Abstract
Background
Severe acute pancreatitis is a potentially life-threatening disease. Despite being a common disorder, acute pancreatitis lacks a specific treatment. The present study aimed to examine the effects of probiotics on pancreatic inflammation and intestinal integrity in mice with acute pancreatitis.
Methods
Male ICR mice were randomly divided into 4 groups (n = 6 per group). The control group received two intraperitoneal (i.p.) injections of normal saline as a vehicle control. The acute pancreatitis (AP) group received two i.p. injections of L-arginine 450 mg/100 g body weight. AP plus probiotics groups received L-arginine to induce acute pancreatitis as above. In the single-strain and mixed-strain groups, mice received 1 mL of Lactobacillus plantarum B7 1 × 108 CFU/mL and 1 mL of Lactobacillus rhamnosus L34 1 × 108 CFU/mL and Lactobacillus paracasei B13 1 × 108 CFU/mL by oral gavage, respectively for 6 days starting 3 days prior to the AP induction. All mice were sacrificed 72 h after L-arginine injection. Pancreatic tissue was obtained for histological evaluation and immunohistochemical studies for myeloperoxidase, whereas ileal tissue was used for immunohistochemical studies for occludin, and claudin-1. Blood samples were collected for amylase analysis.
Results
Serum amylase levels and pancreatic myeloperoxidase levels in the AP group were significantly higher than in controls and significantly decreased in probiotic groups compared with the AP group. Ileal occludin and claudin-1 levels were significantly lower in the AP group than in controls. Ileal occludin levels significantly increased, whereas ileal claudin-1 levels did not significantly change in both probiotic groups as compared with the AP group. The pancreatic histopathology showed significantly higher degree of inflammation, edema, and fat necrosis in the AP group, and these changes improved in mixed-strained probiotic groups.
Conclusions
Probiotics, particularly the mixed-strain ones, attenuated AP via the reduction of inflammation and the maintenance of intestinal integrity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-03998-7.
Keywords: Acute pancreatitis, Probiotics, Mice, Intestinal integrity
Background
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases that require hospital admission [1–3]. The most common causes of AP are gallstones and alcohol consumption [1]. Pathology of AP begins with the early conversion of pancreatic enzymes from inactive to active forms inside the acinar cells which leads to autodigestion of the pancreatic tissue. This results in the release of various proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and IL-8, and the development of pancreatic inflammation [4–6]. TNF-α also stimulates the inducible nitric oxide synthase (iNOS) in the vascular smooth muscle cells of the pancreas [7, 8]. Moreover, the excessive release of inflammatory mediators also leads to systemic inflammatory response syndrome (SIRS) and/or multiple organ dysfunction syndromes (MODS) [9]. As the clinical course of AP progresses to the late phase, local complications may appear [1]. Superimposed bacterial infection may ensue leading to the increased morbidity and mortality [2, 10].
There are many hypotheses regarding the mechanism of which infections reach the pancreas, the widely accepted one is the bacterial translocation from the intestinal tract [11–13]. Gut bacteria subsequently stimulate nucleotide-binding oligomerization domain-containing protein 1 (NOD1) and promote neutrophil priming, which in turn leads to local and systemic inflammatory responses [14]. In experimental models of AP, measures that reduced bacterial translocation prevented infectious complications of AP and improved survival [12, 15]. Nevertheless, the use of antibiotic prophylaxis in severe AP has never been shown to be effective in preventing infectious complications or reducing mortality in human studies [1, 2]. Therefore, non-antibiotic therapy should be considered. With the knowledge that bacterial translocation will occur if the protective mechanisms of the intestine which consist of both physical and immunological barriers have failed, alternative management that can strengthen the intestinal integrity might have beneficial effects in alleviating AP [16].
Probiotics are living microorganisms that when ingested in specific numbers will provide health benefits [17–19]. Examples of probiotics are bacteria, especially lactic acid bacteria and Bifidobacteria [18]. The lactic acid bacteria, such as Lactobacillus plantarum (L. plantarum) and Lactobacillus rhamnosus (L. rhamnosus), are believed to be effective in preserving the colonic microflora balance, eliminating toxic components, and clearing pathogens [19]. However, the use of probiotic prophylaxis in patients with AP remains controversial as some animal and human studies demonstrated potential benefits of probiotics in AP [11, 12, 20–23], whereas others have shown that probiotic prophylaxis increased the risk of bowel ischemia and mortality in patients with severe AP [24]. Therefore, the aims of this study were to evaluate the beneficial effects and mechanism of probiotic prophylaxis in a mouse model of L-arginine induced AP and to compare these effects between single- and mixed-strain probiotics. L. plantarum B7, L. rhamnosus L34, and L. paracasei B13 could be cultivated in our own laboratory and have been shown to have anti-inflammatory effects and reduce intestinal permeability in other conditions, such as alcoholic hepatitis [25], sepsis [26], or Clostridioides difficile infection [27]. As a result, we aimed to evaluate the effects of these strains of Lactobacillus in AP as well.
Methods
L-arginine preparation
L-arginine (L-arg) powder (Sigma Aldrich, St. Louis, MO, USA) was dissolved in 0.9% saline, and the pH was adjusted to 7 with NaOH. Two doses of 450 mg/100 g BW of L-arg were administered through intraperitoneal (i.p.) injections with an interval of 1 h.
Animal protocols and specimens’ collection
Twenty-four male ICR mice, five weeks of age, and 25–30 g of weight were purchased from Nomura Siam International Co., Ltd., Bangkok, Thailand. All mice were kept under standard conditions (the room temperature at 25 ± 1ºC, room humidity 50%, and a 12-h dark–light cycle with the light on from 7:00 a.m. to 7:00 p.m.) and fed with a standard diet and water ad libitum. All mice received proper care in accordance with Ethical Principles and Guidelines for the Use of Animals by the National Research Council of Thailand, and the study was approved by Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (Approval number: 024/2562). This study was reported in accordance with ARRIVE guidelines. After one week of acclimatization, mice were randomly allocated into four groups (6 mice per group).
