Abstract
Variability in transplant access exists, but barriers to referral and evaluation are underexplored due to lack of national surveillance data. We examined referral for kidney transplantation evaluation and start of the evaluation among 34 857 incident, adult (18–79 years) end-stage kidney disease patients from 690 dialysis facilities in the United States Renal Data System from January 1, 2012 through August 31, 2016, followed through February 2018 and linked data to referral and evaluation data from nine transplant centers in Georgia, North Carolina, and South Carolina. Multivariable-adjusted competing risk analysis examined each outcome. The median within-facility cumulative percentage of patients referred for kidney transplantation within 1 year of dialysis at the 690 dialysis facilities in Network 6 was 33.7% (interquartile range [IQR]: 25.3%−43.1%). Only 48.3% of referred patients started the transplant evaluation within 6 months of referral. In multivariable analyses, factors associated with referral vs evaluation start among those referred at any time differed. For example, black, non-Hispanic patients had a higher rate of referral (hazard ratio [HR]: 1.22; 95% confidence interval [CI]: 1.18–1.27), but lower evaluation start among those referred (HR: 0.93; 95% CI: 0.88–0.98), vs white non-Hispanic patients. Barriers to transplant varied by step, and national surveillance data should be collected on early transplant steps to improve transplant access.
Keywords: clinical research/practice, dialysis, disparities, epidemiology, ethnicity/race, health services and outcomes research, kidney transplantation/nephrology, patient referral
1 |. INTRODUCTION
Relative to dialysis, kidney transplantation is the preferred treatment for the majority of the >700 000 US patients with end-stage kidney disease (ESKD).1 The dialysis facility plays an important role in patient access to kidney transplantation, because the majority of ESKD patients start treatment on dialysis in the United States, with only 9.8% of kidney transplant recipients preemptively transplanted.2 Among the 97% of patients who start on dialysis, most receive care in an in-center hemodialysis facility.1 Variation in standardized transplantation ratios has been reported across both dialysis facilities and geographic regions,3 and the Centers for Medicare and Medicaid Services (CMS) proposed the proportion of prevalent dialysis patients waitlisted as a new quality metric for dialysis facilities in 2018 to address this variation.4 However, waitlisting and transplantation may not be the best metrics to evaluate transplant access at the dialysis facility level because access is affected by transplant center waitlisting practices, center aggressiveness, and organ supply.5 Furthermore, the proposed CMS quality metrics do not take into account the important steps in the transplant process prior to waitlisting, such as educational practices in the dialysis facility, referral from a dialysis facility to a transplant center to undergo evaluation, and start of the transplant evaluation.
Although variation in these important transplant steps have been documented—including dialysis facility education practices,6,7 referral for kidney transplantation in the state of Georgia,5 and geographic variability in waitlisting,8 other than single-center studies, little is known about start of the transplant evaluation process. Single-center studies have documented that financial barriers, perceived knowledge about transplant, and psychosocial factors may play a role in absenteeism at the transplant evaluation following referral.9–12 However, it is unknown if the reasons for variation in waitlisting and transplantation more broadly are due to lower referral, or other causes related to the patient not starting the evaluation process.
As part of our community-based Southeastern Kidney Transplant Coalition, which includes all nine transplant centers in End Stage Renal Disease (ESRD) Network 6 (Georgia, North Carolina, and South Carolina), large dialysis organizations, national advocacy organizations, clinicians, and patients, we developed a voluntary data registry of early transplant steps.13,14 We previously reported that transplant referral varied from 0% to 76% among ~300 dialysis facilities in Georgia and that the factors associated with delayed referral are unique from factors associated with waitlisting. The purpose of this paper is to extend these results by examining referral to transplant centers in three states (Georgia, North Carolina, and South Carolina), and by also examining start of the transplant evaluation—a step that has thus far been unexamined outside of single-center studies. We also sought to describe the dialysis facility- and patient-level factors associated with referral and evaluation start in this region of the country with the lowest rates of kidney transplantation in the nation.3
2. |. METHODS
2.1 |. Data sources
Patient-level referral and evaluation data were collected from transplant referral forms and electronic medical records from all nine adult transplant centers in Georgia, North Carolina, and South Carolina from January 1, 2012 through February 2018, including Augusta University Medical Center (Augusta, GA), Emory Hospital (Atlanta, GA), Piedmont Hospital (Atlanta, GA), Carolinas Medical Center (Charlotte, NC), Duke Transplant Center (Durham, NC), University of North Carolina Hospital (Chapel Hill, NC), Wake Forest Baptist Hospital (Winston Salem, NC), Vidant Medical Center (Greenville, NC), and Medical University of South Carolina (Charleston, SC). Each center uploaded data securely to a submission portal at Network 6, the data coordinating center.
We linked referral and evaluation outcome data to ensure complete follow-up and identify a subpopulation of dialysis patients that were not referred for transplant evaluation to the United States Renal Data System (USRDS) surveillance database (incident ESKD patients between January 1, 2012 and August 31, 2016). Facility-level characteristics (at time of dialysis start) were obtained through the annual USRDS facility survey.
The 2010–2014 American Community Survey data was linked with USRDS data by patients’ residential 5-digit ZIP code at time of dialysis initiation to obtain characteristics of patients’ residential neighborhood, as defined by patient 5-digit ZIP code tabulation area.
This study was approved by the institutional review board at Emory University (IRB00079596).
2.2 |. Study population and exclusion criteria
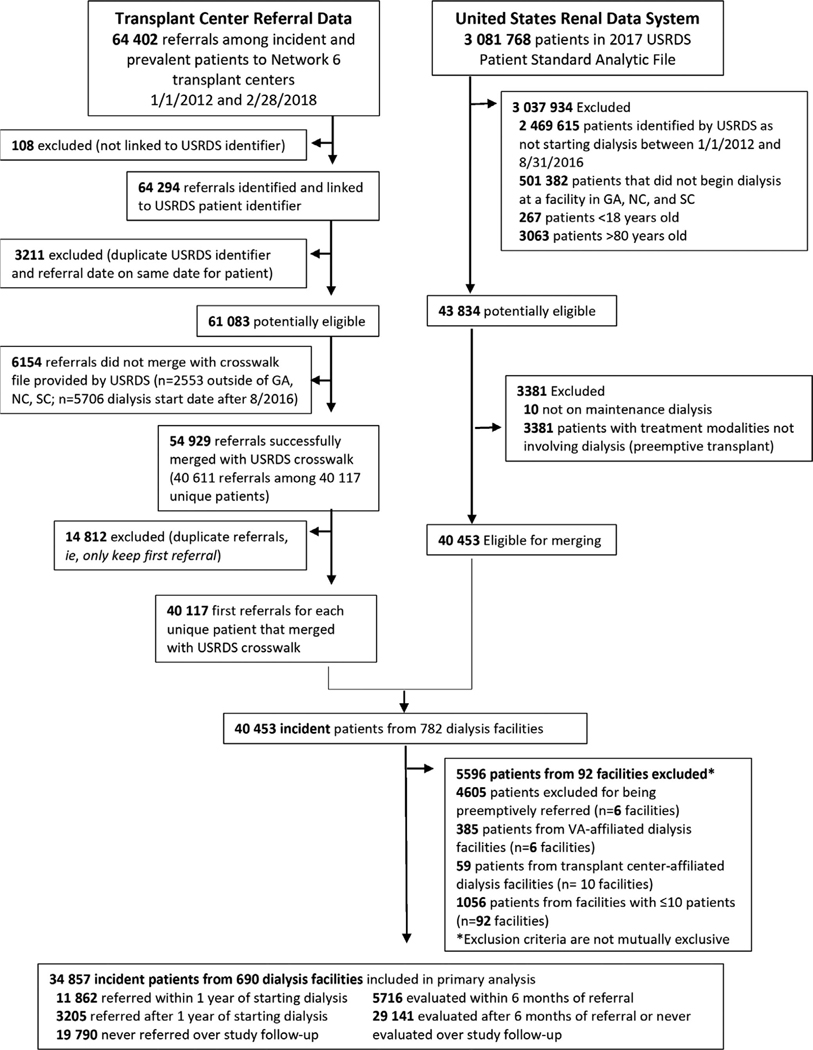
The study cohort included incident ESKD patients (ages 18–79) between January 1, 2012 and August 31, 2016 receiving services in Network 6, identified from the USRDS database, linked with referral and evaluation start outcomes from transplant centers with follow-up data on these outcomes through February 2018. Patients were assigned to the first facility that provided dialysis services. Preemptively referred, evaluated, and waitlisted patients were excluded. We also excluded very small dialysis facilities (treating ≤10 patients per facility) (Figure 1).
