Abstract
Cardiovascular diseases (CVD) remain the leading cause of death in developed societies, and “midlife” (50–64 yr) and older (65+) men and women bear the great majority of the burden of CVD. Much of the increased risk of CVD in this population is attributable to CV dysfunction, including adverse changes in the structure and function of the heart, increased systolic blood pressure, and arterial dysfunction. The latter is characterized by increased arterial stiffness and vascular endothelial dysfunction. Conventional aerobic exercise training, as generally recommended in public health guidelines, is an effective strategy to preserve or improve CV function with aging. However, <40% of midlife and older adults meet aerobic exercise guidelines, due in part to time availability-related barriers. As such, there is a need to develop evidence-based time-efficient exercise interventions that promote adherence and optimize CV function in these groups. Two promising interventions that may meet these criteria are interval training and inspiratory muscle strength training (IMST). Limited research suggests these modes of training may improve CV function with time commitments of ≤60 min/wk. This review will summarize the current evidence for interval training and IMST to improve CV function in midlife/older adults and identify key research gaps and future directions.
Keywords: aging, interval training, inspiratory muscle strength training
AGING AND CARDIOVASCULAR DISEASE
Cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in both men and women in developed, as well as many developing, societies (8, 57). Advancing age is by far the strongest independent risk factor for CVD; thus, midlife (50–64 yr of age) and older (65+ years of age) adults bear the great majority of the burden of CVD (57, 110). The growth of this age cohort predicts an epidemic of new CVD unless steps are taken to reduce CVD risk (50). Thus, establishing effective evidence-based strategies to decrease CVD risk in midlife and older adults is a major biomedical research priority.
There are many reasons why age increases the risk for CVD. Certainly, the “traditional” CVD risk factors, such as obesity, elevated fasting blood glucose, and blood lipids, worsen with aging (58, 59, 132). Additionally, much of the rise in risk is mediated by adverse changes to the heart, including ventricular fibrosis, left ventricular hypertrophy, diastolic dysfunction, and an increased prevalence of atrial fibrillation (68). A progressive increase in systolic blood pressure (SBP) with aging also contributes importantly, whereas diastolic blood pressure remains constant and then declines after approximately age 50 (15, 92). However, much of the increased CVD risk with aging is attributable to the development of arterial dysfunction, due in part to stiffening of the large elastic arteries (aorta and carotid arteries), and vascular endothelial dysfunction (67). Age-related arterial stiffening contributes to increases in SBP with aging but also to detrimental changes in cardiac morphology and function (15, 92). Vascular endothelial dysfunction is characterized primarily by decreased bioavailability of the provasodilatory molecule nitric oxide (NO), driven by increased oxidative stress and inflammation (105). Along with its direct action as a vasodilator, NO has numerous other CV-protective effects, including antithrombotic, anti-inflammatory, and antiproliferative actions; as such, decreased endothelium-derived NO bioavailability is an initiating pathological step for CVD. Cumulatively, adverse changes in the heart, increases in SBP, and arterial dysfunction comprise cardiovascular dysfunction and increase the risk not only for CVD but also other common conditions of aging, including exercise intolerance (23), chronic kidney disease (48), cognitive impairment (123, 134), and dementia (64, 93) (Fig. 1).
Fig. 1.
Age-related cardiovascular dysfunction increases risk for clinical disorders. Continuous aerobic exercise can prevent or reverse cardiovascular dysfunction. High-intensity interval training and inspiratory muscle strength training may have beneficial cardiovascular effects.
CONVENTIONAL AEROBIC EXERCISE TRAINING AND CV FUNCTION WITH AGING
Conventional aerobic exercise training, defined as moving the large muscles in a rhythmic pattern for a sustained (continuous) period of time (91), as generally recommended in public health guidelines, is one of the most well-established strategies to reduce risk for CVD (80, 103) and other conditions of aging (72, 91) (Fig. 1). Consistent with this body of evidence, the 2018 Physical Activity Guidelines for Americans call for a minimum of 150 min/wk of moderate-intensity aerobic activity [3.0–5.9 metabolic equivalents (METS)] or 75 min/wk of vigorous-intensity aerobic activity (≥6.0 METS) (91).
Conventional aerobic exercise training in accordance with current guidelines also is associated with increased cardiorespiratory fitness and improvements in CV function in most midlife and older adults (5, 91). Some adults do not exhibit improvements in cardiorespiratory fitness with guideline-based training (i.e., so called “nonresponders”); however, higher intensities and volumes of training reduce the incidence of nonresponse (99). Cardiorespiratory fitness is a clinically important risk factor for CVD (65), and improvements in cardiorespiratory fitness elicited by continuous aerobic exercise training may play a role in reducing CVD risk by influencing both cardiac and vascular function. Thus, continuous forms of aerobic exercise training have beneficial effects on cardiac function (98), including enhanced ventricular compliance and distensibility (9), higher maximal stroke volume (13), and improved left ventricular diastolic filling (53). Likewise, continuous aerobic exercise decreases SBP by 2–4 mmHg in nonhypertensive adults, while evoking larger reductions of 5–8 mmHg in those with hypertension (129).
Conventional aerobic exercise training also preserves arterial function with aging by suppressing large elastic artery stiffening and vascular endothelial dysfunction (26, 78, 79, 104, 119). Many of the benefits of aerobic exercise for improving CV function are mediated by reducing oxidative stress and inflammation and enhancing mitochondrial content/function (30, 33, 41, 73, 90, 100, 103, 126).
Further benefits from aerobic exercise are conferred by improvements in autonomic function (i.e., increased parasympathetic nervous system activity and region-specific changes in sympathoadrenal activity), which may contribute to the lower resting heart rate and blood pressure characteristic of aerobically trained individuals. The mechanisms through which aerobic exercise modulates autonomic control have been reviewed in detail elsewhere (38, 106).
Despite a preponderance of evidence demonstrating the beneficial effects of continuous aerobic exercise training modalities for preserving CV function with aging, adherence to physical activity guidelines is poor, particularly in subgroups with lower education and income. Some analyses estimate that only 20–40% of midlife and older adults are sufficiently active to meet current weekly physical activity recommendations (61, 102), whereas others suggest much lower rates of adherence (<5%; see Ref. 122). Therefore, there is a biomedical research need for both physiological studies to establish efficacy and behavioral studies to establish viable strategies to promote physical activity adherence.
A major barrier to physical activity in midlife and older adults is a perceived lack of time (118). As emphasized in a recent National Institutes of Health funding opportunity announcement (PA-18–849), this barrier is a particular problem for many midlife men and women, because this is the period of life in which both family and professional responsibilities tend to peak (31, 91). As such, there is a compelling need to develop time-efficient evidence-based alternative approaches to conventional (continuous) aerobic exercise training for maintaining CV health with aging. Ideally, such time-efficient interventions should have the potential for promoting strong adherence while also improving CV function.
With these criteria in mind, interval-based aerobic exercise training and inspiratory muscle strength training (IMST) represent two promising modes of time-efficient physical training that may improve CV function in midlife and older adults (Fig. 1). However, despite some intriguing findings to date on these forms of training (reviewed below), there are still many knowledge gaps that must be filled before these interventions can be fully recommended for improving CV function with aging. Therefore, this review has two primary goals. The first is to summarize the current knowledge regarding the efficacy of interval training and IMST to enhance CV function and promote adherence in the context of healthy aging. However, because research is limited in healthy midlife and older adults, we will attempt to provide additional insight by also discussing results from young adults or populations with CV disease and dysfunction where available. Second, we identify research gaps and associated future directions that need to be addressed to fully understand the efficacy of these interventions for improving CV function in midlife and older adults.
INTERVAL TRAINING
Definitions
Broadly speaking, interval training is defined as alternating bouts of intense effort with periods of recovery within a single training session (Fig. 2). Within interval training, high-intensity interval training (HIIT) is often characterized as vigorous but submaximal efforts that elicit at least 80% of maximum heart rate (128). A commonly studied HIIT protocol is 4 × 4-min intervals interspersed with 3 min of active recovery between exercise bouts (121). HIIT is typically distinguished from a more vigorous version, sprint interval training (SIT), which involves ‘all out’ efforts or an absolute workload that exceed one’s maximal rate of oxygen consumption (V̇o2max; see Ref. 128). An example SIT protocol is four to six repeats of 30-s maximal cycling efforts (repeated Wingate tests) with 4.5 min of recovery between exercise bouts (11). It is important to note that the definitions of HIIT and SIT have not been universally adopted, and studies published using the HIIT acronym may more appropriately be described as SIT, or vice versa.
