Background:
Patients with recurrent or persistent ovarian cancer often have poor prognoses, and their optimal treatment regimen remains unclear. Inhibition of angiogenesis is a valuable strategy for treating ovarian cancer, and the drug pazopanib is a potent, multitarget tyrosine kinase inhibitor. However, treatment with pazopanib in combination with chemotherapy remains controversial. We performed a systematic review and meta-analysis to clarify the efficacy and side effects of pazopanib combined with chemotherapy in the treatment of advanced ovarian cancer.
Methods:
The PubMed, Embase, and Cochrane databases were systematically searched for relevant randomized controlled trials published up to September 2, 2022. The primary outcomes of eligible studies included overall response rate (ORR), disease control rate, 1-year progression-free survival (PFS) rate, 2-year PFS rate, 1-year overall survival (OS) rate, 2-year OS rate, and adverse events.
Result:
Outcomes from a total of 518 recurrent or persistent ovarian cancer patients from 5 studies were analyzed in this systematic review. Pooled results showed that pazopanib plus chemotherapy, when compared with chemotherapy alone, significantly improved the ORR (pooled risk ratio=1.400; 95% CI, 1.062-1.846; P = 0.017) but not the disease control rate, 1-year PFS, 2-year PFS, 1-year OS, or 2-year OS. Moreover, pazopanib increased the risk of neutropenia, hypertension, fatigue, and liver dysfunction.
Conclusion:
Pazopanib plus chemotherapy improved patient ORR but did not improve survival; it also increased the occurrence of several adverse events. Further large-sample clinical trials are needed to verify these results to guide pazopanib use in patients with ovarian cancer.
Key Words: pazopanib, chemotherapy, ovarian cancer, anti-angiogenesis
Ovarian cancer is the leading cause of gynecologic cancer-related death and the fifth most common malignancy, thus it is a serious threat to women’s health worldwide.1 The first-line combination chemotherapy treatment platinum/taxane is ~70% to 80% effective; however, the majority of patients ultimately relapse.2–5 Specifically, recurrence is observed in almost 25% of early-stage cases and more than 80% of advanced stages.6 Although there are several active antitumor therapies for the treatment of recurrent ovarian cancer—including chemotherapy, targeted therapy, antivascular therapy, and immunotherapy—median survival after recurrence is <3 years, highlighting the urgent need for testing novel agents in this population.7 Angiogenesis plays a pivotal role in the growth and progression of several malignant tumors. It has been demonstrated that tumors are unable to grow larger than 1 to 2 mm3 without new blood vessel development.8 In the neoangiogenesis process, vascular endothelial growth factor (VEGF) pathway has been most widely researched, which starts the development of new vessels, whereas platelet-derived growth factor (PDGF) and fibroblast growth factor maintain the process.9 The over expression of VEGF and PDGF has been linked to multiple cancers.10–12 Several anti-angiogenic agents have been researched in the context of ovarian cancer. For example, bevacizumab was found to be effective in improving the progression-free survival (PFS) of patients with recurrent ovarian cancer, and has been approved for the first-line and second-line treatment of advanced ovarian cancer.13,14 In the Phase III PAOLA-1/ENGOT-ov25 trial, the addition of maintenance olaparib to bevacizumab demonstrated a significant PFS benefit in patients with newly diagnosed, advanced ovarian cancer.15 On the basis of these promising results, additional randomized controlled trials (RCTs) were designed to evaluate the effect of other anti-angiogenesis therapies on ovarian cancer, including pazopanib.
Pazopanib is a multitarget tyrosine kinase inhibitor of VEGF receptors 1, 2, and 3; PDGF receptors α and β; fibroblast growth factor receptors; and proto-oncogene receptor tyrosine kinase.16 Pazopanib has been studied as a maintenance therapy after first-line chemotherapy in women with ovarian cancer, and it was reported to improve median PFS.17 However, in the randomized phase II TAPAZ trial, ovarian cancer patients in their first year of bevacizumab maintenance therapy were assigned either weekly paclitaxel plus pazopanib or standard weekly paclitaxel. Results of this trial showed that adding pazopanib to paclitaxel did not improve efficacy, but instead increased toxicity and compromised chemotherapy delivery.18 Several additional clinical trials have evaluated the effect of pazopanib with or without chemotherapy in treating persistent or recurrent ovarian cancer7,16,19; however these studies were Phase I or II clinical trials with discordant results.
In this study, we synthesized the results of several clinical trials—including their reported overall response rate (ORR), disease control rate (DCR), 1-year PFS rate, 2-year PFS rate, 1-year overall survival (OS) rate, 2-year OS rate, and adverse events (AEs)—to provide more objective data and inform the optimal clinical use of pazopanib plus chemotherapy.