Control group
Mice were given two i.p. injections of normal saline, at an interval of 1 h as a vehicle control.
Acute pancreatitis (AP) group
Mice were given two i.p. injections of 450 mg/100 g BW of L-arg dissolved in normal saline, at an interval of 1 h for one day to induce AP.
Single-strain (S) group
Mice were given two i.p. injections of 450 mg/100 g BW of L-arg dissolved in normal saline, at an interval of 1 h. They received 1 mL of L. plantarum B7 1 × 108 colony-forming unit (CFUs)/mL once daily via oral gavage for 6 days starting 3 days prior to the AP induction.
Mixed-strain (M) group
Mice were given two i.p. injections of 450 mg/100 g BW of L-arg dissolved in normal saline, at an interval of 1 h. They received 1 mL of mixed-strain probiotics (L. rhamnosus L34 1 × 108 CFUs/mL and L. paracasei B13 1 × 108 CFUs/mL) once daily via oral gavage for 6 days starting 3 days prior to the AP induction.
The dosages of L-arg and probiotics that we used in this study was acquired from the results of our pilot study. We did not combine L. plantarum with L. rhamnosus and L. paracasei as our pilot study have shown that L. plantarum inhibited the growth of other bacteria.
All mice were sacrificed 72 h after L-arg induction (day 3) by overdose injection of i.p. thiopental sodium. The abdomen was opened, and the whole pancreas was rapidly removed and washed with cold normal saline. Then, the ileocecal valve was identified, and 4 cm of distal ileum was removed and flushed with normal saline to remove adherent bacteria. Pancreatic and ileal tissues were fixed in 10% formalin solution immediately after collection at a room temperature for histological examination and immunohistochemical studies. Blood samples were drawn by cardiac puncture and allowed to clot by being kept at a room temperature for 2 h. Subsequently, blood samples were centrifuged at 1,000 × g for 20 min. After centrifugation, serum was collected for serum amylase analysis.
Serum amylase analysis
The level of serum amylase was evaluated via the colorimetric method, using the biochemical analyzer Reflotron® Plus. The collected serum was applied onto a reagent strip designed specifically for quantitative amylase determination. Results were expressed as enzyme concentration in unit per liter (U/L).
Immunohistochemistry for pancreatic myeloperoxidase (MPO) and ileal tight junction proteins
For the immunohistochemistry (IHC) of pancreatic MPO (a marker of inflammation) and ileal tight junction proteins (occludin and claudin-1), tissue microarray (TMA) technique was used. The paraffin-embedded blocks (donor blocks) were retrieved, sectioned, and stained with hematoxylin and eosin (H&E). Then, the experienced pathologist identified and marked the appropriate area on the slides. The areas on the donor block that corresponded to marked areas on the slide were cored 4–6 times. Cores were then placed in an empty paraffin block (TMA block) to make the recipient block which was used for further analyses.
The selected paraffin block was then sectioned at 5-μm thickness. The slides were deparaffinized by immersing in xylene and alcohol and then rehydrated with wash buffer. For MPO, the slides were incubated overnight with MPO goat polyclonal primary antibody at the dilution of 1:500 (R&D Systems, USA) at 4 °C. After that, they were incubated with donkey anti-goat IgG H&L (HRP) secondary antibody at the dilution of 1:500 (Abcam, UK) for 60 min at a room temperature. The slides were incubated with high sensitivity Streptavidin-HRP conjugate (HSS-HRP) for 30 min and incubated with DAB chromogen solution for 15 min. For occludin and claudin-1, the slides were incubated with occludin rabbit polyclonal primary antibody at the dilution of 1:500 (Novus Biologicals, USA) and claudin-1 rabbit polyclonal antibody at the dilution of 1:200 (Novus Biologicals, USA) at a room temperature for 1 h and incubated at 4 °C in a humidified chamber overnight. The slides were then incubated with the goat anti-rabbit IgG H&L (HRP) secondary antibody at the dilution of 1:1000 (Abcam, UK) at a room temperature for 1 h. The TMA slides were scanned using the Aperio ScanScope System (Aperio, Technology; Vista, CA) at the 40 × magnification to provide a high-resolution digital image. The analysis using the positive pixel count algorithm was performed. Positive and negative control tissues were analyzed to ensure the appropriate settings. Algorithm parameters were reported as negative, weakly positive, positive, and strongly positive. The data of pancreatic MPO, ileal occludin, and ileal claudin-1 were reported as the sum of positive and strongly positive pixels per the total number of counted pixels. The average levels of these parameters were calculated for all experimental groups [26].
Pancreatic histopathology
After formalin fixation, the pancreatic tissue was embedded in paraffin and sectioned at 5-μm thickness. The sections were then deparaffinized with xylene and rehydrated with alcohol. Subsequently, the sections were stained with H&E. The glass slides are ready for light microscopy evaluation. An experienced pathologist evaluated all samples while being blinded to the experimental groups. Slides were evaluated for inflammation, edema, and fat necrosis and graded according to the criteria by De Cock, et al. [28]. This scoring system was selected since pancreatic inflammation and edema/fat necrosis were the main findings in acute interstitial pancreatitis of which our model represented. Inflammation grades depended on the degree of neutrophil infiltration ranging from 0–3 with 0 = No neutrophil present, 1 = mild or < 25%, 2 = moderate or 25–50%, and 3 = severe or > 50% neutrophil infiltration of the total pancreatic parenchyma. Edema and fat necrosis were also graded from 0–3 with 0 = No edema nor necrosis, 1 = mild or < 25%, 2 = moderate or 25–50%, and 3 = severe or > 50% edema or fat necrosis of the total pancreatic parenchyma.
