Abstract
Cysteine sulfinic acid (Cys-SO2−) is a non-enzymatic oxidative post-translational modification (PTM) that has been identified in hundreds of proteins. However, the effects of cysteine sulfination are in most cases poorly understood. Cys-SO2− is structurally distinctive, with long sulfur-carbon and sulfur-oxygen bonds, and with tetrahedral geometry around sulfur due to its lone pair. Cys-SO2− thus has a unique range of potential interactions with the protein backbone which could facilitate protein structural changes. Herein, the structural effects of cysteine oxidation to the sulfinic acid were investigated in model peptides and folded proteins using NMR spectroscopy, circular dichroism, bioinformatics, and computational studies. In the PDB, Cys-SO2− shows a greater preference for α-helix than Cys. In addition, Cys-SO2− is more commonly found in structures with ϕ > 0, including in multiple types of β-turn. Sulfinate oxygens engage in hydrogen bonds with adjacent (i or i + 1) amide hydrogens. Over half of sulfinates have at least one hydrogen bond with an adjacent amide, and several structures have hydrogen bonds with both adjacent amides. Alternately, sulfur or either oxygen can act as an electron donor for n→π* interactions with the backbone carbonyl of the same residue, as indicated by frequent S---C=O or O---C=O distances below the sums of their van der Waals radii in protein structures. In peptides, Cys-SO2− favored α-helical structure at the N-terminus, consistent with helix dipole effects and backbone hydrogen bonds with the sulfinate promoting α-helix. Cys-SO2− has only modestly greater polyproline II helix propensity than Cys-SH, likely due to competition from multiple side chain-backbone interactions. Cys-SO2− stabilizes the i+1 position of a β-turn relative to Cys-SH. Within proteins, the range of side chain-main chain interactions available to Cys-SO2− compared to Cys-SH provides a basis for potential changes in protein structure and function due to cysteine oxidation to the sulfinic acid.
Graphical Abstract

Introduction
Cysteine undergoes oxidative post-translational modification (PTM) to disulfide (-SSR), S-nitrosyl (-SNO), sulfenic acid (-SOH), sulfinic acid (-SO2H), and sulfonic acid (-SO3H) forms (Figure 1), among others.1–9 The sulfinic acid modification, which exists as the deprotonated sulfinate at physiological pH (Figure 1b), has been identified in a wide range of proteins, including in hundreds of human proteins.10–12 Oxidation of cysteine to the sulfinic acid (sulfination) is dynamic, with levels increasing in response to higher concentration of oxidants, and reversible, via ATP-mediated reduction by sulfiredoxin.13,14
Figure 1. Structure and oxidation states of cysteine.
(a) Cysteine is oxidized to multiple forms by a variety of oxidants. Sulfenic acids (R-S-OH) can be non-enzymatically reduced by thiols. In contrast, sulfinic acids (R-SO2H) in proteins can only be enzymatically reduced, by sulfiredoxins in an ATP-dependent manner. (b) Cysteine sulfenic acid, cysteine sulfinic acid, and cysteine sulfinate. While Cys-SO2− is referred to as the sulfinic acid, under physiological conditions, the sulfinate is the dominant ionization state. (c) Potential local interactions of the cysteine sulfinate include hydrogen bonds with the amide hydrogens of the same residue (left), the following residue (middle), or both (right). (d) Cysteine sulfinate is structurally distinct from both cysteine and aspartate. The sulfinate has longer bonds than the equivalent bonds of the carboxylate, and the sulfinate sulfur has pyramidal geometry (∠O-O-Cβ-S = 39°), in contrast to the planar geometry (∠O-O-Cβ-Cγ = 0°) of the carboxylate carbon. The tetrahedral geometry of the sulfinate results in the oxygens being prochiral. The sulfinic acid pKa is approximately 2 pK units lower than that of a carboxylic acid. Bond lengths are from structures optimized by the MP2 method with the 6–311++G(3d,3p) basis set in implicit water. (e) The three-dimensional structures of leucine, cysteine sulfinic acid, and aspartic acid indicate their distinct geometries. Structures are shown as the acetylated amino acid amides in the major ionization states at pH 7. Notably, Cys-SO2− is sterically similar to leucine, in contrast to line structure representations, which imply a false similarity between cysteine sulfinate and aspartate.
Cysteine sulfination is implicated in diverse biological processes, but the mechanisms by which this PTM functions are often unclear. Sulfination of cysteine 215 results in activation of chaperone activity in the protein DJ-1, which is protective against Parkinson’s Disease.15–17 Cysteine sulfination inhibits peroxyredoxins, which are reduced by sulfiredoxins, thus balancing antioxidant activity with redox signaling. 13,18–20 Cysteine sulfinate, along with a cysteine sulfenic acid and an unmodified cysteine, acts as a crucial ligand for an active-site metal in a class of nitrile hydratases, where oxidation of the ligands may tune the electronic properties and catalytic activity of the metal.21 Alternately, sulfination of a Zn-binding cysteine in matrix metalloprotease-7 (MMP-7, matrilysin) is believed to disengage that residue from the metal, allowing proteolysis of the N-terminal fragment and activation of the protein.22 The biological relevance of cysteine sulfinate has not in most cases been thoroughly characterized, for reasons including its relatively recent recognition as a broadly-distributed PTM, challenges in the detection of sulfinates, difficulties in producing specifically sulfinated proteins, and the inherent oxidative instability of the sulfinate.23–26 Thus, the roles and mechanisms of cysteine sulfinic acid remain relatively underexplored.
The sulfinate moiety is unusual in biological contexts, and has several key differences from the superficially similar carboxylate (Figure 1d–e). Sterically, Cys-SO2− resembles leucine more than aspartate (Figure 1e). Cys-SO2− is unique in both structure and electronic properties compared to all canonical amino acids. The sulfinate sulfur has a lone pair, which results in a sulfur geometry with a pyramidal structure (∠O-O-Cβ-S = 39°). The sulfur lone pair is nucleophilic, as are both oxygens.2,8,23,27 Additionally, sulfur is larger than carbon, resulting in longer bonds from C to S, and from S to both oxygens, compared to the equivalent C-C or C-O bonds of a carboxylate (Figure 1d). The unique geometry and electronic properties of the sulfinate suggest a number of possible modes by which Cys-SO2− could modulate protein structure and function.
Protein secondary structure is significantly dependent on the inherent conformational preferences of the amino acids.28–34 PTMs can alter both the conformational preferences and the available interaction modes of a residue.35–43 Thus, altering a key residue can change protein structure, and therefore function. We have previously observed that phosphorylation of serine or (especially) threonine induces the formation of a strong intraresidue hydrogen bond between the phosphate and the backbone amide hydrogen, and that phosphoserine or phosphothreonine can significantly increase α-helicity in model peptides when located near the N-terminus of the α-helix.37,43 Cysteine exhibits low propensities for α-helix, polyproline type II (PPII) helix, β-turn, and β-strand structures.28–34,44 Local backbone interactions that are possible for Cys-SO2− (Figure 1c), as well as a negative charge at physiological pH, might lead to a change in structural bias that is capable of altering protein structure, compared to that in Cys. Herein, we examine the structural preferences of Cys-SO2−, both to understand its biological roles and to explore the potential of Cys-SO2− in protein design. Greater understanding of the structural impacts of oxidation to Cys-SO2− may provide insights in the mechanisms of oxidative protein signaling, including in disease states such as Parkinson’s Disease and cancers.
Experimental
Bioinformatics
Protein structural data for cysteine sulfinic acid were retrieved from the Protein Data Bank on May 14, 2019. Protein structural data for cysteine were retrieved from the Protein Data Bank on September 18, 2019. Protein structures containing cysteine sulfinic acid (ligand ID: CSD) were filtered for 90% sequence identity, with additional selection for unique protein chains, resulting in 171 structures with 179 unique CSD residues from a total of 407 structures. Perl scripts were written and applied to identify the residues of interest and to calculate interatomic angles and distances. Full details of the data generated as well as the scripts are available in the Supporting Information.
Peptide synthesis and characterization
Peptides were synthesized by standard Fmoc solid-phase peptide synthesis protocols using Rink amide resin. Peptides were acetylated on the N-terminus unless noted otherwise (Table S4). All peptides contained C-terminal amides. Synthetic procedures and characterization data are in the Supporting Information.
Cysteine oxidation
Cysteine was oxidized to the sulfinic acid form by two methods. Oxidation via methoxybenzyl (Mob) sulfone. Peptides PPII-C4-SO2− and β12-C6-SO2− were synthesized as described previously, using Mob-protected cysteine, oxidation to the sulfone, and Mob deprotection using strong acid.45 Direct oxidation to sulfinic acid. Peptides α14-CX-SO2− were synthesized from the equivalent peptides α14-CX-SH by direct oxidation of cysteine. A solution of 1.2 mM H2O2 in sulfination buffer (5 mM phosphate, 25 mM NaCl, pH 10) was chilled on ice, then peptide was added to a final concentration of 20 μM. The reaction mixture was vortexed briefly, then incubated at 40 °C for 20 minutes. The reaction mixture was then quenched with excess DTT, and the product was purified by HPLC. Details are in the Supporting Information.
Circular dichroism spectroscopy
Circular dichroism (CD) spectra were collected on a Jasco J-810 spectropolarimeter. CD spectra were collected at 0.5 °C or at 25 °C, as indicated, either in 5 mM phosphate buffer with 25 mM KF (α-helical and PPII model peptides, pH as indicated) or in 100 mM acetate buffer pH 3.8 (β-hairpin model peptides). Spectra were typically acquired with 40–50 μM peptide, prepared by dilution from peptide stock solutions. In the cases of the peptides α14-C1-SO2−, α14-C2-SO2−, and α14-C5-SO2−, which were not stable to exposure to air, this approach was not possible. Instead, these peptides were synthesized, purified by HPLC, sparged with argon, flash frozen, lyophilized, then reconstituted into 500 μL argon-sparged buffer. 50 μL of this solution was immediately injected on an analytical HPLC to confirm purity, and the remainder of the solution was sealed in a cuvette under argon and CD spectra acquired immediately. Sample concentrations for these peptides were determined by NMR spectroscopy, via integration of the tyrosine aromatic hydrogens, compared to a standard solution of formic acid.
