Abstract
Background:
Progressive improvement in the accuracy of profiling of hormone receptors in breast cancer provides the basis for targeted endocrine therapy, a major pillar of multimodal breast cancer treatment. However, the disparity in findings from comparatively smaller sample-sized studies in West Africa has led to somewhat conflicting conclusions and recommendations.
Objectives:
This study investigates the immunohistochemical (IHC) profile of breast cancer specimens for estrogen receptor (ER), progesterone receptor (PR), human epidermal receptor-2 (HER2)/neu, and Ki-67 in a tertiary hospital in Ibadan, Nigeria over 12 years.
Materials and Methods:
We reviewed 998 IHC reports, documented clinicopathologic parameters, computed patterns of biomarkers, and stratified them based on the American Society of Clinical Oncology/College of American Pathologists recommendations. Descriptive analysis including frequency, mean, and median were generated from the data extracted.
Results:
Out of the 998 cases, 975 (97.7%) were females and 23 (2.3%) were males. The mean age was 48.84 ± 11.99 years. Open biopsies were the most common types of specimens (320, 41.6%): lumpectomy and incisional biopsy of ulcerated, fungating or unresectable tumours. In those cases, 246 (32.0%) were samples of breast-conserving or ablative surgical extirpation (mastectomy/wide local excision/quadrantectomy), and 203 (26.4%) were obtained by core needle biopsies. Invasive ductal carcinoma was the most common histopathological type (673, 94.5%). The majority of graded tumours were intermediate grade (444, 53.5%). Four hundred and sixty-nine (48.4%) were ER positive, 414 (42.8%) were PR positive, and 180 (19.4%) were HER2/neu positive. Three hundred and thirty-four (34.0%) were triple-negative. Eighty-nine cases had Ki-67 staining done, and of these 61 (68.5%) had positive nuclear staining.
Conclusion:
Steroid hormone receptors and HER-2/neu proportions in our cohort are likely to be more representative than the widely varied figures hitherto reported in the sub-region. We advocate routine IHC analysis of breast cancer samples as a guide to personalized endocrine therapy.
Keywords: Breast cancer, estrogen receptors, HER-2/neu, Ibadan, immunohistochemistry, Ki-67, rogesterone receptors
Introduction
Hormonal therapy, a major pillar of integrated and personalized breast cancer care, has witnessed significant metamorphosis over the past century since the ground-breaking finding of Sir Beaston.[1] His observation that hormonal manipulation held immense potential in breast cancer treatment has been further explicated. Accurate profiling of hormone receptors expressed in breast cancer provides evidence-based guidance to targeted endocrine therapy, which provides one of the pillars for personalized breast cancer care.[2,3]
The estrogen receptor (ER), a member of the nuclear protein superfamily,[3] functions via a multi-domain nuclear transcription by which it forms complexes with co-activators or co-repressors[4] to modulate transcription. Tamoxifen, the first generation of selective ER modulators (SERMs), is known to act through the latter pathway. Although SERMs have proven to be potent endocrine therapy for breast cancer, it is believed that resistance is ultimately inevitable.[4] The multimodal mechanism of newer-generation aromatase inhibitors (AIs) in blocking ER makes them a more effective hormonal therapy modality.[4] However, in SERM- and AI-refractory cases, pure antiestrogens (also called selective oestrogen receptor degraders, SERDs) can be considered, although the use of the only FDA-approved SERD, fulvestrant is limited by its poor bioavailability.[5] Progesterone receptors (PRs) serve as a surrogate marker of ER activity and a weaker predictor of endocrine therapy response.[3] Based on literature, hormone receptor status is a major determinant of breast cancer responsiveness to endocrine therapy, both in terms of overall and relapse-free survival.[2,6,7]
Human epidermal receptor-2 (HER-2) is a 185-kDa member of the transmembrane growth factor receptor family, and tends to function as a choice dimerization partner for other HER members. Its overexpression culminates in sustained trigger of signal transduction, proliferation overdrive, and immortalization of aberrant tumour cells bearing it. In trastuzumab-naïve patients, HER2-positivity has been identified as an independent unfavourable prognostic factor in breast cancer. Conversely, retrospective studies predict positive response to endocrine therapy and adjuvant chemotherapy in HER2-enriched tumours.[8]
All proliferating cells express Ki-67, and its strong association with regulation of the cell cycle, tendency to drive aggressive tumour phenotype as well as its prognostic implication in breast cancer management are well described in literature. This is particularly important when the value is greater than 10%,[9,10,11,12,13] especially in younger women with basal-like, high grade, larger size, steroid hormone negative tumours.[14,15] Furthermore, high Ki-67 value assessed via real-time polymerase chain reaction and Immunohistochemistry (IHC) has been found to be a predictor of better response to neoadjuvant chemotherapy in locally advanced breast cancer. [16]
Various studies on breast cancer hormone receptor status in the West African sub-region have been somewhat conflicting. While three studies involving subjects from Nigeria, Senegal and Ghana reported ER positivity of 24%,[17] 25%,[18] 25%,[19] and 26.2%[20], other researchers from Nigeria and Mali found ER positive proportions of 58%,[21] 58.1%,[22] 59%,[23] and 65%,[24] in their studies. These studies had a total number of cases studied ranging between 103[22] and 507.[19] This is noted despite the fact that the cited studies share fairly comparable clinico-epidemiologic parameters, methods and period of study. A recent survey of pathology services from population-based cancer registries in SSA alluded to other potential factors affecting quality of results; including faulty machines, inconsistent power supply, suboptimal technical support and unavailability of consumables.[25]
In Nigeria, as in most other Low- and Middle-Income Countries (LMICs), IHC for receptor status is not routinely determined,[26] although recent evidence suggests this pattern is improving.[25] The fact that many centres with histopathology services lack personnel and infrastructure for it, coupled with the relatively high cost are some of the limiting factors against IHC.[26] The IHC uptake in confirmed breast cancer cases range from 18.7% to 31% in some local studies in Nigeria.[26,27] Thus, a significant proportion of dedicated laboratories like ours are research-driven,[19,28] which confers an additional advantage of cost subsidy, therefore permitting many more samples to be analysed.
