Abstract
Introduction
Treatment landscape for advanced/metastatic NSCLC (aNSCLC) has evolved considerably over the past few decades with the advent of targeted therapies for epidermal growth factor receptor-mutated (EGFRm+) aNSCLC treatment. This study described real-world patient and disease characteristics, treatment and practice patterns, and clinical, economic, and patient-reported outcomes (PROs) in patients with EGFRm+ aNSCLC.
Methods
Data were derived from the Adelphi NSCLC Disease Specific Programme™ (DSP™), a point-in-time survey conducted between July and December 2020. The survey included oncologists and pulmonologists, and their consulting patients (with physician-confirmed EGFRm+ aNSCLC) from nine countries: the US, Brazil, the UK, Italy, France, Spain, Germany, Japan, and Taiwan. All analyses were descriptive.
Results
Overall, 542 physicians reported data for 2857 patients (mean age 65.6 years), and most patients were female (56.0%), white (61.0%), and had stage IV disease at initial diagnosis (76.0%), and adenocarcinoma histology (89.0%). Most patients received EGFR-tyrosine kinase inhibitors (TKI) therapy in first- (91.0%), second- (74.0%), and third-line (67.0%). The most common tumor samples and methods for EGFR detection were EGFR-specific mutation detection tests (44.0%) and core needle biopsy (56.0%). Median time to next treatment was 14.0 (IQR 8.0–22.0) months and disease progression was the main physician-reported reason for early discontinuation. The most common physician-reported disease symptoms were cough (51.0%), fatigue (37.0%), and dyspnea (33.0%). In patients assessed for PROs, mean EQ-5D-5L index and FACT-L health utility scores were 0.71 and 83.5, respectively. On average, patients lost 10.6 h of work/week for approximately 29.2 weeks due to EGFRm+ aNSCLC.
Conclusion
This real-world multinational data set showed that most patients with EGFRm+ aNSCLC were treated per the country relevant clinical guidelines, with progression as the main reason for early treatment discontinuation. For the included countries, these findings may offer a useful benchmark for decision makers to determine future allocation of healthcare resources for patients with EGFRm+ aNSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02530-0.
Keywords: Advanced non-small cell lung cancer, Disease Specific Programme™, EGFR mutation, Patient-reported outcomes, Point-in-time, Real-world, Survey, Treatment patterns
Key Summary Points
| As the epidermal growth factor receptor-mutated (EGFRm+) advanced non-small cell lung cancer (aNSCLC) treatment landscape continues to evolve, and the number of approved and recommended targeted treatments continues to grow, it is important to understand implications on real-world practice and patients. |
| This study used the Adelphi Disease Specific Programme™ (DSP™) methodology to describe real-world patient and disease characteristics, testing and treatment patterns, clinical and economic outcomes, physician practice patterns and rationale, and patient-reported health-related quality of life (HRQoL), across nine countries. |
| Overall, EGFRm+ aNSCLC treatment patterns were in line with international treatment guideline recommendations for targeted therapy with EGFR–tyrosine kinase inhibitors (EGFR-TKIs), although regional variation was observed in practice and resource use patterns, suggesting differing approaches to EGFRm+ aNSCLC management. |
| The burden of lung cancer symptoms remains high. On average, patients lost about 10.6 h of work per week for about 29 weeks in the 1 year prior to data capture. |
| According to physicians, inadequate tissue was the main barrier to EGFR testing and disease progression was the main reason for early treatment discontinuation. |
| For included countries, these findings may offer a useful benchmark for decision makers to determine future allocation of healthcare resources for patients with EGFRm+ aNSCLC. |
Introduction
Lung cancer remains the leading cause of cancer deaths worldwide, accounting for approximately 1.8 million deaths in 2020 [1]. Non-small cell lung cancer (NSCLC) accounts for more than 82.0% of all lung cancer cases and is often diagnosed at advanced stages IIIB or IV [2–4]. Among the different genetic drivers of advanced or metastatic NSCLC (aNSCLC) cases, epidermal growth factor (EGFR) mutation is the second most frequent genetic driver [5]. The recommended standard of care for EGFR-mutated aNSCLC (EGFRm+ aNSCLC) is first-, second-, or third-generation EGFR–tyrosine kinase inhibitors (EGFR-TKIs) [6], which have demonstrated favorable efficacy and safety over cytotoxic chemotherapy in this setting. However, acquired resistance to EGFR-TKIs is inevitable [7, 8], and the 5-year survival rate for patients with EGFRm+ aNSCLC ranges from 14.6% to 23.8% [9, 10]. More recently, anti-angiogenic antibodies, ramucirumab or bevacizumab, in combination with erlotinib, have been incorporated in the clinical guidelines [6] based on landmark trials demonstrating clinical benefit with these combinations over erlotinib monotherapy in patients with EGFRm+ aNSCLC [11, 12].
As the EGFRm+ aNSCLC treatment landscape continues to evolve, and the number of approved and recommended targeted treatments continues to grow, it is important to understand implications on real-world practice and patients. Earlier studies have described real-world EGFRm+ aNSCLC treatment and practice patterns; however, most are out of date and were conducted before regulatory approval and market uptake of osimertinib in the first-line (1L) setting. In addition, most previous studies have focused on US populations [13–17] and therefore may not be applicable to the rest of the world (e.g., Asia, with higher prevalence of EGFRm+ aNSCLC) [18–20]. Lastly, studies determining physician rationale for EGFRm+ aNSCLC treatment decisions or patient perspectives on disease burden, management, and treatment are also limited [21, 22].
To address these knowledge gaps, this study used the Adelphi Disease Specific Programme™ (DSP™) methodology to describe real-world EGFRm+ aNSCLC patient and disease characteristics, testing and treatment patterns, clinical and economic outcomes, physician practice patterns and rationale, and patient-reported health-related quality of life (HRQoL), across nine countries in North and South America (the United States [US] and Brazil), Europe (the United Kingdom [UK], Italy, France, Spain, and Germany), and Asia (Taiwan and Japan).
Methods
Study Design and Survey Sample
Data were derived from the Adelphi NSCLC DSP™ with data collected between July and December 2020 from the US, Brazil, the UK, Italy, France, Spain, Germany, Taiwan, and Japan. The UK, France, Italy, Spain, and Germany are referred to as Europe 5 (EU5). DSP™ are large, multinational, point-in-time surveys conducted with physicians and their patients presenting in real-world clinical practice that describe current disease management, disease-burden impact, and associated treatment effects (clinical and physician-perceived). A complete description of the survey methods used for this DSP™ has been previously published and validated [23–25].
Data sources included: (1) a physician interview or physician survey to capture physicians’ attitudes; (2) a patient record form (PRF) completed by physicians to document individual patient characteristics, disease history, symptoms, biomarker status, current/prior treatments received, adverse events (AEs), healthcare resource utilization, and supportive therapies; and (3) a voluntary patient self-reported questionnaire (PSC) for patient-reported characteristics, symptoms, outcomes/HRQoL, treatment costs, and health status. Participating physicians included oncologists and pulmonologists (and respiratory surgeons in Japan) treating patients with aNSCLC. Per the inclusion criteria, physicians were eligible for inclusion if they were personally responsible for the management and systemic treatment decisions for patients with aNSCLC and were required to be consulting at least three patients with aNSCLC each month. Per the patient-level inclusion criteria in the patient record form, adult patients with a physician-confirmed diagnosis of aNSCLC, who were not participating in a clinical trial during the survey, were eligible for inclusion in the survey.
Participating physicians were asked to complete the physician survey, and to then select prospective patients presenting with aNSCLC, completing a PRF for each patient selected at the point of consultation. The physician surveys and individual PRFs were administered online, with data anonymized at entry.
Physicians were asked to collect data for their next 13 consecutively consulting eligible patients according to the following quota: six consulting patients agnostic of biomarker status (i.e., aNSCLC generally), then the next five consulting patients with confirmed EGFRm+ aNSCLC (EGFRm+ oversample group), and the next two consulting patients with confirmed rearranged during transfection fusions (RET fusion+) aNSCLC (RET+ oversample group, excluding Japan). The overall sample of patients with EGFRm+ aNSCLC in this analysis (n = 2857) was derived from patients with EGFRm+ from the general sample and the EGFRm+ oversample, i.e., all patients included in this DSP™ had EGFRm+ aNSCLC.
Patients for whom a PRF was completed were then given the voluntary PSC by the physicians immediately after completing their consultation at the physician’s office. The PSCs were completed by patients without assistance from the physician and returned in a sealed envelope to ensure anonymity of data.
The data collected were divided as follows: (1) pre-index period: used to capture clinical background including initial diagnosis of NSCLC, treatment (e.g., neo/adjuvant treatment), and management of early-stage NSCLC; (2) index date: date of diagnosis of aNSCLC; and (3) post-index period: data collection on aNSCLC treatment and associated outcomes. In the study, post-index (variable period following the index date) was the time of data collection and the other time points were completed retrospectively, i.e., no follow-up periods were there in this point-in-time study (Supplementary Fig. S1).
Using a check box, patients provided informed consent for use of their anonymized and aggregated data for research and publication in scientific journals. Data were collected in such a way that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt.
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines [26] and although did not require ethical committee approval, the study materials and protocol were reviewed and approved by the Western Institutional Review Board (study protocol number AG8759). In addition, the survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [27], and Health Information Technology for Economic and Clinical Health Act legislation [28].
Study Measures
The primary objectives and measures were to describe (1) patient demographics (age, sex, ethnicity, and smoking status); (2) socioeconomic status (employment, household income, insurance) and economic burden (healthcare resource utilization); (3) clinical characteristics (disease duration, staging at advanced diagnosis, time from advanced diagnosis to treatment, symptoms, metastatic sites, comorbidities, and Eastern Cooperative Oncology Group [ECOG] performance status); (4) treatment patterns (line of therapy, treatment sequence, treatment discontinuation reasons, treatment duration for each line, and supporting medication); and (5) molecular testing patterns (testing at advanced diagnosis, testing method, sample type, and barriers to molecular testing reported by the physicians testing molecular alterations). The corresponding data were collected using the PRF, except for socioeconomic status and testing patterns. Socioeconomic data were collected using the PSC. Data for molecular biomarker testing patterns and physicians’ perceptions on testing patterns data were collected using the PRF and physician survey, respectively. Early treatment discontinuation was defined as failure to complete the full treatment course as intended by the physician, based on a physician response of “no” to the question “Was the full course completed as intended?” on the PRF.
Secondary objectives and corresponding measures of interest included (1) clinical outcomes (in terms of time to next treatment [TTNT], time to treatment failure (TTF), full response [FR], partial response [PR], and no response [NR]), type and severity of aNSCLC symptoms, and AEs of treatment; (2) economic outcomes (frequency and reason for hospitalization, duration of hospitalization, healthcare resource utilization, and loss of productivity for employed patients); and (3) patients’ perception of disease burden and management (loss of productivity, attitude toward diagnosis, perceptions toward treatment decision, out-of-pocket expenses, health status, and HRQoL). Tumor response was defined as the physician’s assessment and documentation of change in disease burden, and the type of change was classified using predefined categories of FR (full resolution of disease), PR (some reduction in overall disease burden), and NR (increase or no change in overall disease burden). TTNT was defined as the time from start of treatment to start of the next line of treatment. TTF was defined as the time from start of treatment to date of progression or start of the next line of treatment, whichever occurred first.
HRQoL was determined using standardized patient-reported outcomes (PROs) questionnaires: European Quality of Life 5 Dimension 5 Level (EQ-5D-5L) and EQ–visual analog scale (EQ-VAS) for general health status [29] and Functional Assessment of Cancer Therapy–Lung (FACT-L) and Functional Assessment of Cancer Therapy–General (FACT-G) questionnaires for HRQoL [30, 31]. The EQ-5D-5L descriptive system comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and each dimension has five response levels: no problems, slight problems, moderate problems, severe problems, unable to/extreme problems. The FACT-G questionnaire includes the Physical Well Being (PWB), Social/family Well-Being (SWB), Emotional Well-Being (EWB), and Functional Well-Being (FWB) HRQoL domains, and the FACT-L consists of nine items involving common lung cancer symptoms, such as shortness of breath, weight loss, tightness in chest. Each item on the FACT questionnaires is rated on a five-point Likert scale, from 0 (not at all) to 4 (very much). For the EQ-5D-5L and FACT questionnaires, higher scores (e.g., higher total score in EQ-5D-5L) indicate higher HRQoL.
Statistical Analysis
This paper presented data for patients with EGFRm+ aNSCLC. Descriptive analyses were used for primary and secondary outcome measures: continuous variables were summarized by mean (standard deviation [SD]) and median (interquartile range [IQR], minimum, and maximum), whereas categorical variables were summarized as the number and percentage of subjects in each category. Patients were excluded from specific analyses if their values were missing for any particular variable, but they were considered eligible for other analyses. No imputation of missing values was attempted. Numbers of non-missing values were reported. TTNT, TTF, and time from aNSCLC diagnosis to initiation of treatment were reported as median and interquartile range (IQR). Patients with ongoing treatment at the time of data collection were censored at the data collection date.
Results were presented for the overall EGFRm+ aNSCLC sample and by country. Data from the UK, Italy, France, Spain, and Germany were combined to present the data for the EU5. All analysis were conducted using the software package IBM SPSS Data Collection Survey Reporter Version 7.5 and STATA® Version 16 (StataCorp LP, College Station, US).
Results
Between July and December 2020, 542 physicians participated in the survey (46.0% (n = 251) in the EU5, 21.0% (n = 116) in Japan, 13.0% (n = 71) in the US, 10.0% (n = 53) in Brazil, 9.0% (n = 51) in Taiwan) and comprised 349 (64.0%) oncologists, 170 (31.0%) pulmonologists, and 23 (4.0%) respiratory surgeons (Supplementary Table S1). The physicians reported data for 2857 patients (i.e., completed PRFs), of whom 942 (33.0%) were eligible for PRO analysis. Table 1 summarizes demographic and clinical characteristics of the overall study cohort and by each of the nine countries.
Table 1.