NMR spectroscopy
NMR spectra were collected on a Brüker Avance 600 MHz NMR with a TXI probe (peptides PPII-C4-SH and PPII-C4-SO2−), or on a Brüker Avance III 600 MHz NMR spectrometer with a 5-mm Brüker SMART probe (other peptides). Peptide samples were dissolved in 90% H2O/10% D2O with either deuterated acetate buffer, pH 3.8 (peptides β12-C6-SH, β12-C6-SO2−, and β12-N6), 5 mM phosphate buffer with 25 mM NaCl (peptides PPII-C4-SH and PPII-C4-SO2−), or 5 mM phosphate buffer with 25 mM KF (peptides α14-C1-SH, α14-C1-SO2−, α14-C2-SH, α14-C2-SO2−, α14-C5-SH, α14-C5-SO2−,α14-C10-SH, and α14-C10-SO2−). Water suppression was accomplished by excitation sculpting. Additional details are in the Supporting Information.
Results
Bioinformatics analysis of cysteine sulfinic acid in protein crystal structures
Cysteine sulfinates have a range of potential interaction modes of the side chain with the protein backbone. The differences between Cys-SO2− and Cys provide a basis for potential oxidative structural control of protein structure and function. In order to understand the conformational preferences of Cys-SO2−, we investigated the structures of cysteine sulfinic acid residues in the Protein Data Bank (PDB). Cys-SO2− (ligand ID: CSD) is present in over 400 structures in the PDB. Interatomic distances, bond angles, dihedral angles, and approximate Ramachandran regions for each cysteine sulfinic acid and its adjacent residues were measured for a non-redundant subset of these structures (179 unique CSD residues from 171 proteins with less than 90% sequence identity). Due to the pKa of cysteine sulfinic acid (~ 2), nearly all of the identified CSD residues are likely to be in the sulfinate ionization state, even if the protein context substantially raises the pKa, although the absence of resolution at a proton level precludes definitive analysis.
To provide a context for how Cys-SO2− might change structure, the Cys-SO2− data were compared to those of cysteine residues, using a set of structures with less than 30% sequence identity and resolution of at least 1.1 Å, with cystine residues (disulfides) excluded, resulting in 1166 unique unmodified CYS residues from 802 proteins. These structures inherently represent significant populations of both cysteine thiol and cysteine thiolate, with the ionization state typically difficult to determine at the resolution of protein crystal structures. These structures were analyzed for their main chain and side chain torsion angles and for side chain-main chain interactions.
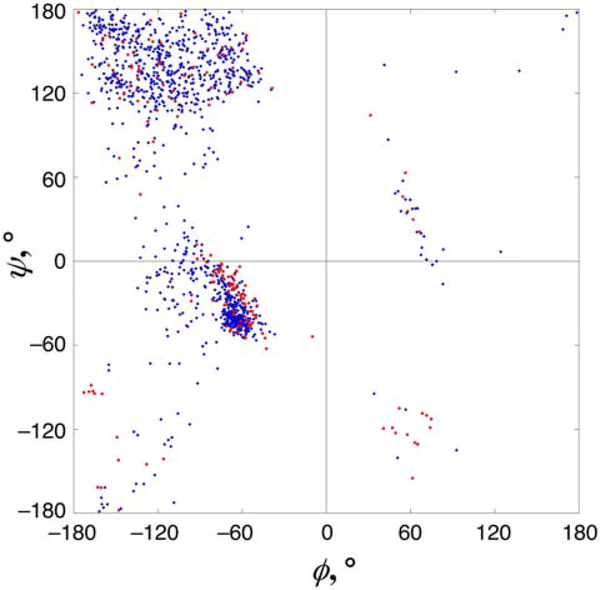
The data from the PDB indicated distinct structural preferences for Cys-SO2− compared to unmodified cysteine. The Ramachandran plot of unmodified versus sulfinated cysteine indicates a significant reduction in occupancy of the β/PPII region of the plot for Cys-SO2− compared to Cys, a moderate increase in the α-helical region, and a large relative increase in structures with ϕ > 0 (Figure 2). In general, the right side of the Ramachandran plot is lightly populated by non-Gly residues due to steric restraints.46–49 Positive ϕ angles are generally associated with β-turns.44 Cysteine sulfinic acid was four times more likely to have a positive ϕ than unmodified cysteine in structures examined (18 out of 179 Cys-SO2− residues versus 32 out of 1166 Cys residues). Glycine was the most common residue adjacent to Cys-SO2− (with at least one adjacent glycine in 30% of structures), although Cys-SO2− with ϕ > 0 was observed less often when glycine was adjacent. Cys-SO2− only rarely has similar backbone torsion angles to those of immediate neighbors (Table S1). From these data, we infer that cysteine sulfinates are usually not contained within repeating structures (β-strands or most helix types). These data instead suggest that Cys-SO2− favors turns or other liminal positions such as helical termini. Consistent with this interpretation, 26% of Cys-SO2− annotated in PDB structures as “helix” have backbone torsion angles outside the α-helical Ramachandran region, and fewer than half of Cys-SO2− residues annotated as “helix” were in similar Ramachandran regions to their neighboring residues.
Figure 2. Ramachandran plot of cysteine and cysteine sulfinic acid residues in the PDB.
Structural data for 179 cysteine sulfinic acid residues (red circles) from 171 proteins at < 3.0 Å resolution with less than 90% sequence identity were used to determine the backbone torsion angle preferences for cysteine sulfinic acid in proteins. Structural data for cysteine (blue diamonds) are based on a set of 1166 non-disulfide cysteines from 802 structures at ≤ 1.1 Å resolution with less than 30% sequence identity. Cysteine thiol/thiolate is in the β/PPII region in 54% of structures; in the α-helical region (αR) in 39% of structures; and in the left-handed α-helical region (αL) in 2% of structures. In contrast, cysteine sulfinate is in the β/PPII region in 41% of structures, in the α-helical region (αR) in 45% of structures, in the left-handed α-helical region (αL) in 3% of structures, and in the right-handed PPII region (centered on ϕ = +60°, ψ = − 120°) in 7% of structures. A small cluster of Cys-SO2− around (ϕ = −170°, ψ = −90°) represents a class of nitrile hydratases which use the sulfinate sulfur as a ligand for metal. Individual Ramachandran plots for cysteine and cysteine sulfinic acid are in the Supporting Information (Figure S28).
The structural preferences of cysteine sulfinic acid are potentially explained by local interactions of the sulfinate with the backbone (Figure 3, Figure 4).50,51 Cys-SO2− was observed to frequently engage in a hydrogen bond with its own (i) amide HN or with the following-residue (i+1) backbone amide HN. A sulfinate oxygen was within hydrogen bonding distance of the i amide (O---N distance < 3.20 Å) in 42% of Cys-SO2− residues, and in 20% of structures a sulfinate oxygen was within hydrogen bonding distance of the i+1 amide NH (Figure 4a–b). Nine structures (5%) had one oxygen within 3.20 Å of each of the adjacent amide nitrogens (Figure 3c), an interaction mode uniquely accessible to cysteine sulfinate.
Figure 3. Representative structures of cysteine sulfinic acid observed in the Protein Data Bank.
Cysteine sulfinic acid can adopt structures from multiple areas of Ramachandran space, driven by different interactions of the sulfinate side chain with the protein backbone. (a) Cys-SO2− (C126) from 1u9c (+69°, −108°) engages in a hydrogen bond between the pro-S oxygen and the amide NH of the following residue. This structure, and several others with similar backbone dihedral angles [(ϕ, ψ) ~ (+60°, −120°)] and an O---H-Ni+1 hydrogen bond, are observed as the i+1 residues of type II’ β-turns. (b) Cys-SO2− (C63) from 1dnc51 (−43°, −62°) acts as a hydrogen bond acceptor in an interaction with its own amide NH, interrupting an α-helix in conjunction with a proline at residue i+2. The sulfinate also engages in a sulfur-aromatic interaction with a bound flavin (3 S---C distances ≤ 3.5 Å). (c) In PDB 2act,50 the sulfinate of C25 (−55°, −30°) engages in hydrogen bonds with both the i and i+1 amide hydrogens, here as N-capping interactions at the N-terminus of an α-helix. (d) In PDB 4g2o, the sulfinate sulfur of C195 (−57°, +161°) engages in a short n→π* interaction with the main chain carbonyl carbon (S---C distance = 2.90 Å).
Figure 4. Side chain-main chain interaction distances of cysteine sulfinates in the PDB.
Distances between the oxygen or sulfur atoms of the cysteine sulfinate side chain and the denoted atoms of the protein backbone are indicated. (a) Minimum distances between either sulfinate oxygen and the cysteine backbone amide nitrogen. (b) Minimum distances between either sulfinate oxygen and the amide nitrogen immediately C-terminal to that cysteine. (c) Minimum distances between either sulfinate oxygen and the main chain carbonyl carbon of the cysteine. (d) Distances between the sulfinate sulfur and the carbonyl carbon of the cysteine main chain. The tetrahedral geometry (including the lone pair) around sulfur results in the oxygens being prochiral; separate analysis of the prochiral oxygens is included in the Supporting Information (Figures S31–S33).
In addition to its preference to hydrogen bond with the backbone amide, Cys-SO2− also was observed to act as an electron donor for n→π* interactions52–54 with its own carbonyl. The n→π* interactions of the sulfinate group may occur via sulfur54 (Figure 3d, Figure 4d) or via either oxygen (Figure 4c, Figure S33, Figure S34g–h). An n→π* interaction involves orbital overlap from a donor lone pair (n) into an acceptor antibonding orbital (π*), resulting in a close approach of the two atoms due to electron delocalization.52–56 The n→π* interaction in proteins is most widely associated with electron donation from a lone pair of the carbonyl oxygen of one residue into the antibonding orbital of the carbonyl carbon of the following residue, but other n→π* interactions can involve side-chain atoms as donors or acceptors.53,54,57–59 These interactions stabilize both PPII structure and α-helix structure.34,52,56,60,61
From the sulfinate moiety of Cys-SO2− in proteins, sulfur was the more common donor atom for n→π* interactions, with 41% of structures displaying S---C=O distances less than the sum of the van der Waals radii (3.5 Å) between the sulfinate sulfur and the backbone carbonyl carbon of the same residue (Figure 4d). Close sulfinate oxygen-carbonyl distances (< 3.22 Å) were also common, observed in 16% of structures (Figure 4c, Figure S33). Due to the proximity of the sulfinate S and O, often both atoms were close to the same carbon, and in these cases oxygen was more commonly observed to exhibit appropriate geometry for orbital overlap (Table S1). By comparison, unmodified cysteine had sub-van der Waals S---C=O distances in 49% of structures (Figure S27). Close S---C=O distances in cysteine were associated with β/PPII region backbone geometry (Table S3). Changes in sulfur-carbon n→π* interactions may be one factor involved in the reduced population of Cys-SO2− in β/PPII Ramachandran space.
