STRUCTURED ABSTRACT
Objective
To determine if Pyk2-deficiency increases midpalatal suture bone mass and preserves sutural integrity after maxillary expansion.
Setting and Sample
Thirty-six male Pyk2 knockout (KO) and control (WT) mice at 6-weeks of age.
Materials and Methods
Mice received nickel-titanium spring expanders delivering 0g (no intervention control), 10g or 20g force for 14 days. High-resolution micro-CT was used to determine bone volume/tissue volume (BV/TV), sutural width and intermolar width. Effects on osteoclasts, chondrocytes and suture morphology were determined by histomorphometry.
Results
Pyk2-KO controls (0g) had 7% higher BV/TV compared with WT controls. Expanded Pyk2-KO maxillae also exhibited 12% (10g) and 18% (20g) higher BV/TV than WT mice. Although bone loss following expansion occurred in both genotypes, BV/TV was decreased to a greater extent in WT maxillae (−10% at 10g; −22% at 20g) compared with Pyk2-KO maxillae (−11% only at 20g) (p<0.001). Expanded WT maxillae also showed a greater increase in sutural width, intermolar width and fibrous connective tissue width compared with expanded Pyk2-KO maxillae. Moreover, osteoclast number was increased 77% (10g) and 132% (20g) in expanded WT maxillae, but remained unchanged in expanded Pyk2-KO, compared to their respective controls. Cartilage area and chondrocyte number were increased to the same extent in expanded WT and Pyk2-KO sutures.
Conclusions
These findings suggest that midpalatal suture expansion increases osteoclast formation in WT but not Pyk2-KO mice, leading to higher BV/TV in expanded Pyk2-KO maxillae. These studies suggest Pyk2-targeted strategies may be beneficial to increase bone density and preserve sutural integrity during maxillary expansion.
Keywords: orthodontic, mechanical force, rapid maxilla expansion, bone remodeling, osteoclast, osteoblast
INTRODUCTION
Rapid maxillary expansion (RME) increases the transverse dimension of the mid-face and corrects posterior cross-bite to relieve dental crowding in patients with a narrow maxillary dental arch.1,2 This technique uses an expander to separate the midpalatal suture, induce new bone deposition at the suture-bone margins and widen the maxillary arch. A limitation is that the rate of RME often exceeds the rate of bone formation within the suture. The prolonged suture gap leads to a high potential of treatment relapse because the connective tissues between the separated bones tend to pull the maxillary bones back into their original positions if insufficient or immature bone occupies the suture gap.3 To minimize the potential for relapse, a long retention period of 3 to 6 months is recommended to allow sufficient time for the restoration of bone mineral density (BMD) within the expanded suture.4,5 To shorten the retention period, and obtain ideal RME treatment effects in patients with constricted maxillary arches, additional strategies are required to increase bone formation in the suture.1,2,4,5
Bone formation is known to be more active in the midpalatal suture after application of an expansion force, compared with sutures without any intervention.6–8 Previous reports have shown that bone morphogenic protein 2 (BMP-2) can promote bone formation in the expanded suture and decrease the relapse ratio.9,10 Further, targeting osteoclasts with the bisphosphonate, etidronate, was shown to reduce suture relapse in rat sagittal sutures combined with mechanical retention.11 However, current therapies are associated with negative health sequelae. For example, BMP-2 expression is associated with suture fusion12,13 and heterotopic bone formation14, while bisphosphonates are linked with osteonecrosis of the jaw.15 Therefore, to increase bone formation and reduce sutural relapse following RME, new approaches targeting the molecules involved in suture remodeling are needed.
The tyrosine kinase Pyk2 is important for the actions of both osteoclasts16–19 and osteoblasts.20–22 In Pyk2 genetic knockout (Pyk2-KO) mice, osteoclast activity is inhibited19 while osteoblast activity is increased,22 leading to significantly elevated bone mass, trabecular number and trabecular thickness, compared to wild-type (WT) mice.19,22 Further, genetic deletion or inhibition of Pyk2 impedes osteoclast actin ring formation and bone resorption.16–18 Moreover, Pyk2 inhibition increased bone mass in ovariectomized rats22 and prevented glucocorticoid-induced bone loss in mice,23 largely due to anti-osteoclastic effects.
Based on the role of Pyk2 in regulating bone mass,19,22 we hypothesized that Pyk2-deficiency may increase bone mineral density in the midpalatal suture after RME and preserve sutural integrity. In the current study, we analyzed the effects of Pyk2-deficiency on morphological and histological changes in midpalatal sutures after 14-day maxillary expansion in mice.
MATERIALS AND METHODS
Animals
Pyk2-KO mice were originally provided by Pfizer (Groton, CT, USA)21,22 and have been maintained on a C57BL/6 background in our colony. Mice were bred as heterozygotes to generate experimental and wild-type (WT) littermates. All animal protocols were approved by the Institutional Animal Care and Use Committee in compliance National Institutes of Health guide on the care and use of laboratory animals. Experimental mice were housed in cages with up to 4 mice, and fed with the same ground meal diet 5001 and transgenic dough diet bacon flavor (Bioserv S3472) with Dietgel boost gel (Clear H20) and water at libitum. Husbandry conditions included 12 h light/dark cycles at controlled temperatures.
Suture expansion model
Custom manufactured nickel-titanium (NiTi) springs were used for midpalatal suture expansion (G&H Orthodontics, Franklin, IN). Spring expanders of 0.008” and 0.010” NiTi wire were designed to deliver 10g and 20g force, respectively. The forces exerted by the compressed/activated springs were confirmed using a Lutron FG-5000 digital force gauge (Taipei, Taiwan) and shown to deliver 10±1g force (10g) and 20±2g force (20g).