METHODS
Search Strategy
The systematic review and meta-analysis were designed to compare the efficacy and safety of pazopanib plus chemotherapy in treating persistent or recurrent ovarian cancer. We conducted systematic computerized searches of PubMed, Embase, and Cochrane databases for reports dated up to September 2, 2022. Only articles and abstracts published in English were considered, and all years from database inception to the search date were included. Case reports, case series, and review articles were excluded. The keywords included “ovarian cancer”, “pazopanib”, “votrient”, and “GW786034”.
Inclusion and Exclusion Criteria
The inclusion criteria were: (1) adult patients histologically diagnosed with persistent or recurrent ovarian cancer, fallopian tube carcinoma, or primary peritoneal carcinoma; (2) patients received at least 1 platinum-based chemotherapy regimen; (3) RCTs compared the efficacy and safety profile of adding pazopanib to systematic chemotherapy in patients with ovarian cancer; (4) studies were published in English; and (5) the most recent or the most complete report was included when the same investigator reported results from the same patient population.
The exclusion criteria were: (1) in vitro or in vivo experiments; (2) case reports, case series, or cases less than 10; (3) non-English studies; and (4) data from the same project or center will be selected as 1 for further meta-analysis.
Literature Screening and Data Extraction
Two researchers (Y.P.Z. and J.C.) independently screened titles and abstracts based on the inclusion and exclusion criteria. Disagreements between the researchers were resolved by discussion. In the event they could not reach a consensus, a third investigator (H.M.F.) was consulted to resolve the dispute, and the final decision was made by majority vote. If inclusion could not be determined by the abstract, or if the abstract did not contain the appropriate data, the full text was evaluated.
A standard form was designed for data extraction, and the data were collected according to the following information: study characteristics (author, publish year, country, institution, study design, etc.), patient characteristics (treatment, total sample size, median age, sex, etc.), and outcome assessment (ORR, DCR, toxicity, and survival status). Only the most frequent toxicity events were analyzed.
Quality Assessment
Two investigators (X.Y.W. and H.W.) performed the quality assessment independently and disagreements were resolved by consensus or consulted with a third reviewer(G.Q.P.). The methodological quality of the included RCTs was estimated according to the Cochrane Risk of bias tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions,20 which rated high, low, or unclear risk of bias (some concerns) to the following domains: sequence generation, allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting, and other potential sources of bias. We created the risk of bias summary using the Review Manager Version 5.4 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020).
Statistical Analysis
Statistical analyses were performed using Stata 12.0 software (Stata Corporation, College Station, TX). Dichotomous data, including DCR, ORR, 1-year PFS, 2-year PFS, 1-year OS, 2-year OS, and toxicities, were compared with a RR. Next, 95% CIs were calculated for each estimate and presented in forest plots. The I2 statistic and x2 test were used for heterogeneity assessment (I2 ≥ 50% indicating the presence of heterogeneity). The random effect model was used when there was significant heterogeneity (I2 value >50% or P < 0.05) between studies; otherwise, the fixed effect model was used.21
In addition, the Begg test and funnel plots were used to assess the publication bias of the enrolled studies. A 2-sided P-value < 0.05 was set as the metric indicating a significant difference. A funnel plot was used to estimate potential publication bias, with an asymmetric plot suggesting possible bias. Funnel plot asymmetry was assessed by Egger linear regression test by using a standardized estimate of the size effect as the dependent variable and inverse of the standard error as the independent variable.22 The significance of the intercept was determined by the t test suggested by Egger, and P < 0.05 was considered a statistically significant publication bias.
RESULTS
Literature Selection
A total of 71 studies published from December 28, 2009 through September, 2022 were found using the predefined search strategy. The literature screening process is shown in the flowchart in Fig. 1. After screening the abstracts and titles, the full text of 27 studies were scanned and a total of 5 studies were ultimately included in the systematic review. These studies were analyzed to compare the efficacy and safety of pazopanib plus chemotherapy in treating persistent or recurrent ovarian cancer.
FIGURE 1.
