Abstract
Tsetse flies transmit trypanosomes, parasites that cause devastating diseases in humans and livestock across much of sub-Saharan Africa. Chemical communication through volatile pheromones is common among insects; however, whether and how such chemical communication occurs in tsetse flies remain to be elucidated. We identified methyl palmitoleate (MPO), methyl oleate, and methyl palmitate as compounds that are produced by the tsetse fly Glossina morsitans and elicit strong behavioral responses. MPO evoked a behavioral response in male but not virgin female G. morsitans. G. morsitans males mounted females of another species, G. fuscipes, when they were treated with MPO. We further identified a subpopulation of olfactory neurons in G. morsitans that increase their firing rate in response to MPO and showed that infecting flies with African trypanosomes alters the chemical profile and mating behavior. The identification of volatile attractants in tsetse flies may be useful for reducing disease spreading.
One-Sentence Summary:
Methyl palmitoleate is a volatile sex attractant in tsetse flies.
Chemical communication among insects is used to identify and locate suitable mating partners. Pheromones are used to recognize a conspecific in a habitat that may contain thousands of the world's millions of insect species. Volatile pheromones have been identified and their mechanisms of action elucidated in a wide diversity of species (1).
However, little is known about chemical communication among tsetse flies. One pheromone has been identified in the tsetse fly Glossina morsitans morsitans (G. morsitans), 15,19,23-trimethylheptatriacontane (morsilure); but this molecule consists of a chain of 37 carbon atoms and its relative vapor pressure is vanishingly low (2-7).
Tsetse flies spread disease across much of sub-Saharan Africa. They can carry African trypanosomes, which they transmit when they bite humans or animals. In humans these parasites cause African Sleeping Sickness, and in livestock they cause a disease called nagana, which has had a devastating effect on the agricultural and economic development of Africa (8, 9).
The primary means of controlling these diseases is to control the tsetse flies that spread them. The use of traps and targets containing attractive odorants derived from animal hosts has been shown to be an effective approach for controlling disease spread (10). Because pheromones have been successfully used in the control of many other insects (11), the identification of airborne chemical communication among tsetse could be useful to enhance further their control.
Tsetse flies are also of great intrinsic biological interest. Rather than laying eggs like most other insects, females give birth to larvae (12). All embryonic and larval stages occur within the female’s uterus, where progeny is nourished by maternal milk. Once laid, larvae burrow into the substrate and pupate within 30 minutes. After eclosion, male and female flies feed exclusively on vertebrate blood. It is an open question whether their chemical communication is also unusual.
The antenna of tsetse contains several classes of olfactory sensilla, including trichoid sensilla (13). Sensilla in the medial portion of the G. morsitans antenna respond to a variety of monomolecular odorants (14). The antennae of other tsetse species have also been shown to respond to natural mixtures of plant or host animal volatiles (15, 16).
G. morsitans mating is initiated rapidly
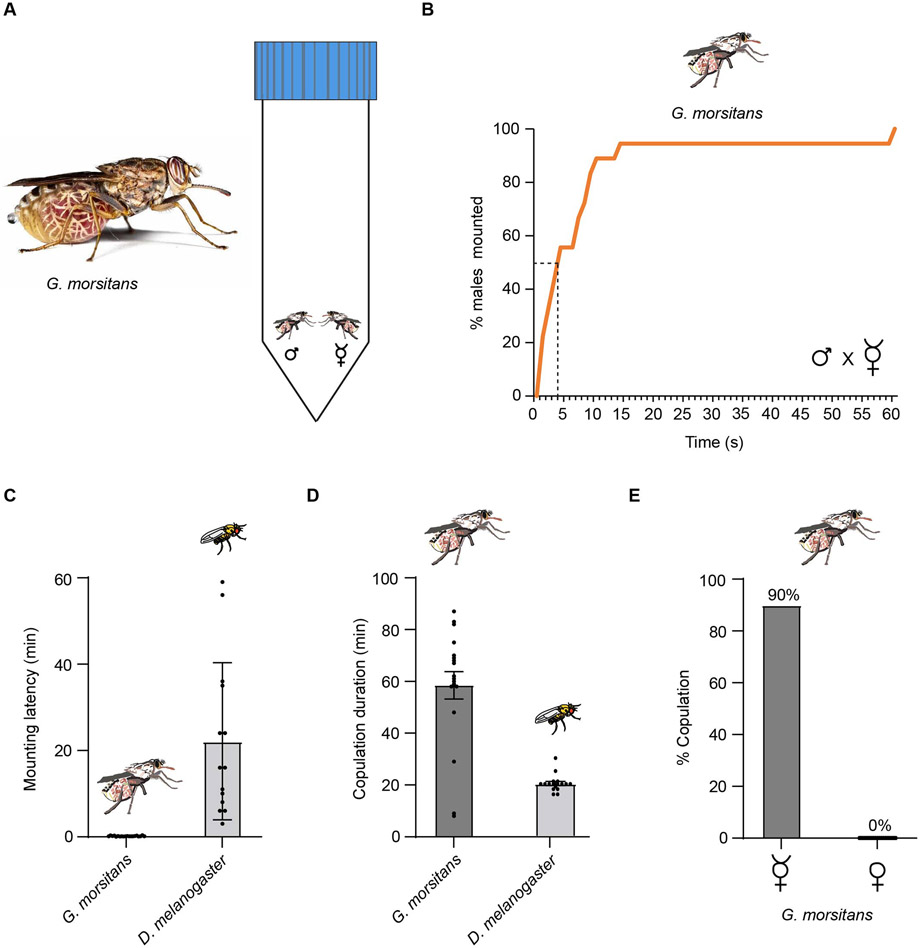
In order to identify chemical factors affecting tsetse mating behavior, we first placed a single male and a single virgin G. morsitans female together in a tube and found that the male quickly mounted the female (Figs. 1A,B). The half-time to mounting was on the order of 5 seconds. Nearly all of 20 pairs began copulating within 15 seconds.
Figure 1. Mating of G. morsitans.
(A) The mating assay. A male and a female are placed together in a 50 ml tube, 11.5 cm in height. (B) Percentage of G. morsitans males that had mounted a virgin female in the mating assay as a function of time. n=20. (C) Copulation latencies of G. morsitans and D. melanogaster males with a virgin female. 14/20 pairs of D. melanogaster and 18/20 pairs of G. morsitans mated; n=14 for D. melanogaster and n=18 for G. morsitans. Error bars are SEM. (D) Copulation duration. n=14 for D. melanogaster and n=18 for G. morsitans. Error bars are SEM. (E) Percentage of copulation of G. morsitans males with either virgin females or mated females. n=20
In a second experiment, we compared directly the copulation of G. morsitans to that of Drosophila melanogaster in the same assay. The mean latency of G. morsitans was two orders of magnitude faster than that of D. melanogaster: 0.15 min ± 0.02 min, v. 22.14 min ± 4.9 min (Fig. 1C, p<0.0001, Mann-Whitney test). G. morsitans also showed a much longer duration of copulation: 58.5 min ± 5.3 min, compared to only 20.3 ± 1.0 min for D. melanogaster (Fig. 1D, p<0.0001, Mann-Whitney test). These latencies and durations were calculated from the pairs that had initiated mating within one hour, i.e. 18/20 pairs for G. morsitans and 14/20 pairs for D. melanogaster.
