Abstract
The early stages of lentivirus infection of dendritic cells have been studied in an in vivo model. Maedi-visna virus (MVV) is a natural pathogen of sheep with a tropism for macrophages, but the infection of dendritic cells has not been proven, largely because of the difficulties of definitively distinguishing the two cell types. Afferent lymphatic dendritic cells from sheep have been phenotypically characterized and separated from macrophages. Dendritic cells purified from experimentally infected sheep have been demonstrated not only to carry infectious MVV but also to be hosts of the virus themselves. The results of the in vivo infection experiments are supported by infections of purified afferent lymph dendritic cells in vitro, in which late reverse transcriptase products are demonstrated by PCR. The significance of the infection of afferent lymph dendritic cells is discussed in relation to the initial spread of lentivirus infection and the requirement for CD4 T cells.
Maedi-visna virus (MVV) is the prototype virus for the Lentivirus genus of the Retroviridae family (14). MVV shares many characteristics with the lentivirus human immunodeficiency virus (HIV), including the establishment of persistent infection associated with chronic active lymphoproliferation, which in sheep affects primarily the lungs, joints, central nervous system, and mammary glands (7, 12). Unlike the immunodeficiency viruses, MVV does not produce severe immunodeficiency. Given that a good immune response is mounted to MVV (3, 5, 6), this makes the persistence of the virus and its inevitable fatality more surprising.
The recovery of virus from infected sheep has always been from cells of the monocyte/macrophage lineage. These are the classical cell hosts in vivo for MVV (22, 23, 52), although using in situ PCR amplification and RNA in situ hybridization techniques, MVV DNA and RNA have been identified in other cell types, including bronchiolar and mammary epithelial cells (8, 68; C. G. Vitali, E. Sanna, G. Braca, L. Boreo, G. Rossi, and A. Leoni, Abstr. 3rd European Workshop on Ovine and Caprine Retroviruses, abstr. 23, 1997). There is one report which suggests that blood dendritic cells may be infected with MVV (23).
In vitro and in vivo infection of dendritic cells with HIV is now firmly established, and evidence indicates that dendritic cell infection is important in the development of protective immune responses, in the spread and persistence of virus, and in the immune dysfunction which characterizes AIDS (11). Dendritic cells are central players in the initiation of immune responses, being the most potent of antigen-presenting cells and also being necessary for the priming of native T cells. Immature dendritic cells strategically patrol the peripheral tissues, where they are specialized to acquire antigens. As they migrate to the draining lymph nodes, they mature into effective antigen-presenting cells and consequently can present an antigenic snapshot of the periphery to T cells in the draining lymph nodes. Use of a rhesus macaque model to study early infection events with the lentivirus simian immunodeficiency virus indicates that dendritic cells may be the first cells to encounter the virus and become infected and are the primary cells for dissemination (67). Infection of dendritic cells with MVV would help to provide insight into the failure of the immune response to clear virus and the initial dissemination of infection into lymphoid and peripheral tissues.
We chose to investigate the possible infection of the afferent lymph subset of dendritic cells because these are the most relevant type with regard to early peripheral infection and the initial establishment of an immune response. In addition, relatively large numbers of these cells can be purified ex vivo by afferent lymph cannulation, eliminating the need for artificial culture and minimizing the potential for the induction of phenotypic changes. Efferent cannulation of prefemoral and popliteal lymphatics is an established model to study the early events in lymphoid tissue following MVV infection (5). By cannulating pseudoafferent lymphatic vessels, we were able to infect sheep subcutaneously and intradermally in the drainage area and directly sample the flow of dendritic cells migrating in afferent lymph on their way to the local lymph node. This in vivo model maximizes the physiological and immunological relevance of the data.
MATERIALS AND METHODS
Experimental animals and in vivo infections.
Adult Finnish Landrace crossed sheep, 2 to 5 years old, were supplied by the Moredun Research Institute, Edinburgh, Scotland. All sheep used were tested MVV seronegative prior to starting the experiments. The prefemoral lymph nodes were surgically removed, and no less than 8 weeks later the consequent pseudoafferent lymphatic vessel was surgically cannulated (29, 35). After a minimum of 4 days of postoperative recovery, sheep were inoculated subcutaneously and intradermally in the prefemoral lymph node drainage area with 106 50% tissue culture infective doses (TCID50) of autologous MVV. Afferent lymph was collected two to three times daily, and cell populations were analyzed over the period of patent cannulation by flow cytometry, cocultivation assays, immunocytochemistry, and in situ hybridization.
Autologous virus production.
Autologous virus was prepared by infecting autologous ovine skin cell monolayers derived from individual sheep skin biopsies with MVV strain EV1 (63) as described elsewhere (3). The titer of each virus stock was determined on skin fibroblasts, and the concentration was calculated by the quantal method of Reed and Muench (51). Heat-inactivated virus was prepared by incubation at 56°C for 30 min, and inactivation was confirmed by virus titration.
Cocultivation assays.
Infectious virus was detected by cocultivation of serial dilutions of afferent lymph cells or lymph plasma on ovine skin fibroblast monolayers in Dulbecco modified Eagle medium with 5% fetal calf serum (FCS) as described previously (3). Infection was scored by the presence of syncytia, and representative samples were also stained for MVV Gag p15 by immunocytochemistry using monoclonal antibody 415 (kindly donated by D. J. Houwers), detected with peroxidase-conjugated rabbit immunoglobulins (Ig) to mouse Ig (Dako) and 3-amino-9-ethyl carbazole for color development. The nature of the samples meant that it was not always possible to use the same number of starting cells in the serial dilutions; therefore, the sensitivity of the assay varied. Where no infectious virus was detected, the sensitivity of the assay is expressed as the maximum TCID50 which might have been detected if the dilution series had commenced with one serial dilution fold more cells and if 100% of wells with these cells contained virus.
Metrizamide density gradient.
Afferent lymph samples were enriched for large granular cells by centrifuging the cell suspension over a discontinuous gradient of 14.5% (wt/vol) metrizamide (Nyegaard) at 800 × g for 30 min at 4°C (10, 28). Low-density cells at the interface were harvested and used as a source of partially purified dendritic cells, referred to as metrizamide gradient cells.
Immunocytochemistry and in situ hybridization.
Cytospins of afferent lymph cells were paraformaldehyde fixed and blocked in phosphate-buffered saline (PBS) with 0.01% Tween 80, 2% normal rabbit serum, and 2% normal sheep serum before incubation with primary monoclonal antibody. After three washes in PBS–0.01% Tween 80, the slides were incubated with alkaline phosphatase-conjugated rabbit Ig to mouse Ig, and a color reaction was developed with Sigma fast red. Monoclonal antibodies used were VPM19 for major histocompatibility complex (MHC) class I (31) and CC20 for CD1b, generously provided by Chris Howard, Institute for Animal Health, Compton, United Kingdom (37). Digoxigenin-labeled sense and antisense riboprobes were prepared from a PCR-amplified fragment of 1514 MVV gag kindly given by Franziska Lechner, Institute of Veterinary Virology, University of Bern (bases 822 to 1564 [66]) and from a PCR product of tat EV1 MVV (MVV EV1 bases 5696 to 5981 [63]). In situ hybridization was performed as described previously (43) except that proteinase K was used at a concentration of 10 μg/ml, the tat probe was used at 0.1 ng/ml, and the gag probe was used at 0.5 ng/ml. Sense probes were always included as negative controls and always produced no signal from the samples.
