Abstract
Acute kidney injury (AKI) is associated with a worse prognosis in coronavirus disease 2019 (COVID-19) patients. Identification of AKI, particularly in COVID-19 patients, is important for improving patients’ management. The study aims to assess risk factors and comorbidities of AKI in COVID-19 patients. We systematically searched PubMed and DOAJ databases for relevant studies involving confirmed COVID-19 patients with data on risk factors and comorbidities of AKI. The risk factors and comorbidities were compared between AKI and non-AKI patients. A total of 30 studies involving 22385 confirmed COVID-19 patients were included. Male (OR: 1.74 (1.47, 2.05)), diabetes (OR: 1.65 (1.54, 1.76)), hypertension (OR: 1.82 (1.12, 2.95)), ischemic cardiac disease (OR: 1.70 (1.48, 1.95)), heart failure (OR: 2.29 (2.01, 2.59)), chronic kidney disease (CKD) (OR: 3.24 (2.20, 4.79)), chronic obstructive pulmonary disease (COPD) (OR: 1.86 (1.35, 2.57)), peripheral vascular disease (OR: 2.34 (1.20, 4.56)), and history of nonsteroidal anti-inflammatory drugs (NSAID) (OR: 1.59 (1.29, 1.98)) were independent risk factors associated with COVID-19 patients with AKI. Patients with AKI presented with proteinuria (OR: 3.31 (2.59, 4.23)), hematuria (OR: 3.25 (2.59, 4.08)), and invasive mechanical ventilation (OR: 13.88 (8.23, 23.40)). For COVID-19 patients, male gender, diabetes, hypertension, ischemic cardiac disease, heart failure, CKD, COPD, peripheral vascular disease, and history of use of NSAIDs are associated with a higher risk of AKI.
Keywords: 2019-nCoV disease, acute kidney injury, COVID-19, SARS-CoV-2 infection
1. Introduction
The emergence of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), infected over 40 million people and was declared a pandemic on 11 March 2020, by the World Health Organization (WHO) [1]. The majority of COVID-19 patients were asymptomatic or presented with mild upper respiratory illness symptoms. However, COVID-19 can lead to the development of a more severe condition, leading to death [2]. As of October 29, 2020, there have been 44,774,763 confirmed cases of COVID-19, causing 1,179,225 deaths worldwide [3].
The previous meta-analysis revealed that acute kidney injury (AKI) was associated with a worse prognosis in COVID-19 patients [4]. AKI developed in 5%–15% of COVID-19 patients and carried a high mortality rate [2,3,4,5]. Patients who recovered from AKI were still independently associated with long-term mortality, cardiovascular events, and the development of chronic kidney disease (CKD) [6]. Several risk factors, including advanced age, male gender, cardiovascular disease, hypertension, diabetes, chronic kidney disease (CKD), and chronic liver disease, were reported to be at higher risk for SARS-CoV-2 infection with a poor prognosis [7,8]. Even though the relationship between AKI and severity or mortality has been established, the risk factors and comorbidities of AKI in COVID-19 patients remain unclear. Hence, we performed a systematic review with meta-analysis aiming to provide information about risk factors and comorbidities of AKI, particularly in COVID-19 patients.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
We conducted a systematic search on PubMed and DOAJ databases from inception to 10 October 2020, for articles using the keywords “COVID-19”, “2019-nCoV disease”, “SARS-CoV-2 infection” in combination with “acute kidney injury”, “acute kidney failures”, “acute renal failures”, “acute renal injury”. Additional studies were retrieved by manual screening of the reference lists of other meta-analyses and systematic reviews identified. The search was limited to original research with full text available in English. AKI was defined according to the 2012 KDIGO definition, which is an increase in serum creatinine ≥ 0.3 within 48 h, or an increase in serum creatinine ≥ 1.5 times baselines within 7 days, or a decrease in urine volume < 0.5 mL/kg/h for 6 h [9]. Prospective cohort, retrospective cohort, case–control, and cross-sectional studies involving adult patients with COVID-19, containing data on risk factors and comorbidities of AKI, met the inclusion criteria.
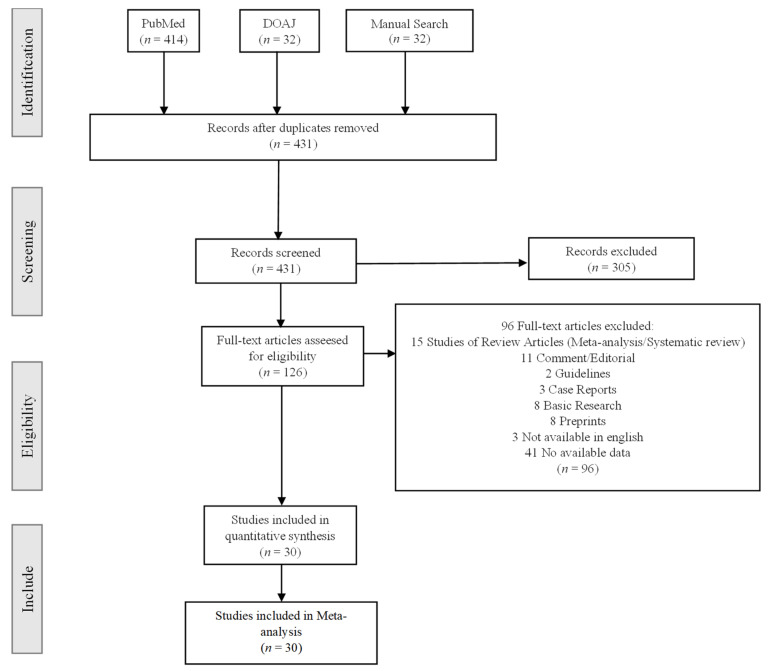
The following studies were excluded: duplicate publications, reviews, editorials, case series, case reports, guidelines, basic research, preprint papers, studies that do not provide risk factors or comorbidities data, and studies that include children as subjects. Four reviewers (A.A.H., V.A.G., F.R.I., and R.A.) independently screened the titles and abstracts for articles and read the full text for the potentially eligible studies. Conflicts were discussed and resolved by the third, fourth, and fifth senior reviewers (D.M.H., A.T., and M.T.). The selection process is shown with a flow chart (Figure 1).
Figure 1.
Selection process on studies.
2.2. Data Extraction and Quality Assessment
Relevant information including the first author, year of publication, study design, location, age, gender, number of populations, AKI prevalence, mortality rate, and proportion of patients requiring renal replacement therapy (RRT) was identified and extracted. Four reviewers (A.A.H., V.A.G., F.R.I., and R.A.) independently extracted data on risk factors and comorbidities, such as the prevalence of males, advanced age, diabetes mellitus, hypertension, obesity, smokers, ischemic cardiac disease, heart failure, cerebrovascular disease, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), peripheral vascular disease, chronic liver disease, cancer, and HIV (human immunodeficiency virus) status. A history of chronic use of drugs was also retrieved. The available characteristics were extracted, including laboratory results and concomitant treatments.
