Introduction:
Coronavirus disease 2019 (COVID-19) began to spread in December 2019 and was declared a pandemic by WHO on 11 March 2020. Pulmonary embolism (PE) is a known sequel to COVID-19 infection. Many patients showed worsened symptoms of thrombotic events of pulmonary arteries during the second week of the disease for which computed tomography pulmonary angiography (CTPA) is recommended. The most frequent complications in critically ill patients are prothrombotic coagulation abnormalities and thromboembolism. So, this study aimed to assess the prevalence of PE in patients with COVID-19 infection and to evaluate the relation to disease severity on CTPA findings.
Methods:
This cross-sectional study was performed to evaluate the patients who tested positive for COVID-19 and underwent CTPA. COVID-19 infection in participants was confirmed by a PCR of nasopharyngeal or oropharyngeal swab samples. Frequencies of computed tomography severity scores and CTPA were calculated and compared with clinical and laboratory findings.
Results:
The study included 92 patients with COVID-19 infection. Positive PE was found in 18.5% of the patients. The mean age of the patients was 59.83±13.58 years with an age range of 30–86 years. Among the total participants, 27.2% underwent ventilation, 19.6% died during treatment, and 80.4% of them got discharged. PE was developed in patients who did not receive prophylactic anticoagulation, which is statistically significant (P≤0.001). There was also a significant relationship between mechanical ventilation and CTPA findings.
Conclusions:
The authors conclude from their study that PE is one of the complications of COVID-19 infection. Rising D-dimer during the second week of disease alerts clinicians to do CTPA to exclude or confirm PE. This will help in the early diagnosis and treatment of PE.
Keywords: COVID-19, computed tomography pulmonary angiography, computed tomography severity score, pulmonary embolism
Introductions
Highlights
Pulmonary embolism (PE) is a known sequel to coronavirus disease 2019 (COVID-19) infection.
Many patients showed worsened symptoms of thrombotic events of pulmonary arteries during the second week of the disease, for which computed tomography pulmonary angiography (CTPA) is recommended.
Rising D-dimer during the second week of disease alerts clinicians to do CTPA to exclude or confirm PE.
Background
Coronavirus disease 2019 (COVID-19) presents as asymptomatic carriers in patients requiring assisted ventilator support1,2. Computed tomography (CT) is one of the main diagnostic tools for the early diagnosis and treatment of COVID-19 pneumonia3. One of the known sequels of COVID-19 infection is pulmonary embolism (PE)4. Many patients showed symptoms of thrombotic events of pulmonary arteries, with worsened symptoms, especially during the second week of the disease, and for this CT pulmonary angiography (CTPA) is recommended5. In COVID-19 patients who have signs of clinical severity and high D-dimer levels, CTPA can be performed to rule out PE6. Coagulation parameters like elevated levels of D-dimer and fibrin degradation products significantly correlate with mortality in patients with COVID-197. Both inflammation storm and coagulation activation caused by high D-dimer levels have been associated with increased mortality8,9. So, this study aimed to assess the presence of PE in patients with COVID-19 infection with severe respiratory symptoms.
Methods
This was a retrospective single-center cross-sectional study conducted on patients who tested positive for COVID-19 and underwent CTPA from November 2020 to April 2021. We used a census sampling method in this study. The patients were reviewed to identify those who had COVID-19 infection confirmed by PCR of nasopharyngeal or oropharyngeal swab samples. Ethical approval was taken from the Ethical Review Board of Nepal Health Research Council with reference number 681. Only the patients who tested positive for COVID-19 with PCR testing were included in the study sample. Data were first collected in a proforma by extracting them from hospital patient records and electronic medical record systems. The data were then tabulated in Microsoft Excel spreadsheets and analyzed with the help of SPSS (Statistical Package for Social Science) version 16.
The work has been reported in line with the STROCSS criteria.
Results
The study included 92 patients proven by PCR to have COVID-19 infection. Seventeen patients representing 18.5% of the patients, showed positive PE; among them, 18.8% (3) were female and 18.4% (14) were male. The mean age of the patients was 59.83±13.58 years with an age range of 30–86 years (Fig. 1). There were four (4.3%) smokers, 78 (84.8%) nonsmokers, and 10 (10.9%) ex-smoker.
Figure 1.
