Abstract
Purpose
Plasmalogens (Plgs) are highly abundant lipids in the retina, and their deficiency leads to severe abnormalities during eye development. The first acylation step in the synthesis of Plgs is catalyzed by the enzyme glyceronephosphate O-acyltransferase (GNPAT), which is also known as dihydroxyacetone phosphate-acyltransferase (EC 2.3.1.42). GNPAT deficiency produces rhizomelic chondrodysplasia punctata type 2, a genetic disorder associated with developmental ocular defects. Despite the relevance of retinal Plgs, our knowledge of the mechanisms that regulate their synthesis, and the role of GNPAT during eye development is limited.
Methods
Using the Xenopus laevis model organism, we characterized by in situ hybridization the expression pattern of gnpat and compared it to glycerol 3-phosphate acyltransferase mitochondrial (gpam or gpat1) during eye neurogenesis, lamination, and morphogenesis. The Xenopus Gnpat was biochemically characterized in a heterologous expression system in yeast.
Results
During development, gnpat is expressed in proliferative cells of the retina and lens, and post-embryogenesis in proliferative cells of the ciliary marginal zone and lens epithelium. In contrast, gpam expression is mainly restricted to photoreceptors. Xenopus Gnpat expressed in yeast is present in both soluble and membrane fractions, but only the membrane-bound enzyme displays activity. The amino terminal of Gnpat, conserved in humans, shows lipid binding capacity that is enhanced by phosphatidic acid.
Conclusions
Enzymes involved in the Plgs and glycerophospholipid biosynthetic pathways are differentially expressed during eye morphogenesis. The gnpat expression pattern and the molecular determinants regulating Gnpat activity advance our knowledge of this enzyme, contributing to our understanding of the retinal pathophysiology associated with GNPAT deficiency.
Keywords: cataracts, eye development, lipid metabolism, glycerophospholipids, DHAPT, GPAT, peroxisome, yeast, ether lipids
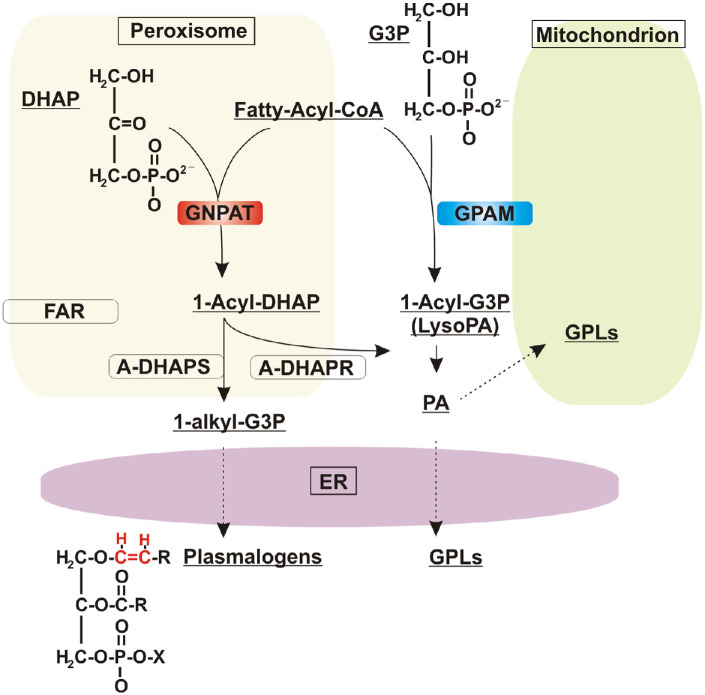
Plasmalogens (Plgs) are ether glycerophospholipids (GPLs) that contain a vinyl-ether bond in the sn-1 position of the glycerol backbone. In the retina, as opposed to other neuronal tissues, Plgs represent an enriched component of the total pool of GPLs.1 The first step in the biosynthetic pathway for the production of ether lipids in vertebrates, which include Plgs, platelet-activating factor, and glycosylphosphatidylinositol (GPI) anchors, is catalyzed by glyceronephosphate O-acyltransferase (GNPAT), which is also known as dihydroxyacetone phosphate acyltransferase (DHAPAT) (EC 2.3.1.42).2 This enzyme is responsible for the acylation of dihydroxyacetone phosphate (DHAP) to form 1-acyl DHAP (Fig. 1). Defects in ether lipid synthesis lead to human disease, particularly in the eye. For example, loss of function mutations in human GNPAT are associated with a severe disorder called rhizomelic chondrodysplasia punctata type 2 (RCDP2), characterized by decreased levels of Plgs.3,4 Clinical manifestations of this disorder include bone abnormalities, growth and intellectual disabilities, and ocular developmental defects.1,5,6 A mouse model deficient in GNPAT mimics these severe human phenotypic alterations, including defects in central nervous system myelination and early-onset cataract development.2,7 Despite its relevance, not much is known about GNPAT expression and cellular distribution during early eye formation or the mechanisms that regulate its activity. This knowledge is critical to better understand the retinal pathophysiology associated with GNPAT deficiency1 and possible treatments.
Figure 1.
De novo synthesis of alkyl and acyl phospholipids by GNPAT and GPAM. GNPAT and GPAM enzymes catalyze the initial transfer of an acyl chain from acyl-CoA to the sn-1 position of DHAP or G3P, respectively. In peroxisomes, GNPAT generates 1-acyl DHAP, which is channeled to the next enzyme in the pathway, acyl-DHAP-synthase (ADHAPS), for the formation of the first ether lipid intermediate, 1-alkyl-DHAP. Both 1-alkyl DHAP and 1-acyl DHAP are substrates of a reductase (ADHAPR) that produces their G3P counterparts. 1-Alkyl-G3P is then consumed in the ER, where the final enzymatic reactions leading to the synthesis of plasmalogens occur, introducing the characteristic vinyl-ether bond (red) in the sn-1 position of the glycerol backbone. GPAM, localized to the outer mitochondrial membrane, produces 1-acyl-glycerol-3-phosphate (1-acyl-G3P; also referred to as lysophosphatidic acid), which is converted to PA by a second acylation step. PA is the precursor of all acyl GPLs, which are the main structural components of cellular membranes. A-DHAPR, acyl/alkyl-DHAP reductase. FAR, fatty acyl-CoA reductase.
Defects in eye development, which include congenital cataracts, are common pathologies seen immediately or a few months after birth in more than 70% of patients with RCDP2.1,8–10 A mouse model deficient in GNPAT shows eye defects after birth, including microphthalmia, cataracts, anterior lenticonus, a persistent hyaloid artery, and alterations in the retinal pigment epithelium (RPE).2,7 Thus, loss of function mutations in both human and mouse suggest that GNPAT is likely required during eye development. The spatial–temporal expression of GNPAT during eye development, however, is unknown. The link between GNPAT and developmental eye defects prompted us to investigate the role of this enzyme in a simple, well-established model for retinal development,11 the embryonic eye of the South African clawed frog Xenopus laevis.
The biosynthesis of Plgs initiates in peroxisomes, where GNPAT catalyzes the first acylation to transfer a long acyl chain from acyl-CoA to the sn-1 position of DHAP (Fig. 1). This event is followed by the actions of alkylglycerone phosphate synthase (AGPS)/alkyl-DHAP synthase (ADHAPS), which replaces the acyl group for an alkyl group by using a fatty alcohol produced by the reductases fatty acyl-CoA reductase 1 (FAR1) and FAR2. The final peroxisomal step consists of the reduction of the ketone group at the sn-2 position by acyl/alkyl DHAP reductase (ADHAPR)1,12 (Fig. 1). Of note, ADHAPR also localizes to the endoplasmic reticulum (ER), where the final steps in Plg biosynthesis occur.13 After the ether precursor is produced in peroxisomes, the biosynthetic pathways of Plg and GPL biosynthesis converge in the ER, with enzymatic steps being shared between both branches (Fig. 1).
The pathway for de novo synthesis of GPLs starts with the acylation of glycerol 3-phosphate (G3P) by glycerol 3-phosphate acyltransferase mitochondrial (GPAM) to form 1-acyl G3P (also known as lysophosphatidic acid [LPA]). This is the rate-limiting and committed step of the pathway for the synthesis of GPLs, which are the most abundant components of cellular membranes (Fig. 1). Two co-existing systems of glycerol-3-phosphate acyltransferases (GPATs) are known, and they localize to mitochondria (GPAM and GPAT2; glycerol-3-phosphate acyltransferase 2, mitochondrial) and the ER (GPAT3 and GPAT4; glycerol-3-phosphate acyltransferase 3 and 4, respectively).14 GNPAT and GPATs belong to the same superfamily that contains a signature acyltransferase domain (pfam01553).15,16 A phylogenetic tree of GPATs/GNPAT homologs shows a robust grouping of GNPATs in a separate clade from mitochondrial GPATs, supporting their common origin.17
We previously identified the genes that code for the entire set of acyltransferases involved in the de novo biosynthesis of glycerolipids, including gnpat from X. laevis.18 Whole-mount in situ hybridization (ISH) on early-stage embryos showed gnpat signal in the spinal cord, notochord, and eye primordia during neurulation. A similar pattern was observed for gpam; however, we do not know whether and how these genes are expressed during eye morphogenesis, retinal neurogenesis, and eye lamination. Furthermore, although gnpat deficiency results in eye defects as described above, no such phenotypes have been associated with gpam mutations. Here, we analyze the expression of gnpat in detail as the eye develops and compare its expression pattern with gpam that is not involved in Plg biosynthesis. gpam expression initiates as the retina laminates and is mainly restricted to differentiating photoreceptors, whereas prior to neuronal differentiation gnpat is expressed both in the optic cup and in proliferating lens forming cells (PLFCs). gnpat expression continues post-embryonically in proliferative cells of the ciliary marginal zone and lens epithelium. To better understand potential roles for Gnpat in proliferative cells, we have taken advantage of the biochemical approaches possible in yeast and performed a biochemical characterization of the amphibian enzyme. At the molecular level, Xenopus Gnpat behaves as a peripheral membrane protein, but only the membrane-bound enzyme displays activity, suggesting lipid-interaction is an important property of the enzyme. Protein–lipid overlays and liposome flotation assays revealed that the amino terminus of Gnpat has a lipid-binding capacity that is enhanced in the presence of phosphatidic acid (PA). Thus, the analysis of gnpat expression during retinal neurogenesis and lamination and its biochemical characterization contribute to our understanding of the retinal pathophysiology associated with GNPAT deficiency.
Materials and Methods
Embryos and Reagents
The Animal Care and Use Committee of the University of Calgary approved procedures involving frogs and embryos. Animal treatment followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Embryos were obtained from chorionic gonadotropin (Intervet Canada Ltd., Kirkland, QC, Canada)-induced eggs and in vitro fertilization according to standard procedures (see protocols at www.xenbase.org/). X. laevis embryos were staged according to Nieuwkoop and Faber using Xenbase (www.xenbase.org/; RRID:SCR_003280). Embryos were maintained at 16°C or 22°C in Marc's modified Ringer's (MMR) solution (100 mm NaCl, 2 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 5 mm HEPES pH 7.4), under light cycles of 12 hours on/12 hours off (light, ∼800 lux; ∼1.2 × 10–4 W/cm2) until the indicated developmental stage. Yeast extract, peptone, yeast nitrogen base, and [γ−32P]-adenosine triphosphate (ATP) were obtained from MP Biomedicals (Irvine, CA, USA). [14C]-Glycerol 3-phosphate disodium salt was obtained from American Radiolabeled Chemicals (St. Louis, MO, USA). Dimyristoyl phosphatidic acid (DMPA); 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC); 1-palmitoyl-2-oleoyl-glycero-3-phosphoserine (POPS); and 1-myristoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphoethanolamine (NBD-PE) were purchased as lyophilized powders from Avanti Polar Lipids (Alabaster, AL, USA). Palmitoyl dihydroxyacetone phosphate (1-acyl DHAP), ammonium salt was obtained from Echelon Biosciences (Salt Lake City, UT, USA).