In contrast to the rapid copulation that ensued when a male G. morsitans encountered a virgin female, no copulation was observed when a male was paired with a non-virgin female (0%, n=20)(Fig. 1E).
Thus, tsetse showed copious mating behavior in this paradigm, with males mounting virgin females quickly and copulating for approximately an hour. Based on these data, we hypothesized that multiple sensory modalities could contribute to tsetse mating behavior, and we investigated whether olfaction played a role.
Female but not male hexane extracts attract G. morsitans males
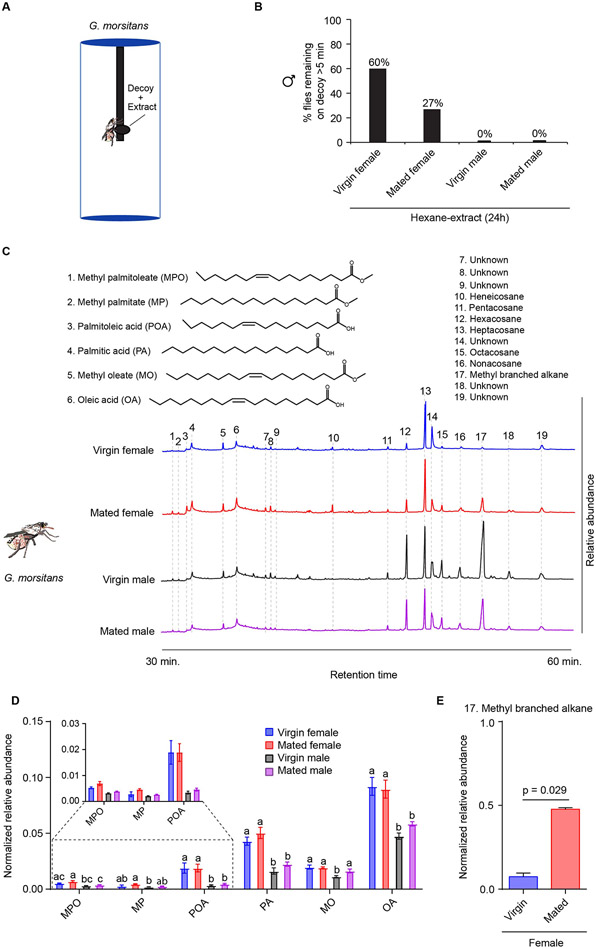
We established a behavioral paradigm to measure the attraction of G. morsitans males to a tsetse-sized decoy (Fig. 2A) (5). We dosed the decoy with a chemosensory stimulus of interest, placed it in a chamber with a single male, and observed for one hour. In some cases males landed on the decoy and stayed attached, often for the remainder of the experiment. We scored this response as the percentage of male flies that landed on the decoy and remained attached for at least five minutes.
Figure 2. G. morsitans hexane extracts contain candidate attractants.
(A) The decoy paradigm. The decoy consists of a knot in a length of black yarn, dosed with extract. (B) Percentage of males that landed on the decoy, when dosed with a 24 hour extract, and remained on it continuously for more than 5 minutes. n=15. (C) Examples of total ion current chromatograms of 24 hour hexane extracts. Peak numbers correspond to the identified compounds. Additional data are shown in Fig. S1. (D) Normalized relative abundance of compounds identified in 24 hour hexane extracts. Normalization is to the sum of the areas of all peaks. One-way ANOVA followed by Tukey’s multiple comparison test; n = 4. Values indicated with different letters are significantly different. Error bars are SEM. (E) Normalized relative abundance of methyl branched alkane (compound # 17) in virgin and mated females. Mann-Whitney test; n = 4.
We prepared extracts from virgin females, mated females, virgin males, and mated males by soaking flies in hexane with gentle shaking for 10 minutes. This procedure has been used previously to isolate pheromones from the cuticle of other insects (17-20). None of these extracts elicited attraction in this paradigm (Fig. S1A). We then considered the possibility that tsetse flies might contain attractive compounds that were temporarily sequestered beneath the surface of the fly, either in internal oenocytes or in internal glands such as those of other insects that synthesize and eventually secrete attractive pheromones (21-23). Accordingly, we prepared hexane extracts by soaking flies in hexane with gentle shaking for 24 hours, a procedure that has been successfully used to isolate pheromones from Drosophila and other insects (24-27).
Extracts from virgin females elicited a behavioral response in 60% (9 of 15) of cases (Fig. 2B). Mated female extracts elicited a response in 27% (4 of 15) of cases. Neither male extract evoked any response in male tsetse flies. This gender-specificity in the effect of extracts suggested a sexually dimorphic composition of effective compounds. In order to identify these compounds, we carried out a Gas Chromatography-Mass Spectrometry (GC-MS) analysis of the extracts.
G. morsitans hexane extracts contain candidate attractants
We injected G. morsitans extracts into a GC-MS instrument and among the compounds identified were three fatty acids and three fatty acid methyl esters that are pheromones in other insects (22) (28) (29) (26, 30-32). (Fig. 2C) (4). These compounds were methyl palmitoleate (MPO), methyl palmitate (MP), palmitoleic acid (POA), palmitic acid (PA), methyl oleate (MO), and oleic acid (OA).
The relative abundance of these six compounds in the 24 hour extracts depended on sex and mating status (Fig. 2D); POA was more abundant in females than in males; PA, MO and OA were more abundant in females than in virgin males. We did not detect the six compounds from an extract that was prepared by a brief immersion of flies in hexane for 10 minutes (Fig. S1B).
We note that the abundance of a certain methyl branched alkane was higher in mated females than in virgin females (Fig. 2E; p = 0.029, Mann-Whitney test; n = 4), suggesting that this compound, which was present at relatively high levels in males, was transferred from males to females during copulation. We speculated that this compound could reduce the attractiveness of mated females, thereby contributing to the lack of copulation observed among previously mated females (Fig. 1E). It could also explain why male extracts do not elicit a response from males in our decoy paradigm (Fig. 2B). Transfer of other compounds, some inhibitory, during mating has previously been shown or suggested in species of Drosophila (33-36).
Taken together, these results suggested the possibility that some of the six new compounds might have a behavioral effect on tsetse flies.