Patient characteristics: overall and by country
| Patient characteristics | Overall (n = 2857) |
US (n = 422) |
BR (n = 313) |
UK (n = 279) |
IT (n = 287) |
FR (n = 285) |
ES (n = 302) |
DE (n = 284) |
EU5a (n = 1437) |
TW (n = 390) |
JP (n = 295) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age in years, mean (SD) | 65.6 (10.6) | 64.9 (10.5) | 65.5 (11.3) | 65.6 (10.0) | 65.2 (10.6) | 65.9 (10.8) | 64.4 (10.9) | 65 (8.2) | 65.2 (10.2) | 65.6 (11.1) | 68.8 (10.3) |
| Sex, n (%) | |||||||||||
| Male | 1246 (44.0) | 172 (41.0) | 150 (48.0) | 113 (41.0) | 136 (47.0) | 118 (41.0) | 129 (43.0) | 172 (61.0) | 668 (47.0) | 152 (39.0) | 104 (35.0) |
| Female | 1611 (56.0) | 250 (59.0) | 163 (52.0) | 166 (60.0) | 151 (53.0) | 167 (59.0) | 173 (57.0) | 112 (39.0) | 769 (54.0) | 238 (61.0) | 191 (65.0) |
| Ethnicity, n (%) | |||||||||||
| Caucasian/White | 1755 (61.0) | 262 (62.0) | 222 (71.0) | 211 (76.0) | 271 (94.0) | 238 (84.0) | 287 (95.0) | 264 (93.0) | 1271 (88.0) | 0 | 0 |
| Asianb | 730 (26.0) | 21 (5.0) | 0 | 23 (8.0) | 1 (< 1.0) | 14 (5.0) | 0 | 0 | 38 (3.0) | 378 (97.0) | 293 (99.0) |
| Hispanic-Latino | 59 (2.0) | 16 (4.0) | 0 | 7 (3.0) | 8 (3.0) | 12 (4.0) | 12 (4.0) | 4 (1.0) | 43 (3.0) | 0 | 0 |
| African American | 83 (3.0) | 53 (13.0) | 30 (10.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Othersc | 260 (9.0) | 70 (17.0) | 91 (29.0) | 38 (14.0) | 7 (2.0) | 21 (7.0) | 3 (1.0) | 16 (6.0) | 85 (6.0) | 12 (3.0) | 2 (1.0) |
| Tumor stage at initial diagnosis, n (%) | |||||||||||
| I | 53 (2.0) | 1 (< 1.0) | 10 (3.0) | 2 (1.0) | 2 (1.0) | 1 (< 1.0) | 4 (1.0) | 2 (1.0) | 11 (1.0) | 4 (1.0) | 27 (9.0) |
| II | 98 (3.0) | 6 (1.0) | 29 (9.0) | 3 (1.0) | 4 (1.0) | 2 (1.0) | 3 (1.0) | 25 (9.0) | 37 (3.0) | 8 (2.0) | 18 (6.0) |
| IIIA | 88 (3.0) | 10 (2.0) | 29 (9.0) | 1 (< 1.0) | 5 (2.0) | 1 (< 1.0) | 2 (1.0) | 11 (4.0) | 20 (1.0) | 12 (3.0) | 17 (6.0) |
| IIIB | 250 (9.0) | 58 (14.0) | 34 (11.0 | 5 (2.0) | 22 (8.0) | 13 (5.0) | 3 (1.0) | 63 (22.0) | 106 (7.0) | 33 (8.0) | 19 (6.0) |
| IIIC | 170 (6.0) | 36 (9.0) | 26 (8.0) | 3 (1.0) | 19 (7.0) | 22 (8.0) | 5 (2.0) | 34 (12.0) | 83 (6.0) | 13 (3.0) | 12 (4.0) |
| IVA | 807 (28.0) | 130 (31.0) | 93 (30.0) | 62 (22.0) | 68 (24.0) | 81 (28.0) | 64 (21.0) | 80 (28.0) | 355 (25.0) | 148 (38.0) | 81 (27.0) |
| IVB | 1368 (48.0) | 181 (43.0) | 88 (28.0) | 202 (72.0) | 149 (52.0) | 165 (58.0) | 221 (73.0) | 69 (24.0) | 806 (56.0) | 172 (44.0) | 121 (41.0) |
| Histology, n (%) | |||||||||||
| Adenocarcinoma | 2553 (89.0) | 392 (93.0) | 232 (74.0) | 271 (97.0) | 262 (91.0) | 258 (91.0) | 287 (95.0) | 182 (64.0) | 1260 (88.0) | 384 (98.0) | 285 (97.0) |
| SCC | 189 (7.0) | 19 (5.0) | 37 (12.0) | 6 (2.0) | 16 (6.0) | 15 (5.0) | 7 (2.0) | 79 (28.0) | 123 (9.0) | 3 (1.0) | 7 (2.0) |
| Large cell carcinoma | 93 (9.0) | 7 (2.0) | 43 (14.0) | 1 (1.0) | 4 (1.0) | 10 (4.0) | 8 (3.0) | 20 (7.0) | 43 (3.0) | 0 | 0 |
| Other | 13 (1.0) | 2 (0) | 0 | 1 (1.0) | 1 (< 1.0) | 1 (0) | 0 | 2 (1.0) | 5 (0.3) | 3 (1.0) | 3 (1.0) |
| Don’t know | 9 (0) | 2 (0) | 1 (0) | 0 | 4 (1.0) | 1 (0) | 0 | 1 (< 1.0) | 6 (< 1.0) | 0 | 0 |
| n = 2728 | n = 377 | n = 303 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 221 | |
| Average CCI score, mean (SD)c | 7.5 (2.2) | 7.1 (2.6) | 7.4 (2.8) | 8.1 (1.0) | 7.6 (2.2) | 7.7 (1.8) | 8.1 (1.1) | 6.2 (3.0) | 7.5 (2.1) | 7.7 (2.0) | 7.4 (2.0) |
| Smoking history, n (%) | |||||||||||
| Yes | 1293 (45.0) | 202 (48.0) | 176 (56.0) | 137 (49.0) | 119 (41.0) | 126 (44.0) | 115 (38.0) | 201 (71.0) | 698 (49.0) | 114 (29.0) | 103 (35.0) |
| No | 1513 (53.0) | 212 (50.0) | 137 (44.0) | 138 (50.0) | 156 (54.0) | 157 (55.0) | 179 (59.0) | 81 (29.0) | 711 (50.0) | 275 (71.0) | 178 (60.3) |
| Don’t know | 51 (2.0) | 8 (2.0) | 0 | 4 (1.0) | 12 (4.0) | 2 (1.0) | 8 (3.0) | 2 (1.0) | 28 (2.0) | 1 (< 1.0) | 14 (4.7) |
| Line of therapy, n (%) | |||||||||||
| 1st | 2364 (83.0) | 381 (90.0) | 278 (89.0) | 226 (81.0) | 234 (82.0) | 235 (83.0) | 266 (88.0) | 217 (76.0) | 1178 (82.0) | 310 (80.0) | 217 (73.6) |
| 2nd | 409 (14.0) | 34 (8.0) | 30 (10.0) | 52 (19.0) | 51 (18.0) | 47 (17.0) | 35 (12.0) | 62 (22.0) | 247 (17.0) | 54 (14.0) | 44 (14.9) |
| 3rd | 84 (3.0) | 7 (2.0) | 5 (2.0) | 1 (< 1.0) | 2 (1.0) | 3 (1.0) | 1 (< 1.0) | 5 (2.0) | 12 (1.0) | 26 (7.0) | 34 (11.5) |
| Exon mutation, n (%) | |||||||||||
| Exon 19 deletion | 1070 (38.0) | 160 (38.0) | 74 (24.0) | 123 (44.0) | 91 (32.0) | 100 (35.0) | 95 (32.0) | 97 (34.0) | 506 (35.0) | 176 (45.0) | 154 (52.2) |
| Exon 21 point mutation | 468 (16.0) | 37 (9.0) | 52 (17.0) | 51 (18.0) | 60 (21.0) | 27 (10.0) | 36 (12.0) | 8 (3.0) | 182 (13.0) | 130 (33.0) | 67 (22.7) |
| RET results, n (%) | |||||||||||
| RET+ | 83 (3.0) | 8 (2.0) | 40 (13.0) | 5 (2.0) | 1 (< 1.0) | 2 (1.0) | 7 (2.0) | 13 (5.0) | 28 (2.0) | 5 (1.0) | 2 (0.7) |
| RET− | 824 (29.0) | 285 (68.0) | 62 (20.0) | 50 (18.0) | 76 (27.0) | 157 (55.0) | 67 (22.0) | 78 (28.0) | 428 (30.0) | 17 (4.0) | 32 (11.0) |
| Unknown | 1950 (68.0) | 129 (31.0) | 211 (67.0) | 224 (80.0) | 210 (73.0) | 126 (44.0) | 228 (75.0) | 193 (68.0) | 981 (68.0) | 368 (94.0) | 261 (88.0) |
| ALK results, n (%) | |||||||||||
| ALK+ | 124 (4.0) | 23 (6.0) | 38 (12.0) | 2 (1.0) | 21 (7.0) | 2 (1.0) | 10 (3.0) | 21 (7.0) | 56 (4.0) | 4 (1.0) | 3 (1.0) |
| ALK− | 2220 (78.0) | 349 (83.0) | 169 (54.0) | 269 (96.0) | 250 (87.0) | 269 (94.0) | 262 (87.0) | 179 (63.0) | 1229 (86.0) | 287 (74.0) | 186 (63.0) |
| Unknown, n(%) | 513 (18.0) | 50 (12.0) | 106 (34.0) | 8 (3.0) | 16 (6.0) | 14 (5.0) | 30 (10.0) | 84 (30.0) | 152 (11.0) | 99 (25.0) | 106 (36.0) |
| ROS1 results, n (%) | |||||||||||
| ROS1+ | 60 (2.0) | 5 (1.0) | 19 (6.0) | 3 (1.0) | 13 (5.0) | 3 (1.0) | 1 (< 1.0) | 12 (4.0) | 32 (2.0) | 3 (1.0) | 1 (0.3) |
| ROS1− | 1827 (64.0) | 328 (78.0) | 112 (36.0) | 206 (74.0) | 212 (74.0) | 232 (81.0) | 251 (83.0) | 141 (50.0) | 1042 (73.0) | 210 (54.0) | 135 (46.0) |
| Unknown | 970 (34.0) | 89 (21.0) | 182 (58.0) | 70 (25.0) | 62 (22.0) | 50 (18.0) | 50 (17.0) | 131 (46.0) | 363 (25.0) | 177 (45.0) | 159 (54.0) |
| PD-L1 results, n (%) | |||||||||||
| PD-L1+ | 602 (21.0) | 106 (25.0) | 73 (23.0) | 76 (27.0) | 50 (17.0) | 74 (26.0) | 63 (21.0) | 62 (22.0) | 325 (23.0) | 40 (10.0) | 58 (19.7) |
| PD-L1− | 1608 (56.0) | 245 (58.0) | 123 (39.0) | 176 (63.0) | 199 (69.0) | 186 (65.0) | 203 (67.0) | 143 (50.0) | 907 (63.0) | 195 (50.0) | 138 (47.0) |
| Unknown | 647 (23.0) | 71 (17.0) | 117 (37.0) | 27 (10.0) | 38 (13.0) | 25 (9.0) | 36 (12.0) | 79 (28.0) | 205 (14.0) | 155 (40.0) | 99 (34.0) |
ALK anaplastic lymphoma kinase, BR Brazil, CCI Charlson Comorbidity Index, DE Germany, ES Spain, EU5 5 countries from the European Union, FR France, IT Italy, JP Japan, PD-L1 programmed death ligand, RET rearranged during transfection, ROS, c-ros oncogene 1, TW Taiwan, UK United Kingdom, US United States
aIncludes the UK, IT, FR, ES, and DE
bIncludes Han/Chinese, Japanese, and Southeast Asian
cIncludes Asian-Indian subcontinent, Asian-Other, Black, Mulatto, Asian, Chinese, Mixed Race, Middle Eastern, Afro-Caribbean, Indigenous Taiwanese, Not answered, and Other
cThe sample sizes present in the columns for average CCI score are not applicable to any other parameter
Patient Characteristics
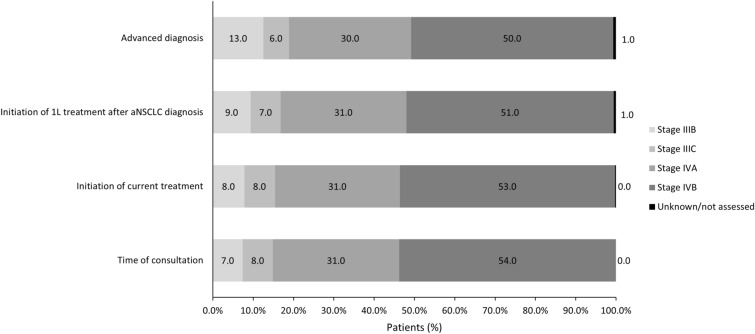
Patient characteristics were largely similar across countries (Table 1). Overall, mean age was 65.6 years (standard deviation [SD] 10.6 years) and the majority were female (56.0%, except in Germany with approx. 40.0% being female) and white (61.0%, except in Japan and Taiwan with > 95.0% Asian patients) with the mean (SD) Charlson comorbidity index score of 7.5 (2.2) (n = 2728). Most patients had stage IV disease at initial diagnosis (76.0%) and had adenocarcinoma histology (89.0%). At advanced diagnosis 80.0% of the patients had stage IV disease (Table 1, Fig. 1, and Supplementary Table S2). In the overall patient population (n = 2857), the most common EGFR mutations were exon 19 deletions (38.0%) or exon 21 mutations (16.0%). Co-mutations with ALK, RET, and ROS1 aberrations were reported in 4.0%, 3.0%, and 2.0%, respectively, and PD-L1 expression was reported in 21.0% (Table 1). Most patients had no history of prior surgery, radiotherapy, or neo/adjuvant therapy (Table 3). In the overall patient population, 53.0% (n = 1513) had no smoking history ranging from 71.0% in Taiwan to 29.0% in Germany (Table 1).
Fig. 1.
Tumor staging at different time points: overall population. All data are presented as %. 1L first line, aNSCLC advanced non-small cell lung cancer
Table 3.
