Abstract
Background
Airway epithelial cells from patients with COPD show suboptimal innate immune responses to nontypeable Haemophilus influenzae (NTHi) and Toll-like receptor (TLR)2 ligands despite expressing TLR2 similar to normal airway epithelial cells, but the underlying mechanisms are poorly understood.
Methods
Normal or COPD mucociliary-differentiated airway epithelial cells were treated with TLR2 agonists or infected with NTHi and expression of β-defensin (HBD)2 was examined. Interleukin-1 receptor-associated kinase (IRAK)-1 and microRNA (miR)146a were genetically inhibited in normal and COPD airway epithelial cell cultures, respectively, and HBD2 responses to TLR2 ligands were determined. IRAK-1 expression in lung sections was determined by immunofluorescence microscopy.
Results
Compared to normal, COPD airway epithelial cell cultures showed impaired expression of HBD2 in response to TLR2 agonists or NTHi infection. Apical secretions from TLR2 agonist-treated normal, but not COPD, airway epithelial cells efficiently killed NTHi. Knockdown of HBD2 significantly reduced NTHi killing by apical secretions of normal airway epithelial cells. Compared to normal, COPD cells showed significantly reduced expression of IRAK-1 and this was associated with increased expression of miR146a. Inhibition of miR146a increased the expression of IRAK-1, improved the expression of HBD2 in response to TLR2 agonists in COPD cells and enhanced the killing of bacteria by apical secretions obtained from TLR2 agonist-treated COPD cells. Bronchial epithelium of COPD patients showed reduced expression of IRAK-1.
Conclusions
These results suggest that reduced levels of IRAK-1 due to increased expression of miR146a may contribute to impaired expression of TLR2-induced HBD2 in COPD airway epithelial cells.
Short abstract
Attenuated IRAK-1 expression due to increased expression miR146a is one of the mechanisms underlying the dysregulated TLR2 signalling and impaired innate immune responses in COPD airway epithelial cells https://bit.ly/3y6s2jG
Introduction
Acute exacerbations are the primary cause of morbidity and mortality in COPD [1]. Respiratory infections are responsible for 80% of the acute exacerbations in COPD, half of which are associated with bacterial infections. Nontypeable Haemophilus influenzae (NTHi) is one of the bacteria frequently detected during acute exacerbations in these patients [2]. Previous literature has demonstrated impaired innate immune responses to NTHi infection in COPD airway epithelial cells [3] and lung macrophages [4, 5].
Airway epithelial cells produce antimicrobial peptides and pro-inflammatory cytokines in response to bacterial infection, thus contributing to innate immunity. Bronchial epithelial cells from COPD patients express reduced levels of antimicrobial peptides, including human β-defensin (HBD)2, in response to NTHi or Pseudomonas aeruginosa infection [3, 6]. Moreover, HBD2 expression was reduced in the bronchial airway epithelium of COPD patients [7]. HBD2 has antimicrobial activity against NTHi [8] and activation of Toll-like receptor (TLR)2 signalling stimulates HBD2 expression in airway epithelial cells [9]. Previously, we demonstrated that despite expressing TLR2 similar to normal airway epithelial cells, COPD cells show attenuated interleukin (IL)-8 response to TLR2 agonists [10]. These observations indicate that dysregulated TLR2 signalling rather than TLR2 expression may contribute to impaired innate immune responses to NTHi in COPD airway epithelial cells, but the underlying mechanisms are not well known.
Upon recognition of a ligand, TLR2 heterodimerises with either TLR1 or TLR6, which initiates recruitment of myeloid differentiation primary response gene 88 (MyD88) adaptor to the TLR interaction domain. MyD88 then complexes with IL-1 receptor-associated kinase (IRAK) family members, and this complex is referred to as Myddosome [11]. During Myddosome formation, IRAK-4 phosphorylates IRAK-1, initiating activation, autophosphorylation and release of IRAK-1 from Myddosome [12]. Release of IRAK-1 is essential for ubiquitination of tumour necrosis factor receptor-associated factor (TRAF) leading to activation of IκB kinase (IKK) complex and translocation of NF-κB or AP-1 translocation via activation of serine threonine-specific protein kinases [13]. TLR2 signalling in airway epithelial cells stimulates HBD2 via activation of NF-κB [9]. There is no defect in IKK-driven NF-κB activation in the bronchial epithelium of COPD patients [14]. Based on this literature, we hypothesised that dysregulated TLR2 signalling in COPD airway epithelial cells may be due to defects in the expression or activation of adaptor proteins, MyD88 or IRAKs.
MicroRNA (miR) is a class of noncoding small RNA molecules. miRs carry out their biological functions by binding to the 3′ untranslated regions of their target mRNAs, thereby repressing translation, degrading the target mRNAs or inducing target mRNAs. Previous studies have demonstrated that several miRs, including miR-21, miR-146, miR-155 and Let-7 family target TLRs or adaptor proteins in TLR signalling pathways (reviewed in [15]). Profiling of miRs in whole lungs identified several differentially expressed miRs between healthy smokers and smokers with COPD with upregulated expression of miR146a in COPD patients [16]. miR146a inhibits MyD88-dependent TLR and IL-1 receptor (IL-1R) signalling via attenuation of IRAK-1 and TRAF-6 expression [17], and degradation of signal transducer and activator of transcription-1 [18]. Therefore, we postulated that increased expression of miR146a may contribute to dysregulation of TLR2 signalling in COPD airway epithelial cells.
In this study, we examined the mechanisms underlying the dysregulation of TLR2 signalling that stimulates HBD2 expression in mucociliary-differentiated COPD bronchial epithelial cell cultures.
Materials and methods
Bronchial epithelial cell cultures
Bronchial tissue segments from normal lung donors and explanted lungs from COPD patients were collected at the time of lung transplantation. COPD patients provided written informed consent forms to use their explanted lungs for research (IRB# 4407). Donors of normal lungs had standing consent to use their organs for organ transplant and research after their demise. The collection and use of the tissue samples were approved by University of Michigan (IRB# HUM00050876) and Temple University (IRB# 23903) institutional review boards. All methods were performed in accordance with the relevant guidelines and regulations. Patient characteristics are provided in supplementary table S1. Airway basal cells from bronchial segments were isolated and expanded in the BronchiaLife medium (Lifeline Cell Technology, Frederick, MD, USA). The basal cells at passage 1 were cultured in 12-mm transwells at an air/liquid interface to promote mucociliary differentiation, as described previously [10, 19]. We seed ∼90 000 live cells·cm−2 in a transwell, and this seeding strategy did not indicate difference in growth rate between COPD and normal cells.
Inhibition of HBD2, IRAK-1 and miR146a in COPD airway epithelial cells
Normal airway basal cells were transduced with lentiviral vectors expressing IRAK-1, DEFB4 or nontargeting (NT) short hairpin (sh)RNA (ThermoFisher Scientific, Waltham, MA, USA), as described previously [19]. Transduced cells were selected on puromycin, and then grown at air/liquid interface to promote mucociliary differentiation. We did not observe differences in growth between control and gene-specific shRNA transduced cells.
