Abstract
Background
Sleep can have consequential effects on people’s health and well-being, and these effects may vary among younger and older adults.
Purpose
The goal of the present study was to investigate how sleep relates to physiologic and stress responses in daily life across adulthood.
Methods
We used an Ecological Momentary Assessment method in a large sample of participants (N = 4,359; Mage = 46.75, SD = 12.39; 69.30% male, 29.85% female) who completed morning sleep diaries, reported subjective stress, and recorded their heart rate and blood pressure for 21 days. Sleep was assessed with self-reports of duration, efficiency, and quality.
Results
Using multilevel modeling, between-person analyses showed that sleep duration, efficiency, and quality were negatively related to morning heart rate and stress, such that people who slept longer, more efficiently, or better experienced lower heart rate and stress compared to those who slept shorter, less efficiently, or worse. Within-person analyses showed that sleep duration, efficiency, and quality predicted morning heart rate, blood pressure (though less consistently), and stress. That is, people experienced lower heart, blood pressure, and stress following nights when they slept longer, more efficiently, or better than they typically did. These within-person relationships were moderated by age, such that the effects of better and longer sleep on lower morning heart rate, blood pressure, and stress were stronger among younger than older adults.
Conclusion
These findings suggest that daily variations in sleep show immediate associations with stress and physiologic responses, but these daily variations have a stronger relationship among younger compared to older adults.
Keywords: Sleep , Blood pressure , Stress , Aging , Ecological Momentary Assessment
Following a night of longer or better sleep, people experience lower stress, lower heart rate, and lower blood pressure, and these relationships are stronger among younger than older adults.
Introduction
Short sleep duration is a major health issue as roughly a third of American adults report getting less than the recommended 7 hours of sleep per night [1]. The effects of limited sleep duration on cognitive, behavioral, physiological, and health outcomes have been well documented in prior research [2–4]. Additionally, poor subjective sleep quality has been associated with higher daytime stress and emotional lability [5–8] as well as increased rates of hypertension and poorer overall cardiovascular health [9,10]. The present study examined the relationships between several facets of sleep and heart rate, blood pressure, and stress.
In taking stock of the literature, it is important to consider the array of methods that have been used to study sleep. Much of this research has relied on cross-sectional studies to assess various indicators of sleep as an individual difference [11,12]. Longitudinal studies measure sleep at different stages of people’s lives to assess changes over time [13,14]. In other studies, sleep has been manipulated to examine causal effects of sleep loss [15]. Finally, a growing body of research has relied on Ecological Momentary Assessment (EMA) [16] methods by asking participants to provide reports about their sleep quantity and quality daily over the course of several days or weeks [17,18]. Each of these methods has its own strengths and weaknesses and can address fundamentally distinct questions that provide nuanced depictions of the nature of sleep and its effects on outcomes.
The present research uses an EMA method to advance our understanding of sleep and how it is related to physiologic responses and reports of stress in daily life. (We use the term physiologic responses to refer to within-person variation in physiology, whereas the term physiological outcomes refer to between-person variation in aggregated states.) One advantage of assessing these outcomes in daily life is that participants are not forced to rely on extensive recall, which is often fraught with heuristics and biases [19,20]. Furthermore, repeated assessments in daily life can capture a random sample of time points, which allows for the generalization of the findings to a larger population of time points in someone’s life. In the present study, we sampled morning responses which allowed us to generalize the findings to typical mornings of people’s lives. Hence, the data are considered ecologically valid [16,21]. Finally, of critical importance for our study, EMA methods allow for the examination of between- and within-person relationships. These distinct levels of analysis are mathematically orthogonal and represent distinct psychological processes [22–24]. The first aim of the present study was to examine both between-person and within-person relationships with a large and diverse sample of participants.
Prior cross-sectional studies have shown that people who do not receive adequate amounts of sleep have a higher risk of hypertension [10]. This association could be attributed to the fact that a habitual lack of sleep could influence the sympathetic nervous system, circadian rhythm, and concentrations of hormones [25,26]. Cross-sectional studies have also shown that people who report lower subjective sleep quality and shorter sleep duration experience higher levels of negative affect on average [6]. Moreover, longitudinal studies similarly document lagged effects from short sleep duration to increased risk of hypertension [10], and daily diary studies have shown that shorter sleep duration predicts higher stress on the following day [27,28]. In light of these findings, we hypothesized that sleep duration, efficiency, and subjective quality would be negatively related to heart rate, blood pressure, and stress at between- and within-person levels of analysis.
In addition to examining between-person and within-person relationships between sleep and physiologic and stress states, we also consider the important moderating influence of age. Heart rate and blood pressure change throughout the lifespan [29] as does the quality and duration of people’s sleep [30]. Moreover, the influence of someone’s sleep on their physiology and stress in daily life may vary as people get older. Research has shown that older adults are less hindered cognitively and emotionally in response to experimentally-induced sleep deprivation compared to younger adults [31–33]. These effects may be attributed to the fact that older adults have higher baseline levels of cognitive deficits, and older adults change their emotion regulation strategies such that they attempt to minimize negative affective experiences to a greater extent than younger adults [33]. These mechanisms could also influence people’s physiologic responses as they get older. Compared to younger adults, sleep in older adults is often marked on average by reduced slow wave sleep and more minutes awake during the night (i.e., more fragmented sleep), which may affect physiologic responses in the morning [34].
Therefore, we aimed to examine the moderating effect of age on (a) the between-person relationships between average sleep duration and quality and physiologic outcomes and stress and (b) the within-person relationships between daily sleep duration and quality and physiologic responses and stress. The moderation effects address two distinct processes. The first considers whether the benefits of being someone who tends to sleep better/longer on physiologic and stress outcomes are the same for individuals who are younger versus older. For example, it may be that people who typically sleep better have lower heart rate, but that is primarily true for younger individuals, and as people age the benefits of typically having good quality sleep are reduced. The second considers whether the effects of sleeping better or worse than one typically does confer similar physiologic and psychological benefits across the lifespan. For example, it may be that heart rate is lower after a particularly good night of sleep compared to a poor night of sleep, but this is only true for people who are younger. In contrast, for people who are older, there may be less differentiation in morning heart rate when comparing a good night with a bad night of sleep.