Thirty-six male 6-week Pyk2-KO and WT mice, each weighing 22–25 grams, were randomly assigned into 3 treatment groups: 0g (no expander), 10g or 20g expansion force. We used 6 mice per group/genotype each for micro-CT and histomorphometry, which was based on our sample size calculation, predicting 80% power to detect a difference of 3% in the ratio of bone volume to tissue volume, assuming two-sided tests each conducted at a 5% significance level and assuming the within-group standard deviation is 1.7%. The spring expander ends were inserted between the first and second molars underneath the proximal contact points. The appliances were bonded bilaterally on the maxillary molars with light cured composite resin (Transbond, 3M Unitek, CA) (Figure 1). During the 14-day expansion period, the springs were generally well tolerated and no long-term adverse systemic reactions were observed.
FIGURE 1.
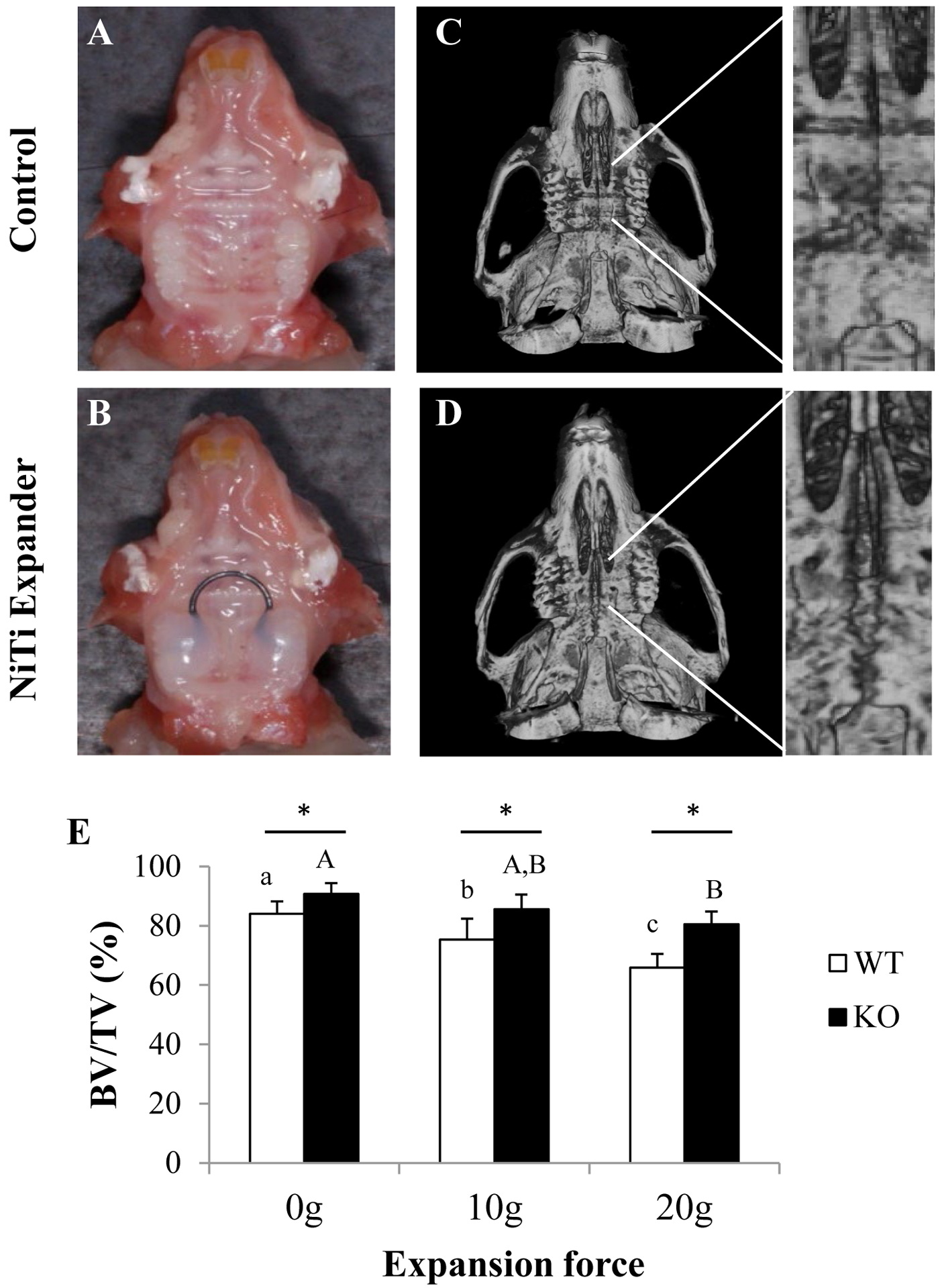
Micro-CT analysis of expanded and control maxillae. A, C) Intraoral view of a maxilla without expander (0g) or with the NiTi expander bonded to first and second molars. B, D) Representative images of the occlusal view of reconstructed skulls after 14 days in control or expanded maxillae. Insets are higher magnification micro-CT images. E) Graph showing percentage (%) BV/TV of WT and Pyk2-KO maxillae treated with the 10g and 20g expanders, or controls. Error bars indicate mean ± standard deviation (SD). Statistically significant differences between force groups in WT mice (lower case letters) or between force groups in Pyk2-KO mice (upper case letters) are indicated. Asterisks (*) indicate significance between genotypes for the same force group (p<0.05).
Micro-computed tomography
Intact maxillae were scanned using high-resolution micro-CT at a resolution of 8 μm pixel size, using 59kV/167μA and rotation angle of 180° with a rotation step of 0.45° analyzed using high-resolution micro-CT (Skyscan 1172, Kontich, Belgium).24 Projection images were reconstructed using the NRecon software. Morphometric 3D analyses were performed by selecting the palatal bone regions to obtain the bone volume fraction. The region of interest (ROI) consisted of the midpalatal suture bony edges, as well as their bilateral extensions for 200 μm on each side with anterior and posterior boundaries at the notches of the palatal process. The sutural width was the distance between the two bony edges of the midpalatal suture, while the intermolar width was the distance between the canals of palatal roots of the first molars.