This study examines the immunohistochemical profile of breast cancer specimens for ER, PR, HER2/neu, and Ki-67 in a tertiary health institution in Ibadan, Nigeria over 12 years.
Materials and Methods
Patients
The study is a retrospective review of 998 patients with breast carcinoma who had samples of core needle biopsy, lumpectomy, wide local excision (WLE) and mastectomy submitted for histopathology and immunohistochemistry service at the University College Hospital, Ibadan, over a 12-year period, January 2005 to December 2016. Data were extracted from the databases of the Surgical Oncology unit and Breast Cancer Registry in the Department of Surgery, Department of Pathology and the Institute for Medical Research and Training of our centre. A total 1118 cases were submitted for IHC after a breast cancer histopathological diagnosis over the 12-year period. The inclusion criteria are age of the patient, gender, hormonal receptor status that was used for therapeutic decision, histopathological type, and tumour grade. Of the 1118, 120 cases with incomplete documentation of demographic parameters were excluded from the study and the analysis.
The gender, age, steroid hormone receptor status (ER and PR) and HER-2/neu status, histologic type (WHO classification), and tumour grade (Scarf-Bloom Richardson system) were retrieved. Each case was re-evaluated and rescored using the American Society of Clinical Oncology (ASCO) scoring for uniformity to meet the inclusion criteria of satisfactory dataset with regard to IHC scoring. Ki-67 analysis was carried out in our centre only in 2016. All the data were entered into a spread sheet.
Laboratory IHC protocol
Pre analytical phase
Typically, the cold ischaemic time is shortened based on the protocol established for IHC service in the hospital. The incisional and excisional biopsies are fixed immediately in 10% neutral buffered formalin (NBF). The mastectomies are grossed within a maximum of 30 minutes and representative sections are placed in 10% NBF. We do not allow tissues more than 24 hours in fixative before processing.
Analytical phase
The protocol used for IHC of Formalin Fixed Paraffin Embedded tumour tissue in our hospital follows manufacturer’s instructions and protocol. After sectioning, this typically involved de-paraffinizing the slides in xylene twice, 5 minutes each. The slides are then transferred into 100% alcohol for two times, 3 minutes each, and then transferred through 95% and 70% alcohol preparations, twice and once respectively for 3 minutes each. Slides are then rinsed with wash buffer twice, 5 minutes each.
Antigen retrieval is then performed to unmask the antigenic epitope, the most commonly used antigen retrieval buffer being a citrate pH 6.0 and EDTA pH 9.0. Blocking buffer (e.g., 10% foetal bovine serum in phosphate-buffered saline or 3% H2O2) is then added onto the sections of the slides, incubated in a humidified chamber at room temperature for 15minutes, drained off and washed in wash buffer. After this, appropriately diluted primary antibody and biotinylated + streptavidin HRP secondary or polymeric-HRP anti-mouse/anti-rabbit are added to the sections on the slides sequentially, with incubation in a humidified chamber following each stage.
3,3′-Diaminobenzidine substrate solution (freshly made just before use: 0.05% 3,3′-Diaminobenzidine – 0.015% H2O2 in phosphate-buffered saline) is used to reveal the colour of antibody staining and slides are then counterstained by immersing sides in Gill’s Haematoxylin for 10–20 seconds. Slides are rinsed, dried, dehydrated serially through alcohol (95%–100%), cleared in xylene and mounted. The colour of the antibody staining in the tissue sections is observed under microscopy. Slides can be stored at room temperature permanently.
