SUMMARY
OBJECTIVE:
This study aimed to investigate if there is any correlation between the quantitative computed tomography and the impulse oscillometry or spirometry results of post-COVID-19 patients.
METHODS:
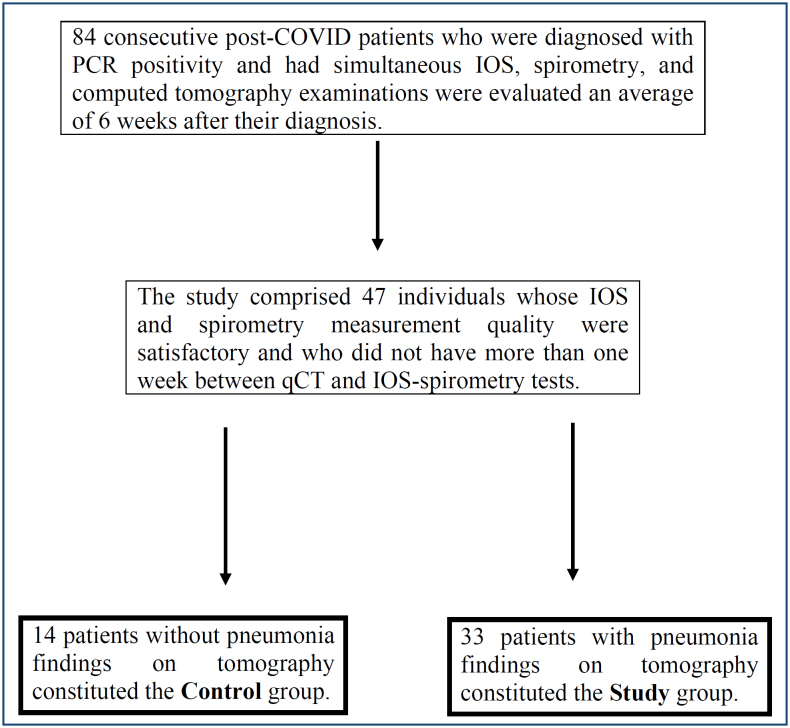
The study comprised 47 post-COVID-19 patients who had spirometry, impulse oscillometry, and high-resolution computed tomography examinations at the same time. The study group consisted of 33 patients with quantitative computed tomography involvement, while the control group included 14 patients who did not have CT findings. The quantitative computed tomography technology was used to calculate percentages of density range volumes. The relationship between percentages of density range volumes for different quantitative computed tomography density ranges and impulse oscillometry-spirometry findings was statistically analyzed.
RESULTS:
In quantitative computed tomography, the percentage of relatively high-density lung parenchyma, including fibrotic areas, was 1.76±0.43 and 5.65±3.73 in the control and study groups, respectively. The percentages of primarily ground-glass parenchyma areas were found to be 7.60±2.86 and 29.25±16.50 in the control and study groups, respectively. In the correlation analysis, the forced vital capacity% predicted in the study group was correlated with DRV%[(-750)-(-500)] (volume of the lung parenchyma that has density between (-750)-(-500) Hounsfield units), but no correlation with DRV%[(-500)-0] was detected. Also, reactance area and resonant frequency were correlated with DRV%[(-750)-(-500)], while X5 was correlated with both DRV%[(-500)-0] and DRV%[(-750)-(-500)] density. Modified Medical Research Council score was correlated with predicted percentages of forced vital capacity and X5.
CONCLUSION:
After COVID-19, forced vital capacity, reactance area, resonant frequency, and X5 correlated with the percentages of density range volumes of ground-glass opacity areas in the quantitative computed tomography. X5 was the only parameter correlated with density ranges consistent with both ground-glass opacity and fibrosis. Furthermore, the percentages of forced vital capacity and X5 were shown to be associated with the perception of dyspnea.
