Background:
Since Aspirin’s adverse effects are dose-dependent, and evidence supporting the use of low-dose (LD) Aspirin in preventing venous thromboembolism (VTE) after total hip arthroplasty (THA) is weak, the authors do not know what the minimal effective dosage of Aspirin is to prevent VTE. This study aimed to compare the rates of 90-day symptomatic VTE following THA and total knee arthroplasty in healthy patients taking LD Aspirin vs. high-dose (HD) Aspirin for 6 weeks postoperatively.
Materials and methods:
A prospective cohort of patients with THA and total knee arthroplasty was conducted at two tertiary centres. Symptomatic VTE within 90 days of index arthroplasty was the primary outcome; gastrointestinal bleeding (GIB) and mortality were secondary outcomes.
Results:
The final analysis included 312 consecutive patients: 158 in the LD group and 154 in the HD group. Two groups were similar regarding preoperative data, including sex, age, BMI, smoking, diabetes mellitus, Hgb and platelet count, and type of surgery. The LD group had one deep vein thrombosis (0.6%), and the HD group had two (1.3%) (P=0.62). Neither group had PTE. Therefore, VTE rates are the same as deep vein thrombosis rates and similar between the groups (0.6% vs. 1.3%, P=0.62)
Regarding GIB due to anticoagulant therapy, no patient in the LD group reported GIB, whereas two (1.3%) patients in the HD group reported GIB within 90 days of arthroplasty. GIB rates did not differ significantly between groups (P=0.24). Considering VTE + GIB combined, the HD groups showed a higher rate of complications (N=4, 2.6%) than the LD groups (N=1, 0.6%) but not statistically significant (P=0.21).
Conclusions:
Prophylactic administration of Aspirin with low doses (81 mg BID) and high doses (325 mg BID) for six weeks is equally effective at reducing VTE in total joint arthroplasty patients and had similar adverse effects.
Level of Evidence:
Therapeutic Level II
Keywords: Aspirin, gastrointestinal bleeding, high-dose, low-dose, total Joint arthroplasty, venous thromboembolism
Introduction
Highlights
Aspirin with low doses (162 mg) and high doses (650 mg) is equally effective.
Both doses showed similar adverse effects.
90-day mortality and gastrointestinal bleeding were similar between the two groups.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are common and effective treatments for end-stage degenerative joint disease, including osteoarthritis 1. Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE), is one of the leading causes of preventable morbidity, mortality, and healthcare expenditures2. As a result of the prolonged surgery and reduced mobility postoperatively, all patients undergoing total joint arthroplasty (TJA) are at risk for VTE. Thus, anticoagulation is administered up to 5–6 weeks after surgery to reduce VTE risk3. Among people who are anticoagulated, rates of VTE are variable following THA and TKA: up to 5% for DVT and 2% for PTE4.
Various anticoagulants can be used to prevent VTE, including simple oral agents (Aspirin), injectable anticoagulants (low molecular weight heparin), and novel oral anticoagulants (dabigatran and rivaroxaban). In the search for ideal chemoprophylaxis, a number of challenges are still being addressed, such as bleeding and wound-related issues, administration route, titration, and blood monitoring5,6. Aspirin is a cheap oral drug that does not require blood testing and is well-tolerated and safe7. A large body of evidence supports Aspirin’s efficacy in preventing postoperative VTE2,4,8–11. Nevertheless, using Aspirin as the sole prophylactic antithrombotic agent in orthopaedic patients has remained controversial despite decades of successful and safe use. The majority of studies included “standard risk” patients, and the controversy is more prominent among other patient populations12. Over the past decades, PTE rates after TJA have not decreased markedly13, and potent anticoagulants contribute only to a lower incidence of asymptomatic VTE14–16. Since PTE persists as an essential complication, the role of potent anticoagulants has been debated17,18. Administrating potent anticoagulants can increase the risk of bleeding, wound complications, and hospitalization days, compared to a simple Aspirin19–21.
