Abstract
Early Career Members of Assembly 2 (Respiratory Intensive Care) attended the 2022 European Respiratory Society (ERS) International Congress in Barcelona, Spain. The conference covered acute and chronic respiratory failure. Sessions of interest to our Assembly members and to those interested in respiratory critical care included the state-of-the-art session on respiratory critical care, the journal session (ERS/Lancet) on acute respiratory distress syndrome (ARDS) phenotyping into precision medicine, and sessions on specificity of coronavirus disease 2019 ARDS and its post-critical care. A symposium on treatment of acute respiratory failure in patients with COPD and innovations in mechanical ventilation either in the intensive care unit or at home were also reported upon. These sessions are summarised in this article.
Short abstract
The Early Career Members of Assembly 2 (Respiratory Intensive Care) attended the #ERSCongress 2022 in Barcelona. They report on sessions related to the theme of this assembly: in particular, ARDS and mechanical ventilation. https://bit.ly/3QwQVxy
Introduction
Assembly 2 of the European Respiratory Society (ERS) encompasses the broad fields of respiratory critical care. Our assembly is divided into two groups, those of acute critical care and of non-invasive support. Currently, Christian Karagiannidis heads our assembly, João Winck has the role of secretary, and Christoph Fisser is our Early Career Representative. The acute critical care subgroup is chaired by Ignacio Martin-Loeches, with Ana Cysneiros as secretary. The non-invasive support group is chaired by Marieke Duiverman, and Claudia Crimi is secretary. At the time of publication, we have 1672 Assembly 2 members, 36% of whom are early career members and 50% of them are female. In this review, we present highlights from the ERS International Congress 2022 of interest to Assembly 2 members and those interested in critical care and mechanical ventilation. The sessions we have reported on include the symposia on acute respiratory distress syndrome (ARDS) phenotypes, non-invasive ventilation (NIV) in hypoxaemic respiratory failure and new developments in mechanical ventilation and weaning, and the guidelines session on high-flow nasal cannula (HFNC) in adults with acute respiratory failure.
ARDS: the path to precision medicine (a joint ERS/Lancet session)
Lorraine Ware (Nashville, TN, USA) began the session by highlighting that ARDS is a heterogenous clinical syndrome. Different methods have been employed to subclassify ARDS based on aetiology, severity, radiological distribution and biological markers [1]. The hyperinflammatory phenotype characterised by Calfee et al. [2] has demonstrated a higher mortality and differential response to treatment. Further advances in ARDS phenotyping may hold promise for future personalised medicine.
Danny McAuley (Belfast, UK) emphasised the limitations of current Berlin definition of ARDS. He suggested incorporating ultrasound to identify lung infiltrates, using the ratio of oxygen saturation measured by pulse oximetry to inspiratory oxygen fraction (SpO2/FIO2 ratio) as a non-invasive marker of oxygenation and removing minimal positive end-expiratory pressure (PEEP) requirement with the advent of high flow nasal oxygen. The cornerstone of ARDS management includes lung protective ventilation, prone positioning and restrictive fluid strategy [3–5]. The recent REST trial explored extracorporeal CO2 removal (ECCO2R) to further lower tidal volumes, without mortality benefit [6]. Additionally, there is an urgent need to assess the long-term outcomes post-ARDS.
Martin Kneyber (Groningen, the Netherlands) presented the paediatric-specific definition and management of ARDS [7]. Interestingly, in the paediatric population, studies have not shown a strong correlation between tidal volume and mortality [8]. However, mortality was higher in children managed with lower PEEP than recommended by the ARDSNet protocol [9]. Increased driving pressure was associated with prolonged time to extubation [10]. More randomised controlled trials are needed to guide individualised therapy in paediatric ARDS.
Michael A. Matthay (San Francisco, CA, USA) concluded the session with lessons learned from past clinical trials. Moving forward, he recommended designing ARDS trials that reduce heterogeneity by targeting treatable traits, yet applicable to diverse global population [11]. A global definition of ARDS was proposed at an international consensus conference to address these goals.
Take-home message
Given the heterogeneity of ARDS, further phenotyping into treatable traits is key to precision medicine in both adults and children.
Beyond COVID-19: translating COVID-19 treatment successes to all-cause ARDS
Tiffanie Jones (Philadelphia, PA, USA) reflected on the need to identify and translate coronavirus disease 2019 (COVID-19) biomarkers into ARDS' treatable traits [12, 13]. Alveolar/endothelial injury contributes to ARDS at different stages [14]; the receptor for advanced glycation endproducts (RAGE) is defined as an alveolar/epithelial injury marker, and its soluble form (sRAGE), a possible treatable trait, is associated with the risk of ARDS [15–19]. Anti-RAGE therapies have been tested in preclinical models with success [20, 21].
Manu Shankar-Hari (Edinburgh, UK) described a molecular signature with a cytokine pattern connected to each ARDS phenotype [22–24]. Targeting inflammation positively impacts mortality. As such, interleukin (IL)-6 antagonist reduced mortality in patients with COVID-19 [25], anti-TNF-α therapy increased survival in septic patients [26], and reparixin appeared to be effective for the treatment of patients with COVID-19 pneumonia [27]. The success of a therapy might be associated with the dominant activated pathway at the moment of the treatment, according to the patient's phenotype.
Jurjan Aman (Amsterdam, the Netherlands) defined the necessity to measure vascular stability and leakage as a critical player for ARDS due to COVID-19 [14, 28–31]. In this regards, angipoietin-2 has been suggested as a marker [32]. To improve alveolocapillary function, imatinib (a tyrosine kinase inhibitor) has been used in COVID-19 patients [33]. Imatinib trials, COUNTER-COVID and INVENT-COVID, exhibited an improvement in the clinical outcome in severe COVID-19 and reduced extravascular lung water index (EVLWi) in subgroups of ARDS due to COVID-19 patients, measured by pulse contour cardiac output monitoring [33–36].
Trials in critical care are challenging due to heterogeneity of the patient population and inefficiencies in obtaining data, including long-term outcomes. Carolyn Calfee (San Francisco, CA, USA) introduced adaptive trials (trials with pre-planned capabilities to adjust design factors) as a proposal to deal with the ARDS phenotype heterogeneity, the stratified randomisation and a response adaptation [37–39]. They had a considerable impact during COVID-19: for example, RECOVERY evaluated 10 treatments in 47 879 participants in 199 sites, and I-SPY-COVID is an adaptive platform for a phase 2 clinical trial to identify agents with potential therapeutic benefit [40, 41].
Take-home messages
The need for finding new biomarkers and treatable traits to develop new therapies targeted against ARDS is evident. Most treatments of ARDS are directed against inflammation, but we must not miss the treatment for vascular leakage.
Adaptive clinical trials have proven to be a useful tool in finding targeted treatments for ARDS.