Mucociliary-differentiated COPD bronchial epithelial cell cultures were transduced basolaterally with a lentiviral vector expressing miRZip scrambled hairpin RNA control or miRZip 146a microRNA inhibitor using TransDux Max transducing reagent (System Biosciences, Mountain View, CA, USA). Briefly, basolateral medium was replaced with fresh culture medium containing TransDux Max enhancer and then lentiviral particle expressing miRZip scrambled hairpin RNA control or miRZip 146a microRNA inhibitor (50 µL medium containing 5×106 plaque-forming units equivalent to 5 MOI (multiplicity of infection)) was added to the basolateral medium. Cell cultures were incubated for 3 days and the expression of miR146a target IRAK-1 was determined by Western blot analysis. Inhibition of miR146a had no effect on cell growth.
Infection of cell cultures with NTHi
A clinical isolate of NTHi, 5P54HI was isolated at the time of exacerbation from COPD patients (kindly provided by Timothy Murphy, University at Buffalo, Buffalo, NY, USA). For infection, 5P54HI was subcultured on a chocolate agar plate and incubated overnight at 37°C/5% carbon dioxide. Bacterial colonies were suspended in PBS and centrifuged at 1000×g for 5 min. Finally, the bacterial pellet was suspended in PBS, and optical density at 600 nm was adjusted to 1, which corresponds to 1–3×109 CFU·mL−1 by dilution plating. The apical surface of the cell cultures was washed once with 0.15% sodium bicarbonate and rinsed with PBS to remove the mucus and other secretions. Cell cultures were transferred to a new receiver plate containing fresh medium with no antibiotics, infected with 50 μL of NTHi (1–3×107 CFU·mL−1 = ∼3 MOI) suspension or treated with 50 μL PBS (control) and incubated for 24 h. The cells were washed and lysed in 0.1% sterile triton X-100; lysates were serially diluted and plated to determine the bacterial density. The MOI of 3 and the incubation time were chosen based on our initial experiment, in which one normal and one COPD culture with MOI of 0.3, 3 and 10 were incubated for 3, 16, 24 and 48 h, and determined DEFB4 expression and cell death. MOI of 3 was found to be optimal in stimulating DEFB4 without causing cell death. NTHi at MOI of 3 stimulated DFEB4 as early as 3 h with maximum expression observed at 24 h post-infection in normal cells. However, in COPD cells there was an increasing trend in the expression of DEFB4 at 24 h. There was no cell death until 24 h. At 48 h we observed cell death in both normal and COPD, but it was much higher in COPD cell cultures.
Treatment with TLR2 ligands
TLR2 ligands Pam3CSK4 and FSL-1 were purchased from InvivoGen (San Diego, CA, USA) and dissolved in sterile PBS. The apical surface of the cell cultures was washed and then the wells were transferred to new receiver plates, as described earlier, and treated with 50 μL of 1 μg·mL−1 Pam3CSK4 or 10 ng·mL−1 FSL-1 as recommended by the manufacturer, or PBS (control), and incubated up to 24 h.
Killing of bacteria by apical secretions
After appropriate treatment, 500 μL PBS was added to the apical surface of the cultures and incubated for 15 min; the apical secretions were collected and homogenised by mixing using a pipette. NTHi (100 μL containing 1×106 CFU) was added to 100 μL apical secretions, incubated for 2 h at 37°C, serially diluted and plated on chocolate agar plates to determine the viable bacterial counts.
HBD2 ELISA
Apical secretions and basolateral medium were used to determine the protein levels of HBD2 by ELISA following manufacturer's instructions (MyBioSource.com, San Diego, CA, USA). The sensitivity of the assay was 15.6 pg·mL−1. IL-8 protein levels were determined in the basolateral medium by ELISA (R&D Systems, Minneapolis, MN, USA).
RNA isolation and quantitative PCR
After appropriate treatment, cell cultures were washed three times with PBS and lysed in 0.7 mL TRIzol. Total RNA was isolated from TRIzol lysates of the cell cultures (Zymo Research, Irvine, CA, USA); cDNA was synthesised using a high-capacity cDNA reverse transcription kit (ThermoFisher Scientific) and subjected to probe-based quantitative (q)PCR. Primetime probe-based assays were purchased from Integrated DNA Technologies (Coralville, IA, USA). Expression of genes of interest was presented as a fold change over a housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). miR146a expression was determined by using a TaqMan microRNA assay kit (ThermoFisher Scientific) and the results were expressed as a fold change over RNU44 (control), which is stably expressed in mammalian cells.
Western blot analysis
Total proteins from normal and COPD cell cultures were isolated by lysing cells with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. Equal amounts of protein (80 μg to detect TLR2 and 50 μg to detect the rest of the proteins) were subjected to electrophoresis; proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin, and then cut based on the molecular weight standards in order to probe with different antibodies at the same time. Membranes were probed with antibodies to TLR2 (1:200 dilution), IRAK-1 (1:1000), IRAK-4 (1:2000), IRAK-M (1:2000), MyD-88 (1:1000) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) or GAPDH (1:20 000 for blots with 50 μg total protein and 1:50 000 for blots with 80 μg total protein) (Sigma Aldrich, St. Louis, MO, USA). Anti-mouse IgG conjugated with horseradish peroxidase (HRP) (1:15 000 for detection of all the primary antibodies except GAPDH, for which we used 1:50 000 dilution; BioRAD, Hercules, CA, USA) and chemiluminescent substrate, SuperSignal West Atto ultimate sensitivity substrate (ThermoFisher Scientific) or Western blotting luminol reagent (Santa Cruz Biotechnology). The blots were developed using Chemidoc imaging system (BioRAD) and exported as TIFF image. Specific bands were quantified by densitometry using ImageJ and expressed as fold change over GAPDH.
Immunofluorescence microscopy
Paraffin lung sections containing secondary branching of bronchi were deparaffinised and subjected to immunofluorescence staining with IRAK-1 antibody (Santa Cruz Biotechnology) using tyramide signal amplification kit (ThermoScientific), as described previously [20]. Briefly, deparaffinised sections were subjected to antigen retrieval in a boiling citric acid buffer, endogenous peroxidase activity quenched with 3% hydrogen peroxide, and blocked in 5% normal horse serum. The sections were incubated with IRAK-1 antibody (1:2000 dilution). The bound antibody was detected using antirabbit polymeric IgG conjugated with HRP (VectorLabs, Burlingame, CA, USA) and tyramide signal amplification kit, counterstained with 4′,6-diamidino-2-phenylindole, and visualised under the fluorescence microscope. Sections stained with nonspecific IgG served as controls, and these sections were used to determine the exposure time to detect specific signals. The IRAK-1 antibody used in this study gives a single band by Western blot analysis of airway epithelial cell lysates.
Statistical analysis
Statistical significance for normally distributed data was assessed by unpaired t-test (for comparisons between two groups) or by ANOVA with the Tukey–Kramer post hoc test (for comparisons between three or more groups). If the data were not normally distributed, we used the Mann–Whitney test to compare between two groups, and ANOVA on ranks with the Kruskal–Wallis H-test for comparing three or more groups. For paired analysis, the statistical significance was determined by Wilcoxon signed-rank test. A p-value <0.05 was considered statistically significant.
