For short‐acting GLP‐1RAs, such as exenatide and lixisenatide, delayed gastric emptying is the main mechanism of suppression of post‐prandial hyperglycemia 18 , 19 , 20 . In contrast, for long‐acting GLP‐1RAs, such as liraglutide, exenatide‐LAR (long‐acting release), dulaglutide and semaglutide, increasing insulin secretion and suppressing glucagon are the main mechanism of suppression of post‐prandial hyperglycemia 21 . Although the GLP‐1RA effect to delay gastric emptying is preserved in the use of short‐acting GLP‐RA, which stimulates GLP‐1 receptors intermittently, this effect diminishes when long‐acting GLP‐1RA is administered for a long term 22 , 23 . Considering these differences, short‐acting GLP‐1RA treatment might cause more gastric residue in an esophagogastroduodenoscopy than long‐acting GLP‐1RA treatment. However, in the present study, GLP‐1RAs prescribed for 11 patients with gastric residue were all long‐acting GLP‐1RAs and their median duration of GLP‐1RA treatment was 57 months (interquartile range 26.5–64). This might be because more long‐acting GLP‐1RAs (n = 199) were prescribed in our clinic than short‐acting GLP‐1RAs (n = 6). The present result suggests that we have to pay attention to the possibility of gastric residue in an esophagogastroduodenoscopy, even when patients are treated with long‐acting GLP‐1RA for a long term.
Although there is no consensus about preparation for gastric residue in an esophagogastroduodenoscopy among the high‐risk group now, it was reported that metoclopramide 24 , domperidone 25 and erythromycin 26 accelerated gastric emptying. It was also reported that enteral nutrient formula 27 and mosapride 28 were effective for a preparation for capsule endoscopy. Therefore, if gastric residue was ever observed in an esophagogastroduodenoscopy examination, especially in patients with diabetes receiving GLP‐1RA treatment, it might be preferable to have a longer fasting time, use of these prokinetic drugs or enteral nutrient formula to avoid gastric residue.
Several limitations of the present study should be acknowledged. First, this study was a single‐center observational study and the number of patients with gastric residue was small; therefore, it might not represent diabetes patients with gastric residue in Japan. To generalize our findings, a further multicenter study is required. Second, diabetes duration, diabetic neuropathy and diabetic retinopathy were not used for calculating the propensity score, because we were unable to obtain this information from electrical medical records. Finally, there remains the possibility that prescription from other hospitals affected gastrointestinal motility.
In summary, the present matched pair case–control study shows for the first time that GLP‐1RA treatment is associated with gastric residue in an esophagogastroduodenoscopy in Japanese patients with diabetes.
Funding
None.
Disclosure
The authors declare no conflict of interest.
Approval of the research protocol: Approval by the Committee of Ethics in the Institute of Medical Science, Asahi Life Foundation.
Informed Consent: Obtained in the form of opt‐out on our website.
Approval date of Registry and the Registration No. of the study: 13 September 2022, No. 14201.
Animal Studies: N/A.
Supporting information
Figure S1 Patient selection flow for propensity score matching for the analysis among patients whose fasting blood glucose level was measured in the morning on the day of the esophagogastroduodenoscopy.
Figure S2 Patient selection flow for propensity score matching for the analysis among patients without glucagon‐like peptide‐1 receptor agonist treatment.
Table S1 Details of each clinical characteristic of 17 patients with gastric residue in an esophagogastroduodenoscopy before propensity score matching.
Table S2 Comparisons of clinical characteristics between patients with and without gastric residue among those who were treated with glucagon‐like peptide‐1 receptor agonist.
Table S3 The characteristics before and after propensity score matching among patients with weekly injectable glucagon‐like peptide‐1 receptor agonist treatment and patients without any glucagon‐like peptide‐1 receptor agonist treatment.
Table S4 Outcome in the unmatched and propensity score‐matched groups among patients with weekly injectable glucagon‐like peptide‐1 receptor agonist treatment and patients without any glucagon‐like peptide‐1 receptor agonist treatment.
Tabel S5 The characteristics before and after propensity score matching among patients whose fasting blood glucose level was measured in the morning on the day of the esophagogastroduodenoscopy.
Table S6 Outcome in the unmatched and propensity score‐matched groups among patients whose fasting blood glucose level was measured in the morning on the day of the esophagogastroduodenoscopy.
Table S7 The characteristics before and after propensity score matching among patients without glucagon‐like peptide‐1 receptor agonist treatment.
Table S8 Outcome in the unmatched and propensity score‐matched groups among patients without glucagon‐like peptide‐1 receptor agonist treatment.
Acknowledgments
We acknowledge staff members, especially Nobuhiro Tachibana and Rieko Ichihashi, for their skilled assistance.