Method
Participants and Procedure
Data, materials, and analyses are available at https://osf.io/2czqp/. Participants were volunteers who downloaded the MyBPLab (https://mybplab.com) app via Google Playstore on their smartphone. The website notes that the study is designed to learn about stress, emotional experiences, blood pressure, and heart rate in daily life. The app requires a compatible phone (e.g., Samsung S9) with an embedded optic sensor (photoplethysmograph) that could be used to estimate blood pressure and heart rate (see [35] for validation study). We designed the study so that participants received notifications on their phones three times per day for a 21-day cycle. At each check-in (once in the morning, afternoon, and evening), participants received immediate feedback about their heart rate and blood pressure levels and were asked several other questions about their present situation. In addition to these daily questions, all participants completed basic demographic measures as part of the onboarding process and participants could also choose to complete additional demographic and individual difference questionnaires. The data analyzed in this paper included those who participated from March 15, 2019, until July 1, 2021. The study was approved by the Human Research Protection Program at the University of California, San Francisco (IRB # 19-27169). Additional analyses of some of the data that were unrelated to the present study have been published previously [36].
For the analyses presented in this paper, we were interested in participants who provided relevant demographic information, sensor readings for heart rate and blood pressure, and daily measures of sleep and stress. Because the sleep questions were asked only in the morning, we restricted our analyses to measures that were completed during the morning check-ins. The sleep questions were initially administered only once every three days, such that 15% of the participants received a maximum of seven check-ins with sleep questions. They completed an average of 3.64 check-ins (SD = 1.03). The procedure was later altered such that 85% of the participants received sleep check-ins every morning. They completed an average of 7.42 (SD = 4.52) sleep check-ins. We decided to include participants who provided their age and at least three-morning check-in reports of their sleep. (We used all available data, which meant that some participants completed more than three check-ins.) Because the goal of an EMA study is to capture a random sample of time points from the larger population of time points of someone’s life, we aimed to capture as reasonable of a sample as possible [21]. One or two completions seemed inadequate, whereas any limit higher than three seemed to be overly restrictive and led to a high rate of deletions. For instance, a requirement of five check-ins would have reduced the sample by roughly 50%. In addition, three check-ins were necessary to calculate within-person slopes when examining within-person differences in sleep.
For the descriptive statistics, we describe the entire pool of participants (i.e., anyone included in any of the analyses). In total, 4,359 participants (Mage = 46.75, SD = 12.39, Range 18–83; 69.30% male, 29.85% female) who were fairly diverse in age and race/ethnicity completed the study. Full descriptions are presented in Tables 1 and 2. Although the app was only available in English, the app was globally available and could be directly downloaded in the following countries’ Google Playstores: USA, UK, Australia, Canada, India, Singapore, Hong Kong, and New Zealand.
Table 1.
Participant Demographics
| Averages (SD) | |||||
|---|---|---|---|---|---|
| N | Percentage | Sleep duration | Sleep efficiency (%) | Subjective sleep quality | |
| Gender | |||||
| Male | 3,021 | 69.30% | 6.66 (1.09) | 90.92 (7.28) | 2.90 (0.46) |
| Female | 1,301 | 29.85% | 6.63 (1.26) | 89.17 (8.69) | 2.84 (0.48) |
| Other | 37 | 0.85% | 6.81 (1.06) | 88.22 (9.48) | 2.75 (0.49) |
| Age | |||||
| 18–29 years old | 335 | 7.69% | 6.70 (1.26) | 89.66 (9.68) | 2.80 (0.48) |
| 30–39 years old | 944 | 21.66% | 6.58 (1.26) | 89.67 (8.58) | 2.80 (0.47) |
| 40–49 years old | 1,320 | 30.28% | 6.57 (1.12) | 90.28 (7.94) | 2.84 (0.46) |
| 50–64 years old | 1,382 | 31.70% | 6.68 (1.04) | 90.73 (6.96) | 2.94 (0.45) |
| 65+ years old | 378 | 8.67% | 6.97 (1.08) | 91.70 (5.71) | 3.07 (0.45) |
| Country | |||||
| United States | 2,780 | 64.10% | 6.64 (1.19) | 90.24 (7.90) | 2.90 (0.47) |
| United Kingdom | 523 | 12.06% | 6.68 (1.02) | 90.26 (8.24) | 2.80 (0.47) |
| Australia | 312 | 7.19% | 6.58 (1.28) | 89.58 (8.66) | 2.82 (0.47) |
| Canada | 308 | 7.10% | 6.85 (0.93) | 91.41 (5.40) | 2.89 (0.40) |
| Other | 414 | 9.55% | 6.58 (1.05) | 90.92 (7.55) | 2.90 (0.45) |
| Education | |||||
| Elementary School (No High School) | 99 | 2.32% | 6.63 (1.20) | 90.08 (7.18) | 2.85 (0.54) |
| High School or GED | 569 | 13.34% | 6.66 (1.21) | 89.54 (9.67) | 2.84 (0.50) |
| Some college | 976 | 22.88% | 6.55 (1.16) | 89.95 (7.94) | 2.87 (0.49) |
| 2-Year degree | 468 | 10.97% | 6.49 (1.36) | 89.80 (8.33) | 2.84 (0.46) |
| 4-Year degree | 1,108 | 25.97% | 6.72 (1.06) | 90.78 (6.91) | 2.90 (0.44) |
| Graduate School | 1,046 | 24.52% | 6.76 (1.03) | 90.99 (7.20) | 2.91 (0.42) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 2,919 | 67.32% | 6.76 (1.12) | 90.56 (7.56) | 2.88 (0.46) |
| Non-Hispanic Black | 238 | 5.49% | 6.15 (1.24) | 89.20 (7.45) | 2.90 (0.55) |
| Non-Hispanic Asian | 311 | 7.17% | 6.43 (1.00) | 90.73 (7.28) | 2.84 (0.44) |
| Hispanic/Latinx | 367 | 8.46% | 6.43 (1.21) | 90.06 (8.90) | 2.86 (0.48) |
| Multiple Races | 128 | 2.95% | 6.19 (1.33) | 87.41 (11.53) | 2.82 (0.50) |
| Other | 373 | 8.60% | 6.68 (1.02) | 90.75 (7.15) | 2.93 (0.45) |
| Hypertension present | 1,313 | 30.56% | 6.62 (1.12) | 90.03 (7.80) | 2.87 (0.46) |
| Hypertension absent | 2,983 | 69.44% | 6.66 (1.15) | 90.52 (7.79) | 2.88 (0.47) |
Table 2.