Post analytical phase/immunohistochemistry assessment
American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) scoring system is adopted in our centre and was used for all the IHC. It is a semi-quantification of the staining of the tumour cells. The scoring of ER and PR was based on the staining intensity (as either weak, moderate, or intense) and the percentage of tumour cells showing a nuclear immunostaining for ER and PR (range: 0%–100%). ER and PR are adjudged positive when ≥1% of tumour cells have nuclear immunoreactivity,[29,30] while the degree of staining intensity as highlighted above is also documented but not considered for ASCO scoring. HER-2 was evaluated based on the intensity of cell membrane staining and percentage of membrane positive tumour cells to give a score that range from 0 to 3+.[31] HER-2 is considered positive when ˃10% of the tumour cells show strong circumferential staining of tumour cells corresponding to a score of 3+. A weak to moderate complete membrane staining in more than 10% of tumour cells is scored 2+ or equivocal while HER2 is negative when there is no staining, weak incomplete staining and membrane staining in less than 10% of tumour cells [Figure 1]. All HER2 that were equivocal are not further assessed due to the lack of fluorescence in situ hybridization (FISH) facilities.
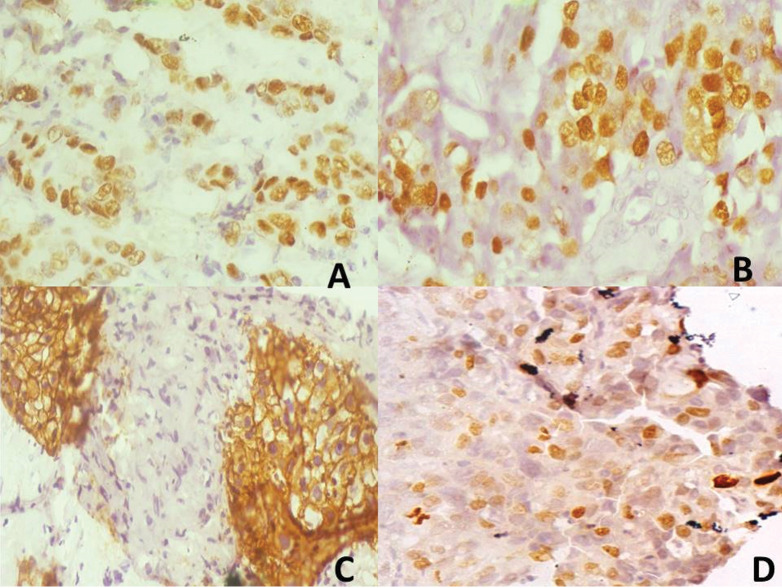
Figure 1.
Composite photomicrographs of (a) (×400): Representative oestrogen receptor (ER) positive tumour (3+). (b) (×400): Representative progesterone receptor (PR) positive tumour (3+). (c) (×400): Representative human epidermal receptor-2 (HER-2) positive tumour (3+). (d) (×400): Representative Ki-67 positive tumour, proliferative index about 35%
Ki-67 score was expressed as the percentage of the number of immunostained nuclei among the total number of nuclei of tumour cells regardless of the immunostaining intensity. The counting was performed in three randomly selected fields of the breast tissue section at ×400 magnification. Ki-67 score ranged from 0% to 100%. The expression of proliferation index Ki-67 is categorized into three groups: low (Ki-67 ≤ 14%), intermediate (Ki-67: 15%–30%), and high (Ki-67 > 30%) according to the recommendations of the St Gallen International Consensus of Experts.[32,33]
Ethical issues and data analysis
The study was conducted in compliance with the guidelines of the Helsinki declaration on biomedical research in human subjects. Confidentiality of the identity of the patients and personal health information was maintained.
Data were statistically analysed using the IBM SPSS Statistics (Version 20.0, IBM Corp, Armonk, NY), and the analysed data are presented using tables and charts. Descriptive analysis in terms of frequency, mean, and median were generated from the data extracted. In addition, patterns of biomarkers were documented in terms of clinico-pathological parameters and Ki-67 expression.
Results
A total of 998 patients were included in the study. Table 1 shows their biodata. The mean age was 48.84 ± 11.99 years, with a range of 20–92 years. Median age was 48.00 years, with 35.6% of the patients in the 41–50 category.
Table 1.