Specimen Histomorphometry
Serial 5μm paraffin-embedded sections of decalcified maxillae were prepared in the coronal plane. Hematoxylin and Eosin staining (H&E) was used to compare changes in suture morphology. Multinucleated osteoclasts were detected by expression of Tartrate Resistant Acid Phosphatase (TRAP). Osteoclasts in the ROI, as previously defined in the coronal plane of the micro-CT images were identified based on TRAP+ staining. The ratio of osteoclast surface (Oc.S) relative to the bone surface (BS) was calculated using Bioquant Osteo software (Nashville, TN) as previously reported25 and following published guidelines.26 Hypertrophic chondrocytes, stained with Alcian blue, were distinguished morphologically by their enlarged cell size and distinct nucleus. The cartilage area was defined as the metachromatic regions on Alcian blue stained sections. The width, height and area of the fibrous suture region in the midpalatal suture were measured by tracing the interface of chondrocytes and fibrous suture. The width was defined as the gap between chondrocytes on parallel bone fronts. The height was the distance between chondrocytes on the nasal and oral sides. The fibrous suture area was the entire fibrous region surrounded by chondrocytes. All images were observed under an inverted microscope (Leica DMI 4000B, Leica microsystems) and analyzed with ImageProPlus software (Media Cybernetics, Bethesda, MD).
Statistical analysis
The Statistic Package for Social Science v22 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. All specimens were blinded to the examiner. The same calibrated examiner repeated the micro-CT and histomorphometric measurements within one month for the reliability analysis. The repeatability coefficients were calculated using the analysis of variance. The coefficients were found to be very close to 1.00. Two-way analysis of variance (ANOVA) was used to evaluate the treatment effects. Post-hoc Tukey pair-wise comparisons were used for differences between Pyk2-KO and WT mice groups. Statistical significance was set at p<0.05. Statistically significance between force groups within a genotype are indicated by matching lower case letters, while asterisks (*) indicate significance between genotypes.
RESULTS
Bone density is preserved in expanded Pyk2-KO midpalatal sutures
Compared to WT mice, Pyk2-KO mice exhibited 7% higher BV/TV in the midpalatal suture at baseline (0g control) (p<0.02), and showed a significantly higher BV/TV after the 14-day expansion period with both the 10g (12%) (p<0.02) and 20g (18.2%) force expanders (p<0.0001) (Figure 1). Both WT and Pyk2-KO mice exhibited some degree of bone loss in the midpalatal suture following expansion. However, expanded WT maxillae showed a greater decline in BV/TV in both the 10g (−10%) (p<0.02) and 20g (−22%) groups, relative to 0g (p<0.01). In comparison, Pyk2-KO sutural BV/TV was reduced only in the 20g group (−11%), compared to the 0g group (p<0.001).
Reduced sutural and intermolar width in expanded Pyk2-KO sutures