FIGURE 1.
Flow diagram of study inclusion and exclusion criteria for study population (2012–2018)
2.3 |. Study variables
This study examines two primary outcomes: referral and evaluation start. Referral date was defined as the date when one of the nine transplant centers in Network 6 received a referral form for a kidney transplant evaluation. Referrals were examined overall (ie, transplant center receipt of the referral form at any time during the study period) as well as referral within 1 year of dialysis start, since dialysis facilities are required to educate ESKD patients about transplant within 60 days of dialysis start. We considered a first referral within 1 year of initiating dialysis as a proxy for access to appropriate care, as in our prior work.5 Although patients can be referred more than once (to the same center or a different center), we restricted the analyses to the first referral event in the study period. Evaluation start was defined as the date when a patient physically initiated a required component of the transplant evaluation. We examined evaluation start overall as well as evaluation within 6 months of the patient’s first referral date among those referred for transplant. Six months was chosen because the median time from referral to evaluation start in prior work was ~3 months; in main analyses we also examined evaluation start at any time (among all those referred). The start of an evaluation was defined by the transplant center and included first visit to the transplant center, visit to a satellite clinic, or attendance at a required transplant education course among those referred.
Patient- and dialysis facility-level characteristics were obtained from the CMS-2728 form and the facility file, respectively, within the USRDS database at the time of ESKD start. Patient characteristics included age, sex, race/ethnicity, attributed cause of ESKD, body mass index (BMI), and comorbidities. Pre-ESKD nephrology care (yes, no, missing), and primary health insurance were also examined. Patient 5-digit ZIP code data from the American Community Survey data were used to calculate neighborhood characteristics, including poverty (percentage of ZIP code below poverty), average percentage black, and average percentage of high school graduates. Dialysis facility-level variables included profit status, facility type (freestanding or not), facility size, and patient to social worker ratio.
2.4 |. Statistical analyses
Dialysis facilities were stratified into tertiles based on the number of referrals aggregated at the facility level, and group differences in facility and aggregated patient characteristics were compared using analysis of variance or chi-square tests. For multivariable patient-level analyses, covariates that were either significant in bivariable analyses or clinically relevant were included. For our main analysis, we examined the time to each event (censoring for death or end of study period) by calculating cause-specific hazard ratios (HR) and respective 95% confidence intervals (CIs). Informative censoring of death was accounted for by incorporating Stabilized Inverse Probability Weighting (SIPW), where the numerator of SIPW was the overall probability of death and the denominator of SIPW was the predicted probability of death for each individual conditioned on the patient’s covariate patterns. A total of 22% of patients had at least one missing covariate. As the missing pattern was arbitrary, we used random forest and predictive mean matching (n = 5) to impute missing values. SAS 9.4 (SAS Institute Inc., Cary, NC) and R software version 3.6.1 was used for data management and analyses. Two-sided P values were calculated and P < .05 was considered statistically significant.
3 |. RESULTS
3.1 |. Study population
There were N = 43 834 adult (18–79 years of age) incident ESKD patients in Georgia, North Carolina, or South Carolina between January 1, 2012 and August 31, 2016; n = 10 were not included in the USRDS annual data report and 3381 patients received a preemptive transplant and were excluded. A total of 40 453 of these patients were merged with 40 117 first referrals from either incident or prevalent ESKD patients in Network 6. Within this merged cohort, 4605 patients were preemptively referred, 385 patients were from Veterans Affairs-affiliated facilities, 59 patients were affiliated with transplant centers (and were not on maintenance dialysis), and 1056 patients were from facilities with ≤10 patients and were excluded, leaving a total cohort of 34 857 patients from 690 dialysis facilities in the study population (Figure 1).
3.2 |. Dialysis facility characteristics and early transplant access: referral and evaluation start
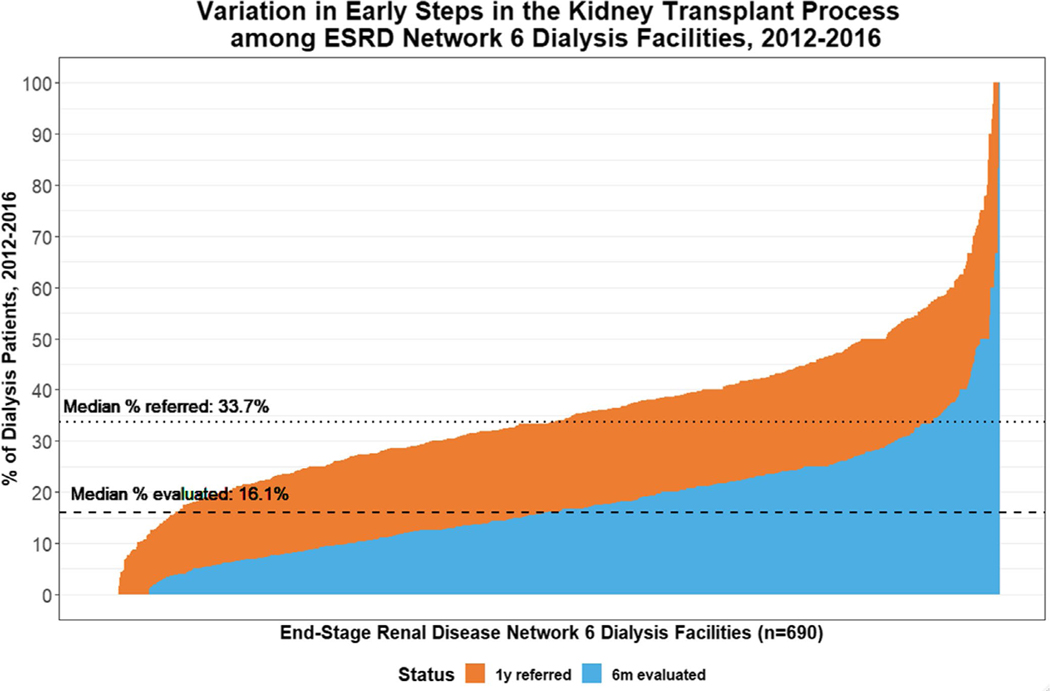
The median within-facility cumulative percentage of patients aged 18–79 referred for kidney transplantation within 1 year of starting dialysis at one of the 690 dialysis facilities in Georgia, North Carolina, and South Carolina dialysis facilities between 2012–2016 was 33.7% (interquartile range [IQR]: 25.3%−43.1%; minimum: 0%; maximum: 100%). The median proportion of patients who started the transplant evaluation within 6 months of referral (among all incident ESKD patients) was 16.1% (95% IQR: 9.5%−23.6%; minimum: 0%, maximum: 100%) (Figure 2). There were 12 facilities with no referrals within 1 year after start of dialysis and 35 facilities with no evaluations within 6 months after referral. Dialysis facility size was not associated with referral or evaluation start (Figure S1).