Statistical analysis
Statistical analysis was calculated by SPSS Statistics, Version 22.0. (Armonk, NY, USA.). All results were expressed as mean ± standard error of the mean (SEM). The difference between groups was determined by one-way analysis of variance (ANOVA) and LSD post-hoc test. Descriptive statistics were used for the histological examination of the pancreas. Differences were considered significant at p < 0.05.
Results
Serum amylase levels
Serum amylase levels of the control and AP groups were 6,118.67 ± 428.94 U/L and 22,991.67 ± 3,720.21 U/L, respectively. Serum amylase in the AP group was significantly higher than in the control group (p < 0.001). Serum amylase in S and M groups were 14,681.67 ± 2,648.28 U/L and 7,730.00 ± 1,833.02 U/L, respectively. Serum amylase in both S and M groups decreased significantly compared with the AP group (p = 0.027 and < 0.001, respectively). Serum amylase levels were similar between S and M groups. Results of serum amylase were illustrated in Fig. 1.
Fig. 1.
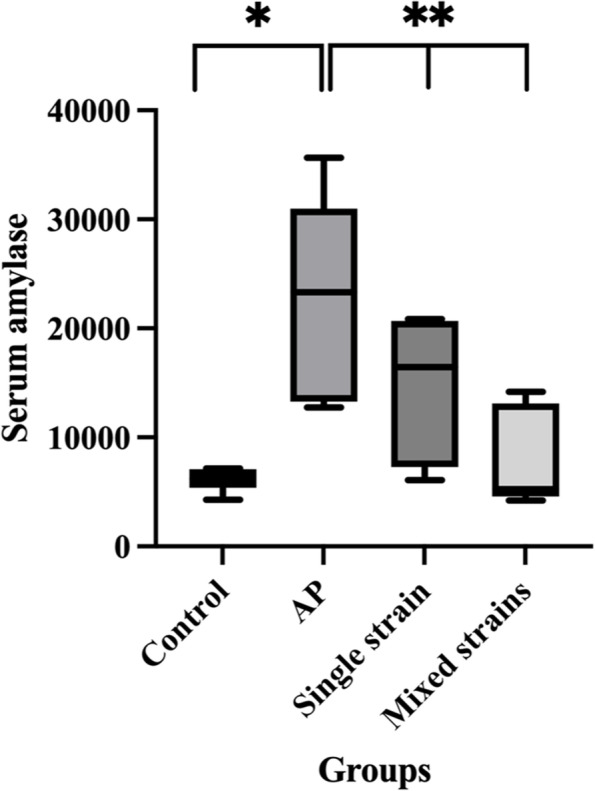
Serum amylase levels in all groups. *p < 0.05 compared with the control group; **p < 0.05 compared with the AP group
Pancreatic myeloperoxidase (MPO)
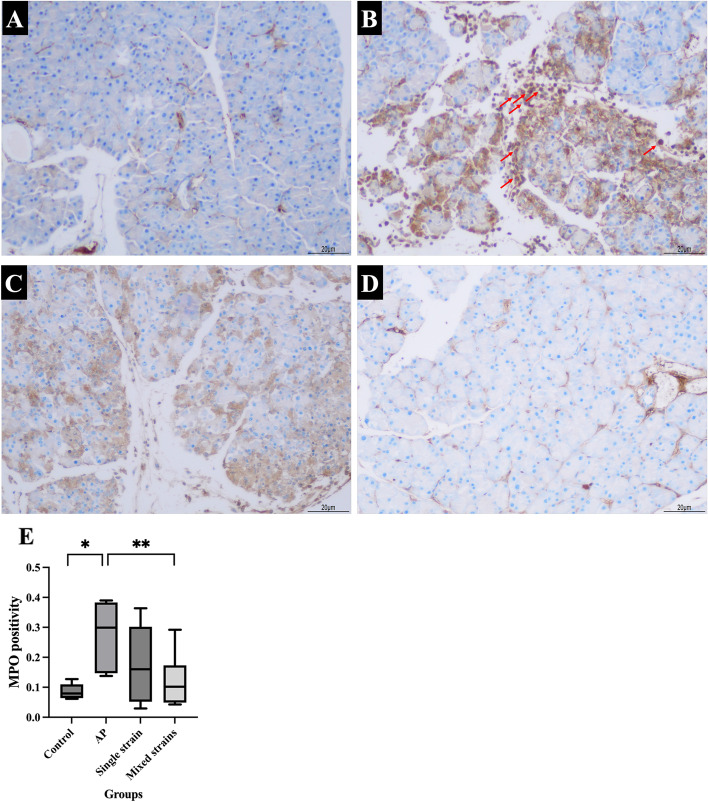
Pancreatic MPO levels of the control and AP groups were 0.0860 ± 0.0104 and 0.2761 ± 0.0457, respectively. The MPO level in the AP group was significantly higher than in the control group (p = 0.004). The MPO levels in S and M groups were 0.1757 ± 0.0565 and 0.1204 ± 0.0378, respectively. The MPO level in the M group decreased significantly compared with the AP group (p = 0.015). MPO levels were similar between S and M groups. The results of pancreatic MPO and representative images of immunohistochemistry for MPO were illustrated in Figs. 2A-E.
Fig. 2.
A-E. Representative images of immunohistochemistry for MPO (100 ×) and levels of MPO positivity in all groups. A IHC for the control group; B IHC for the AP group; C IHC for the S group; D IHC for the M group; E MPO levels in each group
Figure 2A-D showed that inflammatory cells (red arrows) increased in the AP group and decreased in mice that received probiotics. Arrows indicated inflammatory cell infiltration in the pancreatic tissue (cells with brown-stain nuclei). *p < 0.05 compared with the control group; **p < 0.05 compared with the AP group.
