Abstract
Hepatitis C virus (HCV) or HCV–low-density lipoprotein (LDL) complexes interact with the LDL receptor (LDLr) and the HCV envelope glycoprotein E2 interacts with CD81 in vitro. However, E2 interactions with LDLr and HCV interactions with CD81 have not been clearly described. Using sucrose gradient-purified low-density particles (1.03 to 1.07 g/cm3), intermediate-density particles (1.12 to 1.18 g/cm3), recombinant E2 protein, or control proteins, we assessed binding to MOLT-4 cells, foreskin fibroblasts, or LDLr-deficient foreskin fibroblasts at 4°C by flow cytometry and confocal microscopy. Viral entry was determined by measuring the coentry of α-sarcin, a protein synthesis inhibitor. We found that low-density HCV particles, but not intermediate-density HCV or controls bound to MOLT-4 cells and fibroblasts expressing the LDLr. Binding correlated with the extent of cellular LDLr expression and was inhibited by LDL but not by soluble CD81. In contrast, E2 binding was independent of LDLr expression and was inhibited by human soluble CD81 but not mouse soluble CD81 or LDL. Based on confocal microscopy, we found that low-density HCV particles and LDL colocalized on the cell surface. The addition of low-density HCV but not intermediate-density HCV particles to MOLT-4 cells allowed coentry of α-sarcin, indicating viral entry. The amount of viral entry also correlated with LDLr expression and was independent of the CD81 expression. Using a solid-phase immunoassay, recombinant E2 protein did not interact with LDL. Our data indicate that E2 binds CD81; however, virus particles utilize LDLr for binding and entry. The specific mechanism by which HCV particles interact with LDL or the LDLr remains unclear.
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide. Approximately 85% of people infected with HCV remain persistently viremic, and approximately 20% to 50% of these individuals ultimately develop cirrhosis (12, 21, 50). Of those with HCV-related cirrhosis, approximately 5% develop hepatocellular carcinoma (21, 50). In the United States, an estimated 4 million people are infected, and HCV is the leading cause of liver transplantation (21). Extrahepatic manifestations, including cryoglobulinemia and B-lymphocyte proliferative disorders, which are characterized by polyclonal B-cell activation and autoantibody production, are also associated with HCV infection (3, 13, 14, 39). Hepatocytes represent the primary site of HCV replication in vivo. Although explanted peripheral blood mononuclear cells (PBMCs) contain HCV RNA (4, 30, 40), it is unclear if HCV replication occurs in PBMCs in vivo (1, 16, 23, 52). No efficient cell culture system has been described for HCV, but in vitro studies have shown that several human cells including primary PBMC cultures (10) and cell lines of hepatocyte and lymphoid origin (42–45) are permissive for HCV replication.
Currently, the mechanism of HCV cell entry is not clear. Two cell surface receptors interact with HCV or HCV E2 protein in vitro, leading to speculation that either may represent the HCV cellular receptor (2, 15, 29, 35). The HCV envelope glycoprotein E2 was shown to specifically bind to human CD81 (15, 35). CD81 is a member of the tetraspanin superfamily of cell surface molecules and is expressed on virtually all nucleated cells (24). It is highly expressed on germinal-center B cells (15, 26, 35), although the level of expression within a single tissue varies during development and in response to cellular activation (24). Expression of CD81 on B cells was found to be critical for inducing optimal interleukin-4 and antibody production during T helper 2 (Th2) responses, suggesting that CD81 may interact with a ligand on T-helper cells (25). As part of a complex on B cells that includes CD19, CD21, and Leu13, CD81 can provide costimulatory signals that lower the threshold required for B cells to respond to antigen (26). Therefore, it was hypothesized that binding of HCV to CD81 on B cells in vivo lowers the activation threshold of these cells, facilitating the production of autoantibodies found in HCV-associated cryoglobulinemia (15, 35, 39). These studies suggested that E2 binding to CD81 may be responsible for the binding of HCV to target cells in vivo. However, only one study provides any evidence that viral particles bind CD81 in vitro or in vivo (35).
Thomssen et al. (48, 49) and others (36, 55) identified an association between HCV and low density lipoproteins (LDL) in human sera and subsequently demonstrated an interaction between HCV or HCV-LDL complexes with the cellular low-density lipoprotein receptor (LDLr). Seipp et al. demonstrated that persistent HCV replication occurred in cell lines of hepatocyte origin if they were maintained under conditions that upregulated LDLr expression (42). More recent studies demonstrated that HCV did not bind LDLr-deficient fibroblasts but that the expression of recombinant human LDLr in these cells promoted virus binding (29). Recently the LDLr was reported to promote viral entry for several members of the flavivirus family, including HCV and GB virus type C (also called hepatitis G virus) (2). Interactions between the HCV E2 protein or other viral proteins with either LDL or the LDLr have not been described.
The purpose of this study was to characterize interactions of both HCV and the viral envelope protein E2 with the LDLr and CD81. We used a lymphoid cell line (MOLT-4) which had previously been shown to support HCV replication in vitro (44) and human fibroblast cell lines with and without the LDLr to evaluate HCV binding. Our data provide further evidence of an association between HCV and LDL in human plasma and demonstrate that the LDLr is primarily responsible for HCV binding and entry. Although there is clearly specific binding of the envelope glycoprotein E2 to human CD81, our data do not suggest that CD81 is involved in cell binding or entry of infectious HCV particles.
(This work was presented in part at the IX International Symposium on HCV and Related Flaviviruses, Bethesda, Md., 6 June 1999.)
MATERIALS AND METHODS
HCV preparations.
