Abstract
Many AAA+ ATPases assemble as hexamers to unfold protein substrates using a hand-over-hand, threading mechanism, but the Bcs1 AAA+ ATPase facilitates mitochondrial membrane translocation of the folded, iron-sulfur Rieske protein. Two reports now reveal that Bcs1 adopts an unusual heptameric configuration and provide insights into a non-canonical translocation mechanism.
Protein translocation is a fundamental process used by all cells to move proteins across membrane compartments. In eukaryotes, most proteins are translocated as unfolded, extended chains as seen with the Sec61 translocon at the endoplasmic reticulum or the import of mitochondrial proteins through TOM/TIM complexes. Yet, some proteins must be transported in a folded state and therefore must use a different mode of translocation. The assembly of the ubiquinol-cytochrome c reductase (bc1 complex, complex III) represents such a pathway that depends on the trafficking of the folded Rieske iron-sulfur protein (Rip1, ISP) across the mitochondrial inner membrane1,2. Rip1 is a nuclear-encoded protein that is initially imported into the mitochondrial matrix in an unfolded state by the TOM/TIM23 complex. Within the matrix, the precursor Rip1 undergoes a series of maturation steps that culminates in the incorporation of a 2Fe-2S cluster into its globular C-terminal domain3,4 (Fig. 1). The mature, folded protein is then transported across the inner membrane, where it remains tethered with its C-terminal domain exposed to the intermembrane space. Membrane-bound Rip1 is then incorporated into a precursor intermediate of the bc1 complex.
Figure 1. Topogenesis of the Rieske iron-sulfur protein, Rip1, by the Bcs1 AAA+ ATPase.
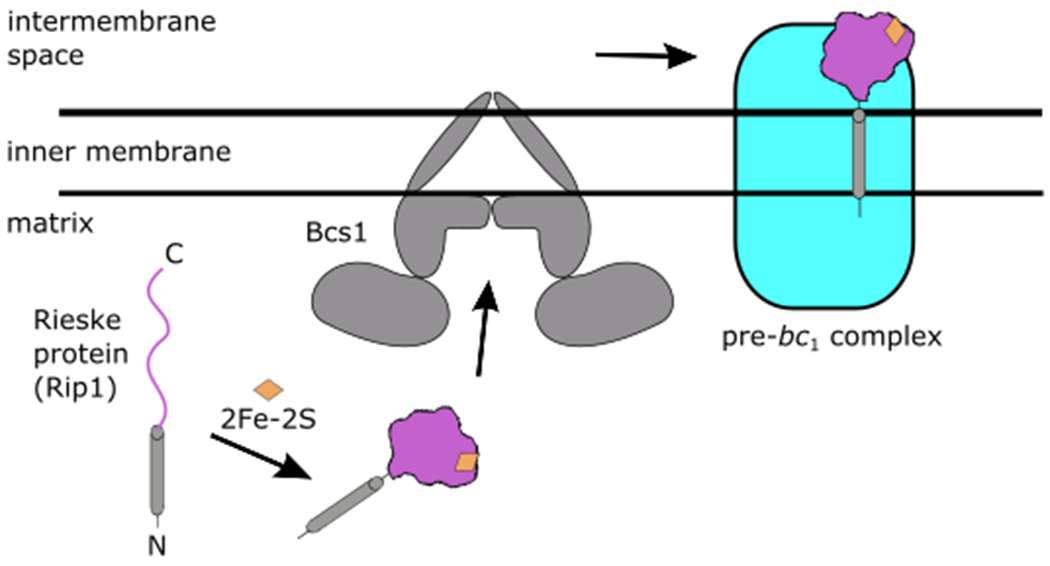
A precursor form of Rip1 is imported into the mitochondrial matrix by the TOM/TIM23 complex (not depicted). After a series of maturation steps, a 2Fe-2S cluster (orange) is incorporated within the folded C-terminal domain of Rip1 (purple). Bcs1 facilitates the translocation of Rip1 from the matrix into the inner membrane, where it is incorporated into the mitochondrial respiratory bc1 pre-complex with its C-terminal domain exposed to the intermembrane space.
The translocation of Rip1 across the inner membrane is mediated by the AAA+ ATPase Bcs15,6. The broad family of AAA+ ATPases is best characterized as protein unfolding translocases, and several recent structures of substrate-bound AAA+ ATPases indicate a hand-over-hand mechanism in which substrates are unraveled and threaded through a central pore7–13. However, such a mechanism does not fit with understanding how Bcs1 translocates a folded substrate. Two studies now report the cryo-EM structures of Bcs1, revealing an unusual homo-heptameric architecture that stands in contrast to classical, hexameric AAA+ assemblies14,15. These structures suggest that Bcs1 uses an alternative mechanism to facilitate the movement of folded Rip1 out of the matrix.