The hard palate of mice consists of a midpalatal suture between the first and second molars, a transverse palatine suture, and an interpalatine suture at the posterior end.27 Our micro-CT studies confirmed robust broadening of the WT and Pyk2-KO midpalatal sutures, with other sutures appearing largely unaffected (Figure 2). In the un-expanded WT and Pyk2-KO control mice, no difference in sutural width was observed. However, both genotypes showed an increase in suture width after expansion. Compared to WT mice, Pyk2-KO mice showed a smaller increase in sutural width following 10g and 20g expansion (Figure 1C). Specifically, sutural width of WT maxillae was increased 4.3-fold (10g) and 5.4-fold (20g), whereas sutural width of Pyk2-KO mice was increased 3.3-fold (10g) and 4.7-fold (20g) (p<0.001), compared to genotype-matched controls.
FIGURE 2.
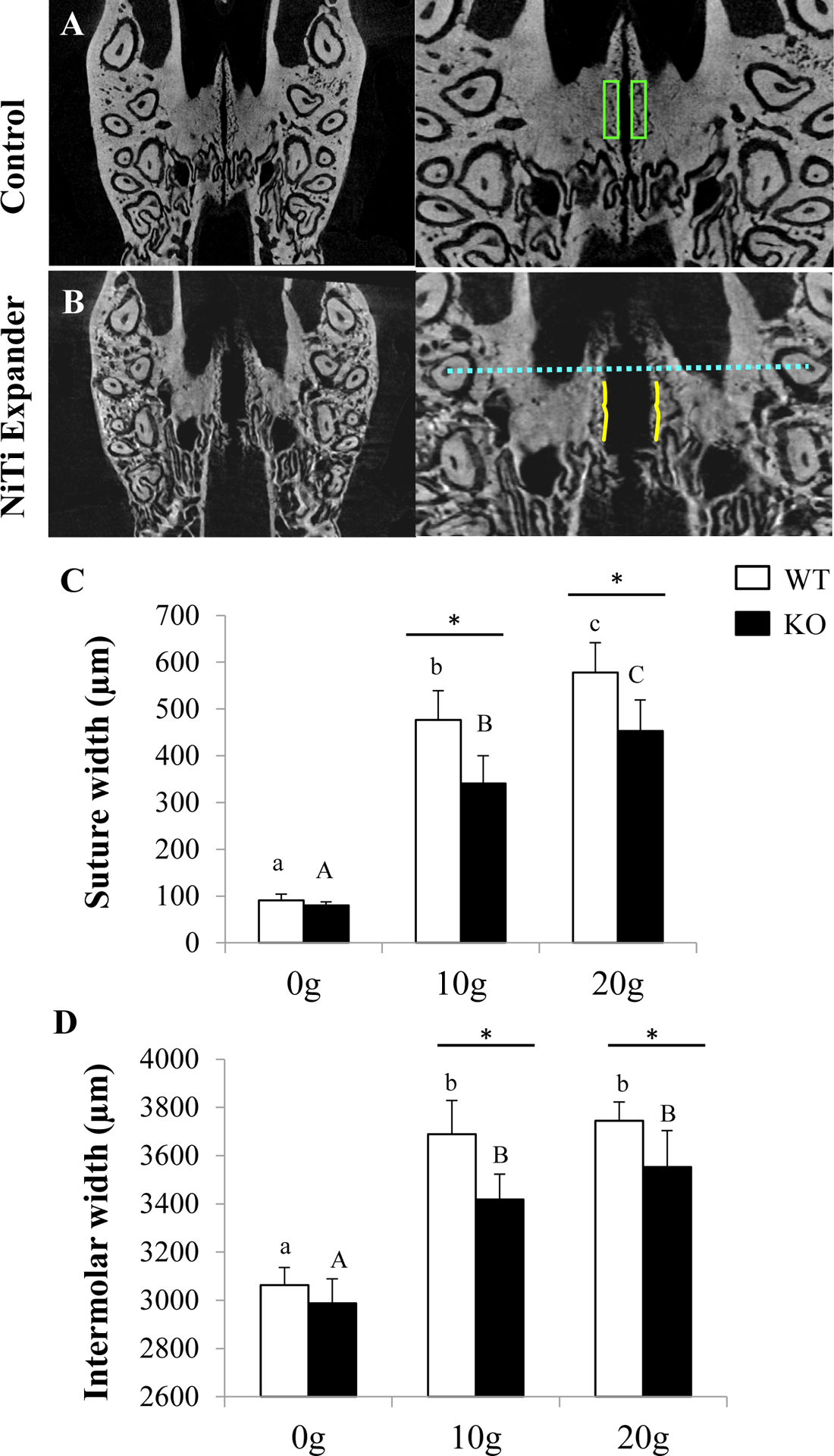
Suture width and intermolar width before and after expansion. A-B) Representative images of the transverse plane of a control or expanded Pyk2-KO maxilla showing the region of interest used for BV/TV measurements (green boxes) (see Figure 1), suture width (between solid yellow lines) and intermolar width (dashed blue line). C-D) Analysis of suture width and intermolar width in WT and Pyk2-KO mice with 0g, 10g and 20g expanders for 14-days. The mean ± SD is shown.
Consistent with these findings, Pyk2-KO intermolar widths were increased 14% (10g) and 19% (20g), whereas WT intermolar widths were increased 20% (10g) and 22% (20g), compared to genotype-matched controls (p<0.001). There was no difference in intermolar width between the 10g or 20g forces in either genotype (Figure 1D). Overall, a more blunted expansion response and higher BV/TV was observed in Pyk2-KO maxillae, compared to WT maxillae.
Pyk2-KO do not show an increase in osteoclasts following expansion
Histomorphometry was used to examine the cellular mechanism of sutural expansion. Multinucleated TRAP-positive osteoclasts were identified by histomorphometry in the sutures of both Pyk2-KO and WT mice (Figure 2). No significant difference in Oc.S/BS was observed between Pyk2-KO and WT mice at baseline (0g). Following expansion, WT maxillae exhibited a 77% (10g) and 132% (20g) increase in Oc.S/BS (p<0.02), compared to 0g. In contrast, Pyk2-KO mice showed no significant change in Oc.S/BS in either the 10g or 20g groups, compared to 0g. When comparing the genotype effects between forces, expanded Pyk2 maxillae exhibited lower Oc.S/BS at 10g (−31%) and 20g (−22%), compared with WT mice. These findings suggest the spring expanders induced osteoclast formation in WT, but not Pyk2-KO maxillae, leading to higher BV/TV in Pyk2-KO maxillae following expansion.
Expanded Pyk2-KO maxillae had reduced midpalatal fibrous connective tissue
H&E staining of expanded Pyk2-KO and WT maxillae revealed a layer of fibrous connective tissue between the front edges of their midpalatal suture (Figure 4). Spindle-shaped fibroblasts and fibrous tissue were also identified in the suture gap. In addition, a layer of chondrocytes arranged parallel to the expanded suture was observed. The horizontal width of the fibrous connective tissue was similar between non-expanded WT and Pyk2-KO mice. Following expansion, WT sutures were significantly wider than Pyk2-KO sutures at both the 10g and 20g forces (p<0.0001). The fibrous tissue width was increased 8-fold (10g) and 10-fold (20g) in WT sutures, whereas Pyk2-KO sutures showed an increase of only 6-fold (10g) and 8-fold (20g), compared to their respective 0g controls. Within a genotype, no differences between the 10g and 20g force groups were observed. The fibrous tissue area in the middle of the suture was also significantly higher in WT mice, compared to Pyk2-KO mice in the 10g group, but no change in fibrous tissue height was observed in either genotype.
FIGURE 4.
