Introduction
Ras pathway-activating mutations are present in up to 44% of acute lymphoblastic leukemia (ALL) cases, and are frequently associated with chemoresistance and worse outcomes (1, 2). Activating mutations in KRAS make up nearly half of this group of alterations. These data underscore the importance of improving therapeutic approaches to KRAS-mutated ALL. However, only a few recent studies have successfully targeted forms of mutant KRAS, long considered to be undruggable (3). Therapies against downstream effectors of Ras signaling have yielded mixed results, likely due to the ability of Ras to compensate through multiple signaling pathways (4).
To improve effective therapies for RAS-mutated ALL, robust syngeneic mouse models are required. Currently available Ras mutation-driven mouse models of ALL have a variety of drawbacks, including variable penetrance, prolonged latency to disease onset, and/or technically challenging requirements for transduction and ablative conditioning and transplantation (5–7). Here, we report successful generation of a novel endogenous, syngeneic mouse model of Ras-mutated ALL. We crossed Mb1Cre/+ mice, which express Cre in most B- and a small percentage of T-lineage cells (8), with KrasLSL-G12D/+ mice. Resulting KrasLSL-G12D/+. Mb1Cre/+ mice developed T-ALL/T-lymphoblastic lymphoma (LLy), with a median latency to death of 97 days and near-complete penetrance. The disease demonstrated variable infiltration of the bone marrow, spleen, and thymus, and displayed an aberrant CD4+CD8+CD44+CD25+/− immunophenotype. The disease was sensitive to standard ALL chemotherapeutic agents, and transplantable, with rapid generation of disease in immunocompromised recipients. This novel transgenic mouse model has numerous beneficial features for advancing the study of KRAS-driven T-ALL/T-LLy.
Materials and Methods (see also Supplemental Materials and Methods)
Mice
B6.C(Cg)-Cd79atm1(cre)Reth/EhobJ (Mb1-Cre) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). B6.129S4-Krastm4Tyi/J (LSL-KrasG12D) mice were provided by Lawrence Donehower, and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice by Michele Redell. As shown in Supplemental Figure 1A, KrasLSL-G12D/+ males were bred with Mb1Cre/+ females (both 12-30 weeks old) to generate KrasLSL-G12D/+.Mb1Cre/+ (n=37) mice and control genotypes (Kras+/+.Mb1Cre/+. n=57, KrasLSL-G12D/+.Mb1+/+ n=22, and Kras+/+.Mb1+/+ n=20). Mice were monitored for declining health, weight loss, and CD4+CD8+ cells in peripheral blood. Investigators were not blinded to the mouse genotypes. All animal experiments were performed with approval of the Baylor College of Medicine Institutional Animal Care and Use Committee.
Genotyping
The Mb1-Cre transgene was assessed with primers 29589 (5’-ACTGAGGCAGGAGGATTGG-3’), 30016 (5’-CTCTTTACCTTCCAAGCACTGA-3’), and 30017 (5’-CATTTTCGAGGGAGCTTCA-3’). LSL-KrasG12D polymerase chain reaction (PCR) genotyping was performed with primers y116 (5’-TCCGAATTCAGTGACTACAGATG-3’), y117 (5’-CTAGCCACCATGGCTTGAGT-3’), and y118 (5’-ATGTCTTTCCCCAGCACAGT-3’). For each primer set, thermocycling conditions were: 95°C 5 min; 40 cycles of 94°C 45 sec, 57°C 30 sec, 72°C 45 sec; followed by 72°C 7 min and holding at 4°C.