KEYWORDS: COVID-19, Oscillometry, Tomography, Spirometry
INTRODUCTION
Computed tomography (CT) abnormalities may last for months following COVID-19 pneumonia. Patients with pneumonia who develop sequelae require clinical, radiological, and functional follow-up 1 . It has been shown that CT data can be utilized to evaluate patients for disease severity and follow-up 2,3 . The most common radiographic findings are ground-glass opacities (GGO), consolidation, and fibrosis. Thin-section spiral volumetric CT is a common imaging modality used in the diagnosis and follow-up of COVID-19 pneumonia patients 4 . Quantitative CT (qCT) was reported to be used to evaluate the extent of COVID-19 pneumonia and in follow-up of the patients 5 . Spirometry and lung diffusion tests are recommended in routine clinical follow-ups of patients, especially in severe disease 6 . Furthermore, sound wave-based tests [forced oscillation technique and impulse oscillometry (IOS)] are employed to evaluate obstructive and restrictive disorders, particularly in obstructive diseases 7 . In obstructive diseases, IOS was shown to be more sensitive than spirometry in identifying minor airway obstruction 8 . Another study in patients who recovered from COVID-19 showed that IOS might detect aberrant findings even when spirometry was normal 9 .
This study aimed to investigate the functional equation of qCT results in patients with COVID-19 pneumonia, as well as their relationship with IOS and spirometry values. Our hypothesis was that relatively high-density lung fields in qCT due to COVID-19 involvement would correlate with IOS and spirometry parameters. As far as we know, no research has been undertaken to explore the correlation of qCT and IOS measurements.
METHODS
Study population
Institutional review board approval was obtained for the study from University of Health Sciences, Ankara Ataturk Sanatorium Training and Research Hospital. A retrospective analysis was performed on 84 consecutive post-COVID-19 patients who applied to our center between November 1, 2020, and January 31, 2021, whose follow-ups were performed using CT, IOS, and spirometry because of the prolongation of their symptomatic periods (to an average of 6 weeks) after the conclusion of therapy. Patients who had more than one week between their qCT and IOS-spirometry dates, as well as those who had poor IOS and spirometry measurement quality, were excluded from the study. Poor measurement quality was defined according to the American Thoracic Society – European Respiratory Society (ATS/ERS) guidelines recommendations for spirometry and ERS task force recommendations for IOS 10,11 . A total of 47 patients’ data were retrieved. The study group included 33 patients who had qCT results consistent with COVID-19 pneumonia, while the control group included 14 patients who did not have COVID-19-related CT findings (Figure 1). The predicted percentages of FEV1, FVC, and FEV1/FVC from spirometry measurements were recorded for the control and study groups. The IOS parameters reactance area (AX), resonant frequency (Fres), R20, R5, R5-20, and X5 were recorded, and the predicted percentages of these values were determined using Shulz et al.’s reference formulae for Caucasians 12 . Percentages of volumes of certain predefined density ranges (DRV%), within a maximum density of 0 Hounsfield units (HU) and a minimum density of -850 HU, were obtained from qCT using a computer program. The modified Medical Research Council (mMRC) score was used to assess the patients’ dyspnea perception scores.
Figure 1. Patient selection.
Impulse oscillometry and spirometry measurements
All patients first underwent IOS, followed by spirometry (Carefusion Vyntus Jaeger IOS, Germany). Initially, the patient was informed about the measurement technique that would be used in order to improve compliance with the test. Oscillometric tests were performed with the patients sitting comfortably and straight, with the head and neck in a neutral or slightly extended posture, and with no forward head flexion. A nasal clip was used to close the nose, and individuals were asked to grip the mouthpiece of the device tightly with their lips and externally support their cheeks with their palms while breathing normally. It was checked visually by the chest physician, who performed the test to see if there was leakage from the edges of the mouthpiece and nose clip and whether the tongue was in the correct position. The tests were repeated at least three times, and the best results were recorded when the coherence at 5 Hz was greater than 0.8 or the coherence at 20 Hz was greater than 0.9 13 . Swallowing, laryngeal closure, leaks around the mouthpiece, inappropriate location of the nasal clip, irregular breathing, and acute hyperventilation during the test are reasons for invalidating the data. Most of these events may be detected by the flow signal, which should be displayed on the screen during measurement. During and after the test, the practitioner controlled each of these conditions visually. The measurements were taken in accordance with the ERS recommendations 11 . Spirometry was used to measure forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and FEV1/FVC in accordance with the ATS-ERS recommendations 10 .