FIGURE 2.
Percentage of patients referred for kidney transplantation (orange) within 1 y of dialysis start and the percentage of patients who start the evaluation (blue) among incident end-stage kidney disease (ESKD) patients within dialysis facilities in Georgia, North Carolina, and South Carolina: 2012–2016, followed through February 2018 [Color figure can be viewed at wileyonlinelibrary.com]
Among the 690 dialysis facilities examined, 84.5% were for profit, 98.8% were freestanding, and the mean patient to social worker ratio was 80.9 (95% CI: 77.6–84.1) in the facility. Facilities in the lowest vs highest tertile of referral performance had a higher mean age (60.4 [95% CI: 60.0–60.9] vs 58.6 years [95% CI: 58.0–59.1]), fewer mean number of black or African American patients (49.5% [46.3–52.6] vs 59.1% [95% CI: 56.0–62.3]), fewer patients with hypertension as attributed cause of ESKD (34.3% [95% CI: 32.1–36.5] vs 40.1% [95% CI: 37.6–42.6]), and significantly more comorbidities with the exception of hypertension, diabetes, and obesity. In addition, facilities in the lowest tertile of referral performance had a higher proportion of patients with Medicaid (26.1% [95% CI: 24.3–27.9] vs 22.2% [95% CI: 20.5–23.8]), a lower proportion of black patients in a neighborhood (29.8% [95% CI: 27.5–32.1] vs 40.1% [95% CI: 37.7–42.6]), a lower proportion of patients in a neighborhood who were high school graduates (81.6% [95% CI: 81.0–82.2] vs 83.8% [95% CI: 83.3–84.4]), and a higher proportion of for-profit dialysis facilities (88.5% vs 81.7%), compared to facilities in the highest tertile of referral performance. Higher referral performance was observed among moderate-sized facilities (patients 26–54 patients per facility) compared to other facility sizes, such as 79+ patients (42.6% vs 20.0%, P < .001) (Table 1).
TABLE 1.
Characteristics of dialysis facilities with low, moderate, and high referral for kidney transplantation within 1 y of dialysis start in Georgia, North Carolina, and South Carolina: 2012–2016
| Tertile of transplant referral, median % of patients (range) |
|||||
|---|---|---|---|---|---|
| Selected dialysis facility characteristics | All dialysis facilitiesa | Lowest 21.9 (0–28.6) | Middle 33.9 (28.7–39.9) | Highest 48.3 (39.9–100.0) | P valueb |
| Facilities, n | 690 | 234 | 226 | 230 | |
| Total number of patients, n (%) | 34 857 | 11 871 (34.0) | 12 951 (37.2) | 10 035 (28.8) | |
| Facility incident patient-level characteristics | |||||
| Mean age in years, mean (95% CI) | 59.6 (59.3–59.9) | 60.4 (60.0–60.9) | 59.8 (59.4–60.2) | 58.6 (58.0–59.1) | <.001 |
| Male sex, mean % (95% CI) | 54.9 (54.0–55.7) | 54.7 (53.0–56.3) | 55.5 (54.4–56.6) | 54.5 (53.0–56.1) | .62 |
| Race/ethnicity, mean (95% CI) | |||||
| White, non-Hispanic | 39.3 (37.6–41.1) | 44.2 (41.1–47.3) | 39.2 (36.4–42.1) | 34.5 (31.5–37.5) | <.001 |
| White, Hispanic | 2.0 (1.8–2.3) | 1.7 (1.3–2.1) | 2.1 (1.7–2.4) | 2.4 (1.8–3.0) | .09 |
| Black or African American | 54.6 (52.8–56.4) | 49.5 (46.3–52.6) | 55.1 (52.1–58.1) | 59.1 (56.0–62.3) | <.001 |
| Other race/ethnicity | 1.8 (1.4–2.2) | 1.8 (1.2–2.4) | 1.9 (1.1–2.6) | 1.7 (1.2–2.3) | .96 |
| Attributed cause of ESKD, mean % (95% CI) | |||||
| Diabetes | 43.4 (42.3–44.5) | 43.8 (41.8–45.8) | 44.7 (43.1–46.4) | 41.7 (39.5–44.0) | .10 |
| Hypertension | 36.9 (35.6–38.2) | 34.3 (32.1–36.5) | 36.2 (34.2–38.2) | 40.1 (37.6–42.6) | .001 |
| Glomerulonephritis | 6.1 (5.7–6.5) | 6.6 (5.8–7.4) | 6.1 (5.5–6.8) | 5.6 (4.9–6.4) | .18 |
| Other | 9.2 (8.7–9.7) | 9.9 (9.0–10.8) | 9.2 (8.4–9.9) | 8.6 (7.5–9.6) | .11 |
| Facility % of incident patient clinical and laboratory measures | |||||
| Comorbidities, mean % (95% CI) | |||||
| Obese (BMI ≥ 35 kg/m2) | 25.0 (24.3–25.8) | 25.3 (23.8–26.7) | 25.5 (24.6–26.5) | 24.2 (22.8–25.6) | .33 |
| Congestive heart failure | 27.1 (26.0–28.1) | 29.9 (28.1–31.8) | 26.0 (24.4–27.6) | 25.2 (23.3–27.1) | <.001 |
| Atherosclerotic heart disease | 9.2 (8.4–10.0) | 11.9 (10.4–13.5) | 9.0 (8.2–9.8) | 6.7 (5.6–7.9) | <.001 |
| Other cardiac disease | 16.9 (15.9–17.9) | 21.0 (19.2–22.8) | 15.4 (13.7–17.0) | 14.3 (12.7–15.9) | <.001 |
| Cerebrovascular disease (stroke) | 9.1 (8.6–9.6) | 11.2 (10.2–12.2) | 9.0 (8.2–9.8) | 7.1 (6.3–7.9) | <.001 |
| Peripheral vascular disease | 8.2 (7.6–8.9) | 10.5 (9.2–11.7) | 8.0 (7.0–9.0) | 6.1 (5.2–7.1) | <.001 |
| Hypertension | 89.0 (88.2–89.7) | 88.2 (86.8–89.7) | 89.5 (88.5–90.5) | 89.2 (87.8–90.6) | .36 |
| Diabetes | 59.2 (58.2–60.2) | 60.0 (58.2–61.7) | 59.7 (58.1–61.2) | 58.0 (56.1–59.9) | .24 |
| Chronic obstructive pulmonary disease | 8.9 (8.3–9.5) | 11.0 (9.8–12.3) | 8.9 (8.0–9.8) | 6.8 (5.9–7.7) | <.001 |
| Tobacco use | 8.8 (8.2–9.4) | 10.3 (9.0–11.6) | 8.9 (8.1–9.7) | 7.2 (6.3–8.1) | <.001 |
| Cancer | 6.0 (5.5–6.4) | 7.0 (6.2–7.8) | 5.8 (5.2–6.5) | 5.1 (4.2–6.0) | .003 |