Treatments received in each line: overall and by country
| Targeted Treatment for EGFR+ aNSCLC | Overall | US | BR | UK | IT | FR | ES | DE | EU5a | TW | JP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-drug treatment | n = 2857 | n = 422 | n = 313 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 295 |
| Surgery | 74 (3.0) | 15 (4.0) | 7 (2.0) | 5 (2.0) | 11 (4.0) | 2 (1.0) | 3 (1.0) | 9 (3.0) | 30 (2.0) | 14 (4.0) | 9 (3.0) |
| Radiotherapy | 573 (20.0) | 103 (24.0) | 73 (23.0) | 49 (18.0) | 60 (21.0) | 49 (17.0) | 37 (12.0) | 44 (15.0) | 239 (17.0) | 101 (26.0) | 57 (19.0) |
| Surgery and radiotherapy | 118 (4.0) | 9 (2.0) | 8 (3.0) | 6 (2.0) | 7 (2.0) | 9 (3.0) | 2 (1.0) | 64 (23.0) | 88 (6.0) | 10 (3.0) | 2 (1.0) |
| None | 2092 (73.0) | 295 (70.0) | 225 (72.0) | 219 (78.0) | 209 (73.0) | 225 (79.0) | 260 (86.0) | 167 (59.) | 1080 (75.0) | 265 (68.0) | 227 (77.0) |
| Neoadjuvant therapy received | n = 192 | n = 24 | n = 15 | n = 11 | n = 18 | n = 11 | n = 5 | n = 73 | n = 118 | n = 24 | n = 11 |
| 40 (21.0) | 6 (25.0) | 2 (13.0) | 0 | 7 (39.0) | 0 | 1 (20.0) | 19 (26.0) | 27 (23.0) | 5 (21.0) | 0 | |
| Adjuvant therapy received | n = 192 | n = 24 | n = 15 | n = 11 | n = 18 | n = 11 | n = 5 | n = 73 | n = 118 | n = 24 | n = 11 |
| 106 (55.0) | 17 (71.0) | 9 (60.0) | 2 (18.0) | 13 (72.0) | 2 (18.0) | 2 (40.0) | 43 (59.0) | 62 (53.0) | 14 (58.0) | 4 (36.0) | |
| 1L advance treatment | n = 2857 | n = 422 | n = 313 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 295 |
| Chemotherapy | 311 (11.0) | 47 (11.0 | 79 (25.0) | 5 (2.0) | 18 (6.0) | 18 (6.0) | 9 (3.0) | 88 (31.0) | 138 (10.0) | 13 (3.0) | 34 (12.0) |
| Targeted | 2586 (91.0) | 378 (90.0) | 232 (74.0) | 273 (98.0) | 262 (91.0) | 268 (94.0) | 294 (97.0) | 232 (82.0) | 1329 (92.0) | 382 (98.0) | 265 (90.0) |
| 1st/2nd generation EGFR-TKI | 1413 (49.0) | 122 (29.0) | 122 (39.0) | 232 (83.0) | 124 (43.0) | 157 (55.0) | 156 (52.0) | 89 (31.0) | 758 (53.0) | 310 (79.0) | 101 (34.0) |
| 3rd generation EGFR-TKI | 957 (33.0) | 220 (52.0) | 61 (19.0) | 39 (14.0) | 128 (45.0) | 104 (36.0) | 126 (42.0) | 89 (31.0) | 486 (34.0) | 37 (9.0) | 153 (52.0) |
| EGFR-TKI and anti-VEGF | 41 (1.0) | 5 (1.0) | 1 (< 1.0) | 0 | 3 (1.0) | 0 | 1 (< 1.0) | 2 (1.0) | 6 (< 1.0) | 24 (6.0) | 5 (2.0) |
| Other targeted therapy | 175 (6.0) | 31 (7.0) | 48 (15.0) | 2 (1.0) | 7 (2.0) | 7 (2.0) | 11 (4.0) | 52 (18.0) | 79 (5.0) | 11 (3.0) | 6 (2.0) |
| Immunotherapy | 76 (3.0) | 15 (4.0) | 31 (10.0) | 0 | 6 (2.0) | 3 (1.0) | 2 (< 1.0) | 14 (5.0) | 25 (2.0) | 2 (1.0) | 3 (1.0) |
| Other | 43 (2.0) | 15 (4.0) | 2 (1.0) | 2 (1.0) | 4 (1.0) | 3 (1.0) | 4 (1.0) | 11 (4.0) | 24 (2.0) | 1 (< 1.0) | 1 (< 1.0) |
| Best supportive care | 349 (12.0) | 47 (11.0) | 109 (35.0) | 5 (2.0) | 21 (7.0) | 20 (7.0) | 10 (3.0) | 89 (31.0) | 145 (11.0) | 14 (4.0) | 34 (12.0) |
| 2L advance treatment | n = 493 | n = 41 | n = 35 | n = 53 | n = 53 | n = 50 | n = 36 | n = 67 | n = 259 | n = 80 | n = 78 |
| Chemotherapy | 136 (28.0) | 8 (20.0) | 6 (17.0) | 9 (17.0) | 13 (25.0) | 8 (16.0) | 5 (14.0) | 11 (16.0) | 46 (18.0) | 44 (55.0) | 32 (41.0) |
| Targeted | 367 (74.0) | 34 (83.0) | 22 (63.0) | 47 (89.0) | 38 (72.0) | 41 (82.0) | 29 (81.0) | 60 (90.0) | 215 (83.0) | 42 (53.0) | 54 (69.0) |
| 1st/2nd generation EGFR-TKI | 67 (14.0) | 5 (12.0) | 8 (23.0) | 4 (8.0) | 5 (9.0) | 6 (12.0) | 9 (25.0) | 13 (19.0) | 37 (14.0) | 5 (6.0) | 12 (15.0) |
| 3rd generation EGFR-TKI | 236 (48.0) | 24 (59.0) | 13 (37.0) | 37 (70.0) | 29 (55.0) | 33 (66.0) | 20 (56.0) | 28 (42.0) | 147 (57.0) | 24 (30.0) | 28 (36.0) |
| EGFR-TKI and anti-VEGF | 3 (1.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3.0) | 1 (1.0) |
| Other targeted therapy | 61 (12.0) | 5 (12.0) | 1 (3.0) | 6 (11.0) | 4 (8.0) | 2 (4.0) | 0 | 19 (28.0) | 31 (12.0) | 11 (14.0) | 13 (17.0) |
| Immunotherapy | 54 (11.0) | 4 (10.0) | 7 (20.0) | 8 (15.0) | 4 (8.0) | 4 (8.0) | 2 (6.0) | 10 (15.0) | 28 (11.0) | 5 (6.0) | 10 (13.0) |
| Other | 6 (1.0) | 2 (5.0) | 0 | 0 | 1 (2.0) | 0 | 0 | 1 (1.0) | 2 (1.0) | 2 (3.0) | 0 |
| Best supportive care | 142 (29.0) | 9 (22.0) | 9 (26.0) | 9 (17.0) | 13 (25.0) | 8 (16.0) | 5 (14.0) | 11 (16.0) | 46 (18.0) | 46 (58.0) | 32 (41.0) |
| 3L advance treatment | n = 84 | n = 7 | n = 5 | n = 1 | n = 2 | n = 3 | n = 1 | n = 5 | n = 12 | n = 26 | n = 34 |
| Chemotherapy | 29 (35.0) | 0 | 2 (40.0) | 0 | 1 (50.0) | 0 | 0 | 0 | 1 (8.0) | 10 (38.0) | 16 (47.0) |
| Targeted | 56 (67.0) | 5 (71.0) | 5 (100.0) | 1 (100.0) | 0 | 1 (33.0) | 1 (100.0) | 4 (80.0) | 7 (58.0) | 18 (69.0) | 21 (62.0) |
| 1st/2nd generation EGFR-TKI | 18 (21.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 (27.0) | 11 (32.0) |
| 3rd generation EGFR-TKI | 24 (29.0) | 5 (71.0) | 3 (60.0) | 1 (100.0) | 0 | 1 (33.0) | 1 (100.0) | 3 (60.0) | 6 (50.0) | 6 (23.0) | 4 (12.0) |
| EGFR-TKI and anti-VEGF | 1 (1.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.0) | 0 |
| Other targeted therapy | 13 (15.0) | 0 | 2 (40.0) | 0 | 0 | 0 | 0 | 1 (20.0) | 1 (8.0) | 4 (15.0) | 6 (18.0) |
| Immunotherapy | 14 (17.0) | 2 (29.0) | 0 | 0 | 1 (50.0) | 1 (33.0) | 0 | 2 (40.0) | 4 (33.0) | 2 (8.0) | 6 (18.0) |
| Other | 2 (2.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.0) | 1 (3.0) |
| Best supportive care | 30 (36.0) | 0 | 2 (40.0) | 0 | 1 (50.0) | 1 (33.0) | 0 | 0 | 2 (17.0) | 10 (38.0) | 16 (47.0) |
All data are presented as n (%), unless specified. n = 2857, unless specified
BR Brazil, DE Germany, ER emergency room, ES Spain, EU5 5 countries from the European Union, FR France, ICU intensive-care unit, IT Italy, JP Japan, SD standard deviation, TW Taiwan, UK United Kingdom, US United States
Chemotherapy: carboplatin, cisplatin, paclitaxel, Nab-paclitaxel, docetaxel, gemcitabine, vinorelbine, pemetrexed, irinotecan, S-1
Targeted therapy: afatinib, erlotinib, gefitinib, osimertinib, dacomitinib, cetuximab, necitumumab, crizotinib, alectinib, ceritinib, brigatinib, lorlatinib, dabrafenib + trametinib, bevacizumab, nintedanib, ramucirumab, entrectinib
Immunotherapy: pembrolizumab, nivolumab, atezolizumab, avelumab, durvalumab
Other: any additional treatments not mentioned
1st generation EGFR-TKI included TKI, erlotinib, gefitinib; 2nd generation EGFR-TKI included afatinib, dacomitinib; 3rd generation EGFR-TKI included osimertinib
aIncludes the UK, IT, FR, ES, and DE
bReported in > 3.0% of the patients
At the start of 1L systemic therapy, the mean (SD) time to aNSCLC diagnosis from data collection was 14.5 (16.3) months (Table 2), and most (77.0%) patients had an ECOG performance status of 0 or 1 at advanced diagnosis (Supplementary Table S3). Among the patients with stage IV disease at data capture, the median number of primary metastatic (n = 2396) sites was 2 (IQR 1–2) (Supplementary Fig. S2), with contralateral lung, bone, and distant lymph nodes as the most common sites. About 14.0% had brain/central nervous system metastases at diagnosis of advanced disease (Supplementary Table S4). Most patients were retired (44.0% physician-reported and 48.0% patient-reported) and had health insurance (95.0%) (Supplementary Fig. S3). Supplementary Table S5 summarizes annual household income for eligible patients by country.
Table 2.
Disease timeline: overall and by country
| Overall (n = 2857) |
US (n = 422) |
BR (n = 313) |
UK (n = 279) |
IT (n = 287) |
FR (n = 285) |
ES (n = 302) |
DE (n = 284) |
EU5a (n = 1437) |
TW (n = 390) |
JP (n = 295) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time between early-stage and advanced-stage diagnosis | n = 208 | n = 12 | n = 53 | n = 7 | n = 7 | n = 2 | n = 9 | n = 37 | n = 62 | n = 23 | n = 58 |
| Months, mean (SD) | 26.8 (17.9) | 28.1 (13.2) | 26.3 (16.4) | 45.1 (46.8) | 18 (7.5) | 42.5 (12.8) | 20.1 (12.5) | 31.7 (14.8) | 30.4 (20.9) | 19.9 (16) | 25.9 (16.6) |
| Time to advanced diagnosis from data collection | n = 2630 | n = 374 | n = 276 | n = 244 | n = 239 | n = 274 | n = 285 | n = 271 | n = 1313 | n = 390 | n = 277 |
| Months, mean (SD) | 14.5 (16.3) | 13.9 (14.5) | 14.4 (15.6) | 14.2 (13.7) | 14.0 (14.5) | 11.3 (12.1) | 9.7 (10.4) | 9.7 (10.2) | 11.7 (12.3) | 20.0 (18.6) | 20.6 (25.9) |
| Time between advanced diagnosis and initiation of the 1st line of advanced treatment | n = 2547 | n = 355 | n = 254 | n = 237 | n = 238 | n = 271 | n = 266 | n = 265 | n = 1277 | n = 389 | n = 272 |
| Months, mean (SD) | 1.6 (4.3) | 2.4 (5.0) | 2.2 (3.3) | 1.4 (2.7) | 1.5 (2.7) | 0.9 (1.1) | 0.8 (1.6) | 1.4 (4.7) | 1.2 (2.9) | 1.3 (4.1) | 2.2 (8.2) |
| Time since 1st line of advanced treatment | n = 2603 | n = 363 | n = 269 | n = 240 | n = 249 | n = 278 | n = 266 | n = 274 | n = 1307 | n = 389 | n = 275 |
| Months, mean (SD) | 12.8 (15.5) | 10.8 (12.4) | 11.9 (15.4) | 12.8 (13.3) | 12.3 (14.1) | 10.1 (11.6) | 9.2 (10.7) | 8.3 (9.3) | 10.4 (12.0) | 18.8 (18.1) | 18.6 (24) |
| Time since metastatic diagnosis | n = 490 | n = 42 | n = 33 | n = 48 | n = 42 | n = 46 | n = 36 | n = 80 | n = 252 | n = 84 | n = 79 |
| Months, mean (SD) | 13.4 (17.5) | 14.2 (14.8) | 15.9 (14.0) | 8.0 (7.9) | 8.5 (7.4) | 7.7 (7.2) | 6.5 (3.9) | 7.2 (7.3) | 7.6 (7.0) | 16.4 (16.2) | 27.1 (31.4) |
| Time between advanced and metastatic diagnosis | n = 99 | n = 8 | n = 41 | n = 2 | n = 6 | n = 7 | n = 3 | n = 12 | n = 30 | n = 8 | n = 12 |
| Months, mean (SD) | 10.8 (12.3) | 13.5 (21.3) | 8.1 (13.8) | 13.6 (12.1) | 13.1 (9.3) | 16.8 (11.8) | 10.5 (6.7) | 10 (7.7) | 12.5 (9.1) | 14.3 (9.8) | 11.6 (6.4) |
BR Brazil, DE Germany, ES Spain, EU5 5 countries from the European Union, FR France, IT Italy, JP Japan, SD standard deviation, TW Taiwan, UK United Kingdom, US United States
aIncludes the UK, IT, FR, ES, and DE
Treatments
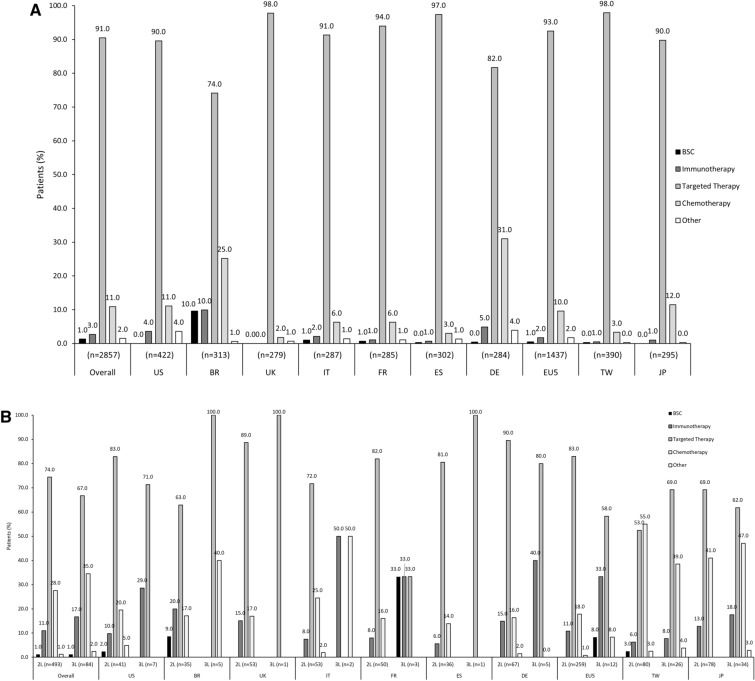
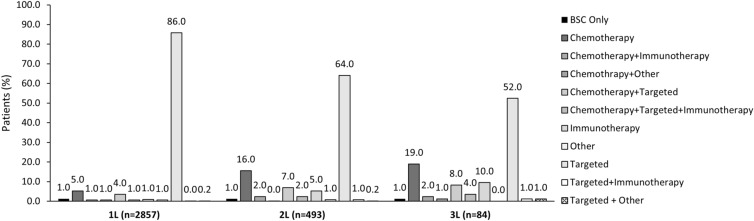
Among the overall patient population (n = 2857), 2819 (99.0%) received 1L systemic therapy for EGFRm+ aNSCLC and 38 (1.0%) received best supportive care only (Fig. 2a); 487 (99.0%, n = 493) and 81 (99.0%, n = 84) patients were treated systemically in second line (2L) and third line (3L) respectively (Fig. 2b). In 1L, most patients (91.0%) received targeted agents, 11.0% received chemotherapy, 3.0% received immunotherapy, and 2.0% received other systemic treatments (Fig. 2a). Similar trends were observed in subsequent lines (2L and 3L) and across most countries (Fig. 2b), although chemotherapy and immunotherapy were used relatively more often in some countries (including Brazil, Germany, Taiwan, and Italy) and in subsequent lines than the 1L setting (Figs. 2a, b). Of note, observed variations in patterns of subsequent therapy (especially 3L treatments) across countries were limited by small numbers of eligible patients in some individual countries. Figure 3 and Supplementary Table S6 present the treatment combinations by overall population and for each country. Table 3 presents the targeted treatment breakdown by generation for overall population and each country.