Computational analysis of cysteine sulfinic acid conformational preferences
In order to identify the underlying causes for the structural preferences observed for Cys-SO2− in protein structures, minimal Ac-Cys-SO2−-NHMe or Ac-Cys-SO2−-NMe2 structures were generated from the original protein contexts, and these structures were subjected to geometry optimization. The analyzed structures represent 8 unique interaction modes, chosen from regions throughout Ramachandran space (Figure 5, Figure S34).50,62–66 These minimal structures were geometry optimized using density functional theory (DFT) methods.67–69 Each interaction mode observed in PDB structures was recapitulated in these calculations, indicating that each represents an inherent local energy minimum of the cysteine sulfinate, even in the absence of interactions provided by the protein structure. The wide range of favorable interactions available to the sulfinate in a minimal peptide context indicates the potential for stabilizing protein structures in diverse contexts.
Figure 5. Local backbone interactions involving cysteine sulfinate.
Cys-SO2− was observed to interact with the i and i+1 backbone amides in 8 distinct modes. Structures of minimal peptides Ac-Cys(SO2−)-NHMe were geometry optimized in Gaussian, with the M06–2X DFT method and the 6–311++G(2d,2p) basis set in implicit water, using structures from the PDB exhibiting the respective interactions and regions of Ramachandran space as initial geometries. (a) In PDB 5gk0,66 the pro-R sulfinate oxygen acts as a hydrogen bond acceptor for HN of the same residue. (b) In PDB 2nqt,63 the pro-S sulfinate oxygen engages in a hydrogen bond with HN of the same residue. (c) In PDB 3a9c,64 the pro-R sulfinate oxygen engages in a hydrogen bond with HN of the following residue. (d) In PDB 1u9c, the pro-S sulfinate oxygen engages in a hydrogen bond with HN of the following residue. (e) In PDB 2act,50 the pro-R sulfinate oxygen engages in a hydrogen bond with HN of the same residue and the pro-S sulfinate oxygen engages in a hydrogen bond with HN of the following residue. (f) In PDB 4g2o, the sulfinate sulfur acts as an electron donor for an n→π* interaction with its own carbonyl. (g) In PDB 1oki,62 the pro-R sulfinate oxygen acts as an electron donor for an n→π* interaction with its own carbonyl. (h) In PDB 4bpy,65 the pro-S sulfinate oxygen acts as an electron donor for an n→π* interaction with its own carbonyl. This structure was analyzed as Ac-Cys(SO2−)-NMe2 because the cysteine sulfinate is followed by a proline in the source protein structure. Details of geometry optimization calculations, as well as the equivalent structures in the original protein contexts (Figure S34), are in the Supporting Information.
Natural bond orbital (NBO)70 analysis was conducted, with a focus on structures with n→π* interactions. Orbital overlap between the sulfinate lone pairs and the carbonyl was observed for all donor atoms (pro-R oxygen, pro-S oxygen, sulfur), indicating favorable contribution of electron delocalization to these conformations (Figure 6). The three observed n→π* interaction modes were further explored in the simplified model compound −O2S–CH2-CH2–C(O)–NMe2. This structure was subjected to geometry optimization for each sulfonate-carbonyl interaction mode to identify the local minima. Energies of each interaction mode were then calculated by the MP2 method. In addition, a torsion angle scan of the molecule was conducted around the C-S bond (Figure 6d) to confirm that the observed preferences represent all local minima and to determine the energy barriers to bond rotation. The energy minima correspond to each of the n→π* interaction modes. The S---C=O interaction exhibited the lowest energy. In this context, with the χ1 torsion angle equivalent to +60° in Cys-SO2−, the pro-S oxygen had a minimum O---C sulfinate-carbonyl distance of 3.06 Å, the pro-R oxygen reached a minimum sulfinate-carbonyl O---C distance of 2.92 Å, both well below the 3.22 Å sum of the van der Waals radii. The minimum sulfur to carbonyl carbon distance was 3.24 Å, again well below the sum of the van der Waals radii of 3.50 Å.
Figure 6. n→π* interactions between the sulfinate sulfur or the sulfinate oxygens and the main chain carbonyl.
(a-c) Natural bond orbital (NBO) analysis of side chain-main chain interactions of the sulfinate group with the intraresidue carbonyl. Orbital overlap between the lone pairs (n) of (a) the pro-S sulfinate oxygen, (b) the pro-R sulfinate oxygen, or (c) the sulfinate sulfur and the antibonding orbital (π*) of their respective backbone carbonyls is indicative of n→π* interactions. Minimal peptide structures (a) Ac-Cys(SO2−)-NMe2 or (b, c) Ac-Cys(SO2−)-NHMe were generated from cysteine sulfinates in the PDB with side chain to carbonyl distances below the sums of their respective van der Waals radii. Minimal peptides were based on (a) 4bpy, (b) 1oki, and (c) 4g2o. These structures were geometry optimized using the M06–2X DFT method and the 6–311++G(2d,2p) basis set in implicit water. The resultant structures exhibited interaction distances and torsion angles similar to those observed in the protein crystal structures. (d) Torsion angle scan of the propylsulfinate-3-dimethylamide (−O2S-CH2-CH2-C(O)-NMe2) C-C-S-Opro-S dihedral angle. The torsion angle scan was conducted with the M06–2X DFT method and the 6–311++G(3d,3p) basis set in implicit water. The relative energies of the local minima were independently subjected to geometry optimization using the MP2 method and the 6–311++G(3d,3p) basis set in implicit water. Minima correspond to structures with a short Opro-S---C=O distance (60°, Erel = 1.6 kcal mol−1), a short Opro-R---C=O distance (180°, Erel = 0.6 kcal mol−1), and a short S---C=O distance (300°, Erel = 0.0 kcal mol−1), consistent with favorable oxygen-carbonyl or sulfur-carbonyl n→π* interactions.
Cysteine sulfinates were observed to have distinct structural preferences in protein structures. To determine how these local preferences might affect secondary structure propensities, three sets of peptides were designed based on the structural motifs that Cys-SO2− might promote (Figure 7). As a hydrogen bond acceptor, a sulfinate could nucleate α-helical structure at the helix N-terminus.71–74 In contrast, strong side chain-main chain interactions would be expected to disfavor α-helix in the interior of the α-helix.35,43 Side chains which can engage in a hydrogen bond with the amide HN of the same residue have previously been shown to promote polyproline type II (PPII) helix structure, an important structural motif in recognition and signaling.32,34,75–79 In addition, Cys-SO2− was observed in both type I’ and type II’ β-turns in the PDB, and in particular was significantly more likely to adopt ϕ > 0 than Cys, suggesting that it may have a special propensity for such structures. Type II’ β-turns in the PDB were specifically associated with a hydrogen bond between the sulfinate and the i+1 amide HN. For all of these structural motifs, the change from unmodified cysteine to cysteine sulfinic acid could potentially provide a basis for oxidative control of protein structure.
Figure 7. Peptide sequences.
Peptides were designed based on protein structural motifs, including model α-helices, a model of type II polyproline helix, and a β-hairpin model, in order to determine the structural preferences of the cysteine sulfinate. All peptides were synthesized with cysteine, cysteine sulfinic acid, or the parent residue used at the indicated site in prior studies.34,43,80,96 The α-helix and polyproline helix model peptides were acetylated on the N-termini. All peptides contained C-terminal amides.
Cysteine sulfinic acid in α-helix model peptides
Baldwin model α-helical peptides were designed, with Cys-SO2− or unmodified cysteine at the N-terminus, near the C-terminus, and at interior positions of the peptides, in order to determine the relative effects of the sulfinic acid modification on α-helicity as a function of helical position.43,73,80,81 Peptides were synthesized and characterized by circular dichroism (Figure 8, Table 1) and NMR spectroscopy (Figure 9, Table 2, Figures S14–S21). As indicated by the magnitudes of the CD minima at 222 and 208 nm, Cys-SO2− resulted in an increased population of α-helix compared to Cys-SH at position 1, 2, or 5 of a 14-residue α-helix. In contrast, Cys-SO2− exhibited lower α-helix propensity than Cys-SH at position 10 (Figure 8d, Table 1). The ability of cysteine sulfinate to stabilize an α-helix at its N-terminus is consistent with its overrepresentation at the N-terminus of α-helices in the PDB, the backbone torsion angles of Cys-SO2− observed in the PDB, and its ability to engage in hydrogen bonds with the adjacent amides.
Figure 8. Cysteine sulfinate effects on α-helicity as a function of position in the α-helix.
Circular dichroism spectra of peptides with cysteine substitution at position (a) 1, (b) 2, (c) 5, or (d) 10 in the 14-residue α-helical portion of the peptide Ac-AKAAAAKAAAAKAAGY-NH2. Data are shown with cysteine sulfinate (red diamonds, pH 7), cysteine (dark green inverted triangles, pH 4, or light green triangles, pH 8.5), and control peptide (blue circles, pH 4). Spectra were acquired at the indicated pH in 5 mM aqueous phosphate buffer with 25 mM KF at 0.5 °C. Spectra of peptides with unmodified cysteine were acquired with 0.1 mM TCEP. Data represent the average of at least 3 independent trials. Error bars indicate standard error.
Table 1.
