Abstract
Background:
Hydatid disease of bone shows a well-defined, multiloculated lytic lesion with the appearance of a bunch of grapes. The presenting symptoms are pain and swelling with or without pathological fracture. The treatment options include surgery followed by a long duration of albendazole. Removal of the involved bone is required to decrease the chances of recurrences.
Case Report:
In our study, we have included a case of 28-year-old woman presented with complaints of pain and difficulty in weight bearing over her right lower limb for 2.5 months. Radiograph suggested an eccentric lytic lesion in midshaft of tibia and biopsy revealed granulosus cyst wall, nucleate germinal layer, the brood capsule, and protoscolices with visible hooklets. Patient was subjected to surgery with the excision of cyst along with extended curettage of bone creating a bone defect around the lesion and with anterolateral platting with coverage of bone defect by allogenic bone grafting. Patient was kept on above knee slab with non-weight-bearing mobilization for 6 weeks. Postoperative chemotherapy with Albendazole was given for 3 months. Patient was followed up every 6 weeks for 3 months and every month thereafter on outpatient basis. Return to work and patient satisfaction were excellent.
Conclusion:
Definitive Surgical management with Preoperative and postoperative chemotherapy seems to be effective to avoid recurrence. The bone defect caused by the disease or surgery can be managed with a bone graft either of autograft or allograft.
Keywords: Allogenic bone graft, echinococcosis, hydatid cyst, skeletal hydatosis, tibia
Introduction
It is caused by Echinococcus granulosus larvae. Dogs are the definitive host whereas cattle are the intermediate hosts. The disease occurs due to accidental infection of humans with eggs of E. granulosus followed by the development of larvae.[1,2]
It can develop in any part of the body, with the most common site being liver involving 70% of cases. Bone involvement is as low as 0.5%–2.5% of all human hydatidosis. It remains asymptomatic for a long duration and thus diagnosis is made at later stages and radiology reveals extensive disease. Clinical feature depends on the anatomic location of the bone involved. The treatment regime is similar to oncologic therapy rather than the simple surgical excision in the case of visceral hydatidosis.[3,4]
Like in other visceral organs, pericyst formation does not occur in bone. Thus, it proliferates aggressively along the areas of least resistance likely in bone canals. With time the disease spreads within the bone tissue to reach the cortex and might spreads into surrounding tissues.[5] Hydatid disease of bone shows well-defined, multiloculated lytic lesion with the appearance of a bunch of grapes. The additional features include expansion of bone, thinning of cortex, and spread into the adjacent tissue. The osteoclastic activity is likely due to impairment of blood supply caused due to pressure erosion and local necrosis.[6]
The presenting symptoms are pain and swelling with or without pathological fracture. As it lacks characteristic clinical features it may mimic tuberculosis, chronic osteomyelitis, aneurysmal bone cyst, giant cell tumor, solitary cyst, chondrosarcoma, or fibrocystic disease.[5] The treatment options include surgery followed by a long duration of albendazole. Removal of the involved bone is required to decrease the chances of recurrences. The bone defects are covered by either bone graft or bone cement due to its added effect of thermal necrosis.[7,8]
This paper was aimed to present the case of hydatid cyst of midshaft tibia, which was successfully managed with excision of cyst along with extended curettage and allogenic bone grafting and review of literatures available on skeletal hydatosis.
Case Presentation
A 28-year-old woman presented with complaints of pain and difficulty in weight bearing over her right lower limb for 2.5 months. visual analog scale score was 8/10. There was a mild swelling over her right leg during the period of 2.5 months. The patient had neither a history of any comorbidities (diabetes, hypertension) nor any significant surgical history. There was no significant history of trauma or tuberculosis contact. The patient did not have any complaints of fever, fatigue, malaise, or weight loss.
On clinical examination, there was localized tenderness present on the anterior aspect of the middle 1/3rd of the leg. There was no local rise in temperature. Both knee and ankle range of motion was within normal limits. The rest of clinical evaluation revealed no other significant findings.