Fig. 2.
Representative duration and intensity of sprint interval training (SIT), high-intensity interval training (HIIT), and moderate-intensity continuous training (MICT) aerobic exercise protocols.
Effects of Interval Training Paradigms on CV Function
Work-matched interval training.
Many studies that compare interval training with continuous aerobic exercise use work-matched designs in which average intensity, energy expenditure, and/or total session duration are the same between interval- and continuous-exercise protocols. Work-matched protocols have the advantage of removing the potentially confounding effects of differing energy expenditure on physiological adaptations. Work-matched interval training usually involves some time savings compared with moderate-intensity continuous training, which may reduce the time-availability barrier faced by busy midlife adults. However, the time savings are not as great as with time-efficient low-volume interval training (see Time-Efficient Low-Volume Interval Training). Nevertheless, much of what has been learned about the efficacy of interval training for improving CV function has come from work-matched study designs and will be reviewed here.
Cardiorespiratory fitness.
Work-matched interval training effectively increases cardiorespiratory fitness in midlife and older adults with comorbidities, and this improvement is often larger in magnitude than that observed in response to moderate-intensity continuous aerobic exercise training (45, 128). In contrast, the effects of interval training on cardiorespiratory fitness have not been investigated as extensively as in healthy midlife and older adults. In one study, adults aged 59–75 who performed all-extremity (i.e., combined upper- and lower-body rhythmic exercise) interval training 4 days/wk for 8 wk improved their V̇o2max by 11% compared with no improvement in a group performing moderate-intensity continuous training (55).
Cardiac function.
The influence of interval training on cardiac function primarily has been investigated in patients with heart disease, as reviewed elsewhere (19, 39, 87). For example, 12 wk of interval training 3 days/wk improves left ventricular end-diastolic diameter in heart failure patients with reduced ejection fraction (32) and reverses ventricular remodeling and improves left ventricular systolic function in patients with postinfarction heart failure (131). Importantly, for the present discussion, 8 wk of all-extremity interval training (4 days/wk) significantly improves ejection fraction at rest in older adults without heart failure or other clinical disease (55). These studies support the notion that interval training has the potential to improve age- and/or disease-related cardiac dysfunction (Fig. 3).
Fig. 3.
Likely mechanisms through which interval training improves cardiovascular function. SIT, sprint interval training; HIIT, high-intensity interval training; ROS, reactive oxygen species.
Systolic blood pressure.
Work-matched interval training also induces clinically significant reductions in SBP in healthy midlife and older adults (Fig. 3). Three months of interval walking 4 days/wk lowered SBP by 8 mmHg in previously sedentary adults with a mean age of 54, whereas no changes were observed in an age-matched sedentary control group (69). Similarly, in a randomized interval walking study, 5 mo of interval walking 4 days/wk lowered SBP by 10 mmHg in healthy midlife to older adults, whereas SBP was decreased by only 3 mmHg in a group that performed the same frequency of moderate-intensity continuous walking (81). Moreover, 16 wk of uphill interval walking 3 days/wk lowered SBP by 9 mmHg in adults with metabolic syndrome, a group at elevated risk of CVD (121). This reduction in SBP was potentially linked to decreased oxidative stress, as indicated by a reduction in plasma oxidized low-density lipoprotein (LDL) concentrations (121) (Fig. 3). Taken together, work-matched interval training, when implemented in midlife and older adults, can reduce SBP, and the magnitude of the reduction exceeds that obtained with moderate-intensity continuous aerobic exercise training.
Vascular function.
Work-matched interval training also has been reported to improve arterial function in certain groups (Fig. 3). In heart failure patients, 12 wk of interval walking three times per week improved endothelial function [~9% improvement in brachial artery flow-mediated dilation (FMD)], likely by decreasing oxidative stress (Fig. 3) and increasing antioxidant defenses (131). This improvement in endothelial function was significantly greater than the ~4% increase in FMD observed in patients who performed moderate-intensity continuous training 3 days/wk (131). Similarly, in adults with metabolic syndrome, 16 wk of interval training three times per week elicited a 9% improvement in FMD, which was greater than the 5% increase observed in response to 3 days/wk of continuous moderate-intensity aerobic exercise training (121). The improvements in endothelial function were associated with improved sensitivity to insulin and lower blood glucose concentrations, along with the aforementioned reductions in plasma oxidized LDL (121). However, a potentially important consideration of the results of these studies is that FMD was performed using the upper-arm occlusion technique (121, 131). The latter may produce a dilation that is less mediated by NO than the lower-arm occlusion approach (29). Thus, the improvements in endothelial function with work-matched interval training cannot necessarily be attributed to improvements in NO bioavailability. Taken together, these studies demonstrate the potential benefits of interval training on vascular endothelial function in groups with increased CVD risk, although the role of NO requires further elucidation.
The effects of interval training on arterial stiffness are somewhat unclear at the moment. In young adults, 6 wk of interval training (3 days/wk) decreased arterial stiffness and improved baroreflex control of heart rate (94) (Fig. 3). Similarly, 16 wk of interval training (3 days/wk) decreased arterial stiffness and plasma norepinephrine, a marker of sympathetic nerve activity, in young women (17) (Fig. 3). In contrast, in older adults, 8 wk of moderate-intensity continuous exercise (4 days/wk) decreased arterial stiffness, as indicated by a reduction in carotid-femoral pulse wave velocity (PWV; see Ref. 62), whereas the interval training group, which performed 4 × 4-min intervals with 3-min active recovery periods four times per week, exhibited no change in PWV. Intervention studies assessing the effects of 3 mo of continuous aerobic exercise training find improvements in arterial stiffness in healthy midlife adults (78, 119); however, results are not as consistent in older (>65 yr) adults (85, 89). Presently, it is unknown if interval training might induce consistent decreases in arterial stiffness in older men and women.
Time-Efficient Low-Volume Interval Training
There is accumulating evidence that work-matched interval training may improve CV function in midlife and older healthy adults and/or clinical populations. Again, although work-matched protocols have some time-saving benefits compared with conventional aerobic exercise, the required duration may still pose a barrier to exercise adherence. Because time availability is a primary deterrent to exercise adherence (75, 82), there is growing interest in the potential for so-called “low-volume” (i.e., time-efficient) interval training protocols to elicit benefits despite requiring a relatively low time commitment, e.g., ~30 min/wk or less. Such protocols involve higher exercise intensities compared with conventional aerobic exercise training programs, often requiring the maximal or supramaximal efforts associated with SIT regimens. To date, very few studies have investigated the influence of low-volume interval training on CV function in healthy midlife and older adults. The published literature on this form of interval training is summarized below.
Cardiorespiratory fitness.
Low-volume time-efficient interval training protocols improve cardiorespiratory fitness in healthy young adults by ~8% on average (42). The effect of low-volume (high-intensity) interval training on cardiorespiratory fitness in midlife and older adults has not been established. A recent study found that 6 wk of low-volume interval training, 3 days/wk, improved V̇o2max by ~3% and ~8% in midlife and older women and men, respectively (112). In another small study that included six midlife to older adults, 6 wk of SIT, 2 days/wk (requiring <30 min/wk), improved V̇o2max by 8% (3). Thus, low-volume interval training has been shown to improve cardiorespiratory fitness in young adults, and there is preliminary evidence for efficacy in midlife and older adults.
Cardiac function and SBP.
Despite the ability of low-volume interval training protocols to improve cardiorespiratory fitness, improvements in cardiac function and reductions in SBP have not been consistently demonstrated in response to these protocols (49, 108). It is thought that larger training volumes may be required to induce changes in these domains of CV function (40). However, 6 wk of SIT performed two times per week lowered SBP by 7 mmHg in older adults with stage 2 systolic hypertension (3), suggesting potential efficacy of low-volume interval training in those with high initial levels of SBP, although the mechanisms remain to be determined.
Arterial function.