Plasma was obtained from patients with HCV-related chronic liver disease. Plasma samples were tested by a commercially available HCV RNA quantitation method as previously described (Roche Monitor assay) (46). All patients tested positive for HCV antibodies (EIA 2.0 antibody tests; Abbott Laboratories, North Chicago, Ill.) and for HCV RNA by reverse transcription-PCR (RT-PCR) as previously described (38). Plasma was prepared from anticoagulated blood samples by centrifugation at 600 × g for 15 min, and HCV particles were separated by sucrose gradient centrifugation as previously described (54, 55). Gradient fractions were evaluated for HCV RNA by an in-house RT-PCR method as previously described (46). Fractions containing HCV low-density particles (1.04 to 1.07 g/ml) and intermediate-density particles (1.12 to 1.18 g/ml) were pooled, pelleted at 156,000 × g for 16 h at 4°C, and resuspended in phosphate-buffered saline (PBS), and aliquots were frozen at −80°C. RNA was extracted from pelleted fractions, and the relative end-point dilution dilution titer of HCV RNA was measured by RT-PCR (56). Negative-control preparations (mock) were simultaneously prepared using HCV antibody and HCV RNA-negative plasma in sucrose gradients, and fractions of corresponding density were pelleted and stored. This study was approved by the University of Iowa Institutional Review Board, and all subjects provided informed consent.
Proteins and antibodies.
Purified recombinant HCV envelope glycoprotein E2 (Ala 384 to Lys 715) and the nonstructural proteins NS3/NS4 (Asp 1569 to Pro 1931) expressed in CHO cells were obtained from Austral Biologicals (San Remo, Calif.). Human and mouse soluble CD81 was kindly provided by Shoshana Levy (Stanford University). LDL was obtained from Sigma (St. Louis, Mo.).
Anti-human LDLr monoclonal antibody (MAb) (clone C7) and anti-human LDL MAb (clone 4G3) were used in the studies (28, 51). Polyclonal goat anti-human LDL was obtained from Sigma. Anti-human CD81 MAb (clone JS64) was obtained from RD Inc. (Flanders, NJ). Anti-HCV E2 MAb was obtained from Austral Biologicals. Nonspecific mouse immunoglobulin G (IgG1) (Zymed, San Francisco, Calif.) was used as an isotype control. Anti-HCV polyclonal serum was obtained from an HCV-seropositive patient who was HCV RNA negative due to interferon therapy (38, 46). The negative control serum used in HCV-binding studies was obtained from an HCV RNA- and antibody-negative individual. Mouse IgG binding to cells was detected using an anti-mouse IgG (labeled with either Oregon green or Texas red), and human IgG binding to cells was detected with anti-human IgG labeled with Oregon green (Molecular Probes, Eugene, Oreg.). Alkaline phosphatase-labeled antispecies antibodies (Sigma) were used for solid-phase assays.
Cell lines.
MOLT-4 cells, a CD4+ T lymphoblastoid cell line, and mouse L cells were obtained from the American Type Culture Collection (Manassas, Va.). Human foreskin fibroblasts (FSF) and LDLr-deficient foreskin fibroblasts from a patient with familial hypercholesterolemia were also used in this study as described previously (20). MOLT-4 cells were cultured in RPMI 1640, and mouse L cells and human fibroblasts were cultured in Dulbecco modified Eagle medium. Media were supplemented with 10% fetal calf serum (FCS), 100 U of penicillin per ml, 100 μg of streptomycin sulfate per ml, and 2 mM l-glutamine. In some experiments, LDLr expression was induced by incubating cells for 48 h in RPMI 1640 or Dulbecco modified Eagle medium without FCS or in medium containing 10% lipoprotein-deficient human serum (LPDS), prepared as previously described (20).
RNA extraction and RT-PCR.
HCV RNA was isolated from patient plasma or gradient fractions using the RNA extraction method described by Chomczynski and Sacchi (8). Oligonucleotide primers for amplification of the HCV 5′ nontranslated region were used as described previously (38). DNA products (250 bp) were separated on 1.5% agarose gels and visualized by ethidium bromide staining.
HCV particle-binding assay.
MOLT-4 cells were washed in PBS containing 1% LPDS and 0.05% NaN3 and resuspended in either the HCV or mock-virus preparations. The cells were incubated for 60 min at 4°C, washed with PBS, and incubated in PBS plus 10% goat serum for 30 min at 4°C to prevent nonspecific antibody binding. The cells were washed and incubated with HCV antiserum or control serum (1:100 in PBS) for 60 min at 4°C. Antibody binding was detected using goat anti-human IgG–Oregon green (10 μg/ml) for 45 min at 4°C. The cells were washed twice, fixed in PBS containing 4% paraformaldehyde, and analyzed using flow cytometry (FACScan; Becton Dickinson). In cases where the fluorescence of the isotype control was higher than that of the mock-virus control, the specific binding of HCV was normalized by subtracting the value of the isotype control.
Solid-phase immunoassay.
Nitrocellulose (Schleicher & Schuell, Keene, N.H.) was placed into 48-well plates, and proteins of interest (10 μg/ml) were directly applied to the membrane and blocked with 5% nonfat dry milk in PBS for 30 min at room temperature. Membranes were subsequently incubated with either LDL, E2, or soluble CD81 (10 μg/ml in PBS) for 45 min at room temperature followed by three washes in PBS. Protein-protein interactions were detected with polyclonal or monoclonal anti-E2 and anti-LDL monoclonal antibodies at 10 μg/ml followed by incubation with alkaline phosphatase (AP)-labeled anti-mouse IgG or anti-human IgG. AP was detected with freshly prepared 5-bromo-4-chloro-3-indolyl phosphate–nitroblue tetrazolium substrate.
Confocal microscopy.
MOLT-4 cells were incubated in RPMI 1640 without FCS for 48 h to upregulate expression of the LDL receptor. Cells were washed in PBS and incubated with the mock-virus or HCV preparations for 45 min at 4°C. Following two washes in PBS, the cells were incubated for 30 min at 4°C in PBS containing 10% goat serum and were again washed twice in PBS. HCV or LDL binding was detected by simultaneous incubation with human polyclonal anti-HCV serum (1:100) and mouse anti-LDL MAb (10 μg/ml) for 45 min at 4°C, followed by fluorescence-labeled antispecies antibodies (10 μg/ml in PBS). The cells were analyzed for HCV colocalization with LDL by using confocal laser microscopy (Zeiss, Jena, Germany) as previously described (53).