Computed tomography
CT examinations were performed using a multi-detector spiral CT scanner (Philips Ingenuity 128 slice) in a single breath hold during deep inspiration. All CT scans obtained after intravenous contrast administration were excluded because contrast material could interfere with density measurements of lung parenchyma. CT acquisitions were performed utilizing a 120 kV tube voltage and current modulation technique, and images were acquired with a 1.5 mm reconstruction thickness and a “B filter.”
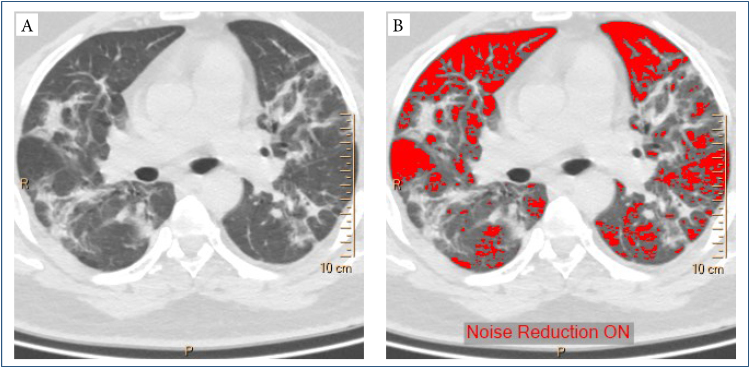
The obtained thin-section volumetric CT images were quantitatively analyzed using the Philips IntelliSpace Portal software, and all steps of this analysis were supervised by a 20 year experienced radiologist. This program can determine the overall volume of parenchyma areas and the volume below a particular threshold density value after automatically recognizing both lungs and their lobes on CT images. The percentages of parenchymal volumes in the whole lung volume that were below the predetermined threshold density values were measured by using density mask technique, a qCT technique that is widely used to quantify emphysema. In this technique, all voxels (the volume element of a CT slice that corresponds to a pixel of CT image) that have a density less than a predefined threshold are identified and masked by a color (Figure 2). Since the volume of a single voxel of a CT slice is known (it is pixel area multiplied by slice thickness), it is possible to calculate the total volume of all “masked densities.”
Figure 2. (A) Computed tomography image and (B) density mask with -750 Hounsfield units.
After measuring parenchymal volumes that have densities below seven predefined threshold density levels (0, -500, -600, -700, -750, -800, and -850 HU), we obtained volumes of lung parenchyma regions that have densities between certain thresholds by simply subtracting the volume of the lower threshold value from the volume of the higher one. In this way, we obtained an absolute volume value of a parenchymal density range, and when we divided it into total lung volume, we got the percentage of a certain density range volume (DRV%), such as DRV%[(-750)-(-500)], which means the percentage of lung parenchyma areas that have a density between -750 and -500 HU.
Although different density ranges are utilized in the literature for lung fibrosis and ground glass opacities, in our study, DRV%[(-500)-0] was accepted to represent parenchymal areas including fibrosis, atelectasis, and consolidation, and DRV%[(-750)-(-500)] was accepted to represent GGO 14,15 .
Correlation between predicted percentages of IOS parameters (AX, Fres, R20, R5, R5-20, and X5), spirometry measurements (FEV1, FVC and FEV1/FVC), and qCT results (DRV%[(-850)-0]) were statistically analyzed.