MPO, MP, and MO attract G. morsitans males
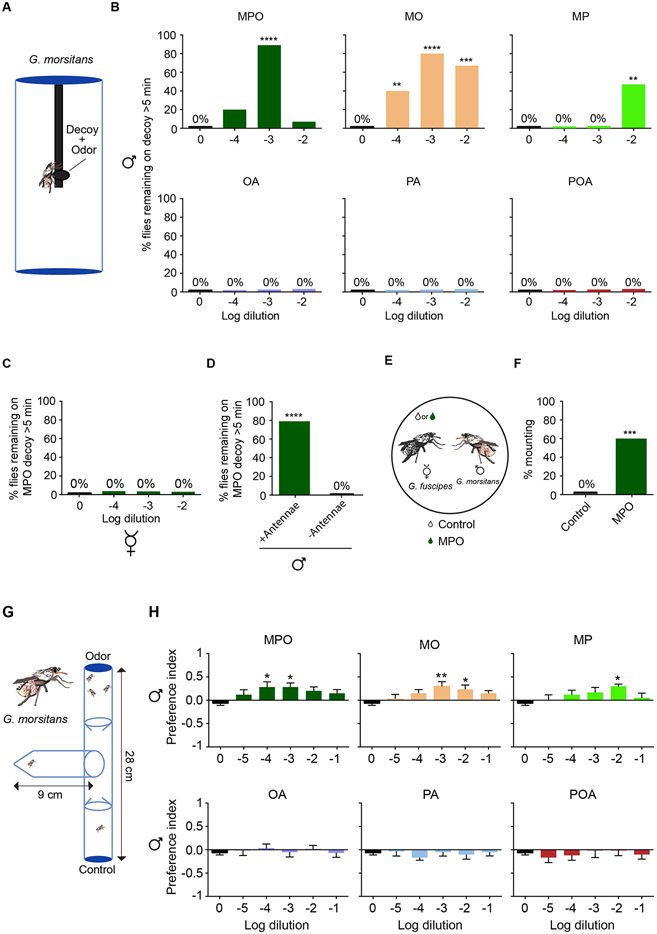
We measured the response of G. morsitans males to the tsetse-sized decoy perfumed with each of the six compounds (Fig. 3A). MO, MPO, and MP all elicited a strong response (Fig. 3B). MPO elicited a response in 87% (13 of 15) of cases when tested at a 10−3 dilution. MO elicited a response at concentrations spanning three orders of magnitude. The other three compounds did not elicit a response. We also tested nine other compounds, including three known attractants of G. morsitans, 1-octen-3-ol, 4-methylphenol, and acetone (10), and found that none of these compounds elicited a response in this paradigm at a 10−2 dilution (Fig. S2).
Figure 3. MPO acts as an aphrodisiac pheromone in G. morsitans males.
(A) The decoy paradigm. The decoy is dosed with a candidate pheromone or odor. (B) Percentage of males that landed on the decoy and remained on it continuously for more than 5 minutes. n=15. Chi-squared test. (C) Percentage of G. morsitans virgin females that stayed for more than 5 minutes on a decoy dosed with a range of dilutions of methyl palmitoleate. n=15. (D) Dependence of G. morsitans response to decoy on antennae. The decoy was dosed with a 10−3 dilution of MPO in paraffin oil. n=15. Chi-squared test. (E) Schematic of a test with a perfumed G. fuscipes virgin female and a G. morsitans male. (F) Percentage of mounting when a G. fuscipes virgin female is perfumed with a 10−3 dilution of MPO, compared to a female perfumed with diluent alone. n=15. Chi-squared test. (G) The T-maze paradigm. Flies are initially in the tube shown at the left. The apparatus is placed horizontally on a benchtop. (H) Behavioral responses of G. morsitans males in the T-maze paradigm. *p<0.05,**p<0.01, Wilcoxon Signed Ranked Test, n=12. Error bars are SEM.
We further investigated MPO, which elicited the greatest response in the decoy paradigm. We tested virgin females with MPO and found no response at any concentration (Fig. 3C). Thus, MPO elicited a male-specific response in this paradigm. Surgical removal of the antennae eliminated the response, suggesting that the response relied on the olfactory system (Fig. 3D).
In insect chemical ecology, compounds are described as arrestants, aphrodisiacs, and/or attractants (37) based on the elicited behavior. Compounds can belong to more than one category. The long periods of time that males spend on a decoy dosed with MPO are reminiscent of the behavior elicited by an arrestant. However, we tested the possibility that MPO also acted as aphrodisiac and attractant.
We paired a G. morsitans male with a G. fuscipes female perfumed with MPO diluted 10−3 in hexane (Fig. 3E). G. fuscipes is a close relative of G. morsitans and accounts for the greatest number of cases of human African trypanosomiasis (38). In control experiments using hexane solvent alone, G. morsitans males made no attempts to mate with G. fuscipes females during a one hour observation period, i.e. 0% (Fig. 3F; n=20). By contrast, when paired with a G. fuscipes female perfumed with MPO, in 60% (9 of 15) of cases the G. morsitans male mounted the G. fuscipes females in an apparent attempt to copulate (Fig. 3F). In no case did the coupling persist; in all cases the female separated from the male shortly after mounting. These data suggest that MPO acts as an aphrodisiac for G. morsitans males.
To test whether the compounds identified acted as attractants, we measured olfactory attraction in a T-maze assay (Fig. 3G). In this paradigm flies made a choice between two tubes, of which one contained a volatile odor and the other contained a diluent control. Flies that entered either tube rarely left. We tested all six compounds in this assay at concentrations spanning five orders of magnitude. MO, MPO, and MP all elicited an attractive response at one or more concentrations (Fig. 3H).
In summary, MPO elicited behavior expected of an arrestant, an aphrodisiac, and an attractant. MO and MP also elicited behavioral responses from males. Next, we asked whether there are olfactory neurons in the G. morsitans antenna that might mediate these responses.
G. morsitans olfactory neurons respond to extracts and to MPO
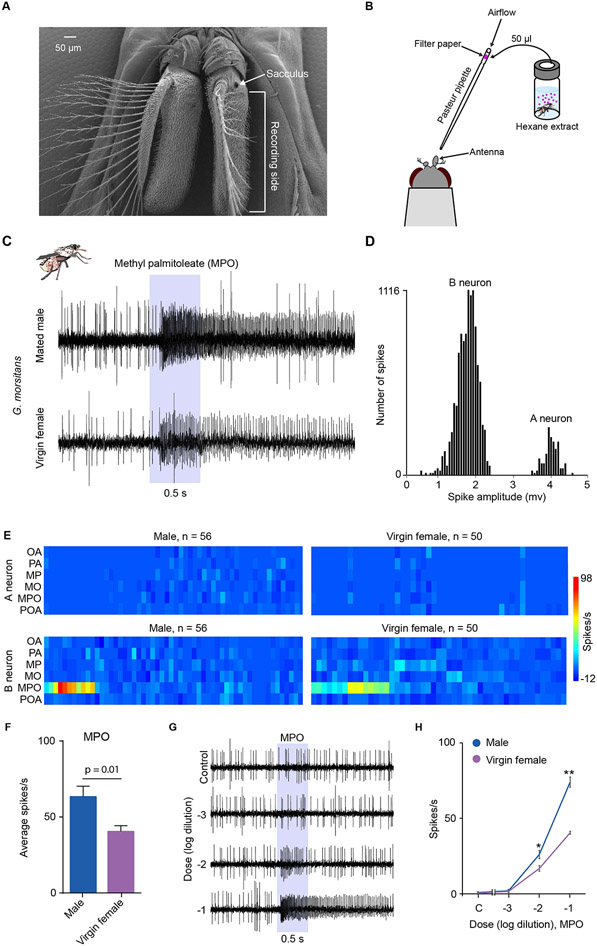
We first asked whether the extracts themselves elicited electrophysiological responses from ORNs of the tsetse antenna (Fig. 4A). We focused on trichoid sensilla because in Drosophila volatile pheromones elicit responses primarily from trichoid sensilla (26).