Ileal occludin and claudin-1
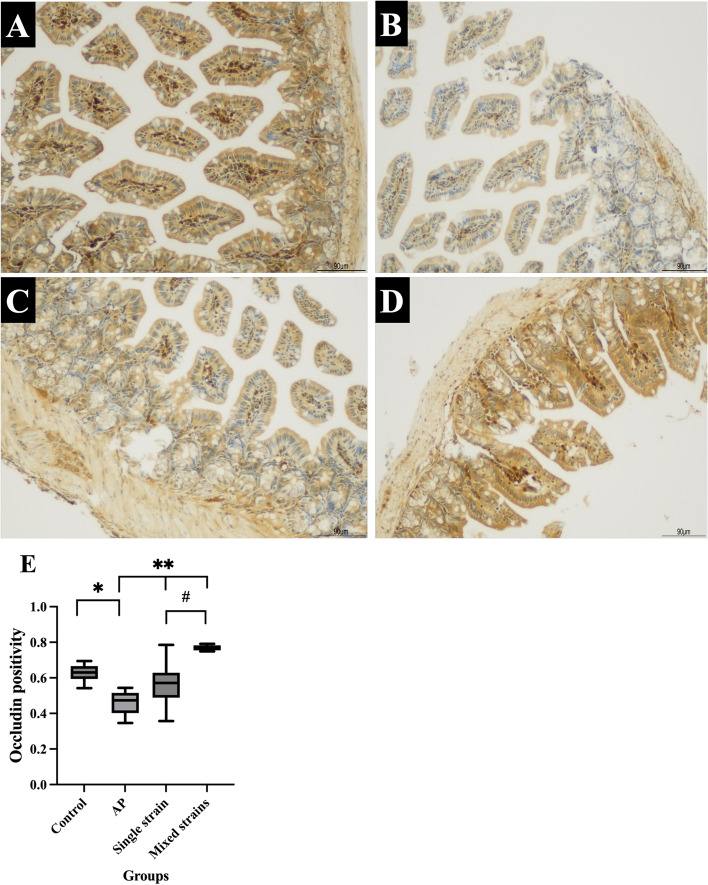
Ileal occludin levels of the control and AP groups were 0.6277 ± 0.0210 and 0.4608 ± 0.0284, respectively. The occludin level in the AP group was significantly lower than in the control group (p = 0.002). The occludin levels in S and M groups were 0.5657 ± 0.0557 and 0.7693 ± 0.0067, respectively. The occludin levels in both S and M groups increased significantly compared with that in the AP group (p = 0.037 and < 0.001, respectively). The occludin level was significant higher in the M group than in the S group (p < 0.001). The results of ileal occludin and representative images of immunohistochemistry for occludin were illustrated in Fig. 3A-E.
Fig. 3.
A-E. Representative images of immunohistochemistry for occludin (100 ×) and levels of occludin positivity in all groups. A IHC for the control group; B IHC for the AP group; C IHC for the S group; D IHC for the M group; E Occludin level in each group
Figure 3A-D showed that occludin positivity (brown stain) significantly decreased in the ileum of AP mice as compared with control mice and increased in mice that received probiotics. *p < 0.05 compared with the control group; **p < 0.05 compared with the AP group; #p < 0.05 compared with the single-strain group.
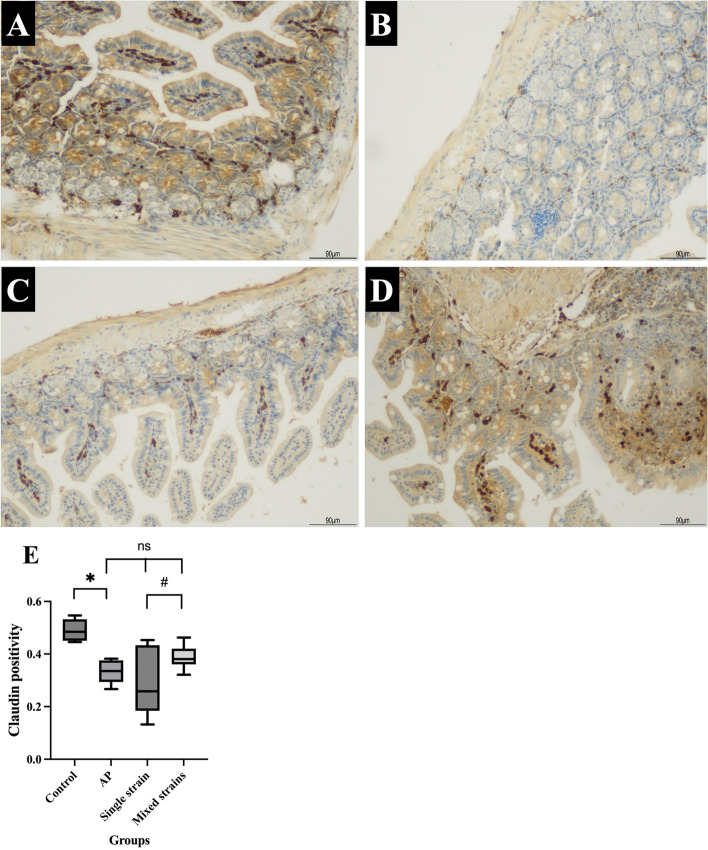
The ileal claudin-1 levels of the control and AP groups were 0.4904 ± 0.0178 and 0.3327 ± 0.0190, respectively. The claudin-1 level in the AP group was significantly lower than in the control group (p = 0.002). The claudin-1 levels in S and M groups were 0.2884 ± 0.0534 and 0.3876 ± 0.0189, respectively; these levels were not statistically different from the AP group (p = 0.327 and p = 0.227, respectively). The claudin-1 level was significantly higher in the M group than in the S group (p = 0.036). The results of ileal claudin-1 and representative images of immunohistochemistry for claudin-1 were illustrated in Fig. 4A-E.