Results
COPD airway epithelial cells are attenuated in reducing bacterial load
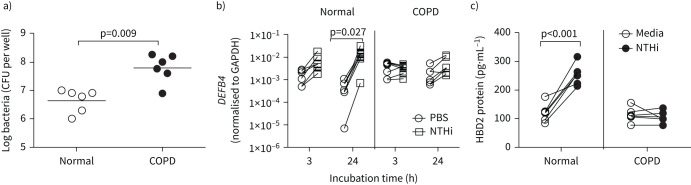
Mucociliary-differentiated normal and COPD airway epithelial cell cultures were apically infected with NTHi at a MOI of 3, incubated for 24 h and bacterial density was measured. Compared to normal, COPD cells showed significantly more bacteria (figure 1a), indicating the inability of COPD cells to control bacterial replication.
FIGURE 1.
COPD airway epithelial cell cultures infected with nontypeable Haemophilus influenzae (NTHi) show defects in killing bacteria and β-defensin (HBD)2 expression. COPD and normal mucociliary-differentiated bronchial epithelial cell cultures (n=6 normal and n=6 COPD) were infected with NTHi or treated with PBS (control) and incubated for 3 or 24 h. a) Cells were washed at 24 h post-infection, lysed in Triton X-100 and bacterial density in the cell lysates was determined; b) total RNA was isolated and the expression of DEFB4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined by reverse transcriptase quantitative PCR using gene-specific primers at 3 and 24 h. DEFB4 expression was normalised to GAPDH; c) HBD2 protein levels were determined in the apical wash at 24 h post-infection. a) Data represent range with median and the statistical significance calculated by Mann–Whitney test. b) and c) paired analysis with Wilcoxon signed-rank test was performed to determine the statistical significance.
HBD2 expression in response to NTHi infection is reduced in COPD cell cultures
Mucociliary-differentiated normal and COPD airway epithelial cell cultures were infected as described earlier, and mRNA expression of DEFB4, a gene encoding human HBD2, was determined at 3 or 24 h post-infection. Under unstimulated conditions, both normal and COPD cell cultures showed similar expression of DEFB4 (supplementary figure S1A). Normal airway epithelial cell cultures showed an increasing trend in DEFB4 at 3 h post-infection, but at 24 h post-infection DEFB4 expression increased significantly (figure 1b). In contrast, COPD cultures did not show any significant changes in the expression of DEFB4 either at 3 or 24 h post-NTHi infection. In addition, the expression of DEFB4 was significantly lower in NTHi-infected COPD cell cultures than the similarly infected normal cultures at both time points (supplementary figure S1B). At 48 h infection, there was significant cell death, particularly in COPD cell cultures; therefore, we did not determine the expression of DEFB4. Next, we examined the HBD2 protein levels in the apical secretions and basolateral medium at 24 h post-infection. A detectable level of HBD2 was observed only in the apical secretions at 24 h post-infection, but not in the basolateral medium. Consistent with mRNA expression, normal, but not COPD, cells showed significant increase in HBD2 levels post-NTHi infection (figure 1c). In the subsequent experiments, HBD2 protein was measured only in the apical secretions.
In addition, we determined the expression of another antimicrobial peptide, cathelicidin, which is expressed by airway epithelial cells. NTHi infection did not stimulate cathelicidin in both normal and COPD cell cultures (supplementary figure S2). Therefore, this study was focused on HBD2 expression.
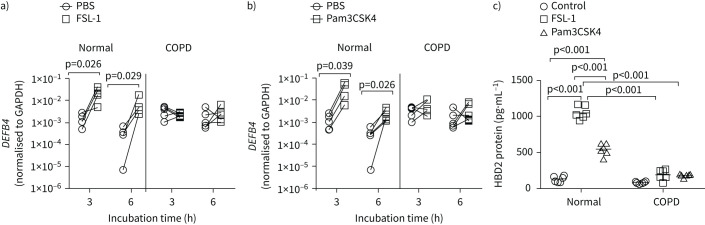
COPD cell cultures show attenuated HBD2 in response to TLR2 agonists
Since NTHi binds to TLR2, and TLR2 activation contributes to HBD2 expression in response to bacterial infection, we assessed whether treating with TLR2 ligands stimulates HBD2 in airway epithelial cells. We used the synthetic TLR2 agonists Pam3CSK4 and FSL-1, which respectively stimulate TLR2/TLR1 and TLR2/TLR6 heterodimerisation, the first step in TLR2 activation. Initially, we conducted a time-course experiment with FSL-1 using one normal and one COPD culture to determine the optimal time for DEFB4 expression. Maximum expression of DEFB4 was observed at 3 h in normal cultures, which was maintained up to 6 h and then returned to basal levels by 24 h. In contrast, HBD2 protein levels were maximal at 6 h, which was maintained until 24 h and returned to basal levels by 48 h. However, in COPD cells, there was no significant increase in the expression of DEFB4 or levels of HBD2 protein at all the time points examined. Since the expression of DEFB4 is maintained up to 6 h and HBD2 levels are maintained up to 24 h post-FSL-1 treatment in normal cells, we chose to determine the expression of DEFB4 at 3 and 6 h post-treatment, and HBD2 protein levels at 24 h post-treatment. Both FSL-1 and Pam3CSK4 stimulated the expression of DEFB4 in normal, but not in COPD, epithelial cell cultures (figure 2a and b). Consistent with the mRNA expression, both FSL-1 and Pam3CSK4 induced the protein expression of HBD2 only in normal cells and not in COPD cells (figure 2c). These results indicate that TLR2-agonist-stimulated DEFB4/HBD2 expression is dysregulated in COPD.
FIGURE 2.
Toll-like receptor (TLR)2 agonists-induced β-defensin (HBD)2 is attenuated in COPD epithelial cell cultures. Mucociliary-differentiated COPD and normal bronchial epithelial cell cultures (n=6 normal and n=6 COPD) were treated with Pam3CSK4, FSL-1 or PBS (control). a and b) After 3 or 6 h of incubation total RNA was isolated and the expression of DEFB4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined by reverse transcriptase quantitative PCR. The data represent DEFB4 expression levels normalised to GAPDH. Paired analysis with Wilcoxon signed-rank test was performed to determine the statistical significance. c) After 24 h incubation, HBD2 protein levels were measured in the apical wash. Data represent range with median, and the statistical significance was calculated by ANOVA on ranks with Kruskal–Wallis post hoc H-test.
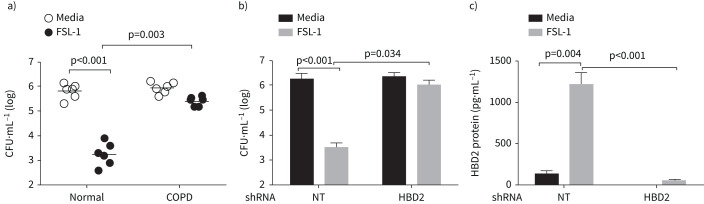
Apical secretions from COPD cell cultures are defective in killing NTHi
HBD2 has antimicrobial effects against NTHi [8]. Therefore, we examined the antibacterial effects of apical secretions from unstimulated and FSL-1-stimulated normal and COPD cell cultures against NTHi. FSL-1 was chosen for this set of experiments because it induced the highest amounts of HBD2 protein in normal cell cultures (figure 2c). Apical secretions from untreated normal and COPD cells showed similar antimicrobial activity against NTHi (figure 3a). Apical secretions from FSL-1-stimulated normal cultures reduced the viable bacterial counts by 2–3 logs, while secretions from similarly stimulated COPD cultures reduced the viable counts by ≤1 log. These results indicate that antimicrobial factors stimulated by TLR2 agonist plays a role in killing NTHi, and this is defective in COPD airway epithelial cell cultures.