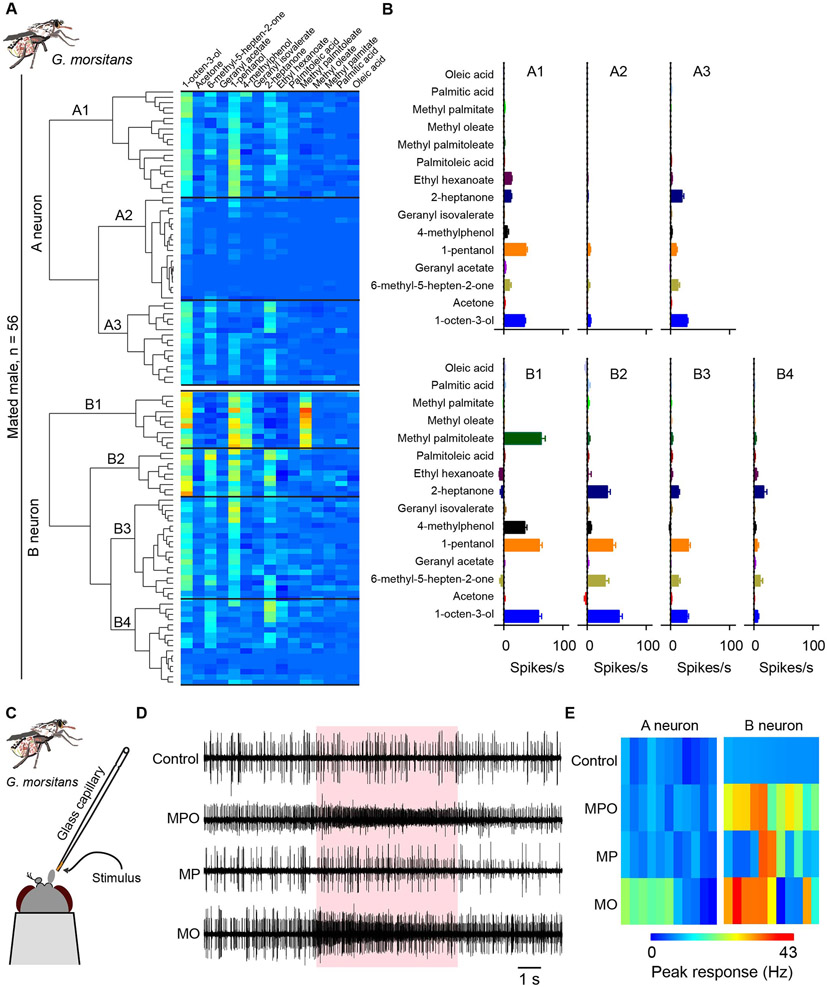
Figure 4. MPO activates ORNs in certain antennal sensilla.
(A) Scanning electron micrograph of antennae showing the region from which electrophysiological recordings were taken from trichoid sensilla. (B) The stimulus delivery system. (C) Example traces of electrophysiological responses of trichoid sensilla in males and virgin females to a 10−1 dilution of methyl palmitoleate. Shaded area indicates the 0.5s odor stimulus. (D) The bimodal distribution of spike amplitudes in trichoid sensilla that respond to MPO in mated males. “A” and “B” indicate subpopulations of spikes attributed to neurons A and B. (E) Heatmap of electrophysiological responses of trichoid sensilla to compounds identified in body-wash extracts. Each rectangle shows the response magnitude of one neuron, in spikes/s, each from a different trichoid sensillum, when tested with a 10−1 dilution of each compound. (F) Mean responses to a 10−1 dilution of MPO in males and virgin females. Mann-Whitney test, n=11 for males and n=15 for virgin females. Error bars are SEM. (G) Example traces of electrophysiological responses of trichoid sensilla in virgin females to a range of dilutions of methyl palmitoleate. In the control trace the stimulus was the diluent control. (H) Dose-response curves of trichoid sensilla to a range of dilutions of methyl palmitoleate. *p < 0.05; **p < 0.01, Mann-Whitney test; n = 5. Mean ± SEM. "C" = control diluent.
Using single-sensillum electrophysiology and a modified stimulus delivery system in which an airflow carried odors to the antenna from a source 24 cm away (Fig. 4B), we recorded from trichoid sensilla located on the lateral face of the antenna (Fig. 4A). We tested hexane extracts from virgin females, mated females, virgin males, and mated males.
Each of the four extracts elicited responses from a number of sensilla, and each of the four groups of flies (virgin female, mated female, virgin male, mated male) contained sensilla that responded to extracts (Fig. S3). Overall, 15% (12 of 82) of the tested sensilla responded to a least one extract with a response of ≥10 spikes/s.
We next tested the six compounds against trichoid sensilla of the antenna. We tested trichoid sensilla from males (n=56) and virgin females (n=50). Delivering the compounds via an airflow using the system shown in Fig. 4B, we found that MPO elicited excitatory responses from a subset of sensilla (Fig. 4C). The responding sensilla have two ORNs, one designated the "A" neuron, which produces spikes of large amplitude, and one, the "B" neuron, which produces spikes of small amplitude (Fig. 4D). The responses to MPO were from the B neuron (Fig. 5C,E). Responses were observed in sensilla of both males and virgin females (Fig. 4C,E); 20% of tested male sensilla (11 of 56) and 26% of female sensilla (13 of 50) responded with >20 spikes/s at the tested dose. We observed few if any responses from the tested trichoid sensilla with the other five compounds using this delivery system (Fig. 4E).
Figure 5. ORNs that respond to MPO also respond to known G. morsitans attractants.
(A) Heatmap based on hierarchical cluster analysis of responses of male trichoid sensilla to a panel of 9 odorants and the 6 newly identified compounds. In the heatmap, each horizontal row represents 1 trichoid sensillum, and each vertical column represents 1 of the olfactory stimuli. The classification was carried out with Ward’s method. The 9 odorants were diluted 10−2 in paraffin oil; the six compounds were diluted 10−1 in paraffin oil. (B) Response profiles of neuronal classes in trichoid sensilla; means ± SEM. n values range from 9-20, as shown in panel A. (C) The close-range stimulus delivery system. The stimulus is placed ~1 mm from the antenna, at the end of a glass capillary. (D) Example traces of electrophysiological responses of male trichoid sensilla to MPO (neat), MP (neat), and MO (10−1 dilution). MPO and MP elicit an increase in the frequency of the small spikes (B neurons); MO elicits an increase in the frequency of both small and large spikes (B and A neurons). (E) Heatmaps of electrophysiological responses of A and B neurons to MPO (neat), MP (neat), and MO (10−1 dilution) in males. Responses are peak responses.
The response magnitudes to MPO were greater in males than in virgin females (Fig. 4F). To determine if responses were dose-dependent we tested a range of concentrations. Since trichoid sensilla were heterogeneous in their responses to MPO we carried out this analysis on two individual sensilla, one in males and one in virgin females, which are located on the proximal portion of the antenna and had produced the highest responses in our survey. Increasing doses produced increasing responses in both cases (Figs. 4G,H).
In order to identify and classify the ORNs that respond to MPO, we tested the sensilla with a panel of volatile compounds. Trichoid sensilla in G. morsitans on the opposite side of the antenna (the medial side) respond to odorants (14). We therefore included in our analysis of the trichoid sensilla nine odorants in addition to the six compounds from the tsetse extract. The nine odorants included three that are known to attract G. morsitans: 1-octen-3-ol, acetone, and 4-methyl phenol (also known as p-cresol) (10).
We then classified the ORNs based on their responses to the 15 compounds, using a hierarchical cluster analysis. Neurons classified as “A” fell into three functional classes, whereas neurons classified as “B” fell into four functional classes, in both males (Fig. 5A) and females (Fig. S4). In some cases the classes appeared qualitatively similar but with different response magnitudes (e.g. B2 and B3 in Fig. 5B). The response profiles of most male ORNs, e.g. B1, have female counterparts that appear similar. (In the case of B1, the male and female counterparts were indistinguishable; p>0.05, ANOSIM test).