Bcs1 contains an N-terminal transmembrane helix, followed by a middle or “Bcs1-specific” domain, and a C-terminal AAA+ ATPase cassette (Fig. 2). Like other AAA+ ATPases, the Bcs1 AAA+ cassette consists of a large and small ATPase domain, which together form the nucleotide-binding site. Yet, phylogenetic analysis places Bcs1 as the sole member of an outlier clade among AAA+ enzymes16. Both Kater et al.14 and Tang et al.15 reveal that the large AAA+ ATPase domain of Bcs1 is expanded by an additional two β-strands, which alters its interactions with neighboring structural elements. The middle, “Bcs1-specific” domain is unique to Bcs1 and adopts a novel fold comprised of a four-stranded beta-sheet, two helices, and a long beta-hairpin that connects with the large ATPase domain and joins the four-stranded sheet on the adjacent subunit. Collectively, the interconnecting middle and AAA+ domains form a wide, sevenfold symmetric cavity within the matrix14,15. By taking the form of a heptamer instead of a hexamer, the matrix cavity is large enough to accommodate the folded C-terminal domain of Rip1.
Figure 2. Structure of the Bcs1 heptamer.
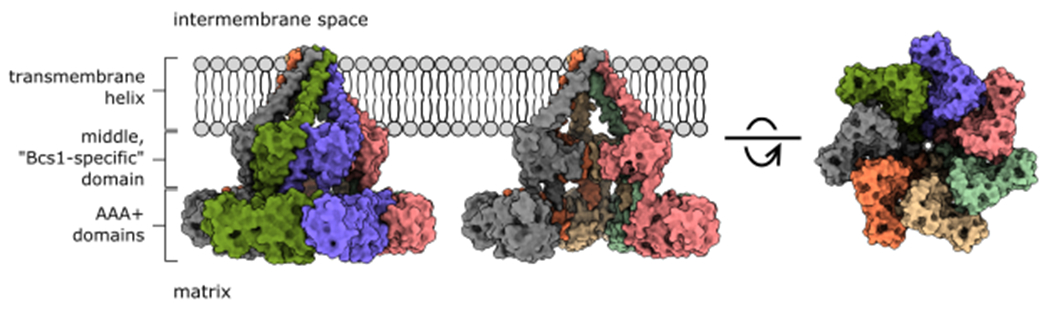
Surface representation of the Bcs1 homo-heptamer in an ADP-bound state. The AAA+ and middle domains form a cavity within the matrix. Each Bcs1 subunit contributes a transmembrane helix that forms a basket-like cavity within the inner membrane. Both cavities are large enough to accommodate the folded C-terminal domain of Rip1 and are separated by a seal of pore loops within the middle domain.
The two studies offer distinct insights by investigating conformational changes under different nucleotide states. Kater et al. observed that the cavity entrance within the AAA+ pore shifts between a widened or narrowed opening in an ADP-bound or apo state, respectively, as a result of concerted movement among the AAA+ domains14. In contrast, Tang et al. report a widened opening in both ADP and apo states, whereas their ATPγS-bound structure led to a constriction of the pore15. Despite the differences in observed conformational states between the two reports, it is clear that nucleotide driven conformational changes are coordinated between the AAA+ ATPase and middle domains. The coordinated movements may play a direct role in modulating substrate accessibility, which is supported by observations that missense mutations in either AAA+ or middle domains cause respiratory deficiency17.
Intriguingly, Kater et al. describe the presence of a seal separating the matrix cavity and inner membrane14. The seal is formed by a collar of hydrophobic pore loops stemming from the middle domains. A potential gating mechanism can be envisioned by remodeling the pore loops to allow Rip1 passage. Indeed, 3D classification of their apo dataset revealed a population of particles with a closed seal, as observed in their ADP-bound state, and another population of particles with a rearranged middle domain that opens the seal. The proposed gating mechanism is supported by the structure of ATPγS-bound Bcs1 reported by Tang et al.15 Described as a putative “post-translocation” state, their structure revealed an even more striking rearrangement of the middle and AAA+ domains that induces a pronounced contraction of the matrix cavity15. The contraction greatly reduces the volume within the matrix cavity and is accompanied by a constriction within the AAA+ pore, as described above. In this closed arrangement, the matrix cavity is no longer able to accommodate the folded substrate and the constricted AAA+ ring would prevent Rip1 from slipping back into the matrix.
Within the inner membrane, Kater et al. show that each Bcs1 promoter contributes an amphipathic transmembrane helix inserted at an angle such that all seven helices converge at the opposite leaflet of the membrane14 (Fig. 2). The transmembrane helices thereby form an unusual aqueous, basket-like chamber within the inner membrane. Thus, Bcs1 appears to form two distinct cavities: one exposed to the matrix where Rip1 is loaded, and the other within the inner membrane to release the translocated protein. Importantly, both cavities are large enough to accommodate folded Rip1 and are separated by the middle domain seal. Based on these observations, the authors propose an airlock-like mechanism of substrate transport in which adjustable pore openings within the AAA+ ring and the middle domain seal act as separate “doors” to support Rip1 translocation without compromising membrane permeability. After translocation, rearrangement of the transmembrane helices would be necessary to permit handoff to the bc1 pre-complex. Although the details of such rearrangements are currently unresolved, the transmembrane helices were poorly ordered in the structures by Tang et al. and suggest extensive conformational heterogeneity within this region15.