Descriptive Statistics and Correlations Among Demographic and “Baseline” Physiologic and Stress Variables
| Variable | N | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 4,359 | 46.75 | 12.39 | |||||||
| 2. Education | 4,266 | 4.18 | 1.48 | −0.02 | ||||||
| 3. Subjective socioeconomic statusa | 3,955 | 5.82 | 2.19 | 0.12 | 0.20 | |||||
| 4. BMI | 4,277 | 29.65 | 6.62 | 0.01 | –0.10 | –0.10 | ||||
| 5. Heart rate (bpm) | 4,236 | 61.89 | 9.31 | –0.15 | –0.07 | –0.09 | 0.18 | |||
| 6. Systolic blood pressure (mmHg) | 3,229 | 126.48 | 16.27 | 0.13 | –0.07 | –0.01 | 0.23 | 0.12 | ||
| 7. Diastolic blood pressure (mmHg) | 3,229 | 80.88 | 11.89 | –0.05 | –0.01 | –0.01 | 0.13 | 0.18 | 0.69 | |
| 8. Stress (1 to 5 scale) | 3,632 | 1.66 | .87 | –0.11 | 0.04 | –0.05 | 0.01 | 0.07 | 0.05 | 0.07 |
a1 to 10 with 10 indicating highest relative income.
Note: For these descriptive statistics, we identified the check-in in which participants reported their lowest heart rate during the course of the study to capture the best analog of a “baseline” recording. We used the heart rate, blood pressure, and stress reports during this particular check-in and examined their correlations with the demographic variables and other physiologic and stress variables. Correlations with absolute values >0.03 were significant at p < .05; absolute values >0.04 significant at p < .01; absolute values >0.05 significant at p < .001.
Person-Level Measures
In addition to standard demographic questions about age, gender, and race/ethnicity, we obtained highest education achieved, subjective socioeconomic status, height and weight (we calculated body mass index [BMI]), and hypertension status. These variables were included as person-level covariates in analyses that examined between-person relationships. Education was assessed with the question, “What is the highest level of education that you completed?” Responses were reported on a 6-point scale (1 = elementary school or no high school diploma, 2 = high school or GED, 3 = some college, 4 = 2-year college degree, 5 = 4-year college degree, 6 = graduate school degree). Subjective socioeconomic status was assessed with a measure that asked participants to place themselves on a ladder that represented where they stand compared to others in their country [37]. The highest scores (1) represented the wealthiest people whereas the bottom (10) represented the poorest people. Scores were reverse-coded such that higher scores indicated greater wealth. We used this measure as opposed to a specific currency because of the intended global reach of this study and the importance of relative subjective socioeconomic status. BMI was calculated with the following formula: weight (pounds)/height (inches)2 × 703. Unrealistically high or low values for weight (<80 pounds or >500 pounds) and height (<36 inches or >84 inches) were treated as missing as were unrealistically high or low BMI values (<15 or >60). Hypertension was assessed during the initial onboarding where participants indicated the presence or absence of various chronic illnesses or conditions.
Daily Measures
At each check-in, participants provided a heart rate and blood pressure response by placing their index finger over the optic sensor for 30–45 s. We encouraged participants to calibrate their blood pressure with an external cuff at the start of the study, and as further incentive (and for health safety reasons), participants were only able to view their blood pressure levels if they provided a calibration value. Because of the importance of calibration in the BP algorithm [35], we only include participants with calibrated blood pressure values. Extreme values of heart rate (HR; <30 and >200), systolic blood pressure (SBP; <80 and >210), and diastolic blood pressure (DBP; <50 and >180) were set to missing. (5.44% of the participants recorded at least one extreme value, and only 1.67% recorded more than three extreme values, which were set to missing.) Check-in blood pressure values also were omitted if the participant responded affirmatively to the question “have you exercised in the last 30 minutes?” given exercise acutely raises blood pressure. Next, participants answered questions about where they were, who they were with, and whether they had experienced any particularly stressful events since their last check-in (yes/no). If they answered no to the acute stress question, they responded to a statement reflecting their chronic stress: “I feel stressed, anxious, overwhelmed” on a 5-point scale (1 = not at all, 5 = extremely). We used this latter measure of stress as our primary subjective stress response, given the vast majority of responses (86.38%) did not indicate a recent acute stressor during the morning check-ins.
Every one to three mornings they were asked a few questions about their sleep. (Sleep questions were initially asked every third day but in September 2019 we switched to everyday.)
We aimed to measure sleep duration, efficiency, and subjective sleep quality by selecting a few items from the core consensus sleep diary [38]. To assess sleep duration, participants were asked what time they tried to go to sleep last night (lights out) and what time they woke up today. 250 (0.32% of responses) sleep and wake-up times were deemed unrealistic and were treated as missing. They were also asked how long it took them to fall asleep (in minutes; sleep onset latency) and how long they were awake during the night (in minutes; wake after sleep onset). We created a sleep duration variable by calculating the difference between the time they reported waking up from the time they reported going to bed and subtracting the number of minutes it took to fall asleep as well as the minutes they were awake during the night. We also created a sleep efficiency variable by dividing total sleep time by the total amount of time spent in bed*100. Finally, to assess subjective sleep quality, participants were asked, “How would you rate the quality of your sleep last night?” Responses were recorded on a 4-point scale (1 = very bad, 2 = fairly bad, 3 = fairly good, 4 = very good).