State-of-the-art: respiratory critical care
Carolyn Calfee (San Francisco, CA, USA) presented the clinical implications of phenotyping ARDS. Several ways of phenotyping have been proposed and have demonstrated acceptable results in different settings according to the aetiology or the severity of the underlying disease (e.g. COVID-19 ARDS [42]). Biomarker models have identified a hyperinflammatory phenotype [1, 2, 43, 44] that have been validated in different cohorts [45, 46]. This might help in the future treatment because hyperinflammatory ARDS might respond to higher PEEP or corticosteroids [42, 46]. However, prospective studies evaluating this concept are still needed.
Laurent Brochard (Toronto, ON, Canada) discussed the clinical implications of patient self-inflicted lung injury (p-SILI). Experimental studies have demonstrated p-SILI [47, 48]; however, in clinical practice it is still a concept. There is indirect clinical evidence of p-SILI such as a high expired tidal volume that is independently associated with NIV failure in patient with acute hypoxaemic respiratory failure [49]. Monitoring techniques including airway occlusion pressure (Pocc) [50] could help to better understand it, although oesophageal pressure (Poes) is the gold standard [51]. Clinical implications hypothesises that mortality is higher with NIV than with HFNC due to p-SILI [52]. Partial neuromuscular blockade, ventilation with higher PEEP and higher FIO2 presents as a promising treatment for p-SILI [53–55].
Lise Piquilloud (Lausanne, Switzerland) commented on the advanced respiratory monitoring in acute respiratory failure. Current recommendations do not address potential phenotypes, chest wall compliance or p-SILI risk [56]. A well targeted Poes ventilation strategy potentially improves ARDS outcomes, especially in patients with lower APACHE-II scores [57, 58]. Respiratory drive and effort monitoring is relatively easy and indicates patient demand during assisted mechanical ventilation [50, 59]. Pocc at 100 ms (P0.1) is a not-so-new technique but useful [60–64] and might predict relapse in COVID-19 ARDS patients [65].
Stefano Nava (Bologna, Italy) presented whether HFNC or NIV should be used for acute hypoxaemic respiratory failure. There are contradictory results on this matter. While the FLORALI study found lower mortality with HFNC in the severe subgroup of patients [52], it failed to reduce therapeutic escalation compared to standard oxygen therapy in mild hypoxaemic COVID-19 patients [66]. Helmet NIV reduced the incidence of intubation compared with HFNC in COVID-19 [67] and other studies found them to be equivalent [68]. Nonetheless, a trial of NIV might be offered to treat de novo acute hypoxaemic respiratory failure, when treated by an experienced team [69].
Take-home messages
ARDS phenotypes are already treated differently, in terms of severity and COVID-19 versus non-COVID-19 distress.
New methods for identifying molecular phenotypes using biomarkers and/or clinical data have been developed, although clinical testing is still needed.
COVID-19 acute respiratory distress syndrome
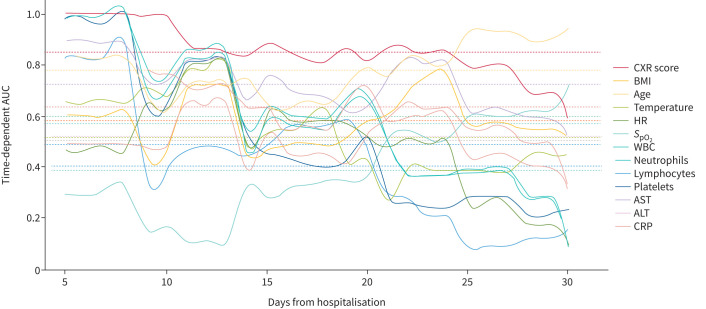
The usefulness of day 1 chest X-ray score for predicting mortality and intensive care unit (ICU) admission in COVID-19 patients was reported by Trieu-Nghi Hoang-Thi (Ho Chi Minh City, Vietnam). In 219 patients hospitalised for COVID-19 pneumonia, a simple severity score based on lung consolidation observed on chest X-ray, with a maximal score of 24, was assessed. Each point increase in this score increased the risk of death over time by 1.33 (HR 1.33, 95% CI 1.10–1.62) and was a strong predictor of mortality in the first 25 days (figure 1). This score had a good sensitivity and specificity and could be a useful tool in hospitals where computed tomography is not readily available [70].
FIGURE 1.
Time-dependent area under receiver operating characteristics curve (AUC) for predicting death within 30 days among 13 clinical parameters. CXR: chest X-ray; BMI: body mass index; HR: heart rate; SpO2: oxygen saturation measured by pulse oximetry; WBC: white blood cells; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CRP: C-reactive protein. Reproduced and modified from [70] with permission.
Elizabeth Rohrs and Thiago Bassi (Burnaby, BC, Canada) presented reports on the value of temporary transvenous diaphragm neurostimulation (TTDN) in ARDS. The studies were conducted on a model of moderate ARDS in deeply sedated pigs. TTDN significantly reduced the total mechanical work of breathing by 19%. Neurostimulation of the diaphragm with each breath modulated the neuroinflammation associated with moderate ARDS by attenuating the proportion of pro-inflammatory microglia in the hippocampus compared to mechanical ventilation alone [71]. Finally, TTDN resulted in 41% less atelectasis and improved homogeneity of alveolar expansion in pigs with moderate ARDS [72]. These results support TTDN as a new tool to improve ARDS outcomes.
Leila Atmowihardjo (Maastricht, the Netherlands) introduced the efficacy and safety of intravenous imatinib in invasively ventilated patients with moderate to severe COVID-19 ARDS, in a multicentre randomised double-blind, phase 2 study. Imatinib was administered twice daily versus placebo and EVLWi (primary outcome) was measured once daily by Pulse Contour Cardiac Output monitoring. 33 patients, mainly men, with a moderate ARDS, were included in each group. There was no significant effect of imatinib on variation of EVLWi or on clinical outcomes between day 1 and day 7, but a biological sub-phenotype of patients (n=20) has been identified, characterised by high levels of alveolar epithelial injury markers that had a decrease of EVLWi over time and needs further characterisation. There were no safety concerns in this population.
Ofir Deri (Tel Hashomer, Israël) reported on the outcomes of patients with COVID-19-associated respiratory failure being registered on the lung transplantation list (n=20), in a single-centre retrospective study. Among these 20 patients (12 males), median age was 49.5 (43.8–57.5) years and median body mass index was 30.5 (28.9–31.1) kg·m−2. Four patients underwent lung transplantation and seven died while waiting on the list. The surviving patients were younger (p=0.016) and spent less time under extracorporeal membrane oxygenation (ECMO) (p=0.044).
Jessica Gonzalez Gutierrez (Lleida, Spain) presented an overview and follow-up in a post-COVID-19 consultation of critically ill patients (i.e. with ICU admission) in a prospective observational cohort. At 12-month follow-up (n=97), one-third of patients needed to continue follow-up due to low diffusing capacity of the lung for carbon monoxide, chest computed tomography abnormalities or persistent symptoms, leading to a high use of healthcare resources.
Take-home messages
A new score, based only on the first-day chest X-ray, may be useful in hospitalised patients with COVID-19 if computed tomography is not available.
Temporary transvenous neurostimulation of the diaphragm improved ARDS outcomes in preclinical studies and needs to be evaluated in patients.