There is some evidence to suggest that time-efficient low-volume interval training is an adequate stimulus to induce benefits in arterial function. Reductions in arterial stiffness, estimated from decreases in brachial-ankle PWV, and improved endothelial function, measured with brachial artery FMD, have been reported in obese preadolescent boys after 12 wk of interval training (3 days/wk; see Ref. 16). Consistent with these findings, 12 wk of low-volume interval training (2 days/wk) improved endothelial function in patients with coronary artery disease (21) (Fig. 4), and 6 wk of SIT training (4 times per week) reduced carotid-femoral PWV (aortic stiffness) in healthy young adults, potentially by increasing NO bioavailability, assessed as plasma nitrate/nitrite levels (49). In both studies (21, 49), the improvements in vascular function in response to low-volume interval training were equivalent to those induced by moderate-intensity continuous training. However, low-volume interval training has not improved arterial function in all cases. For example, endothelial function was improved in young healthy subjects after 12 wk of moderate-intensity continuous training (3 days/wk) but was unchanged in a group that performed very low-volume SIT (3, 20-s intervals/session) for the same treatment duration and frequency (108). Collectively, these results support the idea that low-volume interval training may improve arterial function in healthy adults and in patients with CVD (Fig. 3), although there may be a volume of exercise training below which the training stimulus is inadequate to induce favorable adaptations.
Fig. 4.

Improvement in vascular endothelial function, assessed via brachial artery flow-mediated dilation (FMD), following 6 wk of either moderate-intensity continuous training (MICT) or low-volume sprint interval training (SIT) in patients with coronary artery disease. Data are means ± SE. *P < 0.05 compared with baseline. Data are from Currie et al. (21).
Participant Adherence
The psychosocial responses to interval training, and how these responses influence adherence, have been reviewed in depth previously (84, 117). In general, affect (the outward expression of feelings and emotion) is similar to or more negative when performing a bout of interval training compared with performing continuous aerobic exercise (117). However, affect is the same following interval or continuous training (117), indicating the relatively negative feelings do not persist after completing an interval training session. Exercise enjoyment is similar or greater following interval training compared with continuous aerobic exercise, which may be linked to stronger participant preference for interval versus continuous training (84, 117). These overall positive findings suggest that interval training is a viable strategy to promote exercise adherence.
Adherence rates (i.e., percent of total possible sessions successfully completed) to interval training by midlife and older adults in studies where participation is supervised by researchers generally exceeds 70% (74, 109, 116), which is generally higher than participation to conventional aerobic exercise. This high rate of adherence might be explained, at least in part, by the favorable psychosocial attitudes toward interval training (84). However, adherence in supervised studies bears little resemblance to the real world, where adherence to interval training may be significantly lower. Therefore, more research on the adherence of midlife and older adults to interval training under free-living conditions is needed.
One concern about interval training is that some sedentary adults may not perceive it as appropriate for individuals of their age and baseline fitness level. Indeed, there is evidence that even adults who have successfully participated in interval training still view it as only appropriate for very fit individuals (63). Additional studies supporting the safety and efficacy of interval training in older adults may overcome this barrier to initiation/adoption of interval training.
Overall, the promising rates of adherence from supervised studies and predominantly positive findings regarding the psychosocial perceptions of interval training support further investigation of the potential for these paradigms to improve CV function in midlife and older adults. To date, most research on the psychosocial responses and adherence to interval training has been done on adults younger than 50 yr old. Therefore, more investigation is still needed in midlife and older adults. Some psychosocial barriers to initiating interval training programs may exist and will need to be addressed.
Interval Training: Research Gaps
Interval training, particularly low-volume time-efficient paradigms, shows strong promise for promoting adherence and improving CV function in midlife and older adults. However, presently there are several limitations that will require further investigation before low-volume interval training can be considered an evidence-based strategy for improving CV health in this population (Fig. 5).
Fig. 5.
Research gaps and future directions for interval training research. RCT, randomized controlled trial; CV, cardiovascular; CVD, cardiovascular diseases; PM, postmenopausal; TM, treadmill.
Although time-efficient low-volume interval training protocols have been shown to increase cardiorespiratory fitness and CV function in some cohorts, the efficacy of these time-efficient protocols to elicit improvements in midlife and older healthy adults, as well as those with risk factors and/or clinical CVD, needs to be established in a randomized controlled trial with a sufficiently large sample size. Determining the dose-response characteristics of interval training that can improve CV function will be an important goal in such studies.
Some of the more significant barriers to initiation/adoption of interval training by older adults revolve around issues of safety and the risk of adverse events. This concern is fueled by the knowledge that intense exercise transiently increases the risk of adverse CV events (120). However, numerous investigations show that interval training, even when performed at very high intensities, generally is safe for healthy adults and for clinical populations (37, 54, 97, 128). Although individual medical evaluation before participating in exercise is advised, it appears that the safety concerns associated with interval training are not greater than those associated with continuous moderate-intensity aerobic exercise. That said, concerns for implementing interval training in older adults persist. For example, one report suggests that seniors have a transient increase in the risk of falls after an interval training session (27). Others found older adults require a longer period for recovery after an interval training session than young adults (i.e., 5 versus 3 days) (51). Such age-related differences in recovery periods, if confirmed, might influence the design of interval training programs for young versus older adults. In general, more information is needed on the adherence, safety, and tolerability of interval training for various populations of midlife and older adults. Nevertheless, current research suggests that interval training is safe for most individuals.
As mentioned previously, a barrier to participating in interval training is a perception that it is only appropriate for highly fit adults and not for sedentary individuals seeking health benefits (63). However, there are promising accessible modes of interval training that are likely appropriate and less intimidating for older adults to perform. Recently, stair-climbing protocols have been established for use in healthy young adults (4, 56) and patients with diabetes (43), and improve cardiorespiratory fitness in the former group (4, 56). Stairs are very accessible, and intervals can be done at one’s own pace. Moreover, stairs are a part of daily living. As such, it should be possible to incorporate short sporadic bouts of high-intensity stair climbing into daily activities to promote adherence and improvements in cardiorespiratory fitness (56, 114). Thus, stair climbing represents a promising, but presently understudied, mode of interval training to improve CV function in older adults.
Walking was the interval exercise mode of choice for older adults in the Generation 100 study, a large 5-yr population-based study comprised of adults 70–76 yr old at baseline (95). However, the Generation 100 study used an interval training protocol requiring nearly 40 min/session to complete (115). Similar time-intensive interval walking protocols have been shown to improve cardiorespiratory fitness and lower SBP in older adults (81), suggesting efficacy for interval walking. However, we are not aware of any study that has examined the efficacy of a time-efficient interval walking program to improve CV function in healthy midlife and older adults. In such trials, it will be important to determine whether older adults can achieve sufficiently high workloads while walking to accrue benefits from low-volume interval training. Modifications, such as uphill walking, may be necessary to allow midlife and older adults to achieve the requisite workloads/intensities.
Compared with the current knowledge about continuous aerobic training, very little is known about the effect of work-matched and time-efficient interval training on resting SBP. Mechanistic studies are also needed to further elucidate how interval training improves CV function, since our mechanistic understanding currently lags behind our knowledge of efficacy for functional outcomes. A greater understanding of the mechanistic signals will help create interval training programs that elicit the largest physiological benefit with a level of effort and time commitment that promote adherence. Application of new omics-based analyses offers a particularly exciting avenue to understand how interval training signals changes in CV function (96).
Finally, there is a need to investigate mixed training models that use both moderate-intensity continuous aerobic training and interval training that address the benefits and limitations of both forms of exercise. These mixed training models should also be extended to include resistance exercise, which may also be done in an interval format. For example, a CrossFit style strength-training program was recently shown to improve insulin sensitivity in overweight midlife adults with type 2 diabetes (34). Resistance training is a key component of physical activity guidelines for older adults (5, 91), given the clinical importance of maintaining strength, power, and muscle mass to prevent age-related sarcopenia and preservation of physical function. As such, we need to understand how to optimally combine continuous and interval aerobic exercise and resistance exercise to protect CV health, while maintaining lean mass and neuromuscular function for healthy overall aging.