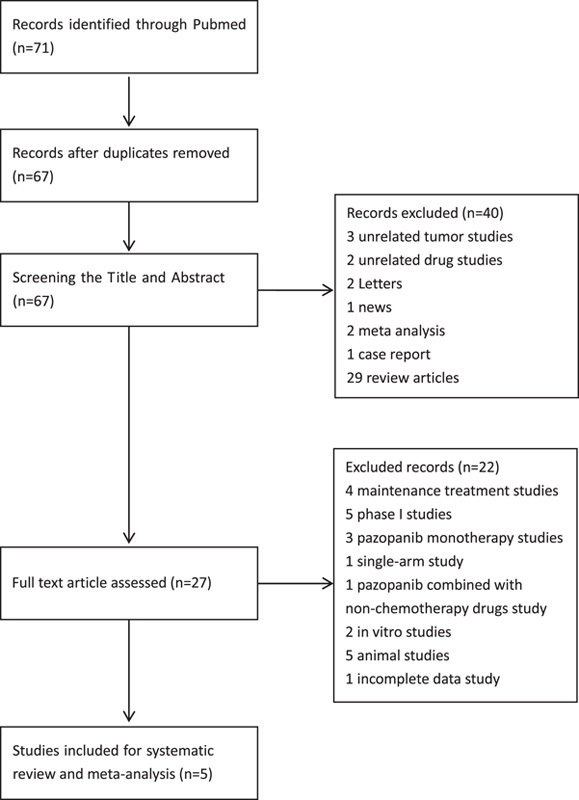
The flowchart of the study selection process for the meta-analysis.
General Characteristics of the 5 Studies
The 5 clinical trials in this meta-analysis included 282 cases in the pazopanib combined with chemotherapy group, and 236 subjects in the chemotherapy alone group. All 5 of the selected trials were prospective RCTs. The sample size across the studies ranged from 73 to 148. Three studies compared pazopanib with paclitaxel chemotherapy regimen, one study compared pazopanib with etoposide plus cyclophosphamide chemotherapy regimen, and the last study compared pazopanib with gemcitabine chemotherapy regimen. The 2 studies that did not include paclitaxel chemotherapy were classified as “other” chemotherapy treatments. Detailed information for the included studies is shown in Table 1.
TABLE 1.
Characteristics of the 6 Trials Included in the Meta-analysis
| Name | Year | Study design | Study phase | Treatment group (N) | Control group (N) | Treatment line |
|---|---|---|---|---|---|---|
| Richardson et al7 | 2018 | RCT | 2 | Paclitaxel plus pazopanib (54) | Paclitaxel (52) | 1-3 |
| Joly et al18 | 2022 | RCT | 2 | Paclitaxel plus pazopanib (79) | Paclitaxel (37). | ≥2 |
| Sharma et al19 | 2021 | RCT | 2 | Etoposide plus cyclophosphamide plus pazopanib (37) | etoposide plus cyclophosphamide (38) | ≥2 |
| Pignata et al16 | 2015 | RCT | 2 | Paclitaxel with pazopanib (37) | Paclitaxel (36) | 1-3 |
| Duska et al23 | 2020 | RCT | 2 | Gemcitabine plus pazopanib (75) | Gemcitabine (73) | 1-3 |
RCT indicates randomized controlled trial.
Assessment of Study Quality and Risk of Bias
A total of 518 patients were included across all 5 studies. All studies reported ORR, 1-year PFS, 2-year PFS, and 1- and 2-year OS, but DCR was not reported in 1 study. We detected some risks of bias in the included studies. The most detected domains of bias were the ones arising from the performance bias16,18,19,23 and allocation concealment.18,19,23 Figure 2 represents the risk of bias summary and the risk of bias graph.
FIGURE 2.
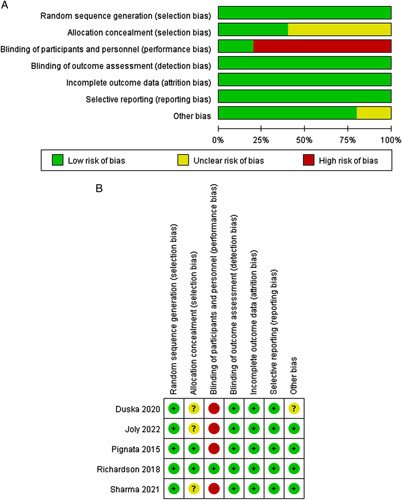
Risk of bias. A, Graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies. B, Summary: review authors’ judgments about each risk of bias item for each included study.
ORR and DCR
The meta-analysis comparing ORR and DCR are shown in Fig. 3 and Fig. 4. Results of statistical tests indicated low heterogeneity for ORR (I2 = 31.5%, P = 0.212) and DCR (I2 =38.0%, P = 0.184). Regarding ORR, patients treated with pazopanib plus chemotherapy had a higher effectiveness rate compared with chemotherapy alone (risk ratio [RR]=1.400; 95% CI, 1.062-1.846; P = 0.017). However, combination treatment did not exhibit an advantage in DCR relative to chemotherapy alone (RR=1.12; 95% CI, 0.989-1.270; P = 0.075).