Fig. 4.
A-E. Representative images of immunohistochemistry for claudin-1 (100 ×) and levels of claudin-1 positivity in all groups. A IHC for the control group; B IHC for the AP group; C IHC for the S group; D IHC for the M group; E Claudin-1 level in each group
Figure 4A-D showed a profound decrease in claudin-1 positivity (brown stain) in the ileum of AP mice as compared with control mice. However, there were no significant changes in the claudin-1 levels in both probiotic groups. *p < 0.05 compared with the control group; #p < 0.05 compared with the single-strain group; ns = non-significant.
Pancreatic histopathology
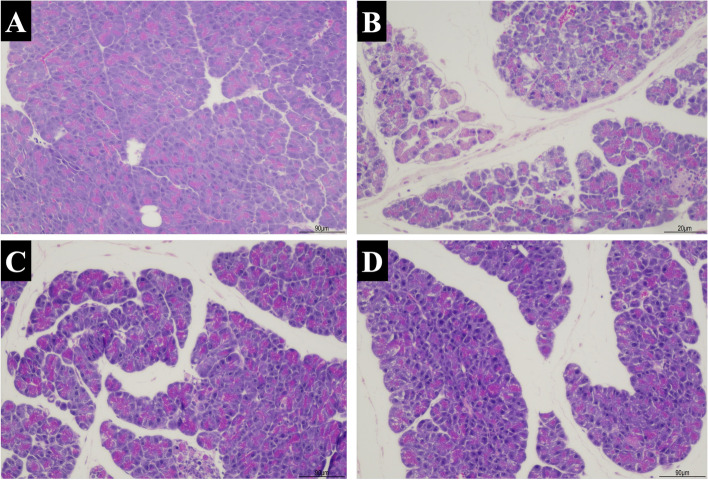
The pancreatic histopathology of the control group revealed normal architecture with some congestions, but no inflammation was observed. In contrast, diffuse inflammation, edema, and acini destruction were found in the AP group. The AP group had higher histopathological scores compared with the control group. Both S and M groups showed the improvement of inflammation, edema and fat necrosis compared with the AP group. The M group had lower histopathologic scores than the S group. The representative images of pancreatic histopathology and histological scores of all groups were reported in Figs. 5A-D, 6, and Table 1, respectively.
Fig. 5.
A-D. Representative images of pancreatic histopathology by H&E staining (100 ×) in all groups. A The Control group with normal histology; B AP group with diffuse inflammation, marked edema, and acini destruction; C S group with mild inflammation and edema; D M group with improved pathological changes
Fig. 6.
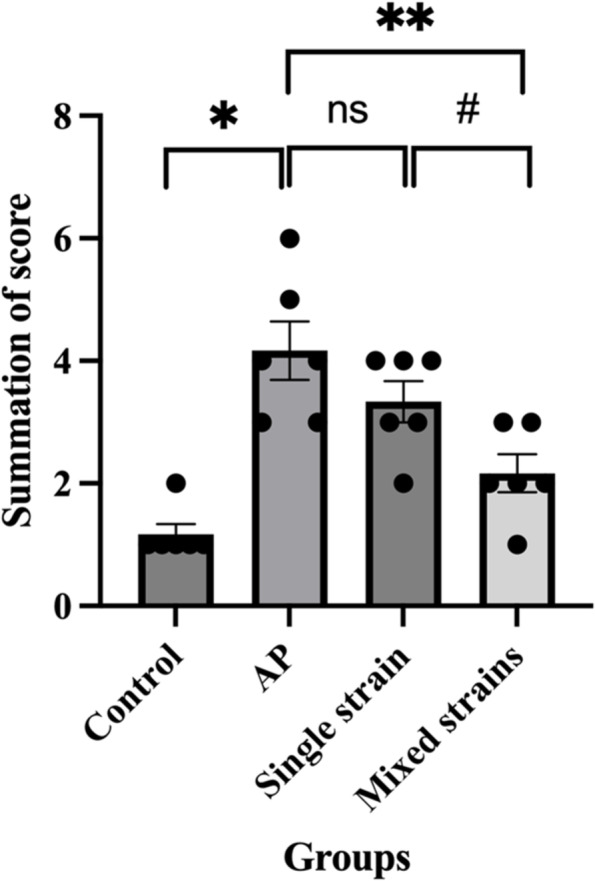
Showed the summation of pancreatic pathological scores (inflammation score + edema and fat necrosis score) in each group. Data are presented as mean ± SEM. Black dots represent the number of mice with that score in each group. *p < 0.05 compared with the control group; **p < 0.05 compared with the AP group; #p < 0.05 compared with the single-strain group; ns = non-significant
Table 1.