ISH Followed by Immunohistochemistry
Transverse cryostat sections (12 µm) of embryos fixed with 4% paraformaldehyde (PFA) (F8775; Sigma-Aldrich, St. Louis, MO, USA) in MEMFA solution, comprised of 0.1-M 3-n-morpholino propanesulfonic acid (MOPS, M8899; Sigma-Aldrich), 2-mM ethylene glycol tetra-acetic acid (EGTA, E3889; Sigma-Aldrich), and 1-mM BD's MgSO4 (B29117; B D Pharmaceutical Works, West Bengal, India) in diethylpyrocarbonate (DEPC)-treated water (Thermo Fisher Scientific, Waltham, MA, USA), were used for ISH and immunohistochemistry. ISH probes were generated from pCRII-TOPO vectors containing partial sequences of X. laevis gnpat.L and gpam.S (pcRII-TOPO-gnpat 5′-end and pcRII TOPO-gpam 5-end; NCBI accession numbers NM_001092675.1 between nucleotides 156 and 690 and NM_001097918.1 between nucleotides 93 and 504, respectively). Of note, the gnpat probe likely recognized the gnpat.S homolog (NCBI accession number XM_018265232.2; 93% identity in the nucleotide sequence), as no similarity was found when the probe sequence was aligned with any of the gpats. Similarly, the gpam.S probe likely hybridized with the gpam.L homolog (NCBI accession number XM_018224957.2; 95% identity at nucleotide level) but not with other gpat mRNAs, as no similarity in their sequence was found when compared with the probe sequence. After DNA linearization with specific restriction enzymes, the polymerases SP6 or T7 (Roche, Basel, Switzerland) were used to generate the corresponding sense or antisense probes (Supplementary Table S1).
The methods for single ISH, as well as ISH followed by immunohistochemistry, were reported previously.19 Briefly, for single ISH, sense and antisense riboprobes were synthesized using nucleotides labeled with digoxigenin (DIG; Roche) and were detected using an anti-DIG antibody (1:2000; Roche) conjugated with alkaline phosphatase. The staining process used 5-bromo-4-chloro-3-indolyl phosphate (BCIP; Roche) and nitro blue tetrazolium chloride (NBT; Roche) substrates in a NTMT solution (100-mM NaCl; 100-mM Tris-Cl, pH 9.5; 50-mM MgCl2; and 1% TWEEN 20). The same developing time was used for sections at different developmental stages in order to detect qualitative differences in expression levels. Immunohistochemistry after ISH was performed by incubating for 1 hour with antibodies that recognize rhodopsin (clone 4D2, mouse 1/200, MABN15; MilliporeSigma, Burlington, MA, USA), calbindin (rabbit polyclonal 1/200, CB38; Swant, Marly, Switzerland), or Islet1/2 (mouse monoclonal, 1/100, clone 394D5; Developmental Studies Hybridoma Bank, Iowa City, IA, USA), and then with an Alexa Fluor-tagged secondary antibody (1:1000; green 488 nm or red 555 nm; Molecular Probes, Eugene, OR, USA). For regular immunohistochemistry, 12-µm sections from stage 39 electroporated tadpoles were stained with an Invitrogen mouse anti-green fluorescent protein (GFP) monoclonal (3E6) antibody (1/1000, A11122; Thermo Fisher Scientific) and a BD Pharmingen rabbit polyclonal anti-activated capase-3 antibody (1/500, 559565; BD Biosciences, Franklin Lakes, NJ, USA), followed by the corresponding Alexa Fluor-tagged secondary antibody.
Blastomeres Injections and Eye Electroporation
Fertilized eggs were treated with 2% l-cysteine (Calbiochem 243005; MilliporeSigma) solution in MMR at pH 8 to remove the jelly coat from the eggs. For embryo-wide overexpression of acyltransferases, single or both blastomeres of two-cell stage embryos were injected using a Picospritzer II (General Valve, Fairfield, NJ, USA) with a borosilicate glass needle pulled on an electrode puller. Glass needle pumped 10 nL of solution with pCMV SPORT6 Xl-Gnpat (IC: 6946416; Open Biosystems, Huntsville, AL, USA) or pCMV SPORT6 Xl-Gpam (IC: 8070414; Open Biosystems) together with pCS2–GFP (1:1 molar ratio; 40 ng/mL total DNA; approximately 2.5 × 104 copies of each plasmid). A StrataGene QuikChange Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA, USA) was used according to the manufacturer's protocol to introduce mutations for amino-acid changes. Eye-targeted expression was achieved by injecting DNA into the corners of the two-animal pole cells of four-cell blastomeres as indicated by the fate map of X. laevis. Electroporation was performed as described previously.20 A borosilicate glass needle was used with a Picospritzer II to inject DNA solution into the right eye of anesthetized stage 27/28 embryos. Two custom-made platinum-wire electrodes, spaced 4 mm apart, were placed on either side of the eyes of the embryo, and a Grass Technologies (West Warick, RI, USA) S44 stimulator was used to apply 10 square, 50-ms, 50-V pulses spaced 1 second apart.
Yeast Strains, Plasmids, and Growth Conditions
Detailed information on plasmids and yeast strains used in this study is provided in Supplementary Tables S1 and S2, respectively. All primers used are listed in Supplementary Table S3. The X. laevis gnpat and gpam were subcloned into Invitrogen pYES2.1 TOPO TA yeast vector with V5 and His6 C-end tags, from previous plasmids based on pCS2–MT.18 All other constructs were generated using Invitrogen Gateway cloning technology,21 producing entry clones and destination plasmids as indicated in Supplementary Table S1. Plasmid sequences were confirmed by standard DNA sequencing (University of Calgary, Calgary, AB, Canada). All plasmids were propagated in the Escherichia coli strain DH5α, whereas BL21(DE3) was used to express the amino end Gnpat (N1-129 His6-tagged). E. coli cells were grown at 37°C in lysogeny broth medium (1% tryptone; 0.5% yeast extract; 1% NaCl, pH 7.0). For the selection of the transformant cells carrying plasmids, the growth medium was supplemented with antibiotics as required (e.g., ampicillin, 100 µg/mL; kanamycin, 50 µg/mL; chloramphenicol, 25 µg/mL). Expression of the amino end Gnpat (N1-129 His6-tagged) was induced with 0.3-mM isopropyl β-d-thiogalactoside for 4 hours at 25°C. Conditional lethal strains lacking Sct1 and Gpt2 were generated by classical yeast genetic crosses of single deletion haploids (CMY strains; see Supplementary Table S2) transformed with plasmids encoding the indicated acyltransferases and followed by tetrad dissection. Yeast strains were grown in rich medium containing 2% glucose or galactose as indicated, or in selective synthetic defined minimal medium lacking uracil and/or leucine as indicated (Sabouraud's dextrose: 0.67% yeast nitrogen base without amino acids, 2% glucose or galactose, and 0.1% tryptophan, 0.1% methionine, 0.1% lysine, 0.1% arginine, 0.1% histidine, and 0.02% adenine to fulfill strain auxotrophies). Unless otherwise indicated, yeast cultures were grown at 30°C with shaking (200 rpm). The standard lithium acetate transformation protocol22 was used, and cells carrying plasmids of interest were grown on selective media.
Yeast Subcellular Fractionation
Yeast subcellular fractionation was performed to obtain a membrane fraction as described previously.23 Briefly, cells were grown to stationary phase for 24 hours and re-diluted in fresh media for 4 hours to allow the culture to resume growth and reach log phase. Twenty A600 units of cells were harvested, washed once with water, and resuspended in cold 350 µL of TNE buffer comprised of 50-mM Tris-HCl, pH 7.4, containing 150-mM NaCl, 5-mM EDTA, cOmplete EDTA-Free Protease Inhibitor Cocktail (Roche), 1-mM phenylmethylsulfonyl fluoride (PMSF), and 3 µg/mL pepstatin. A 1:1 volume of acid-washed glass beads was added to each sample, and cells were then lysed by vortexing (five times for 30 seconds each with 30-second intervals on ice). Glass beads were then rinsed with 0.5 mL of TNE buffer, and the combined lysate was subjected to a clearance step by centrifugation at 500g in a pre-cooled microcentrifuge. The supernatant was centrifuged at 450,000g for 15 minutes at 4°C using a Beckman Coulter Optima TL-100 Ultracentrifuge and TLS 100.2 rotor (Beckman Coulter, Brea, CA, USA). The supernatant (soluble fraction) was collected, and the pellet (microsomal fraction) was resuspended in 0.5 mL of TNE buffer containing protease inhibitors and then homogenized 15 times with a Dounce homogenizer. The TNE lysis buffer was replaced with phosphate buffer for Ni-NTA purification (50-mM NaH2PO4, 300-mM NaCl, 10-mM imidazole, 0.05% TWEEN 20), or TES buffer (10-mM TES, pH7.5; 250-mM sucrose; 50-mM NaF; 1-mM EDTA, pH8) when activity assays were performed. Protein concentration was determined using the BCA assay (Thermo Fisher Scientific) with bovine serum albumin as a standard.
Western Blot Analysis
Samples were resuspended in 1× gel loading buffer (0.1-M Tris HCl, pH 6.8; 4% SDS; 0.2% bromophenol blue; 20% glycerol) and boiled for 1 minute. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by standard methods. Proteins were separated by 8% resolving gel containing trichloroethanol (TCE) to visualize proteins without staining.24 Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (MilliporeSigma) using a Bio-Rad Laboratories (Hercules, CA, USA) transfer system at 100 V for 1 hour, and then stained with red Ponceau (Sigma-Aldrich) to confirm transfer. For western blot analysis, the following antibodies were used: Invitrogen monoclonal anti-V5 (R96025; Thermo Fisher Scientific) and anti-GFP (11814460001; Sigma-Aldrich), and subsequently horseradish peroxidase-conjugated secondary antibody (Invitrogen Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, HRP, G-21040; Thermo Fisher Scientific) followed by detection using enhanced chemiluminescence. An Amersham Imager 600 (GE Healthcare, Chicago, IL, USA) was used for visualization and imaging.
Gnpat Enzymatic Assay
[32P]-DHAP was synthesized prior to enzymatic assay as described by Jones and Hajra.25 The synthesis of [32P]-DHAP was performed in a 1-mL reaction containing 300-mM Tris-HCl buffer, pH 7.5; 100-mM MgCl2; 100-mM cold ATP; 1.6 µCi [γ−32P]-ATP; and 100-mM dihydroxyacetone (DHA). The reaction was started by adding 10 µL (0.8 U) of glycerokinase (from Candida mycoderina, EC 2.7.1.30) suspension (1 g/L; Boehringer Mannheim, Indianapolis, IN, USA). The reaction incubated at 37°C for 1 hour. In these conditions, the kinase reaction goes to completion and is irreversible. Activity of the Xenopus Gnpat was assayed as described by Racenis et al.26 In brief, the formation of 32P-labeled 1-acyl DHAP was measured from the reactants [32P]-DHAP and palmitoyl-CoA. The reaction mixture contained 75-mM Tris-HCl, pH 7.5; 8.3-mM MgCl2; 8.3-mM NaF, 1 mg fatty acid-free BSA; 0.42-mM [32P]-DHAP (50 µL of the synthesis reaction); and 60-mM palmitoyl-CoA or oleoyl-CoA as indicated. Tris-HCl was replaced by 75-mM MES (2-[N-morpholino]ethanesulfonic acid) buffer for assays conducted at pH 5.5. Amounts of enzyme ranged from 20 to 80 µg total protein (yeast lysate in TES buffer). Enzymatic reactions were incubated for 10 minutes at 37°C and then stopped by adding 2.5 mL chloroform: methanol (1:2, v/v). After a clearing step of 5 minutes at 1500g, the liquid was transferred to a clean tube followed by the addition of 0.75 mL chloroform and 0.75 mL 2-M KCl in 0.2-M H3PO4 with vortexing. Phase separation was allowed, and tubes were centrifuged 5 minutes at 1000g in a bench-top centrifuge. The top aqueous layer was aspirated, and the remaining organic phase was washed twice with 2.5 mL chloroform/methanol/water (1/12/12 v/v/v), each time aspirating the aqueous phase. The organic phase (0.6 mL) was collected from the bottom of the tube and transferred to a scintillation vial. Then, 4 mL of scintillation cocktail (EcoLite Liquid Scintillation Cocktail; MP Biomedicals, Santa Ana, CA, USA) was added. Radioactivity was measured using a Beckman Coulter LS6500 Multipurpose Scintillation Counter.