A sub-phenotype of invasively ventilated COVID-19 patients may benefit from imatinib and need further characterisation.
Post-critical care long COVID: reducing the physical and emotional toll
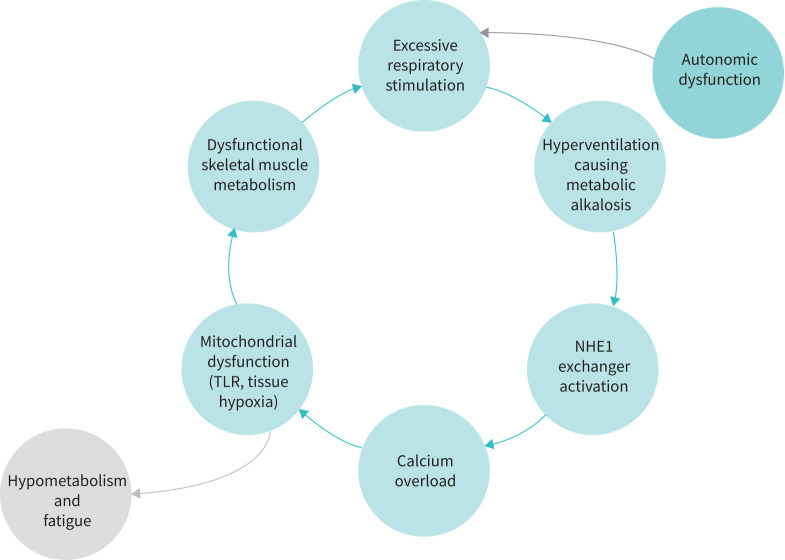
The pathophysiology of post-ICU COVID-19 symptoms was presented by Negin Hajizadeh (New York, NY, USA). 25% to 75% of post-ICU COVID-19 patients reported new disabilities, mainly fatigue, exertional dyspnoea and mental health problems, often irrespective of the severity of the acute illness [73]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) not only causes direct alveolar damage, but also promotes aberrant angiogenesis and microthrombi formation [28]. However, COVID-19 lung lesions only partly explain the dyspnoea and fatigue of some of these patients. Indeed, skeletal muscle wasting promoted by inflammatory cytokines (IL-6 and TNF-α) and myalgic encephalomyelitis due to mitochondrial dysfunction could contribute to the post-COVID-19 hyperventilation syndrome (figure 2) [74].
FIGURE 2.
Proposed hyperventilation syndrome and post-acute COVID-19 dyspnoea vicious cycle. NHE1: sodium-hydrogen antiporter 1; TLR: Toll-like receptors.
Nicholas Hart (London, UK) opened his presentation on prolonged mechanical ventilation (PMV) in COVID-19 patients by pointing out the heterogeneity of PMV definitions across the scientific literature, which urgently requires a standardisation [75]. The effects of evolving standard-of-care, vaccination and virus biology greatly reflected on critical care occupancy and the need for PMV. Indeed, data from the Guy's and St Thomas' NHS Hospital (London, UK) showed that the survival of critical COVID-19 patients progressively increased (from 70% of wave one (April 2020) to 87% of wave four (April 2022)). Nevertheless, during waves three and four, the proportion of patients requiring ECMO doubled, mainly due to the more frequent admission of unvaccinated pregnant women. The recovery process should be focused on “reverse the reversible”, treating each sequela due to SARS-CoV-2 infection that could be addressed.
Tracy Vannorsdall (Baltimore, MD, USA) presented a topic called “Managing anxiety, depression and cognitive impairment to promote recovery”. Higher rates of depressive symptoms were noticed after the start of the pandemic and they were more common in those with lower income, less savings and more stressors [76]. Interestingly, the rate of objective cognitive dysfunction was much lower than subjective complaints. Thus, clinicians should also target other factors such as anxiety, depression or fatigue in order to improve objective and subjective functioning. Data from a long COVID clinic showed that 4 months after leaving ICU, patients had significant levels of psychiatric stress and their cognitive scores decreased [77].
Mara Paneroni (Brescia, Italy) talked about rehabilitation modalities to address physical morbidity and support recovery. Multinational task force recommends early, bedside rehabilitation for patients affected by severe COVID-19 [78]. A global protocolised weaning strategy, started early in the ICU and followed by intensive rehabilitation in a specialised centre, accelerated the physical recovery and psychological status in ICU survivors from COVID-19 ARDS [79].
Take-home messages
Long-term impairment is common in post-acute COVID-19 patients.
Discrepancies between COVID-19 lung parenchymal damage and dyspnoea severity can be explained by myalgic encephalomyelitis and hyperventilation syndrome.
The progressive improvement of the standard of care and the evolving virus biology resulted in a reduction of patients requiring intensive treatment and prolonged mechanical ventilation.
Psychiatric and cognitive disorders became more frequent during the SARS-CoV-2 pandemic.
Early physical rehabilitation is crucial for the optimal recovery of COVID-19 patients.
Treatment of acute respiratory failure in COPD patients
Marieke Duiverman (Groningen, the Netherlands) presented NIV as the first-line intervention to relieve work of breathing in patients with COPD presenting with acute hypercapnic respiratory failure. NIV clinical practice varies widely across hospitals [80]. Evidence-based use improves exacerbation outcomes, including mortality, endotracheal intubation, hospitalisation duration, gas exchange and complications [81, 82], whereas delayed NIV implementation increases mortality [83]. The Non-invasive Ventilation Outcomes (NIVO) score serves as an outcome-prediction tool for in-hospital mortality [84, 85]. NIV settings [86] and patient comfort [87] should be optimised to prevent patient–ventilator asynchrony and intolerance. Home NIV should be considered as an earlier intervention for persistent hypercapnia [88, 89].
Paolo Navalesi (Padua, Italy) presented the role of HFNC in facilitating CO2 wash-out [90], relieving work of breathing through PEEP [91] and delivering humidified, warm air [92, 93]. Although better tolerated than NIV [94], HFNC is not an alternative [95–97] but an ancillary treatment during NIV breaks or withdrawal [98]. Regarding compensated hypercapnic respiratory failure, HFNC shows superiority over conventional oxygen therapy in improving hypercapnia [99] and need for NIV [100]. Post-extubation HFNC is recommended, but NIV remains pivotal for high-risk patients [97, 101].
Christian Karagiannidis (Cologne, Germany) illustrated the decreasing number of patients with COPD under invasive mechanical ventilation (IMV) during the COVID-19 pandemic. IMV poses higher risk of mortality, endotracheal intubation, complications and longer hospitalisation duration than NIV [102, 103]. ECCO2R may prevent or shorten duration of IMV by alleviating acidosis and respiratory rate [104, 105] and may improve right ventricle function by reducing pulmonary artery pressure [106]. However, application of ECCO2R raises technical issues related to recirculation rate [107] and bleeding/thromboembolic complications that limit its current use and therefore should only be used in clinical trials [105, 108].