| Facility % of incident patient socioeconomic characteristics | |||||
| Pre-ESKD nephrology care, mean % (95% CI) | 70.7 (69.3–72.1) | 71.4 (69.0–73.8) | 72.9 (70.8–75.0) | 67.9 (65.1–70.7) | .01 |
| Primary health insurance provider, mean % (95% CI) | |||||
| Medicare | 40.3 (39.3–41.3) | 42.1 (40.6–43.7) | 40.6 (39.1–42.0) | 38.1 (36.1–40.1) | .004 |
| Medicaid | 24.0 (23.1–25.0) | 26.1 (24.3–27.9) | 23.8 (22.4–25.1) | 22.2 (20.5–23.8) | .003 |
| Employer group | 17.7 (16.8–18.6) | 14.8 (13.5–16.0) | 18.5 (17.1–19.9) | 19.8 (18.0–21.7) | <.001 |
| Other coverage | 6.2 (5.7–6.6) | 5.7 (5.1–6.4) | 6.2 (5.5–6.9) | 6.6 (5.4–7.7) | .40 |
| No coverage | 9.6 (9.1–10.1) | 8.4 (7.6–9.2) | 9.3 (8.6–10.0) | 11.1 (10.0–12.2) | .002 |
| Facility % of incident patient neighborhood (ZIP code) characteristics | |||||
| Neighborhood poverty (% ZIP code residents below poverty), mean % (95% CI) | |||||
| ≥20% below poverty | 31.1 (29.0–33.1) | 30.9 (27.2–34.6) | 32.2 (28.7–35.8) | 30.1 (26.8–33.4) | .70 |
| Average % black, mean (95% CI) | 34.6 (33.2–36.0) | 29.8 (27.5–32.1) | 34.0 (31.5–36.4) | 40.1 (37.7–42.6) | <.001 |
| Average % high school graduates, mean (95% CI) | 82.8 (82.5–83.2) | 81.6 (81.0–82.2) | 83.1 (82.4–83.7) | 83.8 (83.3–84.4) | <.001 |
| Dialysis facility characteristics | |||||
| For-profit, N (%) | 583 (84.5) | 207 (88.5) | 188 (83.2) | 188 (81.7) | .06 |
| Freestanding facility, N (%) | 682 (98.8) | 230 (98.3) | 224 (99.1) | 228 (99.1) | .67 |
| Facility size (# of patients), N (%) | |||||
| ≤25 | 52 (7.5) | 17 (7.3) | 12 (5.3) | 23 (10.0) | <.001 |
| 26–54 | 248 (35.9) | 87 (37.2) | 63 (27.9) | 98 (42.6) | |
| 55–78 | 163 (23.6) | 48 (20.5) | 58 (25.7) | 57 (24.8) | |
| 79+ | 213 (30.9) | 80 (34.2) | 87 (38.5) | 546 (20.0) | |
| Patient to social worker ratioc | |||||
| Mean (95% CI) | 80.9 (77.6–84.1) | 82.2 (77.1–87.4) | 87.7 (81.6–93.8) | 72.7 (67.1–78.3) | <.001 |
| Quartile, N (%) | |||||
| ≤48:1 (quartile 1) | 155 (22.5) | 46 (19.7) | 41 (18.1) | 68 (29.6) | .07 |
| 48:1–76:1 (quartile 2) | 156 (22.6) | 55 (23.5) | 49 (21.7) | 52 (22.6) | |
| 76:1–108:1 (quartile 3) | 156 (22.6) | 59 (25.2) | 58 (25.7) | 39 (17.0) | |
| ≥108:1 (quartile 4) | 156 (22.6) | 51 (21.8) | 58 (25.7) | 47 (20.4) | |
| Missing | 67 (9.7) | 23 (9.8) | 20 (8.9) | 24 (10.4) | |
BMI, body mass index; CI, confidence interval; ESKD, end-stage kidney disease.
2 (0.29%) facilities missing comorbidity information; 3 (0.43%) facilities missing pre-ESKD nephrology care; 3 (0.43%) facilities missing profit status; 3 (0.43%) facilities were missing freestanding (vs hospital-based) status; 14 (2.0%) facilities missing facility size.
Across tertiles of referral, by analysis of variance or χ2 test.
Number of patients for every 1 social worker. Calculated only among those facilities that have social workers.
3.3 |. Patient characteristics and early transplant access: referral and evaluation start within 6 months of referral
Among the 34 857 patients in the study population, the mean age was 59.5 years (95% CI: 59.4–59.7). A total of 11 862 (34.0%) of patients were referred within 1 year of starting dialysis, and 5716 (48.3%) were evaluated within 6 months of referral. Among those referred within a year of dialysis start, the mean age was 54.0 (95% CI: 53.8–54.2) and lower among those who started the evaluation within 6 months of referral (52.5 years; 95% CI: 52.2–52.9). There was a larger proportion of males referred (58.9%) and evaluated (60.3%) compared to the study population of incident ESKD patients (55.1%), and a larger proportion of black, non-Hispanic patients (61.9% referred; 60.4% evaluated vs 54.9% of incident ESKD patients). Comorbidities were prevalent among the population and in general, the proportions of patients with comorbidities decreased from ESKD to referral to evaluation start. For example, 27.2% of the ESKD population had congestive heart failure, but this declined to 21.6% of the referred population and 19.1% of the population evaluated. Patients with employer-based health insurance were overrepresented in later steps of the transplant process (17.9% of ESKD patients, but 24.5% of referred patients and 28.9% of evaluated patients). In addition, 85.1% of all incident dialysis patients were treated at for-profit dialysis facilities, with 83.3% of referred and 82.8% of evaluated patients treated at for-profit dialysis facilities (Table 2). Among the 9 transplant centers included, the median time from ESKD start to referral (among those referred) was 245 days (IQR: 225–261), and the median time from referral to evaluation start among those who started the transplant center evaluation was 91 days (IQR: 81–107). The median proportion of patients who start the evaluation among those referred at the transplant center level was 55.1% (IQR: 50.6–61.0) and ranged from 17.5% to 71.4%.
TABLE 2.