INSPIRATORY MUSCLE STRENGTH TRAINING
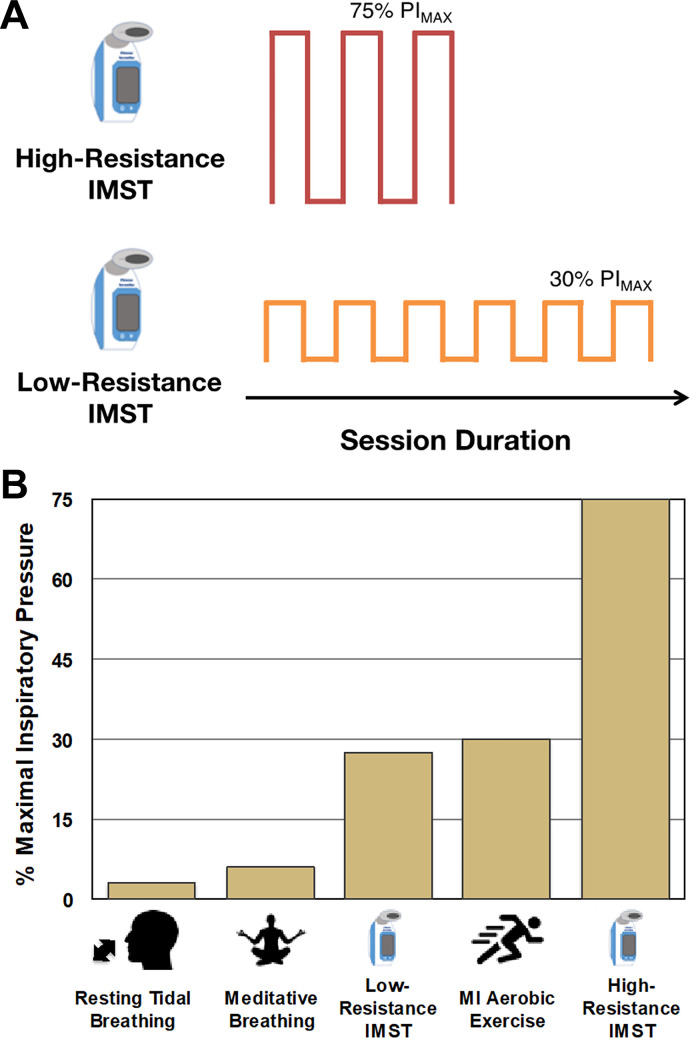
IMST consists of performing repeated inspirations against resistance with unopposed (no resistance) expirations (Fig. 6A). IMST is performed using either a constant resistance or a pressure-threshold device. Constant resistance devices use a narrowed airway to produce resistance. In contrast, the airflow through pressure-threshold devices is regulated by a valve that fully occludes airflow until a prescribed threshold is exceeded, at which point the valve opens to allow airflow. Regardless of the type of training device, IMST requires the user to develop inspiratory pressures that are significantly greater than those produced during tidal breathing or meditative deep breathing, and similar to or much greater than inspiratory pressures produced during maximal aerobic exercise (Fig. 6B) (36, 47, 124). Based on data from young adults, it is the repeated generation of large negative intrathoracic pressures rather than large changes in lung volume that likely serve as the stimulus for IMST-induced changes in CV function (124).
Fig. 6.
A: representative duration and intensity of high- and low-resistance inspiratory muscle strength training (IMST) protocols. B: inspiratory pressures associated with various physical activities. High-resistance IMST is a novel form of physical activity requiring inspiratory pressures much greater than other activities. PIMAX, maximal inspiratory pressure; MI, moderate intensity.
Most research investigating the health-related outcomes of IMST has focused on patients with chronic disease (70, 101, 111, 133) or as an adjunctive therapy for cardiac rehabilitation (18, 52). Within these groups, IMST has been shown to be safe, supporting its use by midlife and older adults.
Time-Intensive IMST
Many IMST studies have used training protocols with time requirements similar to conventional aerobic exercise (i.e., ≥150 min/wk; see Refs. 36, 60, and 76). In general, in these types of protocols, subjects train at or near 30% of their maximal inspiratory pressure (PIMAX), i.e., modest resistance to inspiration. These time-intensive IMST protocols provide little to no time-saving benefit compared with continuous aerobic exercise. However, a majority of what is known about IMST comes from time-intensive protocols and will be discussed here.
Cardiorespiratory fitness.
In heart failure patients, the addition of time-intensive IMST to usual care produced greater improvements in cardiorespiratory fitness, measured with the 6-min walk test (18, 101, 133) and maximal exercise tests (22, 86), compared with standard care alone. Some suggest that IMST improves cardiorespiratory fitness by increasing respiratory muscle efficiency, demonstrated through reductions in the ventilation/V̇co2 slope during graded exercise, which is a marker of ventilatory efficiency (86, 111). Others postulate that IMST-related strengthening of the diaphragm and accessory respiratory muscles improves ventilatory mechanics, which can decrease respiratory muscle metaboreflex activation and the resultant redistribution of blood away from the locomotor muscles (25, 127).
Cardiac function.
Despite efficacy for improving cardiorespiratory fitness, the effect of time-intensive IMST on cardiac function (e.g., left ventricular end-diastolic diameter, ejection fraction) in patients with heart failure has produced largely negative results. Two investigations of 12 wk of IMST found no improvements in cardiac function above standard care in patients with heart failure (2, 86). The effect of time-intensive IMST on cardiac function has not been evaluated in healthy midlife and older adults.
Systolic blood pressure.
To our knowledge, only one study has investigated the influence of low-resistance IMST on SBP as a primary outcome. Eight weeks of IMST performed at 30% PIMAX for 30 min every day lowered daytime SBP, measured with ambulatory monitoring, by ~9 mmHg in midlife and older adults with hypertension; there was no change in nighttime blood pressure. This reduction in SBP was associated with reductions in the low-frequency component of heart rate variability, an indirect (and somewhat controversial) measure of cardiac sympathetic nerve activity (36). In patient populations, IMST has been reported to change indexes of heart rate variability interpreted as reflecting reductions in cardiac sympathetic nerve activity and increases in cardiac parasympathetic nerve activity (1). However, these results remain to be confirmed in healthy midlife and older adults.
Arterial function.
We are not aware of any studies that have assessed the effects of IMST on arterial function. However, a respiratory muscle training protocol implemented in healthy young women that entailed breathing against inspiratory and expiratory resistances evaluated the effect of training on endothelial function. After 8 wk (3 times per week), there was no evidence of a change in endothelial function measured with brachial artery FMD. Note, however, that, because the women exhibited exceptionally high baseline FMD (>10%), they had optimal endothelial function before initiating training (10). Two other studies have evaluated the effect of IMST on markers of endothelial function. One used strain gauge plethysmography reactive hyperemia to assess the effect of 10 wk IMST (3 days/wk) on forearm vascular reactivity in adults with chronic heart failure and found no improvement (71). A second study assessed circulating molecules involved in endothelial activation and markers of oxidative stress in hemodialysis patients before and after 8 wk of IMST performed three times per week and found no changes in markers of endothelial activation (vascular cell adhesion molecule and intercellular adhesion molecule) or oxidative stress (malondialdehyde) (12). Thus, we need more information about the potential for IMST to impact endothelial function and arterial stiffness in any population, including healthy midlife and older adults.
The promising results related to improvements in cardiorespiratory fitness and SBP suggest that time-intensive IMST might be an effective lifestyle intervention to improve CV function. However, as emphasized above, because low-resistance IMST protocols entail time commitments comparable to conventional aerobic exercise, this form of training may not result in better adherence, particularly among busy midlife adults.
Time-Efficient IMST Interventions
More recently, several studies have implemented a high-resistance (~75% PIMAX) IMST protocol comprising 30 breaths/day and a total daily time commitment of just ~5–7 min (24, 70, 124, 125). Healthy adults performing these protocols generally produce inspiratory pressures in the range of 50–70 mmHg, which is at a minimum two to three times greater than inspiratory pressures produced during maximal aerobic exercise (Fig. 6B) (24). Interestingly, many of these higher-resistance time-efficient IMST protocols follow an “interval training” format consisting of five sets of six breaths, with each set interspersed with a 1- to 2-min rest period.