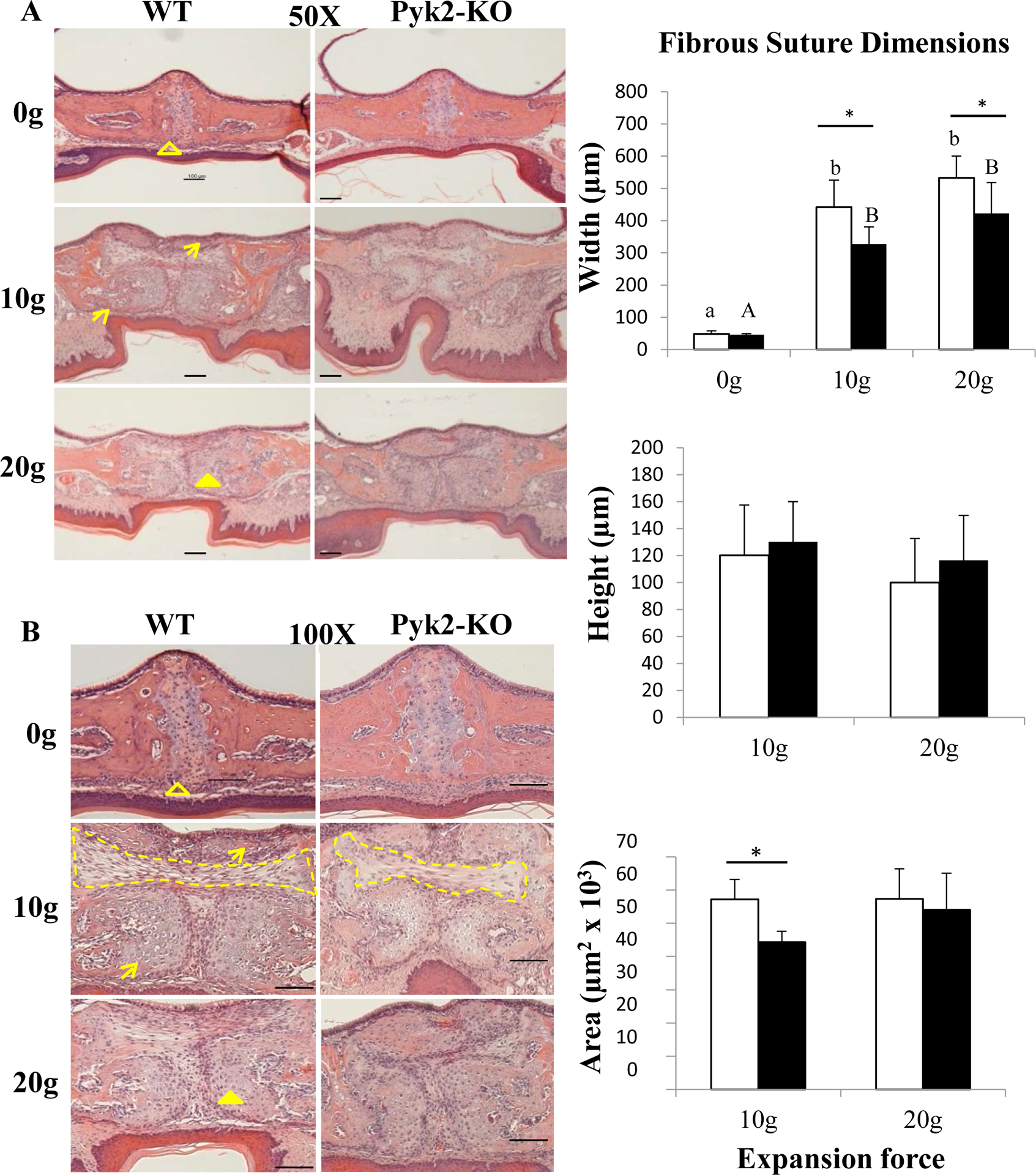
H&E stained serial sections of midpalatal sutures. The open arrowhead indicates the periosteum; closed arrowhead points to chondrocytes; arrows indicate bone formation along the edges of the palatal bone. The dotted line defines the fibrous tissue area surrounded by chondrocytes. Changes in fibrous tissue width, height and area are also shown in the graphs. Scale bars indicate 100 μm. The mean ± SD is shown.
Pyk2-deficiency did not affect secondary cartilage formation
Given the expanded sutures showed elevated fibrous tissue width, we also examined chondrocyte activity by performing Alcian blue staining (Figure 5). Although a thin layer of chondrocytes and cartilage covering the midpalatal suture edges were observed in control WT and Pyk2-KO groups, cartilage area and chondrocyte number were similar between genotypes. After expansion, the pre-existing chondrocytes and cartilage were separated laterally (arrows), and numerous new chondrocytes appeared in the midpalatal suture (arrow heads). Compared to their respective controls, the cartilage area and the number of hypertrophic chondrocytes were similarly increased 4-fold (10g) and 5-fold (20g) in both WT and Pyk2-KO maxillae (p<0.0001). No difference was observed between 10g and 20g force groups within a genotype. These data suggest Pyk2 does not play an observable role in secondary chondrogenesis following mechanical suture expansion.
FIGURE 5.
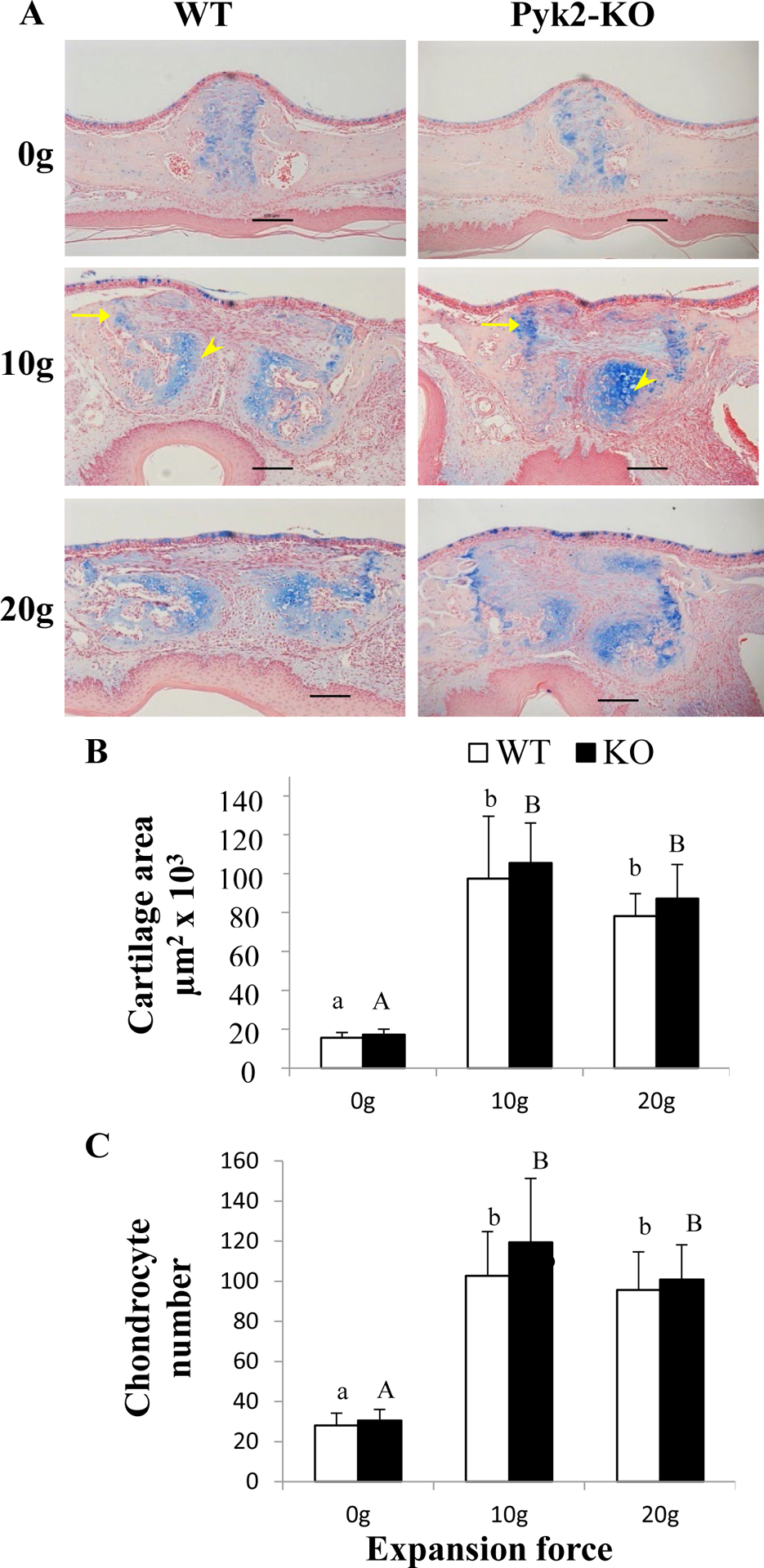
Alcian blue-stained cartilage and chondrocytes in the midpalatal suture. A) Yellow arrows indicate hypertrophic chondrocytes. B) Quantification of cartilage area between genotypes among forces levels. C) Quantification of hypertrophic chondrocytes in control and expanded mice. Scale bars indicate 100 μm. The mean ± SD is shown.
DISCUSSION
The clinical goal of RME is to widen the maxilla transversally, without disturbing the integrity of the midpalatal suture.28–30 A significant decrease in BMD will weaken the suture and will require additional post-expansion (retention) time to allow the suture to recover its original bone density levels. Therefore, higher bone mass in the midpalatal suture is likely to have beneficial effects in reducing the retention period, and preventing relapse following expansion. Given that the Pyk2 is known to be associated with bone remodeling,19,22 we examined if Pyk2-deficiency affects midpalatal suture bone mass and sutural integrity after maxillary expansion.
Compared to WT mice, Pyk2-KO mice showed elevated bone density in the control (0g) group without intervention. As expected for a relatively short treatment period (14-days), sutural expansion was accompanied by bone loss in WT mice, which was more pronounced in the 20g force group compared to 10g. However, in Pyk2-KO mice, bone loss was only observed in the 20g force group, and was overall less than that of WTs. Indeed, Pyk2-KO mice exhibited higher overall bone mass before and after suture expansion, compared with WT mice (Figure 1).