On clinical examination, there was localized tenderness present on the anterior aspect of the middle 1/3rd of the leg. There was no local rise in temperature. Both knee and ankle range of motion was within normal limits. The rest of clinical evaluation revealed no other significant findings. Differential diagnosis includes benign bone tumor (simple bone cyst, fibrous dysplasia, and enchondroma), chronic osteomyelitis, and a brown tumor.
After a routine investigation and following biopsy and radiological findings, a definitive diagnosis of skeletal hydatidosis involving shaft of tibia right side was made, and the patient was subjected to surgery with excision of cyst along with extended curettage of bone creating a bone defect around the lesion and with anterolateral platting with coverage of bone defect by allogenic bone grafting. The patient was then kept on non-weight-bearing mobilization with an above knee slab for 6 weeks. For postoperative chemotherapy, tablet albendazole 400 mg once daily for 3 months was given to the patient from Post-operative day-1.
The patient was able to bear weight without pain after 6 weeks as shown in Figure 1. The final histopathological slide of intraoperative specimen was positive for hydatid disease as shown in Figure 2. The patient was followed up for a period of 6 months at 6 weeks interval. There were no any radiological signs of recurrence over the 6-month period. There was no any complaints of pain or difficulty in weight bearing. Postoperative visual analog scale at 6-month follow-up was 0/10. The knee and ankle ROM was within normal limits and there was no any localized tenderness. The scar mark of surgery has healed with primary intention. Radiologically, there was adequate sign of uptake of allograft. Serology for hydatid disease was also negative for the disease. Return to work and patient satisfaction were excellent.
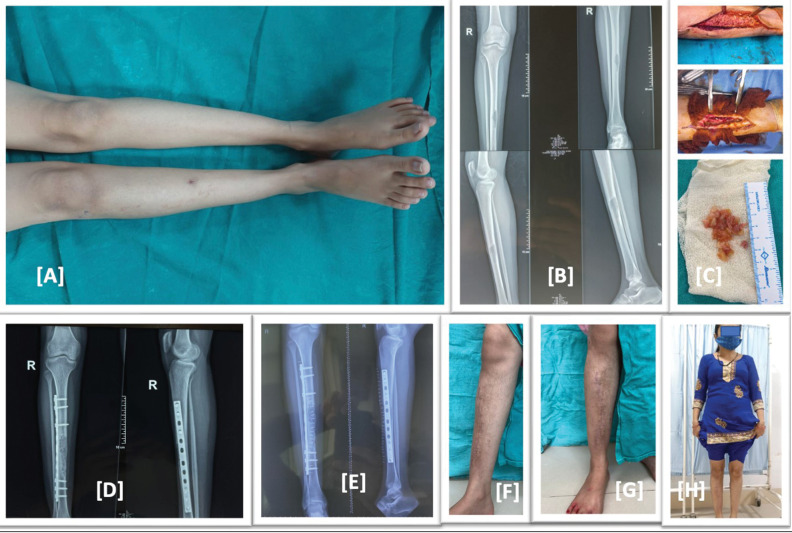
Figure 1.