FIGURE 3.
Secreted β-defensin (HBD)2 from normal epithelial cell cultures shows antibacterial activity against nontypeable Haemophilus influenzae (NTHi). Normal and COPD bronchial epithelial cell cultures (established from n=6 normal and n=6 COPD patients) were treated with FSL-1 or PBS (control) and incubated for 24 h. a) The apical secretions were then collected, incubated with NTHi (1×106 CFU) for 2 h, and the number of viable bacteria was determined by dilution plating. Data represent median with range and the statistical significance analysed by ANOVA on ranks with Kruskal–Wallis post hoc H-test. b) Normal bronchial epithelial cell cultures transduced with nontargeting (NT) or HBD2 short hairpin (sh)RNA were treated with PBS (control) or FSL-1 and the antibacterial activity of apical secretions was measured. c) Levels of HBD2 protein in the apical secretions was measured by ELISA. b) and c) Data represent mean±sd calculated from two independent experiments conducted in triplicate. Statistical significance was calculated by ANOVA with Tukey's post hoc test.
Since FSL-1 stimulated HBD2, we examined the sufficiency of HBD2 in killing NTHi. HBD2 was genetically inhibited by using DEFB4 shRNA in normal airway epithelial cells, stimulated with FSL-1, and the apical secretions were examined for its ability to kill NTHi. Compared to NT-transduced controls, the apical secretions from HBD2 shRNA-transduced cells showed a significantly reduced ability to kill bacteria (figure 3b). HBD2 protein levels in the apical secretion were significantly lower in HBD2 shRNA-transduced cells than in NT shRNA-transduced control cells (figure 3c), indicating the efficient knockdown of HBD2. These results confirm that FSL-1-stimulated HBD2 via TLR2 pathway in airway epithelial cells plays an essential role in killing NTHi.
TLR2 expression is similar in COPD and normal airway epithelial cells
Next, we determined the expression of TLR2 by Western blot analysis. As observed previously [10], we did not observe a difference in the expression of TLR2 between normal and COPD bronchial epithelial cells (supplementary figure S3A and B). These results indicate that the defect in TLR2 signalling is not due to the attenuated expression of TLR2 in COPD.
Expression of IRAK-M is not altered in COPD
IRAK-M is an endogenous inhibitor and inhibits MyD88-dependent TLR signalling by binding to the IRAK-1/IRAK-4 dimer in Myddosome, thereby preventing dissociation of hyperphosphorylated IRAK-1 from the Myddosome and activation of TRAF-6 [21]. By Western blot analysis, there was no difference in the expression of IRAK-M between normal and COPD cultures (supplementary figure S3C and 3D), indicating that IRAK-M may not contribute to the observed dysregulation of TLR2 signalling in COPD bronchial epithelial cells.
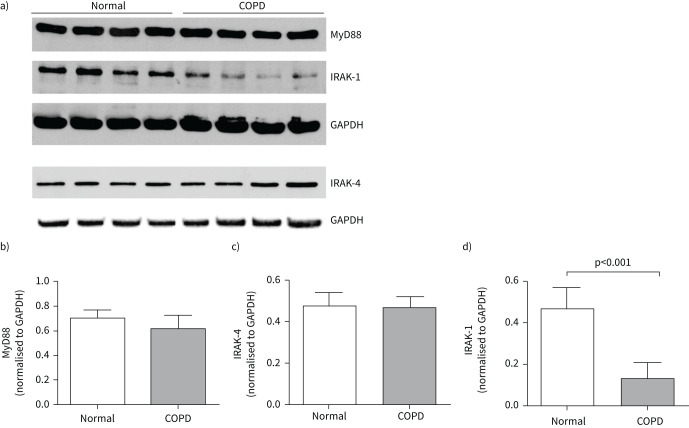
IRAK-1 expression is reduced in COPD bronchial epithelial cells
Next, we examined the expression of adaptor proteins MyD88, IRAK-4 and IRAK-1 between COPD and normal airway epithelial cells by Western blot analysis. There was no difference in the expression of MyD88 or IRAK-4 between COPD and normal cells (figure 4a–c). However, COPD cells showed significantly lower expression of IRAK-1 than normal bronchial epithelial cell cultures (figure 4a and d).
FIGURE 4.
Interleukin-1 receptor-associated kinase (IRAK)-1 expression is reduced in COPD bronchial epithelial cells. a) Total protein isolated from normal and COPD mucociliary-differentiated cultures was subjected to electrophoresis, proteins transferred to nitrocellulose membranes, blocked with 5% bovine serum albumin, and subjected to Western blot analysis with myeloid differentiation primary response gene 88 (MyD88), IRAK-4, IRAK-1 or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. b), c) and d) The intensity of the bands was quantified by ImageJ software and levels of MyD88, IRAK-4 and IRAK-1 were expressed as fold change over GAPDH. Data represent mean±sd calculated from 4–6 normal and COPD cultures, and statistical significance calculated by unpaired t-test.
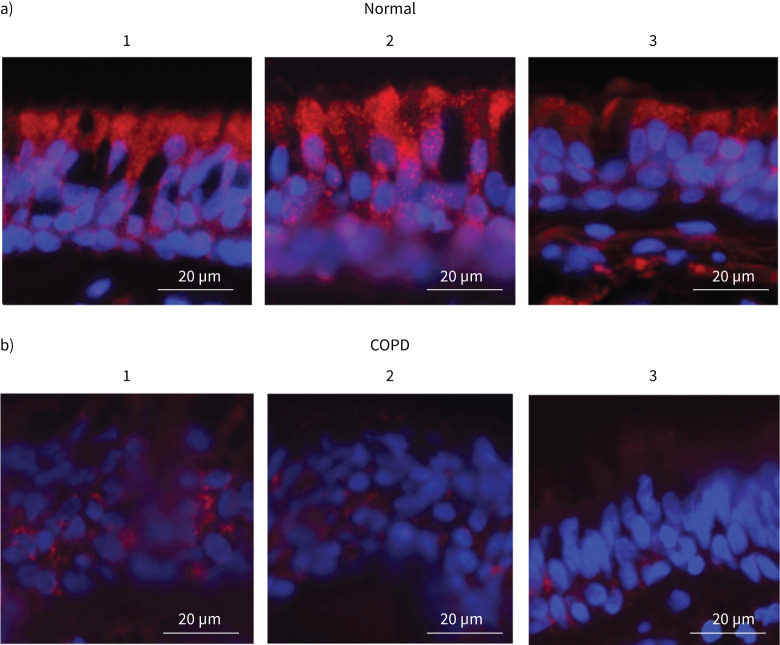
Next, we examined whether the expression of IRAK-1 is also affected in the bronchial epithelium of COPD patients by immunofluorescence microscopy. Immunostaining of lung sections containing secondary bronchi indicated that IRAK-1 expression is substantially reduced in COPD bronchial epithelium when compared with normal (figure 5). Interestingly, there was also a difference in the IRAK-1 localisation pattern; that is while normal bronchial epithelium showed IRAK-1 primarily at the apical side of the epithelium, in COPD it was observed in the cells closer to the basement membrane.