Statistical analysis
In our study, statistical analyses were performed by IBM SPSS version 26.0. To determine if the variables were normally distributed, visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov, Skewness, and Kurtosis tests) were used. Normally distributed independent data were analyzed using the Independent-Samples t-test. Non-normally distributed independent data were analyzed using the Mann-Whitney U test. The correlation between variables that did not show normal distribution was evaluated using Spearman’s test. p<0.05 was considered statistically significant.
RESULTS
A total of 47 patients (32 males and 15 females) with a mean age of 54 years were included in the study. The study and control groups were similar regarding their body mass indices (BMIs), comorbidity rates, and smoking durations. In the control group, the hospitalization rate was 28%, and the average number of hospitalization days was 2.36 days. In the study group, the rate of hospitalized patients was 78%, and the average length of stay was 9.79 days. In addition, the proportion of patients using long-term oxygen therapy (LTOT) was higher in the study group. Detailed demographic data and outcome measures are shown in Table 1 and 2.
Table 1. Demographic data, clinical data, and quantitative computed tomography results of patients.
| All patients n=47 Mean±SD |
Control group n=14 Mean±SD |
Study group n=33 Mean±SD |
p-value | ||
|---|---|---|---|---|---|
| Gender n m/f (%) | 32 (68.1)/15 (31.9) | 6 (42.9)/8 (57.1) | 26 (78.8)/7 (21.2) | 0.017 | |
| Age | 54.23±8.51 | 49.21±5.92 | 56.36±8.62 | 0.007 | |
| Comorbidities y/n | 25 (53.2)/22 (46.8) | 6 (42.9)/8 (57.1) | 19 (57.6)/14 (42.4) | 0.360 | |
| Smoking history (p/y) | 6.71±6.68 | 5.93±6.39 | 7.1±6.90 | 0.664 | |
| BMI | 28.14±4.29 | 26.72±4.39 | 28.75±4.17 | 0.139 | |
| mMRC | 1.80±0.72 | 1,43±,514 | 1,97±,752 | 0.024 | |
| LTOT y/n | 15 (31.9)/32 (68.1) | 1 (7.1)/13 (92.9) | 14 (42.4)/19 (57.6) | 0.003 | |
| qCT (DRV%) | |||||
| [(-500)-0] HU [(-750)-(-500)] HU |
4.49±3.60 | 1.76±0.43 | 5.65±3.73 | <0.001 | |
| 22.80±17.08 | 7.60±2.86 | 29.25±16.50 | <0.001 | ||
BMI: body mass index; DRV%: percentages of density range volumes; HU: Hounsfield units; LTOT: long-term oxygen therapy; m/f: male/female; mMRC: modified Medical Research Council score; n: number; p/y: pack year; qCT: quantitative computed tomography; SD: standard deviation; y/n: yes/no. Statistically significant p-values were given as bold.
Table 2. Spirometry and impulse oscillometry results according to the groups.