(A) Preoperative image, right leg with scar mark of biopsy and mild swelling. (B) Preoperative X-ray showing expansile lytic lesion in mid shaft tibia. (C) Intraoperative images, excised hydatid cyst mass. (D) Immediate postoperative X-ray showing in corporation of allograft and fixation by anterolateral tibial plate. (E) Post operative X-ray at 6 month follow up showing well incorporation of allograft. (F–H) Clinical images at 6 month follow up showing healed scar mark no swelling or deformity and full weight bearing of the patient with full return to activity of daily living
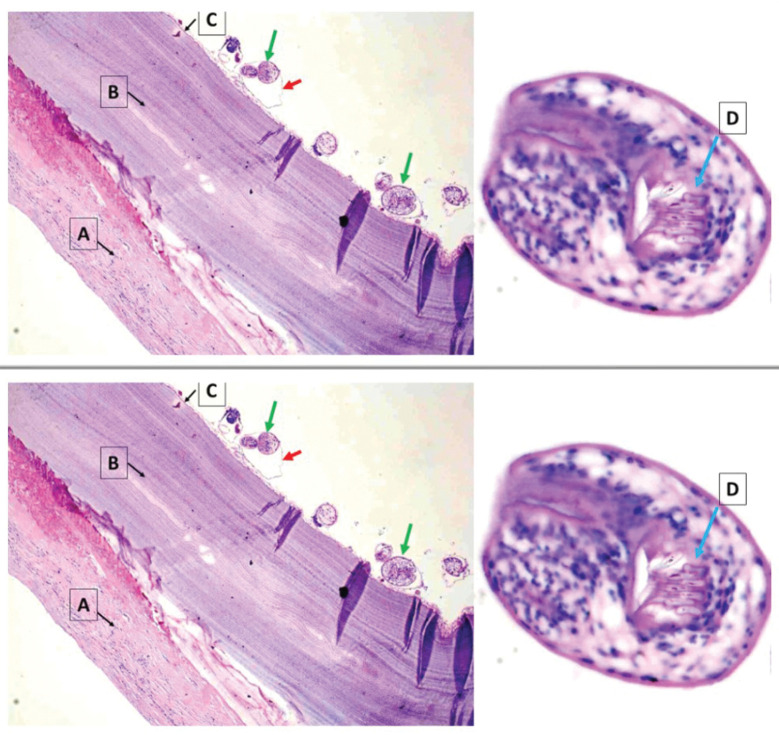
Figure 2.
Hematoxylin and eosin (H&E ×40) stained section shows E. Granulosus cyst wall (A) acellular laminated layer; (B) a nucleate germinal layer; (C) the brood capsule (red arrow), protoscolices (green arrow) with visible hooklets (D)
Discussion
Hydatid disease is caused by a tapeworm infection, Echinococcus, namely E. granulosus and Echinococcus multilocularis, that infects the human species most commonly.[9] Man is the accidental host in this disease. Dogs are the definitive hosts whereas cattle are the intermediate hosts. The definitive hosts, dogs, harvest the adult forms in their gut, where the adult form of tapeworm releases its egg into the feces. These eggs are ingested by the intermediate hosts, namely sheep. After the ingestion of the eggs, the embryo is released in the gut and absorbed into the portal circulation through the duodenal mucosa. The cyst grows in the highly vascular organs such as muscle bed, liver, and lungs of intermediate host. These cysts are ingested by the definitive hosts where the cysts are absorbed in the intestine and grow into protoscolex, scolex, and into an adult form. Humans are infected due to ingestion of eggs of parasite either through food, water or hands contaminated by eggs. After ingestion of the eggs, the embryo is released in the gut and absorbed into the portal circulation through the duodenal mucosa. In the intestinal mucosa, the parasite grows into an adult form and releases egg which are ingested by the intermediate host. A hydatid cyst has three layers, outer pericyst (formed by host cells, limits growth of parasite in visceral organ), middle soft laminated later, and inner germinal layer. In bone infection, the pericyst is absent.[10,11,12] In our case the biopsy revealed acellular laminated layer, nucleate germinal layer, protoscolices, and visible hooklets.