The American College of Chest Physicians (ACCP) and American Association of Orthopedic Surgeons (AAOS) have recommended Aspirin alone as a single agent for the prophylaxis of VTE after joint replacement surgery and a dose of 325 mg BID for six weeks following surgery22,23. On the other hand, 77% of the participants in the International Consensus Meeting (ICM) 2022 agreed that low-dose (LD) Aspirin is the most effective and safest prophylaxis for VTE24. It was unclear until recently what was the optimal dose of Aspirin for VTE prevention25. Few studies suggest that twice-daily (BID) LD Aspirin (81 mg), compared with high-dose (HD) Aspirin (325 mg) for 4-6 weeks, does not differ regarding VTE events after TJA9,25–27. The minimal effective dosage of Aspirin in preventing VTE is unknown since the adverse effects are dose-dependent, and the evidence supporting the use of LD Aspirin in preventing VTE after THA is weak.
To this end, our incentive to conduct this study was due to the paucity of evidence derived from prospective cohort studies on its topic. This study compared the 90-day symptomatic VTE rates among healthy patients following THAs and TKAs with 81 mg BID of Aspirin vs. 325 mg BID of Aspirin for 6 weeks postoperatively. We aimed to secondarily investigate differences in the rate of complications between the two dosing regimens, including 90-day gastrointestinal bleeding events and mortality. We hypothesized that a LD Aspirin would be as effective as a HD Aspirin in preventing VTE and be associated with fewer gastrointestinal bleeding (GIB) events.
Methods
Study design and ethics statement
This study was reported in accordance with STROCSS criteria28. A prospective cohort study of patients with TJA was performed in two tertiary centres of our Medical University between January 2020 and January 2022. The patients were given Aspirin in two different doses to prevent VTE for 6 weeks: 325 mg BID or 81 mg BID. Our university institutional review board (IRB) has approved the study’s protocol and declared it ethically acceptable (Approval ID: IR.TUMS.MEDICINE.REC.1399.293). The participants voluntarily signed informed consent forms and participated in the study.
Participants, inclusion, and exclusion criteria
Our study involved 320 consecutive adult patients in two orthopaedic clinics who were being treated with TKA or THA [Figure 1].
Figure 1.
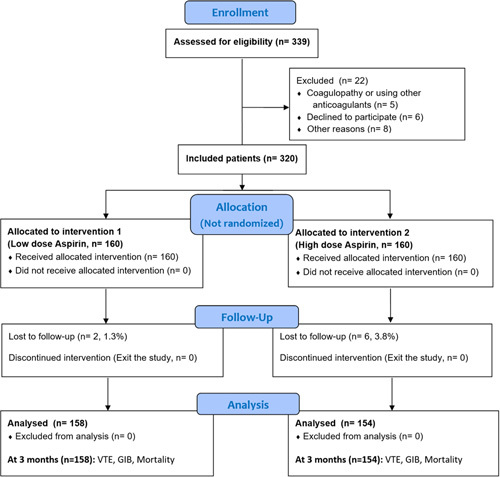
Flow diagram of patients’ enrolment and study assessments. GIB, gastrointestinal bleeding; VTE, venous thromboembolism.
Inclusion criteria
Primary or revision TKA and TJA
Intended to participate and sign a consent
American Society of Anesthesiologists (ASA) score of I–III
Exclusion criteria
Medical history of DVT or PTE
Any coagulopathy (factor V Leiden, antithrombin III deficiencies, Glanzman thrombasthenia, etc.)
Using other medications that interfere with the study outcome (Rivaroxaban, Plavix, Warfarin, etc.)
Active malignancy
A different form of VTE chemoprophylaxis was given rather than Aspirin, Aspirin allergy, or intolerance
No intention of participating or illiterate for successful completion of the follow-up and the forms.
Major comorbidities such as heart failure or renal failure
Unicompartmental knee replacement
Interventions and study protocol
TKA cases were treated using standard conventional PS (posterior stabilizer) TKA. The same surgeon with a fellowship in adult reconstruction performed all TKA procedures using a medial parapatellar approach and a standard midline incision. All of the patients received cemented posterior stabilized prostheses (NexGen LPS-Flex) by Zimmer Biomet (Warsaw). All participants received the same preoperative and postoperative care according to the same protocol. We applied a tourniquet (250 mmHg) and did not employ suction drains. Aspirin (325 or 81 mg/BID) was used postoperatively as a VTE chemoprophylaxis (begin on POD 1). The pain management protocol includes Celecoxib (400 mg) + Pregabalin (75 mg) + Acetaminophen (1g) + Omeprazole (40 mg), taken orally one hour before surgery for all patients. The patients received 2.0 g of injection cephazolin as antibiotic prophylaxis. Patients received 1.5 g of IV tranexamic acid before surgery. Following the closure of the articular capsule, a cocktail was injected intra-articularly, consisting of normal saline (90 mL) + ketorolac (60 mg) + Marcaine (4 mL, 0.5%) + Lidocaine (5 mL, 2.0%) + 3 mg tranexamic acid. During the postoperative period, the following analgesics were administered: celecoxib (400 mg), omeprazole (20 mg), pregabalin (75 mg), acetaminophen (2g), and oxycodone (PRN, maximum 15 mg per day). Patients were mobilized by a walker within 6–8 h of surgery, and range-of-motion exercises were begun. We allowed patients to walk partial weight-bearing using crutches for 4–6 weeks. Brace was not used on any patients. There were no compression devices applied to the patients’ calves after surgery.