Fig. 2.
Treatment patterns: overall population and by country. a 1L. b 2L and 3L. All data are presented as %. 1L first line, 2L second line, 3L third line, BR Brazil, BSC best supportive care, DE Deutschland, ES Spain, EU5 5 countries from the European Union, FR France, IT Italy, JP Japan, TW Taiwan, UK United Kingdom, US United States
Fig. 3.
Treatment combinations: overall population. All data are presented as %. 1L first line, 2L second line, 3L third line, BSC best supportive care
EGFR Testing
Tumor samples used to detect EGFR gene mutations were mainly tissue biopsy specimens (mostly core needle biopsy [56.0%] and bronchial brush biopsy [19.0%]) followed by liquid biopsy specimens (7.0% globally and highest [16.0%] in Germany) (Table 4). Overall, the most common methods used for EGFR detection were EGFR-specific mutation detection test (specifics not captured in the survey) (44.0%; especially in Taiwan, Japan, and Brazil), followed by next-generation sequencing (NGS, 27.0%). However, there was variation in the methods used across countries, with higher usage rates of NGS in the US (54.0% for EGFR mutations and 80.0% for any molecular marker) and France (47.0% and 56.0%, respectively) and relatively lower rates in Taiwan (3.0% and 14.0%).
Table 4.
EGFR testing: type of test, sample used, and physician-reported measures
| Overall | US | BR | UK | IT | FR | ES | DE | EU5a | TW | JP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test used for EGFR | n = 2857 | n = 422 | n = 313 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 295 |
| NGS | 775 (27.0) | 226 (54.0) | 50 (16.0) | 63 (23.0) | 79 (28.0) | 133 (47.0) | 76 (25.0) | 87 (31.0) | 438 (31.0) | 13 (3.0) | 48 (16.0) |
| EGFR mutation detection | 1246 (44.0) | 116 (28.0) | 165 (53.0) | 121 (43.0) | 72 (25.0) | 87 (31.0) | 110 (36.0) | 120 (42.0) | 510 (36.0) | 257 (66.0) | 198 (67.0) |
| FISH | 201 (7.0) | 32 (8.0) | 50 (16.0) | 12 (4.0) | 33 (12.0) | 21 (7.0) | 20 (7.0) | 25 (9.0) | 111 (8.0) | 6 (2.0) | 2 (1.0) |
| IHC | 299 (11.0) | 44 (10.0) | 88 (28.0) | 11 (4.0) | 49 (17.0) | 19 (7.0) | 32 (11.0) | 11 (4.0) | 122 (9.0) | 39 (10.0) | 6 (2.0) |
| DNA sequencing (Sanger) | 77 (3.0) | 6 (1.0) | 15 (5.0) | 7 (3.0) | 15 (5.0) | 1 (< 1.0) | 1 (< 1.0) | 15 (5.0) | 39 (3.0) | 17 (4.0) | 0 |
| RT-PCR | 416 (15.0) | 6 (1.0) | 36 (12.0) | 32 (12.0) | 67 (23.0) | 2 (1.0) | 70 (23.0) | 9 (3.0) | 180 (13.0) | 154 (40.0) | 40 (14.0) |
| Digital PCR | 20 (1.0) | 1 (< 1.0) | 1 (< 1.0) | 4 (1.0) | 2 (1.0) | 0 | 0 | 3 (1.0) | 9 (1.0) | 7 (2.0) | 2 (1.0) |
| Other | 2 (< 1.0) | 1 (< 1.0) | 1 (< 1.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Don’t know | 270 (10.0) | 50 (12.0) | 11 (4.0) | 42 (15.0) | 32 (11.0) | 50 (18.0) | 23 (8.0) | 54 (19.0) | 201 (14.0) | 0 | 8 (3.0) |
| Samples used of EGFR test | n = 2857 | n = 422 | n = 313 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 295 |
| Core needle biopsy | 1592 (56.0) | 263 (62.0) | 136 (44.0) | 217 (78.0) | 165 (58.0) | 188 (66.0) | 233 (77.0) | 93 (33.0) | 896 (62.0) | 225 (58.0) | 72 (24.0) |
| Bronchial brush biopsy | 529 (19.0) | 39 (9.0) | 105 (34.0) | 20 (7.0) | 19 (7.0) | 65 (23.0) | 24 (8.0) | 40 (14.0) | 168 (12.0) | 63 (16.0) | 154 (52.0) |
| Mediastinoscopy | 69 (2.0) | 7 (2.0) | 26 (8.0) | 6 (2.0) | 11 (4.0) | 1 (< 1.0) | 7 (2.0) | 1 (< 1.0) | 26 (2.0) | 7 (2.0) | 3 (1.0) |
| Fine needle aspiration | 334 (12.0) | 81 (19.0) | 38 (12.0) | 19 (7.0) | 48 (17.0) | 12 (4.0) | 30 (10.0) | 73 (26.0) | 182 (13.0) | 25 (6.0) | 8 (3.0) |
| Thoracentesis | 142 (5.0) | 20 (5.0) | 4 (1.0) | 5 (2.0) | 15 (5.0) | 1 (< 1.0) | 6 (2.0) | 2 (1.0) | 29 (2.0) | 69 (18.0) | 20 (7.0) |
| Liquid biopsy | 185 (7.0) | 24 (6.0) | 28 (9.0) | 5 (2.0) | 15 (5.0) | 13 (5.0) | 16 (5.0) | 46 (16.0) | 95 (7.0) | 32 (8.0) | 6 (2.0) |
| Other | 144 (5.0) | 7 (2.0) | 3 (1.0) | 7 (3.0) | 14 (5.0) | 13 (5.0) | 8 (3.0) | 6 (2.0) | 48 (3.0) | 39 (10.0) | 47 (16.0) |
| Don’t know | 139 (5.0) | 34 (8.0) | 8 (3.0) | 11 (4.0) | 24 (8.0) | 10 (4.0) | 9 (3.0) | 38 (13.0) | 92 (6.0) | 0 | 5 (2.0) |
| Requesting EGFR tests (physician-reported) | n = 513 | n = 60 | n = 50 | n = 49 | n = 47 | n = 48 | n = 50 | n = 47 | n = 241 | n = 51 | n = 111 |
| Self (treating physician) | 431 (84.0) | 46 (77.0) | 48 (96.0) | 25 (51.0) | 43 (92.0) | 35 (73.0) | 33 (66.0) | 45 (96.0) | 181 (75.0) | 50 (98.0) | 106 (96.0) |
| Pulmonologist | 62 (12.0) | 7 (12.0) | 4 (8.0) | 8 (16.0) | 8 (17.0) | 10 (21.0) | 3 (6.0) | 3 (6.0) | 32 (13.0) | 5 (10.0) | 14 (13.0) |
| Medical oncologist | 43 (8.0) | 13 (22.0) | 8 (16.0) | 4 (8.0) | 7 (15.0) | 2 (4.0) | 1 (2.0) | 5 (11.0) | 19 (8.0) | 2 (4.0) | 1 (1.0) |
| Pathologist | 104 (20.0) | 14 (23.0) | 3 (6.0) | 29 (59.0) | 7 (15.0) | 15 (31.0) | 21 (42.0) | 1 (2.0) | 73 (30.0) | 5 (10.0) | 9 (8.0) |
| Interventional radiologist | 6 (1.0) | 2 (3.0) | 0 | 1 (2.0) | 1 (2.0) | 0 | 0 | 2 (4.0) | 4 (2.0) | 0 | 0 |
| Surgeon | 16 (3.0) | 1 (2.0) | 4 (8.0) | 2 (4.0) | 4 (9.0) | 1 (2.0) | 0 | 2 (4.0) | 9 (4.0) | 1 (2.0) | 1 (1.0) |
| Radiation oncologist | 12 (2.0) | 1 (2.0) | 1 (2.0) | 3 (6.0) | 4 (9.0) | 0 | 0 | 2 (4.0) | 9 (4.0) | 1 (2.0) | 0 |
| Primary physician/general practitioner | 2 (< 1.0) | 1 (2.0) | 1 (2.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nurse | 4 (1.0) | 1 (2.0) | 2 (4.0) | 1 (2.0) | 0 | 0 | 0 | 0 | 1 (< 1.0) | 0 | 0 |
| Other | 6 (1.0) | 2 (3.0) | 0 | 2 (4.0) | 0 | 1 (2.0) | 1 (2.0) | 0 | 4 (2.0) | 0 | 0 |
| Don’t know | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Barriers to EGFR testing (physician-reported) | n = 513 | n = 60 | n = 50 | n = 49 | n = 47 | n = 48 | n = 50 | n = 47 | n = 241 | n = 51 | n = 111 |
| Inadequate tissue | 348 (68.0) | 37 (62.0) | 34 (68.0) | 36 (74.0) | 34 (72.0) | 28 (58.0) | 37 (74.0) | 28 (60.0) | 163 (68.0) | 44 (86.0) | 70 (63.0) |
| Time delay in getting results | 144 (28.0) | 16 (27.0) | 19 (38.0) | 14 (29.0) | 15 (32.0) | 8 (17.0) | 11 (22.0) | 11 (23.0) | 59 (25.0) | 7 (14.0) | 43 (39.0) |
| Cost per test | 73 (14.0) | 10 (17.0) | 20 (40.0) | 2 (4.0) | 6 (13.0) | 3 (6.0) | 2 (4.0) | 6 (13.0) | 19 (8.0) | 9 (18.0) | 15 (14.0) |
| Reimbursement issue | 73 (14.0) | 11 (18.0) | 18 (36.0) | 4 (8.0) | 1 (2.0) | 2 (4.0) | 2 (4.0) | 7 (15.0) | 16 (7.0) | 8 (16.0) | 20 (18.0) |
| Test availability | 44 (9.0) | 3 (5.0) | 16 (32.0) | 3 (6.0) | 2 (4.0) | 3 (6.0) | 3 (6.0) | 4 (9.0) | 15 (6.0) | 9 (18.0) | 1 (1.0) |
| Patient refusal | 40 (8.0) | 10 (17.0) | 3 (6.0) | 6 (12.0) | 2 (4.0) | 3 (6.0) | 0 | 5 (11.0) | 16 (7.0) | 9 (18.0) | 2 (2.0) |
| Other | 8 (2.0) | 2 (3.0) | 0 | 3 (6.0) | 0 | 1 (2.0) | 0 | 1 (2.0) | 5 (2.0) | 0 | 1 (1.0) |
| None | 94 (18.0) | 16 (27.0) | 3 (6.0) | 9 (18.0) | 10 (21.0) | 13 (27.0) | 5 (10.0) | 12 (26.0) | 49 (20.0) | 3 (6.0 | 23 (21.0) |
| Physicians testing proportion of their patients at advanced diagnosis (physician-reported) | n = 542 | n = 71 | n = 53 | n = 51 | n = 49 | n = 51 | n = 50 | n = 50 | n = 251 | n = 51 | n = 116 |
| 499 (92.0) | 66 (94.0) | 44 (84.0) | 49 (96.0) | 43 (88.0) | 47 (91.0) | 48 (95.0) | 45 (89.0) | 231 (92.0) | 49 (96.0) | 108 (93.0) | |
| Tested for any molecular biomarkers via NGS (physician-reported) | n = 540 | n = 70 | n = 53 | n = 51 | n = 49 | n = 50 | n = 50 | n = 50 | n = 250 | n = 51 | n = 116 |
| 207 (38.0) | 56 (80.0) | 25 (47.0) | 13 (25.0) | 16 (33.0) | 28 (56.0) | 14 (29.0) | 15 (30.0) | 86 (34.0) | 7 (14.0) | 34 (29.0) | |
| Tested for EGFR at advanced stage (physician-reported) | n = 540 | n = 70 | n = 53 | n = 51 | n = 49 | n = 50 | n = 50 | n = 50 | n = 250 | n = 51 | n = 116 |
| 389 (72.0) | 44 (63.0) | 34 (65.0) | 38 (75.0) | 40 (82.0) | 42 (83.0) | 41 (82.0) | 34 (68.0) | 195 (78.0) | 48 (94.0) | 68 (58.0) | |
| Turnaround time for EGFR test results, median (IQR) days | n = 540 | n = 71 | n = 53 | n = 51 | n = 49 | n = 51 | n = 50 | n = 48 | n = 249 | n = 51 | n = 116 |
| 10.0 (7.0–14.0) | 7.0 (5/0–14.0) | 15.0 (10.0–25.0) | 10.0 (7.0–14.0) | 14.0 (8.0–14.0) | 14.0 (10.0–18.5) | 8.0 (7.0–10.0) | 7.0 (6.5–12.0) | 10.0 (7.0–14.0) | 7.0 (7.0–12.0) | 7.0 (7.0–10.0) |
All data are presented as n (%); % rounded off to nearest 0, unless otherwise indicated
EGFR mutation detection test: specifics not captured in the survey
BR Brazil, DE Germany, DNA deoxyribonucleic acid, EGFR endothelial growth factor receptor, ES Spain, EU5 U5 countries from the European Union, FISH fluorescence in situ hybridization, FR France, IHC immunohistochemistry, IT Italy, JP Japan, NGS next-generation sequencing, PCR polymerase chain reaction, RT-PCR reverse-transcription polymerase chain reaction, TW Taiwan, UK United Kingdom, US United States
aIncludes the UK, IT, FR, ES, and DE
EGFR tests were mostly requested by the treating physician, except in the UK where 59.0% of tests were requested by a pathologist. For almost all (96.0%) patients, EGFR mutation results were known before initiation of 1L therapy for aNSCLC. Overall, physicians reported a median turnaround time of 10.0 (IQR 7.0–14.0) days for EGFR test results (Table 4). The most common physician-reported barriers to EGFR testing were inadequate tissue (68.0%, especially in Taiwan [86.0%]) and time delay in getting results (28.0%), although one in three physicians in Brazil also reported test availability, cost, and reimbursement issues as key barriers (Table 4). For the 172 patients with available testing data in the post-progression setting, T790M was tested in 79 patients (46.0%), of whom 56 (71.0%) were T790M positive.