Results
KrasLSL-G12D/+.Mb1Cre/+ mice develop T-ALL/T-LLy with high penetrance and short latency
KrasLSL-G12D/+.Mb1Cre/+ mice developed fatal T-ALL/T-LLy in nearly all cases. No mice developed B-ALL or myeloid leukemia, and none of the control genotypes developed disease. We confirmed Cre-mediated recombination of the KrasLSL-G12D allele in sorted B cells from KrasLSL-G12D/+.Mb1Cre/+ mice, indicating KrasG12D was activated in B cells as intended. T-ALL/T-LLy samples from these same mice also demonstrated Cre-mediated recombination, as well as frequent loss of the wild-type Kras allele (Supplemental Figure 1B). KrasLSL-G12D/+.Mb1Cre/+ mice had significantly enlarged spleens and thymuses compared to age-matched mice of the control genotypes (Figure 1A, 1B). We performed blast immunophenotyping on 13 cases. Twelve demonstrated an aberrant CD4+CD8+ double-positive immunophenotype, and one demonstrated CD4−CD8+ single-positive blasts. All 13 mice had thymic involvement. Spleen involvement was present in 7 mice, with 5 of these also demonstrating blasts in the bone marrow. Three of 13 mice had >25% bone marrow involvement and thus met criteria for ALL (Figure 1C). Five cases were also stained for CD44 and CD25, markers of early T-cell differentiation. Expression of both CD44 and CD25 normally only occurs at the CD4−CD8− double-negative stage, but five of five cases analyzed demonstrated aberrant CD4+CD8+CD44+CD25+/− (Figure 1D). Hematoxylin and eosin stained spleen sections from diseased KrasLSL-G12D/+.Mb1Cre/+ mice demonstrated extensive infiltration of large blasts with immature chromatin, compared to small, mature lymphocytes with condensed chromatin in the control non-leukemic sample (Figure 1E). The median latency to death from disease was 97 days (range 75-139 days) with complete penetrance among the mice that did not die early due to non-leukemic causes (Figure 2A). Thirty-one of 37 KrasLSL-G12D/+.Mb1Cre/+ mice developed T-ALL/T-LLy. The 6 mice that did not manifest T-ALL/T-LLy were all censored at less than 84 days of age (4 had benign causes of death and 2 could not be fully evaluated). The KrasG12D point mutation was verified by Sanger sequencing in thymus samples from T-ALL/T-LLy mice 347, 348, and 349 (data not shown).
Figure 1: KrasLSL-G12D/+.Mb1Cre/+ mice develop T-ALL/T-LLy.
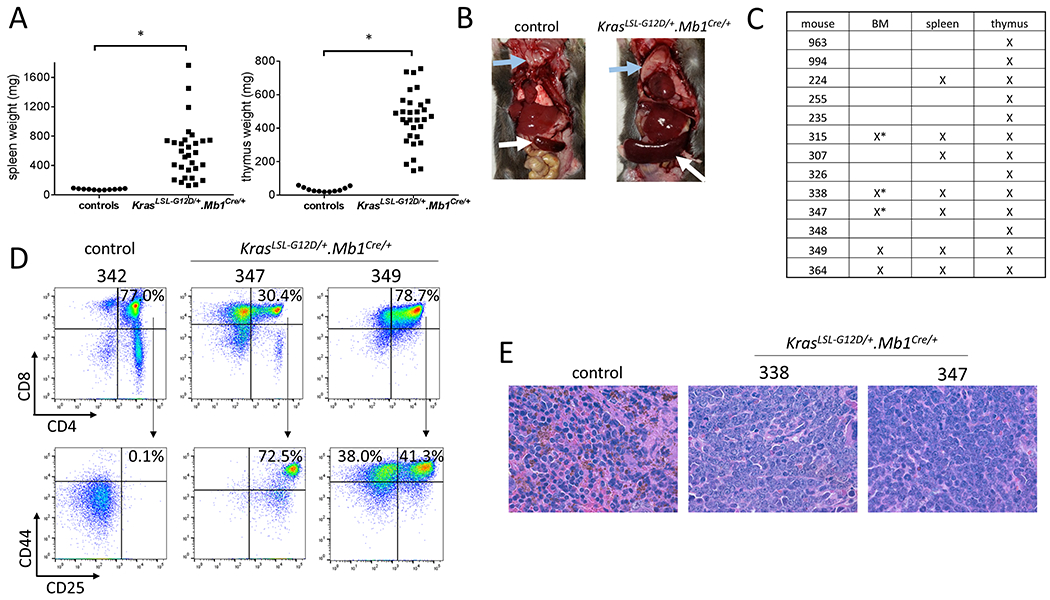
(A) KrasLSL-G12D/+.Mb1Cre/+ mice had enlarged spleens and thymuses compared to age-matched control mice (Welch’s t-test, *p<0.0001). (B) Representative images of a control and a KrasLSL-G12D/+.Mb1Cre/+ mouse. Blue and white arrows indicate thymus and spleen, respectively. (C) Disease involvement of the bone marrow, spleen, and thymus in 13 mice analyzed by flow cytometry. “X” indicates tissue involvement, and * indicates >25% bone marrow involvement, the criterion for T-ALL. (D) KrasLSL-G12D/+.Mb1Cre/+ leukemic blasts from the thymus demonstrated aberrant expression of both early and late T-cell progenitor markers. T-ALL/T-LLy mice 347 and 349 had CD4+CD8+CD44+CD25+/− blasts. Thymocytes from a representative age-matched healthy control mouse (342) are shown for comparison. (E) Hematoxylin and eosin stained spleen sections of leukemic mice 338 and 347 show loss of normal splenic architecture, with diffuse infiltration by large blasts with immature chromatin, compared to a control mouse (100X magnification).