Nonspecific esterase staining.
Cells were cytocentrifuged, then formaldehyde-acetone fixed, and stained for nonspecific esterase activity using the active diazonium salt hexazotized pararosaniline and α-naphthyl acetate (39). Cell nuclei were counterstained with 2% chloroform-washed methyl green.
Flow cytometry and FACS analysis.
Cells were washed in PBS with 0.1% bovine serum albumin and 0.01% sodium azide (omitted for fluorescence-activated cell sorting [FACS]). They were blocked in 10% normal rabbit serum in PBS for 30 min prior to incubation with primary antibody and/or biotinylated antibody for 40 min on ice. After two washes, cells were incubated in isotype-specific, fluorescein isothiocyanate (FITC)-conjugated, anti-mouse antibodies for 20 min and/or streptavidin-phycoerythrin. FACS analysis was performed on a Becton Dickinson FACSort using CellQuest software. FACS sorting was performed on Becton Dickinson FACStar and FACStarplus sorters. Dead cells and erythrocytes were excluded using forward scatter and side scatter electronic gating. Analysis gates for positive staining were set using isotype control antibodies to indicate background staining and autofluorescence. Monoclonal antibodies used were ST4 for CD4 (47), SBU-T8 for CD8 (50), DU2-104 as a pan-B-cell marker (49), CC98 for WC6, a marker known to be expressed on ovine afferent dendritic cells (16), OM1 for CD11c α chain (26, 53), 3.29 for the mannose receptor (a kind gift from A. Lanzavecchia) (62), VPM54 for MHC class II DR α chain (17), VPM36 for MHC class II DQ α chain (17), VPM19 for MHC class I heavy chain (31), CC20 for CD1b (37), and VPM65 for CD14 (25).
In vitro infection and PCR.
Cells were resuspended to 106 cells/ml in DNase-treated MVV EV1 (0.06 TCID50/cell) in medium with 2% FCS. They were incubated at 37°C for 2 h before the cells were diluted to 1.9 × 105 cells/ml in medium containing 10% FCS and returned to the incubator for a further 24 or 48 h.
The medium for afferent lymph cells was supplemented with 10% lymph node conditioned medium prepared as described elsewhere (28). Cells were harvested into PCR lysis buffer (50 mM KCl, 10 mM Tris-HCl [pH 8.3], 2.5 mM MgCl2, 0.1 mg of gelatin/ml, 0.45% Nonidet P-40, 0.45% Tween 20, 10 μg of proteinase K/ml) and incubated for 60 min at 56°C. DNA was extracted from the cell lysates by phenol-chloroform extraction, and the concentration was determined using a PicoGreen double-stranded DNA (dsDNA) quantitation reagent kit from Molecular Probes Inc.
Nested PCR was performed in reaction mix volumes of 20 μl with deoxynucleoside triphosphates at a final concentration of 0.225 mM each, MgCl2 at 2 mM, Taq DNA polymerase at 0.04 U/μl, and each primer at a final concentration of 1 μM. For the first-round PCR, the primers (ACTGTCAGG[A/G]CAGAGAACA[A/G]ATGCC, EV1 nucleotide positions 8914 to 8938, and CTCTCTTACCTTACTTCAGG, complementary to nucleotides 328 to 309 ;[[57;]]), deoxynucleoside triphosphates, and template DNA were heated to 94°C for 1 min 30 s and then held at 80°C while the remaining reaction components were added. This was followed by 30 cycles of 94, 55, and 72°C consecutively, each for 30 s. Then 1 μl from the first reaction was used as the DNA template in the second round of PCR, which used primers AAGTCATGTA(G/T)CAGCTGATGCTT (9049 to 9071) and TTGCACGGAATTAGTAACG (129 to 111) and consisted of 94°C for 1.5 min followed by 30 cycles of 94, 50, and 72°C consecutively, each for 30 s.
Monocyte-derived macrophages.
Monocyte-derived macrophages were prepared from ovine blood and maintained in culture as described elsewhere (45). After 5 days in culture, some cells were harvested as uninfected controls and cytospin preparations were made. Others were infected as described above for PCR. To produce heavily infected controls for immunocytochemistry and in situ hybridization, macrophages were infected with MVV EV1 at a multiplicity of infection of 0.5 TCID50/cell in medium with 2% FCS. After a 2-h incubation at 37°C, additional full maintenance medium was added; cells were monitored for 4 to 5 days before being harvested for cytospins.
RESULTS
Presence of cell-associated MVV in afferent lymph.
The model being used is subcutaneous and intradermal infection of sheep with MVV. This is known to cause infection of the draining lymph node, indicating transport of virus from the skin to the lymph node in the afferent lymph (5). To verify this route and to establish whether virus was cell associated and/or free in lymph plasma, the presence of infectious virus in afferent lymph was monitored by cocultivation of dilutions of lymph plasma or afferent lymph cells with monolayers of indicator ovine skin fibroblasts.
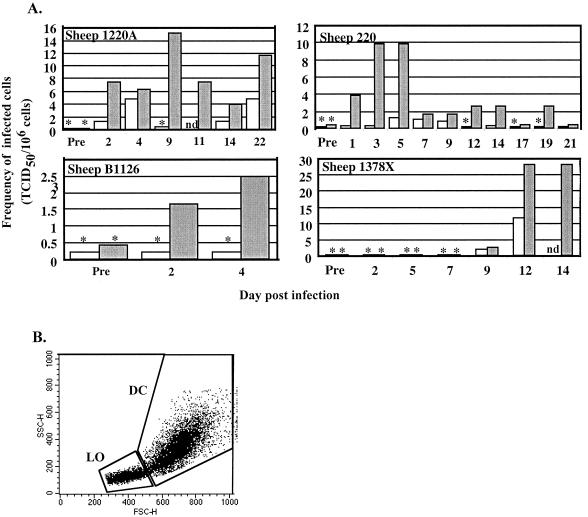
Cell-associated infectious virus was detected in six acutely infected sheep. Figure 1A depicts the data from four sheep over variable periods for which cannulae remained patent. Cells were separated by metrizamide density gradient centrifugation for analysis of the infectious cell populations. Regardless of individual variation in titers and time to appearance of infectivity, in all instances an enrichment for large, granular low-density cells by a metrizamide gradient increased the virus titer (Fig. 1A). Afferent lymph contains memory T lymphocytes, few B cells, up to 10% veiled dendritic cells, and some macrophages (10, 48). Veiled dendritic cells represent the dendritic cell population migrating from the skin. The low-density fraction of cells from the metrizamide gradient purifies both dendritic cells and macrophages (40).
FIG. 1.