FIGURE 5.
Interleukin-1 receptor-associated kinase (IRAK)-1 expression is reduced in the bronchial epithelium of COPD patients. Paraffin lung sections containing secondary branching of bronchi from a) three normal and b) three COPD patients were deparaffinised, subjected to antigen unmasking and incubated with antibody to IRAK-1. The bound antibody was detected by a second antibody conjugated with horseradish peroxidase followed by signal amplification with tyramide conjugated with AlexaFluor 598 and then counterstained with 4′,6-diamidino-2-phenylindole. The exposure time for imaging with fluorescence microscope was adjusted with negative controls (normal lung sections stained with normal IgG instead of IRAK-1 antibody) to capture positive signals (n=3; red: IRAK-1, blue: nuclei).
Knockdown of IRAK-1 reduces FSL-1-mediated HBD2 expression
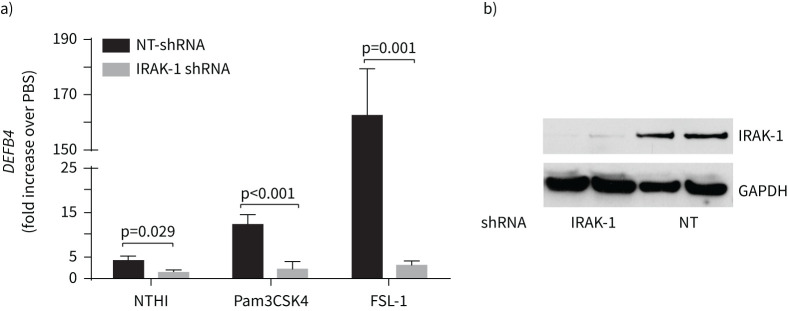
To examine the contribution of IRAK-1 in the expression of HBD2, IRAK-1 was genetically inhibited in normal epithelial cell cultures by using IRAK-1 shRNA. IRAK-1 knockdown significantly reduced NTHi, or TLR2 ligand-induced HBD2 expression (figure 6a). Knockdown of IRAK-1 was confirmed by Western blot analysis (figure 6b). IRAK-1 shRNA did not affect the expression of other IRAK proteins such as IRAK-4 and IRAK-M, indicating the specificity of the IRAK-1 shRNA (supplementary figure S4). These results confirmed the contribution of IRAK-1 in NTHi and TLR2 agonists-induced HBD2 expression.
FIGURE 6.
Interleukin-1 receptor-associated kinase (IRAK)-1 is required for DEFB4 response in airway epithelial cell cultures. a) Normal basal cells transduced with nontargeting or IRAK-1 short hairpin (sh)RNA were grown as mucociliary-differentiated cultures, challenged with nontypeable Haemophilus influenzae (NTHi), or Toll-like receptor (TLR)2 ligands, and examined for DEFB4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression at 3 h post-TLR2 treatment and 24 post-NTHi infection by reverse transcriptase quantitative PCR. The expression levels of DEFB4 mRNA was normalised to GAPDH and presented as a fold change over PBS-treated cultures. Data represent mean±sd calculated from three independent experiments conducted in duplicate using cells from two donors. Statistical significance was calculated by ANOVA with Tukey's post hoc test. b) Total protein isolated from cell cultures was subjected to electrophoresis, proteins transferred to membrane and subjected to Western blot analysis with IRAK-1 or GAPDH antibody to confirm the successful knockdown of IRAK-1.
Mir146a expression is increased in COPD airway epithelial cells
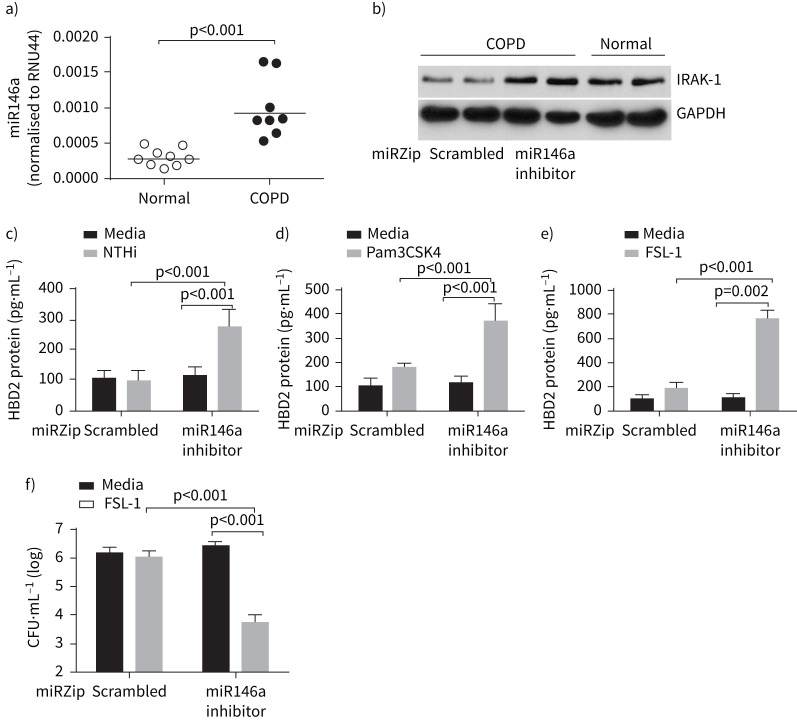
Given the role of miR146a in attenuating the expression of IRAK-1, we examined the expression of miR146a in normal and COPD airway epithelial cells. COPD cells showed a small but significant increase in miR146a expression (figure 7a). Genetic inhibition of miR146a in COPD epithelial cells increased the expression of IRAK-1 (figure 7b), but did not affect the expression of either IRAK-4 or IRAK-M (supplementary figure S5). MiR146a inhibition restored NTHi and TLR2 agonists stimulated HBD2 expression (figure 7c–e). Apical secretions from COPD cells transduced with miR146a inhibitor and stimulated with FSL-1 killed NTHi more efficiently than the secretions from similarly stimulated cells transduced with scrambled hairpin miR (figure 7f). These results indicate the contribution of miR146a in inhibiting HBD2 expression via attenuation of IARK-1 expression in COPD cells.
FIGURE 7.
Enhanced expression of microRNA (miR)146a contributes to the attenuated expression of interleukin-1 receptor-associated kinase (IRAK)-1 and nontypeable Haemophilus influenzae (NTHi)-stimulated β-defensin (HBD)2 in COPD epithelial cells. a) Total RNA isolated from normal and COPD bronchial epithelial cell cultures was subjected to reverse transcriptase quantitative PCR to determine the levels of miR146a and RNU44. Expression of miR146a was normalised to RNU44, a stably expressed miR. Data represent median with range (n=8), and the statistical significance calculated by Mann–Whitney test. b) Total protein isolated from COPD bronchial epithelial cell cultures transduced with scrambled or miR146a inhibitor and normal bronchial epithelial cell cultures were subjected to electrophoresis, proteins transferred to membrane and subjected to Western blot analysis with antibodies to IRAK-1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). c–e) COPD epithelial cells transduced with control or miR146a inhibitor were infected with NTHi, or treated with PBS (control), Pam3CSK4 or FSL-1, and the HBD2 protein levels were measured in the apical secretions by ELISA. f) Apical secretions from FSL-1-treated COPD epithelial cells transduced with control or miR146a inhibitor were incubated with NTHi for 2 h, and the viability of bacteria was measured by dilution plating. Data in c–f represent mean±sd calculated from two independent experiments conducted in triplicate and the statistical significance calculated by ANOVA with Tukey's post hoc test.