The risk of bias was assessed by NIH quality assessment tools. Disagreement was resolved by discussion with senior reviewers (D.M.H., A.T., and M.T.) until a consensus was reached. The third and fourth authors discussed and resolved differences of opinion.
2.3. Data Synthesis and Statistical Analysis
The odds ratio (OR) was used to describe the ratio of the probability of events occurring in acute kidney injury patients (AKI) versus non-acute kidney injury patients (NAKI). Forest plots were created to present the prevalence and the corresponding 95% CI of risk factors and comorbidities. We used the I2 statistic to assess heterogeneity among the studies. I2 values from 0% to 50% indicate low heterogeneity, I2 values between 50% and 75% indicate moderate heterogeneity, and I2 values greater than 75% indicate high heterogeneity. If I2 < 50%, we used the fixed benefit model to pool the data. Contrarily, when I2 > 50%, we used the random-effect model. The threshold of statistical significance was set to 0.05. We used a funnel plot to test publication bias. All analyses and plots were performed and created with Review Manager (version 5.3).
3. Results
3.1. Search Results, Characteristics of the Included Studies, and Methodological Quality
Figure 1 describes the step-by-step method we followed to include studies in our meta-analysis. We initially identified 478 articles in our research. After removing the duplicates, 431 articles remained. From titles and abstract screening, 305 were excluded. A total of 126 potentially eligible articles were assessed by full-text review. Of these 126 studies, 96 articles met the exclusion criteria. A total of 30 studies fulfilled our selection criteria and included the data we need to investigate. A total of 22,385 patients were included in these studies. The main characteristics of patients and these studies are described in Table 1.
Table 1.
The main characteristics of included studies.
| First Author | Published Year | Study Design | Location | Number of Populations | Age | Gender (Male%) | N (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| AKI | Mortality | RRT | |||||||
| Alberici [10] | 2020 | Cohort | Italy | 20 | 57 (44–70) | 16 (80) | 5 (25) | 5 (25) | 9 (17.3) |
| Chan [11] | 2020 | Cohort | USA | 3993 | 64 (56–78) | 2289 (57.32) | 2158 (46) | / | 347 (18.9) |
| Chand [12] | 2020 | Cohort | USA | 300 | 58.2 (12.6) | 182 (60.7) | 230 (76.7) | 157 (52.3) | 117 (39) |
| Cui [13] | 2020 | Cohort | China | 116 | 61.05 (12.9) | 66 (56.89) | 21 (18.1) | 24 (20.6) | / |
| Duduignon [14] | 2020 | Cross Sectional | France | 51 | 63 (57–69) | 39 (76.5) | 26 (50.98) | 14 (27.4) | 10 (39) |
| Fisher [15] | 2020 | Cohort | USA | 3345 | 64.4 (16.4) | 1776 (53.1) | 1903 (56.9) | 775 (23.2) | 574 (17.1) |
| Forminsky [16] | 2020 | Cohort | Italy | 96 | 64 (58.5–70.0) | 80 (83.33) | 72 (75) | 32 (33.33) | 17 (17.70) |
| Grimaldi [17] | 2020 | Cohort | Europe | 414 | 63 (11) | 320 (77.2) | 231 (55.8) | 95 (22.9) | / |
| Hirsch [18] | 2020 | Cohort | USA | 5449 | 64 (52–75) | 3317 (60.9) | 1993 (36.6) | 888 (54.9) | 285 (5.2) |
| Husain [19] | 2020 | Cohort | Europe | 23 | 60 (37–88) | 19 (82.6) | 12 (52.2) | 15 (65.2) | 3 (13) |
| Joseph [20] | 2020 | Cohort | French | 100 | 59 (53–67) | 70 (70) | 81 (81) | 29 (29) | 13 (13) |
| Lee [21] | 2020 | Cohort | USA | 1002 | 66 (53–76) | 619 (62) | 294 (29.3) | 172 (17) | / |
| Lei [22] | 2020 | Cohort | China | 34 | 55 (43–63) | 14 (41.2) | 2 (5.9) | 7 (20.6) | / |
| Lim [23] | 2020 | Cross Sectional | Asia | 160 | 61 (24–98) | 106 (66.25) | 30 (18.8) | 37 (23.12) | 5 (3.1) |
| Liu [24] | 2020 | Cross Sectional | China | 1190 | 57 (47–67) | 635 (53.36) | 51 (4.3) | / | / |
| Mohammed [25] | 2020 | Cohort | Australia | 575 | 65 (36–96) | 356 (62) | 161 (28.0) | 200 (34.7) | 89 (19.4) |
| Naar [26] | 2020 | Cohort | USA | 206 | 60 (47–71) | 134 (65.1) | 148 (71.8) | / | / |
| Naarayan [27] | 2020 | Cohort | USA | 370 | 71 (59–82) | 207 (55.9) | 182 (49.1) | 150 (40.5) | / |
| Nakeshbandi [28] | 2020 | Cohort | USA | 504 | 68 (15) | 263 (52) | 95 (19) | 219 (43) | / |
| Pei [29] | 2020 | Cohort | China | 333 | 56.3 (13.4) | 182 (54.7) | 35 (10.5) | 29 (8.7) | 6 (1.8) |
| Pelayo [30] | 2020 | Cross Sectional | USA | 223 | 70.30 (12.80) | 115 (51.56) | 110 (49.3) | 44 (19.73) | 3 (1.3) |
| Russo [31] | 2020 | Cohort | Italy | 777 | 70 (16) | 458 (59) | 176 (22.65) | 273 (35.13) | 21 (2.7) |
| Soleimani [32] | 2020 | Cohort | Iran | 254 | 66.4 (12.9) | 149 (58.7) | 49 (19.3) | 68 (26.8) | / |
| Taher [33] | 2020 | Cohort | Asia | 73 | 54.3 (13.5) | 44 (60.3) | 29 (39.7) | 13 (17.8) | 7 (9.6) |
| Vee [34] | 2020 | Cross Sectional | Malaysia | 247 | 28 (18–35) | 172 (69.6) | 16 (6.5) | / | 7 (2.83) |
| Wang [35] | 2020 | Cohort | China | 116 | 62 (55–69) | 62 (53.4) | 12 (10.34) | / | / |
| Wu [36] | 2020 | Cohort | China | 1048 | 62.5 (48–77) | 591 (56.34) | 19 (1.8) | 52 (4.96) | 21 (2.00) |
| Xia [37] | 2020 | Cohort | China | 81 | 66.6 (11.4) | 54 (66.7) | 41 (50.6) | 60 (70.4) | 8 (9.9) |
| Yan [38] | 2020 | Cohort | China | 882 | 71 (68–77) | 440 (49.9) | 115 (13.0) | 128 (14.5) | 7 (1.9) |
| Zahid [39] | 2020 | Cohort | USA | 469 | 66 (55–75) | 268 (57.14) | 128 (27.29) | 188 (40.09) | / |
3.2. Data Synthesis
3.2.1. Patient’s Sex
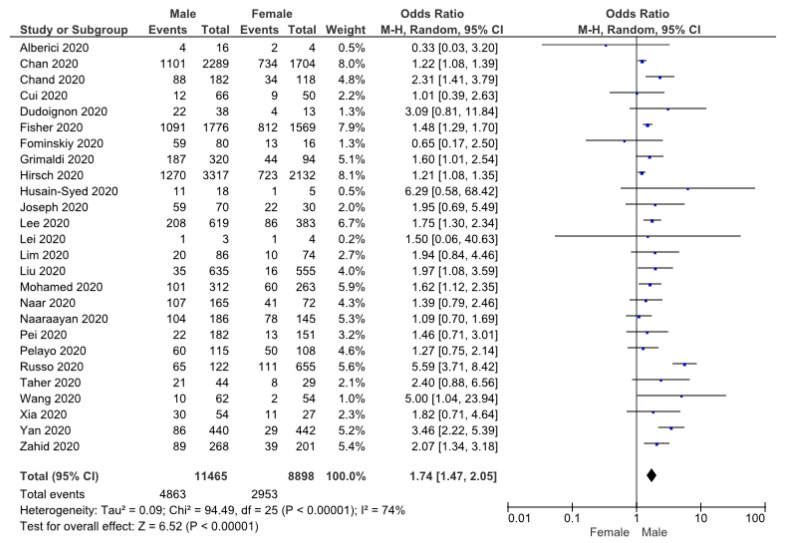
Twenty-six studies of 20,363 patients reported the relationship between male gender and AKI in patients with COVID-19. The results obtained are shown in Figure 2. Due to the high level of heterogeneity, the random-effects model was selected. The meta-analysis demonstrated that male gender was associated with a higher risk of AKI (OR: 1.74 (1.47, 2.05), p < 0.05).