FIGURE 3.
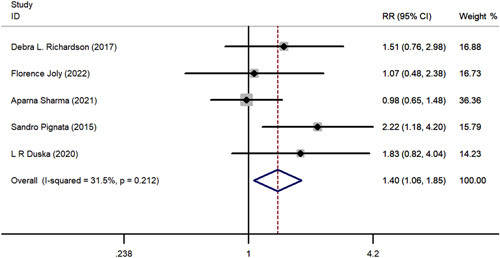
The forest plot comparing of overall response rate between chemotherapy with or without pazopanib. RR indicates risk ratio.
FIGURE 4.
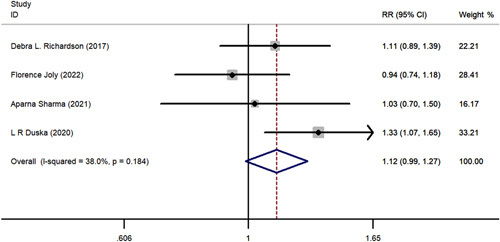
The forest plot comparing of disease control rate between chemotherapy with or without pazopanib. RR indicates risk ratio.
Subgroups based on chemotherapy regimen were also analyzed. Three trials7,16,18 investigated pazopanib plus paclitaxel chemotherapy regimen, and the pooled results revealed a significant improvement in ORR (RR, 1.589; 95% CI, 1.067-2.366; P = 0.023) compared with chemotherapy alone. However, pazopanib used with other chemotherapy regimens did not demonstrate this superiority to chemotherapy alone (RR, 1.216; 95% CI, 0.830-1.782; P = 0.315). Subgroup analysis of DCR revealed pazopanib plus other non-paclitaxel chemotherapy regimens demonstrated an advantage in DCR (RR, 1.229; 95% CI, 1.016-1.486; P = 0.034) relative to paclitaxel chemotherapy alone, but this was not observed in the pazopanib with paclitaxel group (RR, 1.014; 95% CI, 0.864-1.192; P = 0.862). The subgroup analysis results are depicted in Figure 5.
FIGURE 5.
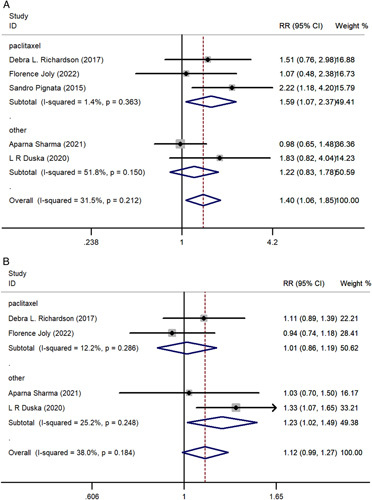
A, The forest plot comparing of overall response rate subgroup analysis. B, The forest plot comparing of disease control rate subgroup analysis. RR indicates risk ratio.
PFS and OS
A comparative meta-analysis was conducted to examine 1-year and 2-year survival of pazopanib plus chemotherapy versus chemotherapy alone using data from the 5 included RCTs. One-year PFS (RR=1.315; 95% CI, 0.841-2.056; P = 0.230), 2-year PFS (RR=3.487; 95% CI, 0.753-16.137; P = 0.110), 1-year OS (RR=1.035; 95% CI, 0.858-1.248 P = 0.720), and 2-year OS (RR=1.339; 95% CI, 0.707-2.535; P = 0.370) all favored pazopanib plus chemotherapy versus only chemotherapy; however, these results were not statistically significant (Figs. 6–9). No apparent heterogeneity was observed among the studies.
FIGURE 6.
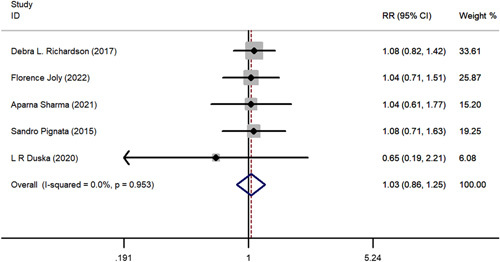
The forest plot comparing of 1-year overall survival between chemotherapy with or without pazopanib. RR indicates risk ratio.
FIGURE 9.