Outcomes
Effectiveness
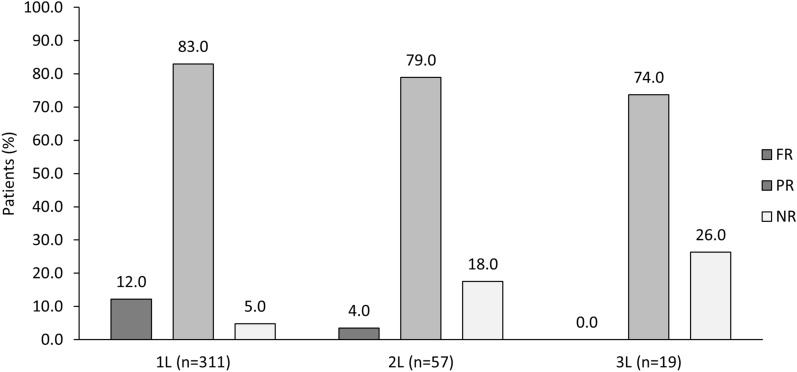
In the 1L setting, the median (IQR) TTNT and TTF were 14.0 (IQR 8.0–22.0) and 13.3 (IQR 7.9–20.9) months, respectively. Of the 2857 patients, a response assessment was given for 311 patients. Of the 2857 with documented response in 1L (n = 311), FR and PR to 1L therapy were reported in 12.0% and 83.0%, respectively, with 5.0% showing no response. Generally similar results were observed across countries, although Brazil had a relatively higher rate of no response (18.0%) (Supplementary Table S7). Response rates for overall population by each line of therapy are presented in Fig. 4. In the overall patient population (n = 2857), the mean (SD) duration of 1L treatment was 14.3 (11.2) months and the median number of treatment lines received was 1 (IQR 1–1). Mean (SD) duration of 2L therapy was 12.3 (10.7) months. The main physician-reported reason for early discontinuation of 1L (n = 223) or 2L therapy (n = 29), in all countries, was disease progression (77.0% for 1L and 83.0% for 2L), followed by side effects (20.0% for 1L and 17.0% for 2L) and lack of response (12.0% for 1L and 7.0% for 2L) (Supplementary Table S7).
Fig. 4.
Response rates after end of treatment: overall population. All data are presented as %. 1L first line, 2L second line, 3L third line, FR full response, NR no response, PR partial response
Disease Symptoms and Side Effects
In the overall population, the top five physician-reported disease symptoms in the overall cohort (n = 2857) were cough (51.0%), fatigue (37.0%), dyspnea (33.0%), persistent cough (24.0%), and weight loss (22.0%) (Table 5). Analysis of patient-reported disease symptoms in the subset of patients with eligible PSC data (n = 962, 34.0% of overall cohort) showed a similar trend. However, patient-reported rates trended higher than physician-reported rates, especially for weight loss (33.0% and 22.0%), low mood (24.0% and 14.0%, respectively), and anxiety (27.0% and 14.0%) (Table 5). Table 5 also includes the patient- and physician-reported symptoms across all counties.
Table 5.
Disease symptoms and side effects: overall and by country
| Overall (n = 2857) |
US (n = 422) |
BR (n = 313) |
UK (n = 279) |
IT (n = 287) |
FR (n = 285) |
ES (n = 302) |
DE (n = 284) |
EU5a (n = 1437) |
TW (n = 390) |
JP (n = 295) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comorbidities at advanced diagnosisb | |||||||||||
| Hypertension | 913 (32.0) | 180 (43.0) | 116 (37.0) | 86 (31.0) | 102 (36.0) | 80 (28.0) | 77 (26.0) | 112 (39.0) | 457 (32.0) | 104 (27.0) | 56 (19.0) |
| Dyslipidemia | 391 (14.0) | 97 (23.0) | 34 (11.0) | 32 (12.0) | 54 (19.0) | 32 (11.0) | 45 (15.0) | 48 (17.0) | 211 (15.0) | 26 (7.0) | 23 (8.0) |
| COPD | 295 (10.0) | 64 (15.0) | 50 (16.0) | 25 (9.0) | 31 (11.0) | 12 (4.0) | 6 (2.0) | 52 (18.0) | 126 (9.0) | 39 (10.0) | 16 (5.0) |
| Diabetes without chronic complications | 256 (9.0) | 32 (8.0) | 25 (8.0) | 31 (11.0) | 40 (14.0) | 15 (5.0) | 43 (14.0) | 36 (13.0) | 165 (12.0) | 25 (6.0) | 9 (3.0) |
| Renal disease | 133 (5.0) | 23 (6.0) | 23 (7.0) | 10 (4.0) | 13 (5.0) | 14 (5.0) | 6 (2.0) | 18 (6.0) | 61 (4.0) | 19 (5.0) | 7 (2.0) |
| Peripheral vascular disease | 109 (4.0) | 16 (4.0) | 19 (6.0) | 12 (4.0) | 12 (4.0) | 14 (5.0) | 16 (5.0) | 11 (4.0) | 65 (5.0) | 7 (2.0) | 2 (1.0) |
| Mild liver disease | 91 (3.0) | 11 (3.0) | 9 (3.0) | 4 (1.0) | 11 (4.0) | 4 (1.0) | 8 (3.0) | 24 (9.0) | 51 (4.0) | 11 (3.0) | 9 (3.0) |
| Current comorbiditiesb | |||||||||||
| Hypertension | 925 (32.0) | 187 (44.0) | 125 (40.0) | 85 (31.0) | 100 (35.0) | 79 (28.0) | 76 (25.0) | 104 (37.0) | 444 (31.0) | 110 (28.0) | 59 (20.0) |
| Dyslipidemia | 402 (14.0) | 104 (25.0) | 44 (14.0) | 31 (11.0) | 48 (17.0) | 32 (11.0) | 47 (16.0) | 46 (16.0) | 204 (14.0) | 27 (7.0) | 23 (8.0) |
| COPD | 310 (11.0) | 68 (16.0) | 63 (20.0) | 25 (9.0) | 31 (11.0) | 12 (4.0) | 5 (2.0) | 52 (18.0) | 125 (9.0) | 40 (10.0) | 14 (5.0) |
| Diabetes without chronic complications | 257 (9.0) | 35 (8.0) | 25 (8.0) | 32 (12.0) | 40 (14.0) | 15 (5.0) | 44 (15.0) | 35 (12.0) | 166 (12.0) | 23 (6.0) | 8 (3.0) |
| Renal disease | 151 (5.0) | 25 (6.0) | 34 (11.0) | 10 (4.0) | 17 (6.0) | 14 (5.0) | 6 (2.0) | 16 (6.0) | 63 (4.0) | 20 (5.0) | 9 (3.0) |
| Peripheral vascular disease | 115 (4.0) | 15 (4.0) | 30 (10.0) | 11 (4.0) | 12 (4.0) | 14 (5.0) | 14 (5.0) | 11 (4.0) | 62 (4.0) | 8 (2.0) | 0 |
| Mild liver disease | 109 (4.0) | 11 (3.0) | 17 (5.0) | 4 (1.0) | 14 (5.0) | 4 (1.0) | 10 (3.0) | 21 (7.0) | 53 (4.0) | 18 (5.0) | 10 (3.0) |
| Physician-reported symptoms at current consultationc | n = 2857 | n = 422 | n = 313 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 295 |
| Cough | 1461 (51.0) | 232 (55.0) | 170 (54.0) | 123 (44.0) | 159 (55.0) | 152 (53.0) | 143 (47.0) | 146 (51.0) | 723 (50.0) | 242 (62.0) | 94 (32.0) |
| Fatigue | 1055 (37.0) | 163 (39.0) | 115 (37.0) | 118 (42.0) | 119 (42.0) | 135 (47.0) | 101 (33.0) | 152 (54.0) | 625 (44.0) | 111 (29.0) | 41 (14.0) |
| Dyspnea/breathing complications | 935 (33.0) | 154 (37.0) | 105 (34.0) | 95 (34.0) | 117 (41.0) | 116 (41.0) | 111 (37.0) | 119 (42.0) | 558 (39.0) | 104 (27.0) | 14 (5.0) |
| Persistent cough | 675 (24.0) | 145 (34.0) | 107 (34.0) | 33 (12.0) | 66 (23.0) | 41 (14.0) | 75 (25.0) | 101 (36.0) | 316 (22.0) | 91 (23.0) | 16 (5.0) |
| Weight loss | 627 (22.0) | 90 (21.0) | 115 (37.0) | 41 (15.0) | 64 (22.0) | 69 (24.0) | 55 (18.0) | 88 (31.0) | 317 (22.0) | 93 (24.0) | 12 (4.0) |
| Loss of appetite | 597 (21.0) | 71 (17.0) | 91 (29.0) | 51 (18.0) | 55 (19.0) | 63 (22.0) | 52 (17.0) | 97 (34.0) | 318 (22.0) | 97 (25.0) | 20 (7.0) |
| Chest pain | 559 (20.0) | 48 (11.0) | 75 (24.0) | 31 (11.0) | 62 (22.0) | 58 (20.0) | 66 (22.0) | 102 (36.0) | 319 (22.0) | 90 (23.0) | 27 (9.0) |
| Bone pain | 522 (18.0) | 46 (11.0) | 86 (28.0) | 39 (14.0) | 69 (24.0) | 54 (19.0) | 58 (19.0) | 75 (26.0) | 295 (21.0) | 73 (19.0) | 22 (8.0) |
| Hoarseness | 375 (13.0) | 47 (11.0) | 71 (23.0) | 17 (6.0) | 49 (17.0) | 25 (9.0) | 45 (15.0) | 77 (27.0) | 213 (15.0) | 38 (10.0) | 6 (2.0) |
| Low mood | 390 (14.0) | 18 (4.0) | 79 (25.0) | 18 (7.0) | 30 (11.0) | 31 (11.0) | 29 (10.0) | 80 (28.0) | 188 (13.0) | 84 (22.0) | 21 (7.0) |
| Anxiety | 405 (14.0) | 42 (10.0) | 74 (24.0) | 22 (8.0) | 44 (15.0) | 47 (17.0) | 38 (13.0) | 31 (11.0) | 182 (13.0) | 99 (25.0) | 8 (3.0) |
| Weak limbs | 363 (13.0) | 31 (7.0) | 66 (21.0) | 7 (3.0) | 68 (24.0) | 27 (10.0) | 26 (9.0) | 60 (21.0) | 188 (13.0) | 62 (16.0) | 16 (5.0) |
| Headache/dizziness | 285 (10.0) | 41 (10.0) | 57 (18.0) | 11 (4.0) | 36 (13.0) | 27 (10.0) | 25 (8.0) | 44 (16.0) | 143 (10.0) | 32 (8.0) | 12 (4.0) |
| None of the above | 378 (13.0) | 29 (7.0) | 26 (8.0) | 41 (15.0) | 29 (10.0) | 31 (11.0) | 26 (9.0) | 6 (2.0) | 133 (9.0) | 59 (15.0) | 131 (44.0) |
| Patient-reported symptoms at current consultation | n = 962 | n = 160 | n = 128 | n = 55 | n = 79 | n = 79 | n = 127 | n = 151 | n = 491 | n = 49 | n = 134 |
| Cough | 543 (56.0) | 105 (66.0) | 79 (62.0) | 15 (27.0) | 42 (53.0) | 47 (60.0) | 75 (59.0) | 97 (64.0) | 276 (56.0) | 31 (63.0) | 52 (39.0) |
| Fatigue | 361 (38.0) | 49 (31.0) | 42 (33.0) | 9 (16.0) | 36 (46.0) | 46 (58.0) | 62 (49.0) | 72 (48.0) | 225 (46.0) | 14 (29.0) | 31 (23.0) |
| Weight loss | 313 (33.0) | 32 (20.0) | 68 (53.0) | 16 (29.0) | 26 (33.0) | 27 (34.0) | 41 (32.0) | 52 (34.0) | 162 (33.0) | 19 (39.0) | 32 (24.0) |
| Loss of appetite | 291 (30.0) | 24 (15.0) | 62 (48.0) | 16 (29.0) | 18 (23.0) | 27 (34.0) | 43 (34.0) | 60 (40.0) | 164 (33.0) | 19 (39.0) | 22 (16.0) |
| Anxiety | 258 (27.0) | 42 (26.0) | 68 (53.0) | 11 (20.0) | 13 (17.0) | 22 (28.0) | 28 (22.0) | 24 (16.0) | 98 (20.0) | 13 (27.0) | 37 (28.0) |
| Chest pain | 242 (25.0) | 22 (14.0) | 46 (36.0) | 1 (2.0) | 18 (23.0) | 15 (19.0) | 44 (35.0) | 52 (34.0) | 130 (27.0) | 13 (27.0) | 31 (23.0) |
| Dyspnea/breathing complications | 238 (25.0) | 48 (30.0) | 34 (27.0) | 18 (33.0) | 7 (9.0) | 16 (20.0) | 26 (21.0) | 78 (52.0) | 145 (30.0) | 10 (20.0) | 1 (1.0) |
| Low mood | 228 (24.0) | 12 (8.0) | 67 (52.0) | 5 (9.0) | 16 (20.0) | 16 (20.0) | 34 (27.0) | 38 (25.0) | 109 (22.0) | 13 (27.0) | 27 (20.0) |
| Persistent cough | 223 (23.0) | 30 (19.0) | 43 (34.0) | 7 (13.0) | 21 (27.0) | 14 (18.0) | 37 (29.0) | 45 (30.0) | 124 (25.0) | 17 (35.0) | 9 (7.0) |
| Bone pain | 195 (20.0) | 20 (13.0) | 56 (44.0) | 5 (9.0) | 15 (19.0) | 12 (15.0) | 26 (21.0) | 33 (22.0) | 91 (19.0) | 17 (35.0) | 11 (8.0) |
| Weak limbs | 182 (19.0) | 22 (14.0) | 44 (34.0) | 2 (4.0) | 18 (23.0) | 7 (9.0) | 23 (18.0) | 23 (15.0) | 73 (15.0) | 12 (25.0) | 31 (23.0) |
| Hoarseness | 166 (17.0) | 7 (4.0) | 44 (34.0) | 4 (7.0) | 11 (14.0) | 6 (8.0) | 20 (16.0) | 49 (33.0) | 90 (18.0) | 9 (18.0) | 16 (12.0) |
| Headache/dizziness | 143 (15.0) | 14 (9.0) | 50 (39.0) | 4 (7.0) | 7 (9.0) | 13 (17.0) | 17 (13.0) | 17 (11.0) | 58 (12.0) | 13 (27.0) | 8 (6.0) |
| Coughing blood | 112 (12.0) | 7 (4.0) | 26 (20.0) | 4 (7.0) | 10 (13.0) | 16 (20.0) | 9 (7.0) | 28 (19.0) | 67 (14.0) | 6 (12.0) | 6 (5.0) |
| Stomach problem | 101 (11.0) | 5 (3.0) | 19 (15.0) | 2 (4.0) | 3 (4.0) | 8 (10.0) | 12 (9.0) | 31 (21.0) | 56 (11.0) | 6 (12.0) | 15 (11.0) |
| Difficulty in swallowing | 64 (7.0) | 0 | 30 (23.0) | 0 | 5 (6.0) | 2 (3.0) | 8 (6.0) | 10 (7.0) | 25 (5.0) | 5 (10.0) | 4 (3.0) |
| Swelling of extremities | 56 (6.0) | 0 | 26 (20.0) | 3 (6.0) | 1 (1.0) | 2 (3.0) | 2 (2.0) | 5 (3.0) | 13 (3.0) | 1 (2.0) | 16 (12.0) |
| Lump in neck/lymph node | 47 (5.0) | 0 | 17 (13.0) | 2 (4.0) | 3 (4.0) | 3 (4.0) | 4 (3.0) | 3 (2.0) | 15 (3.0) | 6 (12.0) | 9 (7.0) |
| None of the above | 65 (7.0) | 11 (7.0) | 2 (2.0) | 5 (9.0) | 7 (9.0) | 5 (6.0) | 6 (5.0) | 3 (2.0) | 26 (5.0) | 3 (6.0) | 23 (17.0) |
All data are presented as n (%), unless specified. n = 2857, unless specified
BR Brazil, COPD chronic obstructive pulmonary disease, DE Germany, ER emergency room, ES Spain, EU5 5 countries from the European Union, FR France, ICU intensive care unit, IT Italy, JP Japan, SD standard deviation, TW Taiwan, UK United Kingdom, US United States
aIncludes the UK, IT, FR, ES, and DE
bReported in > 3.0% of the patients
cReported in > 10.0% of the patients
Among the 733 patients (26.0% of the overall cohort) with available safety data (reported AEs), the majority were grade 1 or grade 2 in severity. The most common any grade AEs were rash (46.0%, 334/733) and diarrhea (45.0%, 333/733), followed by dry skin (32.0%, 235/733), fatigue (28.0%, 204/733), loss of appetite (26.0%, 191/733), and nausea (24.0%, 178/733) (Supplementary Fig. S4). Patterns of disease symptoms and therapy AEs were generally similar across countries as summarized in Supplementary Table S8.