Neurons that responded to MPO, the B1 neurons, also responded to known G. morsitans attractants: two of the strongest responses were to the attractants 4-methylphenol and 1-octen-3-ol (Fig. 5B). These results suggest that MPO may mediate attractive behaviors at least in part via these ORNs.
MO and MP activate trichoid neurons at close range
MO and MP elicited behavioral responses from G. morsitans (Figs. 3B,H), but not electrophysiological responses in our initial analysis (Fig. 4E). We reasoned that MO and MP might elicit physiological responses from sensilla on untested regions of the large and complex tsetse antenna (13). Alternatively, it is possible that given the low volatility of these long-chain compounds, the dosage or odorant dynamics used in the preceding survey were not adequate to elicit responses. To address this latter possibility, we modified the odor delivery system (Fig. 5C). We reduced the distance from the odor source to the antennal preparation, to ~1 mm, and we eliminated the airflow, analogous to an approach successfully used with pheromones in Drosophila (39). This close proximity of odor source to antenna is reminiscent of the proximity of two mating flies in a natural context.
The diluent control produced no firing among the tested male trichoid sensilla, and MPO elicited responses from B neurons but not A neurons (Fig. 5D,E), consistent with our earlier results (Fig. 4C). However, using this new delivery system, MP elicited responses from B neurons, and MO activated both A and B neurons.
These results indicate that MP and MO, as well as MPO, activate olfactory neurons, consistent with their activity in attracting and arresting G. morsitans males.
G. fuscipes differs from G. morsitans in responses to MPO, MP, MO and other odorants
We next examined the response of G. fuscipes trichoid sensilla to MPO, MP, and MO, using the same close-range delivery system used for G. morsitans. We again focused on trichoid sensilla that cover the lateral side of the male antenna. In contrast to our results with G. morsitans, the tested methyl esters elicited little if any response from any tested sensilla when used at the same concentrations (Fig. S5).
Consistent with these physiological results, behavioral testing did not reveal a response to these compounds, in either the decoy paradigm (Fig. S5B) or the T-maze preference paradigm (Fig. S5C). We also measured the abundance of MPO, MP, MO and the other three compounds in virgin female, mated female, virgin male, and mated male G. fuscipes. In almost all cases the relative abundance of these compounds in G. fuscipes was lower than that in G. morsitans (Fig. S6).
The lack of responses to MPO, MP, and MO motivated us to ask whether these G. fuscipes sensilla respond to other odorants, and if so whether the response profiles to these odorants differ from those of G. morsitans. Accordingly, we surveyed the trichoid sensilla on the lateral face of the male antenna with the same 9 odorants tested against G. morsitans.
Strong olfactory responses were recorded in G. fuscipes (Fig. S5D). The A neurons could be divided via a cluster analysis into four distinguishable classes, as opposed to three in G. morsitans; the B neurons fell into four classes in both species. In both species there are classes that responded to none of the tested odorants. Testing with more odorants might allow more detailed classification. Inhibitory responses were observed in G. fuscipes, but not in G. morsitans. One class of B neurons, B4, was strongly inhibited by several odorants, including 6-methyl-5-hepten-2-one, which excited another class of B neuron, B3.
Trypanosome infection affects chemical profiles and mating behavior
We next investigated the impact of trypanosome infection on olfactory physiology, profiles of body-wash extracts, and mating. All previous experiments in this study were conducted with uninfected tsetse flies. We infected G. morsitans with Trypanosoma brucei brucei (strain RUMP 503) under laboratory conditions and compared them to uninfected controls.
We first asked whether olfactory responses underwent major alterations after infection. From infected flies we measured the responses of 27 trichoid sensilla on the lateral surface of the antenna to a panel of 10 compounds, including MPO (Fig. S7A). We then compared neuronal spaces constructed from the neuronal responses of infected (Fig. S7B) and uninfected G. morsitans (Fig. 5). We found many common features and much overall similarity (p=0.08, R=0.0103 for A neurons; p=0.04, R=0.8 for B neurons; ANOSIM based on Bray-Curtis similarity)(Fig. S7B).
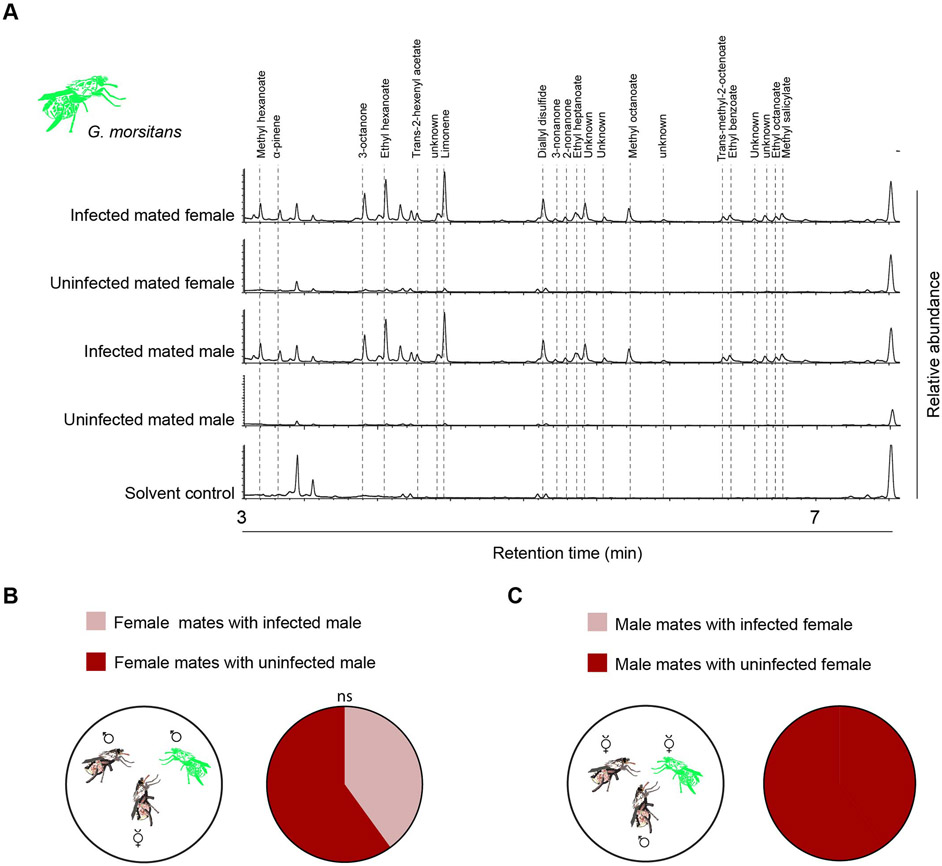
We then compared chemical profiles of infected and uninfected flies. Hexane extracts of infected mated males and females both contained 21 small volatile compounds, including α-pinene, that were not observed in uninfected, control flies or in the solvent control (Fig. 6A). These 21 compounds were specific not only to infected flies, but to mated flies: we did not observe them in either infected or uninfected virgin males or females (Fig. S7C). To test the robustness of these results we individually examined 19-29 flies of each of the eight types (infected and uninfected mated males, infected and uninfected mated females, infected and uninfected virgin males, infected and uninfected virgin females) and found a high degree of consistency: the appearance of these compounds depended on both infection and mating.