MVV is associated with large cells in afferent lymph. (A) Sheep with afferent lymph cannulations were infected with MVV; at variable times postinfection, afferent lymph cells were purified by metrizamide gradient centrifugation. Whole afferent lymph cells and large cells from the metrizamide gradient fraction were analyzed for the presence of infectious virus by cocultivation with indicator skin cells. The frequency of infected cells is shown versus time postinfection. Open histogram, whole afferent lymph cells; closed histogram, metrizamide gradient cells; ∗, no detectable virus, but bar indicates sensitivity of assay, i.e., maximum level of infectivity which theoretically could have been present but undetected; nd, not determined; Pre, samples analyzed preinfection. (B) Forward and side scatter (FSC and SSC) flow cytometry profile of metrizamide gradient afferent lymph cells, with the lymphocyte (LO) and large, granular cell (DC) analysis gates indicated.
The afferent lymph cell populations were analyzed by flow cytometry. Small lymphocytes and larger granular dendritic cells and macrophages were separated using electronic gates, allowing the proportion of each major lymphoid and myeloid population within samples to be determined. A representative flow cytometry plot is shown in Fig. 1B, with the electronic gating indicated. The proportions of large granular cells in unfractionated afferent lymph (6.8% ± 2.8%) and in the low-density fraction of cells from a metrizamide gradient (35.2% ± 16.0%) varied among sheep and samples (data from three sheep). When the frequency of infected cells is adjusted for the percentage of large granular cells in any given sample and plotted for both unfractionated and fractionated afferent lymph, there is good correlation between frequency of infection and proportions of large granular cells (analyzed by linear least squares regression, P < 0.001). The titer of infectious virus in subsets of any one sample was therefore directly related to the proportion of large granular cells in those subsets and suggests that virus in afferent lymph was associated exclusively with large granular cells.
Free infectious virus was never detected in afferent lymph plasma even at times when cell-associated virus was present (data not shown).
Afferent lymph dendritic cells carry infectious MVV.
To investigate whether MVV was associated with the dendritic cells in afferent lymph and/or the small population of macrophages which coenrich on a metrizamide gradient, further phenotypic characterization was required. Afferent lymph cells from MVV-seronegative sheep, purified and electronically gated for large granular cells as in Fig. 1B, were analyzed by flow cytometry for expression of a variety of cell surface antigens found on dendritic cells, macrophages, and B and T lymphocytes. Table 1 shows that the majority of cells in the analysis region expressed the cell surface repertoire characteristic of dendritic and macrophage cells (CD1b, CD11c, MHC class I and II, and WC6; some expressed mannose receptor and CD14), with little expression of T or B lymphocyte markers. It is known that dendritic cells and macrophages share many cell surface antigens such as MHC class II, CD11c, and mannose receptor (26, 27, 62). However, ovine afferent lymph dendritic cells express CD1b but very little CD14 (32, 34), while ovine macrophages express little or no CD1b but low to high levels of CD14 (25, 44, 60).
TABLE 1.
Expression of different surface markers on large granular afferent lymph cells
| Surface marker | No. of replicates screened | % Positive cells (rangea or mean ± SD) |
|---|---|---|
| CD4 | 3 | 0.0–10.1 |
| CD8α/β | 3 | 0.0–14.9 |
| Pan-B cell | 3 | 0.0–7.2 |
| WC6 | 2 | 63.9–78.4 |
| CD11c | 3 | 51.3–89.0 |
| Mannose receptor | 3 | 27.6–41.2 |
| MHC class II DR | 3 | 83.8–98.8 |
| MHC class II DQ | 3 | 83.2–98.7 |
| MHC class I | 10 | 94.2 ± 4.3 |
| CD1b | 10 | 66.0 ± 8.5 |
| CD14 | 10 | 23.9 ± 15.2 |
Due to variability in cell populations, where n ≤ 3 the results are shown as a range.
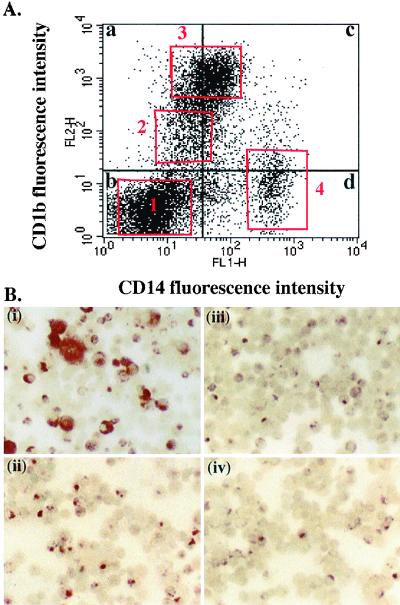
Metrizamide gradient cells were double stained for CD14 and CD1b expression, and the large granular cells were analyzed (Fig. 2A). Four populations were identified: CD14− CD1b−, CD14− CD1blo, CD14lo CD1bhi, and CD14hi CD1b−/lo. These varied slightly in proportion and staining intensity from sheep to sheep, but the mean percentages of large granular metrizamide gradient cells in the four populations were 16.4 ± 6.0, 15.4 ± 4.0, 54.3 ± 11.2, 4.1 ± 4.0, respectively (values from six sheep). We considered the CD1blo and CD1bhi populations to be dendritic cells and the CD14hi population to be macrophages. This left an unknown population of large granular cells which was both CD1b− and CD14−.
FIG. 2.
Phenotype of dendritic cells and macrophages. (A) Metrizamide gradient afferent lymph cells stained for CD14 and CD1b were analyzed in the large, granular cell gate (Fig. 1) for expression of these markers. CD14− CD1b− cells (box 1; 26.7% of cells), CD14− CD1blo cells (box 2; 7.5% of cells), and CD14lo CD1bhi (box 3; 36.2% of cells) were considered dendritic cells, and CD14hi CD1b−ve cells box 4 (12.8% of cells) were considered to be macrophages. (B) Afferent lymph cell populations were separated by FACS using the electronic gates indicated by the quadrant and box markers in panel A. Cytospins of (i) CD14+ (quadrants c and d), (ii) CD14− (quadrants a and b), (iii) CD14lo CD1bhi (box 3), and (iv) CD14− CD1blo (box 2) FACS-sorted cells were stained for nonspecific esterase activity.
Cells from afferent lymph considered to be dendritic cells are heterogeneous with respect to nonspecific esterase activity, being negative or displaying a reticular or punctate pattern of staining (20). In contrast, afferent lymph macrophages display a high level of nonspecific esterase activity throughout the cytoplasm. Metrizamide gradient cells from afferent lymph were separated by FACS into CD14+ and CD14− populations. Cytospin preparations of these cells were stained for nonspecific esterase activity. Macrophages were found only in the CD14+ fraction [Fig. 2B(i)], while dendritic cells were found in both the CD14+ and CD14− populations [Fig. 2B(ii)]. FACS separated CD14− CD1blo and CD14lo CD1bhi populations from afferent lymph both had exclusively dendritic cell patterns of nonspecific esterase activity [Fig. 2B(iii) and (iv)].
