The battle against the opioid epidemic presents a dire challenge. A conundrum, in fact, since the powerful analgesic and euphoric properties of opioids often come with a significant cost, addiction. Where substance abuse and addiction were initially attributed to lacking self-control, its conceptualization as a disorder of the brain mobilized a shift in scientific understanding to unravel underlying neurocircuitries as well as neurotransmitters in fostering drug-dependent behaviors1. Separately, the impact of opioids on the immune system has been widely understood to induce immunosuppression, but it has also been more recently explored in its capacity to stimulate immune activity2–4. As T cells and their derived cytokines have been shown to modulate brain function in different contexts5–9, an evaluation of opioid induced addiction through a neuroimmune lens may be fruitful. Thus, whether opioid use itself impacts the crosstalk between the CNS and the peripheral immune system remains elusive and proves to be a strategic angle to tackle.
In this issue of Cell, Zhu, Yan, and colleagues questioned just that – does opioid dependent alteration to peripheral immunity influence functional remodeling of the CNS to contribute to addictive behaviors? They addressed this by performing CyTOF on blood samples from both healthy human patients and heroin addicts to gauge clinically relevant changes in immune cell populations. In particular, the study noted an increase in Foxp3-expressing regulatory T cells (Tregs) in heroin addicts that intriguingly expressed IFN-γ (Figure 1A). Concordant with prior descriptions of “fragile Tregs” in the tumor microenvironment10, the authors similarly labeled their identified cell subtype as fragile-like Tregs, lending to their maintained expression of Foxp3 with loss of suppressive capacity. Paralleling initial observations made in humans, mice treated with either morphine or heroine exhibited fragile-like Tregs in the blood as well as other tissues following the withdrawal of opioids. As a matter of fact, opioid treatment alone did not directly enforce fragility in Tregs when administered ex vivo. Rather, the authors observed that global hypoxia induced by morphine triggered Hif1α expression and primed Tregs to become functionally fragile.
Figure 1: Fragile-like Tregs pervade through blood into the brain giving rise to opioid-induced dysfunction.
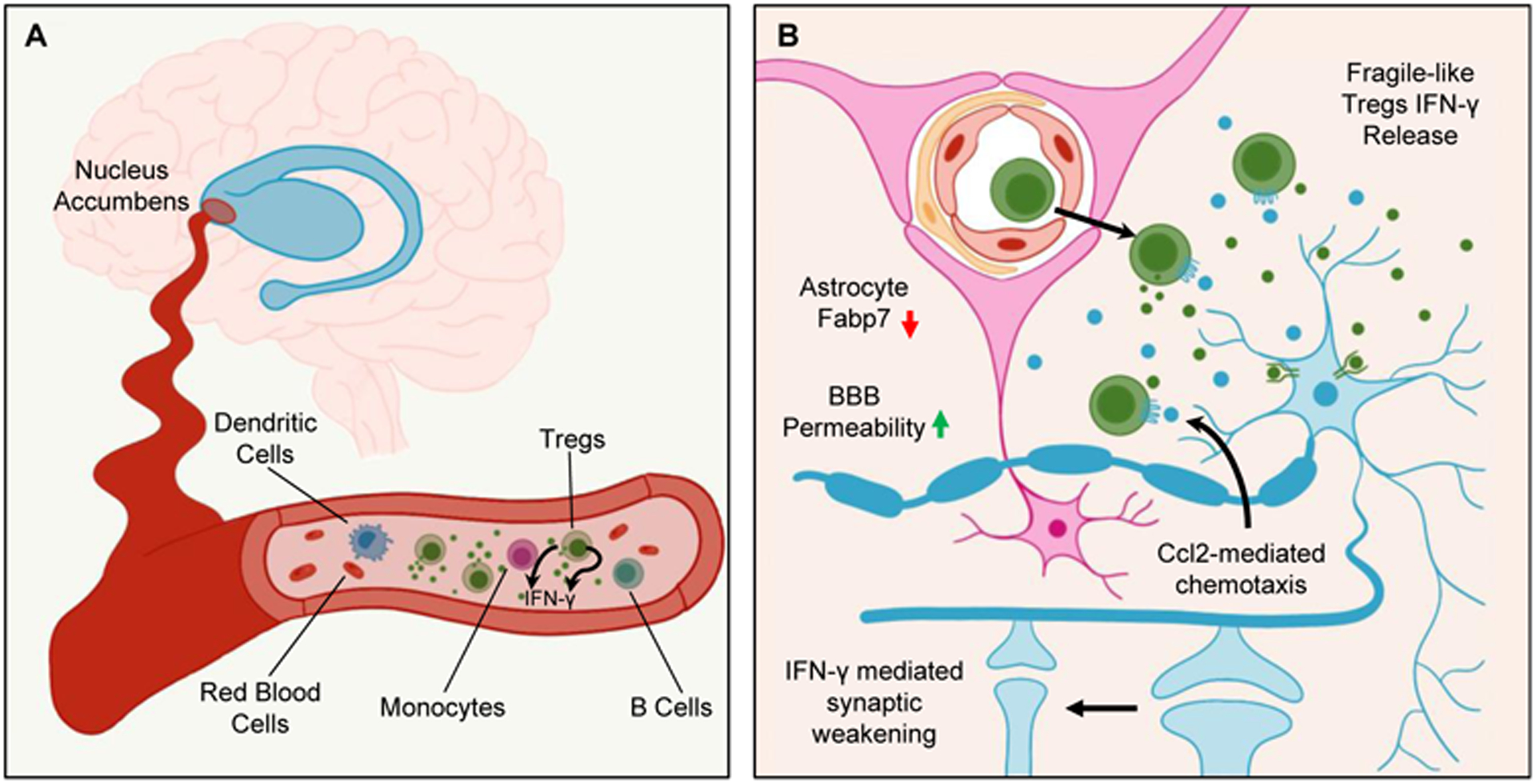
(A) In the blood of both humans and mice, opioids dysregulate the immune system for which this study focuses on Foxp3+ regulatory T cells (Tregs) that falter in their suppression and begin secreting IFN-γ to become “fragile-like.”