Effect of miR146a inhibition on NTHi or TLR2 ligand-induced IL-8 production
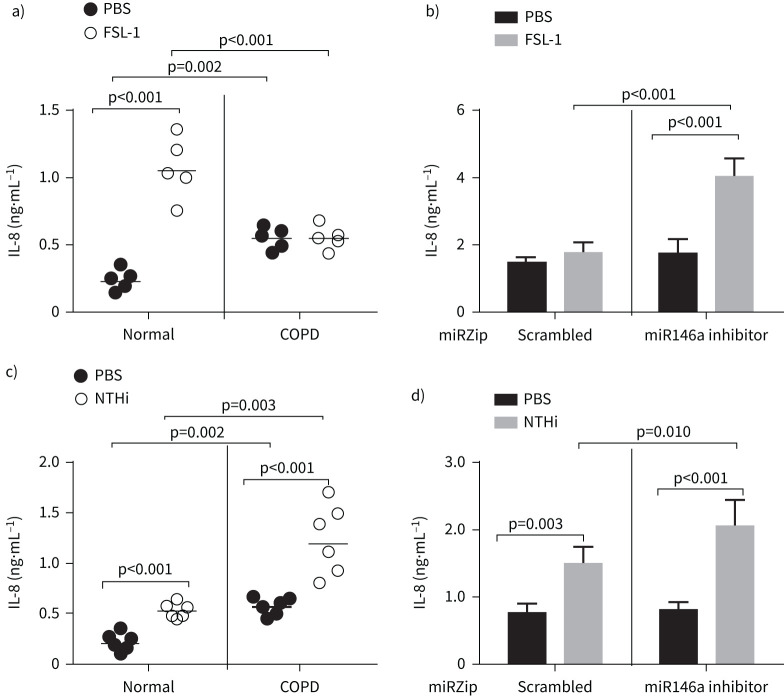
Previously, we demonstrated that compared to normal, COPD cell cultures show reduced IL-8 production in response to TLR2 agonists [10]. To determine the role of miR146a in TLR2 agonist-induced IL-8, we genetically inhibited miRNA in COPD cells and examined for IL-8 response to FSL-1. As observed previously, COPD cell cultures showed higher levels of IL-8 compared to normal under unstimulated conditions (PBS-treated) (figure 8a) [22, 23]. However, normal, but not COPD, cells showed increased expression of IL-8 following treatment with FSL-1, as observed previously [10]. Inhibition of miR146a increased the IL-8 response to FSL-1 in COPD cells (figure 8b) indicating that miR146a not only inhibits TLR2 agonist-induced HBD2, but also the pro-inflammatory response.
FIGURE 8.
Inhibition of microRNA (miR)146a restores Toll-like receptor (TLR)2 agonist-induced interleukin (IL)-8 expression in COPD epithelial cells. a and c) Mucociliary-differentiated normal and COPD epithelial cell cultures were treated with FSL-1 or infected with nontypeable Haemophilus influenzae (NTHi), incubated for 24 h, and IL-8 levels were measured in the basolateral medium using ELISA. Data represent range with medium (n=6) and statistical significance calculated by ANOVA on ranks with Kruskal–Wallis H-test. b and d) COPD epithelial cells (from two donors) transduced with control or miR146a inhibitor were infected with NTHi or treated with PBS (control) or FSL-1, and the IL-8 protein levels were measured by ELISA after 24 h. Data represent mean±sd calculated from two independent experiments conducted in triplicate and the statistical significance was calculated by ANOVA with Tukey's post hoc test.
Next, we examined whether miR146a also affects NTHi-induced IL-8 in COPD cell cultures. Unlike FSL-1, NTHi induced IL-8 expression in COPD cells (figure 8c), which further increased in cells transduced with miRZip miRNA146a inhibitor (figure 8d). These results may indicate that NTHi stimulates IL-8 not only via TLR2 pathway, but also by other pathways.
In summary, these results indicate that increased miR146a negatively regulates the expression of IRAK-1 in COPD airway epithelial cells. In turn, attenuated expression of IRAK-1 may contribute to attenuated expression of HBD2 in response to TLR2 agonists and NTHi infection.
Discussion
Epithelium lining the upper respiratory tract is the first line of defence against inhaled pathogens; therefore, it is equipped with powerful innate immune defence mechanisms. Antimicrobial peptides such as defensins and cathelicidins represent a pivotal part of airway epithelial innate defence mechanisms because of their broad-spectrum activity against bacteria, fungi and enveloped viruses [24]. Inducible antimicrobial peptides in airway mucosa provide protection against bacterial challenge in a mouse model of airway infection [25]. Previous studies have shown that COPD airway epithelial cells are deficient in expressing an inducible antimicrobial peptide, HBD2, which correlated with the defective killing of NTHi by these cells [3, 6]. This report highlights one of the mechanisms underlying the defective HBD2 expression in COPD airway epithelial cells.
TLRs are evolutionarily conserved pathogen recognition molecules, and airway epithelial cells express all TLRs at the protein level, except for TLR8 [26]. TLR2 and TLR4 are required for optimal clearance of bacteria in a mouse model of NTHi infection, bacteria-induced early chemokine and cytokine production and the development of adaptive immune responses [27, 28]. Moreover, in human middle ear epithelial cells, activation of the MyD88-dependent signalling pathway via TLR2, but not TLR4, is required for NTHi-induced expression of HBD2 [29]. Cigarette smoke causes dysregulation of TLR2 signalling by inducing TLR2 hyposensitivity [30]. We have shown previously that TLR2 signalling is dysregulated in COPD tracheobronchial epithelial cell cultures despite expressing TLR2, similar to normal cells [10]. For the first time, this study demonstrates that attenuated expression of IRAK-1 via upregulated expression of miR146a contributes to dysregulated TLR2 signalling in COPD airway epithelial cells.
Here, we show that NTHi induces the expression of DEFB4, a gene encoding HBD2 in normal, but not in COPD mucociliary-differentiated cultures. In addition, we observed significantly attenuated levels of secreted HBD2 in NTHi-infected COPD epithelial cells at 24 h post-infection compared to similarly infected normal cultures. This was associated with a higher bacterial load in COPD cells than normal cultures. In the previous report the difference in HBD2 protein levels between normal and COPD cells was not observed until 72 h post-infection and this may be due to the use of ultraviolet-inactivated NTHi, which is replication deficient [3]. In the present study, we used live NTHi, which can induce HBD2 within 24 h. However, prolonged incubation beyond 24 h caused cell death due to increased bacterial load.