Because time-efficient high-resistance IMST protocols are relatively new, results regarding potential effects on cardiorespiratory fitness, cardiac function, and arterial function are not yet available, although at least one trial assessing such outcomes in healthy midlife and older adults is underway (NCT03266510). Nevertheless, exciting results have recently emerged from a series of small intervention studies conducted by Bailey and colleagues to determine the effects of time-efficient IMST on resting (casual) SBP. They found that 6 wk of high-resistance IMST (30 breaths/day, 5–7 days/wk, 75% PIMAX) lowered SBP by 4–10 mmHg in healthy young men and women with normal levels of SBP (<120 mmHg) at baseline (24, 124) (Fig. 7). Bailey and colleagues observed even larger effects on SBP (−12 mmHg on average) in response to a similar 6-wk 7 day/wk IMST intervention in patients with obstructive sleep apnea (Fig. 7). This marked decrease in resting SBP was associated with a reduction in plasma norepinephrine concentrations, suggesting a decrease in sympathetic nerve activity with high-resistance IMST in this group (125).
Fig. 7.
A: resting brachial artery systolic blood pressure (SBP) before and after 6 wk of time-efficient inspiratory muscle strength training (IMST). B: absolute change in resting SBP following 6 wk of time-efficient IMST or low-resistance sham training. Subjects were either young adults or midlife/older patients with obstructive sleep apnea (OSA). Data are means ± SE. *P < 0.05 compared with baseline. Data are from Vranish and Bailey (124), DeLucia et al. (24), and Vranish and Bailey (125).
It is important to note that the reductions in SBP observed in response to high-resistance IMST are larger than the <5 mmHg reductions typically observed with conventional moderate-intensity aerobic exercise training (20, 130). Decreases in SBP are apparent within the first 3 wk of beginning a time-efficient IMST protocol (125), which may be quicker than reductions elicited by conventional aerobic exercise training (91). The large decrease in SBP over such a short time course makes IMST an intriguing lifestyle intervention to lower SBP.
Whereas conventional aerobic exercise training may improve baroreflex control of heart rate and/or lower resting heart rate in some groups (66, 77), the limited information available on time-efficient IMST suggests no obvious effects on these functions (24, 124, 125). The lack of a clear effect of time-efficient IMST on heart rate could be interpreted as evidence against an augmentation of cardiac parasympathetic tone, as previously reported in response to time-intensive IMST (1). However, it is worth emphasizing here that the influence of time-efficient IMST on resting heart rate and its regulation has not been studied in healthy midlife and older adults. A recent study found that time-efficient IMST reduces blood pressure via reductions in peripheral vascular resistance, with no change in cardiac output (24). This decrease in peripheral vascular resistance suggests the possibility of IMST-mediated changes in the vascular function. In this context, high-resistance IMST generates large negative inspiratory pressures (124), which are greater than those produced during both lower-resistance IMST and maximal aerobic exercise (25, 47). Such large repeated fluctuations in inspiratory pressures during high-resistance IMST protocols may create unique hemodynamic stimulus evoking adaptations in the large elastic arteries (aorta and carotid arteries) and heart (cardiothoracic region), as well as changes in systemic circulatory shear stress that may induce improvements in NO-mediated endothelial function (Fig. 8).
Fig. 8.
Hypothesized acute and chronic physiological changes associated with inspiratory muscle strength training. SBP, systolic blood pressure; NO, nitric oxide.
Participant Adherence
In short-term studies (6 wk) that require subjects to undertake supervised training in a laboratory (24, 124, 125), adherence rates are excellent (i.e., >90%). In one of the first studies to assess home-based training (training sessions performed unsupervised outside the laboratory), adherence to the IMST program (30 breaths, 2 times per day, 12 wk) was 67%. Importantly, when participants were provided with automated internet-based feedback, adherence rates increased to 87% (113). In a second recent study in older adults, adherence to unsupervised training (30 breaths, 2 times per day, 8 wk) was 76% (35). Given the requirement of twice daily training, these rates of adherence are quite promising. Overall, these preliminary data regarding adherence warrant the consideration of IMST as a novel lifestyle intervention to improve CV function in midlife and older adults.
IMST: Research Gaps
Much more information is needed regarding the ability of time-efficient IMST to improve cardiorespiratory fitness, cardiac performance, and/or arterial function (Fig. 9). Of particular importance is the potential for high-resistance time-efficient protocols to improve CV function among midlife and older adults. Although the SBP-lowering effects of IMST reported in young adults and patients with obstructive sleep apnea are promising, these observations require confirmation in healthy midlife and older adults and patients with age-associated clinical disorders, especially chronic CVD.
Fig. 9.
Research gaps and future directions regarding high-resistance inspiratory muscle strength training research. RCT, randomized controlled trial; IMST, inspiratory muscle strength training; CR, cardiorespiratory; CV, cardiovascular; PM, postmenopausal.
Currently there is strong and growing interest in strategies to preserve and/or improve brain health and cognitive performance with aging (6, 44). It now appears certain that age-associated declines in brain structure and cognitive performance are linked to impaired cerebrovascular function (7, 14, 46, 83). Accordingly, research is needed to determine if IMST, like other forms of exercise, confers improvements in cerebrovascular function and whether such improvements are associated with enhanced cognitive performance and brain health. This also should be an important goal for the interval training paradigms involving aerobic exercise modalities discussed earlier in this review.
Along with establishing the efficacy of IMST, the mechanisms responsible for any beneficial effects require elucidation. It is likely that the large inspiratory pressures generated by IMST serve as the stimulus for lowering SBP and producing other CV benefits (124). Results from the initial studies suggest that IMST-associated decreases in sympathetic nerve activity and peripheral resistance may be involved in the impressive reductions in SBP observed (24, 125). However, impairments in CV function with aging are mediated largely by increases in oxidative stress and chronic low-grade inflammation (28, 88). Presently, there is no information on whether IMST improves CV health by reducing oxidative stress and/or inflammation.
How an individual performs IMST may also have an important influence on CV function and training outcomes. To date, most time-efficient IMST protocols have used an interval-like format comprising training sets (6 inspiratory efforts) followed by a brief rest period between sets (24, 124, 125). Such interval-like protocols potentially are more efficacious than training without a break because the user is allowed time to recover and therefore can generate larger, i.e., more negative, inspiratory pressures in each training set.
If high-resistance time-efficient IMST is effective at improving CV function in midlife and older adults, studies evaluating enjoyment, adherence, and safety in real-world settings will be critical to determine IMST’s potential as a safe, effective, and adherable intervention for use by the general public.
Last, there is compelling evidence that conventional moderate-intensity continuous aerobic exercise training may not induce the same CV adaptations in postmenopausal women compared with men of similar age (79, 90, 107). As such, an important goal of future research involving IMST, as well as interval training, is to determine if these novel forms of physical training are efficacious for improving CV function in both midlife/older men and postmenopausal women. If so, it is possible that these interventions may hold advantages for preserving CV health with aging in women.
CONCLUSIONS
Based on the increasing number of midlife and older adults and the high prevalence of CVD in that population, developing novel exercise interventions that improve critical determinants of CV health (cardiac performance, arterial function, SBP, etc.) and promote adherence is an important biomedical research priority. Both interval training and IMST are innovative exercise interventions that can be applied with time-efficient (~30 min/wk) protocols. Initial results regarding time-efficient interval training and IMST on CV function are promising, but much more research is needed on both of these approaches.
GRANTS
This research was supported by an American Heart Association Postdoctoral Fellowship 18POST33990034 (D. H. Craighead).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.H.C. and D.R.S. conceived and designed research; D.H.C., T.C.H., and M.N.H. drafted manuscript; D.H.C., T.C.H., M.N.H., E.F.B., M.J.M., M.J.G., and D.R.S. edited and revised manuscript; D.H.C., T.C.H., M.N.H., E.F.B., M.J.M., M.J.G., and D.R.S. approved final version of manuscript; T.C.H. and M.N.H. prepared figures.