The phenotypic analysis of metrizamide gradient ovine afferent lymph cells shown in Table 1, together with assessment of the morphology of cells stained as cytospin preparations (Fig. 2B), supports the dendritic cell and macrophage classification with CD1b and CD14 (Table 2) and suggests that the unknown CD14− CD1b− population is also dendritic cells. In conclusion, the data demonstrate that ovine afferent lymph dendritic cells can be categorized as three subpopulations: CD14− CD1b−, CD14− CD1blo, and CD14lo CD1bhi. Furthermore, they are distinguishable from afferent lymph macrophages, which are CD14hi CD1b−/lo. Table 2 summarizes the phenotypic criteria established to distinguish dendritic cells from macrophages in afferent lymph.
TABLE 2.
Phenotypic classification for afferent lymph macrophages and dendritic cells
| Afferent lymph cells | Characteristic
|
|||
|---|---|---|---|---|
| Size/granularity | Nonspecific esterase activity | Expressiona
|
||
| CD1b | CD14 | |||
| Dendritic cells | Large/granular | Negative or positive with reticular/punctate distribution | (1) negative, (2) low, (3) high | (1) and (2) negative, (3) low |
| Macrophages | Large/granular | High with homogeneous distribution in cytoplasm | (4) negative or low | (4) high |
Numbers in parentheses refer to FACS analysis boxes shown in Fig. 2A.
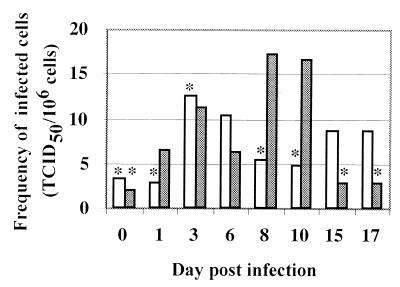
During an in vivo infection, the ability of dendritic cells to carry MVV from tissue to the draining lymph node was assessed by FACS sorting of afferent lymph cells using CD1b and CD14 expression. No functional assay was used to define these cells. CD14− CD1blo and CD14lo CD1bhi dendritic cells were analyzed for infectious virus. Both populations were associated with virus. The highest frequencies of infected cells were detected in the CD1bhi population, and more samples of CD1bhi than of CD1blo cells were positive for virus in this animal (Fig. 3). Infected cells were never detected in cocultures performed using any cell populations from seronegative animals prior to inoculation with MVV (e.g., preinfection or day 0 samples in Fig. 1A and 3). Dendritic cells were therefore involved in the transport of MVV from the periphery to the lymph node.
FIG. 3.
MVV is associated with dendritic cells in afferent lymph. Afferent lymph dendritic cells from a sheep infected with MVV subsequent to cannulation were purified by metrizamide gradient centrifugation followed by FACS sorting. CD14− CD1blo and CD14lo CD1bhi dendritic cells were analyzed for the presence of infectious virus by cocultivation with indicator skin cells. The frequency of infected cells is shown versus time postinfection. Open histogram, CD14− CD1blo dendritic cells; closed histogram, CD14lo CD1bhi dendritic cells; ∗, no detectable virus, but bar indicates sensitivity of assay, i.e., maximum level of infectivity which theoretically could have been present but undetected. Day 0 samples were taken before infection. Mean purity of FACS-separated cells was 91% (range of 67.1 to 97%) after adjustments for autofluorescence and spectral overlap.
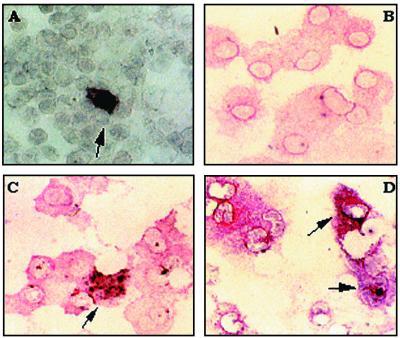
To determine whether dendritic cells were infected with as opposed to carrying virus, we chose to analyze cells for viral mRNA expression by in situ hybridization. This individual cell analysis was preferred due to the low frequency of infectious cells, e.g., 10 to 15 TCID50/106 cells seen in the in vivo infection. Initially we looked at FACS-separated CD14− dendritic cells for both tat and gag RNA expression. Both riboprobes used will detect genomic RNA as well as viral mRNA; however, tat may be expressed earlier in the replication cycle than gag (64) and therefore was considered appropriate for this acute infection model. Cells which were strongly positive for gag (Fig. 4A) or tat (data not shown) RNA were seen. In a total of 3 × 106 cells analyzed during the course of an infection, viral RNA was detected at a rate of approximately 0.3 positive cells per 105 CD14− dendritic cells. This was consistent with the mean virus titer measured by cocultivation assays from the same samples of 0.11 TCID50/105 cells. Due the limited number of samples tested, we are unable to say whether there were more tat than gag RNA-positive cells and therefore whether the tat probe was more sensitive in this infection model. Preinfection samples were always negative with either antisense probe used. Cells were double stained by immunocytochemistry for CD1b to define dendritic cells in unsorted populations used in the in situ hybridizations. The double-staining technique was verified using in vitro MVV-infected macrophages and an antibody detecting MHC class I. The sense tat riboprobe did not produce any signal on heavily infected macrophages (Fig. 4B), while clear signal was seen with the antisense probe at the same time as MHC class I expression (Fig. 4C). The infrequent tat RNA-positive cells detected in afferent lymph from in vivo-infected sheep were shown to be CD1b-positive dendritic cells (Fig. 4D).
FIG. 4.
MVV RNA expression in afferent lymph cells. (A) Cytospins of CD14− FACS-separated afferent lymph dendritic cells from MVV-infected sheep were probed for gag mRNA by in situ hybridization using a digoxigenin-labeled gag riboprobe. Positive controls were skin fibroblasts heavily infected in vitro with MVV; as negative controls, all samples were hybridized with a sense gag riboprobe (data not shown). (B to D) Cytospins of metrizamide gradient cells from MVV-infected sheep were immunostained for CD1b and probed for tat RNA by in situ hybridization using a digoxigenin-labeled riboprobe. MVV-infected monocyte-derived macrophages, used as controls, were immunostained for MHC class I and for tat RNA. Specificity was verified using an isotype control antibody for the immunostaining (data not shown); for the in situ reaction, all samples were hybridized with a sense tat riboprobe. Red staining indicates positive signal for CD1b or MHC class I, and black staining indicates a positive signal from the in situ hybridization reaction. (B) MVV-infected macrophages stained for MHC class I expression and hybridized with sense tat control riboprobe. (C) MVV-infected macrophages stained for MHC class I expression and hybridized with antisense tat riboprobe. (D) Metrizamide gradient afferent lymph cells stained for CD1b expression and hybridized with antisense tat riboprobe. Arrows indicate double-stained cells.
Infection of afferent lymph dendritic cells in vitro.
Theoretically both the gag and the tat riboprobe could have detected genomic viral RNA or DNA. Although unlikely, it remained a possibility that the dendritic cells were not infected but were passively carrying virions. As the time at which virus was detected in afferent lymph cells was variable and the virus titers were low, an in vitro infection system was chosen to assay for late reverse transcription products. During lentivirus replication these are produced only by virions which have started replication inside a host cell, unlike some of the intermediate DNA products of viral replication, which may be detected in extracellular HIV virions (71). Nested PCR was used to amplify a 203-bp sequence of MVV proviral DNA from the 5′ long terminal repeat region. Since the first-round primers span the primer binding site, PCR amplification can occur only when proviral synthesis is almost complete. The presence of a 203-bp product after PCR therefore indicates that virus has uncoated and initiated replication.