Rebecca D'Cruz (London, UK) reflected on COPD exacerbations' detrimental impact on long-term outcomes, with higher mortality in patients requiring mechanical ventilation [109–111]. Interestingly, eosinophilic exacerbations show more favourable outcomes, including need for NIV and mortality [112]. R. D'Cruz highlighted the lung function decline and potential progression to respiratory failure associated with exacerbations [113, 114]. Extrapulmonary sequelae and comorbidities warrant a holistic approach [115–123].
Take-home messages
NIV remains the first-line intervention for acute hypercapnic respiratory failure in patients with exacerbated COPD. The NIVO score is a validated outcome-prediction tool.
HFNC facilitates NIV breaks or withdrawal, showing superiority over conventional oxygen therapy.
ECCO2R alleviates acidosis and respiratory rate, but further clinical trials on its safety and effectiveness are needed.
Extrapulmonary sequelae warrant a holistic approach to COPD exacerbations.
Innovations in non-invasive respiratory support
Nicole Sheers (Heidelberg, Australia) opened the session by presenting a randomised controlled trial that examined the feasibility of setting up NIV at home in patients with motor neuron disease. The patients were randomised to the NIV home model (single-day NIV initiation at home with remote usage monitoring, and weekly telephone follow-up) or a usual care control (single-day in-hospital NIV initiation with in-laboratory polysomnography follow-up). No significant between-group difference was observed in symptoms, quality of life, care burden or adherence.
Ana Díez Izquierdo (Barcelona, Spain) shared data from a retrospective review of 10 children with chronic lung diseases or neurological conditions necessitating home HFNC therapy. The review was conducted over 12 months with the aim of assessing the long-term benefits and safety of HFNC use. During follow-up, it was identified that home HFNC in children resulted in early discharge (40%) and reduction in hospital readmission (30%). This was attributed to the ability to treat exacerbations of disease at home. No adverse events were observed.
Chiara Torregiani (Trieste, Italy) presented a study investigating the utility of forced oscillatory technique as a potential marker of lung compliance in patients with COVID-19. The study enrolled 32 patients with moderate to severe COVID-19 ARDS who underwent NIV and alternated to HFNC. The study identified that forced oscillatory technique measurements can be used to identify abnormal respiratory reactance and could be used to assess patients. Currently, forced oscillatory technique is mainly applied in neonatology to expand the pathophysiological understanding of ARDS and in pre-clinical studies.
David Berlowitz (Heidelberg, Australia) concluded the session with an explanation of an artificial intelligence model which could be used to detect patient–ventilator asynchrony during NIV. The models, which used Multidimensional Matrix Profiles, were able to filter and de-noise polysomnography data to identify asynchronies. Their proposed model had 0.80 sensitivity and specificity for identifying patient–ventilator asynchrony.
Take-home message
Recent research has identified methods to increase the ease of use and optimisation of NIV in both the home and the hospital.
State-of-the-art in home mechanical ventilation
Barbara Garabelli (Milan, Italy) focused her presentation on alternative treatments of NIV support for chronic respiratory failure in neuromuscular diseases. Long-term mechanical ventilation improves survival and increases or maintains health-related quality of life (HRQoL) [124, 125]; however, dependency of the ventilator can affect quality of life especially in patients having continuously a mask on their face. Other types of NIV support can reduce side-effects related to prolonged ventilation such as mouthpiece ventilation or intermittent abdominal pressure ventilation. Mouthpiece ventilation with a volume mode is the preferable choice because it allows air-stacking and there is no leak compensation during patient disconnection [126, 127]. The settings usually suggested are no PEEP or back-up respiratory rate, a low trigger and a tidal volume according to the respiratory abilities and needs of the patient (500–1500 mL). Regarding intermittent abdominal pressure ventilation [128, 129], there is a lack of expert consensus guidelines on its indication, titration, management, and follow-up.
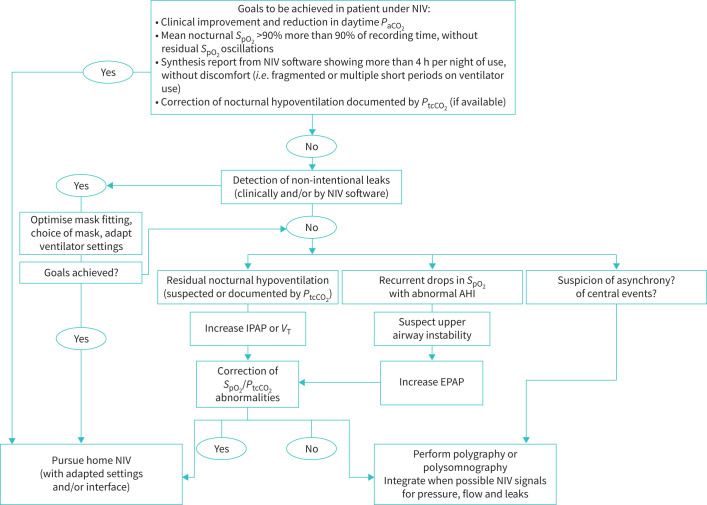
Jean-Paul Janssens (Geneva, Switzerland) highlighted the different structural options for home NIV follow-up that depends on the local healthcare structures, legislations and geographical considerations. Home NIV follow-up should also be tailored for specific groups of patients or situations requiring multidisciplinary assessment. There are numerous tools for home NIV monitoring (e.g. symptom scores, arterial blood gases, nocturnal pulse oximetry) and side-effects of NIV should be systematically assessed using a checklist. A recent study explored different strategies for home NIV monitoring, attempting to efficiently identify patients who were inappropriately ventilated, using ventilator software integrated with overnight capnography [130]. Several goals should be achieved in patients under NIV (figure 3) [131, 132] due to its impact on HRQoL and survival in certain groups of patients [133–135]. Different studies have shown the feasibility, safety and cost-effectiveness of initiating NIV at home [136, 137] with a specialised team of nurses, the use of capnography and telemonitoring.
FIGURE 3.
Goals to be achieved in a patient under home NIV. NIV: non-invasive ventilation; SpO2: oxygen saturation measured by pulse oximetry; PtcCO2: transcutaneous carbon dioxide tension; AHI: apnoea–hypopnoea index; IPAP: inspiratory positive airway pressure; VT: tidal volume; EPAP: expiratory positive airway pressure. Reproduced and modified from [131] with permission.
Take-home messages
NIV supports such as mouthpiece ventilation or intermittent abdominal pressure ventilation can reduce side-effects related to prolonged ventilation.
The impact of NIV support on HRQoL should be tested in clinical trials, especially for intermittent abdominal pressure ventilation.
Logistics for home NIV follow-up are country and healthcare system dependent. The implementation of NIV at home seems feasible and safe.