The THA surgery was performed by three surgeons with fellowships in adult reconstruction either using the direct anterior approach or lateral approach. The bearing surface in all surgeries was metal on a highly cross-linked polyethylene. Fitmore stems (Zimmer, Inc.) and CORAIL implants (DePuy Orthopaedics, Inc.) were used for the femoral component. Hemispherical porous-coated Trilogy cups (Zimmer, Inc.) and porous-coated Pinnacle cups (DePuy Orthopaedics, Inc.) were used for the acetabular component. 2D templating was performed on all patients before surgery using mediCAD software29. The perioperative care was the same for TKA patients described above, except THA patients did not receive cocktail injections and oxycodone.
Outcome measures and data collection
The study’s primary outcome was symptomatic VTE (DVT and PTE) within 90 days of index arthroplasty. In addition, the secondary outcomes were GIB and mortality within 90 days. The routine follow-up for TJA patients at our centre consists of 1 week, 3 weeks, 6 weeks, 3 months, 6 months, 12 months after surgery, and then annually. Clinical manifestations of DVT, such as pain, warmth, redness, swelling, and tenderness in the lower limb, positive Homans sign, and different leg circumferences, were used to screen DVT. PTE was also screened by clinical symptoms like dyspnoea and chest pain. Colour Doppler ultrasound confirmed DVT suspicion, and lung ventilation/perfusion scan or computed tomography-angiography confirmed PTE suspicion. Our orthopaedic resident (PGY-3) conducted the physical examination and VTE screening blinded to the study groups. GIB was defined as bleeding from the upper gastrointestinal tract confirmed by endoscopy.
Hospital length of stay, type of anaesthesia, cemented or cementless prosthesis, and platelet counts before surgery were compiled by the surgeon using the checklist. Blood loss during the surgery is measured by measuring the blood loss directly in volume units. In addition, the patient’s demographics, history of VTE, and comorbidities were recorded. Finally, all parameters were compared between the two groups under prophylactic Aspirin doses of 81 mg and 325 mg.
Statistical analysis
Data were analyzed by SPSS 23.0 (IBM SPSS Inc.). Normality was determined by the Shapiro-Wilk test. Using Student’s t-tests, Mann–Whitney tests, and Analysis of Variance (ANOVA), a comparison of continuous variables by their normality was performed. The nominal variables were also compared using the χ2 and Fisher exact tests. The repeated measures ANOVA test was used to compare the group scores across time. P values of less than 0.05 were considered significant.
Results
The final analysis included 312 consecutive patients: 158 in the LD group (81 mg Aspirin BID) and 154 in the HD group (325 mg Aspirin BID) [Figure 1]. Two groups were similar regarding preoperative data and demographics, including sex, age, BMI, smoking, diabetes mellitus, and platelet count, and type of surgery [Table 1]. In terms of surgical information, the two groups were similar, aside from the THA approach. In the LD groups, direct anterior approach was predominant (75.4%), while in the HD groups, a more lateral approach was prevalent (50.8%, P<0.001).
Table 1.