Summary of circular dichroism data for α-helical peptides.
| peptide | [θ]222 | [θ]208 | [θ]190 | [θ]222/[θ]208 | −[θ]190/[θ]208 | % α-helix |
|---|---|---|---|---|---|---|
| α14-A | −17800 | −18800 | 31500 | 0.95 | 1.68 | 53 |
| α14-C1-SH | −14800 | −18200 | 20600 | 0.82 | 1.13 | 44 |
| α14-C1-S− | −19200 | −19800 | 35000 | 0.97 | 1.77 | 57 |
| α14-C1-SO2− | −18200 | −19700 | 32900 | 0.92 | 1.66 | 54 |
| α14-C2-SH | −16200 | −17400 | 27500 | 0.93 | 1.58 | 48 |
| α14-C2-S− | −26100 | −26000 | 47600 | 1.00 | 1.83 | 77 |
| α14-C2-SO2− | −26900 | −26600 | 51600 | 1.01 | 1.94 | 84 |
| α14-C5-SH | −8400 | −14700 | 3200 | 0.57 | 0.22 | 25 |
| α14-C5-S− | −9400 | −15700 | 6400 | 0.60 | 0.41 | 28 |
| α14-C5-SO2− | −11400 | −15800 | 12100 | 0.72 | 0.76 | 34 |
| α14-C10-SH | −9900 | −14500 | 7700 | 0.68 | 0.53 | 29 |
| α14-C10-S− | −4700 | −10900 | −2600 | 0.43 | 0.13 | 14 |
| α14-C10-SO2− | −7200 | −12500 | 1600 | 0.58 | −0.24 | 21 |
CD data were collected at pH 4 (peptides α14-A and α14-CX-SH), pH 7 (peptides α14-CX-SO2−), or pH 8.5 (peptides α14-CX-S−) in water with 5 mM phosphate buffer and 25 mM NaCl at 0.5 °C. The percent α-helix was calculated as (100% × [θ]222)/(−40000 × (1–2.5/16)). Errors are indicated in the Supporting Information (Table S8).
Figure 9. TOCSY spectra (HN-Hβ region) of peptides, comparing cysteine sulfinates and cysteine thiols.
The HN-Hβ correlations were compared for each peptide with Cys-SH (blue) and the equivalent peptide with Cys-SO2− (red) in the respective TOCSY NMR spectra. (a) Peptides α14-C1; (b) peptides α14-C2; (c) peptides α14-C5; (d) peptides α14-C10; (e) peptides PPII-C4, with minor peaks corresponding to structures with a cis-Pro amide bond; (f) peptides β12-C6. Cysteine sulfinate residues consistently exhibited more upfield and better resolved diastereotopic Hβ chemical shifts, and more downfield amide hydrogen chemical shifts, than those of the corresponding unmodified cysteine residue, consistent with greater order in the sulfinate side chain compared to the thiol. Cysteine sulfinate Hβ had an average Δδ = 0.3 ppm for the diastereotopic Hβ, while peptides with unmodified cysteine had an average Δδ < 0.1 ppm for the diastereotopic Hβ. TOCSY spectra were acquired as described in the Supporting Information. Full TOCSY spectra are in the Supporting information or in ref. 45.
Table 2.
Summary of NMR data in peptides with cysteine compared to peptides with cysteine sulfinic acid.
| peptide | 3JαN, Hz | δ, HN, ppm | δ, Hα, ppm | δ, Hβ, ppm |
|---|---|---|---|---|
| α14-C1-SH | 5.0 | 8.66 | 4.39 | 2.97 |
| α14-C1-SO2− | -a | 8.76 | 4.56 | 2.94, 2.68 |
| α14-C2-SH, | 5.4 | 8.71 | 4.41 | 2.98 |
| α14-C2-SO2− | -a | 8.86 | 4.60 | 2.89, 2.66 |
| α14-C5-SH | 6.0 | 8.49 | 4.41 | 2.98 |
| α14-C5-SO2− | -b | 8.62 | 4.53 | 2.99, 2.58 |
| α14-C10-SH | -b | 8.27 | 4.40 | 2.96 |
| α14-C10-SO2− | -b | 8.49 | 4.50 | 2.96, 2.55 |
| PPII-C4-SH | 7.2 | 8.40 | 4.73 | 2.85, 2.80d |
| PPII-C4-SO2− | 6.3 | 8.59 | -c | 2.72, 2.45 |
| β12-C6-SH | 6.1 | 9.12 | 4.36 | 3.02, 2.90 |
| β12-C6-SO2− | 4.5 | 9.36 | 4.41 | 2.87, 2.74 |
NMR data were acquired as described in the Supporting Information.
HN peaks were broadened for the cysteine sulfinate residues of peptides α14-C1-SO2− and α14-C2-SO2−, precluding measurement of coupling constants.
Resonances were not sufficiently resolved to determine 3JαN.
Hα of the peptide PPII-C4-SO2− was not observed due to water suppression.
The cysteine Hβ of the peptide PPII-C4-SH did not fully resolve, but chemical shifts for the individual resonances are estimated. Notably, the sulfinate Hβ are always upfield of the equivalent resonances in the corresponding thiol peptide, despite the theoretically decreased electron density on the sulfinate sulfur. In addition, resonances for the diastereotopic Hβ of cysteine thiols are not distinct or have less dispersion than those of Hβ in the corresponding sulfinates, indicative of more ordered conformations in the sulfinates.
The largest stabilization of the α-helix by Cys-SO2− was observed at residue 2. The effect at residue 2 here can be attributed both to favorable effects on the helical dipole (changing Lys+ to Cys-SO2−/ Cys-S−) and to the potential for Cys-SO2−/Cys-S− to order the previous residue by hydrogen bonding (α-helix capping).37,43,80 Consistent with these data, Doig has demonstrated that the introduction of Asp or Glu has the greatest effect in increasing α-helicity at residue 2.80 In contrast, after the first turn of the α-helix, side chain-main chain hydrogen bonds disrupt the hydrogen bonding pattern of the α-helix. As expected, Cys-SO2− was less effective at stabilizing the α-helix at position 5, but was still superior to Cys-SH, presumably due to the induction of a shorter α-helix starting at the sulfinate. A similar effect was observed for phosphothreonine/phosphoserine at position 5.43 Furthermore, at position 10, Cys-SO2− destabilizes the α-helix compared to Cys-SH, consistent with the sulfinate disrupting α-helicity in prior residues as at position 5, with insufficient remaining residues for a stable α-helix to form following Cys-SO2−.
NMR spectra of HN for the peptides α14-C1 and α14-C2 exhibited significantly greater dispersion in the sulfinate peptides compared to those of the peptides with thiols, consistent with greater ordering in the peptides with the sulfinates (Figures S14, S16, S18, and S20). Across all of the peptides with Cys-SO2− in this series, the HN chemical shifts of Cys-SO2− were increasingly downfield and with greater dispersion in peptides with increased α-helicity, consistent with the possibility of a hydrogen bond between the sulfinate and amide which stabilizes the α-helix. In the NMR spectra, the greater δ dispersion of Cys Hβ in the sulfinates versus the thiols indicates significantly greater ordering at the side chain for Cys-SO2− than for Cys. Indeed, Cys-SO2− exhibits distinct resonances of the diastereotopic Hβ in all cases, while most peptides with Cys thiol show a single Cys Hβ resonance (Figure 9). These data are consistent with interactions between the sulfinate and the backbone in all cases, with the effects of the sulfinate on α-helicity dependent on the position of Cys-SO2− in the peptide.
Cysteine sulfinic acid in a peptide model of the type II polyproline helix
The PPII helix is a common protein secondary structure which is particularly important in intrinsically disordered proteins and is widely involved in protein-protein interactions. 32,34,75–79 PPII notably lacks hydrogen bonds involving the backbone.82 Instead, PPII is stabilized by a combination of interresidue n→π* interactions and carbonyl-H2O interactions.33,34,75,77,79,83–87 The PPII propensity of Cys-SO2− versus Cys-SH was determined by CD and NMR in the peptide Ac-GPPCPPGY-NH2 (Figure 10, Table 3), which has previously been used to quantify the PPII propensities of all canonical amino acids.34,42,81,88–90 Cys-SH was previously observed to strongly disfavor PPII structure.32,34 Conversely, Cys-SO2− increased the PPII population within this model peptide context. The ability of Cys-SO2− to stabilize PPII was less than that of the electrostatically similar residues phosphoserine (pSer) or asparate (Asp−).32,34,42 The lower PPII propensity of Cys-SO2− was presumably due to its conformational heterogeneity and the multiple potential interactions between the side chain and main chain which were observed for Cys-SO2− in protein structures and in computational models. The low PPII propensity of Cys-SO2− is consistent with its lower population in that region of the Ramachandran plot (Figure 2). The low propensity for PPII structure suggests that the intraresidue hydrogen bond (S-O---H-N for Cys-SO2−) is not the predominant interaction mode of Cys-SO2− in this context, with the multiple modes of side chain-main chain interactions contributing to the observed conformational heterogeneity.
Figure 10. Cysteine sulfinic acid in a type II polyproline helix (PPII).
(a) The peptides Ac-GPPXPPGY-NH2, X = Cys-SO2− (red diamonds), Cys-SH (green squares), or Pro (blue circles), were examined by circular dichroism to determine the relative propensity of the cysteine sulfinic acid for PPII structure, as indicated by the magnitude of the local maximum around 228 nm. Spectra were acquired at 25 °C in 5 mM aqueous phosphate buffer pH 7 with 25 mM KF. Data represent the average of at least 3 independent trials. Error bars indicate standard error. (b) Cysteine sulfinic acid [(ϕ, ψ) = (−77°, +147°)] in the middle of a 3 residue PPII helix (Arg-Cys-SO2−-Phe) in PDB 5g5g.90 The Cys-SO2− side chain does not interact with either the protein backbone or the adjacent side chains in this structure. Intercarbonyl n→π* interactions between consecutive residues, characteristic of PPII structure, are indicated in red.
Table 3.
Summary of circular dichroism data for polyproline II helix model peptides.
| λmax, nm | C4 3JαN, Hz | [θ]228, deg cm2 dmol−1 | [θ] at λmax, deg cm2 dmol−1 | |
|---|---|---|---|---|
| PPII-P4 | 230 | - | 3000 ± 170 | 3070 ± 250 |
| PPII-C4-SH | 232 | 7.2 | −390 ± 260 | 90 ± 180 |
| PPII-C4-SO2− | 231 | 6.2 | 340 ± 120 | 1290 ± 180 |
CD data were collected at pH 4 in an aqueous solution of 5 mM phosphate buffer with 25 mM NaCl at 25 °C. Polyproline II helix is indicated by the magnitude of the local maximum around 228 nm. The intensity ([θ]228) and wavelength (λmax) of this local maximum CD signal are used to determine PPII helix propensity. The 3JαN (vicinal coupling constant between the amide (HN) and alpha (Hα) hydrogens of the same residue) of Cys-SO2− indicates a more compact conformation in ϕ than that of Cys-SH, which is consistent with a greater PPII propensity for Cys-SO2−.