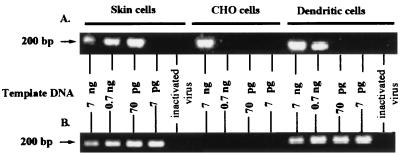
CD14lo CD1bhi dendritic cells separated by FACS from the afferent lymph of uninfected sheep were infected in vitro with a low multiplicity of infectious virus (DNase treated) and incubated for 24 or 48 h. Cells were harvested, and the DNA was extracted for use in the nested PCR described above to detect late reverse transcription products. The assay was quantitated by using serial dilutions of template cellular DNA. Ovine skin cell fibroblasts which are permissive for MVV infection and nonpermissive Chinese hamster ovary (CHO) cells were used as controls for the infection and PCR. CHO cells do not express detectable MVV receptor, as determined by fusion assay with cells expressing MVV env (L. Tiley, personal communication). At 24 h postinfection, skin cells showed detectable viral DNA at 70 pg of input cellular DNA (Fig. 5A). Dendritic cells had detectable viral DNA at 700 pg of input cellular DNA (Fig. 5A). CHO cells did show consistently a viral DNA band at 7 ng of template DNA after 24 h (Fig. 5A), which disappeared by 48 h postinfection (Fig. 5B). This viral DNA band is considered to be genomic viral DNA from high template input. At 48 h, both the skin cells and the dendritic cells showed increased levels of viral DNA per cell equivalent (Fig. 5B). These results were consistent both within and between sheep (two different days' samples from two individual sheep analyzed). Therefore, the level of reverse transcription increased with time postinfection in afferent lymph dendritic cells.
FIG. 5.
In vitro infection of afferent lymph dendritic cells by MVV. CD14lo CD1bhi dendritic cells were separated by FACS from afferent lymph, and skin and CHO cells were harvested from stocks in culture. Cells were infected with DNase-treated MVV (0.06 TCID50/cell) for 2 h, and then additional medium was added for overnight (A) or 48-h (B) incubation. DNA was extracted from the cells, quantified with a PicoGreen dsDNA quantitation reagent kit, and used as template at the given amounts (7 ng to 7 pg) in a nested PCR. Heat-inactivated virus was noninfectious, as determined by cocultivation, and was used at preinactivation titers as above. For these controls, 7 ng of template DNA was used. The expected size for the PCR-amplified early reverse transcription product of EV1 MVV is 203 bp. These results are representative of four experiments using two different sheep. The percentage purity of FACS-separated dendritic cells was 93.1% after the deduction of backgrounds due to autofluorescence and spectral overlap.
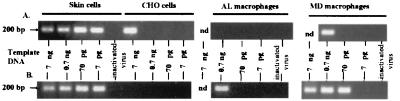
To determine if macrophages behaved in a similar manner, both monocyte-derived and afferent lymph macrophages (CD14hi CD1b−) were infected in vitro and analyzed using the same PCR. Monocyte-derived macrophages had increased levels of viral DNA per cell equivalent with time (Fig. 6). Afferent lymph macrophages were always a minor population, and very low numbers were recovered from FACS separation. The starting input cellular DNA was therefore 0.7 ng rather than 7 ng. At 0.7 ng of input cellular DNA, viral DNA was not detectable at 24 h postinfection but was present by 48 h postinfection (Fig. 6), again showing increased levels of viral reverse transcription with time in the macrophage population. Analysis of the FACS-separated dendritic cell populations for purity indicated that the proportion of macrophages which could have contaminated them (Fig. 5) was less than 5% (data not shown). Therefore, as the dendritic cells showed viral DNA in up to 7 pg of input cellular DNA while the afferent lymph macrophages showed viral DNA only at 700 pg of input cellular DNA, the dendritic cells must be one of the infected cell populations: the macrophages could not account for all the signal seen in dendritic cell samples.
FIG. 6.
In vitro infection of afferent lymph and monocyte derived macrophages. CD14hi CD1b−/lo macrophages were separated by FACS from afferent lymph (AL macrophages), and macrophages were derived in culture from ovine peripheral blood monocytes (MD macrophages). Skin and CHO cells were harvested from stocks in culture. Cells were infected with DNase-treated MVV (0.06 TCID50/cell) for 2 h, and then additional medium was added for overnight (A) incubation or a 48-h (B) incubation. DNA was extracted from the cells, quantified with a PicoGreen dsDNA quantitation reagent kit, and used as template at the given amounts (7 ng to 7 pg) in a nested PCR. Heat-inactivated virus was noninfectious, as determined by cocultivation, and was used at preinactivation titers as above. For these controls, 7 ng (skin cells and CHO cells) or 0.7 ng (AL macrophages and MD macrophages) of template DNA was used. The expected size for the PCR-amplified early reverse transcription product of EV1 MVV is 203 bp. This experiment is representative of four experiments using two different sheep. FACS-separated afferent lymph macrophages were 95.8% pure after deduction of backgrounds for autofluorescence and spectral overlap. nd, not done.
DISCUSSION
The results presented above show that dendritic cells form an important route for transfer of MVV from the site of infection to lymphoid tissue. The dendritic cells not only carry virus but are infected with replicating virus. This was shown by in situ hybridization of in vivo-infected dendritic cells and by PCR amplification of proviral DNA from ex vivo-infected dendritic cells.
This is a novel finding within the MVV field, where isolation and analysis of dendritic cells has been hampered by the lack of markers for ovine dendritic cells. Other investigators characterizing ovine afferent lymph dendritic cells by FACS have not definitively separated these cells from contaminating tissue macrophages. Functional studies have used the low-density large granular cells from afferent lymph as dendritic cell populations, ignoring macrophage contamination (10, 30, 33). As MVV has a known tropism for macrophages, it was necessary in our studies to definitively separate and identify dendritic cells. We have characterized afferent lymph dendritic cells into three distinct populations and distinguished these from macrophages. Ovine macrophages have been characterized by their expression of CD14 (25) and CD11b (27). Here we have used CD14 to differentiate monocytes/macrophages from dendritic cells. Using CD1b and CD14 cell staining, we have managed to sort to high purity afferent lymph dendritic cells. These cells are always large granular cells with high autofluorescence. This means that estimates of purity used 5% background gates, and therefore purity of >95% will never be achieved. However, both double staining by in situ hybridization for viral products and marker expression and infection of purified afferent lymph macrophages show that in the population studied, dendritic cells constituted a major source of virus.
There has been one report suggesting that blood dendritic cells may be infected with MVV (23). These investigators took peripheral blood mononuclear cells (PBMCs) from MVV-infected sheep, depleted them of specific cell subsets using adherence, nylon wool, and panning, and measured cell-associated infectivity in infectious center assays. Depletion of nonadherent MHC class II CD45RA+ cells had the greatest effect in reducing the infectivity of nonadherent PBMCs more than 100-fold (23). The conclusion that the dendritic cell and not the monocyte is the predominant MVV-infected cell type in blood assumed that ovine dendritic cells are CD45RA+ by analogy with human blood dendritic cells (70). The authors used negative selection and an assay based on a reduction in infectivity to identify dendritic cells. The infectivity data for most sheep tested were incomplete, and the infectivities of nonadherent PBMCs and nonadherent PBMCs depleted of MHC class II and CD45RA+ cells were compared in only one animal.