Patients’ demographic and data
| Variable (Mean±SD or n %) | Low dose (N=158) | High dose (N=154) | P |
|---|---|---|---|
| Preoperative data | |||
| Male sex | 53 (33.5) | 58 (37.7) | 0.45 |
| Age (year) | 53.3±18.4 | 55.0±18.9 | 0.43 |
| Body mass index (Kg.m-2) | 27.0±4.3 | 26.9±4.3 | 0.72 |
| Smoke | 36 (22.8) | 22 (14.3) | 0.06 |
| Diabetes mellitus | 24 (15.2) | 21 (13.3) | 0.46 |
| Platelet count (per microlitre) | 252.5±90.0 | 255.2±85.7 | 0.79 |
| Type of surgery | 0.16 | ||
| Primary (281. 90.1%) | 146 (92.4) | 135 (87.7) | |
| Revision (31, 9.9%) | 12 (7.6) | 19 (12.3) | |
| Joint | 0.22 | ||
| Knee (64, 20.5%) | 28 (17.7) | 36 (23.4) | |
| Hip (248, 79.5%) | 130 (82.3) | 118 (76.6) | |
| Operation data | |||
| Anaesthesia | 0.32 | ||
| Spinal | 69 (43.7) | 76 (49.4) | |
| General | 89 (56.3) | 78 (50.6) | |
| Length of stay (day) | 4.6±3.4 | 4.9±2.6 | 0.07 |
| THA approach (248, 100%) | <0.001* | ||
| Direct anterior | 98 (75.4) | 58 (49.2) | |
| Lateral | 32 (24.6) | 60 (50.8) | |
| Type of prosthesis | 0.87 | ||
| Cemented | 62 (39.2) | 59 (38.3) | |
| Cementless | 96 (60.8) | 95 (61.7) | |
THA, total hip arthroplasty.
Indicates significant P value.
The LD group had one DVT (0.6%), and the HD group had two (1.3%) (P=0.62) [Table 2]. Neither group had PTE. Therefore, VTE rates are the same as DVT rates and similar between the groups (0.6% vs. 1.3%, P=0.62) [Figure 2].
Table 2.
Study results (90 days follow-up)
| Variable (Mean±SD or n %) | Low dose (N=158) | High dose (N=154) | P |
|---|---|---|---|
| VTE | 1 (0.6) | 2 (1.3) | 0.62 |
| DVT | 1 (0.6) | 2 (1.3) | 0.62 |
| PTE | 0 | 0 | — |
| GIB | 0 | 2 (1.3) | 0.24 |
| Mortality | 0 | 0 | — |
| Total VTE/bleeding complications | 1 (0.6) | 4 (2.6) | 0.21 |
DVT, deep vein thrombosis; GIB, gastrointestinal bleeding; PTE, pulmonary thromboembolism; VTE, venous thromboembolic.
Figure 2.
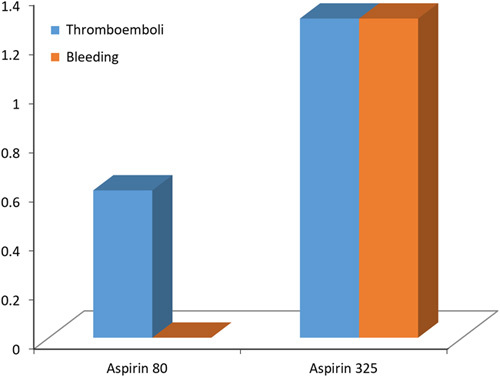
Thromboembolic and haemorrhagic complications in two groups of aspirin.
Regarding GIB due to anticoagulant therapy, no patient in the LD group reported GIB, whereas two (1.3%) patients in the HD group reported GIB within 90 days of arthroplasty. GIB rates did not differ significantly between groups (P=0.24). Considering VTE+GIB combined, the HD groups showed a higher rate of complications (N=4, 2.6%) than the LD groups (N=1, 0.6%) but not statistically significant (0.21). During the study period, no death occurred.
Details on the five patients who had VTE or GIB within 90 days following their arthroplasty are provided in [Table 3].
Table 3.
Information of patients with VTE or GIB
| Sex | Age | BMI | Smoke | Comorbidity | Type of surgery | Anaesthesia | Condition | Aspirin dose | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Male | 79 | 33 | Yes | DM | Primary THA | General | DVT | 81 BID | Conservative |
| Female | 69 | 30 | Yes | — | Primary TKA | Spinal | DVT | 325 BID | Conservative |
| Female | 73 | 27 | Yes | DM | Primary THA | Spinal | DVT | 325 BID | Conservative |
| Male | 64 | 23 | Yes | — | Primary THA | Spinal | GIB | 325 BID | Conservative |
| Male | 51 | 27 | No | — | Primary THA | General | GIB | 325 BID | Conservative |
DM, Diabetes mellitus; DVT, deep vein thrombosis; GIB, gastrointestinal bleeding; THA, total hip arthroplasty; TKA, total knee arthroplasty; VTE, venous thromboembolism.