α-Sarcin coentry studies.
MOLT-4 or mouse L cells (5 × 105) were resuspended with HCV or mock-virus preparations in methionine-deficient medium containing α-sarcin. After a 1-h incubation at 37°C, [35S]methionine (Amersham) was added for 15 min. The cells were washed and lysed, and trichloroacetic acid-precipitable counts were measured. For some experiments, MOLT-4 cells were preincubated for 48 h in serum-free RPMI 1640 or in RPMI 1640 containing 10% LPDS. All experiments were performed in duplicate or triplicate and were repeated at least three times.
RESULTS
Binding of HCV and HCV-E2 to MOLT-4 cells.
To measure HCV binding to cells, plasma from four HCV-infected patients and three controls was fractionated by equilibrium centrifugation on sucrose gradients and HCV RNA was detected in low-density fractions (1.03 to 1.08 g/ml) and in intermediate-density fractions (1.12 to 1.18 g/ml). The concentration of HCV RNA in two of these patients was greater than 106 genome equivalents per ml of plasma. Previous studies demonstrated that low-density HCV particles are associated with infectivity (6, 19) and are thought to represent the complete virion whereas the intermediate-density particles appear to represent nucleocapsids or virus-immune complexes (18, 19, 55). In addition to infectious HCV, LDL is found in low-density fractions of 1.006 to 1.063 g/cm3 and in association with HCV particles (48, 49).
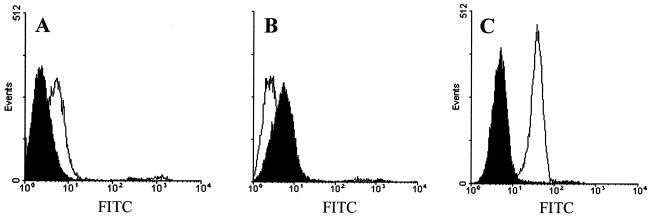
To detect the binding of HCV to MOLT-4 cells, pelleted virus or the corresponding pellet from HCV-negative plasma fractions (Mock) were added to MOLT-4 T cells and virus was identified using human HCV antiserum followed by a fluorescence-labeled secondary antibody. All incubations were performed at 4°C to prevent internalization and receptor cycling. The cells were fixed and examined for cell-bound fluorescence by flow cytometry or confocal microscopy. Low-density HCV particles bound MOLT-4 cells, but intermediate-density HCV particles and Mock fractions did not. The extent of intermediate-density HCV particle binding to MOLT-4 cells was lower than that of the Mock control by this fluorescence-based method (Fig. 1). The relative concentration of HCV RNA in the intermediate-density particles was 10-fold higher than that in the low-density peak when measured by end-point dilution (data not shown). Thus, the difference in binding between the low-density and intermediate-density particles cannot be explained by differences in HCV concentration. Using flow cytometry, we also examined the binding of the HCV envelope glycoprotein E2 to MOLT-4 cells. The HCV nonstructural proteins NS3/NS4 served as the negative control. The results indicated that more than 90% of cells bound HCV-E2 (Fig. 1C).
FIG. 1.
HCV and HCV-E2 binding to MOLT-4 cells. HCV low-density particles (A), HCV intermediate-density particles (B), and HCV-E2 (C) were evaluated for binding to MOLT-4 cells (open graphs). Mock sucrose gradient preparations (shaded graphs [A and B]) or the HCV nonstructural protein NS3/NS4 (shaded graph [C]) served as negative controls. Cell-bound virus or HCV-E2 protein was visualized with HCV-specific antiserum and anti-human IgG Oregon green (FITC).
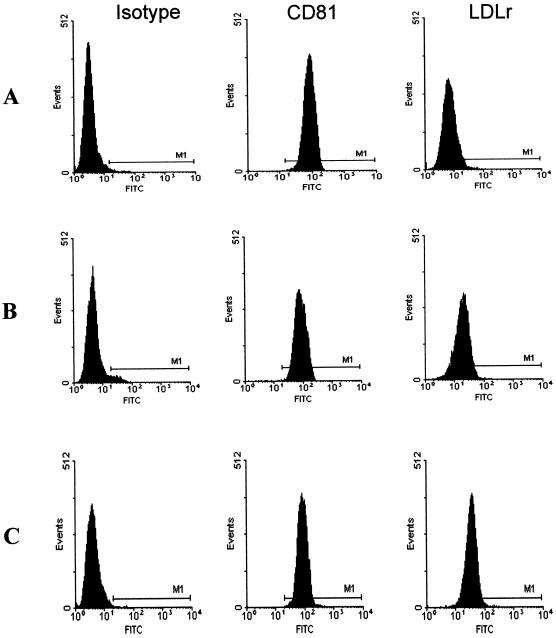
To determine if binding of the low-density HCV particles was correlated with expression of the LDL receptor or CD81, we first examined the regulation of both receptors in different stages of the cell cycle (Fig. 2). MOLT-4 cells were stained for LDLr and CD81 at various time points following cell culture passage. Nonspecific binding was ruled out using a mouse IgG1 isotype control antibody. CD81 was expressed at a constant level (98%), and expression was independent of the cell cycle. LDLr expression (6%) was significantly lower in cells maintained for 48 h in RPMI 1640 containing FCS (Fig. 2A) than in cells grown in lipoprotein-deficient medium for 24 h (52%) or 48 h (91%) (Fig. 2B and C). HCV and HCV-E2 binding was evaluated using cells that expressed low or high densities of LDLr (Table 1). In three independent experiments, HCV binding correlated with LDLr expression, resulting in up to a twofold increase in HCV binding to cells with high levels of LDLr. In contrast to whole virus, the viral envelope glycoprotein E2 bound more efficiently to cells and did not vary significantly in relation to LDLr expression. Upregulation of the LDLr actually resulted in a slight decrease in binding of E2 to MOLT-4 cells (80% versus 98%). The relationship between LDLr expression and HCV low-density particle binding was also shown by virus binding to normal FSF but not to LDLr-deficient FSF (Fig. 3).