Summary of the pancreatic histopathological scores in each group
| Group | N | Inflammation | Edema and fat necrosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Control | 6 | 5 | 1 | 0 | 0 | 0 | 6 | 0 | 0 |
| AP | 6 | 0 | 2 | 3 | 1 | 0 | 0 | 4 | 2 |
| S | 6 | 0 | 4 | 2 | 0 | 0 | 1 | 4 | 1 |
| M | 6 | 3 | 2 | 1 | 0 | 0 | 3 | 2 | 1 |
Inflammation scoring – 0 = No neutrophil present, 1 = mild or < 25%, 2 = moderate or 25–50%, and 3 = severe or > 50% neutrophil infiltration of the total pancreatic parenchyma. Edema and fat necrosis scoring – 0 = No edema nor necrosis, 1 = mild or < 25%, 2 = moderate or 25–50%, and 3 = severe or > 50% edema or fat necrosis of the total pancreatic parenchyma
The numeric represents the number of mice with that histological score
Discussion
Acute pancreatitis (AP) can present in a wide spectrum of disease severities ranging from mild, moderate, and severe AP [29]. Severe AP is defined according to the modified Marshall scoring as AP with persistent organ failure of more than 48 h and usually in conjunction with local complications, such as infected pancreatic necrosis which is resulted from bacterial translocation. Severe AP is strongly associated with high morbidity and mortality rates [2, 10]. Therefore, the prevention of bacterial translocation could potentially attenuate the severity of AP and thus, reducing the mortality rate. Clinical studies have shown that probiotics could reduce the length of hospital stay in patients with AP; however, their effects on mortality and infectious complications remain conflicting [30, 31].
The diagnosis of AP in this study was made based on pancreatic histopathological changes and the elevation of serum amylase. Amylase is an enzyme produced by the acinar cells of the pancreas. In AP, acinar cell injury occurs, and thus, serum amylase is released from the injured acinar cells. Serum amylase rises rapidly 3–6 h after the onset of symptoms and lasts for 3–5 days [2]. In this study, serum amylase was significantly risen in the AP group compared to the control group as expected. Furthermore, mice that received probiotic pre-treatment whether as single- or mixed-strain probiotics had significantly lower serum amylase levels compared with mice in the AP group with a more prominent effect in the mixed-strain probiotic group. Similar to our results, Aykol and colleagues showed the decline of serum amylase levels in rats that received Saccharomyces boulardii and antibiotics compared with antibiotics alone [32]. Li and colleagues similarly showed that the colonization by B. animalis in germ-free and antibiotics-treated mice protected these mice again AP as evidenced by the reduction in serum amylase and pancreatic pathological changes [33]. In contrast, other studies demonstrated that rats receiving Ecologic 641, multiple-strain probiotics that consist of L. acidophilus, L. casei, L. salivarius, L. lactis, B. bifidum, and B. lactis, showed no differences in serum amylase levels compared with control rats [12, 20]. Another study using a different mixture of probiotics containing L. casei, L. rhamnosus, L. acidophilus, and B. longum also showed no differences in serum amylase levels between rats that received and did not receive probiotics [34]. We hypothesized that probiotic administration attenuated the severity of acinar cell damage, and thus, reducing serum amylase levels [32]. However, the inconsistency of findings between studies might be because of the differences in strains and strength of probiotics used.
Mice in the AP group showed significant changes in pancreatic histopathology as compared with those in the control group. Mice receiving mixed-strain probiotics showed an improvement in histological scores; in contrast, mice receiving single-strain probiotics did not show significant improvement. Our results were in contrast with findings from a study by van Minnen and colleagues. The authors found that probiotic supplement, despite reducing late mortality and improving clinical course, did not lead to significant histological improvement compared with placebo. In that study, they used multi-strain probiotics at the concentration of 5 × 109 CFU/mL starting 5 days prior to AP induction compared to our study that used probiotics concentration at 1 × 108 CFU/mL starting 3 days prior to AP induction [11]. Additionally, a meta-analysis of 6 studies on experimental AP demonstrated that probiotic supplement reduced total histopathological scores compared with controls [35]. The differences in histological outcomes could be potentially explained by the different duration of probiotic supplement (from 3 to 14 days prior to AP induction), the species of animals (mice vs. rats), the timing of histological assessment (from 12 h to 4 days after AP induction), probiotic strains and strength (2.4–5.0 × 109 CFU/mL). A previous study suggested that a longer duration of probiotic pre-treatment before AP induction might be needed to see positive results on pancreatic histopathology because a short duration of probiotic supplement might not have a significant impact on gut microbiome [36]. However, higher dose of probiotics and longer duration of therapy did not always translate into better outcomes as evidenced by a study by Horst and colleagues which showed that giving probiotic mixture 1.2 × 109 CFU/mL 14 days prior to the induction of AP led to greater pancreatic tissue damage than in the AP-alone group [34].
Neutrophil recruitment and activation plays a crucial role in the development of pancreatic inflammation and injury in the early phase of AP [37]. As MPO is mainly expressed in neutrophils and is important to neutrophil functions [38], pancreatic tissue MPO was used as a marker of neutrophil infiltration and inflammation in this study. We found that pancreatic MPO levels were significantly higher in the AP group than in the control group and improved with mixed-strain probiotic supplement. These results indicated that probiotic supplement could alleviate pancreatic inflammation, which was in accordance with the reduction in serum amylase and the degree of inflammation on pancreatic histology in the mixed-strain group. Similar to our results, Takauji and colleagues demonstrated that Lactobacillus brevis SBL88-derived long chain polyphosphate significantly reduced the numbers of MPO-positive cells and inflammatory cytokine expression in pancreatic tissues of mice with cerulean-induced AP [36].