Discussion
The main finding of this study was that the administration of both LD (81 mg BID) and HD (325 mg BID) Aspirin for 6 weeks was effective and safe for patients undergoing joint replacement surgery. LD Aspirin not only was not inferior to HD, but also demonstrated an insignificantly lower risk of VTE and GIB. Thus, we considered it reasonable to take LD Aspirin (81 mg BID) into account for the routine practice of TJA. It is important to note that our study is not the first to compare VTE rates between different dosages of Aspirin after TJA. Therefore, we should consider the findings of our study within the context of other published findings.
Patients undergoing arthroplasty surgery have a well-established risk of developing VTE and subsequent PTE that can be fatal7,30,31. In more recent ACCP guidelines from 2012, Aspirin was recommended as an appropriate method of VTE prophylaxis after TJA22. To choose the best anticoagulant and dosage, it is essential to balance agent efficacy and side effects caused by drugs with higher risk profiles6. The network meta-analysis by Tarabichi and colleagues of all levels of evidence studies24 showed that the lowest risk of VTE development was seen with LD Aspirin (100 mg). There was no significant difference between low molecular weight heparin and rivaroxaban when compared to LD Aspirin in their risk of causing VTE [Odds ratio (OR), 95% CI=1.11 (0.33, 3.76) and 1.38 (0.55, 3.45)]. In contrast, VTE is more likely to occur when HD Aspirin (325 mg) is taken [OR = 7.9 (2.60, 24.05)] followed by heparin (OR=5.94 (2.28, 15.47)) as compared to LD Aspirin. Furthermore, when the risk of bleeding events was assessed in all studies, LD Aspirin (81 mg) had the lowest risk estimate. In sum, they found that LD Aspirin effectively reduced the risk of VTE compared with other medications and caused fewer bleeding incidents5,24,32–35. Despite previous research findings, recent literature now disproves the notion that HD Aspirin (325 mg BID) offers greater protection against cardiovascular and cerebrovascular issues than LD Aspirin (75–100 mg BID)36–38. In the Pulmonary Embolism Prevention (PEP) study in 2001, LD Aspirin (80 mg BID) significantly lowered the incidence of DVT and PTE in patients undergoing TJA by at least one-third of place controls39. Still, the AAOS guidelines recommend that HD Aspirin (325 mg BID) be used to prevent VTE following TJA40.
There has been evidence that if taken at doses between 30 and 150 mg, Aspirin inhibits the activity of the platelet COX-1 enzyme36. Several studies directly compare the LD and HD Aspirin as chemoprophylaxis for VTE after TJA9,25–27,41–46. Uvodich and colleagues. compared 90-day incidence of symptomatic VTE on 3512 patients treated with either LD (81 mg BID) or HD (325 mg BID) Aspirin 4–6 weeks after surgery. Neither group showed a difference in symptomatic VTE incidence (0% vs. 0.1%, P=0.79), GIB events (no cases), and mortality (0.3% vs. 0.1%, P=0.24)25. According to Parvizi et al. 27, their prospective cross-over study on 4651 primary TJA did not reveal any differences in VTE between LD and HD groups (0.1% vs. 0.3%, P=0.35). The rates of GIB and ulceration (0.3% vs. 0.4%, P=0.66), as well as acute periprosthetic joint infection (PJI) (0.2% vs. 0.5%, P=0.28), were modestly higher in the HD group, but statistically not significant27. To the best of our knowledge, their study was the only prospective study in the literature search. In the setting of revision surgery, Tang and colleagues‘ study on revision TKA and revision THA patients demonstrated non-inferiority of LD Aspirin vs. HD Aspirin43,45. Only one study found a significant difference in favour of LD Aspirin, that of Merkow et al. 44. On HD Aspirin (325 mg BID), 133 VTE cases were reported among the 9413 TKA compared with 8 cases among the 3453 TKA on HD Aspirin (81 mg BID) (1.41% vs. 0.23%, P<0.001)44. The only systematic review comparing the low and HD Aspirin found that in patients receiving LD or HD Aspirin, there were no significant differences in the rates of symptomatic PTE (0.33% vs. 0.65%, P=0.16), symptomatic DVT (0.52 vs. 0.99%, P=0.23), 90-day mortality (0.33 vs. 21%, P=19), or major bleeding (54% vs. 29%, P=38)26.