Interestingly, the TLR2 agonists Pam3CSK4 and FSL-1, which bind and activate TLR2 induced HBD2 in normal, but not in COPD cells, indicating the contribution of TLR2 to HBD2 expression. Apical secretions from FSL-1-treated normal, but not COPD, cultures effectively killed NTHi. Furthermore, genetic inhibition of HBD2 in normal cells significantly attenuated the ability of apical secretions to kill NTHi, indicating the pivotal contribution of HBD2 to antimicrobial activity against NTHi in airway epithelial cells. Since there was no difference in the expression of TLR2 between normal and COPD bronchial epithelial cell cultures, defects in downstream signalling may contribute to the dysregulation of TLR2 signalling in COPD.
IRAK-M is an endogenous inhibitor of MyD88-dependent TLR signalling. The mRNA expression of IRAK-M is increased in sputum cells obtained from COPD patients, particularly in patients with frequent exacerbations [31]. However, in this study, there was no difference in the expression of IRAK-M between normal and COPD bronchial epithelial cells. The observed discrepancy may be due to differences in the cell types and the microenvironment. The sputum is enriched in innate immune cells, such as alveolar macrophages, monocytes, neutrophils, T-cells and others. Therefore, increased IRAK-M expression may correspond to these cells and not epithelial cells. COPD patients, especially those with a history of exacerbations, are treated with inhaled corticosteroids [32], and this can potentially increase IRAK-M expression, particularly in macrophages and other innate immune cells [33]. The bronchial epithelial cells used in this study were obtained from patients with end-stage lung disease who may have been treated with corticosteroids. The fact that there was no difference in the expression of IRAK-M between normal and COPD cells indicates that corticosteroids’ effect may not persist for an extended period. These observations also suggest that the observed dysregulation of TLR2 in COPD epithelial cells is not due to IRAK-M.
Intriguingly, the assessment of Myddosome adaptor proteins indicated significant reduction in the expression of IRAK-1, but not IRAK-4 or MyD88. Moreover, knockdown of IRAK-1 abrogated TLR2 ligands or NTHi-induced HBD2, indicating the contribution of IRAK-1 in this process. Several mechanisms regulate IRAK-1 expression. For instance, after activating MyD88-dependent signalling, IRAK-1 is rapidly degraded to terminate TLR signalling and maintain homeostasis [34, 35]. TLR2/TLR4 activation induces the expression of miR146a, which acts as a negative feedback regulator of IRAK-1 to dampen the magnitude of innate immune activation [36–38]. Our previous studies have indicated that compared to normal, COPD airway epithelial cells show higher protein expression of IL-6, CXCL-1 and IL-8 under unstimulated conditions [10, 22, 23], indicating persistent activation of TLR or other innate immune signalling pathways. Such persistent activation may modulate the expression of negative regulators of the TLR pathway, such as miR146a, to dampen the excessive activation of innate immune signalling. Consistent with this notion, we observed significantly higher expression of miR146a in COPD than in normal bronchial epithelial cells. Inhibition of miR146a restored IRAK-1 expression and NTHi or TLR2 agonist-stimulated HBD2 and antimicrobial activity in COPD epithelial cells. These observations indicate that the increased expression of miR146a to counteract the excessive TLR signalling to maintain homeostasis may become detrimental during bacterial infections.
It should be noted that IRAK-1 participation in TLR and IL-1R signalling is dependent both on species and cell type. For instance, IRAK-1 plays a predominant role in driving TLR and IL-1R signalling in human but not in murine macrophages [20]. Moreover, TLR signalling in fibroblasts from an infant with fatal inherited IRAK-1 deficiency showed impaired response to TLR ligands but not IL-1β [21]. In contrast, IRAK-1 deficiency did not affect TLR or IL-1R signalling in peripheral blood monocytic cells. Since epithelial cells are nonhaematopoietic cells like fibroblasts, and IRAK-1 expression is reduced in cultured COPD bronchial epithelial cells as well as in COPD bronchial epithelium, it is conceivable that reduced IRAK-1 may significantly contribute to impaired TLR2 signalling, but not IL-1 signalling, in COPD bronchial epithelial cells.
In addition to stimulating HBD2, activation of TLR2 signalling also induces pro-inflammatory cytokines. We observed that unlike HBD2, NTHi-induced IL-8 was not completely attenuated in COPD cells. This is not surprising because NTHi induces pro-inflammatory responses not only via TLR2 signalling, but also by other pathways [39, 40]. However, inhibition of miR146a further enhanced NTHi-induced IL-8 in COPD cells, indicating the contribution of IRAK-1 in this process.
One of the limitations of this study is that the bronchial epithelial cells were obtained from patients with severe lung disease with the forced expiratory volume in 1 s (FEV1) <40% predicted except for one patient (42% pred). Therefore, we could not determine the correlation between IRAK-1 expression with percentage predicted FEV1. Moreover, this population in general are afflicted with a higher frequency and severity of clinical exacerbations; therefore, cells from these patients may show increased dysregulation of TLR2. Bronchial epithelial cells from patients with mild to moderate disease may show variable expression of IRAK-1 and TLR2 desensitisation. Moreover, all the patients had stopped smoking ≥6 months prior to double lung transplantation; therefore, it is hard to predict the effect of smoking. It will be interesting to compare the expression of IRAK-1, dysregulation of TLR2 signalling in patients with mild to moderate disease and current and former smokers, but such studies require prospective collection of tissues, which is beyond the scope of the present study. In summary, although impaired TLR2 signalling in COPD has been known, as far as we know, this is the first report to elucidate one of the underlying mechanisms.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00694-2022.SUPPLEMENT (5.6MB, pdf)
Acknowledgements
We thank Fernando Martinez and Catherine Meldrum (University of Michigan, Ann Arbor, MI, USA), and Nathaniel Marchetti (Temple University, Philadelphia, PA, USA) for providing tracheobronchial tissue segments from COPD and normal subjects.
Provenance: Submitted article, peer reviewed.
Support statement: This work was supported by NIH grant AT007620. Funding information for this article has been deposited with the Crossref Funder Registry.
Author contributions: H. Reddy-Vari designed and conducted the experiments with primary cells and analysed the samples; Y. Kim isolated RNA and analysed the samples by quantitative PCR; C. Rajput performed immunofluorescence on the human lung tissue sections; and U.S. Sajjan conceived the project, performed final analysis of the data and prepared the manuscript.
Data availability statement: All the data are available from the corresponding author on reasonable request.
Conflict of interest: The authors declare that they have no competing interests.