Monitoring of home NIV is mandatory to ensure efficacy and comfort, guided by several goals to be achieved in patients under NIV.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: T. Marín declares payment or honoraria from Fisher & Paykel in the 36 months prior to manuscript submission. S. Tang declares travel and conference fees for the European Respiratory Society International Congress were reimbursed by Queen's University Post-Graduate Medicine Education. M. Patout declares research grants from Fisher & Paykel, Resmed and Asten Santé; consulting fees from Philips Respironics, Resmed, Asten Santé and GlaxoSmithKline; support for attending meetings and/or travel from Asten Santé; participation on a data safety monitoring board or advisory board for Resmed, Philips Respironics and Asten Santé; stock or stock options in Kernel Biomedical; and receipt of equipment, materials, drugs, medical writing, gifts or other services from Philips Respironics, Resmed and Fisher & Paykel, all in the 36 months prior to manuscript submission. C. Fisser declares research grants from the German Heart Research Foundation; and payment or honoraria from CSL Behring, AstraZeneca and Novartis, all in the 36 months prior to manuscript submission; and that they are an Early Career Member Representative for European Respiratory Society Assembly 2 (Respiratory intensive care). All other authors declare no competing interests.
References
- 1.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2: 611–620. doi: 10.1016/S2213-2600(14)70097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 2018; 6: 691–698. doi: 10.1016/S2213-2600(18)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acute Respiratory Distress Syndrome Network , Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 4.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–2168. doi: 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 5.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network , Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354: 2564–2575. doi: 10.1056/NEJMoa062200 [DOI] [PubMed] [Google Scholar]
- 6.McNamee JJ, Gillies MA, Barrett NA, et al. Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical trial. JAMA 2021; 326: 1013–1023. doi: 10.1001/jama.2021.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pediatric Acute Lung Injury Consensus Conference Group . Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16: 428–439. doi: 10.1097/PCC.0000000000000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jager P, Burgerhof JGM, van Heerde M, et al. Tidal volume and mortality in mechanically ventilated children: a systematic review and meta-analysis of observational studies. Crit Care Med 2014; 42: 2461–2472. doi: 10.1097/CCM.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 9.Khemani RG, Parvathaneni K, Yehya N, et al. Positive end-expiratory pressure lower than the ARDS network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med 2018; 198: 77–89. doi: 10.1164/rccm.201707-1404OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Schelven P, Koopman AA, Burgerhof JGM, et al. Driving pressure is associated with outcome in pediatric acute respiratory failure. Pediatr Crit Care Med 2022; 23: e136–e144. doi: 10.1097/PCC.0000000000002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wick KD, Aggarwal NR, Curley MAQ, et al. Opportunities for improved clinical trial designs in acute respiratory distress syndrome. Lancet Respir Med 2022; 10: 916–924. doi: 10.1016/S2213-2600(22)00294-6 [DOI] [PubMed] [Google Scholar]
- 12.McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur Respir J 2019; 53: 1802058. doi: 10.1183/13993003.02058-2018 [DOI] [PubMed] [Google Scholar]
- 13.Bos LDJ, Laffey JG, Ware LB, et al. Towards a biological definition of ARDS: are treatable traits the solution? Intensive Care Med Exp 2022; 10: 8. doi: 10.1186/s40635-022-00435-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leisman DE, Mehta A, Thompson BT, et al. Alveolar, endothelial, and organ injury marker dynamics in severe COVID-19. Am J Respir Crit Care Med 2022; 205: 507–519. doi: 10.1164/rccm.202106-1514OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Curr Diab Rep 2011; 11: 244–252. doi: 10.1007/s11892-011-0198-7 [DOI] [PubMed] [Google Scholar]
- 16.Wick KD, Siegel L, Neaton JD, et al. RAGE has potential pathogenetic and prognostic value in nonintubated hospitalized patients with COVID-19. JCI Insight 2022; 7: e157499. doi: 10.1172/jci.insight.157499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim A, Radujkovic A, Weigand MA, et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann Intensive Care 2021; 11: 50. doi: 10.1186/s13613-021-00836-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabaudon M, Berthelin P, Pranal T, et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci Rep 2018; 8: 2603. doi: 10.1038/s41598-018-20994-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones TK, Feng R, Kerchberger VE, et al. Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201: 47–56. doi: 10.1164/rccm.201810-2033OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blondonnet R, Audard J, Belville C, et al. RAGE inhibition reduces acute lung injury in mice. Sci Rep 2017; 7: 7208. doi: 10.1038/s41598-017-07638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Audard J, Godet T, Blondonnet R, et al. Inhibition of the receptor for advanced glycation end-products in acute respiratory distress syndrome: a randomised laboratory trial in piglets. Sci Rep 2019; 9: 9227. doi: 10.1038/s41598-019-45798-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medzhitov R. The spectrum of inflammatory responses. Science 2021; 374: 1070–1075. doi: 10.1126/science.abi5200 [DOI] [PubMed] [Google Scholar]
- 23.Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med 2021; 385: 628–639. doi: 10.1056/NEJMra1909094 [DOI] [PubMed] [Google Scholar]
- 24.Conway Morris A, Kohler K, Shankar-Hari M. ARDS subphenotypes: searching for Rorschach among the roentgenograms? Thorax 2022; 77: 2–4. doi: 10.1136/thoraxjnl-2021-217428 [DOI] [PubMed] [Google Scholar]
- 25.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group , Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021; 326: 499–518. doi: 10.1001/jama.2021.11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu P, Cui X, Sun J, et al. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: a meta-analysis. Crit Care Med 2013; 41: 2419–2429. doi: 10.1097/CCM.0b013e3182982add [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landoni G, Piemonti L, Monforte AD, et al. A multicenter phase 2 randomized controlled study on the efficacy and safety of reparixin in the treatment of hospitalized patients with COVID-19 pneumonia. Infect Dis Ther 2022; 11: 1559–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–1349. doi: 10.1056/NEJM200005043421806 [DOI] [PubMed] [Google Scholar]