(B) Through both chemokine-mediated migration and hyperpermeability of the blood-brain barrier (BBB), these fragile-like Tregs mobilize into the brain, localizing to the nucleus accumbens (NAc). There, they continue to secrete IFN-γ, and by signaling through IFN-γ receptor R1 (Ifngr1) on neurons in the NAc, induce morphological changes that result in synaptic weakening. Blocking IFN-γ signaling prevented synaptic instability and improved withdrawal symptoms. Altogether, these findings emphasize relevant changes occurring at the neuroimmune interface for opioid-induced impairments.
Interestingly, the expansion of fragile-like Tregs in the periphery was matched with an associated increase of IFN-γ-expressing Foxp3+ fragile-like Tregs in the nucleus accumbens (NAc), a pivotal brain structure implicated in the establishment and maintenance of drug addiction. T cell receptor (TCR) sequencing of T cells present in the NAc further demonstrated increased TCR diversity after morphine treatment, suggesting active infiltration as opposed to local proliferation of T cells. Seeking to understand mechanisms contributing to the infiltration of fragile-like Tregs into the NAc, the authors implicated a role for neuron-derived Ccl2 in recruiting fragile-like Tregs through Ccr2-mediated migration. Moreover, decreased expression of fatty acid binding protein 7 (Fabp7) in astrocytes with morphine administration disrupted blood-brain barrier (BBB) integrity. These observations underscored a role for opioids in compromising the CNS through a two-pronged attack: (1) chemokine secretion and (2) hyperpermeability of the BBB (Figure 1B). These two factors proved critical for drawing fragile-like Tregs from the periphery into the NAc, allowing peripheral immune cells to breach CNS barriers.
Infiltration of morphine-induced, fragile-like Tregs into the NAc would then permit local production of IFN-γ to impact neurocircuitries involved in reinforcing drug-dependent behaviors. Implicating IFN-γ in the pathogenesis of opioid triggered synaptic weakening, the authors elegantly demonstrated through either genetic ablation of IFN-γ in fragile-like Tregs or knockdown of the corresponding IFN-γ receptor R1 (Ifngr1) on neurons that these manipulations could ameliorate withdrawal symptoms. Collectively, this provided a mechanism grounded in IFN-γ signaling through which peripheral immune changes translated into functional changes in the CNS to impart withdrawal-induced behavioral dysfunction (Figure 1B).