Figure 2: KrasLSL-G12D/+.Mb1Cre/+ T-ALL/T-LLy has a short latency, causes disease in secondary recipients, and blasts are sensitive to standard chemotherapy agents.
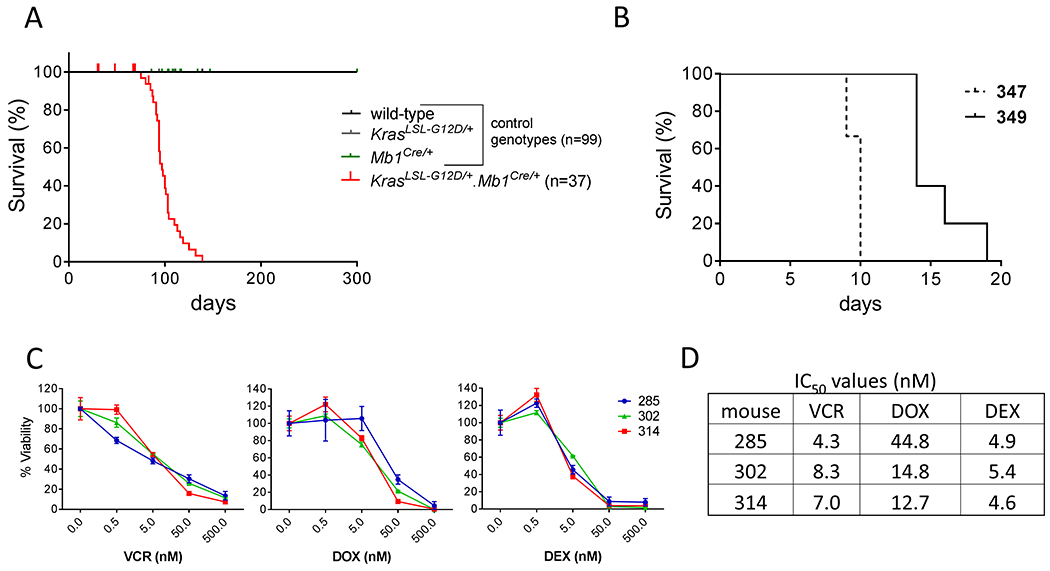
(A) KrasLSL-G12D/+.Mb1Cre/+ mice developed T-ALL/T-LLy and died at a median of 97 days (range 75-139 days). (B) NSG mice transplanted with primary KrasLSL-G12D/+.Mb1Cre/+ T-ALL (mouse 347) and T-LLy (mouse 349) blasts died with median latencies of 10 and 14 days, respectively. (C) Thymic blasts from three leukemic KrasLSL-G12D/+.Mb1Cre/+ mice (285, 302, 314) were incubated for 48 hours with varying concentrations of standard T-ALL/T-LLy chemotherapy agents vincristine (VCR), doxorubicin (DOX), and dexamethasone (DEX), followed by viability determination with ATP assay. Error bars for each point show standard deviation. (D) Each case demonstrated chemosensitivity, with IC50 values in the low nanomolar range.
Notch1 mutations are common in other mouse models of T-ALL/T-LLy (5) and human T-ALL (2). We performed Sanger sequencing of hotspot regions, namely Notch1 exons 26, 27, and 34, in 9 KrasLSL-G12D/+.Mb1Cre/+ T-ALL/T-LLy samples. Six samples demonstrated exon 34 mutations resulting in frameshifts in the PEST domain of Notch1 (Supplemental Figure 1C).
KrasLSL-G12D/+.Mb1Cre/+ leukemic blasts are sensitive to standard chemotherapy agents and cause aggressive disease in secondary recipients
Primary KrasLSL-G12D/+.Mb1Cre/+ blasts (T-ALL from mouse 347 spleen, and T-LLy from mouse 349 thymus) were transplanted into NSG mice. Recipients all developed aggressive and lethal disease, with enlarged spleens and average latency of 10 and 14 days, respectively (Figure 2B). Blasts from the secondary recipients demonstrated the same aberrant immunophenotypes as the primary blasts (primary immunophenotypes shown in Figure 1D).