- 30.Aman J, Weijers EM, van Nieuw Amerongen GP, et al. Using cultured endothelial cells to study endothelial barrier dysfunction: challenges and opportunities. Am J Physiol Lung Cell Mol Physiol 2016; 311: L453–L466. doi: 10.1152/ajplung.00393.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet 2021; 398: 622–637. doi: 10.1016/S0140-6736(21)00439-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villa E, Critelli R, Lasagni S, et al. Dynamic angiopoietin-2 assessment predicts survival and chronic course in hospitalized patients with COVID-19. Blood Adv 2021; 5: 662–673. doi: 10.1182/bloodadvances.2020003736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aman J, Duijvelaar E, Botros L, et al. Imatinib in patients with severe COVID-19: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir Med 2021; 9: 957–968. doi: 10.1016/S2213-2600(21)00237-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Brabander J, Duijvelaar E, Schippers JR, et al. Immunomodulation and endothelial barrier protection mediate the association between oral imatinib and mortality in hospitalised COVID-19 patients. Eur Respir J 2022; 60: 2200780. doi: 10.1183/13993003.00780-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duijvelaar E, Schippers JR, Smeele PJ, et al. Long-term clinical outcomes of COVID-19 patients treated with imatinib. Lancet Respir Med 2022; 10: e34–e35. doi: 10.1016/S2213-2600(22)00052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atmowihardjo L, Schippers JR, Bartelink IH, et al. The INVENT COVID trial: a structured protocol for a randomized controlled trial investigating the efficacy and safety of intravenous imatinib mesylate (Impentri) in subjects with acute respiratory distress syndrome induced by COVID-19. Trials 2022; 23: 158. doi: 10.1186/s13063-022-06055-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016; 375: 65–74. doi: 10.1056/NEJMra1510061 [DOI] [PubMed] [Google Scholar]
- 38.Sinha P, Delucchi KL, McAuley DF, et al. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med 2020; 8: 247–257. doi: 10.1016/S2213-2600(19)30369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha P, Bos LD. Pathophysiology of the acute respiratory distress syndrome: insights from clinical studies. Crit Care Clin 2021; 37: 795–815. doi: 10.1016/j.ccc.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Files DC, Matthay MA, Calfee CS, et al. I-SPY COVID adaptive platform trial for COVID-19 acute respiratory failure: rationale, design and operations. BMJ Open 2022; 12: e060664. doi: 10.1136/bmjopen-2021-060664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.I-SPY COVID Consortium . Clinical trial design during and beyond the pandemic: the I-SPY COVID trial. Nat Med 2022; 28: 9–11. doi: 10.1038/s41591-021-01617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha P, Furfaro D, Cummings MJ, et al. Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med 2021; 204: 1274–1285. doi: 10.1164/rccm.202105-1302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha P, Calfee CS, Cherian S, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med 2020; 8: 1209–1218. doi: 10.1016/S2213-2600(20)30366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy K, Sinha P, O'Kane CM, et al. Subphenotypes in critical care: translation into clinical practice. Lancet Respir Med 2020; 8: 631–643. doi: 10.1016/S2213-2600(20)30124-7 [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Churpek MM, Calfee CS. Machine learning classifier models can identify acute respiratory distress syndrome phenotypes using readily available clinical data. Am J Respir Crit Care Med 2020; 202: 996–1004. doi: 10.1164/rccm.202002-0347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maddali MV, Churpek M, Pham T, et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med 2022; 10: 367–377. doi: 10.1016/S2213-2600(21)00461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mascheroni D, Kolobow T, Fumagalli R, et al. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med 1988; 15: 8–14. doi: 10.1007/BF00255628 [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T, Uchiyama A, Matsuura N, et al. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 2013; 41: 536–545. doi: 10.1097/CCM.0b013e3182711972 [DOI] [PubMed] [Google Scholar]
- 49.Carteaux G, Millán-Guilarte T, De Prost N, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med 2016; 44: 282–290. doi: 10.1097/CCM.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 50.Bertoni M, Telias I, Urner M, et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care 2019; 23: 346. doi: 10.1186/s13054-019-2617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tonelli R, Fantini R, Tabbì L, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am J Respir Crit Care Med 2020; 202: 558–567. doi: 10.1164/rccm.201912-2512OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 53.Yoshida T, Grieco DL, Brochard L, et al. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care 2020; 26: 59–65. doi: 10.1097/MCC.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 54.Volta CA, Alvisi V, Bertacchini S, et al. Acute effects of hyperoxemia on dyspnoea and respiratory variables during pressure support ventilation. Intensive Care Med 2006; 32: 223–229. doi: 10.1007/s00134-005-0012-6 [DOI] [PubMed] [Google Scholar]
- 55.Doorduin J, Nollet JL, Roesthuis LH, et al. Partial neuromuscular blockade during partial ventilatory support in sedated patients with high tidal volumes. Am J Respir Crit Care Med 2017; 195: 1033–1042. doi: 10.1164/rccm.201605-1016OC [DOI] [PubMed] [Google Scholar]
- 56.Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care 2019; 9: 69. doi: 10.1186/s13613-019-0540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarge T, Baedorf-Kassis E, Banner-Goodspeed V, et al. Effect of esophageal pressure-guided positive end-expiratory pressure on survival from acute respiratory distress syndrome: a risk-based and mechanistic reanalysis of the EPVent-2 trial. Am J Respir Crit Care Med 2021; 204: 1153–1163. doi: 10.1164/rccm.202009-3539OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-FIO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2019; 321: 846–857. doi: 10.1001/jama.2019.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012; 38: 395–403. doi: 10.1007/s00134-012-2490-7 [DOI] [PubMed] [Google Scholar]
- 60.Whitelaw WA, Derenne JP, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 1975; 23: 181–199. doi: 10.1016/0034-5687(75)90059-6 [DOI] [PubMed] [Google Scholar]
- 61.Alberti A, Gallo F, Fongaro A, et al. P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 1995; 21: 547–553. doi: 10.1007/BF01700158 [DOI] [PubMed] [Google Scholar]
- 62.Telias I, Damiani F, Brochard L. The airway occlusion pressure (P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not-so-new problem. Intensive Care Med 2018; 44: 1532–1535. doi: 10.1007/s00134-018-5045-8 [DOI] [PubMed] [Google Scholar]
- 63.Beloncle F, Piquilloud L, Olivier P-Y, et al. Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care 2019; 9: 104. doi: 10.1186/s13613-019-0576-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Telias I, Junhasavasdikul D, Rittayamai N, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med 2020; 201: 1086–1098. doi: 10.1164/rccm.201907-1425OC [DOI] [PubMed] [Google Scholar]
- 65.Esnault P, Cardinale M, Hraiech S, et al. High respiratory drive and excessive respiratory efforts predict relapse of respiratory failure in critically ill patients with COVID-19. Am J Respir Crit Care Med 2020; 202: 1173–1178. doi: 10.1164/rccm.202005-1582LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crimi C, Noto A, Madotto F, et al. High-flow nasal oxygen versus conventional oxygen therapy in patients with COVID-19 pneumonia and mild hypoxaemia: a randomised controlled trial. Thorax 2023; 78: 354–361. doi: 10.1136/thoraxjnl-2022-218806 [DOI] [PubMed] [Google Scholar]
- 67.Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA 2021; 325: 1731–1743. doi: 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernández G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016; 316: 1565–1574. doi: 10.1001/jama.2016.14194 [DOI] [PubMed] [Google Scholar]