Previous work has demonstrated that IFN-γ supplied by T cells residing in the brain meninges, membranous coverings wrapping around the brain, act on neuronal circuits to influence social behavior8. As the brain borders harbor a rich niche of immune cells and have captivated the attention for neuroimmunological evaluation11, it remains an intriguing question if in the context of opioid dependence there are also relevant increases in IFN-γ circulating through the meninges. As opioid use has been described to affect sociability12, whether fragile-like Tregs similarly infiltrate and remodel meningeal immunity to effect broader changes on the brain entices further evaluation. It may be that the mobilization of fragile-like Tregs to the meninges rather precedes the authors observed infiltration into the NAc precipitating initial changes to the brain from its borders. It is conceivable then that fragile-like Tregs may also adopt a meningeal route for migration and access into the brain parenchyma13. Such questions arising from this study spark excitement for further investigation – inspecting beyond but closely into the meningeal layers enveloping the brain to understand and to perhaps target relevant opioid-induced immune changes.
Thus, fascinating findings by Zhu, Yan, and colleagues14 in their study expand our arsenal of treatment options to fend against opioid-induced addiction. They show that fragile-like Tregs, cultivated in the periphery, through production of IFN-γ impair function both locally and at a distance in the brain, particularly the NAc. Moreover, as pharmacological and psychosocial methods have been limiting to treat physiological dependence on opioids, deep brain stimulation of the NAc has been sought after as a potential therapeutic with advancements in neurosurgical techniques in recent years15. Albeit deep brain stimulation still poses individuals with an invasive procedure, and to that end, findings by this study highlight an exciting alternative avenue. In fact, it provides opportunities to therapeutically target the NAc at a distance through immunotherapy to combat dysfunction at the neuroimmune interface. Through this study, the authors call attention for a need to surveil beyond the confines of the brain to begin melding connections that intricately intertwine opioid induced neurobiological change and immune dysfunction. It refocuses our attention to the neuroimmune interface to advance our understanding of and design of novel therapeutics for opioid use and abuse.
References
- 1.Koob GF & Volkow ND Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773 (2016). 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballantyne JC & Mao J Opioid therapy for chronic pain. N Engl J Med 349, 1943–1953 (2003). 10.1056/NEJMra025411 [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson MR et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun 22, 1178–1189 (2008). 10.1016/j.bbi.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melik Parsadaniantz S, Rivat C, Rostene W & Reaux-Le Goazigo A Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci 16, 69–78 (2015). 10.1038/nrn3858 [DOI] [PubMed] [Google Scholar]
- 5.Filiano AJ, Gadani SP & Kipnis J How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci 18, 375–384 (2017). 10.1038/nrn.2017.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derecki NC et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 207, 1067–1080 (2010). 10.1084/jem.20091419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi GB et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016). 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filiano AJ et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535, 425–429 (2016). 10.1038/nature18626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro M et al. Meningeal gammadelta T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol 4 (2019). 10.1126/sciimmunol.aay5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overacre-Delgoffe AE et al. Interferon-gamma Drives T(reg) Fragility to Promote Anti-tumor Immunity. Cell 169, 1130–1141 e1111 (2017). 10.1016/j.cell.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rustenhoven J & Kipnis J Brain borders at the central stage of neuroimmunology. Nature 612, 417–429 (2022). 10.1038/s41586-022-05474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier IM, van Honk J, Bos PA & Terburg D A mu-opioid feedback model of human social behavior. Neurosci Biobehav Rev 121, 250–258 (2021). 10.1016/j.neubiorev.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 13.Rustenhoven J & Kipnis J Bypassing the blood-brain barrier. Science 366, 1448–1449 (2019). 10.1126/science.aay0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yongsheng Zhu PY, Rui Wang, Jianghua Lai, Hua Tang, Xu Xiao, Rongshan Yu, Xiaorui Bao, Feng Zhu, Kena Wang, Ye Lu, Jie Dang, Chao Zhu, Rui Zhang, Wei Dang, Bao Zhang, Quanze Fu, Qian Zhang, Chongao Kang, Yujie Chen, Xiaoyu Chen, Qing Liang, and Keijia Wang. Opioid-induced fragile-like regulatory T cells contribute to opioid addiction. Cell 186 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Kuhn J et al. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry 19, 145–146 (2014). 10.1038/mp.2012.196 [DOI] [PubMed] [Google Scholar]