Analytic Overview
First, we present descriptive statistics of the daily measures. Next, we examine between-person associations between aggregated sleep measures and physiologic and stress outcomes. We look at each measure of sleep (i.e., sleep duration, sleep efficiency, sleep quality) as its own predictor and ask whether people who sleep better/longer on average tend to experience lower heart rate, lower blood pressure, and less subjective stress compared to those who do not sleep as well or as long. Extending the research beyond between-person analyses, we then examine the within-person associations between sleep and physiologic and stress responses. For the average person, we ask whether they experience lower heart rate, blood pressure, and stress following nights when they report sleeping particularly better or longer than they typically do. Following these analyses, we examined the moderating effect of age on (a) the between-person relationships between average sleep and average physiologic and stress outcomes and (b) the within-person relationships between these same variables.
Because data collection began before and continued during the Covid-19 pandemic, we examined whether the between-person and within-person relationships between sleep and physiologic and stress outcomes varied before versus during the pandemic. Of the 24 models, the only significant interaction involved the between-person relationship between average sleep quality and heart rate (b = 1.86, t = 2.54, p = .011), such that the relationship was weaker before (b = −0.91, t = −1.78, p = .076) than during (b = −2.73, t = −5.01, p < .001) the pandemic. All other interactions were not significant (ps > .05). Therefore, we collapsed across time-period in the analyses.
Given the nested data structure (morning check-ins nested within individuals), we used multilevel modeling with the lme4 package in R [39]. In a series of two-level models, check-ins were nested within persons. We present unstandardized coefficients, and details of each specific model are presented below. Effect sizes, rb(f) and rw(f), were calculated following recommendations by Rights and Sterba [40]. The rb(f) (rw(f)) statistic is defined as the square root of the proportion of variance explained by between-person (within-person) predictors via fixed slopes and random slope variation/covariation. This is similar to a measure of the square root of the proportion reduction in variance, akin to a correlation [41–43].
Results
Descriptive Statistics of Daily Measures
We first provide descriptive statistics of the daily measures through unconditional models. These models provide estimates of the means and variances of each daily measure. As shown in Table 3, there was more within-person than the between-person variance for most of the sleep measures, whereas most of the variation of the physiologic measures occurred between-persons. Stress varied within- and between-persons in roughly equal amounts. Overall, there was considerable within- and between-person variance for all daily measures to examine analyses at each level.
Table 3.
Descriptive Statistics of Daily Measures
| Variance | ||||||
|---|---|---|---|---|---|---|
| Outcome variable | Total # of entries | Total # of people | Mean | Between-person | Within-person | ICC |
| Sleep duration (hrs) | 21,638 | 3,155 | 6.75 | 1.02 | 1.58 | 0.39 |
| Sleep efficiency (%) | 21,617 | 3,153 | 90.41 | 44.00 | 74.77 | 0.37 |
| Subjective sleep quality | 27,131 | 4,344 | 2.89 | 0.15 | 0.29 | 0.34 |
| Heart rate (bpm) | 26,225 | 4,236 | 72.45 | 108.29 | 57.79 | 0.65 |
| Systolic blood pressure (mmHg) | 19,597 | 3,107 | 128.68 | 214.39 | 44.08 | 0.83 |
| Diastolic blood pressure (mmHg) | 19,597 | 3,107 | 80.68 | 106.20 | 25.00 | 0.81 |
| Stress (1 to 5 scale) | 23,553 | 3,844 | 1.72 | 0.38 | 0.40 | 0.49 |
Between-Person Relationships Among Aggregated Sleep and Physiologic/Stress Outcomes
To examine the between-person relationships between sleep and physiologic and stress outcomes, we created aggregated sleep measures by averaging scores across all check-ins for each participant. We entered the four aggregated sleep measures as uncentered person-level predictors in separate models. Additional person-level covariates (age, gender, education, subjective socioeconomic status, race/ethnicity, BMI, hypertension) were also entered at level 2. Heart rate, blood pressure, and stress were outcome measures at level 1 in separate models. (See Model 1 in Supplemental Materials.)
As presented in Table 4, when examining between participants, longer average sleep duration and higher average sleep efficiency were associated with lower HR and stress, but they did not significantly predict SBP or DBP. Higher average sleep quality was associated with lower HR, lower SBP, lower DBP, and less stress. Significant effect sizes ranged from small to medium in magnitude.
Table 4.
Between-Person Relationships Between Aggregated Sleep Measures and Physiologic/Stress Outcomes
| Predictors | ||||||
|---|---|---|---|---|---|---|
| Sleep duration | ||||||
| DV | N | # Check-ins | b [95% CI] | t | p | r b (f) |
| Heart rate | 2,780 | 18,919 | −1.09 [−1.43, −0.75] | −6.33 | <.001 | 0.13 |
| Systolic blood pressure | 2,160 | 14,616 | −0.13 [−0.68,0.42] | −0.46 | .648 | 0.02 |
| Diastolic blood pressure | 2,160 | 14,616 | −0.13 [−0.53,0.27] | −0.65 | .514 | 0.03 |
| Stress | 2,578 | 17,324 | −0.07 [−0.09, -0.05] | −6.04 | <.001 | 0.11 |
| Sleep efficiency | ||||||
| DV | N | # Check-ins | b [95% CI] | t | p | r b (f) |
| Heart rate | 2,778 | 18,899 | −0.13 [−0.20, −0.08] | −5.02 | <.001 | 0.13 |
| Systolic blood pressure | 2,158 | 14,606 | −0.05 [−0.13, 0.04] | −1.09 | .276 | 0.04 |
| Diastolic blood pressure | 2,158 | 14,606 | −0.02 [−0.08, 0.04] | −0.65 | .515 | 0.02 |
| Stress | 2,574 | 17,307 | −0.01 [−0.02, −0.01] | −8.15 | <.001 | 0.18 |
| Sleep quality | ||||||
| DV | N | # Check-ins | b [95% CI] | t | p | r b (f) |
| Heart rate | 3,795 | 23,535 | −1.78 [−2.51, −1.05] | −4.76 | <.001 | 0.13 |
| Systolic blood pressure | 2,783 | 17,492 | −2.14 [−3.32, −0.97] | −3.57 | <.001 | 0.05 |
| Diastolic blood pressure | 2,783 | 17,492 | −1.47 [−2.33, −0.61] | −3.35 | <.001 | 0.09 |
| Stress | 3,460 | 21,228 | −0.36 [−0.41, −0.31] | −14.47 | <.001 | 0.28 |
Note: These models controlled for age, gender, education, income, race/ethnicity, BMI, and hypertension.