Figure 6. Trypanosome infection changes chemical profile and behavior.
(A) Total ion current chromatograms of 24h hexane extracts of infected and healthy mated G. morsitans. All flies were 14 days old. (B) Healthy females mate with both infected and healthy males. (p > 0.05, Chi-squared test, n=20). All flies are virgins, and all pairs copulated. (C) Healthy males mate with healthy but not infected females. All flies are virgins, and all pairs copulated. n=20.
Finally we investigated whether infection had any effects on mating behavior. We placed a virgin female in a tube with two males, one infected and one uninfected. The female mated with the two kinds of males with equal frequency (Fig. 6B, p>0.05, Chi-squared test, n=20). However, when an uninfected virgin male was placed with two females, one infected and one uninfected, in all of 20 trials the male mated with the uninfected female (Fig. 6C); the infected females were much less receptive to the males.
Discussion
G. morsitans males mounted females very quickly upon coming into each other's vicinity. Although behaviors in laboratory settings are imperfect representations of behaviors in natural settings, in the same laboratory paradigm, pairs of G. morsitans had mounting latencies that were 1/100th those of D. melanogaster. Whereas D. melanogaster males engage in a prolonged and elaborate series of courtship behaviors prior to copulation (40, 41), G. morsitans males were quick to mount.
Once initiated, copulation lasted much longer in G. morsitans than in D. melanogaster. Moreover, the copulation time of G. morsitans, 58 minutes, exceeded that of all but one of 82 species of Drosophila considered in an analysis (42). Our measurement of mean copulation time is consistent with the observation of long-lasting copulation in studies of G. morsitans insemination and ovulation (43), and of interactions between males with decoys (44).
These differences between the two fly species raise questions about whether the molecular, cellular and circuit basis of sexual behavior differs markedly between tsetse flies and Drosophila, whose ecology, physiology, and behavior are quite distinct from those of tsetse flies (40, 41). We have carried out behavioral, chemical, and electrophysiological analysis of tsetse in an effort to gain insight into the underpinnings of tsetse fly sexual behavior.
We have found evidence that volatile compounds can affect mating behavior in tsetse flies. There has been little evidence to support a role for volatile pheromones in these animals. The compound 15,19,23-trimethylheptatriacontane has previously been identified as a contact sex pheromone in G. morsitans (5, 45). Five-day-old females contain more than 4 mg of 15,19,23-trimethylheptatriacontane, and when microgram quantities were placed on dead males they elicited copulatory responses from other males (7). Although widely considered a contact pheromone, this compound was reported in one study to elicit an olfactory response from the antenna (46); however, this finding has been controversial (2, 3), in part because the compound consists of a chain of 37 carbon atoms and its relative vapor pressure has been calculated to be 12 orders of magnitude lower than that of (Z)-9-tricosene, a volatile sex pheromone of houseflies (47).
We have found that G. morsitans flies produce MPO, MO, and MP; these volatile compounds have chain lengths of only 16 carbons, much shorter than the 37 carbon chain length of 5,19,23-trimethylheptatriacontane, or the 23 carbons of of (Z)-9-tricosene. MPO, MO, and MP had an arrestant effect in a decoy assay and an attractive effect in a T-maze assay.
MPO is the compound for which we found the most evidence to support a role as a volatile pheromone in G. morsitans. There are several arguments in favor of such a role. MPO acts as a pheromone in other species (22, 28). It elicited a response from males but not virgin females in the decoy assay. MPO showed characteristics of an aphrodisiac: G. morsitans males mounted G. fuscipes virgin females that were perfumed with MPO, but not with control G. fuscipes females, consistent with a role for MPO in driving male G. morsitans sexual behavior. MPO elicited greater responses from ORNs of males than virgin females. It elicited little if any response from any tested sensilla in G. fuscipes (Fig. S5A). While we cannot exclude the possibility of responses from untested sensilla, we observed no behavioral responses to MPO in G. fuscipes, in either of two paradigms.
However, MPO was recovered from a 24 hour hexane extract but was not detectable in a 10 minute extract, as one might have expected of a cuticular pheromone. The 24 hour extracts have two salient properties. They showed sexual dimorphism in their effects in the decoy paradigm, and the number of peaks in the 24 hour extract did not greatly exceed the number in the 10 minute extracts. Thus, among the universe of small molecules within a fly, very few were detected in the 24 hour extract, and MPO is among them.
We do not know the typical distribution of MPO within the fly. MPO might be synthesized and stored in an internal gland, such as those of Tephritid fruit flies, cockroaches, and a variety of hymenopterans and lepidopterans, which produce pheromones and then release them during certain behaviors, often via a duct to a pore in the cuticle (48-51); in some species a pheromone is released only when needed (48, 52). Alternatively, MPO might reside largely in internal oenocytes that produce pheromones and that deliver them to the cuticle only when the fly is in certain nutritional or behavioral states (53-57). However, the absence of detectable MPO in the cuticular layer of flies fed and maintained under standard laboratory conditions may explain why it has not been identified previously as a pheromone in tsetse.
Insect pheromones are extremely diverse in many ways. Some, such as the classic example of bombykol in moths, act in long-distance attraction, are secreted specifically by females, elicit electrophysiological responses from narrowly tuned male-specific ORNs, and elicit male-specific behavioral responses (58, 59). By contrast, other insect pheromones i) elicit other behaviors (60); ii) are secreted at comparable levels by both sexes (26, 39); iii) elicit electrophysiological responses from broadly tuned ORNs in both sexes (5, 26, 61); or iv) elicit behavioral responses from both sexes (26). The sensitivity of ORNs to their cognate pheromones also varies greatly: one molecule of bombykol is sufficient to elicit a nervous impulse from a male moth ORN (62, 63); by contrast, a 10−2 dilution of cis-vaccenyl acetate was required to elicit responses from its cognate at1 neuron of Drosophila (64), similar to the sensitivity of tsetse trichoid sensilla to MPO that we have observed.
MPO has shown characteristics of both an attractant and an arrestant in laboratory tests. We speculate that as an attractant produced by females it might contribute to the initiation of mating; whereas, as an arrestant it might contribute to the maintenance of mating, preventing its premature termination.
MPO activates neurons that also respond to the olfactory attractants 4-methylphenol and 1-octen-3-ol. These results support the interpretation that MPO activates a circuit that mediates olfactory attraction. Mating encounters of Glossina generally occur on or near hosts (9), and 4-methylphenol, and 1-octen-3-ol are all host odors (10). The circuit might be activated more strongly when both fly and host cues are presented together. We note precedent for the dual response of neurons to pheromones and odorants: in Drosophila the ab9A ORNs respond both to the pheromone (Z)-4-undecenal and to food odors (61). These ORNs express two receptors, one of which responds to (Z)-4-undecenal and the other to food odors.
In addition to promoting or maintaining mounting, MPO could have roles in any of a series of stereotyped sexual behaviors that occur after mounting (65). MPO could also affect female receptivity.
Our results support a role for olfactory cues in tsetse mating behavior. The overall role of olfactory cues in tsetse mating, however, remains to be determined. Multiple sensory modalities are likely to influence mating behavior in tsetse, as in many other animal species (3). Moreover, there is evidence that G. morsitans males from which antennae have been surgically removed are able to mount conspecific females (2). These results are consistent with those from Drosophila, in which cues of several modalities act in mating behavior, but mating does not require the presence of all (66).