Cells isolated post-mortem from the enlarged thymuses of 3 diseased KrasLSL-G12D/+.Mb1Cre/+ mice (285, 302, and 314) were incubated with the standard T-ALL/T-LLy chemotherapy agents vincristine, doxorubicin, and dexamethasone. All three cases demonstrated chemosensitivity, with low-nanomolar IC50 values (Figure 2C, 2D).
Discussion
KrasLSL-G12D/+.Mb1Cre/+ mice develop a highly penetrant, short latency T-ALL/T-LLy, with an aberrant immunophenotype (CD4+CD8+CD44+CD25+/−) similar to those observed in other Kras mutation-driven T-ALL mouse models (5, 7). Expression of the early T cell marker CD44 in our model may be useful for future studies since CD44 expression has been reported to be associated with risk of relapse in primary patient T-ALL samples (9). The aberrant combination of early (CD44 and CD25) and late (CD4 and CD8) T cell markers prevents categorizing as one of the conventional molecular genetic subtypes of T-ALL arrested at a specific T cell differentiation stage (10). Alternative T-ALL classification schemes based instead on mutations contributing to activation of particular cell signaling pathways, classify T-ALL with RAS and/or PTEN mutations as high-risk (11). Our mouse model of T-ALL/T-LLy presented here has similarities to this high-risk T-ALL subtype, and may provide a useful preclinical system to study specific pathogenesis and potential therapies.
Most transgenic mouse models driven by Mb1-Cre yield B-ALL or B-cell lymphoma, and a single study of Mb1-Cre driven LMO2 expression resulted in T-ALL in less than 20% of the mice (12). Thus, the exclusive development of T-lineage disease in our KrasLSL-G12D/+.Mb1Cre/+ model was unexpected. Presumably, since we observed Cre-mediated recombination in both B cells and T-ALL/T-LLy samples, Cre may be expressed in an early lymphoid progenitor cell preceding B/T lineage specification. Hobeika et al (8) showed Mb1-Cre mice demonstrate recombination in most B lineage cells and a small percentage of Thy1+ T cells in the thymus and spleen. Another study found Mb1-Cre-mediated recombination in all pro-B cells and 10% of Ly6D+ common lymphoid progenitors (CLPs), but no recombination in lymphoid-primed multipotent progenitors or Ly6D− CLPs, generally considered the last stage of maturation which contributes to both B and T cell populations (13). However, the Ly6D+ CLP stage has been shown to also generate T cells of the DN3 stage and beyond (14). We hypothesize that Mb1-Cre-mediated recombination occurred at the Ly6D+ CLP stage in our mice, leading to KrasG12D overexpression in these cells and expansion of a descendant population with a derangement in T cell maturation.
Dependable genetically-engineered mouse models are needed for preclinical studies of de novo RAS-mutated T-ALL. One widely-used technique for modeling Ras mutation-driven T-ALL/T-LLy involves treating KrasLSL-G12D/+.Mx1Cre/+ mice with polyinosinic:polycytidylic acid to induce Cre recombination. These mice develop myeloproliferative neoplasms, and secondary transplantation of bone marrow into lethally-irradiated mice is required for T-ALL/T-LLy development (5, 7). This approach results in variability in the type of hematologic malignancy generated and/or a prolonged latency to disease. Finally, similar to xenografting or transduction/transplantation mouse models, the recipient conditioning and transplantation of myeloproliferative cells yield mice less suitable for studies of microenvironment interactions and early stages of leukemogenesis. There is currently only one other mouse model, KrasLSL-G12D/+.LckCre/+, which consistently develops Kras mutation-driven T-ALL with minimal technical manipulation, but its latency to disease is longer (median 121 days) than for KrasLSL-G12D/+.Mb1Cre/+ mice (15).
Compared to other models of Ras mutation-driven T-ALL/T-LLy, the KrasLSL-G12D/+.Mb1Cre/+ model has advantages in terms of high penetrance, short latency, and lack of technical manipulations. Because the blasts demonstrate sensitivity to standard chemotherapy agents, this model may be useful for studying novel targeted therapies in combination with, or in comparison to these agents. It is also well-suited to studies of pro-leukemic interactions between blasts and the native bone marrow microenvironment during leukemogenesis. In sum, this model holds promise as a tool for gaining insights into more effective therapies for RAS mutation-driven leukemias and lymphomas.