FIG. 2.
Flow cytometric characterization of CD81 and LDLr expression on MOLT-4 cells. MOLT-4 cells were grown for 48 h in lipoprotein-rich medium (A) or for 24 h (B) or 48 h (C) in lipoprotein-deficient medium. Background fluorescence was measured with an irrelevant isotype-matched control MAb. CD81 expression was measured with JS64 MAb, and LDLr expression was measured with C7 MAb. Cell-bound antibodies were detected with anti-mouse IgG Oregon green (FITC).
TABLE 1.
HCV and E2 binding to MOLT-4 cells with low or high levels of LDLr expression
| LDLr expression | % Bindinga of:
|
|||||
|---|---|---|---|---|---|---|
| Mock | HCV | NS3/NS4 | E2 | Anti-CD81 | Anti-LDLr | |
| Low | 1.96 | 25.2 | 1.46 | 98.5 | 99.4 | 18.2 |
| High | 2.46 | 54.6 | 1.33 | 80.13 | 99.3 | 91.3 |
Data represent the percent fluorescence of ligand bound to MOLT-4 cells. HCV (low-density particles), Mock, NS3/NS4, and E2 binding was determined using HCV-specific antiserum and anti-human IgG-Oregon green. CD81 expression was measured using an anti-CD81 murine MAb (JS64), and LDLr expression was determined using C7 MAb with anti-mouse IgG-Oregon green. MOLT-4 cells were grown for 48 h in lipoprotein-rich or lipoprotein-deficient medium, resulting in low or high expression, respectively, of the LDLr.
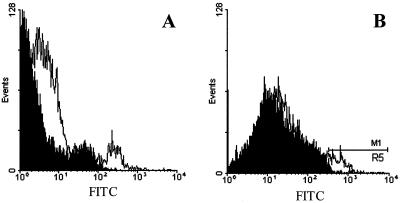
FIG. 3.
HCV binding to FSF. HCV low-density particles were evaluated for binding to FSF (A) and LDLr-deficient FSF (B). Mock sucrose gradient preparations (shaded graphs) served as negative controls. Cell-bound virus was visualized with HCV specific antiserum and anti-human IgG Oregon green (FITC).
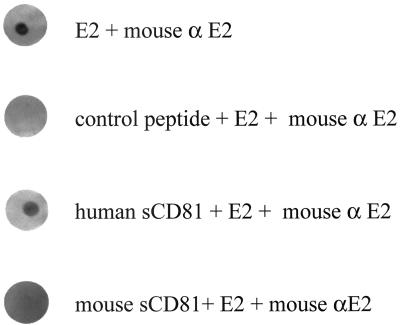
To determine the specificity of HCV and HCV-E2 binding to MOLT-4 cells, competition experiments using LDL (0.05 to 0.5 mg/ml) were performed. HCV binding was inhibited by LDL at 0.5 mg/ml, whereas the binding of E2 was unaffected (Table 2). In contrast, human but not mouse CD81 completely blocked E2 binding to MOLT-4 cells. The specific binding of HCV E2 to human CD81 was further demonstrated using solid-phase immunoassays (Fig. 4). E2 reproducibly bound to human CD81 but not to mouse CD81 or an irrelevant peptide control.
TABLE 2.
Inhibition of HCV and HCV E2 protein binding to MOLT-4 cells by human soluble CD81, mouse soluble CD81, and LDL
| Inhibitor | % Inhibitiona of:
|
|
|---|---|---|
| HCV Low-density particles | HCV E2 protein | |
| Murine soluble CD81 (30 μg/ml) | 7 | 0 |
| Human soluble CD81 (30 μg/ml) | 0 | 100 |
| LDL (0.5 mg/ml) | 27.5 | 1 |
Data represent the percent inhibition of fluorescence of ligand bound to MOLT-4 cells when incubated with inhibitors.
FIG. 4.
Specificity of HCV-E2 interactions with human CD81. Soluble human or mouse CD81 (10 μg/ml) applied to nitrocellulose was incubated with HCV-E2 (10 μg/ml). E2 applied to nitrocellulose served as the positive control, and an irrelevant control peptide served as the negative control. Interactions of E2 with each protein were detected with anti HCV-E2 MAb, anti-mouse IgG-AP, and 5-bromo-4-chloro-3-indolyl phosphate–nitroblue tetrazolium substrate.
HCV, HCV-E2, and LDL interactions.
To determine if HCV was associated with LDL, we evaluated MOLT-4 cells by confocal microscopy following incubation with low-density HCV particles at 4°C. LDL was detected by incubation with mouse anti-LDL followed by a secondary Texas red-labeled antibody, and HCV was identified using HCV antiserum followed by a secondary Oregon green-labeled antibody. MOLT-4 cells incubated with the Mock preparation and stained for HCV and LDL showed only detection of LDL (Fig. 5A). Cells incubated with HCV and HCV E2 protein stained only for HCV showed green fluorescent surface binding (Fig. 5B and C). Colocalization of LDL and HCV was demonstrated in cells incubated with HCV (Fig. 5D to F) but not in cells incubated with the Mock control (Fig. 5A). The LDL concentrations in the original HCV and Mock plasma samples were similar (104 and 105 mg/dl, respectively).
FIG. 5.