The disease mainly involves visceral organ, most commonly the liver (70%). Primary bone hydatidosis is rare and involves 0.5%–2.5%.[3] It might take 10–20 years for hydatid disease of bone to be clinically noted. Thus, the disease is presented in between 4th and 6th decade and women were twice as commonly infected as men. In our case, the patient was 28-year-old woman, with the complaints of pain and difficulty in bearing weight in her leg. The lesion initially starts in epiphysis or metaphysis in long bones and spreads onto diaphysis at later stages. X-ray and CT scan can aid onto diagnosis and the imaging shows unilocular, bilocular, or multilocular cyst.[3]
Merkle et al.[13] did a review of literature on 45 patients with 51 skeletal involvements and found the following distribution: spine 35%, pelvis 21%, femur 16%, 10% tibia, 6% in ribs, 4% in scapula and skull, 2% in humerus, and 2% in fibula and concluded that 60% of osseous lesions occur in spine, pelvis, and hip joint. 28% in long bones such as femur, tibia, and humerus and 8% in ribs and scapula. It is often misdiagnosed as tumor due to progressive changes and cystic appearance on radiographs.[14] In our case, the diaphysis of midshaft tibia was involved where the lesion was expansile, thin-walled, and cystic.
Plain radiograph is considered the investigation of choice. Honeycomb appearance and ill-defined areas of osteolysis are the radiologic features. Periosteal reaction is not seen. CT scan and magnetic resonance imaging are useful in identifying extension of tumor, radiological measurement, and the extent of spread of tumor into the soft tissue.[15]
The treatment option for hydatid disease of the bone is mainly surgery. But preoperative albendazole followed by surgical removal of cyst followed by postoperative albendazole is considered effective.[16] In about 25%–30% of cases antihelminthic therapy alone turned out be ineffective.[5] We have given preoperative albendazole followed by surgery by wide local curettage around the lesion to create a bone defect that was filled with allograft.
Xie et al.[17] did a retrospective study in 2014 with 40 patients, where 24 patients underwent surgery and 16 patients underwent radiotherapy. Relapse was seen in 14 patients who opted surgery whereas only in three patients postradiotherapy. Pain, bone defects, and limb movement disorder were seen in seven patients with surgery whereas hardening of the irradiated limb was seen in two patients with radiotherapy group. Also, the titers of antibodies of parasites were low among radiotherapy group and patient satisfaction was much better among radiotherapy group.
Gautam et al.[18] reported a case with hydatid disease of femur at the site of nonunion subtrochanteric femur fracture presenting with a lytic lesion. Preoperative albendazole followed by curettage and debridement of lesion with exchange nailing, and cement spacer application was done. Following recurrence of the same case 3 months later, the patient was again operated with excision of lesion and cement spacer application, and the patient was kept on chemotherapy for 6 months. The patient was disease-free as shown by X-ray and magnetic resonance imaging, and a femoral shaft allograft was used to reconstruct the bone defect along with the proud nail which was locked proximally and distally. At 6-month follow-up, the patient was well, with complete incorporation of allograft and no limb length discrepancy. Another study where femoral allograft was used for the bone defect after wide resection of lesion caused by hydatid disease of bone was done by Muscolo et al.[19] Two cases were included in the study where wide local excision of the lesion in the femur followed by femoral allograft and fixation of the allograft with implant was done. In patient-1, where the distal femoral diaphysis was involved allograft was fixed by locked intramedullar nail and four cancellous screws at distal osteotomy site. The outcome was excellent. In this case, the patient had not received any antihelminthic therapy preoperatively or postoperatively. In Patient-2, the patient presented with pathological fracture of proximal femur due to hydatid disease. She was also operated with wide local resection and reconstruction by proximal femoral allograft, followed by fixation with plating. This patient received both preoperative and postoperative chemotherapy treatments, and the outcome was also excellent in this patient. In our case, we did an extended curettage around the cystic lesion in the mid shaft tibia, coverage of defect by allograft, and fixation and stabilization by 12-hole narrow plate. The review of available literature is summarized in Table 1.
Table 1.