Figure 2.
Forrest plot showing risk of AKI in COVID-19 male patients [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,29,30,31,33,35,37,38,39].
3.2.2. Patient’s Comorbidity
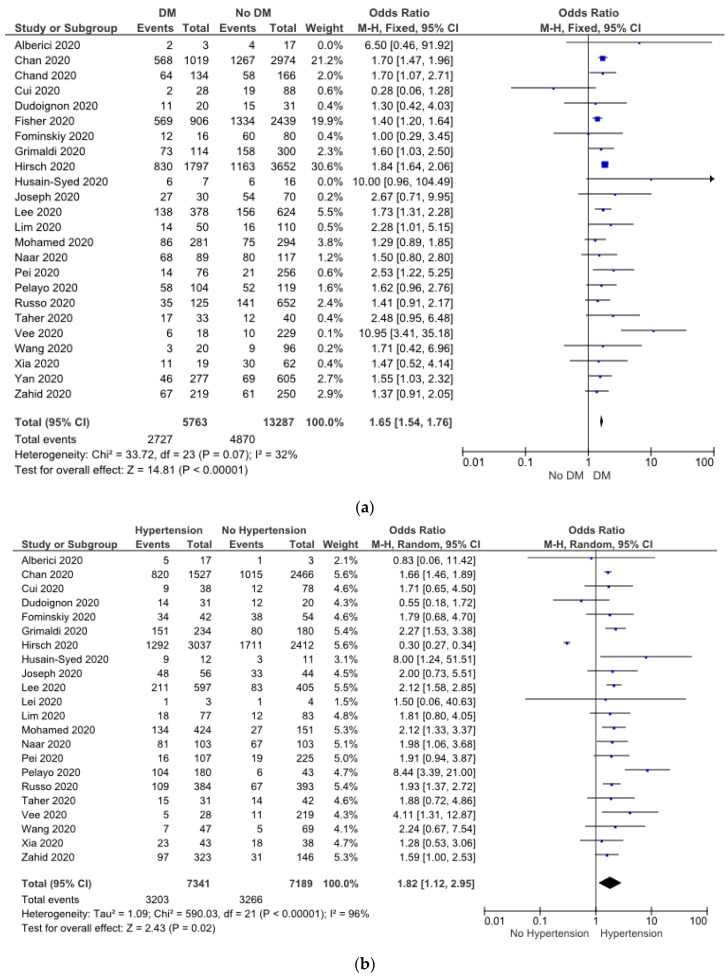
A meta-analysis was performed to investigate the presence of comorbidities potentially associated with the risk of AKI. A total of 24 studies evaluated the role of diabetes, and 22 studies evaluated the role of hypertension in patients with COVID-19 and AKI. The heterogeneity was high in hypertension data; hence, the random-effects model was applied for these studies. Conversely, the fixed-benefit model was used in terms of diabetes due to its low heterogeneity. The meta-analysis showed that COVID-19 patients with diabetes (OR: 1.65 (1.54, 1.76), p < 0.05) (Figure 3a) and hypertension (OR: 1.82 (1.12, 2.95), p < 0.05) (Figure 3b) had a higher risk of AKI.
Figure 3.
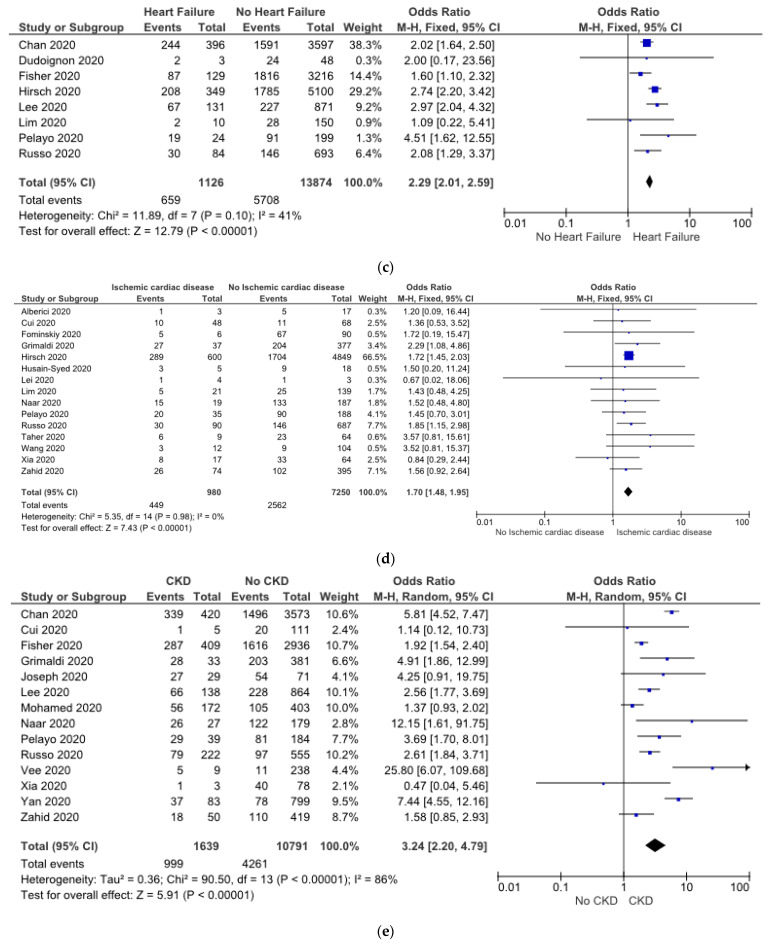
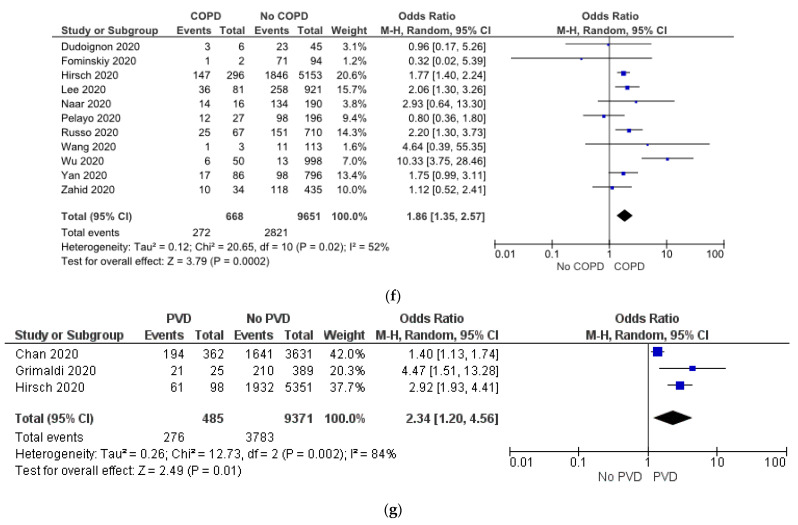
Forrest plot showing association between COVID-19 patients’ comorbidities with risk of AKI. Diabetes (a), hypertension (b), heart failure (c), ischemic cardiac disease (d), CKD (e), COPD (f), and peripheral vascular disease (g) [10,11,12,13,14,15,16,17,18,19,20,21,22,23,25,26,29,30,31,33,34,35,37,38,39].