Ni-NTA Protein Purification for Lipid Overlay Assays
Soluble full-length Xl-Gnpat and recombinant amino terminus N129, both containing a Hisx6 tag, were expressed in bacteria and partially purified by Ni-NTA affinity chromatography. All steps for protein purification were performed at 4°C. E. coli cells expressing the amino end Gnpat (N1-129 His6-tagged) were harvested and resuspended in a phosphate lysis buffer (50-mM NaH2PO4, pH 8.0; 300-mM NaCl; 10-mM imidazole) containing protease inhibitors. Cells were lysed by sonication (Branson 450 Sonifier; Branson Ultrasonics, Brookfield, CT, USA); 5 × 30s pulses at intensity setting of 6. Unbroken cells and cell debris were removed by centrifugation at 12,000 × g for 40 min at 4°C. The cleared cell lysate (4 mL) was mixed gently with 1 mL of a 50% slurry of Ni2+-NTA agarose beads for 1 hour. The Ni2+-NTA agarose/protein mixture was packed in a 10-mL Bio-Rad Poly-Prep column and washed with 100 mL phosphate wash buffer (50-mM NaH2PO4, 300-mM NaCl, 20-mM imidazole). The N129-V5/His6-tagged protein was then eluted from the column in 1-mL fractions (total of 6 mL) with phosphate buffer containing 250-mM imidazole. The eluted protein was concentrated to 500 µL in TES buffer using a Merck Amicon Ultra-15 centrifugal filter unit (10-kDa cut-off; MilliporeSigma) and stored at −80°C. The same purification protocol was applied for the purification of the soluble fraction of Xl-Gnpat expressed in yeast. All buffers (lysis, wash, and elution buffers) used in the purification process contained the following protease inhibitors: Roche cOmplete EDTA-free Protease Inhibitor Cocktail, 1-mM PMSF, and 3 µg/mL pepstatin A.
Protein–Lipid Overlay Assay
The procedure was similar to the one previously described.27 Avanti Polar Lipids stocks were dissolved in chloroform/methanol/water (20/9/1 v/v/v). The indicated amounts of lipids were spotted on a nitrocellulose membrane (GE Healthcare) and allowed to dry at room temperature (RT) for at least 1 hour. Membranes were then blocked for 1 hour at RT in blocking buffer (50-mM Tris-HCl, pH 7.5; 150-mM NaCl; 0.1% [by volume] TWEEN 20; 2 mg/mL fatty acid-free BSA) and then incubated for another hour with the indicated concentrations of V5-tagged proteins. The membranes were then washed and proteins detected, with anti-V5 primary antibody followed by horseradish peroxidase–conjugated secondary antibody, by chemiluminescence using an Amersham Imager 600. Densitometry of the blots was performed using ImageJ (National Institutes of Health, Bethesda, MD, USA). Experiments were repeated at least three times.
Liposome Flotation Assay
For liposome preparation, a known mass of lipid was weighed into a clean glass vial, dissolved in chloroform, and dried in a rotary evaporator to create a lipid film. For liposomes containing a mixture of PA and phosphatidylcholine (PC), the lipid mass was in a ratio of 79% PC to 20% PA, with 1% NBD-PE fluorescent lipid. To ensure that all solvent was removed, the lipid film was placed under vacuum in a desiccator for 1 hour. Lipid films were stored at −20°C. For liposome flotation assays, the dry film was hydrated in 5-mM Tris-HCl, pH7.5, and then vortexed and sonicated to produce multilamellar vesicles (MLVs). The MLV suspension was extruded 21 times through a 100-nm polycarbonate filter (Whatman, Kent, UK) to generate large unilamellar vesicles (LUVs) of 100-nm diameter. The monodispersity of the liposomes was verified using dynamic light scattering (Zetasizer Nano ZS; Malvern Instruments, Malvern, UK). The total phospholipid concentration of the liposome suspension was determined using the Ames assay for detection of inorganic phosphate.28 Liposome binding assays were performed in 5-mM Tris-HCl buffer, pH 7.5, containing liposomes of the indicated composition (1-mM final lipid concentration), and the indicated semi-purified proteins in a total volume of 200 µL. A negative control with no liposomes added was included. Reaction tubes were incubated for 30 minutes at RT. Liposomes were then separated by allowing them to float in sucrose gradient. For this purpose, reactions were mixed with 200 µL of 70% sucrose in 5-mM Tris-HCl, pH 7.5, to yield a 35% final sucrose concentration. This was then overlaid with 400 µL of 25% sucrose followed by a top layer of 200 µL 5-mM Tri-HCl, pH 7.5, buffer. Tubes were centrifuged at 200,000g (Beckman Coulter Optima TL-100 Ultracentrifuge) for 1.5 hours at 20°C in a Beckman Coulter TLS-55 Swinging Bucket Rotor, and a total of four fractions were collected from the top. The fractions were analyzed for the presence of the indicated proteins by western blot using a Bio-Rad Bio-Dot Microfiltration method. For this, a nitrocellulose membrane was fixed in a Bio-Rad Bio-Dot Microfiltration apparatus as per the manufacturer's instructions. The membrane was rehydrated by adding 100 µL of 1× TBS (20-mM Tris-HCl; 150-mM NaCl, pH 7.5) to each well, and vacuum was applied. Each fraction from the sucrose gradient was mixed with an equal volume of 2× Laemmli loading buffer (without bromophenol blue). Samples were loaded into wells and filtered through the nitrocellulose membrane with vacuum. With the vacuum still on, the apparatus was disassembled, and the nitrocellulose membrane was treated for western blotting as described above.
Protein Structural Analysis
Hydrophobicity analysis was performed using the transmembrane helices hidden Markov model (TMHMM) public domain software (https://services.healthtech.dtu.dk/services/TMHMM-2.0/), and amphipathic helices (AHs) were searched using the HeliQuest server (https://heliquest.ipmc.cnrs.fr).
Microscopy and Statistical Analysis
Images from ISH sections and GFP-expressing embryos were taken with a ZEISS AxioCam HRc Color Microscope Camera (Carl Zeiss Microscopy, Jena, Germany) on a ZEISS Stemi SV11 Stereomicroscope. Images were processed for brightness and contrast with Photoshop 7.0 (Adobe, San Jose, CA, USA). Prism 9.0 (GraphPad, San Diego, CA, USA) was used for statistical analysis of the data and graphic preparations. CorelDRAW 10.0 (Alludo, Ottawa, ON, Canada) was used to compile multipaneled figures. Statistical differences (P < 0.05) were tested by using χ2 tests with Yates’ correction or multiple ANOVA followed by the Bonferroni test. Of note, Yates’ correction identifies whether there is an association between two independent categorical variables, each of which is dichotomous, testing the null hypothesis of no difference between the two variables.
Results
gnpat Is Expressed in Proliferative Cells of the Developing Eye and gpam mRNA Is Localized to Differentiating Photoreceptors
We initiated our studies by determining the expression of gnpat by ISH during eye development in X. laevis, an organism for which retinal neurogenesis and lamination are well characterized.29–32 We previously detected expression of gnpat in the proliferating eye primordium at stage 19 by using whole ISH.18 Here, we analyzed expression over the period of retinal neurogenesis, lamination, and circuit formation, performing slide ISH between stages 30/31 and stage 42, when the eye is fully functional.19 Retinal neurogenesis initiates at stages 24/25, with more than 60% of the cells still cycling at stages 30/31,29 as previously determined by incorporation of labeled thymidine. At stages 30/31, proliferation decreases drastically, with only 30% and 10% of retinal cells remaining proliferative by stages 33/34 and 35/36, respectively31; these proliferative cells are present in the retinal periphery. Retinal lamination initiates at stages 33/34 and is clearly evident by stages 35/36,33 whereas the first retinal light-sensitive circuits become functional at stages 37/38.19
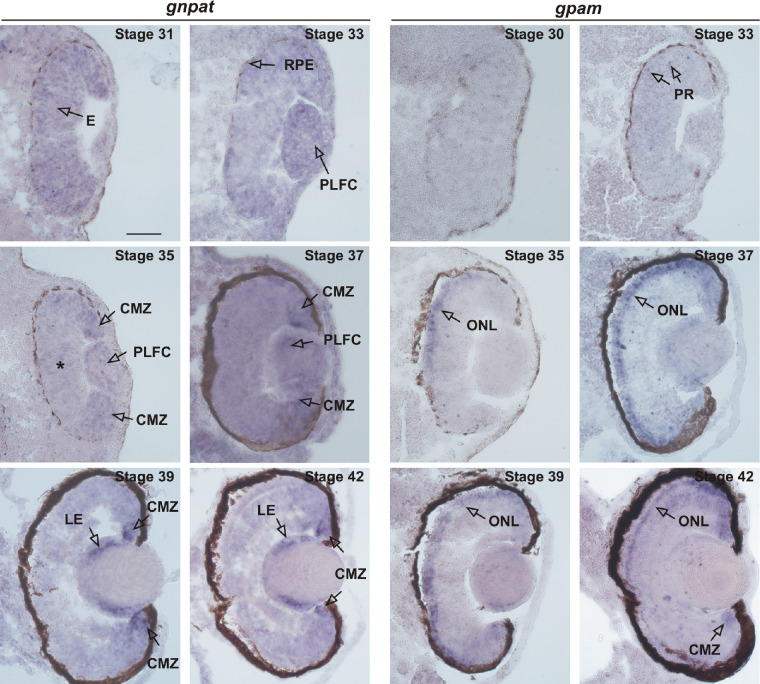
gnpat mRNA expression was broadly detected in the proliferative eye at stage 31. Expression diminished by stage 33 and stage 35, with ISH label becoming restricted to the proliferative retinal margin (Fig. 2; compare stage 31 vs. stages 35/36). This restriction of gnpat expression correlates with the known timing of the termination of retinal neurogenesis. The retinal margin, where gnpat remains expressed at stages 37/38, corresponds to the neurogenic ciliary marginal zone (CMZ) that maintains mitotic activity31,32 (Figs. 2, 3A). The CMZ, characteristic of fish and amphibians, is a self-renewing proliferative neuroepithelium that continuously provides new neurons for the retina over the life span of the organism.35 In addition to expression of gnpat in the CMZ, at stages 37/38 gnpat mRNA was also detected in the PLFCs (Fig. 2). By later stages (39 and 42), when the PLFCs begin synthesizing γ and β crystallins to form the lens,34 gnpat ISH label was restricted to the proliferative lens epithelium (LE) (Fig. 2). Radioactive riboprobes were used previously to show that Gnpat mRNA is present in the RPE of adult rats.35 The black melanin pigment of the RPE from stages 29/30 onward, however, precluded analysis of RPE gnpat expression using the ISH chromogenic reaction; black label was present with both antisense and sense riboprobes (Supplementary Fig. S1). Our results show that, as retinal neurogenesis winds up, gnpat expression becomes restricted to proliferative cells of the CMZ, as well as the PLFCs. Similarly, after the lens is formed, gnpat remains in the proliferative lens epithelium. These data support the idea that gnpat is expressed by proliferative cells of the developing and post-embryonic eye.
Figure 2.
Expression of gnpat and gpam in the eye during development. gnpat: ISH in transverse sections with gnpat-specific antisense riboprobe on slides obtained from X. laevis tadpoles at the indicated embryonic stages. Expression becomes restricted to the CMZ during neuronal differentiation and lamination of the eye (compare stage 31 vs. stages 33/34 and 35/36). At stages 35 and 37, when most retinal cells have exited the cell cycle,29 expression recedes from the central retina (*). At stages 37/38, gnpat mRNA is restricted to the CMZ and PLFCs. At stage 39 and older, after lens formation, gnpat is expressed in the LE. gpam: ISH in transverse sections with gpam antisense riboprobe. Arrows at stages 33/34 potentially indicate newly born photoreceptors (PRs) that have not yet reached the outer retina. At stage 35, the arrow points to photoreceptors located in the dorsal part of the ONL. Note the dorsal–ventral gradient of gpam-expressing cells at stages 35 and 37, whereas expression occurs over the whole ONL by stages 39 and 42. The melanin pigment of the RPE precludes visualization of the ISH signal and is also detected with sense probes (Supplementary Fig. S1). Scale bar: 50 µM.
Figure 3.