Supplementary Material
Acknowledgements
This work was supported by an American Society of Hematology Scholar Award (J.J.J.); grant RP170074 from the Cancer Prevention and Research Institute of Texas (K.R.R.); funding from the Lynch family (K.R.R.); by the National Cancer Institute, National Institutes of Health (R01 CA249867 to K.R.R.); and by the National Cancer Institute, National Institutes of Health (R01 CA207086 to H.D.L.). Flow cytometry assays were performed at the Research Flow Cytometry Core Facility of Texas Children’s Cancer and Hematology Centers with the support from the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672), the NIH (CA125123 and RR024574) and the assistance of Joel M. Sederstrom. Flow assistance was also generously provided by Amos Gaikwad and Tatiana Goltsova. Histology was performed by the BCM Center for Comparative Medicine Comparative Pathology Laboratory with the assistance of Brian Simons. Microscope assistance was generously provided by Debananda Pati and Nenggang Zhang.
Footnotes
Competing Interests
The authors have no conflicts of interest to disclose.
References
- 1.Oshima K, Khiabanian H, da Silva-Almeida AC, Tzoneva G, Abate F, Ambesi-Impiombato A, et al. Mutational landscape, clonal evolution patterns, and role of RAS mutations in relapsed acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2016. Oct 4;113(40):11306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter-Pechanska P, Kunz JB, Hof J, Zimmermann M, Rausch T, Bandapalli OR, et al. Identification of a genetically defined ultra-high-risk group in relapsed pediatric T-lymphoblastic leukemia. Blood cancer journal. 2017. Feb 3;7(2):e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler D, Gmachl M, Mantoulidis A, Martin LJ, Zoephel A, Mayer M, et al. Drugging an undruggable pocket on KRAS. Proceedings of the National Academy of Sciences of the United States of America. 2019. Aug 6;116(32):15823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimple RC, Wang X. RAS: Striking at the Core of the Oncogenic Circuitry. Frontiers in oncology. 2019;9:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindler T, Cornejo MG, Scholl C, Liu J, Leeman DS, Haydu JE, et al. K-RasG12D-induced T-cell lymphoblastic lymphoma/leukemias harbor Notch1 mutations and are sensitive to gamma-secretase inhibitors. Blood. 2008. Oct 15;112(8):3373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sewastianik T, Jiang M, Sukhdeo K, Patel SS, Roberts K, Kang Y, et al. Constitutive Ras signaling and Ink4a/Arf inactivation cooperate during the development of B-ALL in mice. Blood advances. 2017. Nov 28;1(25):2361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wang J, Liu Y, Sidik H, Young KH, Lodish HF, et al. Oncogenic Kras-induced leukemogeneis: hematopoietic stem cells as the initial target and lineage-specific progenitors as the potential targets for final leukemic transformation. Blood. 2009. Feb 5;113(6):1304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proceedings of the National Academy of Sciences of the United States of America. 2006. Sep 12;103(37):13789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaskova M, Mejstrikova E, Kalina T, Martinkova P, Omelka M, Trka J, et al. Transfer of genomics information to flow cytometry: expression of CD27 and CD44 discriminates subtypes of acute lymphoblastic leukemia. Leukemia. 2005. May;19(5):876–8. [DOI] [PubMed] [Google Scholar]
- 10.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer cell. 2002. Feb;1(1):75–87. [DOI] [PubMed] [Google Scholar]
- 11.Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, Lambert J, Beldjord K, Lengline E, et al. Toward a NOTCH1/FBXW7/RAS/PTEN-based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013. Dec 1;31(34):4333–42. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Ramirez I, Bhatia S, Rodriguez-Hernandez G, Gonzalez-Herrero I, Walter C, Gonzalez de Tena-Davila S, et al. Lmo2 expression defines tumor cell identity during T-cell leukemogenesis. The EMBO journal. 2018. Jul 13;37(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luc S, Luis TC, Boukarabila H, Macaulay IC, Buza-Vidas N, Bouriez-Jones T, et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nature immunology. 2012. Feb 19;13(4):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes & development. 2009. Oct 15;23(20):2376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang MY, Wang Q, Gormley AC, Stein SJ, Xu L, Shestova O, et al. High selective pressure for Notch1 mutations that induce Myc in T-cell acute lymphoblastic leukemia. Blood. 2016. Nov 3;128(18):2229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