Immunofluorescence detection of HCV, LDL, and HCV-E2 on MOLT-4 cells. MOLT-4 cells were incubated with low-density Mock fractions (A), low-density HCV (B and D to F), or HCV-E2 (10 μg/ml) (C) at 4°C. HCV and HCV-E2 were localized with human HCV-specific antiserum followed by anti-human IgG-Oregon green (B, C, E, and G), whereas LDL was localized with mouse anti-LDL MAb and anti-mouse IgG-Texas red (A, D, and F). Incubation of cells with HCV low-density particles followed by dual staining showed colocalization of LDL (D) and HCV (E) as yellow fluorescence in the overlay (F). Dual staining of MOLT-4 cells incubated with Mock sucrose gradient fractions is shown in panel A.
To determine if the HCV-LDL interactions involved E2, HCV-E2 binding to the LDL was assessed using solid-phase immunoassays (Fig. 6). HCV E2, the nonstructural proteins NS3/NS4, human or mouse CD81, and a goat anti-LDL-specific antibody (positive control) were each applied to nitrocellulose and incubated with LDL. Bound LDL was detected with a mouse anti-LDL antibody followed by an AP-labeled anti-mouse IgG antibody and nitroblue tetrazolium substrate. LDL was detected only in the anti-LDL control, and no LDL binding was detected using CD81 or E2 proteins.
FIG. 6.
HCV-E2 does not interact with LDL. HCV-E2, HCV NS3/4, human soluble CD81, or mouse soluble CD81 spotted on nitrocellulose were incubated with LDL (10 μg/ml). The positive control consisted of goat-anti LDL polyclonal antibody spotted on nitrocellulose. Interaction of spotted proteins with LDL was detected with mouse anti-LDL MAb and anti-mouse IgG AP followed by 5-bromo-4-chloro-3-indolyl phosphate–nitroblue tetrazolium substrate. Binding was quantitated by densitometry (AlphaImager 2000; Alpha Innotech Corp., San Leandro, Calif.), and results represent density units × 100.
HCV low-density particles promote coentry of α-sarcin.
The protein translation inhibitor α-sarcin does not enter cells with intact cell membranes (31, 32). Previous studies demonstrated that coentry of α-sarcin occurs with binding and penetration of several animal viruses in vitro (11, 31, 32). Depending on the mode of entry, the inhibitor enters the cell via virus-induced changes in membrane permeability or via endocytosis, in which case the inhibitor is set free from the endosome during the uncoating process of the virus (32). To study virus entry, HCV was incubated with MOLT-4 or mouse L cells at 37°C in the presence of α-sarcin. Mouse L cells were used as a negative control, since they do not express human CD81 or the human LDL receptor. Cellular protein synthesis was determined by pulsing cells with [35S]methionine and measuring 35S incorporation into cellular proteins. Incubation of MOLT-4 cells with HCV intermediate-density particles (density, 1.12 to 1.16 g/ml) or Mock control fractions did not result in coentry of α-sarcin, whereas incubation with HCV low-density particles (1.04 to 1.08 g/ml) resulted in protein synthesis inhibition (P < 0.001, paired t test) (Table 3). Coentry of α-sarcin was not seen in mouse L cells incubated with HCV or Mock preparations. As noted above, LDLr expression on MOLT-4 cells was low during log phase and was upregulated during stationary phase or by incubation in LPDS. HCV and α-sarcin coentry occurred significantly more when cells were maintained under conditions that upregulated LDLr expression (Table 4). To determine if the α-sarcin entry correlated with the concentration of HCV applied to cells, serial twofold dilutions of the HCV (low-density particle) preparation or the control were made prior to incubating the MOLT-4 cells with HCV and α-sarcin. Table 5 shows that α-sarcin entry was dose dependent and decreased with decreasing concentrations of HCV.
TABLE 3.
Coentry of HCV and α-sarcin is specific for low-density particlesa
| Infection conditions | Protein synthesis (cpm)b in:
|
|
|---|---|---|
| MOLT-4 | L cells | |
| Mock (1.08 g/ml) | 24,541 | 19,442 |
| Mock (1.16 g/ml) | 22,616 | NTd |
| HCV (1.08 g/ml) | 3,020c | 23,855 |
| HCV (1.16 g/ml) | 18,650 | 17,588 |
| No α-sarcin | 22,371 | 22,760 |
Cells were incubated with low-density (1.08 g/ml) or intermediate-density (1.16 g/ml) fractions from HCV- or mock-infected plasma in the presence or absence of α-sarcin. Cellular protein synthesis was determined 1 h postincubation by labeling with [35S]methionine as described in Materials and Methods.
Data represent [35S]methionine incorporation.
HCV cpm values (1.08 g/ml) were significantly different from Mock (1.08 and 1.16 g/ml), HCV (1.16 g/ml), and no a-sarcin (P < 0.001; paired t test).
NT, not tested.
TABLE 4.
Coentry of HCV and α-sarcin increases with higher levels of LDLr expressiona
| Infection conditions | Protein synthesis (cpm)b in:
|
||
|---|---|---|---|
| MOLT-4 (log) | MOLT-4 (stationary) | MOLT-4 LPDS | |
| Mock (1.08 g/ml) | 24,541 | 21,600 | 22,000 |
| HCV (1.08 g/ml) | 19,877 | 3,020c | 3,800c |
| No α-sarcin | 22,371 | 23,428 | 21,670 |
Cells were incubated with low-density HCV or Mock preparations (1.08 g/ml) in the presence or absence of α-sarcin as described in Materials and Methods. MOLT-4 (log) cells were in the logarithmic phase and demonstrated low levels of LDLr expression. Stationary MOLT-4 cells and MOLT-4 cells grown in LPDS expressed significantly higher levels of LDL receptor (see Fig. 2). Cellular protein synthesis was determined 1 h postincubation by labeling with [35S]methionine as described in Materials and Methods.
Data represent [35S]methionine incorporation.
HCV cpm values in the MOLT-4 stationary and LPDS cells were significantly different from Mock, no α-sarcin, and HCV binding to (log) stage MOLT-4 cells (P < 0.01; paired t test).
TABLE 5.