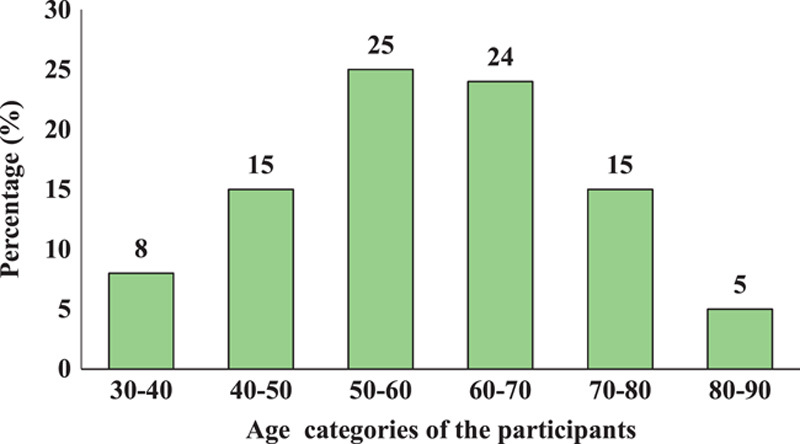
Age-wise distribution of the participants.
COVID-19 Reporting and Data System (CO-RADS) scores were calculated. Among the total participants, 70.7% had a proven CO-RADS score, 22.8% had a very high, and 6.5% had a high CO-RADS score (Fig. 2).
Figure 2.
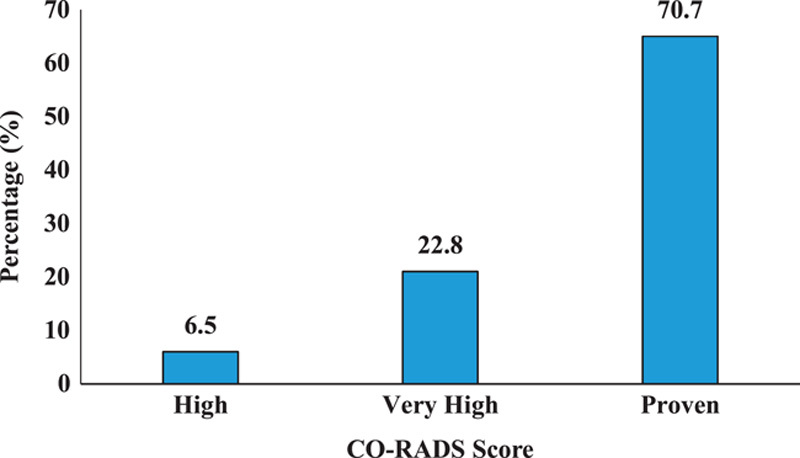
CO-RADS Score of the participants. CO-RADS, COVID-19 Reporting and Data System.
Patients presented with chief complaints of Shortness of breath 84.8% (78), Cough 69.6% (64), and Fever 69.6% (64). Other complaints were chest pain, myalgia, and loose stool. The final diagnosis of participants was COVID-19, COVID-19 with mediastinal lymphadenopathy, pleural effusion, cardiomegaly, hiatal hernia, pneumomediastinum, and subcutaneous emphysema (Table 1).
Table 1.
Final diagnosis of the participants
| Final diagnosis | Frequency (N) | Percent (%) |
|---|---|---|
| COVID-19 | 64 | 69.6 |
| COVID-19 with mediastinal lymphadenopathy | 11 | 12 |
| COVID-19 with pleural effusion | 5 | 5.4 |
| COVID-19 with cardiomegaly | 4 | 4.3 |
| COVID-19 with hiatal hernia | 3 | 3.3 |
| COVID-19 with pneumomediastinum | 2 | 2.2 |
| COVID-19 with subcutaneous emphysema | 2 | 2.2 |
COVID-19, coronavirus disease 2019.
The mean days of hospital stay were 10.85±8.63. There were 76 patients (82.6%) males, while the rest 16 (17.4%) were female patients. The most common comorbidities among the participants were Hypertension (55.4%) followed by Diabetes (30.4%). Among the total participants, 25 (27.2%) underwent ventilation, 18 (19.6%) participants died during treatment, and 74 (80.4%) of them got discharged. However, 17 (18.5%) participants got therapeutic anticoagulants and 75 (81.5%) got prophylactic anticoagulation.