LD Aspirin also showed comparable effects to HD when assessing for other conditions, such as heterotopic ossification (HO) development41. Van Nest and colleagues revealed that Aspirin-treated patients were less prone to develop HO following THA (34.8% vs. 45.5%) and HO following TKA (13.4% vs. 18.4%) compared with non-Aspirin VTE prophylaxis. When comparing low and HD Aspirin, HO formation was lower in LD Aspirin patients (81 mg) vs. HD Aspirin patients (325 mg), although not significantly41. It could show that LD Aspirin is not only sufficient for VTE prophylaxis but is also sufficient to prevent HO. Our study’s results align with the existing literature on LD Aspirin compared with regular-dose Aspirin in the control of VTE after TJA. For otherwise healthy patients, we recommend using LD Aspirin during TJA47.
Our study faced several limitations. First, our sample size is not that large to provide a strong and valid conclusion and is underpowered. Because the sample size was small and only a few patients experienced VTE, it was not possible to identify risk factors and conduct a multivariate analysis. Second, we did not consider wound complications such as dehiscence and infection. Our primary outcome was VTE events; previous studies showed no difference in wound conditions between the different dosages of Aspirin27. Furthermore, our patient sample is inhomogeneous in some respects, and primary TJAs differ significantly from their revision counterparts. Lastly, the study was not randomized, because of which it suffers from selection bias.
Conclusions
According to this prospective comparative study, prophylactic administration of Aspirin with low doses (81 mg BID) and high doses (325 mg BID) for 6 weeks is equally effective at reducing VTE in TJA patients. In addition, 90-day mortality and GIB were similar between groups. Therefore, lower doses are recommended for safety reasons. To confirm these findings, more large-scale randomized clinical trials are needed in the future.
Ethical approval
The study was reviewed and approved by Institutional Review Board of Tehran University of Medical Sciences. (Approval ID: IR.TUMS.MEDICINE.REC.1399.293).
Consent
All the patients obtained informed consent to publish this study and accompanying data. On request, a copy of the written consent is available for review by the Editor-in-Chief of this journal.
Source of funding
There is no source of funding for the research.
Author contribution
S.H.S. contributed to the study conception and design, performed surgeries, and revised the manuscript. P.M. analyzed the data and wrote the first draft of the manuscript and revised it. M.R. contributed to the study design, data collection, and draw figures. B.S. contributed to the study conception and design, performed surgeries, and revised the manuscript. S.M.J.M. supervised the study, performed the surgeries, and edited the final text. All authors commented on previous versions of the manuscript and revised it. All authors read and approved the final manuscript.”
Conflicts of interest disclosure
The authors report no declarations of interest.
Research registration unique identifying number (UIN)
NA.
Guarantor
Seyed Mohammad Javad Mortazavi.
Availability of data and material
The data that support the findings of this study are available from the corresponding author, S.H.S., upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 17 April 2023
Contributor Information
Seyyed Hossein Shafiei, Email: Dr_hshafiei@yahoo.com.
Mohammad Rastegar, Email: Drmrastegar2342@gmail.com.
Peyman Mirghaderi, Email: mirghaderi76@gmail.com.
Babak Siavashi, Email: siavashi@sina.tums.ac.ir.
Seyed Mohammad Javad Mortazavi, Email: smjmort@yahoo.com.