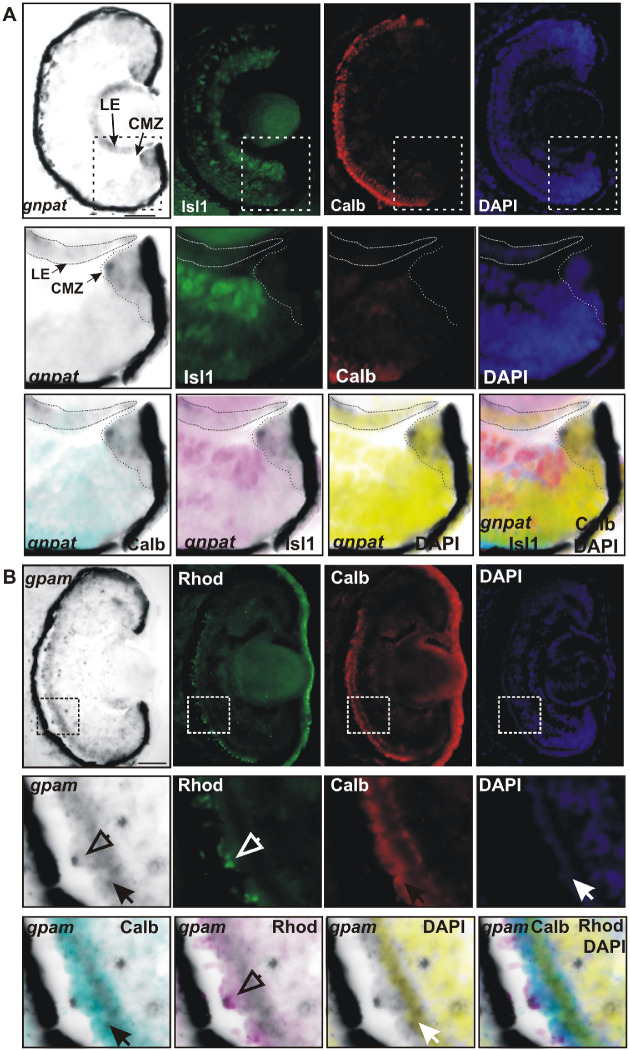
Determination of retinal cells expressing gnpat and gpam. (A) ISH with gnpat-specific riboprobes was followed by immunohistochemistry against calbindin (red; cones) and Islet1 (green; retinal ganglion cells and amacrine cells) on transverse sections of stage 42 eye. Nuclei were revealed by DAPI staining. Higher magnification of the indicated region and merged pictures are shown in the bottom panels. Note that gnpat mRNA in the CMZ is absent from the aadjacent neuronal marker Isl1-expressing cells. In the lens, gnpat expression overlaps with cell nuclei (DAPI staining) of the LE, but it is not expressed in cells that have lost their nuclei (no DAPI staining). Scale bar: 50 µm. (B) ISH against gpam followed by immunohistochemistry against calbindin (red; cones) and rhodopsin (green; rods) on transverse sections of a stage 39 embryo. Nuclei were revealed by DAPI staining. Enlarged magnifications are shown at the bottom. Note that gpam is expressed in the ONL and is co-expressed by both cones (calbindin positive, filled arrow) and rods (rhodopsin positive, open arrows). Scale bar: 50 µm.
For comparison with gnpat, we next investigated the developmental expression of gpam, an acyltransferase that contributes to GPL synthesis with no direct involvement in Plg biosynthesis (Fig. 1). Previously, we identified the full set of gpats (gpat1–4) from Xenopus.18 Importantly, these four isoforms are conserved across all vertebrates.17 Here, we focused on gpam (gpat1), because whole ISH displays high levels of expression in early Xenopus embryos during neurulation, neural tube closure, and forebrain development.18 Indeed, of all the gpats, gpam shows the strongest expression in the eye primordium at stage 23.18
In transverse sections, we could identify the cell types that express gpam at stages 33/34, where scattered cells through the central–dorsal retina, likely putative newborn photoreceptors, expressed gpam (Fig. 2). By stages 35/36, ISH signal extended to the dorsal outer nuclear layer (ONL), where newly born photoreceptors are known to reside (Fig. 2B).32 The restriction of ISH label to the ONL continued as development proceeded, with labeling of photoreceptors across the entire ONL at stage 42 (Fig. 2). gpam is also express in a fraction of the CMZ by stages 39 and 42 which may represent newborn photoreceptors (Fig. 2). This developmental progression of gpam expression matches the characterized processes of specification, migration, and differentiation of X. laevis cone and rod photoreceptors.31,32 Photoreceptor specification, as determined by opsin mRNA expression, initiates as early as stage 31. Cone opsin is the first to appear in a small number of cells. By stages 33/34, mRNAs for both cone and rod opsins are found in the dorsal outer retina,32–34 spreading to ventral outer retina as photoreceptor development proceeds. Thus, gpam expression in the ONL correlates with the emergence of differentiated photoreceptors, when the requirement of membrane components to form the outer-segment discs arises.31,36
Next, we used a combination of ISH followed by immunohistochemistry against specific neuronal markers to confirm the identity of the cell types that express gnpat and gpam. gnpat expression was restricted to the CMZ, with no ISH signal detected in the neighboring postmitotic retinal ganglion cells and amacrine cells, both identified with the Isl1 marker (Fig. 3A). Similarly, cone photoreceptors that express calbindin were negative for gnpat mRNA (Fig. 3A). These results confirm that mRNA levels of gnpat are low or absent in differentiated neurons and are restricted to cells of the proliferative CMZ. This scenario was also true of the lens, where the gnpat ISH signal colocalized with 4′,6-diamidino-2-phenylindole (DAPI) staining of the proliferative lens epithelium but was absent inside the crystalline center, where cells lose their nuclei concomitant with the formation of mature lens fibers (Fig. 3A).34
To determine if both rods and cones express gpam, we took a similar approach to that described above for gnpat. ISH was followed by immunohistochemistry against the rod marker rhodopsin or the cone marker calbindin, with DAPI used to label cell nuclei. Both rods and cones express gpam (Fig. 3B). In cones, gpam mRNA showed a similar distribution to calbindin, which was present throughout the cell (Fig. 3B).37 Interestingly, gpam mRNA in rods was often observed as a pycnotic structure that colocalized with rhodopsin marker in the membranes of the outer segment disc (Fig. 3B).38
Together, these results show that gnpat is likely expressed in proliferative cells. During retinal neuronal differentiation and lamination, gnpat expression becomes restricted both to the PLFC during lens formation and to the proliferative regions of the retina (CMZ and lens epithelium) and is low or absent from postmitotic retinal neurons. In contrast, gpam is expressed by cone and rod photoreceptors in the ONL early in their differentiation and may turn on earlier in newly born photoreceptors that are migrating toward the ONL.
Eye-Specific Overexpression of gnpat and gpam Does Not Affect Eye Development and Embryo-Wide Expression Arrests Development
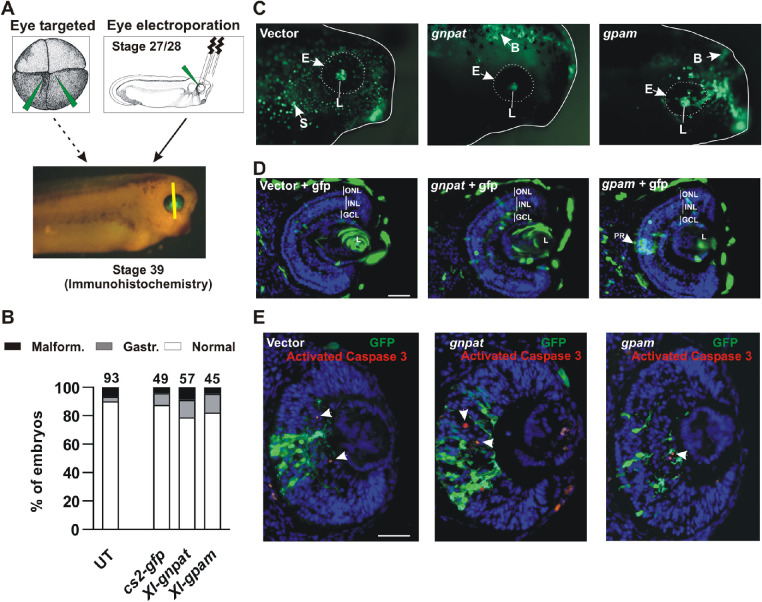
Deficiency of GNPAT in both humans and mice produces developmental eye defects.1,4,7 Whether levels of enzyme activity have to be tightly controlled is unknown. We addressed this issue by overexpressing the enzyme. In this respect, X. laevis is an excellent model, in that overexpression of genes within the eye can be achieved by microinjecting plasmid DNA or mRNA into identified blastomeres of newly fertilized embryos. Microinjection into blastomeres at the one- or two-cell stage produces a general systemic overexpression (Fig. 4A). In contrast, overexpression targeting the eye is achieved by two different techniques: (1) microinjection in the midline corners of the two animal pole cells of the four-cell stage embryo39 (https://www.xenbase.org/entry/ for cell fate map) (Fig. 5A), or (2) through DNA/RNA electroporation of the eye primordium of stage 27/28 embryos (Fig. 5A).
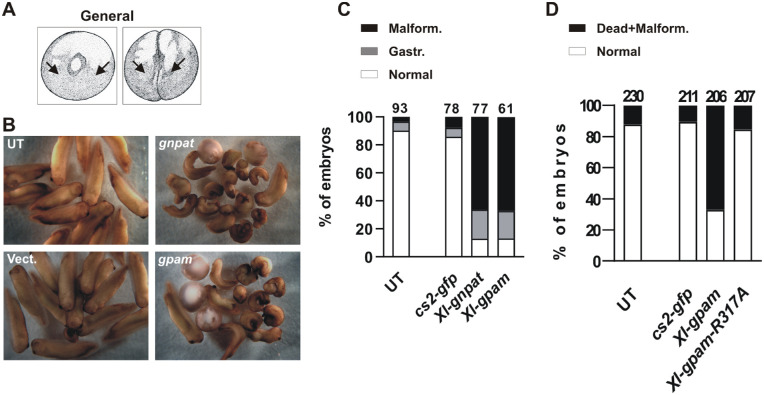
Figure 4.
Gnpat or Gpam general overexpression induces severe defects in early embryonic development. (A) Schematic of stages 1 and 2 (two cells) indicating the microinjection site to produce widespread overexpression. (B) Representative examples showing the developmental defects induced by general overexpression with the indicated plasmids (stages 35/36). (C) Quantitation of normal embryos or those with gastrulation (Gastr.) or embryonic malformation (Malform.) defects after microinjection. (D) Quantitation of embryonic survival of embryos microinjected with Xl-gpam or catalytically inactive Xl-GpamR317A constructs. The total number of embryos analyzed (n) is indicated at the top of each bar (N = 4).
Figure 5.
Retinal lamination appears normal with eye-targeted overexpression or eye electroporation of Gnpat or Gpam. (A) Schematic of stage 3 (four cells; animal view) and stages 27/28 embryos indicating the microinjection site and the electroporation technique used to produce eye-targeted overexpression, respectively. Eye structure and lamination were analyzed at stage 39. The yellow line indicates the approximate location of sections. (B) Quantitation of normal embryos or those with gastrulation (Gastr.) or developmental malformation (Malform.) defects after microinjection of stage 3 embryos targeting the eye. The total number of embryos analyzed (n) is indicated at the top of each bar (N = 4). (C) Representative pictures of GFP expression in lateral views of the head of eye-targeted stage 39 embryos microinjected at the four-cell stage with the indicated plasmid DNA together with pCS2–GFP. E, eye; B, brain; L, lens; S, skin. (D, E) Immunohistochemistry of central retinal sections of stage 39 eyes electroporated at stages 27/28 either for GFP (D) or against GFP and activated caspase 3 (red cells; white arrows) (E). The nuclei were stained with DAPI. GCL, ganglion cell layer; INL, inner nuclear layer; L, lens; PR, photoreceptor. Scale bar: 50 µm.