Review of literature of skeletal hydatosis
| Author | Age/sex | Article | Presenting complaints | Bone involved | Radiograph | Other distant lesion | Biopsy finding | Management | Chemotherapy Outcome | Follow up | Complication | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Babitha et al.[9] | 52/f | Case report | Dull aching pain | Femur | Fracture with Lytic lesion, shaft of femur | Absent | Lamellated linear eosinophilic anucleated membranous structures, granuloma formation and chronic inflammatory reaction, occasional scolices | Interlocking femur nail for pathological shaft of femur fracture | Non union at fracture site No recurrence | 12 months | Non union | |
| Arti | 42/m | Case report | Pain, mild swelling | Fibula | Multiple lytic lesion in mid shaft fibula | Absent | Trilamellar cyst and scolices of E. Granulosus | Wide local excision of cyst 10 cm above and below lesion | Albendazole 400 mg bd for 4 weeks | No recurrence seen | 12 months | – |
| Kalinova et al.[3] | 45/f | Case report | Pain | Tibia | Oval cystic lesion with diameter of 3.5 cm on diaphysis of reaction on cortex | Not mentioned | Osseous tissue with hyaline and germinative membranes, tibia, periosteal lymphocytes, and monocytes | Povidone iodine injection followed by cystectomy | Albendazole 10 mg/kg/day for 12 weeks | No recurrence seen, excellent outcome | 24 months | None |
| Jain | 31/f | Case report | Pain | Pelvis (right iliac fossa) | Ill defined lytic lesion with areas of patchy sclerosis, large solid cystic mass of size 10 × 6.3 cm in right iliac fossa | H/O ovarian hydatid cyst | Osseous tissue with laminated membrane of hydatid cyst mixed with lymphocytes and macrophages | Resection of cystic lesion in sacroiliac joint, reconstruction with allograft and autograft (ribs) with lumbosacroiliac fixation | Albendazole for 1 month | No recurrence | 6 months | |
| Siwach | 51/f | Case report | Pain, swelling and deformity of thigh | Femur, Hemipelvis and sacrum with spinal canal left side, | Segmental pathological fracture left femur with honey comb appearance and multiple osteolytic lesion, complete resorption of femoral head and neck, narrow transition zone without reactive bone formation in whole left femur | absent | Trilamellar hydatid cyst wall and scolices of E. Granulosus | Albendazole 10 mg/kg/day, No surgical intervention was done | Albendazole 10 mg/kg/day given as treatment measures | Died due to sepsis and extensive bedsores | 1 month | Died due to sepsis and extensive bedsores |
| Musculo | 65/f, 33/f | Case report (2 cases) | Case 1:not mentioned Case 2: pathological fracture | Case 1: right femur Case 2: proximal femur | Case 1: multiloculated osteolytic lesion in diaphysis of femur Case 2: pathological fracture, high signal intensity in femoral head, extensive soft tissue compromise laterally | Case 1: not mentioned Case 2: not mentioned | Case 1: not mentioned Case 2: Not dmentioned | Case 1: wide local excision and reconstruction with intercalary allograft, fixation with locked intramedullary nail and four cancellous screws at distal osteotomy site Case 2: wide local excision of proximal femur, proximal femoral prosthesis allograft composite was used to reconstruct the defect, fixation was done with dynamic compression plates and screw | Case 1: no preoperative and postoperative chemotherapy was given Case 2: oral albendazole 15 mg/kg/day preoperatively for 1 months andpostoperatively for 6 months | Case 1: excellent outcome MSTS score 28/30, no recurrence at follow up Case 2: No recurrence, excellent outcome (MSTS score 29/30) | Case 1: 108 months Case 2: 60 months | None in both |