Epidemiologic and clinico-pathologic characteristics
| Characteristic | Variable completeness (%)* | Categories | Number (% of available data) |
|---|---|---|---|
| Age at diagnosis (years) | 100 | ≤30 | 50 (5.0) |
| 31–40 | 199 (19.9) | ||
| 41–50 | 355 (35.6) | ||
| 51–60 | 256 (25.7) | ||
| 61–70 | 80 (8.0) | ||
| 71–80 | 47 (4.7) | ||
| >80 | 11 (1.1) | ||
| Sex | 100 | Female | 975 (97.7) |
| Male | 23 (2.3) | ||
| Period of diagnosis | 100 | 2005–2008 | 254 (25.4) |
| 2009–2012 | 280 (28.1) | ||
| 2013–2016 | 464 (46.5) | ||
| Type of specimen | 96.5 | Core needle | 253 (26.3) |
| Open biopsy | 401 (41.6) | ||
| Mastectomy/WLE/quadrantectomy | 309 (32.1) | ||
| Histological type | 71.3 | Invasive ductal | 673 (94.5) |
| Invasive lobular | 23 (3.2) | ||
| Medullary | 2 (0.3) | ||
| Mucinous | 10 (1.4) | ||
| Tubular | 1 (0.1) | ||
| Mixed | 3 (0.4) | ||
| Tumour grade | 91.2 | Low grade | 174 (21.0) |
| Intermediate grade | 444 (53.5) | ||
| High grade | 212 (25.5) |
*The components left out were either missing, improperly documented or ambiguous
Out of the 769 specimens that had documented modality by which they were obtained, open biopsies were the most common types of specimens (320, 41.6%): lumpectomy and incisional biopsy of ulcerated, fungating or unresectable tumours. 246 (32.0%) were samples of breast-conserving or ablative surgical extirpation (mastectomy/wide local excision/quadrantectomy) and 203 (26.4%) were obtained by core needle biopsies.
Invasive ductal carcinoma was the most common histopathological type (673, 94.5%) followed by invasive lobular carcinoma (23, 3.2%). Others include mucinous, medullary, tubular and mixed subtypes [Table 1]. The most common tumour grade was the intermediate (444, 53.5%) followed by the high grade (212, 25.5%) and then the low grade (174, 21.0%).
Discussion
Literature from the West African sub-region appears to show a common ground in terms of specific epidemiologic characteristics of patients; range and mean ages at diagnosis consistently identified between 20–92 years and 45–49 years, respectively.[21,23,34,35,36,37,38] This similarity has also been demonstrated in different types of studies that include small sample size cross-sectional studies[38,40] as well as in a prospective study of 302 patients in Ivory coast.[41] These findings show disparity and contrast with mean age of 55-58 years reported in the USA[42] and 55.3 ± 14.3 years in a large South African study with a significant white population.[43] Those studies illustrate that most of the breast cancer cases were in post-menopausal women in contrast to most studies from sub-Saharan black African populations who are mainly pre-menopausal.
In our study, over 60% of new cases of breast cancer were diagnosed by age 50 years. These pieces of evidence continue to accumulate and strengthen the argument for screening protocols at an earlier age in indigenous black women. Given that late diagnosis is a standout factor contributing to breast cancer mortality in LMICs with data showing comparable early-stage breast cancer survival outcomes when compared with HICs (78% vs 86%),[21] it is expected that HDI (human development index)-adjusted excess hazards of death in this cohort should further compete fairly with that of HICs, when the economic implications of breast cancer treatment in LMICs are taken into consideration.
Women were unsurprisingly the overwhelming majority in our series; male proportion of 2.3% is in keeping with 1%[39] to 3.8%[23] earlier reported.[44] African and African-American women are less likely to have lobular breast carcinoma subtype compared to their Caucasian counterparts, and the low yield (3.2%) is in keeping with a number of previous findings in black women—4.2%[15] and 2.4%[27], respectively.
Despite the relatively comparable biodata parameters and study designs, data from literature across Nigeria, Senegal, Ivory Coast, Ghana and East Africa have shown a widely variable ER positive status, ranging from a mere 24%, to 65%, with these studies carried out mainly in indigenous black women. This has led to differing treatment recommendations, including contradictory opinions. Omoniyi-Esan et al.[36] in a review of 136 cases in Ile-Ife, south-west Nigeria found ER positivity of 34.6% and suggested that a routine use of antiestrogens is justifiable in such a low-resource setting if IHC is unavailable, even though triple-negative tumours were commoner than receptor-positive groups in their cohort. On the other hand, in another predominantly triple-negative breast cancer cohort with ER+ of 36.8% in north-east Nigeria, Minoza et al.[40] recommended a redirection of treatment of choice from hormonal therapy to cytotoxic chemotherapy, an opinion that had earlier been shared by Zaha in 2014.[30]
A meta-analysis of pooled data of over 4700 breast carcinoma IHC revealed an overall ER+ of 42% in SSA, although there was considerable heterogeneity in ER proportions (I-squared statistic = 97%) between the samples processed prospectively (59%) and archival tissue blocks (30%). [45]
Based on the recommendations of the ASCO/CAP Expert Panel report[29,46] on which the stratification of steroid receptor positivity of our centre is premised, the cut-off point separating positive from negative cases is ≥1% nuclear staining as earlier pointed out, which is the threshold for which endocrine therapy should be considered, justifying the risk-benefit ratio of hormonal therapy. Results were labelled “equivocal” when they were uninterpretable or when sample processing deviated from pre-analytic specifications. Due to variability of computation and interpretation of composite scores such as the AllRed Score across institutions,[47] we therefore adopted the ASCO/CAP three-parameter recommendations to present our findings.