References
- 1.Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev 2010; 19: 113–118. doi: 10.1183/09059180.00002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sriram KB, Cox AJ, Clancy RL, et al. Nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease: a review for clinicians. Crit Rev Microbiol 2018; 44: 125–142. doi: 10.1080/1040841X.2017.1329274 [DOI] [PubMed] [Google Scholar]
- 3.Amatngalim GD, Schrumpf JA, Henic A, et al. Antibacterial defense of human airway epithelial cells from chronic obstructive pulmonary disease patients induced by acute exposure to nontypeable Haemophilus influenzae: modulation by cigarette smoke. J Innate Immun 2017; 9: 359–374. doi: 10.1159/000455193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenson CS, Kruzel RL, Eberhardt E, et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax 2014; 69: 811–818. doi: 10.1136/thoraxjnl-2013-203669 [DOI] [PubMed] [Google Scholar]
- 5.Berenson CS, Wrona CT, Grove LJ, et al. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med 2006; 174: 31–40. doi: 10.1164/rccm.200509-1461OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnason JW, Murphy JC, Kooi C, et al. Human β-defensin-2 production upon viral and bacterial co-infection is attenuated in COPD. PLoS One 2017; 12: e0175963. doi: 10.1371/journal.pone.0175963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pace E, Ferraro M, Minervini MI, et al. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One 2012; 7: e33601. doi: 10.1371/journal.pone.0033601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Andalibi A, Webster P, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis 2004; 4: 12. doi: 10.1186/1471-2334-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Zhang Z, Louboutin JP, et al. Airway epithelia regulate expression of human β-defensin 2 through Toll-like receptor 2. FASEB J 2003; 17: 1727–1729. doi: 10.1096/fj.02-0616fje [DOI] [PubMed] [Google Scholar]
- 10.Xander N, Reddy Vari H, Eskandar R, et al. Rhinovirus-induced SIRT-1 via TLR2 regulates subsequent type I and type III IFN responses in airway epithelial cells. J Immunol 2019; 203: 2508–2519. doi: 10.4049/jimmunol.1900165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motshwene PG, Moncrieffe MC, Grossmann JG, et al. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem 2009; 284: 25404–25411. doi: 10.1074/jbc.M109.022392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawagoe T, Sato S, Matsushita K, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol 2008; 9: 684–691. doi: 10.1038/ni.1606 [DOI] [PubMed] [Google Scholar]
- 13.Watters TM, Kenny EF, O'Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol 2007; 85: 411–419. doi: 10.1038/sj.icb.7100095 [DOI] [PubMed] [Google Scholar]
- 14.Di Stefano A, Caramori G, Oates T, et al. Increased expression of nuclear factor-κB in bronchial biopsies from smokers and patients with COPD. Eur Respir J 2002; 20: 556–563. doi: 10.1183/09031936.02.00272002 [DOI] [PubMed] [Google Scholar]
- 15.Bayraktar R, Bertilaccio MTS, Calin GA. The interaction between two worlds: microRNAs and Toll-like receptors. Front Immunol 2019; 10: 1053. doi: 10.3389/fimmu.2019.01053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzie ME, Crawford M, Cho JH, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 2012; 67: 122–131. doi: 10.1136/thoraxjnl-2011-200089 [DOI] [PubMed] [Google Scholar]
- 17.O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 2011; 11: 163–175. doi: 10.1038/nri2957 [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Zhang X, Ju Y, et al. MicroRNA-146a feedback suppresses T cell immune function by targeting Stat1 in patients with chronic hepatitis B. J Immunol 2013; 191: 293–301. doi: 10.4049/jimmunol.1202100 [DOI] [PubMed] [Google Scholar]
- 19.Jing Y, Gimenes JA, Mishra R, et al. NOTCH3 contributes to rhinovirus-induced goblet cell hyperplasia in COPD airway epithelial cells. Thorax 2019; 74: 18–32. doi: 10.1136/thoraxjnl-2017-210593 [DOI] [PubMed] [Google Scholar]
- 20.Pineau F, Shumyatsky G, Owuor N, et al. Microarray analysis identifies defects in regenerative and immune response pathways in COPD airway basal cells. ERJ Open Res 2020; 6: 00656-2020. doi: 10.1183/23120541.00656-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi K, Hernandez LD, Galán JE, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002; 110: 191–202. doi: 10.1016/S0092-8674(02)00827-9 [DOI] [PubMed] [Google Scholar]
- 22.Ganesan S, Unger BL, Comstock AT, et al. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax 2013; 68: 131–141. doi: 10.1136/thoraxjnl-2012-201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider D, Ganesan S, Comstock AT, et al. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182: 332–340. doi: 10.1164/rccm.200911-1673OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest 2002; 109: 693–697. doi: 10.1172/JCI0215218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans SE, Xu Y, Tuvim MJ, et al. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol 2010; 72: 413–435. doi: 10.1146/annurev-physiol-021909-135909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClure R, Massari P. TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front Immunol 2014; 5: 386. doi: 10.3389/fimmu.2014.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wieland CW, Florquin S, Maris NA, et al. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable Haemophilus influenzae from the mouse lung. J Immunol 2005; 175: 6042–6049. doi: 10.4049/jimmunol.175.9.6042 [DOI] [PubMed] [Google Scholar]
- 28.Lugade AA, Bogner PN, Murphy TF, et al. The role of TLR2 and bacterial lipoprotein in enhancing airway inflammation and immunity. Front Immunol 2011; 2: 10. doi: 10.3389/fimmu.2011.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HY, Takeshita T, Shimada J, et al. Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infect Dis 2008; 8: 87. doi: 10.1186/1471-2334-8-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagaitkar J, Demuth DR, Daep CA, et al. Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS One 2010; 5: e9323. doi: 10.1371/journal.pone.0009323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baines KJ, Fu JJ, McDonald VM, et al. Airway gene expression of IL-1 pathway mediators predicts exacerbation risk in obstructive airway disease. Int J Chron Obstruct Pulmon Dis 2017; 12: 541–550. doi: 10.2147/COPD.S119443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tashkin DP, Strange C. Inhaled corticosteroids for chronic obstructive pulmonary disease: what is their role in therapy? Int J Chron Obstruct Pulmon Dis 2018; 13: 2587–2601. doi: 10.2147/COPD.S172240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyata M, Lee JY, Susuki-Miyata S, et al. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat Commun 2015; 6: 6062. doi: 10.1038/ncomms7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubo-Murai M, Hazeki K, Nigorikawa K, et al. IRAK-4-dependent degradation of IRAK-1 is a negative feedback signal for TLR-mediated NF-κB activation. J Biochem 2008; 143: 295–302. doi: 10.1093/jb/mvm234 [DOI] [PubMed] [Google Scholar]
- 35.Cui W, Xiao N, Xiao H, et al. β-TrCP-mediated IRAK1 degradation releases TAK1-TRAF6 from the membrane to the cytosol for TAK1-dependent NF-κB activation. Mol Cell Biol 2012; 32: 3990–4000. doi: 10.1128/MCB.00722-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saba R, Sorensen DL, Booth SA. MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front Immunol 2014; 5: 578. doi: 10.3389/fimmu.2014.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taganov KD, Boldin MP, Chang KJ, et al. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006; 103: 12481–12486. doi: 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou J, Wang P, Lin L, et al. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 2009; 183: 2150–2158. doi: 10.4049/jimmunol.0900707 [DOI] [PubMed] [Google Scholar]
- 39.Watanabe T, Jono H, Han J, et al. Synergistic activation of NF-κB by nontypeable Haemophilus influenzae and tumor necrosis factor α. Proc Natl Acad Sci USA 2004; 101: 3563–3568. doi: 10.1073/pnas.0400557101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyl KA, Klassert TE, Heinrich A, et al. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. mBio 2014; 5: e01492-14. doi: 10.1128/mBio.01492-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00694-2022.SUPPLEMENT (5.6MB, pdf)