The meta-analysis revealed that heart failure (OR: 2.29 (2.01, 2.59), p < 0.05) (Figure 3c), ischemic cardiac disease (OR: 1.70 (1.48, 1.95), p < 0.05) (Figure 3d), and CKD (OR: 3.24 (2.20, 4.79), p < 0.05) (Figure 3e) were associated with AKI event in COVID-19. No significant heterogeneity was observed in terms of ischemic cardiac disease, whereas the heart failure data showed low heterogeneity. Therefore, the fixed-effect pattern was chosen among these studies. In addition, the random-effect model was used in CKD, because the heterogeneity was high.
Eleven studies consisting of 10,319 patients showed a relationship between COPD and COVID-19 patients with AKI. Moreover, three studies reported that peripheral vascular disease was associated with AKI in COVID-19 patients. The random-effect model was used for meta-analysis because I2 was >50%. The result finds that patients with COPD (OR: 1.86 (1.35, 2.57), p < 0.05) (Figure 3f) and peripheral vascular disease (OR: 2.34 (1.20, 4.56), p < 0.05) (Figure 3g) were at higher risk of AKI.
3.2.3. History Use of NSAID
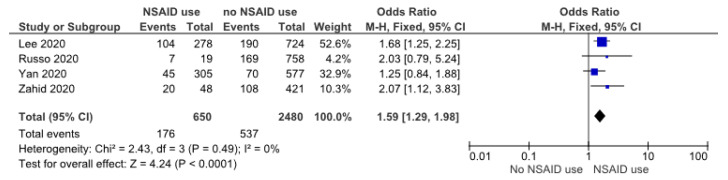
Four studies evaluated the role of the chronic use of NSAIDs in COVID-19 patients with AKI. The data had low heterogeneity; hence, the fixed-effect model was applied. The meta-analysis revealed that the chronic use of this drug was a risk factor for AKI in COVID-19 (OR: 1.59 (1.29, 1.98), p < 0.05) (Figure 4).
Figure 4.
Forrest plot showing risk of AKI in COVID-19 with NSAID use [21,31,38,39].
3.2.4. Mechanical Ventilation Usage
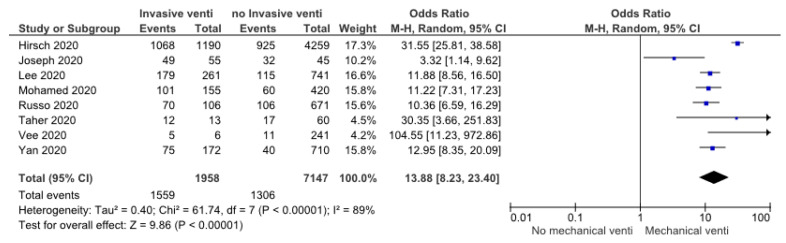
A total of 9105 patients included in eight studies reported the relationship between mechanical ventilation use and AKI in 2019-nCoV disease. The heterogeneity test showed high heterogeneity, so we chose the random-effect model. The meta-analysis result revealed that the use of mechanical ventilation was associated with AKI events in COVID-19 patients (OR: 13.88 (8.23, 23.40), p < 0.05) (Figure 5).
Figure 5.
Forrest plot showing risk of AKI in COVID-19 with mechanical ventilation usage [18,20,21,25,31,33,34,38].
3.2.5. Proteinuria and Hematuria
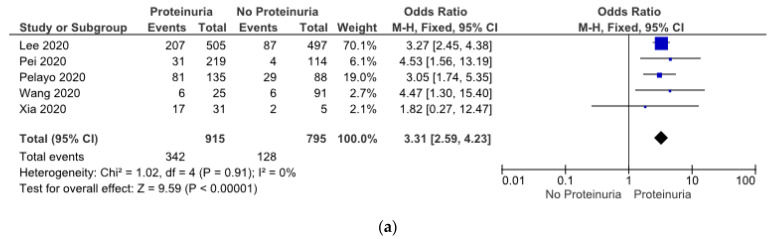
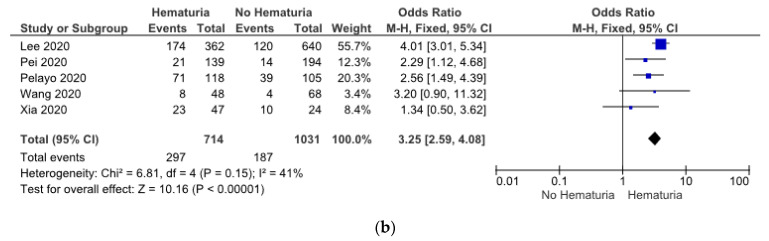
The meta-analysis results found that AKI patients presented with proteinuria (OR: 3.31 (2.59, 4.23), p < 0.05) (Figure 6a) and hematuria (OR: 3.25 (2.59, 4.08), p < 0.05) (Figure 6b). We used a fixed-benefit model for these two parameters, because both of them had low heterogeneity.
Figure 6.
(a) Showing risk of AKI in COVID-19 patients with proteinuria, (b) showing risk of AKI in COVID-19 patients with hematuria [21,29,30,35,37].