Small bowel bacterial overgrowth, mucosal barrier failure, and impaired host defense mechanism are the three main steps involved in the development of bacterial translocation and infectious complications in AP [11, 12]. Early after the onset of AP, neurohormonal substances that are released in response to AP reduce small bowel motility leading to the stasis of luminal contents and overgrowth of potential enteric bacteria including E. coli and Enterococcus spp. [39]. These luminal pathogens along with AP-induced reduction of intestinal blood flow result in mucosal ischemia and reactive oxygen species (ROS) release [21]. Subsequent development of oxidative stress leads to the release of pro-inflammatory mediators and the disruption of epithelial tight junctions resulting in increased epithelial permeability, and eventually pathogenic bacterial translocation [40]. In accordance with previous reports, our findings showed that ileal occludin and claudin-1 levels significantly decreased in mice with L-arg-induced AP indicating the disruption of intestinal integrity. Probiotic supplement with either single or mixed strains of Lactobacillus increased ileal occludin, but not claudin-1 levels compared with the AP group. It is important to note that ileal occludin and claudin-1 levels were significantly higher in the mixed-strain group than in the single-strain one. Similar to our results, Takauji and colleagues found that pre-treatment with Lactobacillus brevis-derived polyphosphate significantly increased colonic occludin expression in cerulein-induced AP mice leading to the reduction in the intestinal permeability. Colonic claudin-1 expression was also augmented in the treatment group albeit not statistically significant [36]. It is hypothesized that probiotics strengthen intestinal barrier through the stimulation of glutathione production in the intestine, which in turn reduces oxidative stress induced mucosal damage [21].
Our study was not without limitations. Firstly, we did not perform gut microbial analysis in each group so we could not state with certainty that probiotic supplement protected against AP through the modulation of gut microbiota. Further studies are needed to confirm this mechanism. Secondly, probiotics were given to mice 3 days prior to the AP induction until 3 days afterward. Pre-treatment protocol might not be completely applicable to the clinical setting as patients usually present to the hospital after AP has already developed. Nevertheless, this protocol could still be applied to patients with recurrent AP from genetic diseases to see if probiotic supplement could prevent pancreatitis episode. Clinical studies are warranted to prove its benefits in this aspect.
Conclusions
Probiotic supplement, particularly the mixed-strain ones, alleviated the severity of L-arg-induced AP through the reduction of inflammatory responses and the improvement of intestinal integrity.
Supplementary Information
Additional file 1: Supplementary 1. - Sample size calculation.
Acknowledgements
None
Abbreviations
- AP
Acute pancreatitis
- BW
Body weight
- CFU
Colony-forming unit
- H&E
Hematoxylin and eosin
- IHC
Immunohistochemistry
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- i.p
Intraperitoneal
- L-arg
L-arginine
- L. plantarum
Lactobacillus plantarum
- M group
Mixed-strain group
- MODS
Multiple organ dysfunction syndromes
- MPO
Myeloperoxidase
- NOD1
Nucleotide-binding oligomerization domain-containing protein 1
- S group
Single-strain group
- SIRS
Systemic inflammatory response syndrome
- TMA
Tissue microarray
- TNF-α
Tumor necrosis factor-α
Authors’ contributions
DW contributed to the conceptualization, acquired the funding, supervised the study, wrote and revised the manuscript. SV conducted the study, performed laboratory tests and statistical analyses, and revised the manuscript. KS conducted the study, performed laboratory tests, and revised the manuscript. ST conducted the study, supplied probiotic emulsion for the study, and revised the manuscript. NK reviewed and scored pancreatic pathology, and revised the manuscript. PS contributed to the conceptualization, supervised the study, and revised the manuscript. MC supervised the study, performed laboratory tests and statistical analyses, wrote the original draft, and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the Grant of Ratchadaphiseksomphot, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (RA64/038) and the Thailand Science Research and Innovation Fund Chulalongkorn University, Grant number CU_FRB65_hea (21) 028_30_09, Bangkok, Thailand.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information file.
Declarations
Ethics approval and consent to participate
All mice received proper care in accordance with Ethical Principles and Guidelines for the Use of Animals by the National Research Council of Thailand, and the study was approved by Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (Approval number: 024/2562). This study was reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tenner S, Baillie J, DeWitt J, Vege SS. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–1415. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 2.Working Group IAP/APA Acute Pancreatitis Guidelines IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–87.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15(12):1427–1430. doi: 10.3748/wjg.15.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siriviriyakul P, Sriko J, Somanawat K, Chayanupatkul M, Klaikeaw N, Werawatganon D. Genistein attenuated oxidative stress, inflammation, and apoptosis in L-arginine induced acute pancreatitis in mice. BMC Complement Med Ther. 2022;22(1):208. doi: 10.1186/s12906-022-03689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko JS, Yang HR, Chang JY, Seo JK. Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-alpha. World J Gastroenterol. 2007;13(13):1962–1965. doi: 10.3748/wjg.v13.i13.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Mufti RA, Williamson RC, Mathie RT. Increased nitric oxide activity in a rat model of acute pancreatitis. Gut. 1998;43(4):564–570. doi: 10.1136/gut.43.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takács T, Czakó L, Morschl E, László F, Tiszlavicz L, Rakonczay Z, Jr, et al. The role of nitric oxide in edema formation in L-arginine-induced acute pancreatitis. Pancreas. 2002;25(3):277–282. doi: 10.1097/00006676-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ge P, Luo Y, Okoye CS, Chen H, Liu J, Zhang G, et al. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: A troublesome trio for acute pancreatitis. Biomed Pharmacother. 2020;132:110770. doi: 10.1016/j.biopha.2020.110770. [DOI] [PubMed] [Google Scholar]