No significant difference was found between the incidence of PE and the sex and comorbidities of the participants. Similarly, mechanical ventilation showed a statistically significant relationship with the incidence of PE, and participants who were on therapeutic and prophylactic anticoagulation showed significant relation with the incidence of PE. However, there was no significant difference between the incidence of PE and the death and discharge of the participants (Table 2).
Table 2.
Clinical characteristics of patients with COVID-19 who had diagnostic chest CTPA and differences in clinical features between patients with positive and negative CTPA
| Clinical characteristics | All patients (n=92), N (%) | CTPA negative (n=75), N (%) | CTPA positive (n=17), N (%) | P |
|---|---|---|---|---|
| Sex | ||||
| Female | 16 (17.4) | 13 (81.3) | 3 (18.8) | 0.97 |
| Male | 76 (82.6) | 62 (81.6) | 14 (18.4) | |
| Comorbidities | ||||
| Hypertension | 51 (55.4) | 41 (80.4) | 10 (19.6) | 0.75 |
| Diabetes | 28 (30.4) | 24 (85.7) | 4 (14.3) | 0.49 |
| COPD | 5 (5.4) | 4 (4.3) | 1 (1.1) | 0.92 |
| DVT | 3 (3.3) | 3 (3.3) | – | 0.40 |
| CAD | 3 (3.3) | 3 (3.3) | – | 0.40 |
| Stroke | 2 (2.2) | 2 (2.2) | – | 0.49 |
| Clinical outcomes | ||||
| Mechanical ventilation | 25 (27.2) | 17 (68) | 8 (32) | 0.04 |
| Discharge | 74 (80.4) | 63 (85.1) | 11 (14.9) | 0.07 |
| Death | 18 (19.6) | 12 (66.7) | 6 (33.3) | 0.07 |
| Anticoagulation therapeutic | 17 (18.5) | – | 17 (100) | <0.001 |
| Anticoagulation prophylactic | 75 (81.5) | 75 (100) | – | <0.001 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CTPA, computed tomography pulmonary angiography; DVT, deep vein thrombosis.
Various laboratory investigations were done. Among the total participants, 63% (58) had high D-dimer with 29.3% (17) positive CTPA, which showed a significant relationship with the incidence of PE. There were a total of 90 (97.8%) participants who had high lactate dehydrogenase (LDH), 36 (39.1%) had high total leukocyte count (TLC), and 73 (79.3%) had high neutrophil count. However, 80.4% (74) had low lymphocytes count. Likewise, 81.5% (75) of participants had high ferritin levels and 96.7% (89) had high CRP levels, and 41.3% (38) had high activated partial thromboplastin clotting time (APTT). Regarding the prothrombin count (PT) and international normalized ratio (INR), 12% (11) had high PT and 10.9% (10) had high INR levels, which showed a significant relationship with the incidence of PE (Table 3).
Table 3.
Differences in laboratory values between patients with positive and negative chest CTPA
| Laboratory values | All patients (n=92), N (%) | CTPA negative (n=75), N (%) | CTPA positive (n=17), N (%) | P |
|---|---|---|---|---|
| D-dimer | ||||
| Normal | 34 (37) | 34 (100) | – | <0.001 |
| High | 58 (63) | 41 (70.7) | 17 (29.3) | |
| LDH | ||||
| Normal | 2 (2.2) | 2 (100) | – | 0.49 |
| High | 90 (97.8) | 73 (81.1) | 17 (18.9) | |
| TLC | ||||
| Low | 7 (7.6) | 7 (100) | – | 0.12 |
| Normal | 49 (53.3) | 42 (85.7) | 7 (14.3) | |
| High | 36 (39.1) | 26 (72.2) | 10 (27.8) | |
| Neutrophil | ||||
| Low | 2 (2.2) | 2 (100) | – | 0.56 |
| Normal | 17 (18.5) | 15 (88.2) | 2 (11.8) | |
| High | 73 (79.3) | 58 (79.5) | 15 (20.5) | |
| Lymphocytes | ||||
| Low | 74 (80.4) | 60 (81.1) | 14 (18.9) | 0.83 |
| Normal | 18 (19.6) | 15 (83.3) | 3 (16.7) | |
| Ferritin | ||||
| Low | 4 (4.3) | 3 (75) | 1 (25) | 0.54 |
| Normal | 13 (14.1) | 12 (92.3) | 1 (7.7) | |
| High | 75 (81.5) | 60 (80) | 15 (20) | |
| CRP | ||||
| Normal | 3 (3.3) | 2 (66.7) | 1 (33.3) | 0.50 |
| High | 89 (96.7) | 73 (82) | 16 (18) | |
| PT | ||||
| Low | 2 (2.2) | 2 (100) | – | 0.04 |
| Normal | 79 (85.9) | 67 (84.8) | 12 (15.2) | |
| High | 11 (12) | 6 (54.5) | 5 (45.5) | |
| INR | ||||
| Low | 1 (1.1) | 1 (100) | – | 0.02 |
| Normal | 81 (88) | 69 (85.2) | 12 (14.8) | |
| High | 10 (10.9) | 5 (50) | 5 (50) | |
| APTT | ||||
| Low | 7 (7.6) | 5 (71.4) | 2 (28.6) | 0.59 |
| Normal | 47 (51.1) | 40 (85.1) | 7 (14.9) | |
| High | 38 (41.3) | 30 (78.9) | 8 (21.1) | |
APTT, activated partial thromboplastin clotting time; CRP, C-reactive protein; CTPA, computed tomography pulmonary angiography; INR, international normalized ratio; LDH, lactate dehydrogenase; PT, prothrombin count; TLC, total leukocyte count.