| All patients n=47 Mean±SD |
Control group n=14 Mean±SD |
Study group n=33 Mean±SD |
p-value | |
|---|---|---|---|---|
| FVC, %pred | 92.19±19.86 | 106.93±13.205 | 85.94±19.002 | <0.001 |
| FEV1, %pred | 91.43±18.54 | 100.57±14.070 | 87.55±19.013 | 0.026 |
| FEV1/FVC, % | 81.30±10.47 | 77.2750±8.40269 | 83.0130±10.90094 | 0.086 |
| Fres, Hz | 16.13±4.31 | 14.25±3.19 | 16.93±4.51 | 0.050 |
| Fres, %pred | 120.99±33.16 | 105.95±31.90 | 127.37±32.04 | 0.042 |
| AX, kPa/L | 0.58±0.41 | 0.48±0.46 | 0.62±0.38 | 0.278 |
| AX, %pred | 180.45±110.30 | 129.67±93.22 | 201.99±111.14 | 0.038 |
| R5, kPa/L/s | 0.35±0.09 | 0.351±0.10 | 0.358±0.09 | 0.560 |
| R5, % | 112.62±29.99 | 100.44±20.280 | 116.54±31.817 | 0.165 |
| R20, kPa/L/s | 0.27±0.07 | 0.275±0.08 | 0.276±0.07 | 0.825 |
| R20, %pred | 101.70±27.21 | 92.85±19.595 | 105.18±29.210 | 0.169 |
| R5-20, kPa/L/s | 0.079±0.043 | 0.076±0.047 | 0.088±0.035 | 0.104 |
| R5-20, % | 126.22±47.45 | 112.39±48.29 | 132.08±46.58 | 0.306 |
| X5, kPa /L/s | -0.122±0.05 | -0.096±0.02 | -0.133±0.06 | 0.035 |
| X5, %pred | 121.74±58.41 | 99.73±20.39 | 131.08±66.58 | 0.044 |
AX: reactance area; FEV1: forced expiratory volume in the first second; Fres: resonant frequency; FVC: forced vital capacity; IOS: impulse oscillometry; pred: predicted; R: respiratory resistance; R5-20: R5-R20; SD: standard deviation; X: respiratory reactance. Statistically significant p-values were given as bold.
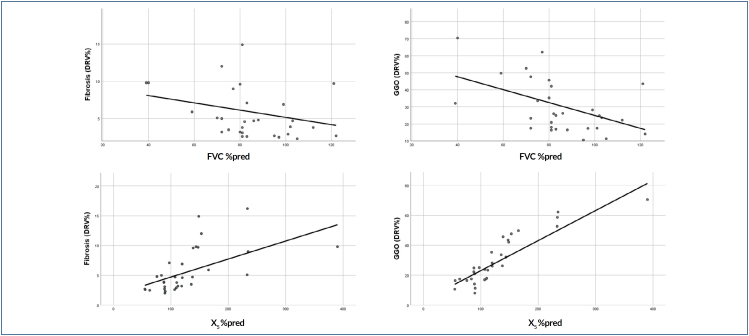
In the correlation analysis, none of the spirometry and IOS parameters in the control group were correlated with any of the qCT-derived DRV% values that were within the range of CT densities between 0 and -750 HU. Predicted FVC percentages were correlated with DRV%[(-750)-(-500)] in the study group, but not with DRV%[(-500)-0] (Table 3; Figure 3). In the correlation analysis between IOS parameters and DRV% values, AX and Fres were correlated with DRV%[(-750)-(-500)], while X5 was correlated with both DRV%[(-500)-0] and DRV%[(-750)-(-500)]. The perception of dyspnea measured by mMRC was correlated with both FVC%pred and X5%pred.
Table 3. Correlations between spirometry-impulse oscillometry parameters with quantitative computed tomography values and dyspnea perception.
| DRV% [(-500)-0] HU |
DRV% [(-750)-(-500)] HU |
mMRC | ||
|---|---|---|---|---|
| FVC, %pred | r | -0.251 | -0.453 | -0.403 |
| p | 0.174 | 0.011 | 0.030 | |
| FEV1, %pred | r | -0.080 | -0.171 | -0.211 |
| p | 0.668 | 0.356 | 0.272 | |
| Fres, Hz | r | 0.002 | 0.057 | 0.190 |
| p | 0.990 | 0.751 | 0.306 | |
| Fres, %pred | r | 0.310 | 0.452 | 0.323 |
| p | 0.079 | 0.008 | 0.077 | |
| X5, kPa/L/s | r | -0.493 | -0.716 | -0.257 |
| p | 0.004 | <0.001 | 0.163 | |
| X5, %pred | r | 0.607 | 0.773 | 0.376 |
| p | 0.001 | <0.001 | 0.037 | |
| AX, kPa/L | r | 0.108 | 0.217 | -0.045 |
| p | 0.549 | 0.225 | 0.808 | |
| AX, %pred | r | 0.304 | 0.430 | 0.334 |
| p | 0.086 | 0.012 | 0.067 | |
| R5-20, kPa/L/s | r | 0.155 | 0.232 | 0.143 |
| p | 0.390 | 0.193 | 0.442 | |
| R5-20, %pred | r | 0.026 | -0.017 | -0.001 |
| p | 0.887 | 0.926 | 0.997 | |
AX: reactance area; DRV%: percentages of density range volumes; FEV1: forced expiratory volume in the first second; Fres: resonant frequency; FVC: forced vital capacity; HU: Hounsfield units; IOS: impulse oscillometry; mMRC: modified Medical Research Council score; pred: predicted; qCT: quantitative computed tomography; R: respiratory resistance; R5-20: R5-R20; X: respiratory reactance. Statistically significant p-values were given as bold.