Selected characteristics of ESKD patients at start of dialysis and at multiple steps in the transplant process: (1) referral for transplant within 1 y; (2) started the evaluation for transplant within 6 mo of referral in Georgia, North Carolina, and South Carolina: 2012–2016
| Among all patients N = 34 857 |
Among patients referred for transplant within 1 y of ESKD start N = 11 826 |
||
|---|---|---|---|
| Patient characteristic | Study population at dialysis starta N = 34 857 | Referred for transplant within 1 y of ESKD start N = 11 862 (34.0%) | Started evaluation for transplant within 6 mo of referral N = 5716 (48.3%) |
| Age, mean (95% CI), y | 59.5 (59.4–59.7) | 54.0 (53.8–54.2) | 52.5 (52.2–52.9) |
| Age category, N (%), y | |||
| 18–29 | 944 (2.7) | 557 (4.7) | 330 (5.8) |
| 30–39 | 2112 (6.1) | 1220 (10.3) | 670 (11.7) |
| 40–49 | 4513 (13.0) | 2362 (19.9) | 1211 (21.2) |
| 50–59 | 7857 (22.5) | 3131 (26.4) | 1520 (26.6) |
| 60–69 | 10 505 (30.1) | 3308 (27.9) | 1528 (26.7) |
| 70–80 | 8926 (25.6) | 1284 (10.8) | 457 (8.0) |
| Male sex, N (%) | 19 216 (55.1) | 6987 (58.9) | 3449 (60.3) |
| Race/ethnicity, N (%) | |||
| White, non-Hispanic | 13 792 (39.6) | 3806 (32.1) | 1835 (32.1) |
| Black, non-Hispanic | 19 147 (54.9) | 7344 (61.9) | 3456 (60.4) |
| White, Hispanic | 719 (2.1) | 314 (2.6) | 198 (3.5) |
| Other race/ethnicity | 649 (1.9) | 268 (2.3) | 163 (2.9) |
| Missing | 550 (1.6) | 130 (1.1) | 64 (1.1) |
| Attributed cause of ESKD, N (%) | |||
| Diabetes | 15 537 (44.6) | 5176 (43.6) | 2363 (41.3) |
| Hypertension | 12 444 (35.7) | 4442 (37.4) | 2155 (37.7) |
| Glomerulonephritis | 2325 (6.7) | 1039 (8.8) | 578 (10.1) |
| Other | 3102 (9.5) | 880 (7.4) | 458 (8.0) |
| Missing | 1208 (3.5) | 325 (2.7) | 161 (2.8) |
| Comorbidities, N (%) | |||
| Obesity (BMI ≥ 35 kg/m2) | 8632 (24.8) | 3016 (25.4) | 1341 (23.5) |
| Congestive heart failure | 9468 (27.2) | 2562 (21.6) | 1093 (19.1) |
| Atherosclerotic heart disease | 3395 (9.7) | 788 (6.6) | 328 (5.7) |
| Other cardiac disease | 5960 (17.1) | 1473 (12.4) | 640 (11.2) |
| Cerebrovascular disease (stroke) | 3174 (9.1) | 751 (6.3) | 288 (5.0) |
| Peripheral vascular disease | 3038 (8.7) | 715 (6.0) | 255 (4.5) |
| Hypertension | 30 668 (88.0) | 10 623 (89.6) | 5113 (89.5) |
| Diabetes | 20 566 (59.0) | 6746 (56.9) | 3135 (54.8) |
| Chronic obstructive pulmonary disease | 3109 (8.9) | 610 (5.1) | 200 (3.5) |
| Cancer | 2106 (6.0) | 375 (3.2) | 172 (3.0) |
| Tobacco use | 3136 (9.0) | 1031 (8.7) | 414 (7.2) |
| Pre-ESKD nephrology care, N (%) | |||
| Yes | 21 673 (62.2) | 7412 (62.5) | 3592 (62.8) |
| No | 8563 (24.6) | 2999 (25.3) | 1449 (25.3) |
| Missing | 4621 (13.3) | 1451 (12.2) | 675 (11.8) |
| Primary health insurance provider, N (%) | |||
| Medicare | 13 985 (40.1) | 3598 (30.3) | 1587 (27.8) |
| Medicaid | 8391 (24.1) | 2654 (22.4) | 1124 (19.7) |
| Employer group | 6230 (17.9) | 2909 (24.5) | 1655 (28.9) |
| Other coverage | 2190 (6.3) | 855 (7.2) | 411 (7.2) |
| No coverage | 3511 (10.1) | 1716 (14.5) | 875 (15.3) |
| Missing | 550 (1.6) | 130 (1.1) | 64 (1.1) |
| Neighborhood poverty (% ZIP code below poverty), N (%) | |||
| 0%−19% (low) | 23 985 (68.8) | 8159 (68.8) | 4007 (70.1) |
| >20% (high) | 10 872 (31.2) | 3703 (31.2) | 1709 (29.9) |
| Average % black, mean (95% CI) | 34.8 (34.5–35.0) | 36.5 (36.1–37.0) | 36.6 (36.0–37.2) |
| Average % high school graduates, mean (95% CI) | 82.8 (82.8–82.9) | 83.1 (83.0–83.3) | 83.5 (83.3–83.7) |
| For-profit, N, % | 29 647 (85.1) | 9882 (83.3) | 4735 (82.8) |
| Freestanding facility, N (%) | 34 676 (99.5) | 11 806 (99.5) | 5687 (99.5) |
| Facility size (# of patients), N (%) | |||
| 11–24 | 563 (1.6) | 200 (1.7) | 100 (1.7) |
| 25–54 | 6731 (19.3) | 2374 (20.0) | 1190 (20.8) |
| 55–78 | 7519 (21.6) | 2727 (23.0) | 1269 (22.2) |
| 79+ | 18 278 (52.4) | 5989 (50.5) | 2864 (50.2) |
| Missing | 1766 (5.1) | 572 (4.8) | 293 (5.1) |
| Patient to social worker ratiob, N (%) | |||
| Mean (95% CI) | 96.8 (96.3–97.2) | 95.9 (95.1–96.6) | 94.7 (93.6–95.8) |
| Quartile, N (%) | |||
| ≤67:1 (quartile 1) | 8083 (23.2) | 2902 (24.5) | 1485 (26.0) |
| 68:1–95:1 (quartile 2) | 8066 (23.2) | 2666 (22.5) | 1302 (22.8) |
| 96:1–122:1 (quartile 3) | 8187 (23.5) | 2543 (21.4) | 1189 (20.8) |
| ≥123:1 (quartile 4) | 7836 (22.5) | 2787 (23.5) | 1266 (22.1) |
| Missing | 2645 (7.6) | 964 (8.1) | 474 (8.3) |
BMI, body mass index; CI, confidence interval; ESKD, end-stage kidney disease.
Additional missing data: 800 (2.3%) missing BMI; 561 (1.6%) missing all comorbidities (excluding BMI); 5 (0.01%) missing facility profit status; 5 (0.01%) missing facility type; 481 (1.4%) missing neighborhood average percent black; 488 (1.4%) missing neighborhood average percentage high school graduates.
Number of patients for every 1 social worker, calculation does not include facilities with 0 social workers.
3.4 |. Multivariable-adjusted analyses
In multivariable-adjusted competing risk analyses, older age was associated with a lower hazard ratio of referral among ESKD patients and starting the evaluation among those referred. For example, for patients aged 60–69 years (representing 30.1% of the study population), the HR of referral was 0.52 (95% CI: 0.48–0.57), and the HR for starting the evaluation among those referred was 0.68 (95% CI: 0.61–0.76), compared to patients aged 18–29 years. Black, non-Hispanic patients had higher referral (HR: 1.22; 95% CI: 1.18–1.27), but lower evaluation start (HR: 0.93; 95% CI: 0.88–0.98) vs white non-Hispanic patients. Comorbidities were generally associated with lower referral and evaluation start. For example, congestive heart failure was associated with lower referral (HR: 0.90; 95% CI: 0.87–0.94) and evaluation start (HR: 0.89; 95% CI: 0.84–0.94), with similar lower rates among patients with cerebrovascular disease and peripheral vascular disease. However, a history of cancer was associated with lower referral (HR: 0.69; 95% CI: 0.63–0.75) but not with evaluation start (OR: 1.00; 95% CI: 0.88–1.14). Pre-ESRD nephrology care was associated with 8% higher referral (HR: 1.08; 95% CI: 1.04–1.12), and a higher rate of evaluation start among those referred (HR: 1.14; 95% CI: 1.09–1.20). Measures of lower socioeconomic status were also associated with lower access. Medicaid (vs Medicare) insurance was associated with lower referral (HR: 0.86; 95% CI: 0.82–0.90) and evaluation start (HR: 0.88; 95% CI: 0.83–0.95); higher neighborhood poverty was associated with lower referral (HR: 0.94; 95% CI: 0.92–0.98) and evaluations (HR: 0.93; 95% CI: 0.88–0.97). Patients treated at for-profit vs not-for-profit facilities had a 13% lower rate of referral (HR: 0.87; 95% CI: 0.83–0.91) and evaluation start (HR: 0.87; 95% CI: 0.82–0.91). A higher patient to social worker ratio was associated with lower referral in some but not all categories (eg, quartile 3 vs quartile 1: HR: 0.88; 95% CI: 0.84–0.92); similarly with evaluation start (eg, quartile 3 vs quartile 1: HR: 0.91; 95% CI: 0.85–0.97). In the model examining evaluation start among those referred, significant differences in evaluation start were observed across the (blinded) transplant center sites, ranging from HR: 0.19 (95% CI: 0.16–0.23) in Center 7 to 1.38 (95% CI: 1.25–1.53) at Center 6 (compared to Transplant Center 1) (Table 3).