Regarding the CT severity score (CTSS), 62.5% (10) of females and 77% (59) of males had severe CT scores. Of those participants who had comorbidities like Hypertension, 74.5% (38) and Diabetes, 85.7% (24) had severe CTSS. Of those participants who were on mechanical ventilation, 92% (23) had severe CTSS which were also statistically significant. Among the total participants, 71.6% (53) who were discharged and 88.9% (16) who were deceased had severe CTSS. Of all the participants who were on therapeutic anticoagulants, 100% (17) and 69.3% (52) of participants who were on prophylactic anticoagulants had severe CTSS and this showed a significant relationship with each other (Table 4).
Table 4.
Clinical characteristics of patients with COVID-19 and differences in clinical features between patients with mild–moderate CTSS and severe CTSS
| Clinical characteristics | All patients (n=92), N (%) | CT score (mild–moderate) (n=23), N (%) | CT score (severe) (n=69), N (%) | P |
|---|---|---|---|---|
| Sex | ||||
| Female | 16 (17.4) | 6 (37.5) | 10 (62.5) | 0.20 |
| Male | 76 (82.6) | 17 (22.4) | 59 (77.6) | |
| Comorbidities | ||||
| Hypertension | 51 (55.4) | 13 (25.5) | 38 (74.5) | 0.90 |
| Diabetes | 28 (30.4) | 4 (14.3) | 24 (85.7) | 0.11 |
| COPD | 5 (5.4) | 4 (4.3) | 1 (1.1) | 0.003 |
| DVT | 3 (3.3) | 2 (2.2) | 1 (1.1) | 0.09 |
| CAD | 3 (3.3) | 1 (1.1) | 2 (2.2) | 0.73 |
| Stroke | 2 (2.2) | – | 2 (2.2) | 0.40 |
| Clinical outcomes | ||||
| Mechanical ventilation | 25 (27.2) | 2 (8) | 23 (92) | 0.02 |
| Discharge | 74 (80.4) | 21 (28.4) | 53 (71.6) | 0.12 |
| Death | 18 (19.6) | 2 (11.1) | 16 (88.9) | 0.12 |
| Anticoagulation therapeutic | 17 (18.5) | – | 17 (100) | 0.008 |
| Anticoagulation prophylactic | 75 (81.5) | 23 (30.7) | 52 (69.3) | 0.008 |
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CT, computed tomography; DVT, deep vein thrombosis.
Among the total participants who had high D-dimer, 64.7% (22) had severe CTSS and among those who had high LDH, 74.4% (67) had severe CTSS. Likewise, regarding participants who had high TLC, 77.8% (28) had severe CTSS and for those with a high neutrophil count, 80.8% (59) had severe CTSS. Similarly, 77.3% (58) of participants who had high ferritin and 74.2% (66) who had high C-reactive protein (CRP) had severe CTSS. Among those participants who had low lymphocyte count, 50% (2) had severe CTSS which were also statistically significant. Of the participants who had high PT, INR, and APTT, among them 63.6% (7), 90% (9), and 76.3% (29) had severe CTSS respectively (Table 5).
Table 5.