The 3JαN of the cysteine sulfinate in the PPII model peptides is smaller than that of the equivalent cysteine thiol (Table 3) and those of most other residues in this context, which indicates a more compact structure in ϕ at this residue, consistent with increased PPII compared to Cys.34,42 In the NMR spectra of the respective cysteine Hβ of the peptides PPII-C4-SH and PPII-C4-SO2−, the significantly greater dispersion of the diastereotopic hydrogens implies greater order in the cysteine sulfinate than the cysteine thiol (Figure 9e). However, multiple interaction modes could each lead to increased order, with only one of these modes expected to stabilize PPII. In addition, in the PDB, Cys-SO2− was observed in a PPII helix in one structure (PDB: 5g5g, Figure 9b). Notably, in this structure the side chain does not interact with either the protein backbone or the adjacent side chains.
Cysteine sulfinic acid in a β-hairpin model peptide
Cys-SO2− was observed at the i+1 position in type I’ and II’ β turns and at the i+2 position of type II and I’ β turns in the PDB (Table S1). Cys-SO2− in type II’ turns consistently exhibited an interresidue hydrogen bond between a sulfinate oxygen and the following amide HN (residue i+2 of the turn). To investigate the ability of the sulfinate to stabilize a β-turn structure, β-hairpin model peptides were synthesized with Cys-SH or Cys-SO2− replacing Asn at position i+1 of an NG type I’ β-turn.44,91–94 The structural effects of Cys-SO2− were compared to those of Cys-SH and of Asn at the i+1 position of the turn.
CD data suggest that Cys-SO2− exhibited a higher population of β-hairpin than Cys-SH, with a CD spectrum more similar to that of Asn (Figure 11).95 Specifically, the peptide β12-C6-SO2− showed a greater local minimum around 218 nm associated with β structure, and a smaller minimum around 195 nm that is associated with random coil.
Figure 11. Cysteine sulfinic acid in a β-turn.
(a) Circular dichroism spectra of the peptides RYVEVXGOKILQ-NH2, X = Asn (blue circles), Cys-SH (green squares), or Cys-SO2− (red diamonds). Spectra were acquired at 25 °C in 100 mM aqueous acetate buffer pH 3.8. Data represent the average of at least 3 independent trials. Error bars indicate standard error. (b) Cysteine sulfinate (+52°, −105°) is the i+1 residue in a type II’ β-turn in PDB 1din,95 engaging in a hydrogen bond with the backbone HN of the following residue.
In order to obtain residue-specific data on structure, peptides were analyzed by 1-D, ROESY, and TOCSY NMR spectroscopy. Collectively, the NMR data indicate that cysteine sulfinate promotes the β-hairpin conformation more effectively than cysteine thiol, as indicated by the more upfield Hα chemical shifts of the β-strand residues (Figure 12). Residues 2–5 and 8–11 showed upfield Hα shifts for all peptides, but peptide β12-C6-SO2− was more similar to peptide β12-N6 than was peptide β12-C6-SH. In addition, greater dispersion of the HN chemical shifts indicated that Cys-SO2− was more favorable in promoting the β-hairpin conformation than Cys-SH (Figure S4). ROESY spectra indicated similar cross-strand interactions of tyrosine in the Cys-SO2− and Cys-SH peptides (Figures S11–S12), as had been observed in the peptide with asparagine.91,96,97 Through-space interactions between Hα were also similar. One exception was a correlation between the respective Hα of E4 and K9 that was observed for β12-C6-SO2−, but not β12-C6-SH (Figure S13). This through-space correlation was also the only strong cross-strand Hα-Hα interaction originally observed for the peptide with NG.91 These data suggest that Cys-SO2− in this peptide context better promotes a β-turn than Cys-SH does, with β-hairpin structure more comparable to Asn.
Figure 12. Hα chemical shifts of the peptides β-N6, β-C6-SH, and β-C6-SO2− compared to random coil values.
Random coil chemical shifts (δ) for each respective residue in the peptides β-N6 (blue), β-C6-SH (green), and β-C6-SO2− (red) were subtracted from the chemical shifts of the residues observed in these peptides. By chemical shift index (CSI) analysis, the Hα chemical shift of each residue correlates with secondary structure, with downfield shifts (Δδ > 0.1 ppm) indicative of β-strand structure and upfield shifts (negative Δδ < −0.1 ppm) indicative of α-helical structure. Compared to random coil values for each residue, the chemical shifts are downfield except for the terminal residues (due to fraying) and the β-turn residues (not expected to be in the extended conformation), with β-N6 generally showing the most positive Δδ, while β-C6-SH generally shows the least positive Δδ. These data indicate that Cys-SO2− is superior to Cys-SH in the i+1 position of the β turn, consistent with Cys-SO2− adopting backbone geometry with ϕ > 0 more readily than Cys-SH. Cys-SO2− is compatible with both type I’ and type II’ β turns, but was more commonly observed in the latter in the PDB. Random coil chemical shift values were from ref. 103. The Cys-SO2− Hα was compared to the random coil chemical shift for cysteine, as there is not a literature value for the random coil chemical shift of cysteine sulfinate.
One key difference between the peptides β12-N6 and β12-C6-SO2− was observed at the i+1 and i+2 residues of the β-turn. Here, the vicinal coupling constant between the HN and Hα (3JαN) was significantly smaller for Cys-SO2−, and significantly larger for Gly, than the comparable values in the peptide β12-N6 (Table 4). The 3JαN of a residue correlates with its ϕ torsion angle.98 The small 3JαN of the cysteine sulfinate and the large 3JαN of the following glycine in the peptide β12-C6-SO2− are consistent with the torsion angles of a type II’ β-turn [(+60°, −120°) and (−80°, 0°) for residues i+1and i+2, respectively].44 Either a type I’ or type II’ β-turn would require ϕ > 0, which Cys-SO2− was more likely to adopt in proteins than Cys-SH was. In the PDB, cysteine sulfinic acid was more commonly found in type II’ β-turns, often with a hydrogen bond between one of the sulfinate oxygens and the i+1 amide NH. The sulfinate appears to favor this β-turn structure, despite the number of competing interactions in which the Cys-SO2− might engage. Globally, the bioinformatics, computational, and experimental data suggest that Cys-SO2− might be particularly effective in promoting the β-turn conformation compared to Cys-SH.
Table 4.
Summary of NMR data for β-hairpin model peptides.
| δ, Hα, ppm | 3JαN, Hz | |||||
|---|---|---|---|---|---|---|
| X = N | X = C-SO2− | X = C-SH | X = N | X = C-SO2− | X = C-SH | |
| R | 4.05 | 4.05 | 4.04 | - | - | - |
| Y | 4.99 | 4.90 | 4.83 | 7.6 | 7.3 | 7.2 |
| V | 4.16 | 4.16 | 4.10 | nr | nr | 9.4 |
| E | 4.67 | 4.56 | 4.56 | 7.6 | nr | nr |
| V | 4.23 | 4.16 | 4.16 | 9.2 | nr | nr |
| X | 4.54 | 4.41 | 4.36 | 5.8 | 4.5 | 6.1 |
| G | 4.00, 3.80 | 3.98, 3.86 | 3.94 | 5.8 | 8.5 | nr |
| O | 4.53 | 4.47 | 4.44 | 8.0 | nr | 8.0 |
| K | 4.44 | 4.41 | 4.37 | 7.0 | nr | 7.0 |
| I | 4.37 | 4.30 | 4.25 | 9.0 | nr | nr |
| L | 4.23 | 4.26 | 4.30 | nr | 6.8 | 6.9 |
| Q | 4.29 | 4.28 | 4.28 | 7.6 | nr | nr |
NMR data were acquired at pH 3.9 in 100 mM deuterated sodium acetate in a solution of 10% D2O in H2O at 4 °C. Resonances indicated as “nr” were not sufficiently resolved to determine coupling constants. Notably, Cys-SO2− has a small 3JαN, which indicates a compact conformation in ϕ at this residue. The 3JαN of this residue and the following glycine are consistent with a type II’ β-turn, while those of the asparagine and glycine in peptide β12-N6 are consistent with either a type I’ or type II’ β-turn (this peptide adopts a type I’ β-turn). In the peptide β12-C6-SH, the diastereotopic glycine Hα do not have distinct chemical shifts, indicating less order round that residue.
Discussion
The structural preferences of cysteine sulfinates were examined within protein structures, via bioinformatics, and within model peptide contexts, via biophysical and computational approaches. Collectively, these data indicate that Cys-SO2− induces significant conformational bias toward interactions with the local backbone, with the sulfinate engaging in side chain-main chain hydrogen bonds and n→π* interactions. Hydrogen bonds between sulfinates and their local protein backbone likely represent new preferred conformations for Cys-SO2− compared to the equivalent cysteines, due to the increase in side chain length of Cys-SO2−, with the added oxygens, and the relatively modest ability of sulfur to act as a hydrogen bond acceptor. Hydrogen bonds between sulfinate oxygens and local backbone amide hydrogens were associated with structures throughout Ramachandran space, and appeared to stabilize both α-helical and β-turn structures. The longer bonds of sulfur to oxygen, and the increased electron density on the oxygens, potentially make cysteine sulfinate a better hydrogen bond acceptor than aspartate. In addition, the unique geometry of Cys-SO2− allows the sulfinate to engage in hydrogen bonds with both the i and i+1 amides simultaneously.
Cys-SO2− in the PDB was observed to engage in numerous n→π* interactions which could impose significant structural bias on the conformations of the side chain, backbone, and adjacent residues. As an electron donor for n→π* interactions, the sulfinate moiety has a unique tridentate nature, with lone pairs on sulfur and on each oxygen capable of interactions. In contrast, unmodified cysteine can only act as an electron donor for n→π* interactions via sulfur.54,99 Because the thiol does not have the wide range of alternative backbone interactions which were observed for the sulfinate, thiols more commonly exhibited the sulfur-carbonyl n→π* interaction. Thus, although n→π* interactions involving sulfur are presumably usually preserved from thiol to sulfinate, these interactions are often apparently abrogated upon sulfination by steric clash with the oxygens or competition from alternate interactions. Newly formed hydrogen bonds and gain or loss of n→π* interactions as a result of cysteine sulfination provide a significant basis for structural changes in proteins.