Entry of α-sarcin into MOLT-4 cells is dependent on the concentration of low-density HCV particlesa
| Infection condition | Protein synthesis (cpm)b at dilution titer of virus preparation of:
|
|||||
|---|---|---|---|---|---|---|
| 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | |
| HCV | 2,040 | 3,011 | 3,325 | 5,877 | 8,955 | 13,545 |
| Mock | 16,337 | 14,261 | 15,925 | 16,440 | 13,855 | NTc |
| No α-sarcin | 15,299 | 14,733 | 8,987 | 14,388 | 15,821 | NT |
Cells were incubated with low-density (1.08 g/ml) particle fractions from Mock and HCV preparations in the presence of α-sarcin. Cells incubated without α-sarcin also served as a negative control (no α-sarcin). Cellular protein synthesis was determined 1 h postincubation by labeling with [35S]methionine as described in Materials and Methods. MOLT-4 cells were in the stationary phase during these experiments.
Data represent [35S]methionine incorporation.
NT, not tested.
DISCUSSION
The mechanism by which HCV binds to and enters cells appears to be complex. Our data confirm a highly specific interaction between HCV E2 protein and cellular CD81; however, viral particles obtained from patient plasma do not appear to have direct interactions with this cellular receptor. This was demonstrated by showing that both E2 and low-density HCV particles bound to MOLT-4 cells, yet human soluble CD81 only competed for binding with HCV E2 and not with low-density HCV particles. In contrast, LDL inhibited only viral binding and did not inhibit E2 binding. The importance of the LDLr in HCV binding was further demonstrated by increasing viral binding and entry, but not E2 binding, under conditions that led to upregulation of the LDLr.
There are several reports indicating that infectious HCV particles in plasma are associated with β-lipoproteins and that binding of HCV to the LDLr may be mediated by very low density lipoprotein (VLDL) or LDL (2, 19, 36, 42, 48, 49). Since the HCV preparations used in our experiments are derived from plasma, the low-density particle fractions in our experiments also contain β-lipoproteins. Our finding that LDL and HCV colocalized on the surface of MOLT-4 cells supports the hypothesis that HCV-LDL interactions occur and that the resulting complex utilizes the LDLr for binding. It is not clear if the intermediate-density fractions in sucrose gradients represent viral nucleocapsids (55), virus-Ig complexes (18, 19), or virions not associated with LDL (2); however, it was previously shown that this population of virus particles was not highly infectious (5, 18, 19). Consistent with these findings, we found that these “intermediate-density” HCV particles did not bind MOLT-4 cells or allow coentry of α-sarcin.
We were unable to demonstrate a specific interaction between E2 and LDL or between E2 and the LDLr by using solid-phase immunoassays; therefore, the interaction between HCV and LDL remains unclear. A potential reason for this is that the recombinant E2 used in our experiments is truncated at the C terminus (deletion of 30 amino acids [positions 716 to 746] within the HCV polyprotein). Deletions in this region may result in conformational changes of the protein, leading to altered binding characteristics, or the LDL or LDLr binding domain could reside in this region of E2. The latter is unlikely, since this is a hydrophobic region that is unlikely to be highly surfaced exposed. An alternative explanation is that HCV binding to LDL or the LDLr may be mediated by viral proteins other than E2. Nonetheless, since several lines of evidence support an interaction between plasma LDL and HCV (2, 29), it seems reasonable to speculate that the LDL-HCV complex binds the LDLr by using the natural ligand (LDL), carrying HCV into the cell (2, 29). Our data support this hypothesis by demonstrating LDL-HCV colocalization by confocal microscopy and by density gradient centrifugation (55). The α-sarcin coentry experiments also reveal a correlation between LDLr expression and viral entry, further supporting data from other groups identifying the LDLr as the major means of HCV entry (2, 29). However, we were unable to block HCV binding to MOLT-4 cells completely with LDL; therefore, our data do not exclude the possibility that HCV may bind cells via additional cell surface proteins. Glycosaminoglycans (GAGs) are important in the cell surface binding of a number of microorganisms (37), including a number of viruses (herpesviruses, human immunodeficiency virus type 1, vaccinia virus, foot-and-mouth disease virus type O, and dengue virus) that bind to heparan sulfate on the cell surface (7, 9, 22, 33, 41). Although the role of proteoglycans in cell entry is unclear, recent reports suggest that HCV attachment may involve cell surface GAGs and possibly CD81 (17, 27, 34, 47). Virus-GAG interactions appear to be weak and reversible unless additional interactions occur, which may involve non-heparan sulfate virus entry receptors (37). Thus, it is possible that HCV or HCV-LDL complex attachment may be promoted by GAGs and that the LDLr is subsequently utilized for cell entry.
ACKNOWLEDGMENTS
This work was supported by a Merit Review and a Career Development Enhancement Award from the Veterans Administration (J.T.S.), NIH grant RO1 AA12671 (J.T.S.), and NIH K08 A101460 (W.N.S.). In addition, The University of Iowa Flow Cytometry Core Program was utilized for these studies.
We thank Shoshana Levy, Stanford University, for providing murine and human soluble CD81. We also thank Douglas LaBrecque, Jinhua Xiang, James McCoy, and Donna Brashear for helpful discussions and the University of Iowa Digestive Diseases nursing staff for assistance with clinical samples.