Figure 3. The relationship among FVC%pred and X5%pred values with fibrosis (DRV%[(-500)-0]) and ground-glass opacities (DRV%[(-750)-(-500)]).
DISCUSSION
In this study, the correlation of qCT-derived DRV% with spirometry and IOS measurement results was investigated in patients with COVID-19 pneumonia 6 weeks after the conclusion of therapy.
Previous studies have shown that patients with COVID-19 can develop a restrictive ventilatory defect associated with the severity of the disease 6 . In another study, CT findings were observed even 3 months after the disease, and a decrease in diffusion capacity was found even when lung volumes were within normal ranges 16 . In both obstructive and restrictive diseases, IOS parameters AX, Fres, and R5 generally increase, while X5 decreases 17,18 . However, studies about COVID-19 are very limited. In our study, FVC%pred was only correlated with DRV%[(-750)-(-500)]. DRV%[(-500)-0] did not correlate with spirometry parameters. This may be because the percentage of DRV%[(-500)-0] is relatively low. Fres and AX values (%pred), which are IOS parameters, are also correlated with DRV%[(-750)-(-500)]. Of these parameters, only X5 is correlated with both DRV%[(-750)-(-500)] and DRV%[(-500)-0]. X5 is associated with elastic recoil as the out-of-phase component of lung impedance. Lung diseases that reduce the elastance of the lung (fibrosis and hyperinflation) lead to more negative X5 13 . It is also a useful parameter for the assessment of the peripheral regions of the lungs. The reactance at 5 Hz is likely to detect small amounts of fibrosis-induced elastic recoil changes. The rate of DRV%[(-500)-0], which is supposed to represent mainly fibrotic areas, was relatively low in our patients, and we believed that X5 might be more sensitive to functional disorders that cannot be detected by FVC.
Studies on the use of IOS in restrictive diseases are relatively few. Soave et al. reported that reactance can be used in the functional follow-up of interstitial lung disease (ILD) 19 . In both obstructive and restrictive diseases, AX, Fres, and R5 increase, and X5 decreases. It has been claimed that a normal R20 level can be used for discrimination in ILD 20 . The mean R20%pred in our patients was also normal. Intrapulmonary airway and alveolar destruction, basal cell proliferation in the airways, and fibrinous exudates have all been seen in autopsy series of COVID-19 patients 21,22 . This shows that some individuals may also have airway obstruction. However, in addition to the normal R20 and R5 percentages in our study group, there was no statistically significant difference when compared to the control group. Although the R5-20 mean was higher in the study group than in the control group, no statistically significant difference was identified between the two groups. Iwamoto et al. reported that the inspiratory X5, being more negative than the expiratory X5, may be a guide to distinguish restrictive diseases from obstructive pathologies, and a single breath reactance measurement would not discriminate 23 . Our study was designed retrospectively, and patients did not have delta X5 results.