Differences in laboratory values between patients with mild–moderate CTSS and severe CTSS
| Laboratory values | All patients (n=92), N (%) | CT score (mild–moderate) (n=23), N (%) | CT score (severe) (n=69), N (%) | P |
|---|---|---|---|---|
| D-dimer | ||||
| Normal | 34 (37) | 12 (35.3) | 22 (64.7) | 0.08 |
| High | 58 (63) | 11 (19) | 47 (81) | |
| LDH | ||||
| Normal | 2 (2.2) | 0 | 2 (100) | 0.40 |
| High | 90 (97.8) | 23 (25.6) | 67 (74.4) | |
| TLC | ||||
| Low | 7 (7.6) | 3 (42.9) | 4 (57.1) | 0.51 |
| Normal | 49 (53.3) | 12 (24.5) | 37 (75.5) | |
| High | 36 (39.1) | 8 (22.2) | 28 (77.8) | |
| Neutrophil | ||||
| Low | 2 (2.2) | 1 (50) | 1 (50) | 0.04 |
| Normal | 17 (18.5) | 8 (47.1) | 9 (52.9) | |
| High | 73 (79.3) | 14 (19.2) | 59 (80.8) | |
| Lymphocytes | ||||
| Low | 74 (80.4) | 15 (20.3) | 59 (79.7) | 0.03 |
| Normal | 18 (19.6) | 8 (44.4) | 10 (55.6) | |
| Ferritin | ||||
| Low | 4 (4.3) | 2 (50) | 2 (50) | 0.41 |
| Normal | 13 (14.1) | 4 (30.8) | 9 (69.2) | |
| High | 75 (81.5) | 17 (22.7) | 58 (77.3) | |
| CRP | ||||
| Normal | 3 (3.3) | 0 | 3 (100) | 0.30 |
| High | 89 (96.7) | 23 (25.8) | 66 (74.2) | |
| PT | ||||
| Low | 2 (2.2) | 0 | 2 (100) | 0.48 |
| Normal | 79 (85.9) | 19 (24.1) | 60 (75.9) | |
| High | 11 (12) | 4 (36.4) | 7 (63.6) | |
| INR | ||||
| Low | 1 (1.1) | 0 | 1 (100) | 0.42 |
| Normal | 81 (88) | 22 (27.2) | 59 (72.8) | |
| High | 10 (10.9) | 1 (10) | 9 (90) | |
| APTT | ||||
| Low | 7 (7.6) | 1 (14.3) | 6 (85.7) | 0.72 |
| Normal | 47 (51.1) | 13 (27.7) | 34 (72.3) | |
| High | 38 (41.3) | 9 (23.7) | 29 (76.3) | |
APTT, activated partial thromboplastin clotting time; CRP, C-reactive protein; CT, computed tomography; INR, international normalized ratio; LDH, lactate dehydrogenase; PT, prothrombin count; TLC, total leukocyte count.
Discussions
Respiratory tract infection is considered a risk factor in the development of PE, especially in hospitalized patients10. This study revealed that the incidence of PE in patients with COVID-19 who underwent CT pulmonary angiography was reported to be 18.5%. These findings were similar to the findings of the study done in the USA and France, Poyiadji et al. 11–13. But in contrast the study done in New York by Kaminetzky et al.14 shows there were 37.1% of positive cases for PE. Also, the study done in Italy, the UK, and Madrid and the study done by Middeldorp et al. revealed that PE ranges from 2.8 to 6.6%15–18.
The mean age of the patients was 59.83±13.58 years with an age range of 30–86 years, according to this study’s findings. This was in concordance with the findings of the study done by Kaminetzky et al.14 and a study done in the UK18. According to this study, patients present with chief complaints of Shortness of breath, Cough, and Fever. This was relatively similar to the findings of the study conducted in Egypt and Italy4,19. The most common comorbidities among the participants were Hypertension (55.4%) followed by Diabetes (30.4%). Likewise, these findings were supported by the study done in New York, USA14.
Among the total participants, 27.2% underwent ventilation, 19.6% of participants died during treatment, and 80.4% of them got discharged. This was in agreement with the findings of the study done in New York, USA14. However, 18.5% of participants got therapeutic anticoagulants, and 81.5% got prophylactic anticoagulation, which was in contrast to the study done by Kaminetzky et al.14 in New York, which showed that only 40% of patients receiving a prophylactic dose of anticoagulation. Statins have been previously described to be associated with decreased rates of venous thromboembolism, as well as decreased risk of recurrent PE20,21.