Aside from the result, several variables did not show any relationship with acute kidney injury in SARS-CoV-2 infection in a meta-analysis. These factors were advanced age (≥65 years old), obesity, smoking, cerebrovascular disease, cancer, chronic liver disease, HIV, and chronic use of aspirin (Figure S1). In addition, we evaluated the published bias for the included studies by funnel plot. The result showed that most of the studies showed no publication bias, implying that the data have been published with both positive and negative results.
4. Discussion
There are several risk factors that can put COVID-19 patients at higher risk of AKI. Our meta-analysis result found that AKI was associated with male gender, diabetes, and hypertension. Males were at higher risk, probably due to higher angiotensin-converting enzyme 2 (ACE2) expression and a less intense immune response [40,41]. Higher estrogen levels in females have a protective effect on the vascular endothelium [24]. The study involving 355 subjects showed that comorbidity with diabetes is an important independent risk factor predicting AKI among COVID-19 patients [42]. Besides, a study reported that hypertension patients were more likely to have inflammatory reactions, organ and tissue damage, and deterioration of the disease [43]. The relationship between diabetes and oxidative stress, atherosclerosis, and endothelial cell dysfunction has been established, as has the association between hypertension and those [44]. These might support the possible mechanism of AKI due to transient renal hypoperfusion and delayed recovery from ischemia–reperfusion injury [45]. Hypertension-mediated organ damage (HMOD) is defined as the structural or functional alteration of the arterial vasculature and/or the organs it supplies that is caused by elevated blood pressure, and end organs include the kidneys [46]. Since these studies were conducted in various countries, our findings may represent the global population. Therefore, we should increase awareness of AKI among male patients with diabetes and hypertension.
Patients with ischemic cardiac disease and heart failure showed signs of subclinical left ventricular impairment or reduced ejection fraction (EF) in severe cases, which may lead to renal hypoperfusion [8]. A meta-analysis including a total of 341 patients revealed that the standardized mean difference of cardiac troponin I (cTnI) levels was higher in those with severe COVID-19, suggesting a direct cardiac attack [47]. Our findings showed that AKI was more prevalent in COVID-19 patients with ischemic cardiac disease and heart failure. However, further studies are needed to determine whether this finding is secondary to COVID-19′s direct cardiac involvement, sepsis, or a proinflammatory state.
Compared with COVID-19 patients with other underlying conditions, those with CKD were admitted to the ICU 12 times more frequently. Moreover, they also had a 9-fold increase in hospital admissions [48]. The risk of AKI in patients with kidney disease ranged from 1.9- to 4.4-fold higher depending on AKI severity [49]. The study design did not distinguish between pre-existing CKD and COVID-19-associated kidney injury. Our results found that patients with CKD were about three times more at risk of AKI progressing. CKD causes marked alterations in the immune system, such as persistent systemic inflammation and acquired immunosuppression, which may lead to clinical deterioration, including AKI [50,51]. Nevertheless, the actual impact of COVID-19 on kidney structure and function in patients with chronic kidney disease still requires further investigation.
We also find that COPD is related to AKI in COVID-19. Viral infections in COPD patients exacerbate the systemic inflammation and slow the recovery of reported symptoms [52,53]. We suggest that COVID-19 in patients with COPD perhaps may have a different inflammatory profile and should be considered distinct, and might contribute to AKI events. Moreover, the meta-analysis also revealed that peripheral vascular disease was associated with AKI in COVID-19. SARS-CoV-2 infection may be directly responsible for peripheral vascular disease, including venous and arterial thrombosis [54]. Global inflammatory response and endothelial damage were the proposed mechanisms; moreover, ACE2 expression was also abundant in the endothelial layer [55].
COVID-19 patients with a history of chronic use of NSAIDs were at higher risk of AKI. The prevalence of NSAID users in COVID-19 patients with AKI was revealed to be up to two times higher than that of non-AKI patients [31,39]. NSAID-induced AKI is associated with hypoperfusion due to vasoconstriction and acute interstitial nephritis (AIN) and has been found to be the major cause of drug-induced AKI. In 15% of all patients with unexplained AKI, the pathogenesis was due to AIN [56]. Several studies confirmed that discontinuation of NSAIDs proved beneficial especially in people whose risk of renal adverse effects is high [57,58]. However, we could not determine the exact etiology of AKI in COVID-19 patients with NSAIDs, whether due to NSAID-induced hypoperfusion or AIN.
As many as 40% of patients admitted to hospitals with COVID-19 have AKI. Accordingly, it commonly presents with dipstick-positive hematuria and mild proteinuria [18]. Hematuria was present in 48% of patients, and proteinuria has been seen in up to 60% of 1002 patients [21]. In another study, hematuria was present in 65%, and proteinuria was present in 77% of COVID-19 patients admitted for acute renal injury. A case series of kidney biopsies on 17 COVID-19 patients had previously been conducted, with AKI and heavy proteinuria being the most common indications for a biopsy. Among those with podocytopathy on kidney biopsy, 71% of patients demonstrated proteinuria [59]. Proteinuria is an indicator of the glomerular capillary wall alteration and its permeability and is thus a useful biomarker of the severity of the glomerular damage [60]. The pathophysiology of COVID-19-associated proteinuria and hematuria could be related to unspecific mechanisms but also to COVID-specific mechanisms, such as direct cellular injury resulting from podocyte damage or podocytopathy [61]. Proinflammatory cytokines conspire to elicit from endothelial cells a change from their homeostatic functions to those that can contribute to thrombosis and local tissue injury. Cytokines such as interleukin (IL)-1a and IL-1b, IL-6, and tumor necrosis factor (TNF)-alpha, among others, contribute critically to normal host defense, but when produced in excess, they can perturb all of the protective functions of the normal endothelium and potentiate pathological processes. The untrammeled production of proinflammatory cytokines contributes to a cytokine storm [62]. Viral injury to podocytes and renin–angiotensin–aldosterone system (RAAS) activation may have contributed to proteinuria. The accumulation of angiotensin II may be responsible for nephron endocytosis and increased glomerular permeability with proteinuria [63]. In our findings, either proteinuria or hematuria showed an independent association with AKI in COVID-19, although more direct investigations would be required to clarify this issue.
Recent investigations involving 81 COVID-19 patients admitted to the ICU discovered that 66 (44%) patients had AKI, with 44% of those having stage III AKI. All patients with AKI stage III had RRT. However, all patients who received RRT and survived their illness eventually fully recovered renal function and returned to their baseline levels [64]. This study revealed that AKI and RRT therapy are frequent in critically ill patients with COVID-19. Those receiving RRT may have a low risk of developing chronic kidney disease. Other meta-analyses, which involved 2401 patients in 15 articles, revealed that the most common complications of COVID-19 were acute respiratory distress syndrome (ARDS) (OR: 100.36 (64.44–156.32), p < 0.05) and shock (OR: 96.60 (23.80–392.14), p < 0.05) [65]. Among our findings on risk factors and comorbidities of AKI in COVID-19, we found that patients requiring mechanical ventilation support carried the highest risk (OR: 13.88 (8.23, 23.40), p < 0.05). These findings suggest that pre-renal factors, including hemodynamic instability, play a major role in AKI pathogenesis. Based on the findings of our meta-analysis, we suggest that the pathogenesis of acute kidney injury in COVID-19 is complex and multifactorial.