First, we overexpressed Xenopus Gnpat or Gpam together with GFP in the early Xenopus embryo. Single-cell or two-cell stage embryos were injected with full-length pCMV SPORT6 Xl-Gnpat (n = 77 embryos; N = 4) or pCMV SPORT6 Xl-Gpam (n = 61 embryos; N = 4) together with pCS2–GFP. Controls were the empty vector microinjected together with pCS2–GFP (n = 78 embryos; N = 4) or untreated (UT) embryos. Injection of both acyltransferase genes resulted in a drastic increase in the numbers of embryos that failed to progress normally after gastrulation, with subsequent embryo malformation and/or early death (Fig. 4B). Approximately 20% of the embryos died shortly after gastrulation (stages 17/18). Death continued over the next day, with almost 70% of the embryos showing severe malformations, most commonly failure of closure of the neural tube (Fig. 4C). Thus, widespread overexpression of Gnpat or Gpam produced a significant decrease in embryo survival, with approximately 90% deaths (normal vs. gastrulation defects + malformation; vector vs. gnpat, z = 8.92 and P < 0.0001; vector vs. gpam, z = 8.37 and P < 0.0001; gnpat vs. gpam, z = 0.23, not significant; χ2 test with Yates’ correction) (Figs. 4B, 4C). In contrast, untreated embryos and those injected with the vector + CS2–GFP appeared normal (Figs. 4B, 4C).
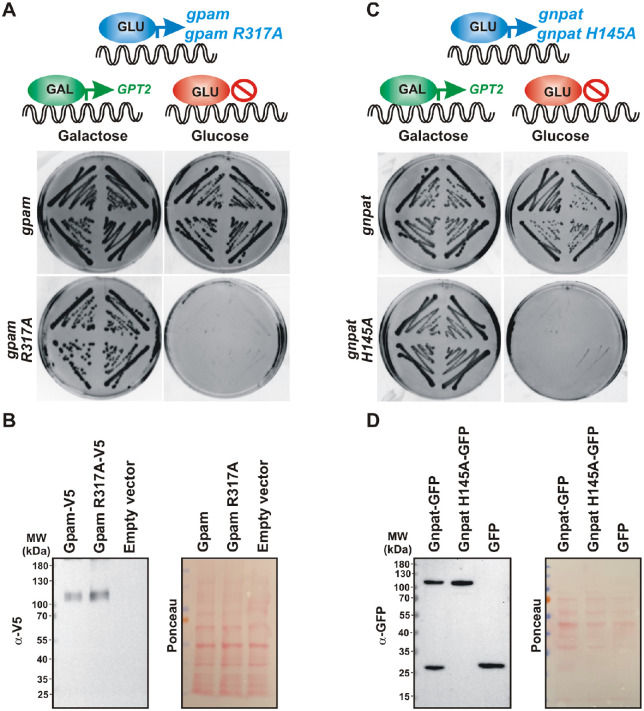
We next analyzed if the developmental defects induced by Gpam overexpression depended on acyltransferase activity. To do that, we compared the survival of embryos microinjected with a construct containing either Xl-gpam or a variant, Xl-gpamR317A, in which arginine (R) 317 was mutated to alanine (A); amino acid 317 is located in the catalytic domain of the enzyme, and, in mice, is known to be essential for acyltransferase activity.40 We confirmed that Xenopus GpamR317A was indeed inactive using a yeast functional assay (see below, Fig. 7). We microinjected embryos with CS2–GFP alone or together with gpam or gpamR317A expression constructs. The embryos expressing the wild-type form of Gpam failed to develop normally and died, whereas those expressing the catalytically dead Gpam showed a survival rate similar to that of GFP-expressing embryos (CS2–GFP vs. gpam: z = 138.6, P < 0.0001; CS2–GFP vs. gpamR317A: z = 1.38, not significant; gpam vs. gpamR317A: z = 10.54, P < 0.0001; χ2 test with Yates’ correction) (Fig. 4D). These data argue for the specificity of cell death to excess acyltransferase activity.
Figure 7.
Catalytically active forms of Xenopus Gpam and Gnpat support life of yeast devoid of endogenous GPATs. A double knock-out sct1Δ gpt2Δ strain expressing GPT2 under the GAL promoter in a LEU2 plasmid (previously introduced in Fig. 6A) was transformed with a URA3-based plasmid containing wild-type gpam or catalytically dead gpamR317A (A) or wild-type gnpat or catalytically dead gnpatH145A (C). Four independent transformants were grown in selective –ura/–leu plates containing galactose and then restreaked on galactose or glucose plates and grown for 3 days at 30°C (A, C). Expression of the catalytically dead enzymes GpamR317A (B) or GnpatH145A (D) confirmed by western blot of wild-type yeast transformed with empty vector or the same plasmids used in A and C. Membranes stained with Ponceau Red dye after transfer are shown for loading control. Primary antibodies against V5 epitope fused to Gpam (B) or GFP fused to Gnpat (D) detected heterologous expression of the proteins.
We were interested in whether normal eye development required tight control of enzyme activity. Thus, we needed to bypass the embryonic lethality that arose with early and general overexpression of Gnpat and Gpam by targeting Gnpat and Gpam overexpression to the eye. We microinjected Gnpat and Gpam plasmids into the central corners of the two-animal pole blastomeres of the four-cell-stage embryo (Fig. 5A) or electroporated the plasmids into the eye at stages 27/28 (Fig. 5A). In contrast to systemic overexpression, the embryos microinjected at the four-cell stage developed normally, with no significant difference in survival from controls (normal vs. gastrulation defects + malformation; CS2–GFP vs. gnpat: z = 0.95, P = 0.34, not significant; CS2–GFP vs. gpam: z = 0.46, P = 0.64, not significant; gnpat vs. gpam: z = 0.16, P = 0.87, not significant; χ2 test with Yates’ correction) (Fig. 5B). Because embryos developed normally, we were able to analyze eye structure, choosing to do our analysis at stage 39 when the main period of retinal cell genesis is over and lamination has occurred. We assessed eyes macroscopically in both fixed embryos (Fig. 5C) and in transverse sections immunostained with an anti-GFP antibody (Fig. 5D). Blastomere injections at the four-cell stage, although targeting the eye, do result in mosaic expression in various tissues, mainly the retina, lens, dorsal brain, and other adjacent neuronal tissues and the skin (Fig. 5C). The macroscopic structure of the eye (Fig. 5C) and lamination in retinal sections (Fig. 5D) were normal in all embryos analyzed (N = 23), including embryos where Gpam was overexpressed in photoreceptors of the ONL, as well as in embryos where Gnpat was overexpressed in the lens or other areas of the retina (Fig. 5D).
We also asked if overexpression of Gnpat and Gpam increased apoptosis in the eye, as determined by expression of the activated caspase 3. Eye electroporation was chosen for these experiments because electroporation (1) is more specific than microinjection in targeting eye alone, (2) completely bypasses lethality because eye electroporation is performed at stages 27/28, and (3) increases the number of retinal transfected cells. Embryos were electroporated at stages 27/28 with Gnpat and Gpam plasmids together with CS2–GFP. By stage 39, central retina sections were immunostained with both GFP and activated caspase 3 antibodies. The proportion of GFP-expressing cells that were immunolabeled for activated caspase 3 was similar in control (CS2–GFP) and for Gnpat and Gpam overexpression (Fig. 5E) (based on the percentage of GFP+/activated caspase 3− cells and GFP+/activated caspase 3+ cells per slide; CS2–GFP: 97.07 ± 4.87 and 2.93 ± 4.87, respectively; Gnpat: 95.89 ± 3.73 and 4.11 ± 3.73, respectively; Gpam: 98.37 ± 2.47 and 1.63 ± 2.47, respectively; no significant differences by χ2 test with Yates’ correction. Note that the total numbers of cells counted were 535, 703, and 570 for CS2–GFP, Gnpat, and Gpam, respectively (n = 10 slides from 10 embryos; N = 2). These data suggest that overexpression of acyltransferases does not induce caspase 3–mediated apoptosis.
The embryonic lethality we observed for Gnpat and Gpam during widespread overexpression was not present with the catalytically dead Gpam, arguing that both of the overexpressed wild-type acyltransferases were active in this experiment. Thus, the developmental lethality observed with overexpression appears to be caused by high acyltransferase activity. In contrast, targeted eye overexpression of the same acyltransferase constructs by microinjection or electroporation did not grossly impact eye development or retinal cell survival.
Biochemical Characterization of X. laevis Gnpat
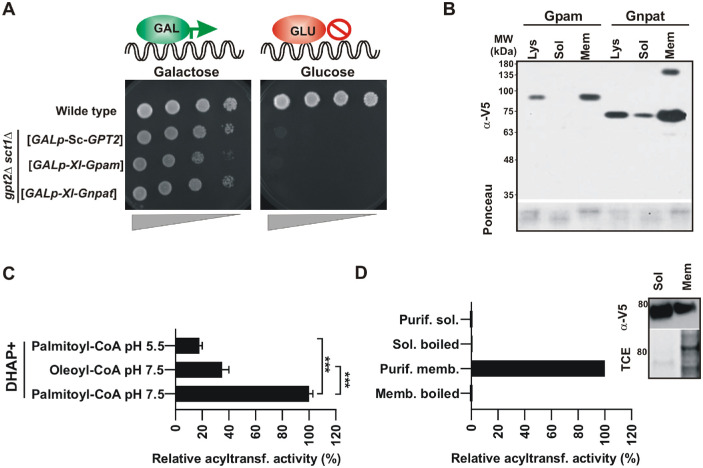
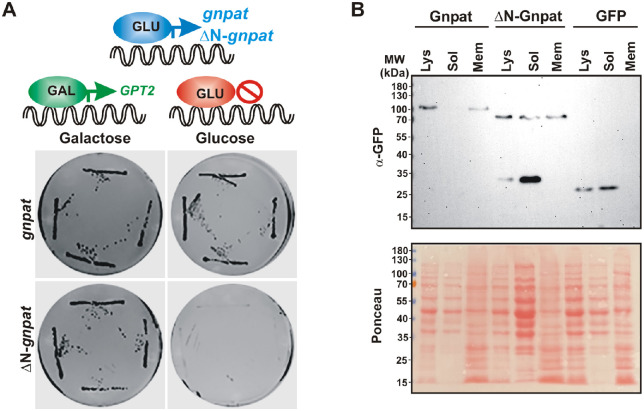
The developmental lethality of blastomere-injected embryos with constructs coding full-length gnpat and gpam suggests that both plasmids express the active enzyme. In order to confirm their acyltransferase activity and to biochemically characterize the Xenopus Gnpat, we used an heterologous expression system in Saccharomyces cerevisiae (referred to as yeast from here on). There are two important advantages of using yeast as a eukaryotic model organism for the biochemical study of GNPATs and GPATs: yeast has a reduced set of acyltransferases and does not synthesize ether lipids. Only two genes code for the entire set of GPATs in yeast (SCT1 and GPT2), and their simultaneous deletion results in synthetic lethality.41 Unlike animal GPATs, yeast enzymes are dual-specificity acyltransferases with both GPAT and GNPAT activities, although their preferences for DHAP differ.42 Therefore, replacement of the endogenous yeast acyltransferases with the Xenopus Gpam or Gnpat provides a platform to test their functionality. Hence, we challenged the X. laevis gnpat and gpam to support life of a yeast devoid of endogenous GPATs. The genes coding for these enzymes were cloned in a yeast vector under a galactose-inducible promoter and were introduced in yeast carrying genomic deletions of the genes coding for GPATs (sct1Δ gpt2Δ double knock-out). The promoter used induces expression when yeast is grown on galactose, but expression is repressed in the presence of glucose. Therefore, if the acyltransferase expressed from the inducible promoter is functional, growth is expected on galactose but not in glucose.
Successful conditional lethal yeast strains were obtained for gnpat, gpam, and the positive control yeast GPT2. As expected for functional acyltransferases, culturing the yeast on galactose allowed for cell growth, whereas shifting cells to glucose-containing medium resulted in cessation of growth (Fig. 6A). The Xenopus proteins produced in yeast were tagged with a V5/His6, and their expression was confirmed by western blot analysis, with Xl-Gnpat and Xl-Gpam bands appearing near their expected molecular weights of 75,895 and 93,039 Da, respectively (plus V5/Hisx6 tag 2500 Da) (Fig. 6B). Furthermore, we performed subcellular fractionation expecting to find both acyltransferases associated with the membrane fraction. To our surprise, however, a considerable amount of Xl-Gnpat–V5/His6 was recovered in the soluble fraction, suggesting it behaves as a peripheral membrane protein, in contrast to the behavior of Xl-Gpam, which is a bona fide integral membrane protein with two transmembrane segments.43 Together, these results show that both Xl-Gnpat and Xl-Gpam were expressed, and the proteins were functionally active in a yeast heterologous system, thus providing an excellent platform for the production of the protein for biochemical studies without the interference of other acyltransferases.
Figure 6.