Although Pyk2-KO mice had higher baseline bone density than WT mice, it did not lead to synostosis or an unsuccessful attempt at expansion. This is important because the higher baseline BMD of Pyk2-KO sutures might have led to more resistance to the expansion forces. In addition, the higher force (20g) expander was correlated with greater sutural opening but lower bone density in both genotypes, suggesting that lower force expanders may initially produce a more stable suture than higher force expanders, which is consistent with published literature.8
To examine the cellular mechanism of preserved bone density in Pyk2-KO mice, we performed histomorphometric analyses. It is known that the sutural gap between the two halves of the expanded maxilla initially fills with fibrous connective tissue.6 As expected, after the 14-day expansion period, we observed dense fibrous connective tissue in the suture gap of both WT and Pyk2-KO mice, consistent with published reports.6 However, the distance was greater in WT mice, compared to Pyk2-KO mice at both force groups. The increase in fibrous tissue area was also found to be correlated with increases in suture width and intermolar width, both of which were higher in WT mice compared to Pyk2-KO mice.
Our data suggest that the genotype differences in bone density after expansion was due to an increase in osteoclast number in WT mice, but not Pyk2-KO mice. The reduced osteoclast activity on bone surfaces suggests Pyk2-KO mice may be more-protected from bone loss during sutural remodeling. This is consistent with our previous report showing that Pyk2-deficient mice have reduced osteoclast activity19 as well as other studies showing that the Pyk2 chemical inhibitor protects against bone loss in ovariectomized rats.22 Further, although we did not investigate root resorption, which is sometimes associated with RME,31,32 the current study and published studies16–18,33–35 demonstrate that Pyk2-deficiency reduces osteoclast activity, arguing against significant root resorption due to higher resistance in boney structures in Pyk2-KO mice.
In mice treated with bisphosphonates, midpalatal expansion not only decreased bone resorption as expected, but also decreased bone formation and led to disorganized sutural architecture.36 One limitation is that our study did not include a retention period to evaluate formation, although overall sutural architecture after expansion was found to be similar to WT sutures. Based on their higher bone density, combined with studies showing that Pyk2 also regulates osteoblast activity37,38 and bone formation,21,22 expanded Pyk2-KO sutures may have higher bone formation and improved suture stability, compared to WT mice, although this remains to be confirmed experimentally.
CONCLUSION
Midpalatal suture expansion in Pyk2-KO mice resulted in increased BMD, with reduced suture width and intermolar width, which was likely due to reduced osteoclast activity. These findings support the potential for Pyk2-targeted strategies to increase bone density during maxillary expansion and other craniofacial applications.
FIGURE 3.