Most instances of Cys-SO2− in the PDB are apparently products of oxidation of proteins during protein purification or crystallization, rather than a result of intentional oxidation to the sulfinic acid. Therefore, the backbone torsion angles we observed for Cys-SO2− likely reflect a combination of the preferences imposed by the sulfinate and the structural contexts in which cysteine is most likely to be oxidized to the sulfinate. The residues adjacent to Cys-SO2− observed herein are presumably indicative of the local sequence context of a cysteine most likely to be oxidized to the sulfinic acid. This interpretation is consistent with the overrepresentation of glycine residues adjacent to Cys-SO2−, as Gly is least likely to interfere have steric interference with the oxidation reactions or with the increase in size of cysteine upon sulfination. Cysteine residues were not observed adjacent to Cys-SO2− in the PDB, potentially due to the ability of Cys to reduce the sulfenic acid that is an intermediate in sulfinic acid formation. In addition to glycine, aromatic residues (F, W, Y) and methionine were all more common in positions adjacent to Cys-SO2− compared to Cys, while polar and charged residues (D, E, K, N, Q, R) were underrepresented. Cys-SO2− could also potentially be generated by oxidation of a cysteine disulfide via the thiosulfinate intermediate, although we could not identify a Cys that was otherwise exclusively annotated as a disulfide.20
Many observed Cys-SO2− in the PDB are likely not functionally relevant. However, in a number of the structures with Cys-SO2− that were analyzed, sulfination has been specifically identified to be functionally important, including in the proteins DJ-1, nitrile hydratases, peroxyredoxins, β-actin, and Pin1.13–17,100,101 In addition, a number of proteins identified via proteomics to be reversibly sulfinated in cells10 were also observed with Cys-SO2− and examined herein, including glyceraldehyde-3-phosphate dehydrogenase, aspartate aminotransferase, and cathepsins. In addition, cysteine proteases were common among structures with Cys-SO2−, suggesting that these proteins might be particularly susceptible to sulfination, presumably due to the lower Cys pKa and high reactivity necessary for their enzymatic activity.
Model peptides indicated that cysteine sulfination can significantly stabilize an α-helix when the sulfinate is near the α-helical N-terminus. Conversely, Cys-SO2− was disruptive to α-helicity near the central residues of the α-helix. These data are consistent with the bioinformatics data, which indicate a greater preference for the α-helical region for Cys-SO2− compared to Cys. The ability of Cys-SO2− to act as a hydrogen bond acceptor for adjacent amide hydrogens can impose local order, while also capping the otherwise unsatisfied hydrogen bond donors at the N-terminus of an α-helix. Notably, pSer/pThr exhibited similar position-dependent effects on α-helicity in the same context.37,38,43 Cys-SO2− was able to favor or disfavor α-helix structure by over 0.5 kcal/mol compared to Cys-SH, depending on its position in the peptide. In the PDB, Cys-SO2− was often found at the N-terminus of α-helices, where its backbone hydrogen bonds could stabilize the helix. Overall, 45% of sulfinates were in the α-helical region (80 out of 179 structures); 72% of cysteine sulfinates with close O---Ni distances consistent with an intraresidue hydrogen bond were in the α-helical region (55 of 76 structures), while close S---C=O or O---C=O distances were significantly underrepresented in the α-helical region (19 of 74 and 5 of 29 structures respectively, less than 25% total). Most Cys-SO2− residues in the α-helical region of the Ramachandran plot had significantly divergent backbone dihedral angles compared to the adjacent residues (Table S1), suggesting that Cys-SO2− is more likely to be near α-helical termini than in the interior of α-helices.
We previously observed increased PPII propensity for residues (Asp−, pSer, pThr) with similar chemical properties to Cys-SO2−.32,34,43 The higher PPII propensity of these residues was attributed to properties they have in common with Cys-SO2−, in particular their ability to engage in intraresidue O---H-N hydrogen bonds. Cys-SO2− was less effective in promoting PPII structure in this model peptide context, although it exhibited greater PPII propensity than Cys-SH. While an intraresidue hydrogen bond can promote PPII, any n→π* interactions between the side chain sulfinate and the backbone carbonyls would compete with the n→π* interactions that stabilize PPII.102 The only structure in the PDB in which Cys-SO2− was observed within a multi-residue PPII helix did not exhibit any interaction of the sulfinate with the backbone or with neighboring side chains (Figure 10b). Collectively, these data suggest that Cys-SO2− exhibits only modest propensity for the polyproline II helix.
Cys-SO2− in proteins was frequently observed in β-turns, present at the i+1 position of type I’ and type II’ β-turns, and at the i+2 position of type I’ and type II β-turns. In β-turns, the sulfinate often engaged in an interaction with the local backbone. At the i+1 position of the β-turn in a peptide model, Cys-SO2− significantly increased the β-hairpin population compared to Cys-SH. However, the peptide β12-C6-SO2− exhibited decreased β-hairpin stability compared to the peptide β12-N6. The reduced β-turn propensity of Cys-SO2− compared to Asn is likely due in significant part to the unique suite of local interactions in which Cys-SO2− can engage. The β-hairpin model peptide H-RYVEVXGOKILQ-NH2 has many hydrogen bond donors (bold) in addition to the i+1 amide, providing competition for a hydrogen bond with the following amide HN, which was consistently observed in type II’ β-turns (Figure 11b). In addition, a short model peptide is solvent exposed, leading to further competition for hydrogen bonds. Overall, sulfination of cysteine increases the preference of the residue to participate in a β-turn. In a protein context which is predisposed to such a conformational change, for example by excluding water or lacking competing hydrogen bond donors, this preference could potentially result in substantial structural changes in a protein due to oxidation.
Across all peptides examined, Cys-SO2− exhibited increased order compared to Cys-SH, as evidenced by the increased dispersion of the chemical shifts of the diastereotopic β hydrogens (Figure 9, Table 2). The Hβ of Cys-SO2− had chemical shifts that differed by 0.1–0.4 ppm in peptides examined herein. In contrast, in all cases except the peptide β12-C6-SH, the Cys-SH β hydrogens did not fully resolve, indicating a lack of order in 5 of the 6 peptide contexts examined. The order observed for cysteine in peptide β12-C6-SH might reflect a S---C=O n→π* interaction, which was common for unmodified cysteine in the β/PPII region, since the thiol is a poor donor or acceptor for hydrogen bonds. Induction of order upon sulfination is strongly evident in varied sequences, suggesting multiple mechanisms by which cysteine sulfination could lead to oxidation-induced changes in protein structure and function.
Conclusions
We have examined the structural preferences of the cysteine sulfinic acid in protein crystal structures and in model peptides. Cys-SO2− was observed to be common in α-helices in protein structures, and to promote α-helical structure at the N-terminus of model peptides. Cys-SO2− adopted β-turn geometries in protein structures, and promoted a β-turn at the i+1 position of a β-turn in a β-hairpin model peptide. Cys-SO2− promotes PPII compared to Cys-SH, but still exhibits only modest PPII propensity. Cys-SO2− especially stabilizes the α-helix near its N-terminus, where it can potentially serve as a hydrogen-bonding N-cap for otherwise unsatisfied amide hydrogen bond donors and can stabilize the α-helical macrodipole. Cys-SO2− can adopt structures across a wide range of Ramachandran space via multiple side chain-main chain non-covalent interactions which are collectively not possible for unmodified cysteine or for other canonical residues. Non-covalent interactions are crucial to protein structure, and the unique steric and electronic properties of Cys-SO2− suggest possibilities for how cysteine oxidation to the sulfinic acid could modify protein function. This work also provides parameters for application in protein design of oxidatively tunable protein structures based on Cys-SO2− and its extensive and unique range of interactions with the protein backbone.
Supplementary Material
Cysteine sulfinic acid exhibits distinct structural preferences compared to Cys
Cysteine sulfinate is Lewis basic at sulfur and at both oxygens
The sulfinate promotes hydrogen bonds with nearby backbone amides
The sulfinate engages in intercarbonyl n→π* interactions via S or O
Cysteine sulfinate promotes α-helix and β-turn conformations compared to Cys
Acknowledgements
This work was supported by NIH (GM093225) and NSF (CBET-1403532 and BIO-1616490). The authors thank Dr. Himal Ganguly for assistance with bioinformatics analysis.