REFERENCES
- 1.de Abreu RM, Rehder-Santos P, Minatel V, Dos Santos GL, Catai AM. Effects of inspiratory muscle training on cardiovascular autonomic control: A systematic review. Auton Neurosci 208: 29–35, 2017. doi: 10.1016/j.autneu.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Adamopoulos S, Schmid J-P, Dendale P, Poerschke D, Hansen D, Dritsas A, Kouloubinis A, Alders T, Gkouziouta A, Reyckers I, Vartela V, Plessas N, Doulaptsis C, Saner H, Laoutaris ID. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: The Vent-HeFT trial: a European prospective multicentre randomized trial. Eur J Heart Fail 16: 574–582, 2014. doi: 10.1002/ejhf.70. [DOI] [PubMed] [Google Scholar]
- 3.Adamson SB, Lorimer R, Cobley JN, Babraj JA. Extremely short-duration high-intensity training substantially improves the physical function and self-reported health status of elderly adults. J Am Geriatr Soc 62: 1380–1381, 2014. doi: 10.1111/jgs.12916. [DOI] [PubMed] [Google Scholar]
- 4.Allison MK, Baglole JH, Martin BJ, Macinnis MJ, Gurd BJ, Gibala MJ. Brief intense stair climbing improves cardiorespiratory fitness. Med Sci Sports Exerc 49: 298–307, 2017. doi: 10.1249/MSS.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 5.American College of Sports Medicine; Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 41: 1510–1530, 2009. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 6.Barnes JN. Exercise, cognitive function, and aging. Adv Physiol Educ 39: 55–62, 2015. doi: 10.1152/advan.00101.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes JN, Corkery AT. Exercise improves vascular function, but does this translate to the brain? Brain Plast 4: 65–79, 2018. doi: 10.3233/BPL-180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 9.Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B, Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol 64: 1257–1266, 2014. doi: 10.1016/j.jacc.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisconti AV, Devoto M, Venturelli M, Bryner R, Olfert IM, Chantler PD, Esposito F. Respiratory muscle training positively affects vasomotor response in young healthy women. PLoS One 13: e0203347, 2018. doi: 10.1371/journal.pone.0203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos NG, Marizeiro DF, Florêncio ACL, Silva ÍC, Meneses GC, Bezerra GF, Martins AMC, Libório AB. Effects of respiratory muscle training on endothelium and oxidative stress biomarkers in hemodialysis patients: A randomized clinical trial. Respir Med 134: 103–109, 2018. doi: 10.1016/j.rmed.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116: 736–745, 2014. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong JSX, Liu S, Loke YM, Hilal S, Ikram MK, Xu X, Tan BY, Venketasubramanian N, Chen CL-H, Zhou J. Influence of cerebrovascular disease on brain networks in prodromal and clinical Alzheimer’s disease. Brain 140: 3012–3022, 2017. doi: 10.1093/brain/awx224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrysant SG, Chrysant GS. The age-related hemodynamic changes of blood pressure and their impact on the incidence of cardiovascular disease and stroke: new evidence. J Clin Hypertens (Greenwich) 16: 87–90, 2014. doi: 10.1111/jch.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuensiri N, Suksom D, Tanaka H. Effects of high-intensity intermittent training on vascular function in obese preadolescent boys. Child Obes 14: 41–49, 2018. doi: 10.1089/chi.2017.0024. [DOI] [PubMed] [Google Scholar]
- 17.Ciolac EG, Bocchi EA, Bortolotto LA, Carvalho VO, Greve JM, Guimarães GV. Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res 33: 836–843, 2010. doi: 10.1038/hr.2010.72. [DOI] [PubMed] [Google Scholar]
- 18.Cordeiro ALL, de Melo TA, Neves D, Luna J, Esquivel MS, Guimarães ARF, Borges DL, Petto J. inspiratory muscle training and functional capacity in patients undergoing cardiac surgery. Braz J Cardiovasc Surg 31: 140–144, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelis J, Beckers P, Taeymans J, Vrints C, Vissers D. Comparing exercise training modalities in heart failure: a systematic review and meta-analysis. Int J Cardiol 221: 867–876, 2016. doi: 10.1016/j.ijcard.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2: e004473, 2013. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie KD, Dubberley JB, McKelvie RS, MacDonald MJ. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc 45: 1436–1442, 2013. doi: 10.1249/MSS.0b013e31828bbbd4. [DOI] [PubMed] [Google Scholar]
- 22.Dall’Ago P, Chiappa GRS, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 47: 757–763, 2006. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 23.Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 73: 2209–2225, 2019. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 24.DeLucia CM, De Asis RM, Bailey EF. Daily inspiratory muscle training lowers blood pressure and vascular resistance in healthy men and women. Exp Physiol 103: 201–211, 2018. doi: 10.1113/EP086641. [DOI] [PubMed] [Google Scholar]
- 25.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol 151: 242–250, 2006. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 26.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 27.Donath L, Kurz E, Roth R, Hanssen H, Schmidt-Trucksäss A, Zahner L, Faude O. Does a single session of high-intensity interval training provoke a transient elevated risk of falling in seniors and adults? Gerontology 61: 15–23, 2015. doi: 10.1159/000363767. [DOI] [PubMed] [Google Scholar]
- 28.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 29.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001. doi: 10.1042/cs1010629. [DOI] [PubMed] [Google Scholar]
- 30.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Ansari W, Lovell G. Barriers to exercise in younger and older non-exercising adult women: a cross sectional study in London, United Kingdom. Int J Environ Res Public Health 6: 1443–1455, 2009. doi: 10.3390/ijerph6041443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen A-I, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk-Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A; SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group . High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 135: 839–849, 2017. doi: 10.1161/CIRCULATIONAHA.116.022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fealy CE, Nieuwoudt S, Foucher JA, Scelsi AR, Malin SK, Pagadala M, Cruz LA, Li M, Rocco M, Burguera B, Kirwan JP. Functional high-intensity exercise training ameliorates insulin resistance and cardiometabolic risk factors in type 2 diabetes. Exp Physiol 103: 985–994, 2018. doi: 10.1113/EP086844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraro FV, Gavin JP, Wainwright T, McConnell A. The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: a randomized, double-blind, placebo-controlled study. Physiol Rep 7: e14076, 2019. doi: 10.14814/phy2.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira JB, Plentz RDM, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol 166: 61–67, 2013. doi: 10.1016/j.ijcard.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 37.Francois ME, Little JP. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr 28: 39–44, 2015. doi: 10.2337/diaspect.28.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Q, Levine BD. Exercise and the autonomic nervous system. Handb Clin Neurol 117: 147–160, 2013. doi: 10.1016/B978-0-444-53491-0.00013-4. [DOI] [PubMed] [Google Scholar]
- 39.Gayda M, Ribeiro PAB, Juneau M, Nigam A. Comparison of different forms of exercise training in patients with cardiac disease: where does high-intensity interval training fit? Can J Cardiol 32: 485–494, 2016. doi: 10.1016/j.cjca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590: 1077–1084, 2012. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging (Albany NY) 8: 2897–2914, 2016. doi: 10.18632/aging.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gist NH, Fedewa MV, Dishman RK, Cureton KJ. Sprint interval training effects on aerobic capacity: a systematic review and meta-analysis. Sports Med 44: 269–279, 2014. doi: 10.1007/s40279-013-0115-0. [DOI] [PubMed] [Google Scholar]
- 43.Godkin FE, Jenkins EM, Little JP, Nazarali Z, Percival ME, Gibala MJ. The effect of brief intermittent stair climbing on glycemic control in people with type 2 diabetes: a pilot study. Appl Physiol Nutr Metab 43: 969–972, 2018. doi: 10.1139/apnm-2018-0135. [DOI] [PubMed] [Google Scholar]
- 44.Gutchess A. Plasticity of the aging brain: new directions in cognitive neuroscience. Science 346: 579–582, 2014. doi: 10.1126/science.1254604. [DOI] [PubMed] [Google Scholar]