Within-Person Relationships Between Sleep and Physiologic and Stress Responses
To examine the within-person relationships between sleep measures and physiologic and stress responses in the morning, we entered HR, SBP, DBP, and stress as outcome measures in separate models. Here, rather than focusing on average levels, we examine within-person variation from day to day. Thus, the individual sleep variables were centered around each individual’s mean and were entered as predictors at level 1 in separate models [44]. We used calibrated data for SBP and DBP, and we used data only from participants who provided their age. (See Model 2 in Supplemental Materials.)
As presented in Table 5, longer sleep duration was associated with lower HR and SBP, and less stress, but was not significantly associated with DBP. (The rw(f) effect size estimates may not correspond intuitively to t-values and p-values. Separate variance estimates are provided in Supplemental Materials.) Higher daily sleep efficiency was associated with lower HR and stress but was not significantly related to either SBP or DBP. Greater daily sleep quality was associated with lower HR, SBP, DBP, and stress. In sum, these analyses show that, in general, as someone sleeps longer or better than they normally do, they experience lower HR and less stress when they wake-up in the morning, compared to mornings when they wake-up after sleeping worse/less. In addition, people have lower blood pressure following nights of higher quality sleep, compared to nights in which they slept worse. As with the between-person effects, daily variations in sleep duration and efficiency were less consistently associated with blood pressure. Significant effect sizes ranged in magnitude from small to medium.
Table 5.
Within-Person Relationships Between Sleep and Physiologic/Stress Responses
| Predictors | ||||||
|---|---|---|---|---|---|---|
| Sleep duration | ||||||
| DV | N | # Check-ins | b [95% CI] | t | p | r w (f) |
| Heart rate | 3,089 | 20,966 | −0.30 [−0.40, −0.20] | −5.88 | <.001 | 0.14 |
| Systolic blood pressure | 2,401 | 16,293 | −0.10 [−0.19, −0.01] | −2.18 | .029 | 0.12 |
| Diastolic blood pressure | 2,401 | 16,293 | −0.00 [−0.08, 0.07] | −0.08 | .937 | 0.13 |
| Stress | 2,861 | 19,169 | −0.05 [−0.06, −0.04] | −10.54 | <.001 | 0.16 |
| Sleep efficiency | ||||||
| DV | N | # Check-ins | b [95% CI] | t | p | r w (f) |
| Heart rate | 3,087 | 20,945 | −0.03 [−0.04, −0.01] | −3.87 | <.001 | 0.10 |
| Systolic blood pressure | 2,399 | 16,282 | −0.01 [−0.02, 0.01] | −1.14 | .254 | 0.01 |
| Diastolic blood pressure | 2,399 | 16,282 | 0.00 [−0.01, 0.01] | 0.30 | .768 | 0.00 |
| Stress | 2,857 | 19,151 | −0.00 [−0.01, −0.00] | −8.07 | <.001 | 0.15 |
| Sleep quality | ||||||
| DV | N | # Check-ins | b [95% CI] | t | p | r w (f) |
| Heart rate | 4,235 | 26,218 | −0.65 [−0.85, −0.44] | −6.13 | <.001 | 0.14 |
| Systolic blood pressure | 3,107 | 19,594 | −0.39 [−0.58, −0.20] | −4.07 | <.001 | 0.09 |
| Diastolic blood pressure | 3,107 | 19,594 | −0.14 [−0.29, −0.00] | −1.97 | .049 | 0.10 |
| Stress | 3,844 | 23,551 | −0.16 [−0.18, −0.14] | −16.68 | <.001 | 0.19 |
Moderating Role of Age on Between- and Within-Person Relationships
In addition to examining between- and within-person relationships among sleep and physiologic and stress outcomes, we examined whether age moderates these relationships. First, we tested whether the between-person relationships varied as a function of participant’s age. We added age and an interaction term as predictors at the person-level to Model 1. (See Model 3 in Supplemental Materials.)
These interactions were not significant (all ps > .07; most ps > .30). Results are presented in Supplemental Materials. Thus, the effect of average levels of sleep on average physiologic/stress outcomes does not vary significantly by age.
As a separate question, we examined whether the within-person relationships between sleep and physiological responses and stress differed by age. To do so, we entered heart rate, blood pressure, and stress as outcomes in separate models. Sleep predictors were person-mean centered and entered at level 1 in separate models, just as they were in Model 2. Age and demographic covariates were entered as predictors at level 2, creating cross-level interactions. (See Model 4 in Supplemental Materials.)
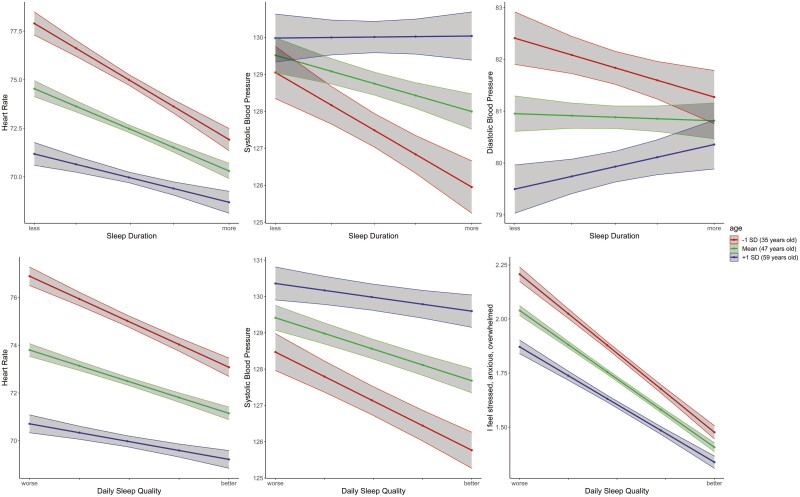
As shown in Table 6, age moderated the within-person relationships between sleep duration and HR, SBP, and DBP but did not significantly moderate the within-person relationship between sleep duration and stress. As depicted in Fig. 1, the within-person relationships between longer sleep duration and lower heart rate and blood pressure were stronger among younger adults than older adults. Age did not significantly moderate any of the within-person relationships between sleep efficiency and physiological responses and stress.