The phenotypic heterogeneity of ruminant afferent lymph dendritic cells has been reported by other investigators (15, 20, 30, 36). Previously, four subpopulations of ovine afferent lymph dendritic cells were defined by CD1 and Fc receptor expression (30); therefore, our finding of three dendritic cell populations by CD1b and CD14 staining is not surprising. Subsets of bovine afferent lymph dendritic cells defined by CD11a and the novel bovine antigen MyD-1, which is a member of the SIRP family of signal regulatory binding proteins and mediates binding to CD4+ T cells, differ in the ability to stimulate T cells (9, 38). Subsets of ovine afferent lymph dendritic cells have not been functionally subdivided, and so it is not known what significance to attribute to the more consistent infection of CD14lo CD1bhi dendritic cells than of CD14− CD1blo cells (Fig. 3).
The highest level of infection in purified macrophage or dendritic cell populations from in vivo-infected animals was <0.1%. This is consistent with the results of other workers using a range of tissue samples and suggests that factors which confine infection to a small minority of cells are also acting in afferent lymph (3, 46, 55). The quantitative and qualitative aspects of HIV infection of dendritic cells in vivo has been the subject of many opposing opinions (11). However, it seems likely that dendritic cells not only act as a reservoir of infection, passing virus to the T cells which they activate, but also stimulate the protective CD4 and CD8 immune responses which characterize asymptomatic infection (41). The low percentage of dendritic cells infected by MVV in vivo in this study precluded functional studies. As gross immunodeficiency is not a feature of MVV infection, any functional defects in infected dendritic cells would probably relate specifically only to MVV.
Afferent lymph plasma from three sheep was assayed for infectious virus by cocultivation and was negative at all time points, including those where cell-associated infectious virus was demonstrated (data not shown). Diluting stock virus to a known titer with autologous serum or lymph plasma for 30 min at room temperature completely abrogated infectivity (data not shown). This effect was not seen in the controls where tissue culture medium was used as a diluent. These results are consistent with the established fact that MVV is predominantly a cell-associated virus and free virus has not been detected in the efferent lymph of experimentally infected animals (3) or in the serum of naturally infected sheep (8), although there are reports of free MVV in cerebrospinal fluid and synovial fluid (8, 55). The antiviral elements of serum and lymph plasma have not been identified for MVV. Possible candidates include collectins such as mannose binding protein and bovine conglutinin, which can both bind to HIV gp120 and inhibit virus infection of T cells (1, 19). Activation of the alternative complement pathway is thought to occur with vesicular stomatitis virus, measles virus, and respiratory syncytial virus and may occur with MVV.
The absence of infectious free virus in fluids from inoculated tissue emphasizes the importance of dendritic cell infection in the initial spread of MVV. As ubiquitous patrollers of the periphery, dendritic cells in lungs would probably perform the same function when sheep become infected through the intranasal route. Afferent lymph dendritic cells migrate to the lymph nodes, where they become short-lived interdigitating dendritic cells in the T-cell paracortex, sited to engage and sample the large number of naive and memory T cells circulating through the node. Evidence from our previous studies indicates that these CD4 T lymphocytes are necessary for transfer of virus from dendritic cells to the macrophages which leave the node in the efferent lymph (18). The mechanism for this is not known, but T cell-dendritic cell interactions are two way: just as dendritic cell activation of T cells is required for productive T-cell infection with HIV (56, 57), so perhaps T-cell enhancement of dendritic cell activation status (2, 13, 61, 65) is necessary to stimulate complete MVV replication in dendritic cells and enable transfer of infection to macrophages. Our in vitro PCR assays have established that MVV can commence replication in afferent lymph dendritic cells, and the cocultivation assays confirmed that in vivo-infected dendritic cells can transfer infection to skin cell lines in vitro. In the absence of CD4 T cells, in vitro culture is known to greatly enhance the levels of infection in macrophages (21), and therefore there remains the possibility that full production of virions in vivo in dendritic cells requires CD4 T cells. In mature dendritic cells, HIV reverse transcription is commenced but not completed and viral transfer is dependent on T-cell interaction (24, 69). Our preliminary evidence suggests that in vitro, MVV replication in dendritic cells is faster in metrizamide gradient cell preparations than in purified dendritic cell populations. The two differ mainly by the presence of memory lymphocytes (data not shown).
It has previously been observed that MVV-infected sheep fail to mount an IgG2 response to the virus (4) and also that such animals show a reduced cutaneous delayed-type hypersensitivity response (58). The definitive identification of MVV-infected afferent lymph dendritic cells presented here could provide an explanation for these observations if the interaction between dendritic cells harboring MVV and CD4 helper T cells was defective in stimulating a Th1 response. It has been shown that infection with a related virus, caprine arthritis encephalitis virus, dysregulates cytokine expression in macrophages (42). The absence of antibody-dependent cellular cytotoxicity to MVV-infected cells (59) may reflect the lack of MVV-specific IgG2 antibodies (Inderpal Singh, personal communication) and represent a mechanism for viral persistence. Dendritic cells could further contribute to viral persistence and spread if the reservoir of infected bone marrow cells which have been described by expression of an undefined macrophage antigen (22) are in fact hematopoietic precursors of both monocytes and dendritic cells (54).
The ability to cannulate sheep and study fresh afferent lymph dendritic cells draining the site of an experimental MVV infection has enabled us to identify these cells as targets for the virus in vivo. This approach maximizes the immunological and pathological relevance of the findings, both in minimizing phenotypic changes induced by culture of dendritic cells and in studying the acquisition of infection in the context of the whole-animal model.
ACKNOWLEDGMENTS
This work was supported by Wellcome Trust program grant 035157. Susanna Ryan was funded by a veterinary fellowship from the BBSRC.
We thank Kristina Eriksson, Elizabeth McInnes, and Paul Tonks for invaluable assistance. We also thank Ray Hicks and Nigel Miller for FACS sorting.