- 45.Hannan AL, Hing W, Simas V, Climstein M, Coombes JS, Jayasinghe R, Byrnes J, Furness J. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: a systematic review and meta-analysis. Open Access J Sports Med 9: 1–17, 2018. doi: 10.2147/OAJSM.S150596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med 29: 737–752, 2013. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985) 85: 609–618, 1998. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 48.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14: 2934–2941, 2003. doi: 10.1097/01.ASN.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa N, Fujie S, Horii N, Miyamoto-Mikami E, Tsuji K, Uchida M, Hamaoka T, Tabata I, Iemitsu M. Effects of Different Exercise Modes on Arterial Stiffness and Nitric Oxide Synthesis. Med Sci Sports Exerc 50: 1177–1185, 2018. doi: 10.1249/MSS.0000000000001567. [DOI] [PubMed] [Google Scholar]
- 50.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 51.Herbert P, Grace FM, Sculthorpe NF. Exercising caution: prolonged recovery from a single session of high-intensity interval training in older men. J Am Geriatr Soc 63: 817–818, 2015. doi: 10.1111/jgs.13365. [DOI] [PubMed] [Google Scholar]
- 52.Hermes BM, Cardoso DM, Gomes TJN, Santos TD, Vicente MS, Pereira SN, Barbosa VA, Albuquerque IM. Short-term inspiratory muscle training potentiates the benefits of aerobic and resistance training in patients undergoing CABG in phase II cardiac rehabilitation program. Rev Bras Cir Cardiovasc 30: 474–481, 2015. doi: 10.5935/1678-9741.20150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howden EJ, Carrick-Ranson G, Sarma S, Hieda M, Fujimoto N, Levine BD. Effects of sedentary aging and lifelong exercise on left ventricular systolic function. Med Sci Sports Exerc 50: 494–501, 2018. doi: 10.1249/MSS.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 54.Hussain SR, Macaluso A, Pearson SJ. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev 24: 273–281, 2016. doi: 10.1097/CRD.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 55.Hwang C-L, Yoo J-K, Kim H-K, Hwang M-H, Handberg EM, Petersen JW, Christou DD. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol 82: 112–119, 2016. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins EM, Nairn LN, Skelly LE, Little JP, Gibala MJ. Do stair climbing exercise “snacks” improve cardiorespiratory fitness? Appl Physiol Nutr Metab 44: 681–684, 2019. doi: 10.1139/apnm-2018-0675. [DOI] [PubMed] [Google Scholar]
- 57.Joseph P, Leong D, McKee M, Anand SS, Schwalm J-D, Teo K, Mente A, Yusuf S. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res 121: 677–694, 2017. doi: 10.1161/CIRCRESAHA.117.308903. [DOI] [PubMed] [Google Scholar]
- 58.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 99: 1165–1172, 1999. doi: 10.1161/01.CIR.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 59.Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am 42: 333–347, 2013. doi: 10.1016/j.ecl.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaminski DM, Schaan BD, da Silva AMV, Soares PP, Lago PD. Inspiratory muscle training in patients with diabetic autonomic neuropathy: a randomized clinical trial. Clin Auton Res 25: 263–266, 2015. doi: 10.1007/s10286-015-0291-0. [DOI] [PubMed] [Google Scholar]
- 61.Keadle SK, McKinnon R, Graubard BI, Troiano RP. Prevalence and trends in physical activity among older adults in the United States: a comparison across three national surveys. Prev Med 89: 37–43, 2016. doi: 10.1016/j.ypmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H-K, Hwang C-L, Yoo J-K, Hwang M-H, Handberg EM, Petersen JW, Nichols WW, Sofianos S, Christou DD. All-extremity exercise training improves arterial stiffness in older adults. Med Sci Sports Exerc 49: 1404–1411, 2017. doi: 10.1249/MSS.0000000000001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinnafick F-E, Thøgersen-Ntoumani C, Shepherd SO, Wilson OJ, Wagenmakers AJM, Shaw CS. In it together: a qualitative evaluation of participant experiences of a 10-week, group-based, workplace HIIT program for insufficiently active adults. J Sport Exerc Psychol 40: 10–19, 2018. doi: 10.1123/jsep.2017-0306. [DOI] [PubMed] [Google Scholar]
- 64.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 322: 1447–1451, 2001. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 66.Komine H, Sugawara J, Hayashi K, Yoshizawa M, Yokoi T. Regular endurance exercise in young men increases arterial baroreflex sensitivity through neural alteration of baroreflex arc. J Appl Physiol (1985) 106: 1499–1505, 2009. doi: 10.1152/japplphysiol.91447.2008. [DOI] [PubMed] [Google Scholar]
- 67.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I. Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 68.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II. The aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- 69.Lalande S, Okazaki K, Yamazaki T, Nose H, Joyner MJ, Johnson BD. Effects of interval walking on physical fitness in middle-aged individuals. J Prim Care Community Health 1: 104–110, 2010. doi: 10.1177/2150131910363598. [DOI] [PubMed] [Google Scholar]
- 70.Langer D, Charususin N, Jácome C, Hoffman M, McConnell A, Decramer M, Gosselink R. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Phys Ther 95: 1264–1273, 2015. doi: 10.2522/ptj.20140245. [DOI] [PubMed] [Google Scholar]
- 71.Laoutaris ID, Dritsas A, Brown MD, Manginas A, Kallistratos MS, Degiannis D, Chaidaroglou A, Panagiotakos DB, Alivizatos PA, Cokkinos DV. Immune response to inspiratory muscle training in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 14: 679–685, 2007. doi: 10.1097/HJR.0b013e3281338394. [DOI] [PubMed] [Google Scholar]
- 72.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 144: 73–81, 2006. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 73.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuki S, Mori M, Tabara Y, Sakurai A, Hashimoto S, Morikawa M, Miyagawa K, Sumiyoshi E, Miki T, Higuchi K, Nose H; Shinshu University Genetic Research Consortium . The factors affecting adherence to a long-term interval walking training program in middle-aged and older people. J Appl Physiol (1985) 118: 595–603, 2015. doi: 10.1152/japplphysiol.00819.2014. [DOI] [PubMed] [Google Scholar]
- 75.McArthur D, Dumas A, Woodend K, Beach S, Stacey D. Factors influencing adherence to regular exercise in middle-aged women: a qualitative study to inform clinical practice. BMC Womens Health 14: 49, 2014. doi: 10.1186/1472-6874-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mello PR, Guerra GM, Borile S, Rondon MU, Alves MJ, Negrão CE, Dal Lago P, Mostarda C, Irigoyen MC, Consolim-Colombo FM. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J Cardiopulm Rehabil Prev 32: 255–261, 2012. doi: 10.1097/HCR.0b013e31825828da. [DOI] [PubMed] [Google Scholar]
- 77.Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol 529: 263–271, 2000. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57: 861–868, 2003. doi: 10.1016/S0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 79.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation 107: e2–e5, 2003. doi: 10.1161/01.CIR.0000048890.59383.8D. [DOI] [PubMed] [Google Scholar]
- 81.Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc 82: 803–811, 2007. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- 82.Normansell R, Smith J, Victor C, Cook DG, Kerry S, Iliffe S, Ussher M, Fox-Rushby J, Whincup P, Harris T. Numbers are not the whole story: a qualitative exploration of barriers and facilitators to increased physical activity in a primary care based walking intervention. BMC Public Health 14: 1272, 2014. doi: 10.1186/1471-2458-14-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci 67: 345–351, 2017. doi: 10.1007/s12576-017-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oliveira BRR, Santos TM, Kilpatrick M, Pires FO, Deslandes AC. Affective and enjoyment responses in high intensity interval training and continuous training: A systematic review and meta-analysis. PLoS One 13: e0197124, 2018. doi: 10.1371/journal.pone.0197124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oudegeest-Sander MH, Olde Rikkert MGM, Smits P, Thijssen DHJ, van Dijk APJ, Levine BD, Hopman MTE. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: a randomized factorial design trial. Exp Gerontol 48: 1509–1517, 2013. doi: 10.1016/j.exger.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palau P, Domínguez E, Núñez E, Schmid J-P, Vergara P, Ramón JM, Mascarell B, Sanchis J, Chorro FJ, Núñez J. Effects of inspiratory muscle training in patients with heart failure with preserved ejection fraction. Eur J Prev Cardiol 21: 1465–1473, 2014. doi: 10.1177/2047487313498832. [DOI] [PubMed] [Google Scholar]
- 87.Pattyn N, Beulque R, Cornelissen V. Aerobic interval vs. continuous training in patients with coronary artery disease or heart failure: an updated systematic review and meta-analysis with a focus on secondary outcomes. Sports Med 48: 1189–1205, 2018. doi: 10.1007/s40279-018-0885-5. [DOI] [PubMed] [Google Scholar]