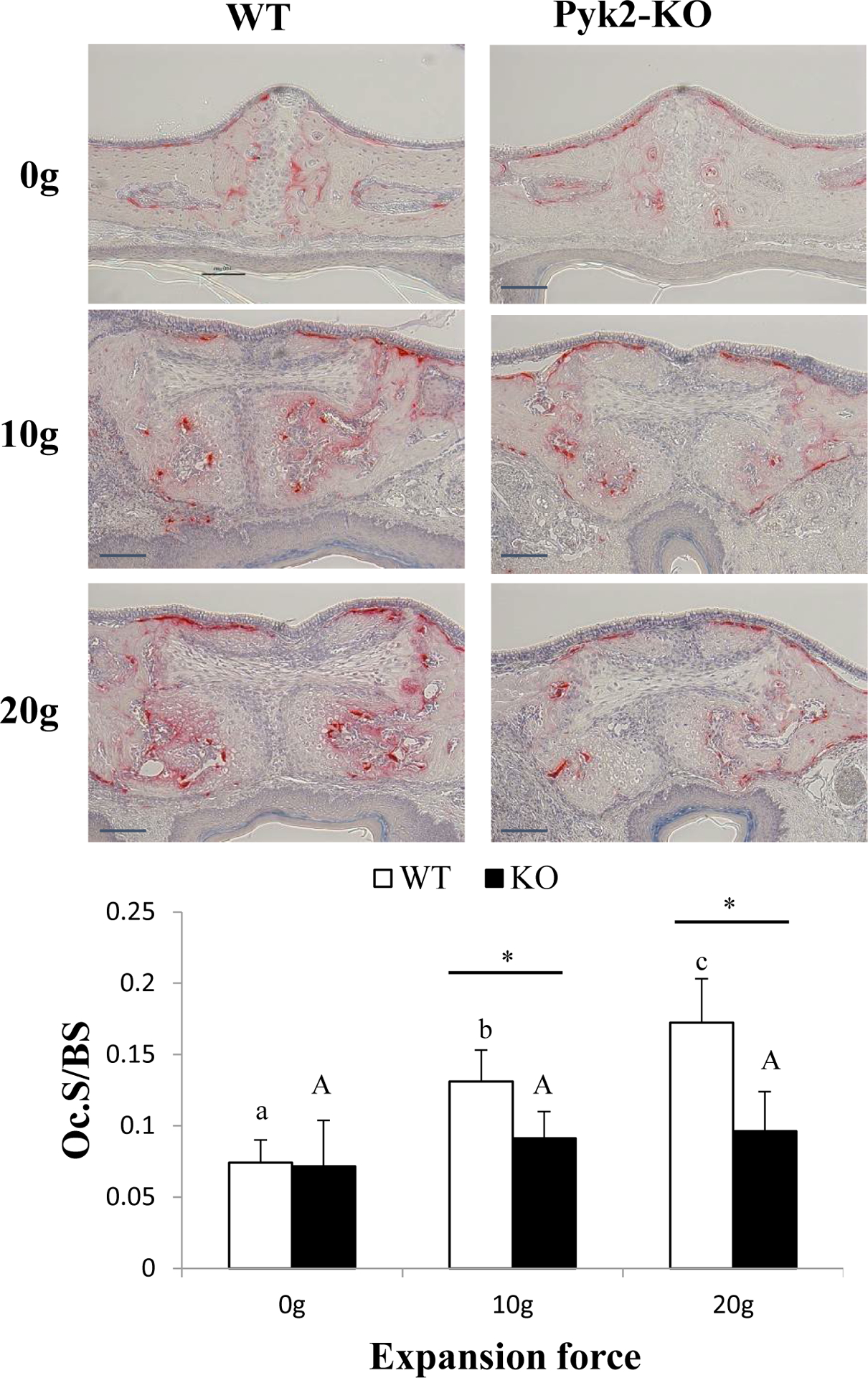
Osteoclast number in control and expanded sutures. Histological staining for TRAP-positive osteoclasts (red) in WT and Pyk2-KO maxillae used to determine Oc.S/BS. Osteoclasts were concentrated in the bone marrow around sutures after expansion. Scale bars indicate 100 μm. The mean ± SD is shown.
ACKNOWLEDGEMENT
This study was funded in part by the NIH (AR060332), the IUPUI Research Support Funds grant, the Indiana Clinical and Translational Sciences Institute (RR025761, TR000006), as well as funds from the IU School of Dentistry, and the Delta Dental Foundation, Dental Masters’ Thesis Award Program.
Footnotes
CONFLICT OF INTEREST
The authors state that there are no conflicts of interest in connection with this article.