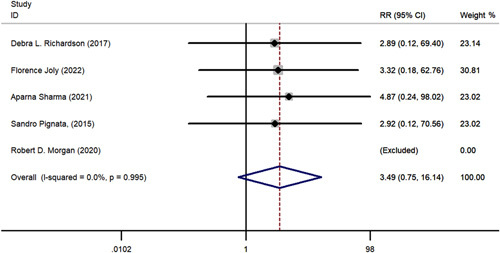
The forest plot comparing of 2-year progression-free survival between chemotherapy with or without pazopanib. RR indicates risk ratio.
FIGURE 7.
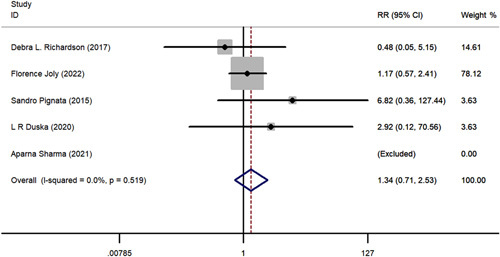
The forest plot comparing of 2-year disease control rate between chemotherapy with or without pazopanib. RR indicates risk ratio.
FIGURE 8.
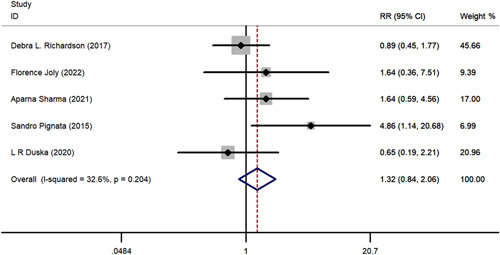
The forest plot comparing of 1-year progression-free survival between chemotherapy with or without pazopanib. RR indicates risk ratio.
Subgroups based on chemotherapy regimen were also analyzed. The 1-year PFS (RR=1.315; 95% CI, 0.814-2.056; P = 0.23), 2-year PFS (RR=3.487; 95% CI, 0.753-16.137; P = 0.110), 1-year OS (RR=1.035; 95% CI, 0.858-1.248; P = 0.720), and 2-year OS (RR=1.339; 95% CI, 0.707-2.535; P = 0.370) showed no significant advantage in the paclitaxel group compared with the “other” chemotherapy group.
Toxicities
We assessed grade 3 and grade 4 toxicities that arose from adding pazopanib to chemotherapy in the treatment of persistent or recurrent ovarian cancer (Table 2). Results indicated that the grade 3 and grade 4 toxicities neutropenia (RR=2.405; 95% CI, 1.722-3.360; P <0.01), hypertension (RR=6.582; 95% CI, 2.741-15.804; P <0.01), fatigue (RR= 3.087; 95% CI, 1.470-6.483; P = 0.003), and liver dysfunction (RR= 2.281; 95% CI, 1.85-4.392; P = 0.014) increased with the addition of pazopanib. Although there was no significant difference in the occurrence of gastrointestinal perforation between the 2 groups, one case of gastrointestinal perforation death was mentioned in the literature. This may have been related to pazopanib and/or the underlying disease.
TABLE 2.
Meta-analysis of the Toxicities in Patients With Achieved Chemotherapy With or Without Pazopanib.
| No. grade 3-4/other | ||||||
|---|---|---|---|---|---|---|
| Toxicity | N | Experimental | Placebo | RR (95% CI) | P | Heterogeneity, Q, I 2 , P |
| Neutropenia | 5 | 100/178 | 35/199 | 2.405 (1.722-3.360) | <0.01 | 7.41, 46.0%, 0.116 |
| Thrombocytopenia | 5 | 23/255 | 9/225 | 1.947 (0.948-3.999) | 0.070 | 4.51,33.5%,0.211 |
| Anemia | 5 | 34/244 | 18/216 | 1.556 (0.903-2.681) | 0.111 | 5.34,25.1%,0.254 |
| Hypertension | 4 | 52/176 | 4/180 | 6.582 (2.741-15.804) | <0.01 | 0.45,0.0%,0.931 |
| Fatigue | 4 | 34/194 | 8/176 | 3.087 (1.470-6.483) | 0.003 | 1.49,0.0%,0.685 |
| Liver dysfunction | 4 | 32/196 | 8/176 | 2.281 (1.85-4.392) | 0.014 | 5.06,40.7%,0.168 |
| Gastrointestinal perforation | 3 | 6/164 | 1/124 | 2.459 (0.542-11.163) | 0.244 | 0.44,0.0%,0.801 |
RR indicates risk ratio.
Publication Bias
Begg funnel plot and Egger test were performed to evaluate the publication bias of the 5 eligible studies. Begg funnel plot of RRs found no asymmetry, and evaluation with Egger test did not find any evidence of significant publication bias (Fig. 10, P > 0.05).
FIGURE 10.