In this study, ER+ was 48.4%, [Table 2] which is lower than the 58%[21] and 59%[23] earlier reported by two studies from our unit. These studies involved 63 and 354 participants, respectively. This larger cohort of almost 1000 patients across strata of gender, clinical stage and specimen types which were reviewed over a 12-year period, arguably presents a more representative population of patients and to our knowledge, the largest cohort reported in West Africa.
Table 2.
Pattern of biomarkers based on IHC
| Characteristic | Variable completeness (%) | Categories | Number (% of sample data)# |
|---|---|---|---|
| ER | 97.1 | Negative | 500 (51.6) |
| Positive | 469 (48.4) | ||
| -out of which - Staining intensity: | 293 (30.2) | ||
| 1+ (1%–9%) | |||
| 2+ (10%–33%) | 66 (6.8) | ||
| 3+ (>33%) | 110 (11.4) | ||
| PR | 97.0 | Negative | 554 (57.2) |
| Positive | 414 (42.8) | ||
| -out of which - Staining intensity: | 267 (27.6) | ||
| 1+ (1%–9%) | |||
| 2+ (10%–33%) | 71 (7.3) | ||
| 3+ (>33%) | 76 (7.9) | ||
| HER2/neu | 92.8 | Equivocal (2+) | 42 (4.5) |
| Negative (0 and 1+) | 704 (76.0) | ||
| Positive (3+) | 180 (19.4) | ||
| Ki-67 (n = 89) | 100 | Equivocal | 13 (14.6) |
| Negative | 15 (16.9) | ||
| Positive: out of which | 61 (68.5) | ||
| Staining intensity: Low (≤14%) | 46 (51.7) | ||
| Intermediate (15%–30%) | 9 (10.1) | ||
| High (>30%) | 6 (6.7) | ||
| Molecular | 98.4 | Lumina A (ER &/or PR+, HER2–) | 364 (37.1) |
| subtypes | Lumina B (ER &/or PR+, HER2+) | 86 (8.8) | |
| Triple negative (ER/PR–, HER2–) | 334 (34.0) | ||
| HER2-enriched (ER/PR-, HER2-) | 91 (9.3) | ||
| Inconclusive (ER/PR+, HER2?; ER/PR?, HER2+/–) | 107 (10.9) |
#Apart from Ki-67, for which the subgroup percentages are with respect to n = 89
While Adebamowo et al.[24] notably concluded that there is no difference between hormone receptor status of breast cancer patients of indigenous African women and other populations, other reports suggest this assertion may be true only for a subset of East African women.[40,48] Our findings therefore support the majority who have found a lower proportion of ER+ tumours.[37,38,39,40,41] Furthermore, an NCI SEER database of over 197,000 breast cancer cases showed a higher stage-adjusted ER-positivity rates in African-Americans while the TJUH database of 2230 patients showed Caucasian versus African-American ER+ values of 63.1% and 51.9%, respectively (P = 0.0003).[15]
Correlates data on ER and PR suggest that both receptors are co-dependent, the latter being the weaker variable in predicting response to endocrine therapy, after adjusting for confounders.[49,50]
At 19.4%, HER-2/neu+ status of our patients is higher than the 4%[37], 5.2%[24] and 10.8%[28] reported in western Nigeria; it tallies with the 19%[47], 19.6%[51], 21.1%[40] and 22%[38] found across West Africa but much lower than the 38.2%[36] documented at Ile-Ife. IHC studies in which archival tissue blocks are retrieved and processed for receptor status are known to present low yield as a result of molecular degeneration over time due to marked fluctuations in temperature of the tissue storage facility, among other factors.
Furthermore, Adebamowo et al. documented unknown/inconclusive outcome for over 15% of the HER-2 results, some of which could have been positive. In a sequential FISH analysis of IHC-equivocal 373 HER-2 results, Agersborg et al. found that 52.3% of the equivocal cases became positive.[52] In our study, the equivocal HER-2 results are FISH-unadjusted as facility for it was unavailable. Opinions are somewhat divided on the cut-off for a “positive” HER-2 status, but current consensus (ASCO/CAP) provides that a uniformly circumferential intense tumour staining of >30%, corresponding to 3+ is adjudged positive, while weakly positive (1+ and 2+) are excluded.[30] This guideline has been adopted in our study; however, studies that capture 1+ and 2+ HER-2 positivity are likely to obtain spuriously inflated figures. This may explain some of the unusually high percentages in local literature.