| Bitar | 26/m | Case report | Pathological fracture following blunt trauma after sports injury | Tibia | Pathological fracture of tibia, well defined cystic lesion involving medullary cavity and scalloping of cortex | None | Foreign body granuloma and sheets of lamellated membrane consistent with hydatid cyst | Curettage of lesion followed by reconstruction with bone graft | Albendazole 10 mg/kg/day bd for every 4 weeks out of 6 weeks for 4 months | 44 months | ||
| Alem-daroglu | 30/m | Case report | Limping, intermittent pain, swelling | Tibia | Multiloculated mixed lytic and sclerotic lesion, bunch of grapes appearance, diffusely expanded bone, endosteal thinning, no obvious deformity or fracture | 5 × 4 cm cystic lesion in spleen | Cyst of variable sizes with smooth outer membrane, daughter embryos seen in inner layer of cyst | Wide local curettage to create an anterior window on tibia, thermal effect of PMMA applied for 3 min and reconstruction with femoral cortical allograft to cover the anterior defect, splenectomy performed in same session Second procedure was done with unreamed locked intramedullary nail and mixture of cancellous allograft and 30 mL demineralized bone matrix used for nonunion | Albendazole 10 mg/kg/day preoperatively for 1 month | No relapse, complete healing Complete union after second procedure at 12 months | 34 months | Non union of allograft on first procedure |
| Schnep-penheim | 54/f | Case report | Pain and swelling | Tibia | Multiple osteolytic lesion with reactive sclerosis | None | Trilamellar cystic wall with scolices of E Granulosus | Wide local curettage and reconstruction of defect with fibular autograft as well as allograft | Postoperative albendazole 10 mg/kg/day and Praziquantal (40 mg/kg/week) | Pain free, asymptomatic and no recurrence, well uptake of allograft | 24 months | None |
| Gnana-sekaran | 25/f | Case report | Discharging sinus on and off | Femur | Cortical thickening and sclerosis with intervening lucencies in diaphysis of femur, mild periosteal reaction with deformity seen | None | Viable and necrotic bones with cyst wall composed of acellular eosinophilic lamellated material surrounded with fibrosis, scolices of E Granulosus with hooklets in germinal layer | Debridement, sequestrectomy and saucerization followed by cotrimoxazole, praziquantel and albendazole, second stage surgery with re debridement with hypertonic saline and hypertonic saline with antibiotic cement spacer for cortical defect | Postoperative praziquantel, albendazole for 6 months | Good wound healing, asymptomatic and no evidence of recurrence | 12 months | Recurrence after first debridement |
Conclusion
Skeletal involvement is rare, accounting for less than 3% of hydatid disease. They are often diagnosed late or misdiagnosed as usual hematological and serological tests for hydatid disease are absent. Radiological features are usually suggestive of a lytic lesion of the bone. Biopsy is helpful in diagnosing skeletal hydatid disease. Preoperative chemotherapy followed by a surgery followed by postoperative chemotherapy seems to be effective to avoid recurrence. The bone defect caused by the disease or surgery can be managed with a bone graft either of autograft or allograft.
Patient’s perspective
Satisfactory with outcome.
Informed consent for publication
Informed consent was obtained from the patient for the publication of this case report. On request, a copy of the written consent is available for review by the Editor-in-Chief of this journal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical review committee statement
Not applicable.
Author contributions
B.B.N.—Planning of study, data management, writing, and revising the manuscript.
A.R.—Data management, manuscript preparation.
S.B.—Planning of study, revising the manuscript.
M.D.—Revising the manuscript.
R.H.P.—Data management.
Acknowledgment
None.