TABLE 3.
Competing risks modeling results for the association of patient- and dialysis facility-level factors with time to referral for kidney transplantation in Georgia and evaluation start within referral among incident ESKD patients in Georgia, North Carolina, and South Carolina: 2012–2016, followed through February 2018
| Covariate | Referred Total N = 34 857 Hazard ratios (95% CI) | Started evaluation among patients referred Total N = 15 067 Hazard ratios (95% CI) |
|---|---|---|
| Patient-level characteristics | ||
| Age, y | ||
| 18–29 | 1 [Reference] | 1 [Reference] |
| 30–39 | 1.0 (0.91, 1.1) | 0.85 (0.76, 0.95) |
| 40–49 | 0.86 (0.79, 0.94) | 0.76 (0.69, 0.85) |
| 50–59 | 0.66 (0.61, 0.73) | 0.72 (0.64, 0.80) |
| 60–69 | 0.52 (0.48, 0.57) | 0.68 (0.61, 0.76) |
| 70–80 | 0.22 (0.20, 0.24) | 0.46 (0.40, 0.52) |
| Female (vs male) | 0.86 (0.83, 0.89) | 0.94 (0.89, 0.98) |
| Race/Ethnicity | ||
| White, non-Hispanic | 1 [Reference] | 1 [Reference] |
| Black, non-Hispanic | 1.22 (1.18, 1.27) | 0.93 (0.88, 0.98) |
| White, Hispanic | 1.06 (0.95, 1.17) | 1.16 (1.02, 1.32) |
| Other | 1.30 (1.16, 1.46) | 1.26 (1.09, 1.46) |
| ESRD cause | ||
| Hypertension | 1 [Reference] | 1 [Reference] |
| Diabetes | 0.99 (0.95, 1.04) | 0.99 (0.93, 1.05) |
| Glomerulonephritis | 1.04 (0.98, 1.11) | 1.11 (1.02, 1.20) |
| Other | 0.77 (0.72, 0.82) | 1.05 (0.96, 1.14) |
| Year of incident ESRD | ||
| 2012 | 1 [Reference] | 1 [Reference] |
| 2013 | 1.08 (1.03, 1.14) | 1.08 (1.01, 1.15) |
| 2014 | 1.24 (1.18, 1.30) | 1.23 (1.15, 1.32) |
| 2015 | 1.11 (1.05, 1.16) | 1.33 (1.24, 1.42) |
| 2016 | 1.09 (1.03, 1.16) | 1.44 (1.33, 1.56) |
| Clinical and laboratory measures | ||
| Congestive heart failure | 0.90 (0.87, 0.94) | 0.89 (0.84, 0.94) |
| Atherosclerotic heart disease | 0.98 (0.92, 1.05) | 0.94 (0.85, 1.03) |
| Other cardiac disease | 0.88 (0.84, 0.93) | 0.99 (0.92, 1.06) |
| Cerebrovascular disease (stroke) | 0.81 (0.76, 0.86) | 0.84 (0.76, 0.93) |
| Peripheral vascular disease | 0.83 (0.77, 0.89) | 0.84 (0.75, 0.93) |
| Hypertension | 1.07 (1.01, 1.13) | 1.02 (0.95, 1.10) |
| Diabetes | 0.97 (0.93, 1.02) | 0.99 (0.93, 1.05) |
| Chronic obstructive pulmonary disease | 0.79 (0.74, 0.85) | 0.77 (0.69, 0.87) |
| Tobacco use | 0.88 (0.83, 0.93) | 0.80 (0.73, 0.87) |
| Cancer | 0.69 (0.63, 0.75) | 1.00 (0.88, 1.14) |
| Socioeconomic characteristics | 1.13 (1.08, 1.19) | |
| Pre-ESRD nephrology care | 1.08 (1.04, 1.12) | 1.14 (1.09, 1.20) |
| Health insurance | ||
| Medicare | 1 [Reference] | 1 [Reference] |
| Medicaid | 0.86 (0.82, 0.90) | 0.89 (0.84, 0.96) |
| Employer group | 1.24 (1.18, 1.30) | 1.23 (1.16, 1.31) |
| Other coverage | 1.04 (0.97, 1.11) | 0.96 (0.88, 1.06) |
| No coverage | 0.99 (0.94, 1.05) | 0.95 (0.88, 1.02) |
| Neighborhood poverty (% ZIP code below poverty) | ||
| 0%−19% (low) | 1 [Reference] | 1 [Reference] |
| ≥20% (high) | 0.94 (0.91, 0.98) | 0.93 (0.88, 0.97) |
| Dialysis facility characteristics | ||
| Patient: social worker ratio | ||
| ≤67:1 (quartile 1) | 1 [Reference] | 1 [Reference] |
| 68:1–95:1 (quartile 2) | 0.90 (0.86, 0.94) | 0.91 (0.85, 0.97) |
| 96:1–122:1 (quartile 3) | 0.88 (0.84, 0.92) | 0.91 (0.85, 0.97) |
| ≥123:1 (quartile 4) | 0.97 (0.93, 1.02) | 0.95 (0.89, 1.01) |
| For-profit (vs nonprofit) | 0.86 (0.82, 0.90) | 1.02 (0.95, 1.08) |
| Transplant center characteristic | ||
| Transplant center site (blinded) | N/A | |
| 1 | 1 [Reference] | |
| 2 | 0.80 (0.72, 0.89) | |
| 3 | 1.29 (1.15, 1.45) | |
| 4 | 0.97 (0.88, 1.07) | |
| 5 | 0.48 (0.43, 0.53) | |
| 6 | 1.38 (1.22, 1.53) | |
| 7 | 0.19 (0.16, 0.23) | |
| 8 | 0.97 (0.86, 1.09) | |
| 9 | 0.79 (0.70, 0.90) | |
CI, confidence interval; ESKD, end-stage kidney disease.
For multivariable patient-level analyses, covariates that were either significant in bivariable analyses or clinically relevant were included in the multivariable model.
4 |. DISCUSSION
In a population of ~35 000 ESKD patients starting dialysis in 2012–2016 in Georgia, North Carolina, and South Carolina, we observed substantial variation in early transplant steps among the 690 dialysis facilities. The median within-dialysis facility proportion of ESKD patients referred within 1 year was 33.7%, and the range was 0% to 100%. Fewer than half of referred patients started the evaluation within 6 months of referral, representing only 16.1% of all incident dialysis patients. This suggests there are important barriers between referral and evaluation that influence transplant access and potential opportunities for dialysis clinicians, transplant center clinicians, and outreach coordinators to improve the referral of appropriate candidates and increase the conversion of referrals to evaluations. In addition, the barriers to referral were not always the same as those associated with starting the transplant evaluation process. The wide variability in transplant referral and evaluation start across dialysis facilities—particularly when national policies are promoting new payment reform for dialysis facilities to increase transplant access—suggests the need to begin monitoring these important early steps in national surveillance data.