REFERENCES
- 1.Anderson O, Sorensen A-M, Holmskov U, Friischristiansen P, Thiel S, Svehag S-E, Bahraoui E, Gluckman J C, Fenouillet E. Bovine conglutinin binds the HIV envelope glycoprotein gp160 and inhibits its binding to cell-membrane CD4. Scand J Immunol. 1990;32:382. [Google Scholar]
- 2.Bennett S R M, Carbone F R, Karamalls F, Flavells R A, Miller J F A P, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature (London) 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 3.Bird P, Blacklaws B, Reyburn H T, Allen D, Hopkins J, Sargan D, McConnell I. Early events in immune evasion by the lentivirus maedi-visna occurring within infected lymphoid tissue. J Virol. 1993;67:5187–5197. doi: 10.1128/jvi.67.9.5187-5197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird P, Reyburn H T, Blacklaws B A, Allen D, Nettleton P, Yirrell D L, Watt N, Sargan D, McConnell I. The restricted IgG1 antibody response to maedi visna virus is seen following infection but not following immunization with recombinant gag protein. Clin Exp Immunol. 1995;102:274–280. doi: 10.1111/j.1365-2249.1995.tb03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blacklaws B, Bird P, McConnell I. Early events in infection of lymphoid tissue by a lentivirus, maedi-visna. Trends Microbiol. 1995;3:434–440. doi: 10.1016/s0966-842x(00)88997-0. [DOI] [PubMed] [Google Scholar]
- 6.Blacklaws B A, Bird P, Allen D, Roy D J, MacLennan I C M, Hopkins J, Sargan D R, McConnell I. Initial lentivirus-host interactions within lymph nodes: a study of maedi-visna virus infection in sheep. J Virol. 1995;69:1400–1407. doi: 10.1128/jvi.69.3.1400-1407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blacklaws B A, Bird P, McConnell I. Pathogenesis and immunity in lentivirus infections of small ruminants. In: Goddeeris B M L, Morrison W I, editors. Cell-mediated immunity in ruminants. Boca Raton, Fla: CRC Press; 1994. pp. 199–212. [Google Scholar]
- 8.Brodie S J, Pearson L D, Zink M C, Bickle H M, Anderson B C, Marcom K A, Demartini J C. Ovine lentivirus expression and disease. Virus replication, but not entry, is restricted to macrophages of specific tissues. Am J Pathol. 1995;146:250–263. [PMC free article] [PubMed] [Google Scholar]
- 9.Brooke G P, Parsons K R, Howard C J. Cloning of two members of the SIRP alpha family of protein tyrosine phosphatase binding proteins in cattle that are expressed on monocytes and a subpopulation of dendritic cells and which mediate binding to CD4 T cells. Eur J Immunol. 1998;28:1–11. doi: 10.1002/(SICI)1521-4141(199801)28:01<1::AID-IMMU1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Bujdoso R, Hopkins J, Young P, Dutia B M, McConnell I. Characterisation of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med. 1989;170:1285–1302. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron P, Pope M, Granelli-Piperno A, Steinman R M. Dendritic cells and the replication of HIV-1. J Leukoc Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 12.Carey N, Dalziel R G. The biology of maedi-visna virus—an overview. Br Vet J. 1993;149:437–454. doi: 10.1016/S0007-1935(05)80110-1. [DOI] [PubMed] [Google Scholar]
- 13.Cella M, Scheidegger D, Palmer Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffin J M. Retroviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1767–1847. [Google Scholar]
- 15.Dutia B M, Hopkins J. Analysis of the CD1 cluster in sheep. Vet Immunol Immunopathol. 1991;27:189–194. doi: 10.1016/0165-2427(91)90099-x. [DOI] [PubMed] [Google Scholar]
- 16.Dutia B M, Hopkins J. Analysis of the monoclonal antibodies comprising WC6. Vet Immunol Immunopathol. 1993;39:193–199. doi: 10.1016/0165-2427(93)90181-3. [DOI] [PubMed] [Google Scholar]
- 17.Dutia B M, Hopkins J, Allington M P, Bujdoso R, McConnell I. Characterisation of monoclonal-antibodies specific for alpha-chains and beta-chains of sheep MHC class-II. Immunology. 1990;70:27–32. [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson K, McInnes E, Ryan S, Tonks P, McConnell I, Blacklaws B. CD4 T cells are required for the establishment of maedi-visna virus infection in macrophages but not dendritic cells. Virology. 1999;258:355–364. doi: 10.1006/viro.1999.9711. [DOI] [PubMed] [Google Scholar]
- 19.Ezekowitz R A B, Kuhlman M, Groopman J E, Byrn R A. A human-serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiskerstrand C E. Ph.D. thesis. Edinburgh, United Kingdom: University of Edinburgh; 1993. [Google Scholar]
- 21.Gendelman H E, Narayan O, Kennedy-Stoskopf S, Kennedy P G E, Ghotbi Z, Clements J E, Stanley J, Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986;58:67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gendelman H E, Narayan O, Molineaux S, Clements J E, Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci USA. 1985;82:7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorrell M D, Brandon M R, Sheffer D, Adams R J, Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992;66:2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O'Doherty F, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta V K, McConnell I, Dalziel R G, Hopkins J. Identification of the sheep homolog of the monocyte cell surface molecule CD14. Vet Immunol Immunopathol. 1996;51:89–99. doi: 10.1016/0165-2427(95)05512-6. [DOI] [PubMed] [Google Scholar]
- 26.Gupta V K, McConnell I, Hopkins J. Reactivity of the CD11/CD18 workshop monoclonal antibodies in the sheep. Vet Immunol Immunopathol. 1993;39:93–102. doi: 10.1016/0165-2427(93)90168-4. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V K, McConnell I, Pepin M, Davis W C, Dalziel R G, Hopkins J. Biochemical and phenotypic characterization of the ovine beta-2 (leukocyte) integrins. J Comp Pathol. 1995;112:339–349. doi: 10.1016/s0021-9975(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 28.Haig D M, Percival A, Mitchell J, Green I, Sargan D. The survival and growth of ovine afferent lymph dendritic cells in culture depends on tumour necrosis factor-alpha and is enhanced by granulocyte-macrophage colony-stimulating factor but inhibited by interferon gamma. Vet Immunol Immunopathol. 1995;45:221–236. doi: 10.1016/0165-2427(94)05341-o. [DOI] [PubMed] [Google Scholar]
- 29.Hall J G. A method for collecting lymph from the prefemoral lymph node of unanaesthetised sheep. Q J Exp Physiol. 1967;52:200–205. doi: 10.1113/expphysiol.1967.sp001902. [DOI] [PubMed] [Google Scholar]
- 30.Harkiss G D, Hopkins J, McConnell I. Uptake of antigen by afferent lymph dendritic cells mediated by antibody. Eur J Immunol. 1990;20:2367–2373. doi: 10.1002/eji.1830201102. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins J, Dutia B M. Monoclonal-antibodies to the sheep analogues of human-CD45 (leukocyte common antigen), MHC class-I and CD5—differential expression after lymphocyte activation in vivo. Vet Immunol Immunopathol. 1990;24:331–346. doi: 10.1016/0165-2427(90)90004-c. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins J, Dutia B M. Workshop studies on the ovine CD1 homolog. Vet Immunol Immunopathol. 1991;27:97–99. doi: 10.1016/0165-2427(91)90086-r. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins J, Dutia B M, Bujdoso R, McConnell I. In vivo modulation of CD1 and MHC class II expression by sheep afferent lymph dendritic cells. J Exp Med. 1989;170:1303–1318. doi: 10.1084/jem.170.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopkins J, Gupta V K. Identification of three myeloid-specific differentiation antigens in sheep. Vet Immunol Immunopathol. 1996;52:329–339. doi: 10.1016/0165-2427(96)05584-5. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins J, McConnell I, Bujdoso R, Munroe A J. Studies of MHC class II products on sheep peripheral and efferent lymph cells. In: Morris B, Miyasaka M, editors. Immunology of the sheep. F. Basel, Switzerland: Hoffmann-La Roche & Co.; 1985. pp. 441–459. [Google Scholar]