References
- 1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007;370:1508–1519. [DOI] [PubMed] [Google Scholar]
- 2. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2020;180:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet 2001;358:9–15. [DOI] [PubMed] [Google Scholar]
- 4. Bala A, Huddleston JI, III, Goodman SB, et al. Venous thromboembolism prophylaxis after TKA: aspirin, warfarin, enoxaparin, or factor Xa inhibitors? Clin Orthop Relat Res 2017;475:2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang RC, Parvizi J, Hozack WJ, et al. Aspirin is as effective as and safer than warfarin for patients at higher risk of venous thromboembolism undergoing total joint arthroplasty. J Arthroplasty 2016;31:83–86. [DOI] [PubMed] [Google Scholar]
- 6. Sharrock NE, Gonzalez Della Valle A, Go G, et al. Potent anticoagulants are associated with a higher all-cause mortality rate after hip and knee arthroplasty. Clin Orthop Relat Res 2008;466:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parvizi J, Ceylan HH, Kucukdurmaz F, et al. Venous thromboembolism following hip and knee arthroplasty: the role of aspirin. J Bone Joint Surg Am 2017;99:961–72. [DOI] [PubMed] [Google Scholar]
- 8. Salzman EW, Harris WH, DeSanctis RW. Reduction in venous thromboembolism by agents affecting platelet function. N Engl J Med 1971;284:1287–1292. [DOI] [PubMed] [Google Scholar]
- 9. Faour M, Piuzzi NS, Brigati DP, et al. Low-dose aspirin is safe and effective for venous thromboembolism prophylaxis following total knee arthroplasty. J Arthroplasty 2018;33:S131–s35. [DOI] [PubMed] [Google Scholar]
- 10. Haas SB, Insall JN, Scuderi GR, et al. Pneumatic sequential-compression boots compared with aspirin prophylaxis of deep-vein thrombosis after total knee arthroplasty. J Bone Joint Surg Am 1990;72:27–31. [PubMed] [Google Scholar]
- 11. Moharrami A, Mirghaderi SP, Hoseini-Zare N, et al. Restoring femoral medial offset could reduce pelvic obliquity following primary total hip arthroplasty, an observational study. Int Orthop 2022;46:2765–2774. [DOI] [PubMed] [Google Scholar]
- 12. Matzko C, Berliner ZP, Husk G, et al. Equivalent VTE rates after total joint arthroplasty using thromboprophylaxis with aspirin versus potent anticoagulants: retrospective analysis of 4562 cases across a diverse healthcare system. Arthroplasty 2021;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cote MP, Chen A, Jiang Y, et al. Persistent pulmonary embolism rates following total knee arthroplasty even with prophylactic anticoagulants. J Arthroplasty 2017;32:3833–39. [DOI] [PubMed] [Google Scholar]
- 14. Johanson NA, Lachiewicz PF, Lieberman JR, et al. Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Am Acad Orthop Surg 2009;17:183–196. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs JJ, Mont MA, Bozic KJ, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Bone Joint Surg Am 2012;94:746–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bozic KJ, Vail TP, Pekow PS, et al. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty 2010;25:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lotke PA, Lonner JH. The benefit of aspirin chemoprophylaxis for thromboembolism after total knee arthroplasty. Clin Orthop Relat Res 2006;452:175–180. [DOI] [PubMed] [Google Scholar]
- 18. Lieberman JR, Cheng V, Cote MP. Pulmonary embolism rates following total hip arthroplasty with prophylactic anticoagulation: some pulmonary emboli cannot be avoided. J Arthroplasty 2017;32:980–86. [DOI] [PubMed] [Google Scholar]
- 19. Nam D, Nunley RM, Johnson SR, et al. Thromboembolism prophylaxis in hip arthroplasty: routine and high risk patients. J Arthroplasty 2015;30:2299–2303. [DOI] [PubMed] [Google Scholar]
- 20. Drescher FS, Sirovich BE, Lee A, et al. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta-analysis. J Hosp Med 2014;9:579–585. [DOI] [PubMed] [Google Scholar]
- 21. Bloch BV, Patel V, Best AJ. Thromboprophylaxis with dabigatran leads to an increased incidence of wound leakage and an increased length of stay after total joint replacement. Bone Joint J 2014;96-b:122–126. [DOI] [PubMed] [Google Scholar]
- 22. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e278S–e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johanson NA, Lachiewicz PF, Lieberman JR, et al. American academy of orthopaedic surgeons clinical practice guideline on. Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Bone Joint Surg Am 2009;91:1756–1757. [DOI] [PubMed] [Google Scholar]
- 24. The ICMVTEGDPAASAWAJARJAOAJA. Recommendations from the ICM-VTE: General JBJS 2022;104::4–162. [DOI] [PubMed] [Google Scholar]