Taken together, the structures reported by Kater et. al and Tang et al. hint at a mechanistic model in which all seven Bcs1 subunits act in concert to translocate the folded substrate out of the matrix14,15. Important lingering questions include defining the nucleotide hydrolysis cycle in mediating this process and whether substrate binding induces different conformational changes. Early structures of substrate-free AAA+ ATPases were generally resolved with uniform nucleotide states distributed throughout symmetric rings, but these are now thought to represent inactive, ground states. More recent structures of substrate-bound complexes reveal an asymmetric, spiral staircase arrangement that contains a mixture of nucleotide states (i.e., ATP, ADP, and apo)7–13. These structures support a model in which ATP hydrolysis at the tail end of the staircase is coupled with ATP binding at the leading end, and indicate a unified hand-over-hand translocation mechanism that appears conserved among AAA+ ATPases. This mechanism also supports more complex substrates beyond linear extensions, including looped, crosslinked, and branched (e.g., polyubiquitylated) chains18–20. Whether a similar mechanism is incompatible with translocation of a folded substrate through the heptameric central pore will need to be addressed in future studies.
Footnotes
Conflict of interest: The author declares no competing interests
References
- 1.Smith PM, Fox JL & Winge DR Biogenesis of the cytochrome bc 1 complex and role of assembly factors. Biochimica et Biophysica Acta - Bioenergetics (2012) doi: 10.1016/j.bbabio.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagener N & Neupert W Bcs1, a AAA protein of the mitochondria with a role in the biogenesis of the respiratory chain. Journal of Structural Biology (2012) doi: 10.1016/j.jsb.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Wagener N, Ackermann M, Funes S & Neupert W A pathway of protein translocation in mitochondria mediated by the AAA-ATPase Bcs1. Mol. Cell (2011) doi: 10.1016/j.molcel.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Cui T-Z, Smith PM, Fox JL, Khalimonchuk O & Winge DR Late-Stage Maturation of the Rieske Fe/S Protein: Mzm1 Stabilizes Rip1 but Does Not Facilitate Its Translocation by the AAA ATPase Bcs1. Mol. Cell. Biol (2012) doi: 10.1128/mcb.00441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nobrega FG, Nobrega MP & Tzagoloff A BCS1, a novel gene required for the expression of functional Rieske iron-sulfur protein in Saccharomyces cerevisiae. EMBO J. (1992) doi: 10.1002/j.1460-2075.1992.tb05474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruciat CM, Hell K, Fölsch H, Neupert W & Stuart RA Bcs1p, an AAA-family member, is a chaperone for the assembly of the cytochrome bc1 complex. EMBO J. (1999) doi: 10.1093/emboj/18.19.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monroe N, Han H, Shen PS, Sundquist WI & Hill CP Structural basis of protein translocation by the Vps4-Vta1 AAA ATPase. Elife 6, e24487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ripstein ZA, Huang R, Augustyniak R, Kay LE & Rubinstein JL Structure of a AAA+ unfoldase in the process of unfolding substrate. Elife 6, e25754 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gates SN et al. Ratchet-like polypeptide translocation mechanism of the AAA+ disaggregase Hsp104. Science (80-. ). (2017) doi: 10.1126/science.aan1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puchades C et al. Structure of the mitochondrial inner membrane AAA+ protease YME1 gives insight into substrate processing. Science (80-. ). 358, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De la Peña AH, Goodall EA, Gates SN, Lander GC & Martin A Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis–driven translocation. Science (80-. ). (2018) doi: 10.1126/science.aav0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooney I et al. Structure of the Cdc48 segregase in the act of unfolding an authentic substrate. Science (80-. ). (2019) doi: 10.1126/science.aax0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twomey EC et al. Substrate processing by the Cdc48 ATPase complex is initiated by ubiquitin unfolding. Science (80-. ). (2019) doi: 10.1126/science.aax1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kater L et al. Structure of the Bcs1 AAA-ATPase suggests an airlock-like translocation mechanism for folded proteins. Nat. Struct. Mol. Biol (2020). [DOI] [PubMed] [Google Scholar]
- 15.Tang WK et al. Translocation Mechanism of Folded Protein is Suggested by Structures of AAA Protein Translocase Bcs1. Nat. Struct. Mol. Biol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frickey T & Lupas AN Phylogenetic analysis of AAA proteins. in Journal of Structural Biology (2004). doi: 10.1016/j.jsb.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Nouet C, Truan G, Mathieu L & Dujardin G Functional Analysis of Yeast bcs1 Mutants Highlights the Role of Bcs1p-Specific Amino Acids in the AAA Domain. J. Mol. Biol (2009) doi: 10.1016/j.jmb.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Han H et al. Structure of Vps4 with circular peptides and implications for translocation of two polypeptide chains by AAA+ ATPases. Elife (2019) doi: 10.7554/eLife.44071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton RE, Siddiqui SM, Kim YI, Baker TA & Sauer RT Effects of protein stability and structure on substrate processing by the ClpXP unfolding and degradation machine. EMBO J. (2001) doi: 10.1093/emboj/20.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodnar NO & Rapoport TA Molecular Mechanism of Substrate Processing by the Cdc48 ATPase Complex. Cell 169, 722–735.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