X . laevis GNPAT and GPAM are functional in yeast. (A) Conditional lethal yeast strains lacking endogenous yeast GPATs Sct1 and Gpt2 (sct1Δ gpt2Δ) were successfully generated by introducing the sequences coding for the indicated acyltransferases in URA3-based plasmids under the galactose inducible promoter. Shift of these strains to glucose represses expression from the plasmid resulting in the lethal phenotype. (B) Expression of Gnpat-V5/His6 and Gpam-V5/His6 analyzed by western blotting using anti-V5 antibodies in lysates and soluble and membrane preparations obtained from subcellular fractionation. (C) Acyltransferase activity assay. Lysates were obtained from a conditional lethal sct1Δ gpt2Δ strain expressing Gnpat-V5/His6 under the GAL promoter. Activity was expressed relative to that obtained with Gnpat lysate at pH 7.5 with DHAP and palmitoyl-CoA as substrates (activity lysate at pH 7.5 = 3 pmol/min/mg protein). ***P < 0.001 ANOVA followed by Bonferroni's test. (D) Gnpat lacks activity when soluble. The western blot and acyltransferase activity assays are shown in C and D, respectively. Note that, for the soluble fraction, the signal of the band corresponding to Gnpat was five times higher than the one corresponding to the membrane fraction in the western blot (activity membrane protein = 1.4 pmol/min/mg protein). Shown are representative results of one of two independent experiments.
Although Gpam has been thoroughly characterized at the biochemical level,41 most Gnpat activity studies were performed before the identification of its coding gene, using whole cell extracts or subcellular fractions containing other acyltransferases.25,44 Taking advantage of our heterologous yeast system expressing a functional Gnpat, we next performed a biochemical characterization of Gnpat, measuring its activity in different conditions. For this purpose, lysates from double knock-out yeast expressing Xl-Gnpat were used as the source of enzyme in DHAP acylation reactions. In the past, activity assays have been performed at pH 5.5 in order to avoid interference from GPAT activity.45 Given that our expression system was devoid of endogenous GNPATs and GPATs, we performed GNPAT activity assays at pH 7.5, which increased activity by more than five times as compared to pH 5.5 (Fig. 6C). The enzyme showed a preference for palmitoyl-CoA versus oleoyl-CoA; therefore, palmitoyl-CoA was used as the acyl donor in additional GNPAT assays. No activity was detected in semipurified soluble Gnpat extracts in contrast to membrane-associated Gnpat, suggesting that association with membranes is necessary for Gnpat activity (Fig. 6D).
Next, we challenged our functional assay by expressing catalytically dead enzymes that carry alanine mutations to replace the conserved arginine (R317) residue localized to the acyltransferase signature motif III17,40 in Xl-Gpam or histidine (H145) critical in motif I of Xl-Gnpat.15,17 The functional assay was adapted in order to evaluate the effect of a catalytically dead Gpam/Gnpat mutant which was expected to fail to support life of a yeast devoid of endogenous GPATs. For this, plasmids carrying wild-type or catalytically dead Xl-Gpam or Xl-Gnpat under a constitutive promoter (expressed regardless of the carbon source) were introduced in the double knock-out sct1Δ gpt2Δ strain expressing GPT2 under the GAL promoter (previously introduced in Fig. 6A). In this way, it is expected that GPT2 would support life when yeast are grown on galactose-containing medium, but only the expression of functional heterologous acyltransferases would support life when cells are grown on glucose (which shuts down GPT2 expression). Indeed, although cells expressing the wild-type Xl enzymes were able to grow on glucose, those expressing Xl-GpamR317A or Xl-GnpatH145A failed to do so despite being expressed at similar levels (Figs. 7A–7D).
Together, our results show that Xenopus Gpam and Gnpat are functional when expressed in yeast. We determined that Gnpat is active when associated with membranes, with a preference for palmitoyl rather than oleoyl-CoA as substrate.
The Amino Terminus of Gnpat Interacts With Lipids and Is Required for Enzyme Function
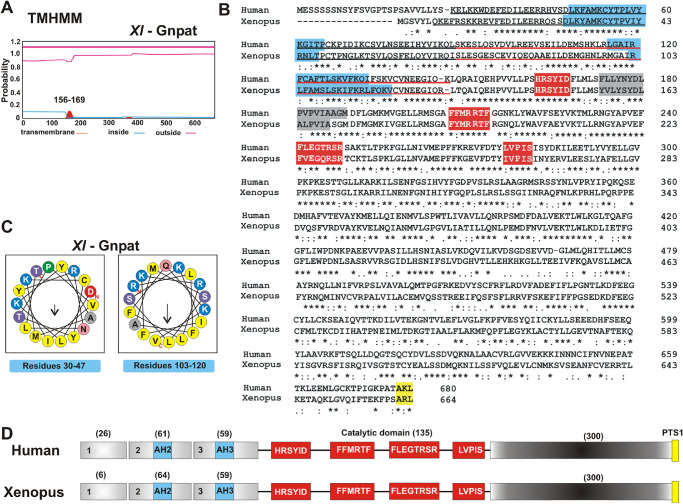
Because Xenopus Gnpat associates with membranes and this association is relevant for its activity, we next determined which part of the protein was responsible for this interaction by analyzing possible hydrophobic regions. Hydrophobicity analysis using TMHMM software indicated one possible hydrophobic region with very low probability (Fig. 8A), corresponding to residues ∼156 to 169 located between motifs I and II in the catalytic domain (Fig. 8B, gray box and red boxes, respectively). Therefore, this hydrophobic patch could potentially correspond to the acyl-CoA binding site, rather than a transmembrane helix. No other conserved lipid binding domains or motifs could be identified within Gnpat. The amino end of human GNPAT is suggested to be responsible for binding to membranes,46 but this has not been demonstrated. Xenopus and human GNPATs display 76% similarity and 58% identity with high conservation in the N-end regions encoded by exons 2 and 3 (Figs. 6B, 6D). Thus, we decided to further investigate the role of the amino terminus of GNPAT by generating a deletion of the sequence encoded by the first three exons, resulting in a protein lacking the first 129 amino acids (ΔN-Gnpat), sparing the four catalytic motifs (Figs. 8B–8D). Interestingly, this truncated Gnpat failed to support life of the double knock-out (sct1Δ gpt2Δ) yeast, despite being expressed at levels comparable to full Gnpat in a wild-type strain (Figs. 9A, 9B). The ΔN-Gnpat was still detected in the membrane fraction, but it showed a higher proportion of degraded protein associated with the soluble fraction, supporting a role for the N-end in stabilizing the membrane-bound protein.
Figure 8.
Comparative protein analysis between Xenopus and human GNPAT. (A–D) Hydrophobicity plot detected by analysis with TMHMM software (A), which is also indicated as a gray shade in the protein alignment in B. The protein alignment (B) was performed between X. laevis (Uniprot Q6GM34) and Homo sapiens GNPAT (Uniprot O15228-1). The HeliQuest analysis (C) shows putative AHs (light blue) associated with regions encoded by exons 2 (AH2) and 3 (AH3), which are also indicated in the protein alignment (B) and the schematic of the protein structure (D). HeliQuest analysis was carried out for the N-terminal region of both proteins encoded by exons 1 to 3, using a window of 18 amino acids. The N-terminus region encoded by exons 1, 2 (black underlined), and 3 (red underlined) is shown in B and D. The middle catalytic region contains the four motifs with signature conserved amino acid sequence (red box), and the peroxisomal targeting signal 1 (PTS1, yellow) is located in the carboxy-end region.
Figure 9.
The amino end of Gnpat is necessary for a functional enzyme. (A) A double knock-out sct1Δ gpt2Δ strain expressing GPT2 under the GAL promoter in a LEU2 plasmid was transformed with a URA3-based plasmid containing wild-type gnpat or truncated Δ129N-gnpat. Four independent transformants were grown in selective –ura/–leu plates containing galactose and then re-streaked on galactose or glucose plates and grown for 3 days at 30°C. (B) Expression of Gnpat-GFP, ΔN-Gnpat-GFP, and GFP in wild-type cells analyzed by western blotting using anti-GFP antibodies in lysates and soluble and membrane preparations obtained from subcellular fractionation. Membranes stained with Ponceau Red dye after transfer are shown for the loading control.
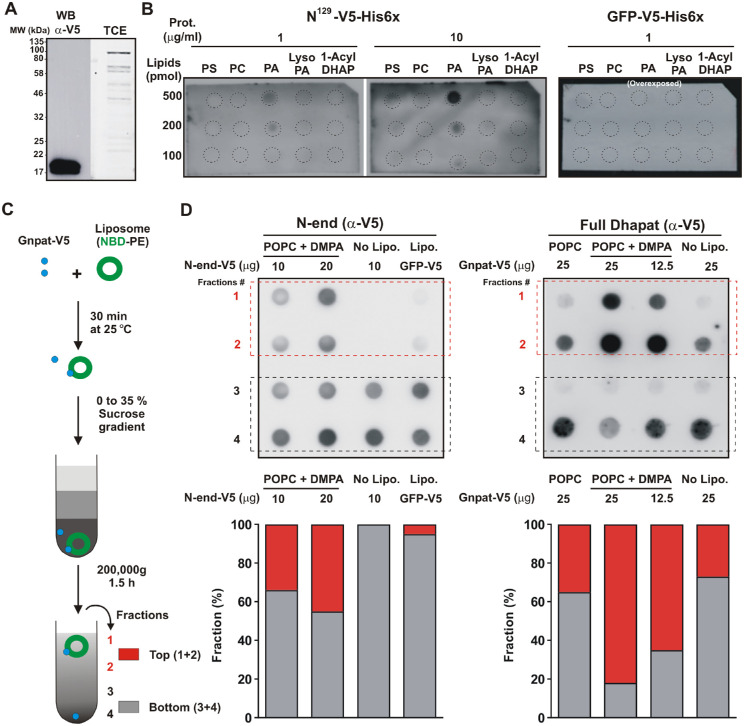
We next investigated the lipid-binding properties of the amino terminus of Xenopus Gnpat by searching first for the presence of possible amphipathic helices (AHs) using the HeliQuest server.47 HeliQuest analysis was carried out for the N-terminal region with a window of 18 amino acids and revealed the presence of two strong putative AHs, spanning residues 30 to 47 and 103 to 120 of the Gnpat, which are also conserved in the human GNPAT (Figs. 10B–10D).
Figure 10.
The GNPAT amino-terminus has binding preference for phosphatidic acid. (A) The N-terminal end of Xl-Gnpat tagged with V5/His6 was expressed in E. coli and purified by Ni-NTA chromatography. Western blot analysis with anti-V5 primary antibodies is shown on the left. Expected molecular weight for the N-terminal end is 18.4 kDa. Total protein stained with TCE is shown (right). (B) Protein lipid overlay. Indicated lipid dots were incubated with proteins and detected as in A. GFP tagged with V5/His6 was used as a negative control. (C) Flow chart illustrating the steps of the flotation assay. (D) Dot-blot analysis of fractions obtained from the top to the bottom of the gradient (1–4) using anti-V5 primary antibody. Densitometry shown below the blots combined fractions 1 and 2 (top) and 3 and 4 (bottom), expressed as a percentage of the total signal from all combined fractions. POPC, palmitoyl oleoyl phosphatidylcholine.
The fact that these predicted AHs displayed a positively charged polar face prompted us to test binding to negatively charged lipids. The product of GNPAT is 1-acyl DHAP, which can be converted to LPA and then to PA (Fig. 1), all negatively charged lipids. Therefore, we conducted protein lipid overlay assays to test binding of the amino terminus (N129Xl-Gnpat) to these lipids. For this purpose, recombinant N129Xl-Gnpat (18.4 kDa) fused to V5 and His6 tags was produced in E. coli and purified (Fig. 10A). GFP-V5/His6 was included as control. Phosphatidylcholine (PC, no net charge) and phosphatidylserine (PS, negatively charged) were also tested. The N129Xl-Gnpat was found to preferentially bind PA (Fig. 10B). This binding depended on both protein and lipid concentrations (Fig. 10B). To confirm the overlay results, we performed flotation experiments using liposomes composed of PC or PC/PA (Fig. 10C). In addition to the recombinant N129Xl-Gnpat, the semi-purified functional full-length Xenopus Gnpat expressed in yeast was included in these experiments. Consistently, it was found that both the full enzyme and its N-end were able to associate with the floating layer that contained liposomes. The presence of 20% PA in the liposomes increased association of the full length Gnpat by almost three times (Fig. 10D). Altogether, these results indicate that the amino terminus of Gnpat has lipid-binding capacity, which may play a role in stabilizing the association of the enzyme with membranes.