- 25. Uvodich ME, Siljander MP, Taunton MJ, et al. Low-dose vs regular-dose aspirin for venous thromboembolism prophylaxis in primary total joint arthroplasty. J Arthroplasty 2021;36:2359–63. [DOI] [PubMed] [Google Scholar]
- 26. Azboy I, Groff H, Goswami K, et al. Low-dose aspirin is adequate for venous thromboembolism prevention following total joint arthroplasty: a systematic review. J Arthroplasty 2020;35:886–92. [DOI] [PubMed] [Google Scholar]
- 27. Parvizi J, Huang R, Restrepo C, et al. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am 2017;99:91–98. [DOI] [PubMed] [Google Scholar]
- 28. Mathew G, Agha R. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 29. Mirghaderi SP, Sharifpour S, Moharrami A, et al. Determining the accuracy of preoperative total hip replacement 2D templating using the mediCAD® software. J Orthop Surg Res 2022;17:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flevas DA, Megaloikonomos PD, Dimopoulos L, et al. Thromboembolism prophylaxis in orthopaedics: an update. EFORT Open Rev 2018;3:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mir Mansour Moazen J, Alireza M, Seyed Peyman M, et al. The role of spinopelvic parameters in total hip arthroplasty: a current concept review. J Orthop Spine Trauma 2022;8:40–3. [Google Scholar]
- 32. Josefsson G, Dahlqvist A, Bodfors B. Prevention of thromboembolism in total hip replacement. Aspirin versus dihydroergotamine-heparin. Acta Orthop Scand 1987;58:626–629. [DOI] [PubMed] [Google Scholar]
- 33. Deirmengian GK, Heller S, Smith EB, et al. Aspirin can be used as prophylaxis for prevention of venous thromboembolism after revision hip and knee arthroplasty. J Arthroplasty 2016;31:2237–2240. [DOI] [PubMed] [Google Scholar]
- 34. Vulcano E, Gesell M, Esposito A, et al. Aspirin for elective hip and knee arthroplasty: a multimodal thromboprophylaxis protocol. Int Orthop 2012;36:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Intermountain Joint Replacement Center Writing Committee. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J Arthroplasty 2012;27:1–9.e2. [DOI] [PubMed] [Google Scholar]
- 36. Eikelboom JW, Hirsh J, Spencer FA, et al. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e89S–e119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hart RG, Harrison MJ. Aspirin wars: the optimal dose of aspirin prevent stroke. Stroke 1996;27:585–587. [DOI] [PubMed] [Google Scholar]
- 38. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pulmonary Embolism Prevention (PEP) trial Collaborative Group, Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000;355:1295–1302. [PubMed] [Google Scholar]
- 40. Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg 2011;19:768–776. [DOI] [PubMed] [Google Scholar]
- 41. Van Nest DS, Clarkson S, Chisari E, et al. Low-dose aspirin administered for venous thromboembolism prophylaxis reduces the incidence of heterotopic ossification in total joint arthroplasty. J Arthroplasty 2021;36:1543–47. [DOI] [PubMed] [Google Scholar]
- 42. Mihara M, Tamaki Y, Nakura N, et al. Clinical efficacy of risk-stratified prophylaxis with low-dose aspirin for the management of symptomatic venous thromboembolism after total hip arthroplasty. J Orthop Sci 2020;25:156–60. [DOI] [PubMed] [Google Scholar]
- 43. Tang A, Zak SG, Waren D, et al. Low-dose aspirin is safe and effective for venous thromboembolism prevention in patients undergoing revision total knee arthroplasty: a retrospective cohort study. J Knee Surg 2022;35:553–59. [DOI] [PubMed] [Google Scholar]
- 44. Merkow DB, Tang A, Iorio R, et al. Low dose aspirin is effective in preventing venous thromboembolism in patients undergoing primary total knee arthroplasty. J Orthop 2021;24:26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang A, Zak S, Iorio R, et al. Low-dose aspirin is safe and effective for venous thromboembolism prevention in patients undergoing revision total hip arthroplasty: a retrospective cohort study. J Arthroplasty 2020;35:2182–87. [DOI] [PubMed] [Google Scholar]
- 46. Faour M, Piuzzi NS, Brigati DP, et al. No difference between low- and regular-dose aspirin for venous thromboembolism prophylaxis after THA. Clin Orthop Relat Res 2019;477:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muscatelli SR, Charters MA, Hallstrom BR. Time for an update? A look at current guidelines for venous thromboembolism prophylaxis after hip and knee arthroplasty and hip fracture. Arthroplasty Today 2021;10:105–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, S.H.S., upon reasonable request.