Footnotes
Supporting Information Available
This material is available free of charge at the journal web page.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Klomsiri C; Karplus PA; Poole LB Cysteine-Based Redox Switches in Enzymes. Antioxid. Redox Signaling 2011, 14, 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Lo Conte M; Carroll KS The Redox Biochemistry of Protein Sulfenylation and Sulfinylation. J. Biol. Chem. 2013, 288, 26480–26488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Paulsen CE; Carroll KS Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mailloux RJ; Jin XL; Willmore WG Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biology 2014, 2, 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Poole LB The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yang J; Carroll KS; Liebler DC The Expanding Landscape of the Thiol Redox Proteome. Mol. Cell. Proteomics 2016, 15, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chauvin JPR; Pratt DA On the Reactions of Thiols, Sulfenic Acids, and Sulfinic Acids with Hydrogen Peroxide. Angew. Chem., Int. Ed. 2017, 56, 6255–6259. [DOI] [PubMed] [Google Scholar]
- (8).Alcock LJ; Perkins MV; Chalker JM Chemical methods for mapping cysteine oxidation. Chem. Soc. Rev. 2018, 47, 231–268. [DOI] [PubMed] [Google Scholar]
- (9).Fass D; Thorpe C Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 293–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Akter S; Fu L; Jung Y; Lo Conte M; Lawson JR; Lowther WT; Sun R; Liu KK; Yang J; Carroll KS Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat. Chem. Biol. 2018, 14, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Paulech J; Liddy KA; Engholm-Keller K; White MY; Cordwell SJ Global Analysis of Myocardial Peptides Containing Cysteines With Irreversible Sulfinic and Sulfonic Acid Post-Translational Modifications. Mol. Cell. Proteomics 2015, 14, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rahuel-Clermont S; Toledano MB Parsing protein sulfinic acid switches. Nat. Chem. Biol. 2018, 14, 991–993. [DOI] [PubMed] [Google Scholar]
- (13).Woo HA; Chae HZ; Hwang SC; Yang KS; Kang SW; Kim K; Rhee SG Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 2003, 300, 653–656. [DOI] [PubMed] [Google Scholar]
- (14).Lei K; Townsend DM; Tew KD Protein cysteine sulfinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene 2008, 27, 4877–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Canet-Aviles RM; Wilson MA; Miller DW; Ahmad R; McLendon C; Bandyopadhyay S; Baptista MJ; Ringe D; Petsko GA; Cookson MR The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 9103–9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Blackinton J; Lakshminarasimhan M; Thomas KJ; Ahmad R; Greggio E; Raza AS; Cookson MR; Wilson MA Formation of a Stabilized Cysteine Sulfinic Acid Is Critical for the Mitochondrial Function of the Parkinsonism Protein DJ-1. J. Biol. Chem. 2009, 284, 6476–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kiss R; Zhu M; Jojart B; Czajlik A; Solti K; Forizs B; Nagy E; Zsila F; Beke-Somfai T; Toth G Structural features of human DJ-1 in distinct Cys106 oxidative states and their relevance to its loss of function in disease. BBA Gen. Subj. 2017, 1861, 2619–2629. [DOI] [PubMed] [Google Scholar]
- (18).Joensson TJ; Murray MS; Johnson LC; Lowther WT Reduction of cysteine sulfinic acid in peroxiredoxin by sulfiredoxin proceeds directly through a sulfinic phosphoryl ester intermediate. J. Biol. Chem. 2008, 283, 23846–23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lowther WT; Haynes AC Reduction of Cysteine Sulfinic Acid in Eukaryotic, Typical 2-Cys Peroxiredoxins by Sulfiredoxin. Antioxid. Redox Signaling 2011, 15, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Perkins A; Parsonage D; Nelson KJ; Ogba OM; Cheong PHY; Poole LB; Karplus PA Peroxiredoxin Catalysis at Atomic Resolution. Structure 2016, 24, 1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Katayama Y; Hashimoto K; Nakayama H; Mino H; Nojiri M; Ono T; Nyunoya H; Yohda M; Takio K; Odaka M Thiocyanate hydrolase is a cobalt-containing metalloenzyme with a cysteine-sulfinic acid ligand. J. Am. Chem. Soc. 2006, 128, 728–729. [DOI] [PubMed] [Google Scholar]
- (22).Fu XY; Kassim SY; Parks WC; Heinecke JW Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [DOI] [PubMed] [Google Scholar]
- (23).Lo Conte M; Lin JS; Wllson MA; Carroll KS A Chemical Approach for the Detection of Protein Sulfinylation. ACS Chem. Biol. 2015, 10, 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lo Conte M; Carroll KS Chemoselective Ligation of Sulfinic Acids with Aryl-Nitroso Compounds. Angew. Chem., Int. Ed. 2012, 51, 6502–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Corpuz N; Schwans JP Generation of a cysteine sulfinic acid analog for incorporation in peptides using solid phase peptide synthesis. Bioorg. Med. Chem. Lett. 2017, 27, 2410–2414. [DOI] [PubMed] [Google Scholar]
- (26).Kuo YH; Konopko AM; Borotto NB; Majmudar JD; Haynes SE; Martin BR Profiling Protein S-Sulfination with Maleimide-Linked Probes. ChemBioChem 2017, 18, 2028–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Griesser M; Chauvin JPR; Pratt DA The hydrogen atom transfer reactivity of sulfinic acids. Chem. Sci. 2018, 9, 7218–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).O’Neil KT; Degrado WF A Thermodynamic Scale for the Helix-Forming Tendencies of the Commonly Occurring Amino-Acids. Science 1990, 250, 646–651. [DOI] [PubMed] [Google Scholar]
- (29).Minor DL; Kim PS Measurement of the Beta-Sheet-Forming Propensities of Amino-Acids. Nature 1994, 367, 660–663. [DOI] [PubMed] [Google Scholar]
- (30).Smith CK; Withka JM; Regan L A Thermodynamic Scale for the Beta-Sheet Forming Tendencies of the Amino-Acids. Biochemistry 1994, 33, 5510–5517. [DOI] [PubMed] [Google Scholar]
- (31).Pace CN; Scholtz JM A helix propensity scale based on experimental studies of peptides and proteins. Biophys. J. 1998, 75, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rucker AL; Pager CT; Campbell MN; Qualls JE; Creamer TP Host-Guest Scale of Left-Handed Polyproline II Helix Formation. Proteins 2003, 53, 68–75. [DOI] [PubMed] [Google Scholar]
- (33).Shi Z; Chen K; Liu Z; Ng A; Bracken WC; Kallenbach NR Polyproline II propensities from GGXGG peptides reveal an anticorrelation with b-sheet scales. Proc. Natl. Acad. Sci. USA 2005, 102, 17964–17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Brown AM; Zondlo NJ A Propensity Scale for Type II Polyproline Helices (PPII): Aromatic Amino Acids in Proline-Rich Sequences Strongly Disfavor PPII Due to Proline-Aromatic Interactions. Biochemistry 2012, 51, 5041–5051. [DOI] [PubMed] [Google Scholar]
- (35).Szilak L; Moitra J; Krylov D; Vinson C Phosphorylation destabilizes a-helices. Nature Struct. Biol. 1997, 4, 112–114. [DOI] [PubMed] [Google Scholar]
- (36).Bienkiewicz EA; Lumb KJ Random-coil chemical shifts of phosphorylated amino acids. J. Biomol. NMR 1999, 15, 203–206. [DOI] [PubMed] [Google Scholar]
- (37).Andrew CD; Warwicker J; Jones GR; Doig AJ Effect of phosphorylation on a-helix stability as a function of position. Biochemistry 2002, 41, 1897–1905. [DOI] [PubMed] [Google Scholar]
- (38).Signarvic RS; DeGrado WF De Novo Design of a Molecular Switch: Phosphorylation-Dependent Association of Designed Peptides. J. Mol. Biol. 2003, 334, 1–12. [DOI] [PubMed] [Google Scholar]
- (39).Lee CW; Ferreon JC; Ferreon ACM; Arai M; Wright PE Graded enhancement of p53 binding to CREB-binding protein (CBP) by multisite phosphorylation. Proc. Natl. Acad. Sci. USA 2010, 107, 19290–19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bah A; Vernon RM; Siddiqui Z; Krzeminski M; Muhandiram R; Zhao C; Sonenberg N; Kay LE; Forman-Kay JD Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 2015, 519, 106–109. [DOI] [PubMed] [Google Scholar]
- (41).Bielska AA; Zondlo NJ Hyperphosphorylation of tau induces local polyproline II helix. Biochemistry 2006, 45, 5527–5537. [DOI] [PubMed] [Google Scholar]
- (42).Brister MA; Pandey AK; Bielska AA; Zondlo NJ OGlcNAcylation and Phosphorylation Have Opposing Structural Effects in tau: Phosphothreonine Induces Particular Conformational Order. J. Am. Chem. Soc. 2014, 136, 3803–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Elbaum MB; Zondlo NJ OGlcNAcylation and Phosphorylation Have Similar Structural Effects in α-Helices: Post-Translational Modifications as Inducible Start and Stop Signals in α-Helices, with Greater Structural Effects on Threonine Modification. Biochemistry 2014, 53, 2242–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hutchinson EG; Thornton JM A revised set of potentials for beta-turn formation in proteins. Protein Sci. 1994, 3, 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Urmey AR; Zondlo NJ Synthesis of Peptides with Cysteine Sulfinic Acid via the Cysteine Methoxybenzyl Sulfone. Peptide Sci. 2019, e24137. [Google Scholar]
- (46).Swindells MB; MacArthur MW; Thornton JM Intrinsic phi,psi propensities of amino-acids, derived from the coil regions of known structures. Nature Struct. Biol. 1995, 2, 596–603. [DOI] [PubMed] [Google Scholar]
- (47).Pal D; Chakrabarti P On residues in the disallowed region of the Ramachandran map. Biopolymers 2002, 63, 195–206. [DOI] [PubMed] [Google Scholar]
- (48).Hollingsworth SA; Karplus PA A fresh look at the Ramachandran plot and the occurrence of standard structures in proteins. Biomol. Concepts 2010, 1, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ting D; Wang GL; Shapovalov M; Mitra R; Jordan MI; Dunbrack RL Neighbor-Dependent Ramachandran Probability Distributions of Amino Acids Developed from a Hierarchical Dirichlet Process Model. PLoS Comput. Biol. 2010, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Baker EN; Dodson EJ Crystallographic Refinement of the Structure of Actinidin at 1.7 a Resolution by Fast Fourier Least-Squares Methods. Acta Cryst. A 1980, 36, 559–572. [Google Scholar]
- (51).Becker K; Savvides SN; Keese M; Schirmer RH; Karplus PA Enzyme inactivation through sulfhydryl oxidation by physiologic NO-carriers. Nature Struct. Biol. 1998, 5, 267–271. [DOI] [PubMed] [Google Scholar]