Ki-67 assay is not readily performed in our country and sub-region. In our analysis of 89 samples, just over two-thirds (68.5%) were Ki-67 positive [Table 3]. Agboola et al.[9] reported Ki-67 positivity of 82.6% in a cohort of 308 Nigerian women, and found poorer breast cancer prognosis compared with UK grade-matched 1902 patients, among whom 66.7% were Ki-67 positive. In their Nigerian series, a significant proportion of the patients were younger than 50 years at diagnosis, were premenopausal, had ductal carcinoma histologic type and had evidence of vascular invasion and lymph node metastasis (P < 0.001). With our total cases less than a third of theirs, we adjudge they may have presented a more representative summary, although the details of their sampling techniques have to be taken into consideration. Furthermore, our data were obtained in the first year of Ki-67 assay learning curve of our laboratory. Agboola et al. concluded that Ki-67 expression is an indicator of unfavourable tumour biology and poor prognosis. In addition to other known parameters with prognostic value including ER, PR and HER-2 status, accurate characterization of this cell proliferation signature holds immense value in prognostication of indigenous black women breast cancer patients.
Table 3.
Proportions of clinico-pathological parameters and KI-67 expression in breast cancer samples
| Variable | Frequency (%) |
|---|---|
| Age (n = 89) | |
| ≤30 | 3 (3.4) |
| 31–40 | 19 (21.3) |
| 41–50 | 28 (31.5) |
| 51–60 | 25 (28.1) |
| 61–70 | 7 (7.9) |
| 71–80 | 6 (6.7) |
| >80 | 1 (1.1) |
| Histological type (n = 57) | |
| Invasive ductal | 55 (96.4) |
| Invasive lobular | 1 (1.8) |
| Mucinous | 1 (1.8) |
| Tumour grade (n = 64) | |
| Low grade | 12 (18.8) |
| Intermediate grade | 43 (67.1) |
| High grade | 9 (14.1) |
| Vascular invasion (n = 44)* | |
| Absent | 15 (34.1) |
| Present | 29 (65.9) |
| ER (n = 86) | |
| Negative | 58 (67.4) |
| Positive | 28 (32.6) |
| PR (n = 86) | |
| Negative | 64 (74.4) |
| Positive | 22 (25.6) |
| HER2/neu (n = 80) | |
| Negative | 68 (85.0) |
| Positive | 12 (15.0) |
| Molecular subtypes (n = 88) | |
| Lumina A | 23 (26.1) |
| Lumina B | 7 (8.0) |
| Triple negative | 44 (50.0) |
| HER2-enriched | 5 (5.7) |
| Inconclusive | 9 (10.2) |
*45 cases had unrecorded vascular invasion status
Among 679 cases in the TJUH registry in which a direct race comparison was made, African-Americans had a significantly higher Ki-67 proliferation expression (42.2% vs 28.7%; P < 0.001).[15] The figures are probably lower because a positive cut-off of 20% was used in that database. For the women of African heritage, increased Ki-67 expression correlated with poor survival outcomes; while there was also increased p53 expression, higher nuclear grades and overall trend of invasive tumour subtypes.
Although Morris et al. did not find any difference in the expression of p21 and bcl-2 between their African–American and Caucasian patients; Agboola et al. found a difference between their Nigeria versus UK cohorts when sub-stratified based on Ki-67 expression. [9,15] Given that these protein biomarkers are not assayed routinely, more research into this area is needed to draw conclusions about their patterns and correlation with other immunohistochemical biomarkers.
Conclusion
At 48.4% and 19.4% respectively, ER and HER-2/neu positive immunoreactivity found in our study is arguably more representative than the widely varied figures hitherto reported in the sub-region. We have presented data from a larger number of samples processed in a dedicated laboratory with reports classified based on the ASCO/CAP recommendations. We strongly advocate routine IHC analysis of all breast cancer samples as a guide to individualized endocrine therapy. Further research to explore manipulating KI-67 as a potential breast cancer treatment in native black women is recommended.