References
- 1. Draznin B, Aroda VR, Bakris G, et al. 9. Pharmacologic approaches to glycemic treatment: Standards of medical Care in Diabetes‐2022. Diabetes Care 2022; 45: S125–s143. [DOI] [PubMed] [Google Scholar]
- 2. Bouchi R, Kondo T, Ohta Y, et al. A proposed algorithm for pharmacotherapy in people with type 2 diabetes. J Japan Diabetes Soc 2022; 65: 419–434 (Japanese). [Google Scholar]
- 3. Kuwata H, Yabe D, Murotani K, et al. Effects of glucagon‐like peptide‐1 receptor agonists on secretions of insulin and glucagon and gastric emptying in Japanese individuals with type 2 diabetes: A prospective, observational study. J Diabetes Investig 2021; 12: 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quast DR, Nauck MA, Schenker N, et al. Macronutrient intake, appetite, food preferences and exocrine pancreas function after treatment with short‐ and long‐acting glucagon‐like peptide‐1 receptor agonists in type 2 diabetes. Diabetes Obes Metab 2021; 23: 2344–2353. [DOI] [PubMed] [Google Scholar]
- 5. Quast DR, Schenker N, Menge BA, et al. Effects of Lixisenatide versus Liraglutide (short‐ and long‐acting GLP‐1 receptor agonists) on esophageal and gastric function in patients with type 2 diabetes. Diabetes Care 2020; 43: 2137–2145. [DOI] [PubMed] [Google Scholar]
- 6. Dahl K, Brooks A, Almazedi F, et al. Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes. Diabetes Obes Metab 2021; 23: 1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab 2021; 23: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hjerpsted JB, Flint A, Brooks A, et al. Semaglutide improves postprandial glucose and lipid metabolism, and delays first‐hour gastric emptying in subjects with obesity. Diabetes Obes Metab 2018; 20: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stark JE, Cole JL, Ghazarian RN, et al. Impact of glucagon‐like Peptide‐1 receptor agonists (GLP‐1RA) on food content during esophagogastroduodenoscopy (EGD). Ann Pharmacother 2022; 56: 922–926. [DOI] [PubMed] [Google Scholar]
- 10. Kobori T, Onishi Y, Iwamoto M, et al. Association of insulin treatment with gastric residue during an esophagogastroduodenoscopy. J Diabetes Investig 2022; 13: 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology 2017; 126: 376–393. [DOI] [PubMed] [Google Scholar]
- 12. Davis MD. Diabetic Retinopathy.In: Current Diagnosis and Management of Chorioretinal Diseases. St Louis: CV Mosby, 1977. [Google Scholar]
- 13. Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol 2001; 2: 169–188. [Google Scholar]
- 14. Linden A, Samuels SJ. Using balance statistics to determine the optimal number of controls in matching studies. J Eval Clin Pract 2013; 19: 968–975. [DOI] [PubMed] [Google Scholar]
- 15. Rayner CK, Samsom M, Jones KL, et al. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001; 24: 371–381. [DOI] [PubMed] [Google Scholar]
- 16. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nauck MA, Quast DR, Wefers J, et al. GLP‐1 receptor agonists in the treatment of type 2 diabetes ‐ state‐of‐the‐art. Mol Metab 2021; 46: 101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meier JJ, Menge BA, Schenker N, et al. Effects of sequential treatment with lixisenatide, insulin glargine, or their combination on meal‐related glycaemic excursions, insulin and glucagon secretion, and gastric emptying in patients with type 2 diabetes. Diabetes Obes Metab 2020; 22: 599–611. [DOI] [PubMed] [Google Scholar]
- 19. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open‐label, non‐inferiority study. Lancet 2008; 372: 1240–1250. [DOI] [PubMed] [Google Scholar]
- 20. Meier JJ, Rosenstock J, Hincelin‐Méry A, et al. Contrasting effects of Lixisenatide and Liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: A randomized, open‐label trial. Diabetes Care 2015; 38: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 21. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 22. Nauck MA, Kemmeries G, Holst JJ, et al. Rapid tachyphylaxis of the glucagon‐like peptide 1‐induced deceleration of gastric emptying in humans. Diabetes 2011; 60: 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Umapathysivam MM, Lee MY, Jones KL, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon‐like peptide 1 receptor on gastric emptying and glycemia. Diabetes 2014; 63: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braverman D, Bogoch A. Metoclopramide for gastroparesis diabeticorum. Diabetes Care 1978; 1: 356–359. [DOI] [PubMed] [Google Scholar]
- 25. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol 2008; 6: 726–733. [DOI] [PubMed] [Google Scholar]
- 26. Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol 1993; 88: 203–207. [PubMed] [Google Scholar]
- 27. Niv E, Ovadia B, Ron Y, et al. Ensure preparation and capsule endoscopy: A two‐center prospective study. World J Gastroenterol 2013; 19: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei W, Ge ZZ, Lu H, et al. Effect of mosapride on gastrointestinal transit time and diagnostic yield of capsule endoscopy. J Gastroenterol Hepatol 2007; 22: 1605–1608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Patient selection flow for propensity score matching for the analysis among patients whose fasting blood glucose level was measured in the morning on the day of the esophagogastroduodenoscopy.
Figure S2 Patient selection flow for propensity score matching for the analysis among patients without glucagon‐like peptide‐1 receptor agonist treatment.
Table S1 Details of each clinical characteristic of 17 patients with gastric residue in an esophagogastroduodenoscopy before propensity score matching.
Table S2 Comparisons of clinical characteristics between patients with and without gastric residue among those who were treated with glucagon‐like peptide‐1 receptor agonist.
Table S3 The characteristics before and after propensity score matching among patients with weekly injectable glucagon‐like peptide‐1 receptor agonist treatment and patients without any glucagon‐like peptide‐1 receptor agonist treatment.
Table S4 Outcome in the unmatched and propensity score‐matched groups among patients with weekly injectable glucagon‐like peptide‐1 receptor agonist treatment and patients without any glucagon‐like peptide‐1 receptor agonist treatment.
Tabel S5 The characteristics before and after propensity score matching among patients whose fasting blood glucose level was measured in the morning on the day of the esophagogastroduodenoscopy.
Table S6 Outcome in the unmatched and propensity score‐matched groups among patients whose fasting blood glucose level was measured in the morning on the day of the esophagogastroduodenoscopy.
Table S7 The characteristics before and after propensity score matching among patients without glucagon‐like peptide‐1 receptor agonist treatment.
Table S8 Outcome in the unmatched and propensity score‐matched groups among patients without glucagon‐like peptide‐1 receptor agonist treatment.