Patient-Reported Outcomes
The mean (SD) EQ-5D-5L index score for the 934 patients with available PSC data was 0.7 (0.2) and mean (SD) EQ-5D VAS for 947 patients was 69.7 (17.6) (Table 6). Some or extreme problems were reported by at least 40.0% of patients across most domains (self-care, 35.0%; mobility, 42.0%; usual activities, 55.0%; anxiety/depression, 64.0%; pain/discomfort, 66.0%) (Supplementary Table S9).
Table 6.
EQ-5D-5L, EQ-VAS, and FACT scores: overall population
| PRO | PRO score (min–max) | |||
|---|---|---|---|---|
| Overall | 1L | 2L | 3L | |
| EQ-5D-5L | n = 934 | n = 764 | n = 138 | n = 32 |
| 0.7 (0.2) | 0.7 (0.3) | 0.7 (0.3) | 0.8 (0.3) | |
| EQ-VAS | n = 947 | n = 776 | n = 138 | n = 33 |
| 69.7 (17.6) | 70.7 (17.4) | 63.7 (17.7) | 71.8 (16.9) | |
| FACT-La | n = 930 | n = 764 | n = 135 | n = 31 |
| 83.5 (21.0) | 83.9 (21.4) | 79.6 (18.9) | 90.5 (16.4) | |
| FACT-Gb | n = 931 | n = 765 | n = 135 | n = 31 |
| 66.1 (17.4) | 66.4 (17.8) | 63.2 (15.6) | 71.5 (13.5) | |
| Trial index outcomesc | n = 935 | n = 768 | n = 136 | n = 31 |
| 51.1 (14.9) | 51.6 (15.0) | 47.5 (14.0) | 56.1 (14.2) | |
All values are presented as mean (SD)
1L first line, 2L second line, 3L third line, EQ-5D-5L European Quality of Life 5 Dimension 5 Level, EQ-VAS EuroQoL visual analog scale, FACT-L Functional Assessment of Cancer Therapy–Lung, FACT-G Functional Assessment of Cancer Therapy–General
aIncludes all 5 individual parameters
bIncludes social/family well-being subscale, emotional well-being subscale, functional well-being subscale, and physical well-being subscale
cIncludes functional well-being subscale, physical well-being subscale, and lung cancer subscale
The mean (SD) FACT-L score was 83.5 (21.0) (Table 6), with subscale mean scores ranging from 13.8 for the emotional well-being subscale to 19.7 for the physical well-being subscale (Supplementary Table S10). Almost all the patients (942/956 patients, 99.0%) felt completely informed by their physicians about their disease and treatment options (reported in patient self-reported questionnaire), and involved in their treatment decisions (88.0%, 831/945), with key treatment goals of maintaining or improving NSCLC symptoms (59.0%, 556/950) and prolonging life (47.0%, 442/950).
Healthcare Resource Utilization
Over the 12 months prior to data capture, almost all patients (98.0%) consulted with the physician involved in data capture with mean (SD) 1.0 (0) consultations for the overall population. Among hospitalized patients (n = 827), the mean (SD) number of hospital visits in the last 12 months was 1.5 (1.5). Other healthcare providers involved in the ongoing management of patients are summarized in Table 7. Patients lost about mean (SD) 10.6 (6.6) h of work per week for approximately mean (SD) 29.2 (25.7) weeks. Approximately one in three patients (31.0%, 288/916) incurred out-of-pocket expenses for NSCLC treatment. Table 7 summarizes the proportion of patients incurring out-of-pocket costs and median (IQR) out-of-pocket costs by country. Out of the 2857 patients, 827 (29.0%) were admitted to the hospital at some point in time for a mean of 1.5 visits. Mean hospital length of stay ranged from 7 to 9 nights. About one of three hospitalizations involved emergency care (e.g., 35.0% for the first hospitalization) and 7.0% of hospitalizations involved intensive care (Table 7). Most of the hospitalizations were to treat a complication, except for biopsy which was the main reason (38.0%) for the first hospitalization (Supplementary Table S11).
Table 7.
Healthcare resource utilization: by country
| Overall (n = 2857) |
US (n = 422) |
BR (n = 313) |
UK (n = 279) |
IT (n = 287) |
FR (n = 285) |
ES (n = 302) |
DE (n = 284) |
EU5a (n = 1437) |
TW (n = 390) |
JP (n = 295) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCPs involved in management in last 12 months | |||||||||||
| Physicians involved in data capture | 2814 (98.0) | 413 (98.0) | 306 (98.0) | 272 (97.0) | 279 (97.0) | 284 (100.0) | 294 (97.0) | 282 (99.0) | 1411 (98.0) | 389 (100.0) | 295 (100.0) |
| Pulmonologist | 1025 (36.0) | 125 (30.0) | 111 (35.0) | 109 (39.0) | 104 (36.0) | 130 (46.0) | 160 (53.0) | 162 (57.0) | 665 (46.0) | 79 (20.0) | 45 (15.0) |
| Oncologist | 842 (29.0) | 138 (33.0) | 90 (29.0) | 74 (27.0) | 141 (49.0) | 85 (30.0) | 84 (28.0) | 112 (39.0) | 496 (35.0) | 93 (24.0) | 25 (8.0) |
| Primary care physician | 772 (27.0) | 126 (30.0) | 31 (10.0) | 85 (30.0) | 104 (36.0) | 129 (45.0) | 95 (31.0) | 139 (49.0) | 552 (38.0) | 37 (9.0) | 26 (9.0) |
| Radio oncologist/radiologist | 650 (23.0) | 94 (22.0) | 69 (22.0) | 40 (14.0) | 98 (34.0) | 77 (27.0) | 63 (21.0) | 94 (33.0) | 372 (26.0) | 90 (23.0) | 25 (8.0) |
| Specialist nurse | 630 (22.0) | 25 (6.0) | 72 (23.0) | 152 (54.0) | 56 (20.0) | 90 (32.0) | 93 (31.0) | 64 (23.0) | 455 (32.0) | 43 (11.0) | 35 (12.0) |
| Specialist cancer surgeon | 357 (12.0) | 32 (8.0) | 64 (20.0) | 21 (8.0) | 60 (21.0) | 31 (11.0) | 44 (15.0) | 59 (21.0) | 215 (15.0) | 36 (9.0) | 10 (3.0) |
| Internist | 279 (10.0) | 12 (3.0) | 36 (12.0) | 20 (7.0) | 32 (11.0) | 24 (8.0) | 38 (13.0) | 59 (21.0) | 173 (12.0) | 48 (12.0) | 10 (3.0) |
| Other | 43 (2.0) | 4 (1.0) | 6 (2.0) | 1 (0) | 6 (2.0) | 5 (2.0) | 3 (1.0) | 3 (1.0) | 18 (1.0) | 10 (3.0) | 5 (2.0) |
| Don’t know | 47 (2.0) | 8 (2.0) | 10 (3.0) | 6 (2.0) | 6 (2.0) | 0 | 5 (2.0) | 10 (4.0) | 27 (2.0) | 1 (0) | 1 (0) |
| Hospitalization in the last 12 months | |||||||||||
| Yes | 827 (29.0) | 74 (18.0) | 110 (35.0) | 36 (13.0) | 62 (22.0) | 79 (28.0) | 39 (13.0) | 122 (43.0) | 338 (24.0) | 165 (42.0) | 140 (48.0) |
| No/unknown | 2030 (71.0) | 348 (83.0) | 203 (65.0) | 243 (87.0) | 225 (78.0) | 206 (72.0) | 263 (87.0) | 162 (57.0) | 1099 (77.0) | 225 (58.0) | 155 (53.0) |
| Admitted through ER | n = 827 | n = 74 | n = 110 | n = 36 | n = 62 | n = 79 | n = 39 | n = 122 | n = 338 | n = 165 | n = 140 |
| 1st visit | 287 (35.0) | 53 (72.0) | 77 (70.0) | 25 (69.0) | 25 (40.0) | 22 (28.0) | 21 (54.0) | 7 (5.0) | 100 (30.0) | 44 (27.0) | 13 (9.0) |
| 2nd visit | 88 (11.0) | 15 (20.0) | 31 (28.0) | 5 (14.0) | 3 (5.0) | 10 (13.0) | 3 (8.0) | 5 (4.0) | 26 (8.0) | 13 (8.0) | 3 (2.0) |
| 3rd visit | 22 (3.0) | 2 (3.0) | 13 (12.0) | 1 (3.0) | 1 (2.0) | 1 (1.0) | 0 | 0 | 4 (1.0) | 4 (2.0) | 0 |
| 4th visit | 10 (1.0) | 0 | 7 (6.0) | 1 (3.0) | 0 | 1 (1.0) | 0 | 0 | 2 (0.6) | 0 | 1 (1.0) |
| 5th visit | 2 (< 1.0) | 0 | 1 (6.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.0) | 0 |
| Admitted through ICU | n = 827 | n = 74 | n = 110 | n = 36 | n = 62 | n = 79 | n = 39 | n = 122 | n = 338 | n = 165 | n = 140 |
| 1st visit | 61 (7.0) | 5 (7.0) | 27 (25.0) | 2 (6.0) | 2 (3.0) | 3 (4.0) | 1 (3.0) | 10 (8.0) | 18 (5.0) | 8 (5.0) | 3 (2.0) |
| 2nd visit | 14 (2.0) | 3 (4.0) | 7 (6.0) | 1 (3.0) | 0 | 1 (1.0) | 3 (8.0) | 1 (1.0) | 6 (2.0) | 0 | 1 (1.0) |
| 3rd visit | 12 (2.0) | 1 (1.0) | 8 (7.0) | 1 (3.0) | 1 (2.0) | 0 | 0 | 0 | 2 (1.0) | 1 (1.0) | 1 (1.0) |
| 4th visit | 2 (< 1.0) | 0 | 2 (2.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5th visit | 1 (< 1.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.0) |
| Mean nights in hospital, mean (SD) | |||||||||||
| 1st visit | n = 679 | n = 47 | n = 92 | n = 27 | n = 42 | n = 57 | n = 31 | n = 98 | n = 255 | n = 157 | n = 128 |
| 7.0 (7.3) | 3.0 (1.6) | 5.6 (4.0) | 5.6 (5.1) | 5.4 (4.1) | 6.2 (6.0) | 5.5 (2.8) | 6.2 (4.7) | 5.9 (4.76) | 7.8 (6.6) | 10.8 (12.3) | |
| 2nd visit | n = 176 | n = 13 | n = 38 | n = 6 | n = 3 | n = 18 | n = 4 | n = 9 | n = 40 | n = 39 | n = 46 |
| 7.5 (7.6) | 4.4 (2.1) | 6.2 (3.6) | 8 (11.7) | 8.0 (5.6) | 5.2 (1.7) | 3.5 (2.1) | 5.1 (3.8) | 5.7 (5.05) | 6.9 (5.5) | 11.6 (12.0) | |
| 3rd visit | n = 69 | n = 3 | n = 20 | n = 1 | n = 1 | n = 2 | n = 0 | n = 2 | n = 6 | n = 22 | n = 18 |
| 8.1 (7.1) | 8.0 (7.8) | 9.0 (5.2) | 6 (0) | 10.0 | 5.5 (0.7) | 0 | 2.0 | 5.2 (2.99) | 6.3 (7.4) | 10.5 (9.1) | |
| 4th visit | n = 34 | n = 1 | n = 9 | n = 0 | n = 0 | n = 1 | n = 0 | n = 0 | n = 1 | n = 15 | n = 8 |
| 6.9 (7.2) | 4.0 | 8.3 (5.0) | 0 | 0 | 4.0 | 0 | 0 | 4.0 | 4.3 (6.4) | 10.9 (9.8) | |
| 5th visit | n = 22 | n = 0 | n = 3 | n = 0 | n = 0 | n = 0 | n = 0 | n = 0 | n = 0 | n = 13 | n = 6 |
| 9.2 (14.2) | 0 | 11.0 (5.0) | 0 | 0 | 0 | 0 | 0 | 0 | 7.0 (15.4) | 13.2 (15.4) | |
| Out-of-pocket expense for NSCLC | n = 916 | n = 156 | n = 128 | n = 51 | n = 77 | n = 73 | n = 121 | n = 138 | n = 460 | n = 49 | n = 123 |
| 288 (31.0) | 82 (53.0) | 46 (36.0) | 1 (2.0) | 7 (9.0) | 3 (4.0) | 7 (6.0) | 40 (29.0) | 58 (13.0%) | 12 (24.0) | 90 (73.0) | |
| Median (IQR) out-of-pocket expense per month | – | $50 (40–100) | R$500 (200–762.5) | – | – | – | – | – | EUR 25 (10–60) | NT$50,000 (30,000–60,000) | ¥20,000 (10,000–44,550) |
All data are presented as n (%), unless specified. n = 2857, unless specified
BR Brazil, DE Germany, ER emergency room, ES Spain, EU5 5 countries from the European Union, FR France, ICU intensive-care unit, IT Italy, JP Japan, SD standard deviation, TW Taiwan, UK United Kingdom, US United States
aIncludes the UK, IT, FR, ES, and DE
bReported in > 10.0% of the patients
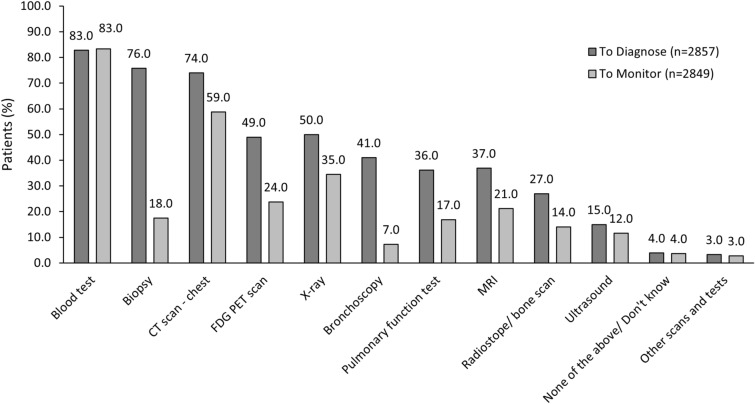
At data capture, most (58.0%) patients had received supportive therapy, and patterns of supportive therapy use were generally consistent across countries as summarized in Table 8. Procedures or tests performed in diagnosis and monitoring of EGFRm+ mutated aNSCLC are summarized in Fig. 5 and Supplementary Table S12. Overall, the most used tests for diagnosis included biopsy (76.0%), chest computerized tomography (CT) scans (74.0%), and X-ray (50.0%). These tests were also commonly used for disease monitoring, albeit at lower rates especially for biopsy testing (18.0% for monitoring and 76.0% for diagnosis). Testing patterns were largely consistent across countries but the use of specific imaging techniques varied between countries: ultrasound use ranged from 2.0% in the UK to 50.0% in Germany, X-ray usage ranged from 27.0% in the US to 81.0% in Japan, magnetic resonance imaging [MRI] use ranged from 16.0% in Japan to 72.0% in Taiwan). In addition, most patients in Japan did not receive biopsy testing for either diagnosis (51.0%) or monitoring (94.0%) of EGFRm+ aNSCLC.