- 36.Howard C J, Brooke G P, Werling D, Sopp P, Hope J C, Parsons K R, Collins R A. Dendritic cells in cattle: phenotype and function. Vet Immunol Immunopathol. 1999;72:119–124. doi: 10.1016/s0165-2427(99)00124-5. [DOI] [PubMed] [Google Scholar]
- 37.Howard C J, Naessens J. Summary of workshop findings for cattle. Vet Immunol Immunopathol. 1993;39:25–48. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- 38.Howard C J, Sopp P, Brownlie J, Kwong L S, Parsons K R, Taylor G. Identification of two distinct populations of dendritic cells in afferent lymph that vary in their ability to stimulate T cells. J Immunol. 1997;159:5372–5382. [PubMed] [Google Scholar]
- 39.Hudson L, Hay F C. Practical immunology. 3rd ed. Oxford, United Kingdom: Blackwell Scientific; 1989. [Google Scholar]
- 40.Knight S C, Farrant J, Bryant A, Edwards A J, Burman S, Lever A, Clarke J, Webster D B. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes both with veiled morphology. Immunology. 1986;57:595–603. [PMC free article] [PubMed] [Google Scholar]
- 41.Knight S C, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 42.Lechner F, Machado J, Bertoni G, Seow H F, Dobbelaere D A E, Peterhans E. Caprine arthritis encephalitis virus dysregulates the expression of cytokines in macrophages. J Virol. 1997;71:7488–7497. doi: 10.1128/jvi.71.10.7488-7497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lechner F, Vogt H R, Seow H F, vonBodungen U, Bertoni G, Zurbriggen A, Peterhans E. Expression of TNF alpha in arthritis caused by caprine arthritis encephalitis virus. Vet Immunol Immunopathol. 1996;54:281–289. doi: 10.1016/s0165-2427(96)05701-7. [DOI] [PubMed] [Google Scholar]
- 44.Lee W C. Ph.D. thesis. Edinburgh, United Kingdom: University of Edinburgh; 1994. [Google Scholar]
- 45.Lee W C, Bird P, McConnell I, Watt N J, Blacklaws B A. The phenotype and phagocytic activity of macrophages during maedi-visna virus infection. Vet Immunol Immunopathol. 1996;51:113–126. doi: 10.1016/0165-2427(95)05508-8. [DOI] [PubMed] [Google Scholar]
- 46.Lujan L, Begara I, Collie D D S, Watt N J. Ovine lentivirus (maedi-visna virus) protein expression in sheep alveolar macrophages. Vet Pathol. 1994;31:695–703. doi: 10.1177/030098589403100610. [DOI] [PubMed] [Google Scholar]
- 47.Mackay C R, Hein W R, Brown M R, Matzinger P. Unusual expression of CD2 in sheep: implications for T cell interactions. Eur J Immunol. 1988;18:1681–1685. doi: 10.1002/eji.1830181105. [DOI] [PubMed] [Google Scholar]
- 48.Mackay C R, Marston W L, Dudler L. Naive and memory T-cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay C R, Marston W L, Dudler L, Spertini O, Tedder T F, Hein W R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T-cells. Eur J Immunol. 1992;22:887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 50.Maddox J F, Mackay C R, Brandon M R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985;55:739–748. [PMC free article] [PubMed] [Google Scholar]
- 51.Mohanty S G, Dutta S K. Veterinary virology. Philadelphia, Pa: Lea & Febiger; 1981. p. 43. [Google Scholar]
- 52.Narayan O, Wolinsky J S, Clements J E, Strandberg J D, Griffin D E, Cork L C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982;59:345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- 53.Pepin M, Cannella D, Fontaine J J, Pittet J C, Le Pape A. Ovine mononuclear phagocytes in situ: identification by monoclonal antibodies and involvement in experimental pyogranuloma. J Leukoc Biol. 1992;51:188–198. doi: 10.1002/jlb.51.2.188. [DOI] [PubMed] [Google Scholar]
- 54.Peters J H, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–278. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 55.Petursson G, Nathanson N, Georgsson G, Panitch H, Palsson P A. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab Investig. 1976;35:402–412. [PubMed] [Google Scholar]
- 56.Pope M, Betjes M G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 57.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pyrah I T G, Watt N J. Immunohistological study of the depressed cutaneous DTH response in sheep naturally infected with an ovine lentivirus (maedi-visna virus) Clin Exp Immunol. 1996;104:32–36. doi: 10.1046/j.1365-2249.1996.d01-661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reyburn H T. Ph.D. thesis. Edinburgh, United Kingdom: University of Edinburgh; 1992. [Google Scholar]
- 60.Rhind S M, Dutia B M, Howard C J, Hopkins J. Discrimination of two subsets of CD1 molecules in the sheep. Vet Immunol Immunopathol. 1996;52:265–270. doi: 10.1016/0165-2427(96)05576-6. [DOI] [PubMed] [Google Scholar]
- 61.Ridge J P, Rosa D F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 62.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sargan D R, Bennet I D, Cousens C, Roy D J, Blacklaws B A, Dalziel R G, Watt N J, McConnell I. Nucleotide sequence of EV1, a British isolate of maedi-visna virus. J Gen Virol. 1991;72:1893–1903. doi: 10.1099/0022-1317-72-8-1893. [DOI] [PubMed] [Google Scholar]
- 64.Sargan D R, Roy D J, Dalziel R G, Watt N J, McConnell I. A temporal study of RNAs produced in maedi-visna virus infection of choroid-plexus cells. Vet Microbiol. 1994;39:369–378. doi: 10.1016/0378-1135(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 65.Schoenberger S P, Toes R E M, Van der Voort E I H, Offringa R, Melief C J M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 66.Sonigo P, Alizon M, Staskus K, Klatzmann D, Cole S, Danos O, Retzel E, Tiollais P, Haase A, Wainhobson S. Nucleotide-sequence of the visna lentivirus—relationship to the AIDS virus. Cell. 1985;42:369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- 67.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1995;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staskus K A, Couch L, Bitterman P, Retzel E F, Zupancic M, List J, Haase A T. In situ amplification of visna virus DNA in tissue sections reveals a reservoir of latently infected cells. Microb Pathog. 1991;11:67–76. doi: 10.1016/0882-4010(91)90095-r. [DOI] [PubMed] [Google Scholar]
- 69.Steinman R M, Granelli-Piperno A, Tenner-Racz K, Racz P, Frankel S, Delgado E, Ignatius R, Pope M. Dendritic cells during infection with HIV-1 and SIV. In: Lotze M T, Thomson A W, editors. Dendritic cells. 1st ed. London, United Kingdom: Academic Press; 1999. pp. 421–434. [Google Scholar]
- 70.Wood G S, Freudenthal P S, Edinger A, Steinman R M, Warnke R A. CD45 epitope mapping of human CD1a+ dendritic cells and peripheral blood dendritic cells. Am J Pathol. 1991;138:1451–1459. [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, Zhang Y, Spicer T P, Abbott L Z, Abbott M, Poiesz B J. Reverse transcription takes place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993;9:1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]