Discussion
Inherent mutations of three genes encoding for the peroxisomal enzymes involved in Plg synthesis (GNPAT, AGPS, and FAR1) (Fig. 1) cause RCDP types 2, 3, and 4, respectively.12 Most young patients with RCDP are diagnosed with “clouding” of the lens (cataracts) that is apparent at birth (congenital) or detected in early infancy. In the last 15 years, an increased awareness of RCDP and the identification of mutations associated with the disease have contributed to a better clinical description and understanding of neurological symptoms associated with the different types of RCDP. Here, we used the well-established model organism X. laevis to expand our knowledge of the first step in the synthesis of Plgs catalyzed by GNPAT, providing molecular insight into why Gnpat deficiency is linked to eye developmental defects. Our results show that Gnpat appears to be expressed in proliferative cells of the neural retina, before cells exit the cell cycle to differentiate into distinct neuronal cell types, and in the lens in PLFCs, until crystalline formation when expression becomes restricted to the proliferative lens epithelium (Figs. 2, 3). We found that this expression differs dramatically from that of a second acyl transferase, gpam, which is expressed in newly generated photoreceptors (Figs. 2, 3). Also, systemic overexpression of Gnpat impaired Xenopus embryo viability (Fig. 4), whereas overexpression of Gnpat in the eye produced no apparent defects (Fig. 5). Finally, biochemical characterization shows that Gnpat is active when associated with membranes, an association that is stabilized by the N-end of the enzyme (Figs. 6789–10). Is not clear at this point if the lipid-binding activity of the N-end comprised of the first 129 amino acids is required for the catalytic activity of full-length Gnpat or if it contains, in addition, a portion of Gnpat required for its proper folding and/or catalytic activity. Future studies will investigate these hypotheses.
Given the cataract phenotype in patients with RCDP2, it is interesting that we found gnpat expressed in PLFCs (stage 37), suggesting an involvement of Plgs and Gnpat when crystalline forms and lens fibers are packed. During lens formation, cells in the posterior half of the lens vesicle differentiate into PLFCs, which go on to form the lens fiber core, whereas cells in the anterior half maintain a proliferative state as a monolayer lens epithelium.48 For the lens to be transparent, fiber cells eliminate their nucleus to form an “organelle-free zone” by a poorly understood process.49 This membrane degradation process associates with an increase of lipoxygenase expression in the lens region where organelle degradation occurs.50 Thus, one might have expected low levels of Gnpat, and likely other acyltransferases, in already differentiated lens cells that lack membrane renewal. In contrast, our results showed expression of gnpat in PLFCs, strongly suggesting Gnpat involvement in protein and lipid packing/composition during lens fiber formation. The change that occurs with age in the protein and lipid composition of lens fibers may provide an answer to the initial role of Gnpat during lens formation. Ethanolamine-based Plgs and sphingomyelin are the two main lipid components in the lens membranes that interact with α- and β/γ-crystallin.51,52 The relative amounts of Plgs and sphingomyelins vary in different regions of the adult lens. The younger fibers, which comprise the cortical region, are enriched in plasmenyl ethanolamines, whereas the central and older regions exhibit more sphingomyelin.52 This distribution suggests that plasmenyl ethanolamines are likely required during lens fiber differentiation and elongation, but sphingomyelin becomes enriched over time in the older central region. The comparative analysis of sphingolipids and plasmenyl ethanolamine between normal lens and lens obtained from patients with cataracts is also supportive of this idea; the content of sphingomyelin increases with age and in the cataract lens, whereas plasmenyl ethanolamines are enriched in young and normal lenses.53 In addition, a model of triparanol-induced cataracts in rats also shows a similar distribution of Plgs and GPLs.54 Is it worth noting that the enrichment in palmitoyl over oleoyl–ethanolamines in the lens agrees with our biochemical data with respect to Gnpat affinity for the two substrates. Thus, the expression of Gnpat in PLFCs and its increased activity with palmitoyl rather than oleoyl strongly support a role during lens fiber formation and elongation, with a likely contribution to the synthesis of plasmenyl ethanolamines. This developmental role of Gnpat during crystalline formation could explain the early manifestations of cataracts in GNPAT-deficient patients.1,8–10 Interestingly, our results also show that, after the lens is formed (e.g., stage 42), Gnpat remains expressed in the proliferative lens epithelium. Similar results were observed previously in mouse lens epithelium cells in vitro,7,51 suggesting that Gnpat helps maintain lens biochemical properties after the initial role in lens formation.
gpam has a distinct eye expression pattern from that of gnpat, one that suggests a role for Gpam in photoreceptor cell differentiation. gpam is developmentally expressed in cones and rods as they differentiate. For rods specifically, ISH followed by immunohistochemistry suggests that there are pycnotic domains that co-express gpam mRNA and rhodopsin. During photoreceptor development, membrane addition in the outer segment increases dramatically, and its rate in mature photoreceptors is affected by light/dark exposure.55 Rhodopsin is trafficked from the ER and then from Golgi structures to the outer segment disc membranes in a manner that depends on the dark/light cycle.56 Of note, the macromolecular structures devoted to the maintenance of the membranes of the photoreceptor outer segment include mitochondria and rough ER, as well as proteins responsible for the de novo biosynthesis of GPLs that are located in the myoid and basal outer region of photoreceptors.57,58 Gpam translation in “subcellular domains” in this region may facilitate the rapid synthesis of GPLs needed for replenishing membranes in areas of high turnover. Additional studies are necessary to determine if Gpam is the main contributor to GPLs during photoreceptor development and if its translation occurs in specific subcellular domains in the outer segments.
GNPAT loss of function in mice and humans results in eye defects that include cataracts, microphthalmia, optic nerve hypoplasia, and dysgenesis of the anterior eye chamber.1,2,7 Using the X. laevis model organism, we tested possible defects associated with gain of function of acyltransferase activity during development. Although targeting the eye did not produce any noticeable morphological malformation, the systemic overexpression of both Gpam and Gnpat produced non-viable embryos, most of which died during neurulation, neural tube closure, and early organogenesis (stages 17–23). Our results using the catalytically dead GpamR317A suggest that the embryonic lethality is likely mediated by an excess of acyltransferase activity. Previously, we found that all Gpam and Gnpat mRNAs show a similar pattern and developmental increase of expression from stages 17 to 23, with the exception of Gpam, whose mRNA levels appear similar throughout development.18 The fact that embryos die when normal acyl transferase expression increases suggests the need for an ideal level of acyl transferase activity, with activity above that level being deleterious. Thus, our gain-of-function results suggest that the pathway for glycerolipid biosynthesis must be regulated tightly during development, mainly during neurulation and early organogenesis (stages 17–23). After this period, our eye-targeted overexpression results suggest that excess acyltransferase activity is not critical developmentally. Of note, after neurulation/early organogenesis the gpats and gnpat show a more distinctive and tissue-specific expression pattern.18
We confirmed the functionality of the gpam and gnpat constructs used in our microinjection experiments by means of a yeast functional assay. This led to a unique opportunity to further characterize Gnpat at the biochemical level, advancing our knowledge of how the enzyme operates. We find Gnpat in both the soluble and membrane fractions, but only the membrane-associated enzyme is active. The biochemical requirement of membranes for Gnpat activity agrees with its expression in PFLCs but not membrane-free differentiated lens cells. Furthermore, whereas it has been proposed that GNPAT activity requires AGPS,59 our results indicate clearly that GNPAT is active in the absence of AGPS, both in vivo and in vitro, as yeast lacks AGPS. In addition, we have shown, using both the protein lipid overlay and flotation assays, that the first 129 amino acid residues of Gnpat bind to lipids, with a preference for PA. Finally, we demonstrated that the amino terminus region is critical to support life of a yeast strain devoid of endogenous GPATs (sct1Δ gpt2Δ). Supportive of the idea that these amino acids are involved in lipid binding is that human GNPAT missing the first 135 amino acids (Δ135GNPAT) is unstable, prone to aggregation, and loses enzymatic activity.46 Further, we find in the amino terminus region of Xenopus Gnpat two amphipathic helices that are conserved in the human GNPAT (Fig. 8B). Of note, ether lipids are not part of the yeast lipidome, and the specific contribution of GNPAT activity from the yeast acyltransferases Sct1 and Gpt2 to lipid metabolism is not fully understood.41,60 Thus, the ability of Gnpat to rescue and support the life of yeast devoid of GPATs could be explained by the ability of yeast cells to reduce 1-acyl DHAP to LPA by the reductase Ayr1, to feed the GPL biosynthetic pathway (see Fig. 1).
The potential application of Plgs to the prevention and treatment of RCDP conditions is very promising.61 Plg precursors that can cross both the blood–retina and the blood–brain barriers and do not show toxicity in humans are being tested currently in Plg-replacement therapy trials.62 1-O-alkyl-sn-glycerol (AG) is the first metabolite produced in the ER from the precursor made in peroxisomes by the GNPAT-AGPS pathway. Supplementing the diet of individuals carrying loss-of-function GNPAT mutations with AG has improved retinal pigmentation and motor tone.63,64 Our work sets the foundation for the use of yeast for the production of specific Plg precursors that could be tested directly in genetic eye developmental conditions recreated in Xenopus. We believe that the combined use of frog and yeast model organisms will lead to a synergistic advancement of our knowledge of many aspects of the ether lipid pathway and its potential applications.
Supplementary Material
Acknowledgments
The authors thank Elesha Hofarth for her technical contributions to the study.
Supported by Discovery grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to VZ (PIN303585-2016) and to SM (PIN03909-2018) and by Discovery Accelerator Supplements from the NSERC to VZ and SM. CCT was the recipient of an NSERC Undergraduate Student Research Award.