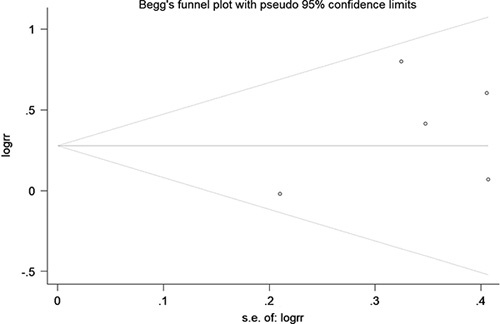
Funnel plot of odds ratio for chemotherapy with or without pazopanib and inverse standard error (SE) of log (odds ratio).
DISCUSSION
The OS of patients with ovarian cancer has improved over the past several decades. The 5-year relative survival rate was just 36% between 1975 and 1997, but it improved significantly to 49% between 2010 and 2016.24 This improvement reflects advances in treatment of ovarian cancer; however, the 5-year survival rate of patients with recurrent ovarian cancer remains low at about 40%.25 Angiogenesis plays an important role in the occurrence and development of ovarian cancer; it is one of the several known malignancies that overexpresses VEGF and its cognate receptor.
Initial studies investigating the use of pazopanib, a potent multitarget tyrosine kinase inhibitor, whose metabolism occurs primarily through CYP450 subtypes and is active against growth factors involved in angiogenesis and tumor microenvironment, in ovarian cancer patients focused on its use as a maintenance therapy for those who had undergone chemotherapy and/or surgery.26 AGO-OVAR16 was a phase III RCT exploring the use of pazopanib for maintenance of stage II to IV ovarian cancer, specifically in cases where the disease has not progressed after first-line therapy. Results indicated that patients in the pazopanib group had a 23% reduced risk of disease progression or death compared with placebo (mPFS 17.9 vs. 12.3 mo; hazard ratio 0.77, 95% CI 0.64-0.91), but unfortunately OS did not improve.27 Pazopanib was later used for the treatment of recurrent ovarian cancer, either as a single agent or in combination with chemotherapy such as paclitaxel or cyclophosphamide. In a phase II clinical trial involving 36 patients with relapsed ovarian cancer, the ORR was 18%.28 The NCCN panel also recommends pazopanib as a potentially active targeted recurrence therapy in patients who had a complete response to initial therapy.29 But the reported results have been inconsistent.
Our study included 5 RCTs involving a total of 518 patients to evaluate the efficacy and safety of pazopanib plus chemotherapy in treating persistent or recurrent ovarian cancer. Each of the five included trials reported the ORR, 1-year PFS, 2-year PFS, 1-year OS, and 2-year OS outcomes, and 4 of the 5 reported DCR. Summary results showed that pazopanib plus chemotherapy significantly increased ORR (RR=1.400; 95% CI, 1.062-1.846; P = 0.017), whereas DCR, 1-year PFS, 2-year PFS, 1-year OS, and 2-year OS trended toward an improvement but were not statistically significant. Therefore, pazopanib plus chemotherapy improves short-term outcomes but contributes to limited improvement in long-term survival. One likely explanation for these results is that long-term survival can be affected by many factors. Previous studies have shown that long-term survival may be associated with mutation frequency (such as the BRCA mutation in breast cancer), sensitivity to chemotherapy, primary complete or optimal cytoreductive surgery, preoperative disease burden, multiple lines of prior therapy, and inconsistent treatment.30–35
Different chemotherapy regimens may also influence the effect of combination treatment. Our subgroup analysis showed that improvements in ORR were primarily associated with pazopanib combined with paclitaxel, although pazopanib combined with paclitaxel showed no benefit for 1-year PFS, 2-year PFS, 1-year OS, or 2-year OS. Paclitaxel is one of the most effective drugs for the treatment of cancer. It works by binding to tubulin of dividing cancer cells, as well as stabilizing microtubules to prevent depolymerization, blocking mitosis, and blocking the cell cycle progression to the G2/M phase. Together, paclitaxel treatment results in cancer cells halting division and subsequently dying. In addition, paclitaxel has been reported to significantly diminish microvessel density and decrease VEGF synthesis in vivo.36 On the basis of our analyses, the clinic use of pazopanib combined with chemotherapy should prioritize paclitaxel; however, larger samples are still needed to further verify these results.