The mMRC dyspnea score has been proposed as a simple and valid method for classifying COPD-related disability 24 . In patients with idiopathic pulmonary fibrosis whose restriction is prominent, the mMRC score has been shown to correlate with major functional parameters of both maximal and submaximal exercise tests, which are known to be associated with disease severity and survival, as well as ventilatory impairment and exercise limitation 25 . Correlation of mMRC score with spirometry and IOS findings shows the importance of functional follow-up of patients and suggests that IOS can be used in the follow-up of patients.
IOS is a test that requires minimal patient cooperation. Oscillometry is fundamentally a different measurement from traditional lung function measurements, spirometry, and lung volumes. IOS detects small airway obstructions more sensitively than spirometry and has a strong correlation with the degree of obstruction. Furthermore, it can reveal the location of the obstruction. However, spirometry was found to be more sensitive in cases of large airway obstruction 8 . Lu et al. showed that IOS may be more sensitive than spirometry in the diagnosis of small airway disease in people with normal lung functions. However, in patients with abnormal lung function, spirometry may be more sensitive than IOS to detect patients with clinical symptoms and CT lesions 26 . It can detect lung involvement in patients with ILDs who have mild or even normal spirometry changes 27 . Our findings showed that IOS parameters, especially X5 value, were associated with some qCT-derived DRV%s. It may be useful to use the IOS test together with spirometry in the functional evaluation of post-COVID patients.
As a result of this study, we hope that general pulmonologists will remember that the findings on quantitative thoracic CT of patients with COVID-19 pneumonia correlated with spirometry and IOS parameters, with the strongest correlation being with X5 from the IOS parameters.
Our study had some limitations. As it was a retrospective study, some results could not be reached, and the number of our patients was small. However, as far as we know, this is the first study to investigate the functional equivalence of qCT findings with IOS measurements.
CONCLUSION
In this study, 6 weeks after COVID-19 pneumonia, the spirometry parameter FVC, as well as the IOS parameters AX and Fres (%predicted), was correlated with the qCT-derived DRV%[(-750)-(-500)]. The percentage of X5 relative to what was predicted was the sole parameter associated with both DRV%[(-750)-(-500)] and DRV%[(-500)-0]. Furthermore, the predicted percentages of FVC and X5 were correlated to the perception of dyspnea. IOS can be used in combination with spirometry to assess pulmonary function in individuals with COVID-19 pneumonia.
Footnotes
Funding: none.
REFERENCES
- 1.Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463–100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi W, Peng X, Liu T, Cheng Z, Lu H, Yang S, et al. A deep learning-based quantitative computed tomography model for predicting the severity of COVID-19: a retrospective study of 196 patients. Ann Transl Med. 2021;9(3):216–216. doi: 10.21037/atm-20-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, et al. Coronavirus disease (COVID-19): spectrum of CT findings and temporal progression of the disease. Acad Radiol. 2020;27(5):603–608. doi: 10.1016/j.acra.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J, Yang H, Lei P, Fan B, Qiu Y, Zeng B, et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J Xray Sci Technol. 2020;28(3):383–389. doi: 10.3233/XST-200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen C, Yu N, Cai S, Zhou J, Sheng J, Liu K, et al. Quantitative computed tomography analysis for stratifying the severity of coronavirus disease 2019. J Pharm Anal. 2020;10(2):123–129. doi: 10.1016/j.jpha.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217–2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellinckx J, Cauberghs M, Boeck K, Demedts M. Evaluation of impulse oscillation system: comparison with forced oscillation technique and body plethysmography. Eur Respir J. 2001;18(3):564–570. doi: 10.1183/09031936.01.00046401. [DOI] [PubMed] [Google Scholar]