We found that infection with trypanosomes changed the chemical profile of mated males and mated females: 21 volatile compounds were identified in the body-wash extracts of infected flies but not in their uninfected counterparts. This finding raises a series of questions, including whether these compounds are synthesized by the fly or by the parasites. Malaria parasites that infect mosquitoes produce a variety of volatile compounds, including α-pinene, which is one of the 21 compounds we identified (67). Although trypanosomes could synthesize the 21 identified compounds directly, infection also causes a wide variety of effects on the gene expression of tsetse flies (68, 69), some of which could promote the production of volatile compounds by the fly. Perhaps the appearance of these compounds requires mating because it allows their transport from the interior of the fly to the external surface. In support of this possibility, evidence indicates that the act of mating exposes pores in the ninth tergite of the male, through which compounds could be released (70).
Previous data have shown that a G. morsitans odor receptor, GmmOr35 responds to α-pinene (13), supporting the possibility that the infection or mating status of a fly could be discerned by another fly via olfaction; α-pinene also elicited antennal responses from three other species of Glossina (16).
Infection with trypanosomes reduced mating receptivity in females. Animals fighting infections may invest less in reproduction, as there is a trade-off between these processes due to limited energy resources (71). Consistent with this argument, fecundity of infected female tsetse flies is reduced (72), and mosquitoes infected with malaria parasites produce fewer eggs (73, 74).
The identification of volatile pheromones in tsetse flies may in the long term have important implications for disease control. One of the most effective means of controlling tsetse flies is with traps and targets, which historically have included attractive host odors. Our results now suggest the possibility of using tsetse odors, in combination with long-range attraction to host odors, for controlling tsetse flies and disease spreading.
Supplementary Material
Acknowledgments:
We are very grateful to Dr. Serap Aksoy for the use of equipment in her laboratory and for providing financial support required to rear the tsetse flies and African trypanosomes used in this study. We thank Dr. Adly M. M. Abd-Alla (International Atomic Energy Agency, Vienna, Austria) for providing G. fuscipes fuscipes pupae.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data are available in the main paper or supplement.
REFERENCES
- 1.e. Mucignat-Caretta C, Neurobiology of Chemical Communication. Mucignat-Caretta C, Ed., (Boca Raton (FL): CRC Press/Taylor & Francis; 2014., Boca Raton FL). [PubMed] [Google Scholar]
- 2.Langley PA, Huyton PM, Carlson DA, Sex pheromone perception by males of the tsetse fly, Glossina morsitans morsitans. Physiological Entomology 12, 425–433 (1987). [Google Scholar]
- 3.WALL R, LANGLEY PA, The mating behaviour of tsetse flies (Glossina): a review. Physiological Entomology 18, 211–218 (1993). [Google Scholar]
- 4.Engl T et al. , Effect of antibiotic treatment and gamma-irradiation on cuticular hydrocarbon profiles and mate choice in tsetse flies (Glossina m. morsitans). BMC Microbiol 18, 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson DA, Langley PA, Huyton P, Sex pheromone of the tsetse fly: isolation, identification, and synthesis of contact aphrodisiacs. Science 201, 750–753 (1978). [DOI] [PubMed] [Google Scholar]
- 6.Hall MJR, Langley PA, The responses of individual males in an isolated population of Glossina morsitans morsitans Westwood (Diptera: Glossinidae) to pheromone-baited decoy ‘females’. Bulletin of Entomological Research 79, 319–334 (1989). [Google Scholar]
- 7.HUYTON PM, LANGLEY PA, CARLSON DA, COATES TW, The role of sex pheromones in initiation of copulatory behaviour by male tsetse flies, Glossina morsitans morsitans. Physiological Entomology 5, 243–252 (1980). [Google Scholar]
- 8.Simarro PP et al. , Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis 6, e1859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash TAM, Africa's Bane: The Tsetse Fly. (London: Collins., 1969). [Google Scholar]
- 10.Torr SJ, Vale GA, Know your foe: lessons from the analysis of tsetse fly behaviour. Trends Parasitol 31, 95–99 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Reddy GV, Guerrero A, New pheromones and insect control strategies. Vitam Horm 83, 493–519 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Benoit JB, Attardo GM, Baumann AA, Michalkova V, Aksoy S, Adenotrophic viviparity in tsetse flies: potential for population control and as an insect model for lactation. Annu Rev Entomol 60, 351–371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chahda JS et al. , The molecular and cellular basis of olfactory response to tsetse fly attractants. PLoS Genet 15, e1008005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soni N, Chahda JS, Carlson JR, Odor coding in the antenna of the tsetse fly Glossina morsitans. Proc Natl Acad Sci U S A 116, 14300–14308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harraca V, Syed Z, Guerin PM, Olfactory and behavioural responses of tsetse flies, Glossina spp., to rumen metabolites. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195, 815–824 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Syed Z, Guerin PM, Tsetse flies are attracted to the invasive plant Lantana camara. Journal of insect physiology 50 1, 43–50 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Snellings Y et al. , The role of cuticular hydrocarbons in mate recognition in Drosophila suzukii. Scientific Reports 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley BR, Chenoweth SF, Nuzhdin SV, Blows MW, Natural Genetic Variation in Cuticular Hydrocarbon Expression in Male and Female Drosophila melanogaster. Genetics 175, 1465 – 1477 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaníčková L et al. , Cuticular hydrocarbons of the South American fruit fly Anastrepha fraterculus: variability with sex and age. J Chem Ecol 38, 1133–1142 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Vernier CL et al. , The cuticular hydrocarbon profiles of honey bee workers develop via a socially-modulated innate process. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra A, Function of the Dufour’s gland in solitary and social Hymenoptera. Journal of Hymenoptera Research 35, 33–58 (2013). [Google Scholar]
- 22.Noushini S et al. , Attraction and Electrophysiological Response to Identified Rectal Gland Volatiles in Bactrocera frauenfeldi (Schiner). Molecules 25, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigglesworth VB, The source of lipids and polyphenols for the insect cuticle: The role of fat body, oenocytes and oenocytoids. Tissue Cell 20, 919–932 (1988). [DOI] [PubMed] [Google Scholar]
- 24.Abril S et al. , Cuticular hydrocarbons correlate with queen reproductive status in native and invasive Argentine ants (Linepithema humile, Mayr). PLoS One 13, e0193115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batista-Pereira LG et al. , Cuticular hydrocarbons of Heterotermes tenuis (Isoptera: Rhinotermitidae): analyses and electrophysiological studies. Z Naturforsch C J Biosci 59, 135–139 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Dweck HK et al. , Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci U S A 112, E2829–2835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurenka RA, Subchev M, Identification of cuticular hydrocarbons and the alkene precursor to the pheromone in hemolymph of the female gypsy moth, Lymantria dispar. Arch Insect Biochem Physiol 43, 108–115 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Ayasse M, Marlovits T, Tengö J, Taghizadeh T, Francke W, Are there pheromonal dominance signals in the bumblebee Bombus hypnorum L (Hymenoptera, Apidae)? Apidologie 26, 163–180 (1995). [Google Scholar]