Table 6.
Cross-Level Interaction Coefficients of Age Moderation of Within-Person Relationships
| Predictors | |||||
|---|---|---|---|---|---|
| Sleep duration | |||||
| DV | N | # Check-ins | b [95% CI] | t | p |
| Heart rate | 2,780 | 18,919 | 0.010 [0.001, 0.018] | 2.27 | .023 |
| Systolic blood pressure | 2,160 | 14,616 | 0.012 [0.003, 0.020] | 2.60 | .009 |
| Diastolic blood pressure | 2,160 | 14,616 | 0.007 [0.000, 0.013] | 2.09 | .036 |
| Stress | 2,567 | 17,226 | 0.000 [−0.001, 0.001] | 0.61 | .540 |
| Sleep efficiency | |||||
| DV | N | # Check-ins | b [95% CI] | t | p |
| Heart rate | 2,778 | 18,899 | 0.001 [−0.001, 0.002] | 0.96 | .339 |
| Systolic blood pressure | 2,158 | 14,606 | 0.001 [−0.000, 0.002] | 1.32 | .188 |
| Diastolic blood pressure | 2,158 | 14,606 | 0.001 [−0.000, 0.002] | 1.49 | .136 |
| Stress | 2,574 | 17,307 | 0.000 [−0.000, 0.000] | .01 | .989 |
| Sleep quality | |||||
| DV | N | # Check-ins | b [95% CI] | t | p |
| Heart rate | 3,795 | 23,535 | 0.023 [0.004, 0.042] | 2.42 | .016 |
| Systolic blood pressure | 2,783 | 17,492 | 0.022 [0.004, 0.040] | 2.45 | .014 |
| Diastolic blood pressure | 2,783 | 17,492 | 0.007 [−0.007, 0.020] | .95 | .345 |
| Stress | 3,460 | 21,228 | 0.002 [0.000, 0.004] | 2.46 | .014 |
Note: These models controlled for age, gender, education, income, race/ethnicity, BMI, and hypertension.
Fig. 1.
Moderating effect of age on the within-person relationships between sleep and physiology and stress. Note: The red line represents those at age 35, one standard deviation below the mean. The green line represents those at age 47, the mean. The blue line represents those at age 59, one standard deviation above the mean.
Regarding sleep quality, age moderated the within-person relationships between sleep quality and heart rate, SBP, and stress (but not DBP). Similar to the patterns of sleep duration, the within-person relationships were stronger among younger adults than older adults. To summarize, the pattern of associations suggests that, on average, changes in sleep from night to night are more impactful on physiology and subjective stress for younger people than older people. Interestingly, the pattern of effects is different for SBP than for HR, DBP, and stress. For HR, DBP, and stress, age differences are most pronounced when people sleep shorter or worse than usual, such that younger individuals more than older individuals experience greater increases in HR, DBP, and stress after a poor/short night of sleep. For SBP, however, age differences are more pronounced when people sleep longer or better than usual, with younger adults more than older adults experiencing greater reductions in SBP after a longer/better night of sleep.
Discussion
Across a large sample of adults who provided morning recordings of their heart rate, blood pressure, subjective stress, and various indicators of their sleep over the course of three weeks, we found that longer sleep duration, higher sleep efficiency, and better sleep quality predicted lower heart rate and stress at between-person and within-person levels of analysis. Although the pattern of results across between- and within-person levels of analysis were similar, it is important to emphasize that these levels of analysis represent distinct processes. For example, between-persons, people who report higher levels of sleep quality on average report lower subjective stress in the mornings compared to those who report lower sleep quality, a finding consistent with cross-sectional research [12]. Within-persons, as someone reports greater sleep quality than they typically do, they report lower subjective stress in the morning, similar to results from daily diary research that has measured sleep’s association with a broader range of negative affective states [7].
A few nuances between the individual sleep indicators warrant discussion. Sleep quality consistently predicted lower heart rate, blood pressure, and stress at between- and within-person levels of analysis. Sleep duration and sleep efficiency predicted lower heart rate and stress at each level of analysis, but they were inconsistently related to blood pressure. This highlights the importance of including a variety of different sleep facets [45–47] and suggests that subjective sleep quality may be a more important indicator of the physiological effects than recall estimates of sleep duration.
Extending our findings beyond the main effects of sleep on physiology and stress, we examined the moderating effects of age at between- and within-person levels of analysis. Age did not moderate any of the between-person relationships but did significantly moderate several of the within-person relationships. Receiving an extra hour of sleep or sleeping particularly better than normal had stronger and more beneficial effects among younger than older adults. Thus, it may be easier for younger adults to improve their short-term cardiovascular health and subjective stress via better sleep than it is for older adults.
These insights about the varying associations between sleep and physiology and stress across the lifespan were supported by several unique strengths of the method employed. By asking participants to provide multiple self-report assessments of their sleep and stress in the morning, we were able to limit recall bias [16,20]. Although daily diary and EMA methods have been frequently utilized in sleep research [48], this study is the first to the best of our knowledge to combine EMA methods with ambulatory assessments of heart rate and blood pressure from a large sample using a digital platform that included a wide range of ages.