Although SARS-CoV-2 mainly targets the respiratory system, renal involvement, particularly AKI, was also discovered in COVID-19 patients. We found that in a large cohort of hospitalized patients at both tertiary and community hospitals, the rates of AKI were higher than those reported in previous studies. AKI has been reported in up to 35%–50% of COVID-19 patients [15,18]. The key mechanisms of acute kidney injury in COVID-19 remain unclear, whether direct kidney attack via ACE2, secondary to a global inflammatory state, hemodynamic instability, or concomitant nephrotoxic medication. Understanding the exact mechanism will have therapeutic implications. A recent study reported that SARS-CoV-2 NP antigen was accumulated in kidney tubules with severe acute tubular necrosis, but without evidence of glomerular pathology or tubule-interstitial lymphocyte infiltration. Recent human tissue RNA sequencing data have demonstrated that ACE2 expression in the kidney tubules is nearly 100-fold higher than in the lungs, suggesting the kidneys as a potential direct organ target [66]. It was known that ACE2 receptors are the major binding site for SARS-CoV-2 in host cells [67,68]. In contrast, a study in China also reported that they did not identify SARS-CoV-2 in any of the 72 urine samples using polymerase chain reaction [69]. There is no official treatment for COVID-19. Generally, the treatment for COVID-19 consists of antiviral, antibacterial, immunomodulatory, and anti-inflammatory. Each therapy has a different mechanism of action, and some drugs have nephrotoxic effects [70,71]. Chloroquine and hydroxychloroquine require special attention especially in patients with kidney dysfunction, because of their renal excretion [72]. Antiviral drugs, namely, lopinavir, ritonavir, and remdesivir, also have the potential for kidney injury [70,71]. There were several cases of renal injury in remdesivir users [73]. In addition to antivirals, intravenous immunoglobulin has a risk of proximal tubular injury [70,71].
Our meta-analysis has several limitations. COVID-19 is a new disease; given the rapid continuous expansion of the COVID-19 literature, many cohorts had relatively short follow-up periods and limitations in their descriptions of details, and there are new cohorts being reported continuously. It should be noted that the majority of studies did not clearly classify the data based on COVID-19 severity. The classification of severity is also different between studies. Hence, we cannot provide a subanalysis based on disease severity. Moreover, some articles included only severe cases. This might result in an overestimation of risk factors due to the inclusion of predominantly severe cases. For this reason, the gross variation of AKI incidence among studies and its association with disease severity cannot be equally compared. In addition, more information, particularly regarding risk factors and comorbidities, was not available to extract. We could not conduct subgroup analysis according to AKI grade, RRT requirement, or other outcomes due to the limited number of data points. Many studies also did not briefly compare risk factors and our desired outcome. The differences in study design, baseline characteristics, and standard laboratory measurements could result in unequal representation. The screening and diagnostic protocol of COVID-19 might also differ in each center due to limited resources. Additionally, baseline values and the sensitivity and specificity of a laboratory test differ across studies, which may affect the outcome. This might affect the number of populations in the included studies, resulting in a less representative analysis. Shock also has a great impact on the development of AKI, either with or without a cytokine storm. Since the original studies did not provide the number of shock patients in both AKI and non-AKI groups, we cannot analyze the direct association between them.
5. Conclusions
AKI carries high morbidity and mortality in COVID-19. There are several risk factors that can put COVID-19 patients at higher risk of AKI, including male gender, diabetes, hypertension, ischemic cardiac disease, heart failure, CKD, COPD, peripheral vascular disease, and history of use of NSAIDs. These factors may have an association with AKI in non-COVID patients as well. Proteinuria, hematuria, and the use of mechanical ventilation are frequently found in AKI patients with SARS-CoV-2 infection, suggesting that these are important characteristics of AKI. The results of several variables might be less representative due to the small sample size of the included studies and the fact that they were conducted in a few countries. Therefore, further investigation is still recommended. Future observational multicenter studies based on the etiology of AKI in COVID-19 patients are required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathophysiology30020020/s1, Figure S1. Forrest plot showing association between risk of AKI in patients with advanced age (≥65 years old) (a), obesity (b), smokers (c), cerebrovascular disease (d), cancer (e), chronic liver disease (f), HIV (g), and chronic use of aspirin (h).
Author Contributions
Conceptualization: A.A.H., V.A.G. and A.T.; data curation: A.A.H., V.A.G., F.R.I., R.A. and A.T.; formal analysis: A.A.H., V.A.G. and F.R.I.; methodology: A.A.H., R.A., D.M.H. and M.T.; writing—original draft: A.A.H., V.A.G., F.R.I. and R.A.; investigation: F.R.I., R.A. and D.M.H.; project administration: D.M.H.; validation: A.T. and M.T.; supervision: A.T. and M.T.; writing—review and editing: D.M.H., A.T. and M.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. In Proceedings of the WHO Director General’s Speeches, 4 March 2020. [(accessed on 20 October 2020)]. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometers COVID-19 Coronavirus Pandemic. [(accessed on 29 October 2020)]. Available online: https://www.worldometers.info/coronavirus/
- 4.Cheruiyot I., Henry B., Lippi G., Kipkorir V., Ngure B., Munguti J., Misiani M. Acute Kidney Injury is Associated with Worse Prognosis In COVID-19 Patients: A Systematic Review and Meta-analysis. Acta Biomed. 2020;91:e2020029. doi: 10.23750/abm.v91i3.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical Course and outcomes of critically ill patients with COVID-19 in Wuhan China. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta S., Chauhan K., Patel A., Patel S., Pinotti R., Nadkarni G.N., Parikh C.R., Coca S.G. The prognostic importance of duration of AKI: A systematic review and meta-analysis. BMC Nephrol. 2018;19:91. doi: 10.1186/s12882-018-0876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y., Sun L.J., Xu M., Pan J., Zhang Y.T., Fang X.L., Fang Q., Cai H.L. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J. Zhejiang Univ. Sci. B. 2020;21:378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA-J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron-Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 10.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A., Maffei C., Possenti S., Zambetti N., Moscato M., et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan L., Chaudhary K., Saha A., Chauhan K., Vaid A., Zhao S., Paranjpe I., Somani S., Richter F., Miotto R., et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2020;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chand S., Kapoor S., Orsi D., Fazzari M.J., Tanner T.G., Umeh G.C., Islam M., Dicpinigaitis P.V. COVID-19-Associated Critical Illness—Report of the First 300 Patients Admitted to Intensive Care Units at a New York City Medical Center. J. Intensive Care Med. 2020;35:963–970. doi: 10.1177/0885066620946692. [DOI] [PubMed] [Google Scholar]