All the trials included in this study compared AEs between the combination treatment and chemotherapy alone groups. Pazopanib plus chemotherapy increased the incidence of neutropenia, hypertension, fatigue, and liver dysfunction. Specifically, the incidence of neutropenia and hypertension largely increased. Although statistical tests found that the incidence of gastrointestinal perforation was not significantly different between the two groups, one reported event indicated that the addition of pazopanib may increase these potentially fatal events. Therefore, pazopanib combination may be avoided if the patient has obvious intestinal infiltration or other circumstances that may increase the probability of perforation. This may be explained by an in vitro study, which showed that pazopanib is metabolized primarily by intestinal epithelial cells and bile ducts.37
In conclusion, our study indicated that pazopanib plus chemotherapy improves ORR compared with chemotherapy alone. Although the increases in 1-year and 2-year survival rates did not reach statistical significance, there was a trend suggesting improvement with combination therapy. With respect to these metrics, combination therapy with pazopanib is a viable option for the treatment of recurrent ovarian cancer with a poor chemotherapy response rate. However, from the perspective of safety analysis, we determined that the addition of pazopanib increases the incidence of a variety of grade 3 to 4 AEs including neutropenia, hypertension, fatigue, and liver dysfunction. Therefore, the use of pazopanib should be carefully considered against these risks. Admittedly, this result is not satisfactory, but considering the low response rate of current treatments for recurrent ovarian cancer, it is of dramatic clinical significance to explore more potential treatment options. In addition, previous studies on pazopanib combined with chemotherapy in treatment of recurrent ovarian cancer were all phase I/II studies with a small sample size, whereas our current study conducted a comprehensive meta-study to provide more significant evidence.
Our study has certain limitations. Pazopanib has a low solubility and thereby a low oral bioavailability (14% to 39%). A threshold steady state pazopanib concentration of at least 40 μmol/L was determined by clinical studies for inhibition of VEGF-induced VEGF receptor 2 phosphorylation, which was achieved by dosing of 800 mg pazopanib daily.26 The doses of pazopanib in the 5 studies ranged from 400 to 800 mg, and the combined chemotherapy regimens were also different, which may have led to bias in the analysis results. The most consequential problem is that only 5 clinical trials were ultimately included in our study, which in turn limited the number of patients. Moreover, incomplete PFS and OS data were obtained. Although the number of included trials was small, they all were RCTS with high availability. Evidence-based medicine is particularly important in this context considering the current poor therapeutic outcomes of recurrent ovarian cancer. Our study provides ample evidence that can guide clinicians in their decision to combine pazopanib with chemotherapy for recurrent ovarian cancer; thus, our study has practical clinical significance.
CONCLUSION
Results of our study indicate that treating patients with persistent or recurrent ovarian cancer using pazopanib plus chemotherapy improves ORR but not survival. We found that the addition of pazopanib also increased the incidence of grade 3 to 4 neutropenia, hypertension, fatigue, and liver dysfunction. Our results provide a higher level of evidence regarding pazopanib use compared with previous studies; however, additional studies are required to confirm and expand upon our results.
ACKNOWLEDGMENTS
The authors thank to all the people who have ever helped them in this paper. The authors’ thanks and appreciations go firstly to Dr Jian Chen, whose guidance and suggestions have given them decisive insight into this study. The authors are also extremely grateful to all their colleagues who have kindly provided them assistance and companionship in the course of preparing this paper. In addition, the authors’ thanks go to their family for their unfailing love and unwavering support. Finally, the authors are really grateful to all those who devote much time to reading this thesis and give them much advice, which will benefit them in their later study.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Yaping Zhang, Email: zyp890307@163.com.
Hongming Fang, Email: fanghongming0412@163.com.
Xiaoyan Wang, Email: wangxiaoyan0412@163.com.
Hui Wang, Email: huiwang0412@163.com.
Guoqiang Pan, Email: guoqiangp@163.com.