Implications and Future Research
These findings point to a few implications and practical applications. For instance, our results suggest that high quality sleep might indirectly or directly influence health issues associated with hypertension [49] and stress [50,51]. Sleep could also help mitigate negative outcomes associated with chronic stress. To confirm the long-term benefits of daily variations in sleep quality and quantity, longitudinal studies would benefit from the integration of EMA methods. Our findings also point to potential avenues for sleep interventions, which could help improve stress reductions and physiologic reactions in daily life. In particular, sleep interventions that specifically target improvements in sleep quality may improve people’s morning heart rate, blood pressure, and subjective stress. Sleep interventions that target sleep quantity may be especially useful in lowering heart rate and subjective stress. Additionally, our large-scale EMA method that captures objective indicators of physiology in daily life has important implications for epidemiology as it can show which groups of people might be most impacted by deficits in sleep. These types of findings could help inform policy makers as they decide on implementations for specific subgroups of the population.
Understanding the precise mechanisms that explain why younger adults benefit from better and more sleep than older adults remains an interesting avenue for future research. The integrity of the cardiovascular system changes with age, with older adults showing greater atherosclerotic development and vascular stiffness, the latter of which contributes to elevated blood pressure [52]. Because of these age-related changes in the vasculature and related reduction in system flexibility [53], it is possible that there is less variability to be explained by modest nightly changes in sleep. In contrast, sleep appears to be a stronger contributor to blood pressure dynamics in younger adults.
Limitations
A few limitations are worth noting. First, although our sample was fairly large and diverse in certain regards, our convenience sample was not representative of the general population. The sample was primarily from the USA, more males than females, and the participants were highly educated, on average. We did not have any control over participant selection, and hence our sample may have been biased towards individuals who would likely download an app that provides blood pressure and heart rate information. These factors are important to bear in mind when considering the generalizability of our findings. Second, we relied solely on self-report measures of sleep, and we did not include measures that captured objective aspects of sleep, such as actigraphy or polysomnography. Each method provides unique information about people’s sleep and integrating them into future research would be fruitful. Third, as in all field studies, measurements of physiological responses are bound to have more errors than measurements obtained in the lab. In this work, blood pressure and heart rate were obtained using an optic sensor and required participants to provide calibrated blood pressure responses. We are not able to ascertain how carefully participants adhered to instructions regarding providing calibrated blood pressure values or the context in which they provided a sensor reading (the app recommends sitting down with the sensor positioned at heart height). Valid heart rate is easier to obtain from an optic sensor than blood pressure, so the effect sizes observed here (e.g., larger effect sizes for associations between sleep quality and heart rate than sleep quality and blood pressure) might be, in part, due to more measurement error in blood pressure.
Conclusion
In summary, longer sleep duration, higher sleep efficiency, and better sleep quality predict lower heart rate, blood pressure, and stress at between- and within-person levels of analysis. The within-person effects of better and longer sleep are more pronounced among younger than older adults. This suggests that sleep has particularly strong temporary benefits on their physiology and well-being in daily life.
Supplementary Material
Contributor Information
David B Newman, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USA.
Amie M Gordon, Department of Psychology, University of Michigan, Ann Arbor, MI, USA.
Aric A Prather, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USA.
Wendy Berry Mendes, Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, CA, USA.
Funding
This research was supported by the National Institute on Aging (R24AG048), National Institute of Mental Health (T32MH019391), and Samsung Electronics CO.
Conflict of Interest
The authors report no conflicts of interest.
Authors’ Contributions DBN: conceptualization, formal analysis, writing-original draft preparation. AMG: conceptualization, writing-review & editing. AAP: conceptualization, writing-review & editing. WBM: funding, investigation, provided resources, supervision, writing-review & editing.
Data Availability
The study and analysis plan were not formally preregistered. De-identified data, analytic code used to conduct the analyses presented in this study, and all materials used to conduct the study are available in a public archive: https://osf.io/2czqp/.
References
- 1. Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB.. Prevalence of healthy sleep duration among adults — United States, 2014. Morb Mortal Wkly Rep. 2016;65(6):137–141. [DOI] [PubMed] [Google Scholar]
- 2. Astill RG, Van der Heijden KB, Van IJzendoorn MH, Van Someren EJW.. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol Bull. 2012;138:1109–1138. [DOI] [PubMed] [Google Scholar]
- 3. Dinges DF. The state of sleep deprivation: from functional biology to functional consequences. Sleep Med Rev. 2006;5:303–305. [DOI] [PubMed] [Google Scholar]
- 4. Lim J, Dinges DF.. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Åkerstedt T, Orsini N, Petersen H, Axelsson J, Lekander M, Kecklund G.. Predicting sleep quality from stress and prior sleep – a study of day-to-day covariation across six weeks. Sleep Med. 2012;13:674–679. [DOI] [PubMed] [Google Scholar]
- 6. Palmer CA, Alfano CA.. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. [DOI] [PubMed] [Google Scholar]
- 7. Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, Buxton OM.. Bidirectional, temporal associations of sleep with positive events, affect, and stressors in daily life across a week. Ann Behav Med. 2017;51:402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sin NL, Wen JH, Klaiber P, Buxton OM, Almeida DM.. Sleep duration and affective reactivity to stressors and positive events in daily life. Health Psychol. 2020;39:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itani O, Jike M, Watanabe N, Kaneita Y.. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 10. Guo X, Zheng L, Wang J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14:324–332. [DOI] [PubMed] [Google Scholar]
- 11. Berry DTR, Webb WB.. State measures and sleep stages. Psychol Rep. 1983;52:807–812. [DOI] [PubMed] [Google Scholar]