- 69.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50: 1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 70.Hoang-Thi T-N, Tran D-T, Tran H-D, et al. Usefulness of hospital admission chest X-ray score for predicting mortality and ICU admission in COVID-19 patients. J Clin Med 2022; 11: 3548. doi: 10.3390/jcm11123548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bassi TG, Rohrs EC, Fernandez KC, et al. Transvenous diaphragm neurostimulation mitigates ventilation-associated brain injury. Am J Respir Crit Care Med 2021; 204: 1391–1402. doi: 10.1164/rccm.202101-0076OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohrs EC, Bassi TG, Fernandez KC, et al. Diaphragm neurostimulation during mechanical ventilation reduces atelectasis and transpulmonary plateau pressure, preserving lung homogeneity and PaO2/FIO2. J Appl Physiol 2021; 131: 290–301. doi: 10.1152/japplphysiol.00119.2021 [DOI] [PubMed] [Google Scholar]
- 73.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. doi: 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wirth KJ, Scheibenbogen C. Dyspnea in post-COVID syndrome following mild acute COVID-19 infections: potential causes and consequences for a therapeutic approach. Medicina 2022; 58: 419. doi: 10.3390/medicina58030419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rose L, McGinlay M, Amin R, et al. Variation in definition of prolonged mechanical ventilation. Respir Care 2017; 62: 1324–1332. doi: 10.4187/respcare.05485 [DOI] [PubMed] [Google Scholar]
- 76.Ettman CK, Abdalla SM, Cohen GH, et al. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open 2020; 3: e2019686. doi: 10.1001/jamanetworkopen.2020.19686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vannorsdall TD, Brigham E, Fawzy A, et al. Cognitive dysfunction, psychiatric distress, and functional decline after COVID-19. J Acad Consult Liaison Psychiatry 2022; 63: 133–143. doi: 10.1016/j.jaclp.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spruit MA, Holland AE, Singh SJ, et al. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from the European Respiratory Society and American Thoracic Society-coordinated International Task Force. Eur Respir J 2020; 56: 2002197. doi: 10.1183/13993003.02197-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lemyze M, Komorowski M, Mallat J, et al. Early intensive physical rehabilitation combined with a protocolized decannulation process in tracheostomized survivors from severe COVID-19 pneumonia with chronic critical illness. J Clin Med 2022; 11: 3921. doi: 10.3390/jcm11133921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fortis S, Gao Y, O'Shea AMJ, et al. Hospital variation in non-invasive ventilation use for acute respiratory failure due to COPD exacerbation. Int J Chron Obstruct Pulmon Dis 2021; 16: 3157–3166. doi: 10.2147/COPD.S321053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017; 49: 1600791. doi: 10.1183/13993003.00791-2016 [DOI] [PubMed] [Google Scholar]
- 82.Osadnik CR, Tee VS, Carson-Chahhoud KV, et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 7: CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jayadev A, Stone R, Steiner MC, et al. Time to NIV and mortality in AECOPD hospital admissions: an observational study into real world insights from National COPD Audits. BMJ Open Respir Res 2019; 6: e000444. doi: 10.1136/bmjresp-2019-000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartley T, Lane ND, Steer J, et al. The Noninvasive Ventilation Outcomes (NIVO) score: prediction of in-hospital mortality in exacerbations of COPD requiring assisted ventilation. Eur Respir J 2021; 58: 2004042. doi: 10.1183/13993003.04042-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elshof J, Duiverman ML, Wijkstra PJ. The NIVO score: can it help to improve noninvasive ventilation in daily clinical practice? Eur Respir J 2021; 58: 2100336. doi: 10.1183/13993003.00336-2021 [DOI] [PubMed] [Google Scholar]
- 86.Oppersma E, Doorduin J, Roesthuis LH, et al. Patient-ventilator interaction during noninvasive ventilation in subjects with exacerbation of COPD: effect of support level and ventilator mode. Respir Care 2020; 65: 1315–1322. doi: 10.4187/respcare.07159 [DOI] [PubMed] [Google Scholar]
- 87.Cammarota G, Simonte R, De Robertis E. Comfort during non-invasive ventilation. Front Med 2022; 9: 874250. doi: 10.3389/fmed.2022.874250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017; 317: 2177–2186. doi: 10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Struik FM, Sprooten RTM, Kerstjens HAM, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014; 69: 826–834. doi: 10.1136/thoraxjnl-2014-205126 [DOI] [PubMed] [Google Scholar]
- 90.Möller W, Celik G, Feng S, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol 2015; 118: 1525–1532. doi: 10.1152/japplphysiol.00934.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care 2011; 56: 1151–1155. doi: 10.4187/respcare.01106 [DOI] [PubMed] [Google Scholar]
- 92.Williams R, Rankin N, Smith T, et al. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit Care Med 1996; 24: 1920–1929. doi: 10.1097/00003246-199611000-00025 [DOI] [PubMed] [Google Scholar]
- 93.Kilgour E, Rankin N, Ryan S, et al. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med 2004; 30: 1491–1494. doi: 10.1007/s00134-004-2235-3 [DOI] [PubMed] [Google Scholar]
- 94.Cortegiani A, Longhini F, Madotto F, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care 2020; 24: 692. doi: 10.1186/s13054-020-03409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alnajada AA, Blackwood B, Mobrad A, et al. High flow nasal oxygen for acute type two respiratory failure: a systematic review. F1000Res 2021; 10: 482. doi: 10.12688/f1000research.52885.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oczkowski S, Ergan B, Bos L, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J 2022; 59: 2101574. doi: 10.1183/13993003.01574-2021 [DOI] [PubMed] [Google Scholar]
- 97.Longhini F, Pisani L, Lungu R, et al. High-flow oxygen therapy after noninvasive ventilation interruption in patients recovering from hypercapnic acute respiratory failure: a physiological crossover trial. Crit Care Med 2019; 47: e506–e511. doi: 10.1097/CCM.0000000000003740 [DOI] [PubMed] [Google Scholar]
- 98.Kim ES, Lee H, Kim SJ, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis 2018; 10: 882–888. doi: 10.21037/jtd.2018.01.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X-Y, Tang X, Wang R, et al. High-flow nasal cannula for chronic obstructive pulmonary disease with acute compensated hypercapnic respiratory failure: a randomized, controlled trial. Int J Chron Obstruct Pulmon Dis 2020; 15: 3051–3061. doi: 10.2147/COPD.S283020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Di Mussi R, Spadaro S, Stripoli T, et al. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Crit Care 2018; 22: 180. doi: 10.1186/s13054-018-2107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med 2012; 185: 152–159. doi: 10.1164/rccm.201106-1094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Girou E, Schortgen F, Delclaux C, et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA 2000; 284: 2361–2367. doi: 10.1001/jama.284.18.2361 [DOI] [PubMed] [Google Scholar]
- 103.Del Sorbo L, Pisani L, Filippini C, et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 2015; 43: 120–127. doi: 10.1097/CCM.0000000000000607 [DOI] [PubMed] [Google Scholar]
- 104.Karagiannidis C, Strassmann S, Schwarz S, et al. Control of respiratory drive by extracorporeal CO2 removal in acute exacerbation of COPD breathing on non-invasive NAVA. Crit Care 2019; 23: 135. doi: 10.1186/s13054-019-2404-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braune S, Sieweke A, Brettner F, et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med 2016; 42: 1437–1444. doi: 10.1007/s00134-016-4452-y [DOI] [PubMed] [Google Scholar]