Our findings that only about one-third of incident ESKD patients are referred for transplant within a year of ESKD are fairly consistent with prior findings in Georgia that found 24.5% referral5 and a recent Canadian study that found 17.3% of incident ESKD referral within 1 year of ESKD start.15 Our reported referral rates were slightly higher, which may be partially explained by our exclusion of very small facilities. The reasons for variation in referral across the 690 dialysis facilities in our study—from 0% to 100%—may be partially due to provider-level practices in referral. Tong et al16 examined 24 studies in a systematic review and found that disparities in nephrologist referral for transplant may be partially explained by provider preferences. Patients with comorbidities, nonadherence, older age, minorities, or with low socioeconomic status were less likely recommended for transplant referral. In a random sample of nephrologists surveyed on 25 hypothetical case-based patient scenarios, nephrologists with an academic affiliation and those within 10 years of fellowship training had higher referral rates.17 In a separate study of 216 nephrologists, Bartolomeo et al18 reported the top reasons for excluding patients for transplant referral as inadequate social support (44%), inadequate education (32%), and patient age >64 (26%) and that practice location in a more rural area, proximity to a transplant center, and the level of education of the nephrologist’s patient population influenced referral. However, distance to transplant center was not associated with referral and evaluation in Georgia, North Carolina, and South Carolina.19
This is the first study to report the proportion of patients evaluated beyond a single-center study. We found that fewer than half of referred patients (48.3%) start the transplant evaluation within 6 months of referral, demonstrating a substantial drop-off of patients that may partially explain disparities in transplant access. Although our registry data were not granular enough to determine causes of dropout, prior single-center studies suggest several explanations for our findings. First, it is possible that referred patients do not understand the steps needed to start the evaluation. In a single-center study of hemodialysis patients undergoing evaluation, more than half of patients did not know their waitlisting status and of these, nearly 90% mistakenly believed they were already waitlisted.20 Second, in studies examining barriers for patients who do not show up for the transplant evaluation, financial concerns and transportation were frequently cited as a concern.9 In a survey of ESKD patients in Georgia referred from 2014–2016, patients with medical mistrust, perceived racism, and those who experienced discrimination were associated with substantially lower evaluation start.10 More interventions are needed to address the challenges that patients may face in the steps between referral and evaluation start.
In our analyses, we report that older age, female sex, Medicaid insurance, and higher neighborhood poverty were associated with lower rates of referral and evaluation start among those referred, similar to prior work.5 Of note, non-Hispanic black patients were more likely referred for transplant but had lower likelihood of evaluation start, compared to non-Hispanic whites. The higher frequency of referral among black ESKD patients is striking, as decades of research identifying racial/ethnic disparities in transplantation 12,21–26 has suggested this disparity is at least partially explained by lower referral for transplant.21,22 These results, when combined with prior literature that shows lower rates of waitlisting for black vs white patients,25,27–29 suggests that the reasons for racial disparities in waitlisting among ESKD patients who have already started on dialysis—at least in this region of the country—may be due to factors that occur after referral. Of note, there are also important racial disparities in transplant access prior to dialysis start, where preemptive transplantation is less common among blacks vs whites, both in the Southeast30 as well as nationally.2,31 Although our study focused on patients who started treatment on dialysis, a higher rate of preemptive referral among white ESKD patients may partially explain the higher referral among black non-Hispanics vs white dialysis patients. Taken together, these results suggest that efforts to tackle the longstanding racial disparities in waitlisting and transplant access12,23,24,30 should focus interventions to reduce disparities on the step after referral (for patients who start on dialysis in the Southeast), and prior to dialysis start.
Dialysis facilities in the lowest tertile of referral performance had a lower patient to social worker ratio—a potentially modifiable characteristic of a dialysis facility. This suggests that having more social workers in a facility could be beneficial. In our region, surveys of primarily dialysis facility social workers reported a high level of comfort with educating patients about transplant, but only 56% reportedly educated patients about transplant annually and fewer than a quarter thought that the majority of patients were interested in transplant.32 Social workers in the Southeast have reported their perception of the primary reason for racial disparity in transplant access is low socioeconomic status.33 In our study, nonprivate insurance and high neighborhood poverty were associated with lower referral and evaluation start. Some patients may not have the means to travel to the hospital or take off work for the transplant evaluation. Based on this study as well as prior single-center studies examining barriers to starting the evaluation,9–12,34 interventions such as the use of telemedicine, rideshare reimbursements by transplant centers, and local or federal policies that provide reimbursement to patients for missed work could help address these barriers. In addition, continued involvement of dialysis facility social worker to address patient-specific barriers for starting the transplant evaluation may be appropriate.
We also identified that patients treated at for-profit vs nonprofit dialysis facilities have a ~14% lower rate of referral, but there were no differences by profit status in starting the transplant evaluation. Gander et al35 reported that nearly 1.5 million US ESKD patients treated at 6511 dialysis facilities between 2000–2016, patients dialyzing at for-profit vs nonprofit facilities had lower rates of waitlisting and receipt of a living or deceased donor kidney transplant. Our results suggest that this disparity—at least in Georgia, North Carolina, and South Carolina—may be due to lower referral among the for-profit dialysis facilities but not to lower rates of starting the transplant evaluation.
Results from this study have important policy implications. The proposed new waitlisting measure for the 2020 ESRD Quality Incentive Program would link a portion of payment directly to performance on this metric.4,36 However, our results suggest that the various factors influencing referral and evaluation start may not be the same factors that influence transplant rates. The executive order on Advancing American Kidney Health released in July 2019 aims to expand transplant access through a number of initiatives, including restructuring payment models for dialysis facilities and nephrologists to incentivize waitlisting and transplantation,36 which has a number of implications for both dialysis facilities and transplant centers.37 Our results suggest that it is pertinent to monitor not just waitlisting and transplant rates with these new policies but also to examine referral and evaluation start. As ESKD care providers and health care systems prepare to change care practices to incentivize transplantation, and as the CMS Statement of Work for ESRD Networks focuses on improving waitlisting,38 it is important to understand all steps in the transplant process that may drive performance payment adjustments. The collection of national surveillance data for transplant referral was proposed nearly two decades ago by a CMS Technical Expert Panel39 and continues to be of importance today,40–42 and yet we still know little about these necessary steps for transplant.
There are limitations to our findings. First, our results may have limited generalizability nationally. The Southeastern United States has the lowest rates of kidney transplantation in the nation, and trends in this region may not reflect national trends. However, given substantial variability that has been reported in other steps of the transplant process across other geographic regions and nationally,2,3,8 it is likely there is also variation in referral and evaluation in other regions. Second, our results apply only to incident dialysis patients; we do not have an accurate denominator of chronic kidney disease patients who may be appropriate candidates for transplant but were not referred. Third, although our multistate collaborative is the only regional data registry in the nation that captures early transplant steps, we do not capture referrals and evaluations that may occur outside of these three states. In a subanalysis, ~10% of listings were outside of the tristate region. Evaluation start data in our study capture only the first encounter the patient has with the transplant center, but we do not have the ability in our data to determine whether the first encounter was a satellite clinic, required educational course, or the medical/psychosocial evaluation appointments, because not all transplant center electronic medical records capture this granular information. Finally, with data from only 9 transplant centers, it is difficult to explore the center-level factors associated with higher vs lower evaluation rates among those referred, although the variability we observed in just one region does suggest there is room for transplant center-level improvement in access.