- 12. Norlander T, Johansson A, Bood SA.. The affective personality: its relation to quality of sleep, well-being and stress. Soc Behav Pers Int J. 2005;33:709–722. [Google Scholar]
- 13. Gami Apoor S, Olson Eric J, Shen Win K, et al. Obstructive sleep apnea and the risk of sudden cardiac death. J Am Coll Cardiol. 2013;62:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon AM, Prather AA, Dover T, Espino-Pérez K, Small P, Major B.. Anticipated and experienced ethnic/racial discrimination and sleep: a longitudinal study. Pers Soc Psychol Bull. 2020;46:1724–1735. [DOI] [PubMed] [Google Scholar]
- 15. de Bruin EJ, van Run C, Staaks J, Meijer AM.. Effects of sleep manipulation on cognitive functioning of adolescents: a systematic review. Sleep Med Rev. 2017;32:45–57. [DOI] [PubMed] [Google Scholar]
- 16. Shiffman S, Stone AA, Hufford MR.. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 17. Broderick JE, Junghaenel DU, Schneider S, Pilosi JJ, Stone AA.. Pittsburgh and epworth sleep scale items: accuracy of ratings across different reporting periods. Behav Sleep Med. 2013;11:173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris AL, Carmona NE, Moss TG, Carney CE.. Testing the contiguity of the sleep and fatigue relationship: a daily diary study. Sleep. 2021;44(5):zsaa252. [DOI] [PubMed] [Google Scholar]
- 19. Bradburn NM, Rips LJ, Shevell SK.. Answering autobiographical questions: the impact of memory and inference on surveys. Science. 1987;236:157–161. [DOI] [PubMed] [Google Scholar]
- 20. Schwarz N: Why researchers should think “real-time”: a cognitive rationale. In: Mehl M R, Conner T S, eds. Handbook of Research Methods for Studying Daily Life. New York: Guilford Press, 2012. 27. [Google Scholar]
- 21. Newman DB, Stone AA.. Understanding daily life with Ecological Momentary Assessment. In: Frank K, Herr PM, Schwarz, Norbert, eds. Handbook of Research Methods in Consumer Psychology. New York: Routledge, 2019. 259–275. [Google Scholar]
- 22. Affleck G, Zautra A, Tennen H, Armeli S.. Multilevel daily process designs for consulting and clinical psychology: a preface for the perplexed. J Consult Clin Psychol. 1999;67:746–754. [DOI] [PubMed] [Google Scholar]
- 23. Newman DB, Nezlek JB, Thrash TM.. The dynamics of searching for meaning and presence of meaning in daily life. J Pers. 2018;86:368–379. [DOI] [PubMed] [Google Scholar]
- 24. Nezlek JB. Multilevel random coefficient analyses of event- and interval-contingent data in social and personality psychology research. Pers Soc Psychol Bull. 2001;27:771–785. [Google Scholar]
- 25. Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R.. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12:63–68. [DOI] [PubMed] [Google Scholar]
- 26. Goncharuk VD, Van Heerikhuize J, Dai J-P, Swaab DF, Buijs RM.. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J Comp Neurol. 2001;431:320–330. [DOI] [PubMed] [Google Scholar]
- 27. Newman DB, Epel ES, Coccia M, Puterman E, Prather AA.. Asymmetrical effects of sleep and emotions in daily life. Affect Sci. 2022;3:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yap Y, Slavish DC, Taylor DJ, Bei B, Wiley JF.. Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: a daily intensive longitudinal study. Sleep. 2020;43:zsz250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrari AU, Radaelli A, Centola M.. Aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. [DOI] [PubMed] [Google Scholar]
- 30. Wolkove N, Elkholy O, Baltzan M, Palayew M.. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duffy JF, Willson HJ, Wang W, Czeisler CA.. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57:1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philip P, Taillard J, Sagaspe P, et al. Age, performance and sleep deprivation. J Sleep Res. 2004;13:105–110. [DOI] [PubMed] [Google Scholar]
- 33. Schwarz J, Axelsson J, Gerhardsson A, et al. Mood impairment is stronger in young than in older adults after sleep deprivation. J Sleep Res. 2019;28:e12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV.. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. [DOI] [PubMed] [Google Scholar]
- 35. Gordon AM, Mendes WB.. A large-scale study of stress, emotions, and blood pressure in daily life using a digital platform. Proc Natl Acad Sci USA. 2021;118:e2105573118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Newman DB, Gordon AM, Mendes WB.. Comparing daily physiological and psychological benefits of gratitude and optimism using a digital platform. Emotion. 2021;21:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh-Manoux A, Marmot MG, Adler NE.. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–861. [DOI] [PubMed] [Google Scholar]
- 38. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bates D, Mächler M, Bolker B, Walker S.. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 40. Rights JD, Sterba SK.. Quantifying explained variance in multilevel models: an integrative framework for defining R-squared measures. Psychol Methods. 2019;24:309–338. [DOI] [PubMed] [Google Scholar]
- 41. Hox JJ. Multilevel Analyses: Techniques and Applications. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- 42. Kreft IG, de Leeuw J.. Introducing Multilevel Modeling. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 43. Raudenbush SW, Bryk AS.. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 44. Enders CK, Tofighi D.. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–138. [DOI] [PubMed] [Google Scholar]
- 45. Gordon AM, Mendes WB, Prather AA.. The social side of sleep: elucidating the links between sleep and social processes. Curr Dir Psychol Sci. 2017;26:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jackowska M, Ronaldson A, Brown J, Steptoe A.. Biological and psychological correlates of self-reported and objective sleep measures. J Psychosom Res. 2016;84:52–55. [DOI] [PubMed] [Google Scholar]
- 47. Jarrin DC, McGrath JJ, Drake CL.. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes. 2013;37:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Konjarski M, Murray G, Lee VV, Jackson ML.. Reciprocal relationships between daily sleep and mood: a systematic review of naturalistic prospective studies. Sleep Med Rev. 2018;42:47–58. [DOI] [PubMed] [Google Scholar]
- 49. Calhoun DA, Harding SM.. Sleep and hypertension. Chest. 2010;138:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kashani M, Eliasson A, Vernalis M.. Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress. 2012;15:45–51. [DOI] [PubMed] [Google Scholar]
- 51. Steptoe A, Kivimäki M.. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. [DOI] [PubMed] [Google Scholar]
- 52. Lakatta EG, Mitchell JH, Pomerance A, Rowe GG.. Human aging: changes in structure and function. J Am Coll Cardiol. 1987;10:42A–47A. [DOI] [PubMed] [Google Scholar]
- 53. Sutton-Tyrrell K, Newman A, Simonsick EM, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study and analysis plan were not formally preregistered. De-identified data, analytic code used to conduct the analyses presented in this study, and all materials used to conduct the study are available in a public archive: https://osf.io/2czqp/.