- 106.Karagiannidis C, Strassmann S, Philipp A, et al. Veno-venous extracorporeal CO2 removal improves pulmonary hypertension in acute exacerbation of severe COPD. Intensive Care Med 2015; 41: 1509–1510. doi: 10.1007/s00134-015-3917-8 [DOI] [PubMed] [Google Scholar]
- 107.Gross-Hardt S, Hesselmann F, Arens J, et al. Low-flow assessment of current ECMO/ECCO2R rotary blood pumps and the potential effect on hemocompatibility. Crit Care 2019; 23: 348. doi: 10.1186/s13054-019-2622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vatani A, Liao S, Burrell AJC, et al. Improved drainage cannula design to reduce thrombosis in veno-arterial extracorporeal membrane oxygenation. ASAIO J 2022; 68: 205–213. [DOI] [PubMed] [Google Scholar]
- 109.Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67: 957–963. doi: 10.1136/thoraxjnl-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lindenauer PK, Dharmarajan K, Qin L, et al. Risk trajectories of readmission and death in the first year after hospitalization for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1009–1017. doi: 10.1164/rccm.201709-1852OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Hirtum PV, Sprooten RTM, van Noord JA, et al. Long term survival after admission for COPD exacerbation: a comparison with the general population. Respir Med 2018; 137: 77–82. doi: 10.1016/j.rmed.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 112.Jabarkhil A, Moberg M, Janner J, et al. Elevated blood eosinophils in acute COPD exacerbations: better short- and long-term prognosis. Eur Clin Respir J 2020; 7: 1757274. doi: 10.1080/20018525.2020.1757274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–852. doi: 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makris D, Moschandreas J, Damianaki A, et al. Exacerbations and lung function decline in COPD: new insights in current and ex-smokers. Respir Med 2007; 101: 1305–1312. doi: 10.1016/j.rmed.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 115.Wang M, Lin EP-Y, Huang L-C, et al. Mortality of cardiovascular events in patients with COPD and preceding hospitalization for acute exacerbation. Chest 2020; 158: 973–985. doi: 10.1016/j.chest.2020.02.046 [DOI] [PubMed] [Google Scholar]
- 116.Shafuddin E, Fairweather SM, Chang CL, et al. Cardiac biomarkers and long-term outcomes of exacerbations of COPD: a long-term follow-up of two cohorts. ERJ Open Res 2021; 7: 00531-2020. doi: 10.1183/23120541.00531-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Donaldson GC, Hurst JR, Smith CJ, et al. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010; 137: 1091–1097. doi: 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 118.Abdulai RM, Jensen TJ, Patel NR, et al. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 433–449. doi: 10.1164/rccm.201703-0615CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vanaparthy R, Mota P, Khan R, et al. A longitudinal assessment of sleep variables during exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis 2015; 12: 299–304. doi: 10.1177/1479972315587517 [DOI] [PubMed] [Google Scholar]
- 120.Spina G, Spruit MA, Alison J, et al. Analysis of nocturnal actigraphic sleep measures in patients with COPD and their association with daytime physical activity. Thorax 2017; 72: 694–701. doi: 10.1136/thoraxjnl-2016-208900 [DOI] [PubMed] [Google Scholar]
- 121.McAuley HJC, Harvey-Dunstan TC, Craner M, et al. Longitudinal changes to quadriceps thickness demonstrate acute sarcopenia following admission to hospital for an exacerbation of chronic respiratory disease. Thorax 2021; 76: 726–728. doi: 10.1136/thoraxjnl-2020-215949 [DOI] [PubMed] [Google Scholar]
- 122.Vikjord SAA, Brumpton BM, Mai X-M, et al. The HUNT study: association of comorbidity clusters with long-term survival and incidence of exacerbation in a population-based Norwegian COPD cohort. Respirology 2022; 27: 277–285. doi: 10.1111/resp.14222 [DOI] [PubMed] [Google Scholar]
- 123.Williams V, Hardinge M, Ryan S, et al. Patients’ experience of identifying and managing exacerbations in COPD: a qualitative study. NPJ Prim Care Respir Med 2014; 24: 14062. doi: 10.1038/npjpcrm.2014.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Markussen H, Lehmann S, Nilsen RM, et al. Health-related quality of life as predictor for mortality in patients treated with long-term mechanical ventilation. BMC Pulm Med 2019; 19: 13. doi: 10.1186/s12890-018-0768-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Markussen H, Lehmann S, Nilsen RM, et al. Factors associated with change in health-related quality of life among individuals treated with long-term mechanical ventilation, a 6-year follow-up study. J Adv Nurs 2018; 74: 651–665. doi: 10.1111/jan.13472 [DOI] [PubMed] [Google Scholar]
- 126.Toussaint M, Chatwin M, Gonçalves MR, et al. Mouthpiece ventilation in neuromuscular disorders: narrative review of technical issues important for clinical success. Respir Med 2021; 180: 106373. doi: 10.1016/j.rmed.2021.106373 [DOI] [PubMed] [Google Scholar]
- 127.Chatwin M, Gonçalves M, Gonzalez-Bermejo J, et al. 252nd ENMC international workshop: developing best practice guidelines for management of mouthpiece ventilation in neuromuscular disorders. March 6th to 8th 2020, Amsterdam, the Netherlands. Neuromuscul Disord 2020; 30: 772–781. doi: 10.1016/j.nmd.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pierucci P, Di Lecce V, Carpagnano GE, et al. The intermittent abdominal pressure ventilator as an alternative modality of noninvasive ventilatory support: a narrative review. Am J Phys Med Rehabil 2022; 101: 179–183. doi: 10.1097/PHM.0000000000001804 [DOI] [PubMed] [Google Scholar]
- 129.Fiorentino G, Annunziata A, Coppola A, et al. Intermittent abdominal pressure ventilation: an alternative for respiratory support. Can Respir J 2021; 2021: 5554765. doi: 10.1155/2021/5554765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Georges M, Rabec C, Monin E, et al. Monitoring of noninvasive ventilation: comparative analysis of different strategies. Respir Res 2020; 21: 324. doi: 10.1186/s12931-020-01586-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Janssens J-P, Michel F, Schwarz EI, et al. Long-term mechanical ventilation: recommendations of the Swiss Society of Pulmonology. Respiration 2020; 99: 867–902. [DOI] [PubMed] [Google Scholar]
- 132.Janssens J-P, Borel J-C, Pépin J-L. Nocturnal monitoring of home non-invasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation. Thorax 2011; 66: 438–445. doi: 10.1136/thx.2010.139782 [DOI] [PubMed] [Google Scholar]
- 133.Tsuboi T, Oga T, Sumi K, et al. The importance of controlling PaCO₂ throughout long-term noninvasive ventilation. Respir Care 2014; 59: 1671–1678. doi: 10.4187/respcare.02829 [DOI] [PubMed] [Google Scholar]
- 134.Sancho J, Servera E, Morelot-Panzini C, et al. Non-invasive ventilation effectiveness and the effect of ventilatory mode on survival in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15: 55–61. doi: 10.3109/21678421.2013.855790 [DOI] [PubMed] [Google Scholar]
- 135.Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014; 2: 698–705. doi: 10.1016/S2213-2600(14)70153-5 [DOI] [PubMed] [Google Scholar]
- 136.Hazenberg A, Kerstjens HAM, Prins SCL, et al. Initiation of home mechanical ventilation at home: a randomised controlled trial of efficacy, feasibility and costs. Respir Med 2014; 108: 1387–1395. doi: 10.1016/j.rmed.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 137.Duiverman ML, Vonk JM, Bladder G, et al. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial. Thorax 2020; 75: 244–252. doi: 10.1136/thoraxjnl-2019-213303 [DOI] [PMC free article] [PubMed] [Google Scholar]