- 13.Cui X., Yu X., Wu X., Huang L., Tian Y., Huang X., Zhang Z., Cheng Z., Guo Q., Zhang Y., et al. Acute Kidney Injury in Patients with the Coronavirus Disease 2019: A Multicenter Study. Kidney Blood Press. Res. 2020;45:612–622. doi: 10.1159/000509517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudoignon E., Moreno N., Deniau B., Coutrot M., Longer R., Amiot Q., Mebazaa A., Pirracchio R., Depret F., Legrand M. Activation of the renin-angiotensin-aldosterone system is associated with Acute Kidney Injury in COVID-19. Anaesth. Crit. Care Pain Med. 2020;39:453–455. doi: 10.1016/j.accpm.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher M., Neugarten J., Bellin E., Yunes M., Stahl L., Johns T.S., Abramowitz M.K., Levy R., Kumar N., Mokrzycki M.H., et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J. Am. Soc. Nephrol. 2020;31:2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fominskiy E.V., Scandroglio A.M., Monti G., Calabrò M.G., Landoni G., Dell’Acqua A., Beretta L., Moizo E., Ravizza A., Monaco F., et al. Prevalence, Characteristics, Risk Factors, and Outcomes of Invasively Ventilated COVID-19 Patients with Acute Kidney Injury and Renal Replacement Therapy. Blood Purif. 2020;50:102–109. doi: 10.1159/000508657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaldi D., Aissaoui N., Blonz G., Carbutti G., Courcelle R., Gaudry S., Gaultier A., D’hondt A., Higny J., Horlait G., et al. Characteristics and outcomes of acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviral strategies: The COVADIS multicentre observational study. Ann. Intensive Care. 2020;10:131. doi: 10.1186/s13613-020-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D., Northwell COVID-19 Research Consortium, & Northwell Nephrology COVID-19 Research Consortium Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain-Syed F., Wilhelm J., Kassoumeh S., Birk H.W., Herold S., Vadász I., Walmrath H.D., Kellum J.A., Ronco C., Seeger W. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol. Dial. Transplant. 2020;35:1271–1274. doi: 10.1093/ndt/gfaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph A., Zafrani L., Mabrouki A., Azoulay E., Darmon M. Acute kidney injury in patients with SARS-CoV-2 infection. Ann. Intensive Care. 2020;10:117. doi: 10.1186/s13613-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J.R., Silberzweig J., Akchurin O., Choi M.E., Srivatana V., Lin J., Liu F., Malha L., Lubetzky M., Dadhania D.M., et al. Characteristics of Acute Kidney Injury in Hospitalized COVID-19 Patients in an Urban Academic Medical Center. Clin. J. Am. Soc. Nephrol. 2021;16:284–286. doi: 10.2215/CJN.07440520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W., Zhan L.Y., Jia Y., Zhang L., Liu D., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim J.-H., Park S.H., Jeon Y., Cho J.H., Jung H.Y., Choi J.Y., Kim C.D., Lee Y.H., Seo H., Lee J., et al. Fatal Outcomes of COVID-19 in Patients with Severe Acute Kidney Injury. J. Clin. Med. 2020;9:1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Zhang L., Chen Y., Wu Z., Dong X., Teboul J.L., Zhang S., Ye X., Liu Y., Wang T., et al. Association of sex with clinical outcomes in COVID-19 patients: A retrospective analysis of 1190 cases. Respir. Med. 2020;173:106159. doi: 10.1016/j.rmed.2020.106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed M.M.B., Lukitsch I., Torres-Ortiz A.E., Walker J.B., Varghese V., Hernandez-Arroyo C.F., Alqudsi M., LeDoux J.R., Velez J.C.Q. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney360. 2020;1:614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naar L., Langeveld K., El Moheb M., El Hechi M.W., Alser O., Kapoen C., Breen K., Christensen M.A., Mokhtari A., Gaitanidis A., et al. Acute Kidney Injury in Critically-ill Patients with COVID-19: A Single-center Experience of 206 Consecutive Patients. Ann. Surg. 2020;272:e280–e281. doi: 10.1097/SLA.0000000000004319. [DOI] [PubMed] [Google Scholar]
- 27.Naaraayan A., Nimkar A., Hasan A., Pant S., Durdevic M., Elenius H., Nava Suarez C., Jesmajian S. Analysis of Male Sex as a Risk Factor in Older Adults With Coronavirus Disease 2019: A Retrospective Cohort Study From the New York City Metropolitan Region. Cureus. 2020;12:e9912. doi: 10.7759/cureus.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakeshbandi M., Maini R., Daniel P., Rosengarten S., Parmar P., Wilson C., Kim J.M., Oommen A., Mecklenburg M., Salvani J., et al. The impact of obesity on COVID-19 complications: A retrospective cohort study. Int. J. Obes. 2020;44:1832–1837. doi: 10.1038/s41366-020-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei G., Zhang Z., Peng J., Liu L., Zhang C., Yu C., Ma Z., Huang Y., Liu W., Yao Y., et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelayo J., Lo K.B., Bhargav R., Gul F., Peterson E., DeJoy Iii R., Salacup G.F., Albano J., Gopalakrishnan A., Azmaiparashvili Z., et al. Clinical Characteristics and Outcomes of Community-And Hospital-Acquired Acute Kidney Injury with COVID-19 in a US Inner City Hospital System. CardioRenal Med. 2020;10:223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo E., Esposito P., Taramasso L., Magnasco L., Saio M., Briano F., Russo C., Dettori S., Vena A., Di Biagio A., et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J. Nephrol. 2020;34:173–183. doi: 10.1007/s40620-020-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soleimani A., Kazemian S., Karbalai Saleh S., Aminorroaya A., Shajari Z., Hadadi A., Talebpour M., Sadeghian H., Payandemehr P., Sotoodehnia M., et al. Effects of angiotensin receptor blockers (ARBs) on in-hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID-19. Am. J. Hypertens. 2020;33:1102–1111. doi: 10.1093/ajh/hpaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taher A., Alalwan A.A., Naser N., Alsegai O., Alaradi A. Acute Kidney Injury in COVID-19 Pneumonia: A Single-Center Experience in Bahrain. Cureus. 2020;12:e9693. doi: 10.7759/cureus.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soh T.V., Dzawani M., Noorlina N., Nik F., Norazmi A. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 (Sars-cov2) patients in hospital tengku ampuan afzan. Med. J. Malaysia. 2020;75:479–484. [PubMed] [Google Scholar]