Limitations
Lack of FISH facilities hampered further characterization of equivocal hormone receptor status.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Trans Med Chir Soc Edinb. 1896;15:153–79. [PMC free article] [PubMed] [Google Scholar]
- 2.Puhalla S, Bhattacharya S, Davidson NE. Hormonal therapy in breast cancer: A model disease for the personalization of cancer care. Mol Oncol. 2012;6:222–36. doi: 10.1016/j.molonc.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 1998;51:227–38. doi: 10.1023/a:1006132427948. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat Res. 2005;591:247–63. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Mottamal M, Kang B, Peng X, Guangdi W. From pure antagonists to pure degraders of the estrogen receptor: Evolving strategies for the same target. ACS Omega. 2021;6:9334–43. doi: 10.1021/acsomega.0c06362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: Evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian breast and colorectal cancer study group trial 5. J Clin Oncol. 2002;20:4621–27. doi: 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, et al. Use of luteinizing-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor positive breast cancer: A meta-analysis of individual patient data from randomized adjuvant trials. The Lancet. 2007;369:1711–23. doi: 10.1016/S0140-6736(07)60778-8. [DOI] [PubMed] [Google Scholar]
- 8.Tubbs RR, Stoler MH, Elsevier . 1st ed. Edinburgh: Churchill Livingstone; 2009. Cell and Tissue Based Molecular Pathology; pp. 360–78. [Google Scholar]
- 9.Agboola AOJ, Banjo AAF, Anunobi CC, Salami B, Agboola MD, Musa AA, et al. Cell proliferation (KI-67) expression is associated with poorer prognosis in Nigerian compared to British breast cancer women. ISRN Oncol 2013. 2013 doi: 10.1155/2013/675051. 675051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aleskandarany MA, Green AR, Rakha EA, Mohammed RA, Elsheikh SE, Powe DG, et al. Growth fraction as a predictor of response to chemotherapy in node-negative breast cancer. Int J Cancer. 2010;126:1761–9. doi: 10.1002/ijc.24860. [DOI] [PubMed] [Google Scholar]
- 11.Dai H, Van’tVeer L, Lamb J, He YD, Mao M, Fine BM, et al. A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Res. 2005;65:4059–66. doi: 10.1158/0008-5472.CAN-04-3953. [DOI] [PubMed] [Google Scholar]
- 12.Finek J, Holubec H, Jr, Topolcan O, Elgrova L, Skalova A, Pecen L. The importance of prognostic factors in premenopausal women with breast cancer. Anticancer Res. 2007;27:1893–6. [PubMed] [Google Scholar]
- 13.De Azambuja E, Cardoso F, De Castro G, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12155 patients. Br J Cancer. 2007;96:1504–13. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jemal A, Clegg LX, Ward E, Ries LAG, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 15.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: A single-institution compilation compared with the national cancer institute’s surveillance, epidemiology, and end results database. Cancer. 2007;110:876–84. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 16.Prihantono P, Hatta M, Binekada C, Sampepajung D, Haryasena H, Nelwan B, et al. Ki-67 expression by immunohistochemistry and quantitative real-time polymerase chain reaction as predictor of clinical response to neoadjuvant chemotherapy in locally advanced breast cancer. J Oncol 2017. 2017 doi: 10.1155/2017/6209849. 6209849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikpatt OF, Ndoma-Egba R. Oestrogen and progesterone receptors in Nigerian breast cancer: Relationship to tumour histopathology and survival of patients. Cent Afr J Med. 2003;49:122–6. [PubMed] [Google Scholar]
- 18.Gukas ID, Jennings BA, Mandong BM, Igun GO, Girling AC, Manasseh AN, et al. Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr J Med. 2005;24:209–13. doi: 10.4314/wajm.v24i3.28220. [DOI] [PubMed] [Google Scholar]
- 19.Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, et al. Population differences in breast cancer: Survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–21. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz T, Stark A, Pang J, Awuah B, Kleer CG, Quayson S, et al. Expression of aldehyde dehydrogenase 1 as a marker of mammary stem cells in benign and malignant breast lesions of Ghanaian women. Cancer. 2013;119:488–94. doi: 10.1002/cncr.27737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayandipo OO, Afuwape OO, Adepoju OJ, Ajiboye JA, Ogundiran TO. Stage-specific five-year survival outcomes in women treated for early stage breast cancer in Ibadan, Nigeria. Niger J Med. 2020;29:152–7. [Google Scholar]
- 22.Togo A, Kante L, Dembele BT, Traore A, Diakite I, et al. Breast cancer in Bamako hospitals: Epidemiologic and diagnostic aspects. Medecine d’ Afrique Noire. 2010;57:249–53. [Google Scholar]
- 23.Ogundiran TO, Ayandipo OO, Ademola AF, Adebamowo CA. Mastectomy for management of breast cancer in Ibadan, Nigeria. BMC Surg. 2013;13:59. doi: 10.1186/1471-2482-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183–8. doi: 10.1007/s10549-007-9694-5. [DOI] [PubMed] [Google Scholar]
- 25.Ziegenhorn HV, Frie KG, Ekanem IO, Ebughe G, Kamate B, Traore C, et al. Breast cancer pathology services in sub-Saharan Africa: A survey within population-based cancer registries. BMC Health Serv Res. 2020;20:1–9. doi: 10.1186/s12913-020-05752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nwafor CC, Keshinro SO. Pattern of hormone receptors and human epidermal growth factor-2 status in sub-Saharan breast cancer cases: Private practice experience. Niger J Clin Pract. 2015;18:553–8. doi: 10.4103/1119-3077.156905. [DOI] [PubMed] [Google Scholar]