Jian Chen, Email: chenjianxsh@163.com.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Rubio Pérez MJ. Effect of the combination of trabectedin and pegylated liposomal doxorubicin in a BRCA2 mutation carrier with recurrent platinum-sensitive ovarian cancer. Case Rep Oncol. 2017;10:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.du Bois A, Quinn M, Thigpen T, et al. 2004. Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl):viii7–viii12. [DOI] [PubMed] [Google Scholar]
- 4.López-Guerrero JA, Romero I, Poveda A. Trabectedin therapy as an emerging treatment strategy for recurrent platinum-sensitive ovarian cancer. Chin J Cancer. 2015;34:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoletti M, Pelizzari G, Gerratana L, et al. Bevacizumab or PARP-inhibitors maintenance therapy for platinum-sensitive recurrent ovarian cancer: a network meta-analysis. Int J Mol Sci. 2020;21:3805–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garzon S, Laganà AS, Casarin J, et al. Secondary and tertiary ovarian cancer recurrence: what is the best management? Gland Surgery. 2020;9:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson DL, Sill MW, Coleman RL, et al. Paclitaxel with and without pazopanib for persistent or recurrent ovarian cancer: a randomized clinical trial. JAMA Oncol. 2018;4:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenhauer EL, Zanagnolo V, Cohn DE, et al. A phase II study of gemcitabine, carboplatin and bevacizumab for the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2014;134:262–266. [DOI] [PubMed] [Google Scholar]
- 9.Musella A, Vertechy L, Romito A, et al. Bevacizumab in ovarian cancer: state of the art and unanswered questions. Chemotherapy. 2017;62:111–120. [DOI] [PubMed] [Google Scholar]
- 10.Eichbaum M, Mayer C, Eickhoff R, et al. The PACOVAR-trial: a phase I/II study of pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant recurrent, pre-treated ovarian cancer. BMC Cancer. 2011;11:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- 12.Monk BJ, Minion LE, Coleman RL. Anti-angiogenic agents in ovarian cancer: past, present, and future. Ann Oncol. 2016;27(suppl 1):i33–i39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO Ovarian Cancer Consensus Conference Working Group. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. 2019;30:672–705. [DOI] [PubMed] [Google Scholar]
- 15.Harter P, Mouret-Reynier MA, Pignata S, et al. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol Oncol. 2022;164:254–264. [DOI] [PubMed] [Google Scholar]
- 16.Pignata S, Lorusso D, Scambia G, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16:561–568. [DOI] [PubMed] [Google Scholar]
- 17.du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32:3374–3382. [DOI] [PubMed] [Google Scholar]
- 18.Joly F, Fabbro M, Berton D, et al. Paclitaxel with or without pazopanib for ovarian cancer relapsing during bevacizumab maintenance therapy: The GINECO randomized phase II TAPAZ study. Gynecol Oncol. 2022;166:389–396. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Singh M, Chauhan R, et al. Pazopanib based oral metronomic therapy for platinum resistant/refractory epithelial ovarian cancer: a phase II, open label, randomized, controlled trial. Gynecol Oncol. 2021;162:382–388. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, vol. 4. John Wiley & Sons; 2011. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 22.Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. Br Med J. 1998;316:471. [PMC free article] [PubMed] [Google Scholar]
- 23.Duska LR, Petroni GR, Varhegyi N, et al. A randomized phase II evaluation of weekly gemcitabine plus pazopanib versus weekly gemcitabine alone in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma. Gynecol Oncol. 2020;157:585–592. [DOI] [PubMed] [Google Scholar]
- 24.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 25.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrero S, Leone Roberti Maggiore U, Aiello N, et al. Pharmacokinetic drug evaluation of pazopanib for the treatment of uterine leiomyosarcomas. Expert Opin Drug Metab Toxicol. 2017;13:881–889. [DOI] [PubMed] [Google Scholar]
- 27.Vergote I, du Bois A, Floquet A, et al. Overall survival results of AGO-OVAR16: a phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2019;155:186–191. [DOI] [PubMed] [Google Scholar]
- 28.Friedlander M, Hancock KC, Rischin D, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119:32–37. [DOI] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer, Version 1. 2023. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- 30.Wang Y, Zhang S, Song Z, et al. Anti-angiogenesis maintenance therapy in newly diagnosed and relapsed ovarian cancer: a meta-analysis of phase III randomized controlled trials. Front Pharmacol. 2021;12:726278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang SYC, Lheureux S, Karakasis K, et al. Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Medicine. 2018;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwase H, Takada T, Iitsuka C, et al. Clinical features of long-term survivors of recurrent epithelial ovarian cancer. Int J Clin Oncol. 2015;20:143–149. [DOI] [PubMed] [Google Scholar]
- 33.Javellana M, Hoppenot C, Lengyel E. The road to long-term survival: Surgical approach and longitudinal treatments of long-term survivors of advanced-stage serous ovarian cancer. Gynecol Oncol. 2019;152:228–234. [DOI] [PubMed] [Google Scholar]
- 34.Hoppenot C, Eckert MA, Tienda SM, et al. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol Oncol. 2018;148:204–212. [DOI] [PubMed] [Google Scholar]
- 35.Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37:2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ai B, Bie Z, Zhang S, et al. Paclitaxel targets VEGF-mediated angiogenesis in ovarian cancer treatment. Am J Cancer Res. 2016;6:1624–1635. [PMC free article] [PubMed] [Google Scholar]
- 37.Messing MJ, Stringer CA, Matthys GM, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119:32–37. [DOI] [PubMed] [Google Scholar]