- 10.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 11.van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141(4):470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.van Baal MC, van Rens MJ, Geven CB, van de Pol FM, van den Brink IW, Hannink G, et al. Association between probiotics and enteral nutrition in an experimental acute pancreatitis model in rats. Pancreatology. 2014;14(6):470–477. doi: 10.1016/j.pan.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP, Isenmann R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol. 2011;46(3):261–270. doi: 10.3109/00365521.2010.531486. [DOI] [PubMed] [Google Scholar]
- 14.Patel BK, Patel KH, Bhatia M, Iyer SG, Madhavan K, Moochhala SM. Gut microbiome in acute pancreatitis: a review based on current literature. World J Gastroenterol. 2021;27(30):5019–5036. doi: 10.3748/wjg.v27.i30.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2003;10(6):415–418. doi: 10.1007/s00534-002-0727-5. [DOI] [PubMed] [Google Scholar]
- 16.Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. Eur J Clin Nutr. 2002;56(Suppl 3):S60–S64. doi: 10.1038/sj.ejcn.1601489. [DOI] [PubMed] [Google Scholar]
- 17.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 18.Marteau P, Shanahan F. Basic aspects and pharmacology of probiotics: an overview of pharmacokinetics, mechanisms of action and side-effects. Best Pract Res Clin Gastroenterol. 2003;17(5):725–740. doi: 10.1016/S1521-6918(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 19.Brown AC, Valiere A. Probiotics and medical nutrition therapy. Nutr Clin Care. 2004;7(2):56–68. [PMC free article] [PubMed] [Google Scholar]
- 20.Lutgendorff F, Trulsson LM, van Minnen LP, Rijkers GT, Timmerman HM, Franzén LE, et al. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1111–G1121. doi: 10.1152/ajpgi.00603.2007. [DOI] [PubMed] [Google Scholar]
- 21.Lutgendorff F, Nijmeijer RM, Sandström PA, Trulsson LM, Magnusson KE, Timmerman HM, et al. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS ONE. 2009;4(2):e4512. doi: 10.1371/journal.pone.0004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oláh A, Belágyi T, Pótó L, Romics L, Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology. 2007;54(74):590–594. [PubMed] [Google Scholar]
- 23.Mangiante G, Colucci G, Canepari P, Bassi C, Nicoli N, Casaril A, et al. Lactobacillus plantarum reduces infection of pancreatic necrosis in experimental acute pancreatitis. Dig Surg. 2001;18(1):47–50. doi: 10.1159/000050096. [DOI] [PubMed] [Google Scholar]
- 24.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 25.Chayanupatkul M, Somanawat K, Chuaypen N, Klaikeaw N, Wanpiyarat N, Siriviriyakul P, et al. Probiotics and their beneficial effects on alcohol-induced liver injury in a rat model: the role of fecal microbiota. BMC Complement Med Ther. 2022;22(1):168. doi: 10.1186/s12906-022-03643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panpetch W, Chancharoenthana W, Bootdee K, Nilgate S, Finkelman M, Tumwasorn S, et al. Lactobacillus rhamnosus L34 attenuates gut translocation-induced bacterial sepsis in murine models of leaky gut. Infect Immun. 2017;86(1):e00700–e717. doi: 10.1128/IAI.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boonma P, Spinler JK, Venable SF, Versalovic J, Tumwasorn S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 2014;14:177. doi: 10.1186/1471-2180-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Cock HE, Forman MA, Farver TB, Marks SL. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol. 2007;44(1):39–49. doi: 10.1354/vp.44-1-39. [DOI] [PubMed] [Google Scholar]
- 29.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 30.Rohith G, Sureshkumar S, Anandhi A, Kate V, Rajesh BS, Abdulbasith KM, et al. Effect of synbiotics in reducing the systemic inflammatory response and septic complications in moderately severe and severe acute pancreatitis: a prospective parallel-arm double-blind randomized trial. Dig Dis Sci. 2023;68(3):969–977. doi: 10.1007/s10620-022-07618-1. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Zhang Y, Yang Q, Lee P, Windsor JA, Wu D. An updated systematic review with meta-analysis: efficacy of prebiotic, probiotic, and synbiotic treatment of patients with severe acute pancreatitis. Pancreas. 2021;50(2):160–166. doi: 10.1097/MPA.0000000000001734. [DOI] [PubMed] [Google Scholar]
- 32.Akyol S, Mas MR, Comert B, Ateskan U, Yasar M, Aydogan H, et al. The effect of antibiotic and probiotic combination therapy on secondary pancreatic infections and oxidative stress parameters in experimental acute necrotizing pancreatitis. Pancreas. 2003;26(4):363–367. doi: 10.1097/00006676-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Xie J, Guo X, Yang G, Cai B, Liu J, et al. Bifidobacterium spp and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses. Gut Microbes. 2022;14(1):2127456. doi: 10.1080/19490976.2022.2127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horst NL, Marques RG, Diestel CF, Matzke BD, Caetano CE, Simões FC, et al. Effects of probiotic supplementation on markers of acute pancreatitis in rats. Curr Ther Res Clin Exp. 2009;70(2):136–148. doi: 10.1016/j.curtheres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooijmans CR, de Vries RB, Rovers MM, Gooszen HG, Ritskes-Hoitinga M. The effects of probiotic supplementation on experimental acute pancreatitis: a systematic review and meta-analysis. PLoS ONE. 2012;7(11):e48811. doi: 10.1371/journal.pone.0048811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takauji S, Konishi H, Fujiya M, Ueno N, Tanaka H, Sato H, et al. Polyphosphate, derived from lactobacillus brevis, modulates the intestinal microbiome and attenuates acute pancreatitis. Dig Dis Sci. 2021;66(11):3872–3884. doi: 10.1007/s10620-020-06747-9. [DOI] [PubMed] [Google Scholar]
- 37.Wan J, Ren Y, Yang X, Li X, Xia L, Lu N. The role of neutrophils and neutrophil extracellular traps in acute pancreatitis. Front Cell Dev Biol. 2020;8:565758. doi: 10.3389/fcell.2020.565758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Wu CT, Li ZL, Xiong DX. Relationship between enteric microecologic dysbiosis and bacterial translocation in acute necrotizing pancreatitis. World J Gastroenterol. 1998;4(3):242–245. doi: 10.3748/wjg.v4.i3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balzan S, de Almeida QC, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22(4):464–471. doi: 10.1111/j.1440-1746.2007.04933.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary 1. - Sample size calculation.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information file.