REFERENCES
- 1.Chrcanovic BR, Custodio AL. Orthodontic or surgically assisted rapid maxillary expansion. Oral Maxillofac Surg. 2009;13(3):123–137. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Mathur R. Maxillary Expansion. Int J Clin Pediatr Dent. 2010;3(3):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson BM, McNamara JA Jr, Bandeen RL, Baccetti T. Changes in soft tissue nasal widths associated with rapid maxillary expansion in prepubertal and postpubertal subjects. Angle Orthod. 2010;80(6):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchi L, Baccetti T, Lione R, Fanucci E, Cozza P. Modifications of midpalatal sutural density induced by rapid maxillary expansion: A low-dose computed-tomography evaluation. Am J Orthod Dentofacial Orthop. 2010;137(4):486–488; discussion 412A-413A. [DOI] [PubMed] [Google Scholar]
- 5.Costa JG, Galindo TM, Mattos CT, Cury-Saramago AA. Retention period after treatment of posterior crossbite with maxillary expansion: a systematic review. Dental Press J Orthod. 2017;22(2):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou B, Fukai N, Olsen BR. Mechanical force-induced midpalatal suture remodeling in mice. Bone. 2007;40(6):1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beederman M, Farina EM, Reid RR. Molecular basis of cranial suture biology and disease: Osteoblastic and osteoclastic perspectives. Genes Dis. 2014;1(1):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herring SW. Mechanical influences on suture development and patency. Front Oral Biol. 2008;12:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu SS-Y, Xu H, Sun J, et al. Recombinant human bone morphogenetic protein-2 stimulates bone formation during interfrontal suture expansion in rabbits. Am J Orthod Dentofacial Orthop. 2013;144(2):210–217. [DOI] [PubMed] [Google Scholar]
- 10.Lai R-F, Zhou Z-Y, Chen T Accelerating bone generation and bone mineralization in the interparietal sutures of rats using an rhBMP-2/ACS composite after rapid expansion. Exp Anim. 2013;62(3):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Sugiyama H, Imoto S, Tanne K. Effects of bisphosphonate on the remodeling of rat sagittal suture after rapid expansion. Angle Orthod. 2001;71(4):265–273. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu Y, Yu PB, Kamiya N, et al. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res. 2013;28(6):1422–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitton A, Hyzy SL, Britt C, Williams JK, Boyan BD, Olivares-Navarrete R. Differential spatial regulation of BMP molecules is associated with single-suture craniosynostosis. J Neurosurg Pediatr. 2016;18(1):83–91. [DOI] [PubMed] [Google Scholar]
- 14.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 2014;14(3):552–559. [DOI] [PubMed] [Google Scholar]
- 15.Ascani G, Campisi G, Junquera Gutierrez LM. Current controversies in classification, management, and prevention of bisphosphonate-related osteonecrosis of the jaw. Int J Dent. 2014;2014:565743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eleniste PP, Du L, Shivanna M, Bruzzaniti A. Dynamin and PTP-PEST cooperatively regulate Pyk2 dephosphorylation in osteoclasts. Int J Biochem Cell Biol. 2012;44(5):790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duong LT. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J Biol Chem. 2003;278(13):11502–11512. [DOI] [PubMed] [Google Scholar]
- 18.Sanjay A, Houghton A, Neff L, et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J Cell Biol. 2001;152(1):181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil-Henn H, Destaing O, Sims NA, et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178(6):1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posritong S, Hong JM, Eleniste PP, et al. Pyk2 deficiency potentiates osteoblast differentiation and mineralizing activity in response to estrogen or raloxifene. Mol Cell Endocrinol. 2018;474:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YH, Hooker RA, Nguyen K, et al. Pyk2 regulates megakaryocyte-induced increases in osteoblast number and bone formation. J Bone Miner Res. 2013;28(6):1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckbinder L, Crawford DT, Qi H, et al. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc Natl Acad Sci U S A 2007;104(25):10619–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato AY, Cregor M, McAndrews K, et al. Glucocorticoid-Induced Bone Fragility Is Prevented in Female Mice by Blocking Pyk2/Anoikis Signaling. Endocrinology. 2019;160(7):1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen MR, Chu TM, Ruggiero SL. Absence of exposed bone following dental extraction in beagle dogs treated with 9 months of high-dose zoledronic acid combined with dexamethasone. J Oral Maxillofac Surg. 2013;71(6):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S, Eleniste PP, Wayakanon K, et al. The Rho-GEF Kalirin regulates bone mass and the function of osteoblasts and osteoclasts. Bone. 2014;60:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katebi N, Kolpakova-Hart E, Lin CY, Olsen BR. The mouse palate and its cellular responses to midpalatal suture expansion forces. Orthod Craniofac Res. 2012;15(3):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seif-Eldin NF, Elkordy SA, Fayed MS, Elbeialy AR, Eid FH. Transverse Skeletal Effects of Rapid Maxillary Expansion in Pre and Post Pubertal Subjects: A Systematic Review. Open Access Maced J Med Sci. 2019;7(3):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu X, Di Carlo G, Cornelis MA, Cattaneo PM. Three-dimensional analyses of short- and long-term effects of rapid maxillary expansion on nasal cavity and upper airway: A systematic review and meta-analysis. Orthod Craniofac Res. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Reyneke JP, Conley RS. Surgical/Orthodontic Correction of Transverse Maxillary Discrepancies. Oral Maxillofac Surg Clin North Am. 2020;32(1):53–69. [DOI] [PubMed] [Google Scholar]
- 31.Kittel PW, Sampson WJ. RME-induced root resorption and repair: a computerised 3-D reconstruction. Aust Orthod J. 1994;13(3):144–151. [PubMed] [Google Scholar]
- 32.Lo Giudice A, Galletti C, Gay-Escoda C, Leonardi R. CBCT assessment of radicular volume loss after rapid maxillary expansion: A systematic review. J Clin Exp Dent. 2018;10(5):e484–e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzzaniti A, Neff L, Sandoval A, Du L, Horne WC, Baron R. Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclasts. Mol Cell Biol. 2009;29(13):3644–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998;102(5):881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakkakorpi PT, Nakamura I, Nagy RM, Parsons JT, Rodan GA, Duong LT. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J Biol Chem. 1999;274(8):4900–4907. [DOI] [PubMed] [Google Scholar]
- 36.Koehne T, Kahl-Nieke B, Amling M, Korbmacher-Steiner H. Inhibition of bone resorption by bisphosphonates interferes with orthodontically induced midpalatal suture expansion in mice. Clin Oral Investig. 2018;22(6):2345–2351. [DOI] [PubMed] [Google Scholar]
- 37.Eleniste PP, Patel V, Posritong S, et al. Pyk2 and Megakaryocytes Regulate Osteoblast Differentiation and Migration Via Distinct and Overlapping Mechanisms. J Cell Biochem. 2016;117(6):1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posritong S, Flores Chavez R, Chu TG, Bruzzaniti A. A Pyk2 inhibitor incorporated into a PEGDA-gelatin hydrogel promotes osteoblast activity and mineral deposition. Biomed Mater. 2019;14(2):025015. [DOI] [PMC free article] [PubMed] [Google Scholar]