Disclosure: G.E. Bertolesi, None; M.F.J. Chilije, None; V. Li, None; C.C. Thompson, None; A. López-Villalobos, None; C.L. Hehr, None; K. Atkinson-Leadbeater, None; V. Zaremberg, None; S. McFarlane, None
References
- 1. Saab S, Mazzocco J, Creuzot-Garcher CP, Bron AM, Bretillon L, Acar N.. Plasmalogens in the retina: from occurrence in retinal cell membranes to potential involvement in pathophysiology of retinal diseases. Biochimie. 2014; 107(part A): 58–65. [DOI] [PubMed] [Google Scholar]
- 2. Gorgas K, Teigler A, Komljenovic D, Just WW.. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006; 1763(12): 1511–1526. [DOI] [PubMed] [Google Scholar]
- 3. Ofman R, Hettema EH, Hogenhout EM, Caruso U, Muijsers AO, Wanders RJ.. Acyl-CoA:dihydroxyacetonephosphate acyltransferase: cloning of the human cDNA and resolution of the molecular basis in rhizomelic chondrodysplasia punctata type 2. Hum Mol Genet. 1998; 7(5): 847–853. [DOI] [PubMed] [Google Scholar]
- 4. Itzkovitz B, Jiralerspong S, Nimmo G, et al.. Functional characterization of novel mutations in GNPAT and AGPS, causing rhizomelic chondrodysplasia punctata (RCDP) types 2 and 3. Hum Mutat. 2012; 33(1): 189–197. [DOI] [PubMed] [Google Scholar]
- 5. Moser HW. Molecular genetics of peroxisomal disorders. Front Biosci. 2000; 5(12): D298–D306. [DOI] [PubMed] [Google Scholar]
- 6. Braverman N, Chen L, Lin P, et al.. Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum Mutat. 2002; 20(4): 284–297. [DOI] [PubMed] [Google Scholar]
- 7. Rodemer C, Thai TP, Brugger B, et al.. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Genet. 2003; 12(15): 1881–1895. [DOI] [PubMed] [Google Scholar]
- 8. Wanders RJA, Schumacher H, Heikoop J, Schutgens RBH, Tager JM.. Human dihydroxyacetonephosphate acyltransferase deficiency: a new peroxisomal disorder. J Inherit Metab Dis. 1992; 15(3): 389–391. [DOI] [PubMed] [Google Scholar]
- 9. Clayton PT, Eckhardt S, Wilson J, et al.. Isolated dihydroxyacetonephosphate acyltransferase deficiency presenting with developmental delay. J Inherit Metab Dis. 1994; 17(5): 533–540. [DOI] [PubMed] [Google Scholar]
- 10. Waterham HR, Ebberink MS.. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim Biophys Acta. 2012; 1822(9): 1430–1441. [DOI] [PubMed] [Google Scholar]
- 11. Mcfarlane S, Lom B.. The Xenopus retinal ganglion cell as a model neuron to study the establishment of neuronal connectivity. Dev Neurobiol. 2012; 72(4): 520–536. [DOI] [PubMed] [Google Scholar]
- 12. Honsho M, Fujiki Y.. Plasmalogen homeostasis – regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 2017; 591(18): 2720–2729. [DOI] [PubMed] [Google Scholar]
- 13. Honsho M, Tanaka M, Zoeller RA, Fujiki Y.. Distinct functions of acyl/alkyl dihydroxyacetonephosphate reductase in peroxisomes and endoplasmic reticulum. Front Cell Dev Biol. 2020; 8: 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gimeno RE, Cao J.. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res. 2008; 49: 2079–2088. [DOI] [PubMed] [Google Scholar]
- 15. Lewin TM, Wang P, Coleman RA.. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate. Biochemistry. 1999; 4(38): 5764–5771. [DOI] [PubMed] [Google Scholar]
- 16. Takeuchi K, Reue K.. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009; 296(6): E1195–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smart HC, Mast FD, Chilije MFJ, Tavassoli M, Dacks JB, Zaremberg V.. Phylogenetic analysis of glycerol 3-phosphate acyltransferases in opisthokonts reveals unexpected ancestral complexity and novel modern biosynthetic components. PLoS One. 2014; 9(10): e110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertolesi GE, Iannattone S, Johnston J, Zaremberg V, McFarlane S.. Identification and expression analysis of GPAT family genes during early development of Xenopus laevis. Gene Expr Patterns. 2012; 12(7-8): 219–227. [DOI] [PubMed] [Google Scholar]
- 19. Bertolesi GE, Hehr CL, McFarlane S.. Wiring the retinal circuits activated by light during early development. Neural Dev. 2014; 9(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atkinson-Leadbeater K, Bertolesi GE, Hehr CL, Webber CA, Cechmanek PB, McFarlane S.. Dynamic expression of axon guidance cues required for optic tract development is controlled by fibroblast growth factor signaling. J Neurosc. 2010; 30(2): 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albert S, Gitler AD, Lindquist S.. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast. 2007; 24(10): 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawai S, Hashimoto W, Murata K.. Transformation of Saccharomyces cerevisiae and other fungi: methods and possible underlying mechanism. Bioeng Bugs. 2010; 1(6): 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marr N, Foglia J, Terebiznik M, Athenstaedt K, Zaremberg V.. Controlling lipid fluxes at glycerol-3-phosphate acyltransferase step in yeast. J Biol Chem. 2012; 287(13): 10251–10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ladner CL, Yang J, Turner RJ, Edwards RA.. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal Biochem. 2004; 326(1): 13–20. [DOI] [PubMed] [Google Scholar]
- 25. Jones KM, Hajra AK.. Assay of dlhydroxyacetone phosphate acyltransferase with [32P]DHAP substrate. Clin Chem. 1994; 40(6): 946–947. [PubMed] [Google Scholar]
- 26. Racenis P V., Lai JL, Das AK, Mullick PC, Hajra AK, Greenberg ML.. The acyl dihydroxyacetone phosphate pathway enzymes for glycerolipid biosynthesis are present in the yeast Saccharomyces cerevisiae. J Bacteriol. 1992; 174(17): 5702–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naufer A, Hipolito VEB, Ganesan S, et al.. pH of endophagosomes controls association of their membranes with Vps34 and PtdIns(3)P levels. J Cell Biol. 2018; 217(1): 329–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ames BN, Dubin DT.. Assays of inorganic phosphate, total phosphate and phosphatases. Anal Methods. 1966; 8: 115–118. [Google Scholar]
- 29. Holt CE, Bertsch TW, Ellis HM, Harris WA.. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988; 1(1): 15–26. [DOI] [PubMed] [Google Scholar]
- 30. Hollyfield JG. Differential growth of the neural retina in Xenopus laevis larvae. Dev Biol. 1971; 24(2): 264–286. [DOI] [PubMed] [Google Scholar]
- 31. Stiemke MM, Hollyfield JG.. Cell birthdays in Xenopus laevis retina. Differentiation. 1995; 58(3): 189–193. [DOI] [PubMed] [Google Scholar]
- 32. Chang WS, Harris WA. Sequential genesis and determination of cone and rod photoreceptors in Xenopus. J Neurobiol. 1998; 35(3): 227–244. [PubMed] [Google Scholar]
- 33. Perron M, Kanekar S, Vetter ML, Harris WA.. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998; 199(2): 185–200. [DOI] [PubMed] [Google Scholar]
- 34. Viet J, Reboutier D, Hardy S, Lachke SA, Paillard L, Gautier-Courteille C.. Modeling ocular lens disease in Xenopus. Dev Dyn. 2020; 249(5): 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Acar N, Gregoire S, Andre A, et al.. Plasmalogens in the retina: in situ hybridization of dihydroxyacetone phosphate acyltransferase (DHAP-AT) – the first enzyme involved in their biosynthesis – and comparative study of retinal and retinal pigment epithelial lipid composition. Exp Eye Res. 2007; 84(1): 143–151. [DOI] [PubMed] [Google Scholar]
- 36. Stiemke M, Landers R, Al-Ubaidi M, Rayborn M, Hollyfield J.. Photoreceptor outer segment development in Xenopus laevis: influence of the pigment epithelium. Dev Biol. 1994; 162(1): 169–180. [DOI] [PubMed] [Google Scholar]
- 37. Chiquet C, Dkhissi-Benyahya O, Chounlamountri N, Szel A, Degrip WJ, Cooper HM.. Characterization of calbindin-positive cones in primates. Neuroscience. 2002; 115(4): 1323–1333. [DOI] [PubMed] [Google Scholar]
- 38. Rakshit T, Senapati S, Sinha S, Whited AM, Park PSH.. Rhodopsin forms nanodomains in rod outer segment disc membranes of the cold-blooded Xenopus laevis. PLoS One. 2015; 10(10): e0141114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang S, Johnson KE, Wang HZP.. Blastomeres show differential fate changes in 8-cell Xenopus laevis embryos that are rotated 90 degrees before first cleavage. Dev Growth Differ. 1998; 40(2): 189–198. [DOI] [PubMed] [Google Scholar]
- 40. Dircks LK, Ke J, Sul HS.. A conserved seven amino acid stretch important for murine mitochondrial glycerol-3-phosphate acyltransferase activity. Significance of arginine 318 in catalysis. J Biol Chem. 1999; 274(49): 34728–34734. [DOI] [PubMed] [Google Scholar]
- 41. Zaremberg V, McMaster CR.. Differential partitioning of lipids metabolized by separate yeast glycerol-3-phosphate acyltransferases reveals that phospholipase D generation of phosphatidic acid mediates sensitivity to choline-containing lysolipids and drugs. J Biol Chem. 2002; 277(41): 39035–39044. [DOI] [PubMed] [Google Scholar]
- 42. Bratschi MW, Burrowes DP, Kulaga A, et al.. Glycerol-3-phosphate acyltransferases Gat1p and Gat2p are microsomal phosphoproteins with differential contributions to polarized cell growth. Eukaryot Cell. 2009; 8(8): 1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonzalez-Baró MR, Granger DA, Coleman RA.. Mitochondrial glycerol phosphate acyltransferase contains two transmembrane domains with the active site in the N-terminal domain facing the cytosol. J Biol Chem. 2001; 276(46): 43182–43188. [DOI] [PubMed] [Google Scholar]
- 44. Hajra AK. Dihydroxyacetone phosphate acyltransferase. Biochim Biophys Acta. 1997; 1348: 27–34. [DOI] [PubMed] [Google Scholar]
- 45. Hajra AK, Larkins LK, Das AK, Hemati N, Erickson RL, MacDougald OA.. Induction of the peroxisomal glycerolipid-synthesizing enzymes during differentiation of 3T3-L1 adipocytes. Role in triacylglycerol synthesis. J Biol Chem. 2000; 275(13): 9441–9446. [DOI] [PubMed] [Google Scholar]
- 46. Piano V, Nenci S, Magnani F, Aliverti A, Mattevi A.. Recombinant human dihydroxyacetonephosphate acyl-transferase characterization as an integral monotopic membrane protein. Biochem Biophys Res Commun. 2016; 481(1-2): 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gautier R, Douguet D, Antonny B, Drin G.. HELIQUEST: a web server to screen sequences with specific α-helical properties. Bioinformatics. 2008; 24(18): 2101–2102. [DOI] [PubMed] [Google Scholar]
- 48. Mochizuki T, Masai I.. The lens equator: a platform for molecular machinery that regulates the switch from cell proliferation to differentiation in the vertebrate lens. Dev Growth Differ. 2014; 56(5): 387–401. [DOI] [PubMed] [Google Scholar]
- 49. Costello MJ, Brennan LA, Mohamed A, Gilliland KO, Johnsen S, Kantorow M. Identification and ultrastructural characterization of a novel nuclear degradation complex in differentiating lens fiber cells. PLoS One. 2016; 11(8): e0160785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M.. A function for lipoxygenase in programmed organelle degradation. Nature. 1998; 395(6700): 392–395. [DOI] [PubMed] [Google Scholar]
- 51. Thai TP, Rodemer C, Worsch J, Hunziker A, Gorgas K, Just WW.. Synthesis of plasmalogens in eye lens epithelial cells. FEBS Lett. 1999; 456(2): 263–268. [DOI] [PubMed] [Google Scholar]
- 52. Borchman D, Yappert MC.. Lipids and the ocular lens. J Lipid Res. 2010; 51(9): 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang L, Grami V, Marrero Y, et al.. Human lens phospholipid changes with age and cataract. Invest Ophthalmol Vis Sci. 2005; 46(5): 1682–1689. [DOI] [PubMed] [Google Scholar]
- 54. Mizuno GR, Chapman CJ, Chipault JR, Pfeiffer DR.. Lipid composition and (Na+ + K+)-ATPase activity in rat lens during triparanol-induced cataract formation. Biochim Biophys Acta. 1981; 644(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 55. Hollyfield JG, Rayborn ME.. Photoreceptor outer segment development: light and dark regulate the rate of membrane addition and loss. Invest Ophthalmol Vis Sci. 1979; 18(2): 117–132. [PubMed] [Google Scholar]
- 56. Hsu YC, Chuang JZ, Sung CH.. Light regulates the ciliary protein transport and outer segment disc renewal of mammalian photoreceptors. Dev Cell. 2015; 32(6): 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baker SA, Haeri M, Yoo P, et al.. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008; 183(3): 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mercurio AM, Holtzman E.. Ultrastructural localization of glycerolipid synthesis in rod cells of the isolated frog retina. J Neurocytol. 1982; 11: 295–322. [DOI] [PubMed] [Google Scholar]
- 59. de Vet EC, Van Den Bosch H. Characterization of recombinant guinea pig alkyl-dihydroxyacetonephosphate synthase expressed in Escherichia coli. Kinetics, chemical modification and mutagenesis. Biochim Biophys Acta. 1999; 1436(3): 299–306. [DOI] [PubMed] [Google Scholar]
- 60. Zheng Z, Zou J.. The initial step of the glycerolipid pathway: identification of glycerol 3-phosphate/dihydroxyacetone phosphate dual substrate acyltransferases in Saccharomyces cerevisiae. J Biol Chem. 2001; 276(45): 41710–41716. [DOI] [PubMed] [Google Scholar]
- 61. Zhou Y, Yu N, Zhao J, Xie Z, Yang Z, Tian B.. Advances in the biosynthetic pathways and application potential of plasmalogens in medicine. Front cell Dev Biol. 2020; 8: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bozelli JC, Epand RM.. Plasmalogen replacement therapy. Membranes (Basel). 2021; 11(11): 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Das AK, Holmes RD, Wilson GN, Hajra AK.. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 1992; 27(6): 401–405. [DOI] [PubMed] [Google Scholar]
- 64. Holmes RD, Wilson GN, Hajra A.. Oral ether lipid therapy in patients with peroxisomal disorders. J Inher Metab Dis. 1987; 10: 239–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.