Table 8.
Supportive treatment patterns in advanced non-small cell lung cancer
| Treatment | Overall | US | BR | UK | IT | FR | ES | DE | EU5a | TW | JP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Supportive therapy | n = 2857 | n = 422 | n = 313 | n = 279 | n = 287 | n = 285 | n = 302 | n = 284 | n = 1437 | n = 390 | n = 295 |
| Bisphosphonates | 476 (17.0) | 55 (13.0) | 100 (32.0) | 40 (14.0) | 39 (14.0) | 42 (15.0) | 51 (17.0) | 52 (18.0) | 224 (16.0) | 69 (18.0) | 28 (10.0) |
| Antiemetics | 538 (19.0) | 108 (26.0) | 88 (28.0) | 28 (10.0) | 44 (15.0) | 43 (15.0) | 22 (7.0) | 106 (37.0) | 243 (17.0) | 66 (17.0) | 33 (11.0) |
| Antidiarrheals | 467 (16.0) | 87 (21.0) | 52 (17.0) | 50 (18.0) | 62 (22.0) | 27 (10.0) | 16 (5.0) | 61 (22.0) | 216 (15.0) | 96 (25.0) | 16 (5.0) |
| Corticosteroids | 457 (16.0) | 18 (4.0) | 78 (25.0) | 23 (8.0) | 65 (23.0) | 47 (17.0) | 44 (15.0) | 66 (23.0) | 245 (17.0) | 95 (25.0) | 21 (7.0) |
| Oxygen | 233 (8.0) | 35 (8.0) | 48 (15.0) | 2 (1.0) | 24 (8.0) | 21 (7.0) | 10 (3.0) | 37 (13.0) | 94 (7.0) | 54 (14.0) | 2 (1.0) |
| Opioids | 367 (13.0) | 47 (11.0) | 76 (24.0) | 14 (5.0) | 54 (19.0) | 28 (10.0) | 36 (12.0) | 39 (14.0) | 171 (12.0) | 60 (15.0) | 13 (4.0) |
| Non-opioid analgesics | 513 (18.0) | 46 (11.0) | 54 (17.0) | 33 (12.0) | 46 (16.0) | 51 (18.0) | 70 (23.0) | 122 (43.0) | 322 (22.0) | 72 (19.0) | 19 (6.0) |
| Erythropoietin | 35 (1.0) | 13 (3.0) | 8 (3.0) | 0 | 4 (1.0) | 0 | 7 (2.0) | 3 (1.0) | 14 (1.0) | 0 | 0 |
| G-CSF | 43 (2.0) | 7 (2.0) | 6 (2.0) | 1 (< 1.0) | 6 (2.0) | 5 (2.0) | 2 (1.0) | 1 (< 1.0) | 15 (1.0) | 9 (2.0) | 6 (2.0) |
| Antihypertensives | 199 (7.0) | 16 (4.0) | 42 (13.0) | 5 (2.0) | 35 (12.0) | 8 (3.0) | 5 (2.0) | 44 (16.0) | 97 (7.0) | 31 (8.0) | 13 (4.0) |
| Antimicrobials | 75 (3.0) | 16 (4.0) | 14 (5.0) | 3 (1.0) | 6 (2.0) | 2 (1.0) | 1 (< 1.0) | 8 (3.0) | 20 (1.0) | 20 (5.0) | 5 (2.0) |
| Blood transfusion | 65 (2.0) | 9 (2.0) | 15 (5.0) | 5 (2.0) | 2 (1.0) | 5 (2.0) | 0 | 2 (1.0) | 14 (1.0) | 26 (7.0) | 1 (< 1.0) |
| Other | 43 (2.0) | 7 (2.0) | 4 (1.0) | 10 (4.0) | 1 (< 1.0) | 2 (1.0) | 2 (1.0) | 0 | 15 (1.0) | 10 (3.0) | 7 (2.0) |
| No therapy received | 1201 (42.0) | 165 (39.0) | 97 (31.0) | 147 (53.0) | 110 (38.0) | 148 (52.0) | 151 (50.0) | 60 (21.0) | 616 (43.0) | 133 (34.0) | 190 (64.0) |
All data are presented as n (%)
BR Brazil, DE Germany, ES Spain, EU5 5 countries from the European Union, FR France, G-CSF granulocyte-colony stimulating factor, IT Italy, JP Japan, TW Taiwan, UK United Kingdom, US United States
aIncludes the UK, IT, FR, ES, and DE
Fig. 5.
Types of tests performed for diagnosis and monitoring: overall population. All data are presented as %. CT computed tomography, FDG-PET fluorodeoxyglucose-positron emission tomography, MRI magnetic resonance imaging
Discussion
Findings from this large multiregion data set reflect real-world treatment practice and outcomes for EGFRm+ aNSCLC across nine countries, during a period when the treatment landscape was rapidly changing with implementation of third-generation EGFR-TKI and dual EGFR-VEGF pathway inhibition as guideline-recommended treatment strategies [6, 32, 33]. Our results set an important baseline for care as evaluation for increased adoption of new regimens and ongoing shifting treatment algorithms are affecting patient outcomes. Overall, EGFRm+ aNSCLC treatment patterns across all regions were in line with international treatment guideline recommendations for systemic therapy with targeted regimens and consistent with the available treatment options covered for reimbursement [6, 32, 33]. However, regional variation was observed in treatment and resource use patterns, suggesting differing approaches to EGFRm+ aNSCLC management. As guidelines and treatment patterns will change with the development of new treatment options, future studies should assess the place in therapy for these newer agents.
Consistent with most EGFR aNSCLC populations, adenocarcinoma was the most common histology in this study, most patients were older, had cardiovascular comorbidities, and contralateral lungs as the main site of metastasis [14, 34–36]. While most (74.0–98.0%) patients received EGFR-TKIs in the 1L setting, reflecting current European Society for Medical Oncology (ESMO) [33], American Society of Clinical Oncology (ASCO) [32], and National Comprehensive Cancer Network (NCCN) guideline [6] recommendations, the relative use of first/second-generation EGFR-TKIs and third-generation TKIs (i.e., osimertinib) varied across countries. First/second-generation EGFR-TKIs were the most common 1L treatment in most of the countries, except in the US and Japan where osimertinib (3rd generation EGFR-TKI) was the most common treatment and in Germany and Italy with similar usage of these regimens. As osimertinib is the preferred 1L treatment option in clinical guidelines [37] our finding suggests differential adoption of innovative therapies, possibly owing to differences in access, reimbursement, or physician behavior across countries. Hence, these and other data in this study should be considered in the context of the country in which the data were collected and may not be globally applicable.
Although clinical guidelines also recommend anti-angiogenic antibodies, ramucirumab or bevacizumab, in combination with erlotinib as alternative 1L treatments [6, 32, 33], there was little use of these regimens (1%) in our data set. This was likely due to the recent approval of ramucirumab plus erlotinib by the EMA (December 2019) and FDA (May 2020) [38, 39] and its incorporation into guidelines [38, 40, 41], which limits the time period for evaluation for these therapies. In spite of specific recommendations, some patients (about 14.0% overall) in our study received chemotherapy and/or immunotherapy instead of EGFR-TKIs, suggesting incomplete adherence to the guideline’s recommendations and further dissemination and clarification may be needed.
In this study, a partial or full response to therapy was reported for 83.0% and 12.0%, respectively, of the overall patient population. As these rates are based on the physician’s own clinical judgment in routine practice, and not based on protocol-driven Response Evaluation Criteria in Solid Tumors (RECIST) criteria, direct comparison with previous studies [42–44] is difficult. In addition, the median TTNT of 14.0 months was within the range (10.6–14.2 months) reported in previous observational studies of patients with EGFRm+ aNSCLC [17, 42]. The most common reason for stopping 1L treatment in this data set was disease progression (77.0%). As most patients received first/second-generation EGFR-TKI monotherapy in this study, the time to progression may change with the use of newer treatment options [45].
The burden of lung cancer symptoms remains high, and HRQoL continues to be impaired for patients with EGFRm+ aNSCLC, highlighting the need for more effective and targeted treatment strategies and symptom control in this setting [46–48]. Consistent with previous studies, coughing, fatigue, breathing complications, and appetite or weight loss were the most common symptoms observed in this data set [49, 50]. However, in our study there seemed to be some discordance between patients and physicians in the assessment of symptoms, especially low mood, anxiety, and weight loss [51]. Future research should investigate associated factors for the discordance as the disagreement could lead to suboptimal management and outcomes for EGFRm+ aNSCLC. A more comprehensive clinical picture of symptom burden captured from both patient perspective (subjective) and physician perspective (objective) could help optimize patient management and outcomes.
The overall mean EQ-5D derived health utility score in this study was 0.7, which is similar to that previously reported for EGFR-TKI trial populations [52, 53]. This suggests that patients with EGFRm+ aNSCLC are generally in good health, as the score is only marginally lower than the average utility score (0.81) of the general UK population of similar age [54]. In addition, the score is similar to that reported in a small observational EGFRm+ aNSCLC cohort (n = 183) with disease progression in Canada (0.70) but lower than the score of 0.81 observed in the subgroup of patients with stable disease in that study [55]. FACT-L scores in our study were also within the range reported in a previous data set of patients with aNSCLC in France and Germany [22], although the absolute values were higher in this study (71.4 vs 83.5, respectively), possibly because our study focused only on patients with EGFRm+ aNSCLC and did not include as many patients receiving later lines of therapy as the previous study had. With limited real-world health utility information available for EGFRm+ aNSCLC, utility and functional scores in this study could be considered in future economic evaluations of EGFR targeted treatments.
In line with previous studies of patients with aNSCLC, resource use was high in this study, including diagnostic testing, treatments, multiple office visits, hospitalizations, and supportive care [56–58]. However, there was regional variation in resource use measures, with more frequent hospitalizations in Japan (48.0%), Germany (43.0%), and Taiwan (42.0%), relative to the other countries (29.0% overall). In addition, the mean length of each hospital stay varied across countries (ranging from 3.0 nights in the US to 10.8 in Japan). While the rate of hospital admission via the emergency room also varied, the lowest rates were observed in Germany (5.0%) and Japan (9.0%) versus the US (72.0%) and overall population (35.0%), further suggesting that clinical preference and practices varied across the countries as reported previously [59, 60]. The costs associated with healthcare resource use in this study were not evaluated; however, hospitalizations are known to be a key driver of healthcare costs in many countries [48, 58]. In terms of productivity, on average, patients in this data set lost about 11 h of work per week for about 29 weeks in the 1 year prior to data capture, and about one in three incurred direct out-of-pocket expenses for aNSCLC.
For most patients, EGFR mutation status was known before initiation of 1L treatment, and turnaround time for testing was within international guidelines recommendations [32, 33, 46]. However, only a small proportion of patients (n = 172, 6.0%) showed disease progression on 1L therapy, of whom 52.0% (n = 90) were retested for molecular markers, despite prevalent use of first/second-generation EGFR-TKIs and osimertinib in 1L and 2L settings, respectively. While this study did not investigate why fewer patients than expected (65.0%)—instead of all patients as recommended by guidelines [33, 46]—underwent testing at the time of disease progression, potential reasons include previously reported difficulties in conducting re-biopsy as a result of patient intolerance or tumor location, failed re-biopsies, and financial limitations [33, 61, 62]. According to the physicians who participated in this study, inadequate tissue was the main barrier to EGFR testing irrespective of timing. These challenges may suggest the need for increased adoption of blood biopsy specimens that are easier to collect and NGS-based approaches with the advantages of sparing tissue samples, detecting EGFR-TKI resistance mutations in plasma ctDNA samples with high sensitivity, avoiding multiple sequential single gene tests for prognostic markers such as co-occurring TP53 mutations, and minimizing delays for patients. Overall, liquid biopsy specimens and NGS were used to detect EGFR mutations for about 6.0% and 27.0% of patients in this study, respectively, although testing rates varied across countries likely owing to differences in access to healthcare systems and economic barriers between countries. Of note, the proportion of T790M positive patients in this study (56 of 79 patients tested, 71.0%) is comparable with that reported earlier [63, 64], although this result should be interpreted cautiously given the small sample size for patients who showed disease progression in this cohort.