In summary, among ESKD patients initiating dialysis in the Southeastern United States, the region with the lowest rates of kidney transplantation in the nation, only 33.7% of patients were referred for transplant within a year of dialysis start, and approximately half of patients do not start the transplant evaluation after referral. National surveillance data should be collected on early transplant steps to help dialysis facilities and transplant centers address the barriers to improve access to transplantation and meet national goals to advance kidney health. Dialysis facility-level interventions are needed to increase referrals among low performing facilities, and transplant center interventions are needed to encourage evaluation start among those referred.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the Southeastern Kidney Transplant Coalition for their significant assistance with this work, and IPRO of ESRD Network 6 which served as the data coordinating center for this study. The authors disclosed receipt of the following financial support for the research, authorship, and or publication of this article: U01MD010611. The data reported here have been supplied in part by the USRDS. Deidentified data are available upon request and with a signed data use agreement to the Southeastern Kidney Transplant Coalition. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Funding information
National Institutes of Health, Grant/Award Number: U01MD010611
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- ESKD
end-stage kidney disease
- GA
Georgia
- HR
hazard ratio
- NC
North Carolina
- SC
South Carolina
- USRDS
United States Renal Data System
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
Deidentified data are available upon request and with a signed data use agreement to the Southeastern Kidney Transplant Coalition.
REFERENCES
- 1.United States Renal Data System. 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 2.King KL, Husain SA, Jin Z, Brennan C, Mohan S. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol. 2019;14(10):1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patzer RE, Plantinga L, Krisher J, Pastan SO. Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. Am J Transplant. 2014; 14(7):1562–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Medicare Program; End-Stage Renal Disease Prospective Payment System, Payment for Renal Dialysis Services Furnished to Individuals with Acute Kidney Injury, End-Stage Renal Disease Quality Incentive Program. In: Services CfMaM, ed. Washington, DC: 2018. [PubMed] [Google Scholar]
- 5.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015;314(6):582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12(2):351–357. [DOI] [PubMed] [Google Scholar]
- 7.Waterman AD, Peipert JD, Goalby CJ, Dinkel KM, Xiao H, Lentine KL. Assessing transplant education practices in dialysis centers: comparing educator reported and medicare data. Clin J Am Soc Nephrol. 2015;10(9):1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant. 2007;7(5 Pt 2):1412–1423. [DOI] [PubMed] [Google Scholar]
- 9.Dageforde LA, Box A, Feurer ID, Cavanaugh KL. Understanding patient barriers to kidney transplant evaluation. Transplantation. 2015;99(7):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamoda RE, McPherson LJ, Lipford K, et al. Association of sociocultural factors with initiation of the kidney transplant evaluation process. Am J Transplant. 2020;20(1):190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6(7):1760–1767. [DOI] [PubMed] [Google Scholar]
- 12.Waterman AD, Peipert JD, Hyland SS, McCabe MS, Schenk EA, Liu J. Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant. Clin J Am Soc Nephrol. 2013;8(6):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patzer RE, Gander J, Sauls L, et al. The RaDIANT community study protocol: community-based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrol. 2014;15(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patzer RE, Paul S, Plantinga L, et al. A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol. 2017;28(3):935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Gill JS, Knoll G, et al. Referral for kidney transplantation in Canadian provinces. J Am Soc Nephrol. 2019;30(9):1708–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong A, Hanson CS, Chapman JR, et al. The preferences and perspectives of nephrologists on patients’ access to kidney transplantation: a systematic review. Transplantation. 2014;98(7):682–691. [DOI] [PubMed] [Google Scholar]
- 17.Tandon A, Wang M, Roe KC, Patel S, Ghahramani N. Nephrologists’ likelihood of referring patients for kidney transplant based on hypothetical patient scenarios. Clin Kidney J. 2016;9(4):611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartolomeo K, Tandon Gandhir A, Lipinski M, Romeu J, Ghahramani N. Factors considered by nephrologists in excluding patients from kidney transplant referral. Int J Organ Transplant Med. 2019;10(3):101–107. [PMC free article] [PubMed] [Google Scholar]
- 19.McPherson L, Barry V, Yackley J, et al. Distance to kidney transplant center and access to early steps in the kidney transplantation process in the Southeastern United States. Clin J Am Soc Nephrol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie A, Hammer H, Lee J, Nnewihe C, Gordon J, Silva P. Lack of listing status awareness: results of a single-center survey of hemodialysis patients. Am J Transplant. 2011;11(7):1522–1526. [DOI] [PubMed] [Google Scholar]
- 21.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280(13):1148–1152. [DOI] [PubMed] [Google Scholar]
- 22.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation–clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343(21):1537–1544, 1532 p preceding 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol. 2011;22(4):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20(6):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12(2):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purnell TS, Luo X, Cooper LA, et al. Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA. 2018;319(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2012;7(9):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK. Disparities in completion rates of the medical prerenal transplant evaluation by race or ethnicity and gender. Transplantation. 2015;99(1):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8(9):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gander JC, Zhang X, Plantinga L, et al. Racial disparities in preemptive referral for kidney transplantation in Georgia. Clin Transplant. 2018;32(9):e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purnell TS, Crews DC. Persistent disparities in preemptive kidney transplantation. Clin J Am Soc Nephrol. 2019;14(10):1430–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browne T, Patzer RE, Gander J, et al. Kidney transplant referral practices in Southeastern dialysis units. Clin Transplant. 2016;30(4):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipford KJ, McPherson L, Hamoda R, et al. Dialysis facility staff perceptions of racial, gender, and age disparities in access to renal transplantation. BMC Nephrol. 2018;19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark CR, Hicks LS, Keogh JH, Epstein AM, Ayanian JZ. Promoting access to renal transplantation: the role of social support networks in completing pre-transplant evaluations. J Gen Intern Med. 2008;23(8):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gander JC, Zhang X, Ross K, et al. Association between dialysis facility ownership and access to kidney transplantation. JAMA. 2019;322(10):957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Department of Health and Human Services. Advancing American Kidney Health. Washington DC, 2019. https://aspe.hhs.gov/system/files/pdf/262046/AdvancingAmericanKidneyHealth.pdf. Accessed October 26, 2019. [Google Scholar]
- 37.Hippen BE, Reed AI, Ketchersid T, Maddux FW. The implications of the advancing American kidney health initiative for kidney transplant centers. Am J Transplant. 2019. 10.1111/ajt.15619 . [DOI] [PubMed] [Google Scholar]
- 38.CMS. ESRD Network Organizations. 2019. https://www.cms.gov/Medicare/End-Stage-Renal-Disease/ESRDNetworkOrganizations/index.html. Accessed October 26, 2019.
- 39.Sehgal AR, Leon J, Stark S. Renal Network. ESRD Special Study: Developing Dialysis Facility–Specific Kidney Transplant Referral Clinical Performance Measures. Washington, DC: Department of Health and Human Services; 2005. [Google Scholar]
- 40.Fowler KJ. Accountability of dialysis facilities in transplant referral: CMS needs to collect national data on dialysis facility kidney transplant referrals. Clin J Am Soc Nephrol. 2018;13(2):193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patzer RE, McPherson L. Variation in kidney transplant referral: how much more evidence do we need to justify data collection on early transplant steps? J Am Soc Nephrol. 2019;30(9):1554–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sehgal AR. Should transplant referral be a clinical performance measure? J Am Soc Nephrol. 2017;28(3):721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data are available upon request and with a signed data use agreement to the Southeastern Kidney Transplant Coalition.