- 88.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association . Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107: 499–511, 2003. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 89.Pierce GL. Aortic stiffness in aging and hypertension: prevention and treatment with habitual aerobic exercise. Curr Hypertens Rep 19: 90, 2017. doi: 10.1007/s11906-017-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10: 1032–1037, 2011. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pinto E. Blood pressure and ageing. Postgrad Med J 83: 109–114, 2007. doi: 10.1136/pgmj.2006.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med 166: 1003–1008, 2006. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 94.Rakobowchuk M, Harris E, Taylor A, Cubbon RM, Birch KM. Moderate and heavy metabolic stress interval training improve arterial stiffness and heart rate dynamics in humans. Eur J Appl Physiol 113: 839–849, 2013. doi: 10.1007/s00421-012-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reitlo LS, Sandbakk SB, Viken H, Aspvik NP, Ingebrigtsen JE, Tan X, Wisløff U, Stensvold D. Exercise patterns in older adults instructed to follow moderate- or high-intensity exercise protocol - the generation 100 study. BMC Geriatr 18: 208, 2018. doi: 10.1186/s12877-018-0900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rognmo Ø, Moholdt T, Bakken H, Hole T, Mølstad P, Myhr NE, Grimsmo J, Wisløff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126: 1436–1440, 2012. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 98.Roh J, Rhee J, Chaudhari V, Rosenzweig A. The role of exercise in cardiac aging: from physiology to molecular mechanisms. Circ Res 118: 279–295, 2016. doi: 10.1161/CIRCRESAHA.115.305250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ross R, de Lannoy L, Stotz PJ. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin Proc 90: 1506–1514, 2015. doi: 10.1016/j.mayocp.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 100.Rossman MJ, LaRocca TJ, Martens CR, Seals DR. Healthy lifestyle-based approaches for successful vascular aging. J Appl Physiol (1985) 125: 1888–1900, 2018. doi: 10.1152/japplphysiol.00521.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saglam M, Arikan H, Vardar-Yagli N, Calik-Kutukcu E, Inal-Ince D, Savci S, Akdogan A, Yokusoglu M, Kaya EB, Tokgozoglu L. Inspiratory muscle training in pulmonary arterial hypertension. J Cardiopulm Rehabil Prev 35: 198–206, 2015. doi: 10.1097/HCR.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 102.Schoenborn CA, Stommel M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am J Prev Med 40: 514–521, 2011. doi: 10.1016/j.amepre.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 103.Seals DR. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 117: 425–439, 2014. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 105: 1323–1332, 2008. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seals DR, Monahan KD, Bell C, Tanaka H, Jones PP. The aging cardiovascular system: changes in autonomic function at rest and in response to exercise. Int J Sport Nutr Exerc Metab 11, Suppl: S189–S195, 2001. doi: 10.1123/ijsnem.11.s1.s189. [DOI] [PubMed] [Google Scholar]
- 107.Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol 597: 4901–4914, 2019. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shenouda N, Gillen JB, Gibala MJ, MacDonald MJ. Changes in brachial artery endothelial function and resting diameter with moderate-intensity continuous but not sprint interval training in sedentary men. J Appl Physiol (1985) 123: 773–780, 2017. doi: 10.1152/japplphysiol.00058.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shepherd SO, Wilson OJ, Taylor AS, Thøgersen-Ntoumani C, Adlan AM, Wagenmakers AJM, Shaw CS. Low-volume high-intensity interval training in a gym setting improves cardio-metabolic and psychological health. PLoS One 10: e0139056, 2015. doi: 10.1371/journal.pone.0139056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sistino JJ, Fitzgerald DC. Epidemiology of cardiovascular disease in the United States: implications for the perfusion profession. A 2017 update. Perfusion 32: 501–506, 2017. doi: 10.1177/0267659117696140. [DOI] [PubMed] [Google Scholar]
- 111.Smart NA, Giallauria F, Dieberg G. Efficacy of inspiratory muscle training in chronic heart failure patients: a systematic review and meta-analysis. Int J Cardiol 167: 1502–1507, 2013. doi: 10.1016/j.ijcard.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 112.Søgaard D, Lund MT, Scheuer CM, Dehlbaek MS, Dideriksen SG, Abildskov CV, Christensen KK, Dohlmann TL, Larsen S, Vigelsø AH, Dela F, Helge JW. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol (Oxf) 222: e13009, 2018. doi: 10.1111/apha.13009. [DOI] [PubMed] [Google Scholar]
- 113.Sørensen D, Svenningsen H. Adherence to home-based inspiratory muscle training in individuals with chronic obstructive pulmonary disease. Appl Nurs Res 43: 75–79, 2018. doi: 10.1016/j.apnr.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 114.Stamatakis E, Johnson NA, Powell L, Hamer M, Rangul V, Holtermann A. Short and sporadic bouts in the 2018 US physical activity guidelines: is high-intensity incidental physical activity the new HIIT? Br J Sports Med 53: 1137–1139, 2019. doi: 10.1136/bjsports-2018-100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stensvold D, Viken H, Rognmo Ø, Skogvoll E, Steinshamn S, Vatten LJ, Coombes JS, Anderssen SA, Magnussen J, Ingebrigtsen JE, Fiatarone Singh MA, Langhammer A, Støylen A, Helbostad JL, Wisløff U. A randomised controlled study of the long-term effects of exercise training on mortality in elderly people: study protocol for the Generation 100 study. BMJ Open 5: e007519, 2015. doi: 10.1136/bmjopen-2014-007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Støren Ø, Helgerud J, Sæbø M, Støa EM, Bratland-Sanda S, Unhjem RJ, Hoff J, Wang E. The effect of age on the V˙O2max response to high-intensity interval training. Med Sci Sports Exerc 49: 78–85, 2017. doi: 10.1249/MSS.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 117.Stork MJ, Banfield LE, Gibala MJ, Martin Ginis KA. A scoping review of the psychological responses to interval exercise: is interval exercise a viable alternative to traditional exercise? Health Psychol Rev 11: 324–344, 2017. doi: 10.1080/17437199.2017.1326011. [DOI] [PubMed] [Google Scholar]
- 118.Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J 50: 499–507, 2002. doi: 10.1177/216507990205001106. [DOI] [PubMed] [Google Scholar]
- 119.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 120.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NAM III, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Heart Association Council on Clinical Cardiology; American College of Sports Medicine . Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115: 2358–2368, 2007. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 121.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118: 346–354, 2008. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 123.Vendemiale G, Romano AD, Dagostino M, de Matthaeis A, Serviddio G. Endothelial dysfunction associated with mild cognitive impairment in elderly population. Aging Clin Exp Res 25: 247–255, 2013. doi: 10.1007/s40520-013-0043-8. [DOI] [PubMed] [Google Scholar]
- 124.Vranish JR, Bailey EF. Daily respiratory training with large intrathoracic pressures, but not large lung volumes, lowers blood pressure in normotensive adults. Respir Physiol Neurobiol 216: 63–69, 2015. doi: 10.1016/j.resp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 125.Vranish JR, Bailey EF. Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep (Basel) 39: 1179–1185, 2016. doi: 10.5665/sleep.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor κB. Clin Sci (Lond) 127: 645–654, 2014. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Welch JF, Archiza B, Guenette JA, West CR, Sheel AW. Sex differences in diaphragmatic fatigue: the cardiovascular response to inspiratory resistance. J Physiol 596: 4017–4032, 2018. doi: 10.1113/JP275794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48: 1227–1234, 2014. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 129.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: e13–e115, 2018. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 130.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 136: 493–503, 2002. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 131.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 132.Wong ND, Lopez VA, Roberts CS, Solomon HA, Burke GL, Kuller L, Tracy R, Yanez D, Psaty BM. Combined association of lipids and blood pressure in relation to incident cardiovascular disease in the elderly: the cardiovascular health study. Am J Hypertens 23: 161–167, 2010. doi: 10.1038/ajh.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeren M, Demir R, Yigit Z, Gurses HN. Effects of inspiratory muscle training on pulmonary function, respiratory muscle strength and functional capacity in patients with atrial fibrillation: a randomized controlled trial. Clin Rehabil 30: 1165–1174, 2016. doi: 10.1177/0269215515628038. [DOI] [PubMed] [Google Scholar]
- 134.Zou Y, Zhu Q, Deng Y, Duan J, Pan L, Tu Q, Dai R, Zhang X, Chu L-W, Lü Y. Vascular risk factors and mild cognitive impairment in the elderly population in Southwest China. Am J Alzheimers Dis Other Demen 29: 242–247, 2014. doi: 10.1177/1533317513517042. [DOI] [PMC free article] [PubMed] [Google Scholar]