- 35.Wang J., Wang Z., Zhu Y., Li H., Yuan X., Wang X., Wang Y., Hu J., Feng C., Liu C., et al. Identify the Risk Factors of COVID-19-Related Acute Kidney Injury: A Single-Center, Retrospective Cohort Study. Front. Med. 2020;7:436. doi: 10.3389/fmed.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu F., Zhou Y., Wang Z., Xie M., Shi Z., Tang Z., Li X., Li X., Lei C., Li Y., et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: A multicenter, retrospective, observational study. J. Thorac. Dis. 2020;12:1811–1823. doi: 10.21037/jtd-20-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia P., Wen Y., Duan Y., Su H., Cao W., Xiao M., Ma J., Zhou Y., Chen G., Jiang W., et al. Clinicopathological Features and Outcomes of Acute Kidney Injury in Critically Ill COVID-19 with Prolonged Disease Course: A Retrospective Cohort. J. Am. Soc. Nephrol. 2020;31:2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Q., Zuo P., Cheng L., Li Y., Song K., Chen Y., Dai Y., Yang Y., Zhou L., Yu W., et al. Acute Kidney Injury Is Associated With In-hospital Mortality in Older Patients With COVID-19. J. Gerontol. Ser. 2020;76:456–462. doi: 10.1093/gerona/glaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahid U., Ramachandran P., Spitalewitz S., Alasadi L., Chakraborti A., Azhar M., Mikhalina G., Sherazi A., Narh J.T., Khattar P., et al. Acute Kidney Injury in COVID-19 Patients: An Inner City Hospital Experience and Policy Implications. Am. J. Nephrol. 2020;51:786–796. doi: 10.1159/000511160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. N. Engl. J. Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Bwire G.M. Coronavirus: Why Men are More Vulnerable to COVID-19 Than Women? SN Compr. Clin. Med. 2020;2:874–876. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S., Fu L., Fei J., Xiang H., Xiang Y., Tan Z., Li M., Liu F., Li Y., Han M., et al. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: A hospital-based retrospective analysis. Medrxiv. 2020 doi: 10.1101/2020.03.24.20042408. [DOI] [Google Scholar]
- 43.Huang S., Wang J., Liu F., Liu J., Cao G., Yang C., Liu W., Tu C., Zhu M., Xiong B. COVID-19 patients with hypertension have more severe disease: A multicenter retrospective observational study. Hypertens. Res. 2020;43:824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma R., Davidoff M.N. Oxidative stress and endothelial dysfunction in heart failure. Congest. Heart Fail. 2002;8:165–172. doi: 10.1111/j.1527-5299.2002.00714.x. [DOI] [PubMed] [Google Scholar]
- 45.Patschan D., Müller G.A. Acute Kidney Injury in Diabetes Mellitus. Int. J. Nephrol. 2016;2016:6232909. doi: 10.1155/2016/6232909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger T., Borghi C., Charchar F., Khan N.A., Poulter N.R., Prabhakaran D., Ramirez A., Schlaich M., Stergiou G.S., Tomaszewski M., et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 47.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CDC COVID-19 Response Team Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, February 12-March 28, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thaha M., Widiana I.G.R. The Role of Inflammation in Chronic Kidney Disease. Ina Kidney. 2019;2:4–13. doi: 10.32867/inakidney.v2i3.33. [DOI] [Google Scholar]
- 51.Vaziri N.D. CKD impairs barrier function and alters microbial flora of the intestine: A major link to inflammation and uremic toxicity Nosratola. Curr. Opin. Nephrol Hypertens. 2012;21:587–592. doi: 10.1097/MNH.0b013e328358c8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aaron S.D., Donaldson G.C., Whitmore A.G., Hurst J.R., Ramsay T., Wedzicha A.J. Time course and pattern of COPD exacerbation onset. Thorax. 2012;67:238–243. doi: 10.1136/thoraxjnl-2011-200768. [DOI] [PubMed] [Google Scholar]
- 53.Williams N.P., Ostridge K., Devaster J.M., Kim V., Coombs N.A., Bourne S., Clarke S.C., Harden S., Abbas A., Aris E., et al. Impact of radiologically stratified exacerbations: Insights into pneumonia aetiology in COPD. Respir. Res. 2018;19:143. doi: 10.1186/s12931-018-0842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dixit M., Doan T., Kirschner R., Dixit N. Significant Acute Kidney Injury Due to Non-steroidal Anti-inflammatory Drugs: Inpatient Setting. Pharmaceuticals. 2010;3:1279–1285. doi: 10.3390/ph3041279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones M., Tomson C. Acute kidney injury and ‘nephrotoxins’: Mind your language. Clin. Med. J. R. Coll. Physicians Lond. 2018;18:384–386. doi: 10.7861/clinmedicine.18-5-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X., Donnan P.T., Bell S., Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrology. 2017;18:256. doi: 10.1186/s12882-017-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akilesh S., Nast C.C., Yamashita M., Henriksen K., Charu V., Troxell M.L., Kambham N., Bracamonte E., Houghton D., Ahmed N.I., et al. Multicenter Clinicopathologic Correlation of Kidney Biopsies Performed in COVID-19 Patients Presenting With Acute Kidney Injury or Proteinuria. Am. J. Kidney Dis. 2021;77:82–93. doi: 10.1053/j.ajkd.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Amico G., Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63:809–825. doi: 10.1046/j.1523-1755.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 61.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Rojas M.A., Vega-Vega O., Bobadilla N.A. Is the kidney a target of SARS-CoV-2? Am. J. Physiol. Renal Physiol. 2020;318:F1454–F1462. doi: 10.1152/ajprenal.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lowe R., Ferrari M., Nasim-Mohi M., Jackson A., Beecham R., Veighey K., Cusack R., Richardson D., Grocott M., Levett D., et al. Clinical characteristics and outcome of critically ill COVID-19 patients with acute kidney injury: A single centre cohort study. BMC Nephrol. 2021;22:92. doi: 10.1186/s12882-021-02296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu L., Jiao R., Zhang A., Chen X., Ning Q., Fang F., Zeng F., Tian N., Zhang Y., Huang Y., et al. A Case of Critically Ill Infant of Coronavirus Disease 2019 With Persistent Reduction of T Lymphocytes. Pediatr. Infect. Dis. J. 2020;39:e87–e90. doi: 10.1097/INF.0000000000002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J., Guo T., Dong D., Zhang X., Chen X., Feng Y., Wei B., Zhang W., Zhao M., Wan J. Defining heart disease risk for death in COVID-19 infection. QJM. 2020;113:876–882. doi: 10.1093/qjmed/hcaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magrone T., Magrone M., Jirillo E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin- Converting Enzyme 2 as a Potential Drug Target—A Perspective. Endocr. Metab. Immune Disord. Drug Targets. 2020;20:807–811. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 68.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA–J. Am. Med. Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassanein M., Radhakrishnan Y., Sedor J., Vachharajani T., Vachharajani V.T., Augustine J., Demirjian S., Thomas G. COVID-19 and the kidney. Cleve. Clin. J. Med. 2020;87:619–631. doi: 10.3949/ccjm.87a.20072. [DOI] [PubMed] [Google Scholar]
- 71.Hassanein M., Thomas G., Taliercio J. Management of acute kidney injury in COVID-19. Cleve. Clin. J. Med. 2020:1–3. doi: 10.3949/ccjm.87a.ccc034. [DOI] [PubMed] [Google Scholar]
- 72.Mahmoudi J., Sadigh-Eteghad S., Salehi-Pourmehr H., Gharekhani A., Ziaee M. Nephrotoxicity of Chloroquine and Hydroxychloroquine in COVID-19 Patients. Tabriz Univ. Med. Sci. 2021;7:113–117. doi: 10.34172/apb.2021.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang F., Liang Y. Potential risk of the kidney vulnerable to novel coronavirus 2019 infection. Am. J. Physiology. Ren. Physiol. 2020;318:F1136–F1137. doi: 10.1152/ajprenal.00085.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.