- (52).Bartlett GJ; Choudhary A; Raines RT; Woolfson DN n->pi* interactions in proteins. Nat. Chem. Biol. 2010, 6, 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Newberry RW; Raines RT The n->pi* interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kilgore HR; Raines RT n ->pi* Interactions Modulate the Properties of Cysteine Residues and Disulfide Bonds in Proteins. J. Am. Chem. Soc. 2018, 140, 17606–17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Bretscher LE; Jenkins CL; Taylor KM; DeRider ML; Raines RT Conformational Stability of Collagen Relies on a Stereoelectronic Effect. J. Am. Chem. Soc. 2001, 123, 777–778. [DOI] [PubMed] [Google Scholar]
- (56).Wenzell NA; Ganguly HK; Bhatt MR; Yap GPA; Zondlo NJ Electronic and steric control of n→π* interactions via N-capping: stabilization of the α-helix conformation without a hydrogen bond. ChemBioChem 2019, 20, 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Choudhary A; Kamer KJ; Raines RT An n ->pi* Interaction in Aspirin: Implications for Structure and Reactivity. J. Org. Chem. 2011, 76, 7933–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Newberry RW; Bartlett GJ; VanVeller B; Woolfson DN; Raines RT Signatures of n ->pi* interactions in proteins. Protein Sci. 2014, 23, 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Newberry RW; Raines RT A Key n ->pi* Interaction in N-Acyl Homoserine Lactones. ACS Chem. Biol. 2014, 9, 880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Choudhary A; Raines RT Signature of n ->pi* interactions in alpha-helices. Protein Sci. 2011, 20, 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Zondlo NJ Fold Globally, Bond Locally. Nat. Chem. Biol. 2010, 6, 567–568. [DOI] [PubMed] [Google Scholar]
- (62).Van Montfort RLM; Bateman OA; Lubsen NH; Slingsby C Crystal structure of truncated human beta B1-crystallin. Protein Sci. 2003, 12, 2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Cherney LT; Cherney MM; Garen CR; Niu CY; Moradian F; James MNG Crystal structure of N-acetyl-gamma-glutamyl-phosphate reductase from Mycobacterium tuberculosis in complex with NADP(+). J. Mol. Biol. 2007, 367, 1357–1369. [DOI] [PubMed] [Google Scholar]
- (64).Nakamura A; Fujihashi M; Aono R; Sato T; Nishiba Y; Yoshida S; Yano A; Atomi H; Imanaka T; Miki K Dynamic, Ligand-dependent Conformational Change Triggers Reaction of Ribose-1,5-bisphosphate Isomerase from Thermococcus kodakarensis KOD1. J. Biol. Chem. 2012, 287, 20784–20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Blundell K; Hough MA; Vijgenboom E; Worrall JAR Structural and mechanistic insights into an extracytoplasmic copper trafficking pathway in Streptomyces lividans. Biochem. J. 2014, 459, 525–538. [DOI] [PubMed] [Google Scholar]
- (66).Mori T; Awakawa T; Shimomura K; Saito Y; Yang D; Morita H; Abe I Structural Insight into the Enzymatic Formation of Bacterial Stilbene. Cell Chem. Biol. 2016, 23, 1468–1479. [DOI] [PubMed] [Google Scholar]
- (67).Zhao Y; Truhlar DG The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- (68).Raghavachari K; Binkley JS; Seeger R; Pople JA Self-Consistent Molecular Orbital Methods. 20. Basis sets for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar]
- (69).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA; Nakatsuji H; Caricato M; Li X; Hratchian HP; Izmaylov AF; Bloino J; Zheng G; Sonnenberg JL; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Montgomery, J. JA; Peralta JE; Ogliaro F; Bearpark M; Heyd JJ; Brothers E; Kudin KN; Staroverov VN; Keith T; Kobayashi R; Normand J; Raghavachari K; Rendell A; Burant JC; Iyengar SS; Tomasi J; Cossi M; Rega N; Millam JM; Klene M; Knox JE; Cross JB; Bakken V; Adamo C; Jaramillo J; Gomperts R; Stratmann RE; Yazyev O; Austin AJ; Cammi R; Pomelli C; Ochterski JW; Martin RL; Morokuma K; Zakrzewski VG; Voth GA; Salvador P; Dannenberg JJ; Dapprich S; Daniels AD; Farkas O; Foresman JB; Ortiz JV; Cioslowski J; Fox DJ: Gaussian 09, Revision D.01. Gaussian, Inc.: Wallingford, CT, 2013. [Google Scholar]
- (70).Glendening CR; Landis CR; Weinhold F Natural bond orbital methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar]
- (71).Serrano L; Fersht AR Capping and Alpha-Helix Stability. Nature 1989, 342, 296–299. [DOI] [PubMed] [Google Scholar]
- (72).Lyu PC; Zhou HX; Jelveh N; Wemmer DE; Kallenbach NR Position-dependent stabilizing effects in a-helices: N-terminal capping in synthetic model peptides. J. Am. Chem. Soc. 1992, 114, 6560–6562. [Google Scholar]
- (73).Doig AJ; Baldwin RL N- and C-Capping Preferences for All 20 Amino-Acids in Alpha-Helical Peptides. Protein Sci. 1995, 4, 1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Aurora R; Rose GD Helix capping. Protein Sci. 1998, 7, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Stapley BJ; Creamer TP A survey of left-handed polyproline II helices. Protein Sci. 1999, 8, 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Kelly MA; Chellgren BW; Rucker AL; Troutman JM; Fried MG; Miller AF; Creamer TP Host-guest study of left-handed polyproline II helix formation. Biochemistry 2001, 40, 14376–14383. [DOI] [PubMed] [Google Scholar]
- (77).Shi ZS; Woody RW; Kallenbach NR Is polyproline II a major backbone conformation in unfolded proteins? Adv. Protein Chem. 2002, 62, 163–240. [DOI] [PubMed] [Google Scholar]
- (78).Jha AK; Colubri A; Zaman MH; Koide S; Sosnick TR; Freed KF Helix, sheet, and polyproline II frequencies and strong nearest neighbor effects in a restricted coil library. Biochemistry 2005, 44, 9691–9702. [DOI] [PubMed] [Google Scholar]
- (79).Adzhubei AA; Sternberg MJE; Makarov AA Polyproline-II Helix in Proteins: Structure and Function. J. Mol. Biol. 2013, 425, 2100–2132. [DOI] [PubMed] [Google Scholar]
- (80).Cochran DAE; Doig AJ Effect of the N2 residue on the stability of the alpha-helix for all 20 amino acids. Protein Sci. 2001, 10, 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Tressler CM; Zondlo NJ (2S,4R)- and (2S,4S)-Perfluoro-tert-butyl 4-Hydroxyproline: Two Conformationally Distinct Proline Amino Acids for Sensitive Application in 19F NMR. J. Org. Chem. 2014, 79, 5880–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Wilhelm P; Lewandowski B; Trapp N; Wennemers H A Crystal Structure of an Oligoproline PPII-Helix, at Last. J. Am. Chem. Soc. 2014, 136, 15829–15832. [DOI] [PubMed] [Google Scholar]
- (83).Adzhubei AA; Sternberg MJE Left-Handed Polyproline-II Helices Commonly Occur in Globular-Proteins. J. Mol. Biol. 1993, 229, 472–493. [DOI] [PubMed] [Google Scholar]
- (84).Chellgren BW; Creamer TP Effects of H2O and D2O on polyproline II helical structure. J. Am. Chem. Soc. 2004, 126, 14734–14735. [DOI] [PubMed] [Google Scholar]
- (85).Whittington SJ; Chellgren BW; Hermann VM; Creamer TP Urea promotes polyproline II helix formation: Implications for protein denatured states. Biochemistry 2005, 44, 6269–6275. [DOI] [PubMed] [Google Scholar]
- (86).Horng JC; Raines RT Stereoelectronic effects on polyproline conformation. Protein Sci. 2006, 15, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Shi LQ; Holliday AE; Bohrer BC; Kim D; Servage KA; Russell DH; Clemmer DE “Wet” Versus “Dry” Folding of Polyproline. J. Am. Soc. Mass. Spec. 2016, 27, 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Pandey AK; Thomas KM; Forbes CR; Zondlo NJ Tunable Control of Polyproline Helix (PPII) Structure via Aromatic Electronic Effects: An Electronic Switch of Polyproline Helix. Biochemistry 2014, 53, 5307–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tressler CM; Zondlo NJ Perfluoro-tert-butyl Homoserine is a Helix-Promoting, Highly Fluorinated, NMR-Sensitive Aliphatic Amino Acid: Detection of the Estrogen Receptor•Coactivator Protein-Protein Interaction by 19F NMR. Biochemistry 2017, 56, 1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Correia MAS; Otrelo-Cardoso AR; Schwuchow V; Clauss K; Haumann M; Romao MJ; Leimkuhler S; Santos-Silva T The Escherichia coli Periplasmic Aldehyde Oxidoreductase Is an Exceptional Member of the Xanthine Oxidase Family of Molybdoenzymes. ACS Chem. Biol. 2016, 11, 2923–2935. [DOI] [PubMed] [Google Scholar]
- (91).Stanger HE; Gellman SH Rules for antiparallel beta-sheet design: D-Pro-Gly is superior to L-Asn-Gly for beta-hairpin nucleation. J. Am. Chem. Soc. 1998, 120, 4236–4237. [Google Scholar]
- (92).Haque TS; Little JC; Gellman SH Mirror-Image Reverse Turns Promote Beta-Hairpin Formation. J. Am. Chem. Soc. 1994, 116, 4105–4106. [Google Scholar]
- (93).Tatko CD; Waters ML The geometry and efficacy of cation-pi interactions in a diagonal position of a designed beta-hairpin. Protein Sci. 2003, 12, 2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Tatko CD; Waters ML Comparison of C-H center dot center dot center dot pi and hydrophobic interactions in a beta-hairpin peptide: Impact on stability and specificity. J. Am. Chem. Soc. 2004, 126, 2028–2034. [DOI] [PubMed] [Google Scholar]
- (95).Pathak D; Ollis D Refined Structure of Dienelactone Hydrolase at 1.8a. J. Mol. Biol. 1990, 214, 497–525. [DOI] [PubMed] [Google Scholar]
- (96).Chung YJ; Christianson LA; Stanger HE; Powell DR; Gellman SH A beta-peptide reverse turn that promotes hairpin formation. J. Am. Chem. Soc. 1998, 120, 10555–10556. [Google Scholar]
- (97).Syud FA; Stanger HE; Gellman SH Interstrand Side Chain-Side Chain Interactions in a Designed -Hairpin: Significance of Both Lateral and Diagonal Pairings. J. Am. Chem. Soc. 2001, 123, 8667–8677. [DOI] [PubMed] [Google Scholar]
- (98).Vuister GW; Bax A Quantitative J Correlation: A New Approach for Measuring Homonuclear Three-Bond JHNHa Coupling Constants in 15N-enriched Proteins. J. Am. Chem. Soc. 1993, 115, 7772–7777. [Google Scholar]
- (99).Newberry RW; VanVeller B; Guzei IA; Raines RT n ->pi* Interactions of Amides and Thioamides: Implications for Protein Stability. J. Am. Chem. Soc. 2013, 135, 7843–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Innes BT; Sowole MA; Gyenis L; Dubinsky M; Konermann L; Litchfield DW; Brandl CJ; Shilton BH Peroxide-mediated oxidation and inhibition of the peptidyl-prolyl isomerase Pin1. BBA Mol. Basis Dis. 2015, 1852, 905–912. [DOI] [PubMed] [Google Scholar]
- (101).Lassing I; Schmitzberger F; Björnstedt M; Holmgren A; Nordlund P; Schutt CE; Lindberg U Molecular and Structural Basis for Redox Regulation of beta-Actin. J. Mol. Biol. 2007, 370, 331–348. [DOI] [PubMed] [Google Scholar]
- (102).Choudhary A; Fry CG; Kamer KJ; Raines RT An n -> pi* interaction reduces the electrophilicity of the acceptor carbonyl group. Chem. Commun. 2013, 49, 8166–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Bundi A; Wüthrich K 1H NMR Parameters of the Common Amino Acid Residues Measured in Aqueous Solutions of the Linear Tetrapeptides H-Gly-Gly-X-L-Ala-OH. Biopolymers 1979, 18, 285–297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.