References
- 1.Torricelli P, Martinelli C, Biagini R, Ruggieri P, De Cristofaro R. Radiographic and computed tomographic findings in hydatid disease of bone. Skeletal Radiol. 1990;19:435–9. doi: 10.1007/BF00241799. [DOI] [PubMed] [Google Scholar]
- 2.Botezatu C, Mastalier B, Patrascu T. Hepatic hydatid cyst—Diagnose and treatment algorithm. J Med Life. 2018;11:203–9. doi: 10.25122/jml-2018-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalinova K, Proichev V, Stefanova P, Tokmakova K, Poriazova E. Hydatid bone disease: A case report and review of the literature. J Orthop Surg (Hong Kong) 2005;13:323–5. doi: 10.1177/230949900501300321. [DOI] [PubMed] [Google Scholar]
- 4.Zlitni M, Ezzaouia K, Lebib H, Karray M, Kooli M, Mestiri M. Hydatid cyst of bone: Diagnosis and treatment. World J Surg. 2001;25:75–82. doi: 10.1007/s002680020010. [DOI] [PubMed] [Google Scholar]
- 5.Song XH, Ding LW, Wen H. Bone hydatid disease. Postgrad Med J. 2007;83:536–42. doi: 10.1136/pgmj.2007.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beggs I. The radiology of hydatid disease. Am J Roentgenol. 1985;145:639–48. doi: 10.2214/ajr.145.3.639. [DOI] [PubMed] [Google Scholar]
- 7.Dathik S, Chopra RK, Talwar J, Pheroz M, Prasad R. Primary hydatidosis of distal femur masquerading malignancy—A rare case. J Clin Orthop Trauma. 2019;10:213–20. doi: 10.1016/j.jcot.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chari PR. Hydatid disease of scapula and upper third of humerus treated by en bloc excision and fibular bone grafting. Indian J Orthop. 2007;41:241–3. doi: 10.4103/0019-5413.33691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babitha F, Priya P, Poothiode U. Hydatid cyst of bone. Indian J Med Microbiol. 2015;33:442–4. doi: 10.4103/0255-0857.158587. [DOI] [PubMed] [Google Scholar]
- 10.Lewall DB, McCorkell SJ. Hepatic echinococcal cysts: Sonographic appearance and classification. Radiology. 1985;155:773–5. doi: 10.1148/radiology.155.3.3890008. [DOI] [PubMed] [Google Scholar]
- 11.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–94. doi: 10.1148/rg.232025704. quiz 536. [DOI] [PubMed] [Google Scholar]
- 12.Reddy IV, Kumar AHA, Samorekar B, Babu BA, Mettu AK. Complicated hydatid cyst of Ulna—A rare case report. J Clin Diagn Res. 2017;11:RD01–3. doi: 10.7860/JCDR/2017/21804.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merkle EM, Schulte M, Vogel J, Tomczak R, Rieber A, Kern P, et al. Musculoskeletal involvement in cystic echinococcosis: Report of eight cases and review of the literature. Am J Roentgenol. 1997;168:1531–4. doi: 10.2214/ajr.168.6.9168719. [DOI] [PubMed] [Google Scholar]
- 14.Arik HO, Arican M, Cetin NK, Sarp U. Primary intraosseous hydatid cyst of femur. Iran Red Crescent Med J. 2015;17:e21070. doi: 10.5812/ircmj.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loudiye H, Aktaou S, Hassikou H, Bardouni AE, Manouar ME, Fizazi M, et al. Hydatid disease of bone. Joint Bone Spine. 2003;70:352–5. doi: 10.1016/s1297-319x(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 16.Liang Q, Wen H, Yunus A, Tian Z, Jiang F, Song X. Treatment experiences of pelvic bone hydatidosis. Int J Infect Dis. 2014;18:57–61. doi: 10.1016/j.ijid.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Chen L, Xie Q, Bao Y, Luo X, Yi C, et al. Surgery or radiotherapy for the treatment of bone hydatid disease: A retrospective case series. Int J Infect Dis. 2015;33:114–9. doi: 10.1016/j.ijid.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Gautam D, Malhotra R, Dubey S. Combination drug chemotherapy and massive skeletal allograft in the management of hydatid disease of femur. BMJ Case Rep 2018. 2018 doi: 10.1136/bcr-2017-223332. bcr2017223332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscolo DL, Zaidenberg EE, Farfalli GL, Aponte-Tinao LA, Ayerza MA. Use of massive allografts to manage hydatid bone disease of the femur. Orthopedics. 2015;38:e943–6. doi: 10.3928/01477447-20151002-92. [DOI] [PubMed] [Google Scholar]