- 8.Mousa H, Kamal E. Impulse oscillation system versus spirometry in assessment of obstructive airway diseases. Egypt J Chest Dis Tuberc. 2018;67(2):106–106. doi: 10.4103/ejcdt.ejcdt_3_18. [DOI] [Google Scholar]
- 9.Lopes AJ, Mafort TT, Cal MS, Monnerat LB, Litrento PF, Ramos I, et al. Impulse oscillometry findings and their associations with lung ultrasound signs in COVID-19 survivors. Respir Care. 2021;66(11):1691–1698. doi: 10.4187/respcare.09193. [DOI] [PubMed] [Google Scholar]
- 10.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 11.Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 12.Schulz H, Flexeder C, Behr J, Heier M, Holle R, Huber RM, et al. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One. 2013;8(5):e63366. doi: 10.1371/journal.pone.0063366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146(3):841–847. doi: 10.1378/chest.13-1875. [DOI] [PubMed] [Google Scholar]
- 14.Cheng T, Li Y, Pang S, Wan H, Shi G, Cheng Q, et al. Normal lung attenuation distribution and lung volume on computed tomography in a Chinese population. Int J Chron Obstruct Pulmon Dis. 2019;14:1657–1668. doi: 10.2147/COPD.S187596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rorat M, Jurek T, Simon K, Guziński M. Value of quantitative analysis in lung computed tomography in patients severely ill with COVID-19. PLoS One. 2021;16(5):e0251946. doi: 10.1371/journal.pone.0251946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):2003448–2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeichi N, Yamazaki H, Fujimoto K. Comparison of impedance measured by the forced oscillation technique and pulmonary functions, including static lung compliance, in obstructive and interstitial lung disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1109–1118. doi: 10.2147/COPD.S198030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii M, Shirai T, Mori K, Mikamo M, Shishido Y, Akita T, et al. Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir Physiol Neurobiol. 2015;207:22–27. doi: 10.1016/j.resp.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Soave S, Bellini F, Nori O, Carnevale A, Contoli M, Papi A, et al. Impulse oscillometry (IOS) in interstitial lung diseases: clinical- functional- radiological correlations. [cited on Mar 20, 2022];European Respiratory Journal [Internet] 2020 56(suppl 64) Available from: https://erj.ersjournals.com/content/56/suppl_64/748 . [Google Scholar]
- 20.Porojan-Suppini N, Fira-Mladinescu O, Marc M, Tudorache E, Oancea C. Lung function assessment by ımpulse oscillometry in adults. Ther Clin Risk Manag. 2020;16:1139–1150. doi: 10.2147/TCRM.S275920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Liu H, Huang H, Li H, Saqi A, Qiang L, et al. Distinct stem/progenitor cells proliferate to regenerate the trachea, intrapulmonary airways and alveoli in COVID-19 patients. Cell Res. 2020;30(8):705–707. doi: 10.1038/s41422-020-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, Cai S, Feng H, Cai B, Lin L, Mai Y, et al. Single-cell analysis reveals bronchoalveolar epithelial dysfunction in COVID-19 patients. Protein Cell. 2020;11(9):680–687. doi: 10.1007/s13238-020-00752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiyama A, Hattori N, Haruta Y, Nakamura I, Nakagawa M, Miyamoto S, et al. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir Med. 2013;107(6):875–882. doi: 10.1016/j.rmed.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manali ED, Lyberopoulos P, Triantafillidou C, Kolilekas LF, Sotiropoulou C, Milic-Emili J, et al. MRC chronic dyspnea scale: relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med. 2010;10:32–32. doi: 10.1186/1471-2466-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu L, Peng J, Zhao N, Wu F, Tian H, Yang H, et al. Discordant spirometry and ımpulse oscillometry assessments in the diagnosis of small airway dysfunction. Front Physiol. 2022;13:892448–892448. doi: 10.3389/fphys.2022.892448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkolaly RM, Ganna SA, Nada DW, Elnaggar MH. Impulse oscillometry, an aid or a substitute? Egypt J Bronchol. 2019 Sep;13(3):416–423. doi: 10.4103/ejb.ejb_98_18. [DOI] [Google Scholar]