- 27.Ukah CO, Emegoakor C, Anyiam DCD, Onyiaorah IV, Onwukamuche ME, Egwuonwu OA, et al. The immunohistochemistry profile of breast cancer in indigenous women of southeast Nigeria. Ann Med Health Sci Res. 2017;7:83–7. [Google Scholar]
- 28.Ugiagbe EE, Olu-Eddo AN, Obaseki DE. Immunohistochemical detection of Her-2/neu overexpression in breast carcinoma in Nigerians: A 5-year retrospective study. Niger J Clin Pract. 2011;14:332–7. doi: 10.4103/1119-3077.86779. [DOI] [PubMed] [Google Scholar]
- 29.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–22. doi: 10.5858/134.6.907. Erratum in: Arch Pathol Lab Med. 2010;134:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol. 2014;5:382–92. doi: 10.5306/wjco.v5.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. American Society of Clinical Oncology/College of American pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 32.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for therapies: Highlights of the St Gallen International expert consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–29. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adesunkanmi ARK, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast. 2006;15:399–409. doi: 10.1016/j.breast.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Huo D, Adebamowo CA, Ogundiran TO, Akang EE, Campbell O, Adenipekun A, et al. Parity and breastfeeding are protective against breast cancer in Nigerian women. Br J Cancer. 2008;98:992–6. doi: 10.1038/sj.bjc.6604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omoniyi-Esan GO, Olaofe OO, Aremu OA, Omonisi AE, Olasode BJ, Adisa OA. Hormonal and Her2 receptor immunohistochemistry of breast cancers in Ile-Ife, Nigeria. Austin J Womens Health. 2015;2:1009. [Google Scholar]
- 37.Titiloye NA, Omoniyi-Esan GO, Adisa AO, Komolafe AO, Afolabi OT, Adelusola KA. Breast cancer in a Nigerian cohort: Histopathology, immunohistochemical profile and survival. Postgrad Med J. 2013;2:83–7. [Google Scholar]
- 38.Imam BA, Okechi OO, Abdullahi K, Abubakar U, Musa AB, Okorie N, et al. Immunohistochemical pattern of breast cancer in Maiduguri, Borno State. JCTI. 2017;5:1–10. [Google Scholar]
- 39.Adisa CA, Eleweke N, Alfred AA, Campbell MJ, Sharma R, Nseyo O, et al. Biology of breast cancer in Nigerian women: A pilot study. Ann Afr Med. 2012;11:169–75. doi: 10.4103/1596-3519.96880. [DOI] [PubMed] [Google Scholar]
- 40.Minoza KG, Yawe KDT, Mustapha Z, Lawan M, Na’aya HU, Nggada HA. Hormonal and HER2 receptor immunohistochemistry of breast cancer in north-eastern Nigeria: A preliminary report. IOSR-JDMS. 2016;15:18–23. [Google Scholar]
- 41.Effi AB, Aman NA, Koui BS, Koffi KD, Traoré ZK, Kouyate M. Immunohistochemical determination of estrogen and progesterone receptors in breast cancer: Relationship with clinicopathologic factors in 302 patients in Ivory Coast. BMC Cancer. 2017;17:1–6. doi: 10.1186/s12885-017-3105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallel I, Khabir A, Boujelbene N, Abdennadher R, Daoud J, Frikha M, et al. EGFR overexpression relates to triple negative profile and poor prognosis in breast cancer patients in Tunisia. J Recept Signal Transduct Res. 2012;32:142–9. doi: 10.3109/10799893.2012.664552. [DOI] [PubMed] [Google Scholar]
- 43.McCormack VA, Joffe M, van den Berg E, Broeze N, dos Santos Silva I, Romieu I, et al. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: A case series. Breast Cancer Res. 2013;15:R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayandipo OO, Ogun GO, Adepoju OJ, Fatunla EO, Afolabi AO, Osuala PC, et al. Impact of axillary node-positivity and surgical resection margins on survival of women treated for breast cancer in Ibadan, Nigeria. eCancer. 2020;14:1084. doi: 10.3332/ecancer.2020.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: A systematic review and meta-analysis. PLoS Med. 2014;11:e1001720. doi: 10.1371/journal.pmed.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol. 2014;25:1004–11. doi: 10.1093/annonc/mdu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 48.Jemal A, Fedewa SA. Is the prevalence of ER-negative breast cancer in the US higher among Africa-born than US-born black women? Breast Cancer Res Treat. 2012;135:867–73. doi: 10.1007/s10549-012-2214-2. [DOI] [PubMed] [Google Scholar]
- 49.Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination Trial. J Clin Oncol. 2008;26:1059–65. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 50.Adeniji KA, Huo D, Khramtsov A, Zhang C, Olopade OI. Molecular profiles of breast cancer in Ilorin, Nigeria. J Clin Oncol. 2010;28:1602. [Google Scholar]
- 51.Titiloye NA, Foster A, Omoniyi-Esan GO, Komolafe AO, Daramola AO, Adeoye OA, et al. Histological features and tissue microarray taxonomy of Nigerian breast cancer reveal predominance of the high-grade triple-negative phenotype. Pathobiology. 2016;83:24–32. doi: 10.1159/000441949. [DOI] [PubMed] [Google Scholar]
- 52.Agersborg S, Mixon C, Nguyen T, Aithal S, Sudarsanam S, Blocker F, et al. Immunohistochemistry and alternative FISH testing in breast cancer with HER2 equivocal amplification. Breast Cancer Res Treat. 2018;170:321–8. doi: 10.1007/s10549-018-4755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]