- 29.Keeling CI, Slessor KN, Higo HA, Winston ML, New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci U S A 100, 4486–4491 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sayed AM, The Pherobase: database of pheromones and semiochemicals. Available from www.pherobase.com. (2022). [Google Scholar]
- 31.González JM, Cusumano A, Williams HJ, Colazza S, Vinson SB, Behavioral and chemical investigations of contact kairomones released by the mud dauber wasp Trypoxylon politum, a host of the parasitoid Melittobia digitata. J Chem Ecol 37, 629–639 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Lloyd HA, Schmuff NR, Hefetz A, Chemistry of the anal glands of Bothriomyrmex syrius forel. Olfactory mimetism and temporary social parasitism. Comp Biochem Physiol B 83, 71–73 (1986). [DOI] [PubMed] [Google Scholar]
- 33.Guiraudie-Capraz G, Pho DB, Jallon JM, Role of the ejaculatory bulb in biosynthesis of the male pheromone cis-vaccenyl acetate in Drosophila melanogaster. Integrative zoology 2 2, 89–99 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Khallaf MA et al. , Mate discrimination among subspecies through a conserved olfactory pathway. Sci Adv 6, eaba5279 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khallaf MA et al. , Large-scale characterization of sex pheromone communication systems in Drosophila. Nat Commun 12, 4165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yew JY, Dreisewerd K, de Oliveira CC, Etges WJ, Male-specific transfer and fine scale spatial differences of newly identified cuticular hydrocarbons and triacylglycerides in a Drosophila species pair. PLoS One 6, e16898 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali MF, Morgan ED, Chemical communication in insect communities: a guide to insect pheromones with special emphasis on social insects. Biological Reviews 65, 227–247 (1990). [Google Scholar]
- 38.Krafsur ES, Tsetse flies: genetics, evolution, and role as vectors. Infect Genet Evol 9, 124–141 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Goes van Naters W, Carlson JR, Receptors and neurons for fly odors in Drosophila. Current biology : CB 17, 606–612 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlou HJ, Goodwin SF, Courtship behavior in Drosophila melanogaster: towards a 'courtship connectome'. Curr Opin Neurobiol 23, 76–83 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickson BJ, Wired for sex: the neurobiology of Drosophila mating decisions. Science 322, 904–909 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Markow T, Evolution of Drosophila mating systems. Evolutionary biology 29, 73–106 (1996). [Google Scholar]
- 43.Saunders DS, Dodd CWH, Mating, insemination, and ovulation in the tsetse fly, Glossina morsitans. Journal of Insect Physiology 18, 187–198 (1972). [Google Scholar]
- 44.WALL R, Tsetse mating behaviour: effects of age and hunger in Glossina morsitans morsitans and G.pallidipes. Physiological Entomology 13, 479–486 (1988). [Google Scholar]
- 45.HUYTON PM, LANGLEY PA, CARLSON DA, SCHWARZ M, Specificity of contact sex pheromones in tsetse flies, Glossina spp. Physiological Entomology 5, 253–264 (1980). [Google Scholar]
- 46.den Otter CJ, Saini RK, Pheromone perception in the tsetse fly Glossina morsitans morsitans. Entomologia Experimentalis et Applicata 39, 155–161 (1985). [Google Scholar]
- 47.Carlson DA et al. , Sex attractant pheromone of the house fly: isolation, identification and synthesis. Science 174, 76–78 (1971). [DOI] [PubMed] [Google Scholar]
- 48.Schal C, Fan Y, Blomquist GJ. (2003). [Google Scholar]
- 49.Scolari F, Valerio F, Benelli G, Papadopoulos NT, Vaníčková L, Tephritid Fruit Fly Semiochemicals: Current Knowledge and Future Perspectives. Insects 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayasse M, Paxton RJ, Tengö J, Mating behavior and chemical communication in the order Hymenoptera. Annu Rev Entomol 46, 31–78 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Z. H. K. a. M. A. D. Ganai Mushtaq A, Pheromones in lepidopteran insects: Types, production, reception and its application. Journal of Pharmacognosy and Phytochemistry 6, 2552–2558 (2017). [Google Scholar]
- 52.Percy-Cunningham JE, MacDonald JA. (1987). [Google Scholar]
- 53.Jensen K et al. , Change in sex pheromone expression by nutritional shift in male cockroaches. Behavioral Ecology 28, 1393–1401 (2017). [Google Scholar]
- 54.Ingleby FC et al. , Genotype-by-environment interactions for cuticular hydrocarbon expression in Drosophila simulans. J Evol Biol 26, 94–107 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Ming QL, Lewis SM, Pheromone production by male Tribolium castaneum (Coleoptera: Tenebrionidae) is influenced by diet quality. J Econ Entomol 103, 1915–1919 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Rantala MJ, Kortet R, Kotiaho JS, Vainikka A, Suhonen J, Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Functional Ecology 17, 534–540 (2003). [Google Scholar]
- 57.Weddle CB, Mitchell C, Bay SK, Sakaluk SK, Hunt J, Sex-specific genotype-by-environment interactions for cuticular hydrocarbon expression in decorated crickets, Gryllodes sigillatus: implications for the evolution of signal reliability. J Evol Biol 25, 2112–2125 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Kaissling K-E, Kasang G, Bestmann HJ, Stransky WDD-C, Vostrowsky O, A new pheromone of the silkworm moth Bombyx mori. The Science of Nature 65, 382–384 (2004). [Google Scholar]
- 59.Daimon T et al. , Female sex pheromone and male behavioral responses of the bombycid moth Trilocha varians: comparison with those of the domesticated silkmoth Bombyx mori. Naturwissenschaften 99, 207–215 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Blomquist GJ, Vogt RG. (2003). [Google Scholar]
- 61.Lebreton S et al. , A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biology 15, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaissling KE, Pheromone Reception in Insects: The Example of Silk Moths Neurobiology of Chemical Communication. Mucignat-Caretta C, Ed., (©2014 by Taylor & Francis Group, LLC., Boca Raton FL, 2014). [PubMed] [Google Scholar]
- 63.Kaissling KE, Priesner E, Olfactory threshold of silk moths. Naturwissenschaften 57, 23–28 (1970). [DOI] [PubMed] [Google Scholar]
- 64.Clyne P, Grant A, O'Connell R, Carlson JR, Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci 3, 127–135 (1997). [DOI] [PubMed] [Google Scholar]
- 65.Huyton PM, Langley PA, Copulatory behaviour of the tsetse flies Glossina morsitans and G.austeni. Physiological Entomology 7, 167–174 (1982). [Google Scholar]
- 66.Yamamoto D, Koganezawa M, Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci 14, 681–692 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Kelly M et al. , Malaria parasites produce volatile mosquito attractants. mBio 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aksoy E et al. , Mammalian African trypanosome VSG coat enhances tsetse's vector competence. Proc Natl Acad Sci U S A 113, 6961–6966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hao Z et al. , Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc Natl Acad Sci U S A 98, 12648–12653 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vigneron A et al. , A fine-tuned vector-parasite dialogue in tsetse's cardia determines peritrophic matrix integrity and trypanosome transmission success. PLoS Pathog 14, e1006972 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurd H, Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol 48, 141–161 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Hu C et al. , Infections with immunogenic trypanosomes reduce tsetse reproductive fitness: potential impact of different parasite strains on vector population structure. PLoS Negl Trop Dis 2, e192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmed AM, Hurd H, Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect 8, 308–315 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Hacker CS, The differential effect of Plasmodium gallinaceum on the fecundity of several strains of Aedes aegypti. Journal of Invertebrate Pathology 18, 373–377 ( 1971). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.