REFERENCES
- 1.Afonao A M R, Jiang J, Penin F, Tareau C, Samuel D, Petit M-A, Bismuth H, Dussaix E, Feray C. Nonrandom distribution of hepatitis C virus quasispecies in plasma and peripheral blood mononuclear cells subsets. J Virol. 1999;73:9213–9221. doi: 10.1128/jvi.73.11.9213-9221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnello V, Abel G, Elfahal M, Knight G B, Zhang X. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnello V, Chung R T, Kaplan L M. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 4.Bouffard P, Hayashi P, Acevedo R, Levy N, Zeldis J. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992;166:1276–1280. doi: 10.1093/infdis/166.6.1276. [DOI] [PubMed] [Google Scholar]
- 5.Bradley D W, McCaustland K, Cook E H. Posttransfusion non-A, non-B hepatitis in chimpanzees: physicochemical evidence that the tubule forming agent is a small, enveloped virus. Gastroenterology. 1985;88:773–779. [PubMed] [Google Scholar]
- 6.Bradley D W, McCaustland K, Krawcyznski K, Spelbring J, Humphrey C, Cook E H. Buoyant density of the factor CIII-derived isolate in sucrose. J Med Virol. 1991;34:206–208. doi: 10.1002/jmv.1890340315. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Maguire M, Hileman R E, Fromm J R, Esko J D, Linhardt R, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;2:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloraform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Chung C, Hsiao J, Chang Y, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cribier B, Schmitt C, Bingen A, Kirn A, Keller F. In vitro infection of peripheral blood mononuclear cells by hepatitis C virus. J Gen Virol. 1995;76:2485–2491. doi: 10.1099/0022-1317-76-10-2485. [DOI] [PubMed] [Google Scholar]
- 11.Cuadras M A, Arias C F, Lopez S. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J Virol. 1997;71:9065–9074. doi: 10.1128/jvi.71.12.9065-9074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuthbert J A. Hepatitis C: progress and problems. Clin Microbiol Rev. 1994;7:505–532. doi: 10.1128/cmr.7.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E, Fosella P V, Pasero G, Bombardieri S. Antibodies against hepatitis C virus in mixed cryoglobulinemia patients. Infection. 1991;6:551–555. doi: 10.1007/BF01726453. [DOI] [PubMed] [Google Scholar]
- 14.Ferri C, Greco F, Longombardo G, Pallo P, Moretti A, Marzo E, Mazzoni A, Pasero G, Bombardieri S, Higuchi R. Association between hepatitis C virus and mixed cryoglobulinemia. Clin Exp Rheumatol. 1991;9:621–624. [PubMed] [Google Scholar]
- 15.Flint M, Thomas J M, Maidens C M, Shotton C, Levy S, Barclay W S, McKeating J A. Functional analysis of cell surface-expressed hepatitis C virus E2 glycoprotein. J Virol. 1999;73:6782–6790. doi: 10.1128/jvi.73.8.6782-6790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong T L, Shindo M, Feinstone S M, Hoofnagle J, DiBisceglie A M. Detection of replicative intermediates of hepatitis C viral RNA in liver and serum of patients with chronic hepatitis C. J Clin Investig. 1991;88:1058–1060. doi: 10.1172/JCI115368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garson J A, Lubach D, Passas J, Whitby K, Grant P R. Suramin blocks hepatitis C binding to human hepatoma cells in vitro. J Med Virol. 1999;57:238–242. [PubMed] [Google Scholar]
- 18.Han J-Q, Schmidt W N, Wu P, Loh P, Neil G, LaBrecque D R, Stapleton J T. Specific binding of hepatitis C virus to the Fc fragment of immunoglobulin molecules. In: Rizzeto M, Purcell R H, Gerin J L, Verme G, editors. Viral hepatitis and liver disease. Turin, Italy: Edizioni Minerva Medica; 1997. pp. 228–231. [Google Scholar]
- 19.Hijikata M, Shimizu Y K, Katyo H, Iwamoto A, Shih J W, Alter H J, Purcell R H. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J Virol. 1993;67:1953–1958. doi: 10.1128/jvi.67.4.1953-1958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs H H, Brown M S, Russell D W, Davignon J, Goldstein J L. Deletion in the gene for the low-density-lipoprotein receptor in a majority of French Canadians with familial hypercholesterolemia. N Engl J Med. 1987;317:734–737. doi: 10.1056/NEJM198709173171204. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle J H. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 22.Jackson T, Ellard F M, Ghazaleh R A, Brookes S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W, King A M Q. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanford R E, Chavez D, Chisari F V, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. Virology. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy S, Todd S C, Maecker H T. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 25.Maecker H T, Do M S, Levy S. CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune responses. Proc Natl Acad Sci USA. 1998;95:2458–2462. doi: 10.1073/pnas.95.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maecker H T, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meola A, Sbardellati A, Bruni Ercole B, Cerretani M, Pezzanera M, Ceccacci A, Vitelli A, Levy S, Nicosia A, Traboni C, Scarselli E. Binding of hepatitis C virus E2 glycoprotein to CD81 does not correlate with species permissiveness to infection. J Virol. 2000;74:5933–5938. doi: 10.1128/jvi.74.13.5933-5938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milne R W, Theolis R Jr, Verdery R B, Marcel Y L. Characterization of monoclonal antibodies against human low density lipoprotein. Arteriosclerosis. 1987;3:23–30. doi: 10.1161/01.atv.3.1.23. [DOI] [PubMed] [Google Scholar]
- 29.Monazahian M, Bohme I, Bonk S, Koch A, Scholz C, Grethe S, Thomssen R. Low-density lipoprotein receptor as a candidate receptor for hepatitis C virus. J Med Virol. 1999;57:223–229. doi: 10.1002/(sici)1096-9071(199903)57:3<223::aid-jmv2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Muller H M, Pfaff E, Goeser T, Kallinowski B, Solbach C, Theillmann L. Peripheral blood leukocytes serve as a possible extrahepatic site for hepatitis C virus replication. J Gen Virol. 1993;74:669–676. doi: 10.1099/0022-1317-74-4-669. [DOI] [PubMed] [Google Scholar]