Strengths of this descriptive study include the large real-world data set obtained from nine geographically diverse countries with different health systems. The data set reflects relatively recent clinical practices for the diagnosis and management of EGFRm+ aNSCLC. Several limitations also apply to this study. While minimal inclusion criteria were applied to increase the generalizability of the data, the study cohort does not represent a true random sample of the broader EGFRm+ aNSCLC population as physician and patient participation was influenced by their willingness to complete the survey. Therefore, selection and survival bias were possible, and our findings may be considered generalizable to consulting patients with EGFRm+ aNSCLC in the countries studied. Similar studies should be conducted to evaluate treatment patterns and patient burden in countries not included in this study. Study data were collected during the COVID-19 pandemic (July–December 2020) and thus may not reflect current practice, as previous reports suggest that cancer care was negatively impacted (e.g., delayed testing and treatment) in different countries by pandemic restrictions [65, 66]. However, our findings may serve as a useful point of reference for future studies in the post-COVID-19 pandemic era. The patient survey was cross-sectional and therefore cannot be used to demonstrate cause and effect. Tumor response and progression outcomes were based on physician assessment in routine clinical practice and not according to RECIST criteria, which limits direct comparison with clinical trial end points. All comparisons between countries were descriptive. As no formal statistical testing was applied, use of comparative terms such as “lower” or “more” does not imply statistical significance.
Conclusion
This large real-world multinational data set showed that most patients with EGFRm+ aNSCLC were treated per the country relevant clinical guidelines for EGFR targeted therapy. However, progression continues to be the main reason for early treatment discontinuation, which along with high patient symptom and associated economic burdens, highlights the need for increased adoption of newer, more effective, and safer treatments that delay disease progression. For the included countries, these findings may offer a useful benchmark for decision makers to determine future allocation of healthcare resources for patients with EGFRm+ aNSCLC. Future research should further evaluate the country-specific variability in clinical practices to identify unmet needs for patients with EGFRm+ aNSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi NSCLC Disease Specific Programme™, subscribed to by multiple pharmaceutical companies of which Eli Lilly and Company was one. The analysis reported here was funded by Eli Lilly and Company in accordance with Good Publication Practice (GPP3) guidelines. The journal’s Rapid Service and Open Access fees were funded by Eli Lilly and Company.
Medical Writing Assistance
Medical writing support under the guidance of the authors was provided by Karan Sharma from Eli Lilly and Company, and funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi NSCLC Disease Specific Programme™, subscribed to by multiple pharmaceutical companies of which Eli Lilly and Company was one. All authors were involved in conception and design and/or analysis and interpretation of data, drafting and revising the article, providing intellectual content of critical importance to the work described, and final approval of the version to be published.
Disclosures
Cliff Molife, Katherine B Winfree, Sangmi Kim, Kaisa-Leena Taipale and Tarun Puri are employees and shareholders of Eli Lilly and Company. Cameron Forshaw and Hollie Bailley are employees at Adelphi Real World who received funding from Eli Lilly and Company for this analysis. Publication of study results was not contingent on the subscriber’s approval or censorship of the manuscript. Yulia D’yachkova is a former employee of Eli Lilly and Company.
Compliance with Ethics Guidelines
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines and as such did not require ethics committee approval, although ethical approval was obtained from the Western Institutional Review Board (study protocol number AG8759). Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996, and Health Information Technology for Economic and Clinical Health Act legislation. Patients provided informed consent for use of their anonymized and aggregated data for research and publication in scientific journals using a check box.
Data Availability
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hollie Bailey at hollie.bailey@adelphigroup.com.
Footnotes
Yulia D'yachkova is a former employee of Eli Lilly and Company.
References
- 1.World Health Organization. Cancer, key facts. 2022. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 7 Dec 2022.
- 2.Cancer.net. Lung cancer - non-small cell: statistics. 2022. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics. Accessed 7 Dec 2022.
- 3.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Leiter A, Kong CY, Gould MK, et al. The benefits and harms of adjuvant chemotherapy for non-small cell lung cancer in patients with major comorbidities: a simulation study. PLoS ONE. 2022;17(11):e0263911. doi: 10.1371/journal.pone.0263911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167–179. doi: 10.1016/j.semcancer.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version5.2021. 2020. NCCN.org. Accessed 6 Aug 2021.
- 7.Tanaka K, Nosaki K, Otsubo K, et al. Acquisition of the T790M resistance mutation during afatinib treatment in EGFR tyrosine kinase inhibitor-naive patients with non-small cell lung cancer harboring EGFR mutations. Oncotarget. 2017;8(40):68123–68130. doi: 10.18632/oncotarget.19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou G, Liu Z, Myers JN. TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem. 2016;117(12):2682–2692. doi: 10.1002/jcb.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JJ, Cardarella S, Lydon CA, et al. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11(4):556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimamura SS, Shukuya T, Asao T, et al. Survival past five years with advanced, EGFR-mutated or ALK-rearranged non-small cell lung cancer-is there a "tail plateau" in the survival curve of these patients? BMC Cancer. 2022;22(1):323. doi: 10.1186/s12885-022-09421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):P625–P635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 13.FDA approves osimertinib as adjuvant therapy for non-small cell lung cancer with EGFR mutations. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-adjuvant-therapy-non-small-cell-lung-cancer-egfr-mutations. Accessed 7 Dec 2022.
- 14.Aguilar KM, Winfree KB, Muehlenbein CE, et al. Treatment patterns by EGFR mutation status in non-small cell lung cancer patients in the USA: a retrospective database analysis. Adv Ther. 2018;35(11):1905–1919. doi: 10.1007/s12325-018-0811-0. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Appius A, Pattipaka T, Feyereislova A, Cassidy A, Ganti AK. Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA. PLoS ONE. 2019;14(1):e0209709. doi: 10.1371/journal.pone.0209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadler E, Espirito JL, Pavilack M, Boyd M, Vergara-Silva A, Fernandes A. Treatment patterns and clinical outcomes among metastatic non-small-cell lung cancer patients treated in the community practice setting. Clin Lung Cancer. 2018;19(4):360–370. doi: 10.1016/j.cllc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Winfree KB, Sheffield KM, Cui ZL, Sugihara T, Feliciano J. Study of patient characteristics, treatment patterns, EGFR testing patterns and outcomes in real-world patients with EGFRm(+) non-small cell lung cancer. Curr Med Res Opin. 2022;38(1):91–99. doi: 10.1080/03007995.2021.1983530. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Matsumura M, Togo K, et al. P69.01 real-world data of EGFR-TKI treatment sequence in non-small cell lung cancer patients in Japan. J Thorac Oncol 2021;16(Suppl 3):S560–1.
- 19.Melosky B, Kambartel K, Hantschel M, et al. Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. Mol Diagn Ther. 2022;26(1):7–18. doi: 10.1007/s40291-021-00563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer S, Roughley A, Rider A, Taylor-Stokes G. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22(1):181–187. doi: 10.1007/s00520-013-1959-4. [DOI] [PubMed] [Google Scholar]
- 22.Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81(2):288–293. doi: 10.1016/j.lungcan.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 24.Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi: 10.2147/DMSO.S120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Pharmaceutical Market Research Association (EphMRA) Code of Conduct. 2019. https://www.ephmra.org/standards/code-of-conduct/. Accessed 4 May 2022.
- 27.US Department of Health and Human Services. Summary of the HIPAA Privacy Rule. 2003. http://www.hhs.gov/sites/default/files/privacysummary.pdf. Accessed 4 May 2022.
- 28.Health Information Technology. Health Information Technology Act. 2022. https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf. Accessed 4 May 2022.
- 29.EQ-5D-5L. 2021. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Accessed 8 Aug 2021.
- 30.Functional Assessment of Cancer Therapy—Lung (FACT-L). 2021. https://www.facit.org/measures/FACT-L. Accessed 8 Aug 2021.
- 31.Functional Assessment of Cancer Therapy – General (FACT-G). 2021. https://www.facit.org/measures/FACT-G. Accessed 8 Aug 2021.
- 32.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29(15):2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 33.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. [DOI] [PubMed]
- 34.Adizie JB, Tweedie J, Khakwani A, et al. Biomarker testing for people with advanced lung cancer in England. JTO Clin Res Rep. 2021;2(6):100176. doi: 10.1016/j.jtocrr.2021.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chantharasamee J, Poungvarin N, Danchaivijitr P, Techawatanawanna S. Clinical outcome of treatment of metastatic non-small cell lung cancer in patients harboring uncommon EGFR mutation. BMC Cancer. 2019;19(1):701. doi: 10.1186/s12885-019-5913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chevallier M, Borgeaud M, Addeo A, Friedlaender A. Oncogenic driver mutations in non-small cell lung cancer: past, present and future. World J Clin Oncol. 2021;12(4):217–237. doi: 10.5306/wjco.v12.i4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott LJ. Osimertinib as first-line therapy in advanced NSCLC: a profile of its use. Drugs Ther Perspect. 2018;34(8):351–357. doi: 10.1007/s40267-018-0536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.FDA approves ramucirumab plus erlotinib for first-line metastatic NSCLC. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ramucirumab-plus-erlotinib-first-line-metastatic-nsclc. Accessed 15 Nov 2022.
- 39.EMA recommends extension of therapeutic indications for ramucirumab. 2022. https://www.esmo.org/oncology-news/ema-recommends-extension-of-therapeutic-indications-for-ramucirumab. Accessed 7 Dec 2022.
- 40.Aybintio. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/aybintio. Accessed 15 Nov 2022.
- 41.Cyramza. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/cyramza. Accessed 15 Nov 2022.
- 42.Ho GF, Chai CS, Alip A, et al. Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer. 2019;19(1):896. doi: 10.1186/s12885-019-6107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 44.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 45.Ge X, Zhang Z, Zhang S. Immunotherapy beyond progression in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2020;9(6):2391–2400. doi: 10.21037/tlcr-20-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Cancer Institute. Online summary of trends in US cancer control measures. 2021. https://progressreport.cancer.gov/after/economic_burden. Accessed 8 Apr 2021.
- 47.Skinner KE, Fernandes AW, Walker MS, Pavilack M, VanderWalde A. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: a real-world observational study. J Med Econ. 2018;21(2):192–200. doi: 10.1080/13696998.2017.1389744. [DOI] [PubMed] [Google Scholar]
- 48.Wood R, Taylor-Stokes G. Cost burden associated with advanced non-small cell lung cancer in Europe and influence of disease stage. BMC Cancer. 2019;19(1):214. doi: 10.1186/s12885-019-5428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leighl NB, Karaseva N, Nakagawa K, et al. Patient-reported outcomes from FLAURA: osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020;125:49–57. doi: 10.1016/j.ejca.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Yoh K, Atagi S, Reck M, et al. Patient-reported outcomes in RELAY, a phase 3 trial of ramucirumab plus erlotinib versus placebo plus erlotinib in untreated EGFR-mutated metastatic non-small-cell lung cancer. Curr Med Res Opin. 2020;36(10):1667–1675. doi: 10.1080/03007995.2020.1808781. [DOI] [PubMed] [Google Scholar]
- 51.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang SX, Walton RN, Hueniken K, et al. Real-world health utility scores and toxicities to tyrosine kinase inhibitors in epidermal growth factor receptor mutated advanced non-small cell lung cancer. Cancer Med. 2019;8(18):7542–7555. doi: 10.1002/cam4.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh J, Bourke S, Dyer M, Devlin N, Longworth L. An analysis of 5-level version of EQ-5D adjusting for treatment switching: the case of patients with epidermal growth factor receptor T790M-positive nonsmall cell lung cancer treated with osimertinib. Value Health. 2022;25(7):1205–1211. doi: 10.1016/j.jval.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Macran S, Weatherly H, Kind P. Measuring population health: a comparison of three generic health status measures. Med Care. 2003;41(2):218–231. doi: 10.1097/01.MLR.0000044901.57067.19. [DOI] [PubMed] [Google Scholar]
- 55.Labbe C, Leung Y, Silva Lemes JG, et al. Real-world EQ5D health utility scores for patients with metastatic lung cancer by molecular alteration and response to therapy. Clin Lung Cancer. 2017;18(4):388–95.e4. [DOI] [PubMed]
- 56.Garon EB, Winfree KB, Molife C, et al. Healthcare resource utilization in advanced non-small-cell lung cancer: post hoc analysis of the randomized phase 3 REVEL study. Support Care Cancer. 2021;29(1):117–125. doi: 10.1007/s00520-020-05459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee DH, Isobe H, Wirtz H, et al. Health care resource use among patients with advanced non-small cell lung cancer: the PIvOTAL retrospective observational study. BMC Health Serv Res. 2018;18(1):147. doi: 10.1186/s12913-018-2946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Beachler DC, Masters E, et al. Health care resource utilization and costs associated with advanced or metastatic nonsmall cell lung cancer in the United States. J Manag Care Spec Pharm. 2022;28(2):255–265. doi: 10.18553/jmcp.2021.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carrato A, Vergnenegre A, Thomas M, McBride K, Medina J, Cruciani G. Clinical management patterns and treatment outcomes in patients with non-small cell lung cancer (NSCLC) across Europe: EPICLIN-Lung study. Curr Med Res Opin. 2014;30(3):447–461. doi: 10.1185/03007995.2013.860372. [DOI] [PubMed] [Google Scholar]
- 60.De Geer A, Eriksson J, Finnern HW. A cross-country review of data collected on non-small cell lung cancer (NSCLC) patients in cancer registries, databases, retrospective and non-randomized prospective studies. J Med Econ. 2013;16(1):134–149. doi: 10.3111/13696998.2012.703631. [DOI] [PubMed] [Google Scholar]
- 61.Hanna N, Johnson D, Temin S. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2022;35:3484–3515. doi: 10.1200/JCO.2017.74.6065. [DOI] [PubMed] [Google Scholar]
- 62.Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651–659. doi: 10.1016/j.cllc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Eide IJZ, Helland A, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study) Lung Cancer. 2020;143:27–35. doi: 10.1016/j.lungcan.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Pereira I, Gaspar C, Pina M, Azevedo I, Rodrigues A. Real-world T790M mutation frequency and impact of rebiopsy in patients with EGFR-mutated advanced non-small cell lung cancer. Cureus. 2020;12(12):e12128. doi: 10.7759/cureus.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43(6):452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Global Oncology Trends 2021. Outlook to 2025. 2023. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2021. Accessed 23 Mar 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hollie Bailey at hollie.bailey@adelphigroup.com.