- 31.Munoz A, Castrillo J L, Carrasco L. Modification of membrane permeability during Semliki Forest virus infection. Virology. 1985;146:203–212. doi: 10.1016/0042-6822(85)90004-2. [DOI] [PubMed] [Google Scholar]
- 32.Otero M J, Carrasco L. Proteins are cointernalized with virion particles during early infection. Virology. 1987;160:75–80. doi: 10.1016/0042-6822(87)90046-8. [DOI] [PubMed] [Google Scholar]
- 33.Patel M, Yanagishita M, Rodriguez G, Bou-Habib D C, Oravecz T, Hascall V C, Norcross M A. Cell surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 34.Petracca R, Falugi F, Galli G, Norais N, Rosa D, Campagnoli S, Burgio V, Di Stasio E, Giardina B, Houghton M, Abrignani S, Gransi G. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74:4824–4830. doi: 10.1128/jvi.74.10.4824-4830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 36.Prince A M, Huima-Byron T, Parker T S, Levine M M. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J Viral Hepatol. 1996;3:11–17. doi: 10.1111/j.1365-2893.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 37.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt W N, Klinzman D, LaBrecque D, Macfarlane D E, Stapleton J T. Direct detection of hepatitis C virus (HCV) RNA from whole blood, and comparison with HCV RNA in plasma and peripheral blood mononuclear cells. J Med Virol. 1995;47:153–160. doi: 10.1002/jmv.1890470208. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt W N, Stapleton J T, LaBrecque D R, Mitros F A, Kirkegaard K, Phillips M J P, Brashear D. Hepatitis C infection and cryoglobulinemia: analysis of whole blood and plasma HCV RNA concentration and correlation with liver histology. Hepatology. 2000;31:737–744. doi: 10.1002/hep.510310326. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt W N, Wu P, Han J-Q, Perino M J, LaBrecque D R, Stapleton J T. Distribution of hepatitis C virus (HCV) RNA in whole blood and blood cell fractions: plasma HCV RNA analysis underestimates circulating virus load. J Infect Dis. 1997;176:20–26. doi: 10.1086/514024. [DOI] [PubMed] [Google Scholar]
- 41.Secchiero P, Sun D, DeVico A L, Crowley R W, Reitz M S, Zauli G, Lusso P, Gallo R C. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J Virol. 1997;71:4571–4585. doi: 10.1128/jvi.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seipp S, Mueller H M, Pfaff E, Stremmel W, Theilmann L, Goeser T. Establishment of persistent hepatitis C virus infection and replication in vitro. J Gen Virol. 1997;78:2467–2476. doi: 10.1099/0022-1317-78-10-2467. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu Y K, Feinstone S M, Kohara M, Purcell R H, Yoshikura H. Hepatitis C virus: detection of intracellular virus particles by electron microscopy. Hepatology. 1996;23:205–209. doi: 10.1002/hep.510230202. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu Y K, Iwamoto A, Hijikata M, Purcell R H, Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proc Natl Acad Sci USA. 1992;89:5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu Y K, Purcell R H, Yoshikura H. Correlation between the infectivity of hepatitis C virus in vivo and its infectivity in vitro. Proc Natl Acad Sci USA. 1993;90:6037–6041. doi: 10.1073/pnas.90.13.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stapleton J T, Klinzman D, Schmidt W N, Wu P, LaBrecque D R, Han J-Q, Perino-Phillips M J, Woolson R, Alden B. Prospective comparison of whole blood and plasma hepatitis C virus RNA detection systems: improved detection using whole blood as the source of viral RNA. J Clin Microbiol. 1999;37:484–489. doi: 10.1128/jcm.37.3.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takikawa S, Ishii K, Suzuki T, Asakura H, Matsuura Y, Miyamura T. Cell fusion activity of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74:5066–5074. doi: 10.1128/jvi.74.11.5066-5074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomssen R, Bonk S, Propfe C, Heermann K H, Kochel H G, Uy A. Association of hepatitis C virus in human sera with beta-lipoproteins. Med Microbiol Immunol. 1992;181:293–300. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- 49.Thomssen R, Bonk S, Thiele A. Density heterogeneities of hepatitis C virus in human sera due to binding of beta-lipoproteins and immunoglobulins. Med Microbiol Immunol. 1993;182:329–334. doi: 10.1007/BF00191948. [DOI] [PubMed] [Google Scholar]
- 50.Tong M J, el-Farra N S, Reikes A R, Co R L. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 51.van Driel I R, Goldstein J L, Sudhof T C, Brown M S. First cysteine-rich repeat in ligand-binding domain of low density lipoprotein receptor binds Ca2+ and monoclonal antibodies, but not lipoproteins. J Biol Chem. 1987;262:17443–17449. [PubMed] [Google Scholar]
- 52.Wang J T, Sheu J C, Lin J T, Wang T H, Chen D S. Detection of replicative form of hepatitis C virus RNA in peripheral blood mononuclear cells. J Infect Dis. 1992;166:1167–1169. doi: 10.1093/infdis/166.5.1167. [DOI] [PubMed] [Google Scholar]
- 53.Wünschmann S, Stapleton J T. Fluorescence-based quantitative methods for detecting human immunodeficiency virus type 1-induced syncytia. J Clin Microbiol. 2000;38:3055–3060. doi: 10.1128/jcm.38.8.3055-3060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiang J, Daniels K J, Soll D R, Schmidt W N, LaBrecque D R, Stapleton J T. Visualization and characterization of GB virus C (hepatitis G virus) particles: evidence for a nucleocapsid. J Viral Hepatol. 1999;6:S16–S22. doi: 10.1046/j.1365-2893.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 55.Xiang J, Klinzman D, McLinden J, Schmidt W N, LaBrecque D R, Gish R, Stapleton J T. Characterization of hepatitis G virus (GB-C virus) particles: evidence for a nucleocapsid and expression of sequences upstream of the E1 protein. J Virol. 1998;72:2738–2744. doi: 10.1128/jvi.72.4.2738-2744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang J, Wünschmann S, Schmidt W N, Shao J, Stapleton J T. Full-length GB virus C (hepatitis G Virus) RNA transcripts are infectious in primary CD4-positive T cells. J Virol. 2000;74:9125–9133. doi: 10.1128/jvi.74.19.9125-9133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]