Similarly, mechanical ventilation showed a statistically significant relationship with the incidence of PE, and participants who were on therapeutic and prophylactic anticoagulation showed significant relation with the incidence of PE. These findings were in line with the results from a study done in China and Kollias et al., which showed that there had been an association between COVID-19-positive patients who are diagnosed with PE and treatment with anticoagulation prophylaxis, such as low molecular weight heparin22,23. However, there was a high rate of PE (52%) in COVID-19-positive patients who were receiving prophylactic anticoagulation and were shown to decrease mortality according to the study done in China and the USA24. But, there was no significant difference between the incidence of PE and the death and discharge of the participants in this study’s findings.
No significant difference was found between the incidence of PE and the sex and comorbidities of the participants. This was similar to the findings of the study done by Kaminetzky et al.14 and a study done in the UK18. Among the total participants, 63% had high D-dimer with 29.3% positive CTPA, which showed a significant relationship with the incidence of PE. Likewise, the various studies done in Spain, the USA, the UK, and France showed that the D-dimer level was higher among the PE-positive group relative to the PE-negative group14,18,25,26. Also, other studies were done by Mestre-Gómez et al.17, Poyiadji et al.13, and Wallace et al.20 showed that D-dimer values were significantly higher in PE patients. The study done in Wuhan, China, revealed that increased D-dimer levels have been associated with increased mortality in adult patients with COVID-199.
This study revealed that there were 82.6% of male while the rest (17.4%) were female patients. This was consistent with the study done in New York, which shows that patients who had COVID-19 comprised a significantly higher fraction of men, which may result from men associated with worse prognoses and higher mortality14. It has also been reported in the literature from Wuhan, China, that the severity of disease with COVID-19 infection was more in men than in women27.
CTSSs were higher among the severe category versus the mild–moderate category. This was in concordance with the findings from the study done in Egypt4. Among those participants who had low lymphocyte count, 50% had severe CT scores which were also statistically significant. This finding was in line with the results of earlier studies published in Wuhan, China28.
Conclusions
We conclude from our study that PE is one of the complications of COVID-19 infection. Rising D-dimer during the second week of disease, which all are alert to do CT pulmonary angiography to exclude or confirm PE. This will help in the early diagnosis and treatment of PE in the future. The incidence of PE and COVID-19 disease severity, either by laboratory or by the CTSS, will help in the early anticipation, diagnosis, and treatment of complicated COVID-19 infection cases.
Limitation
The study was conducted at a single-center health institution; thus, it may not be generalizable to the entire country.
Ethical approval
Taken from Nepal Health Research Council (Reference Number: 681).
Consent
Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
None.
Author contribution
I.A.: study concept, designing, literature review, data analysis, and interpretation of the results and writing in I.A.; P.R.R.: study designing, data analysis, and interpretation of the results; B.P., G.A., A.B., and A.D.: data collection and writing the paper. All authors were involved in manuscript drafting and revising, and approved the final version.
Conflicts of interest disclosure
None of the authors have conflicts of interest for this study.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Dr Pradeep Raj Regmi, Assistant Professor, Department of Radiology, Tribhuvan University Teaching Hospital, Maharajgunj Medical Campus, Maharajgunj, Kathmandu, Nepal. E-mail: pradeep.iom@gmail.com
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgments
The authors are appreciative of the help they received from patients and everyone who were engaged directly or indirectly in the study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 11 April 2023
Contributor Information
Isha Amatya, Email: ishaamatya.iom@gmail.com.
Pradeep R. Regmi, Email: pradeep.iom@gmail.com.
Gauri Adhikari, Email: gauryadhikari@gmail.com.
Bidushi Pokhrel, Email: pbidushi@gmail.com.
Anish Baniya, Email: anisb27@gmail.com.
Anisha Dangol, Email: dangolanishaibin@gmail.com.
References
- 1. Thomas-Rüddel D, Winning J, Dickmann P, et al. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist 2021;70:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeed GA, Gaba W, Shah A, et al. Correlation between chest CT severity scores and the clinical parameters of adult patients with COVID-19 pneumonia. Radiol Res Pract 2021;2021:6697677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hafez MA. The mean severity score and its correlation with common computed tomography chest manifestations in Egyptian patients with COVID-2019 pneumonia. Egypt J Radiol Nucl Med 2020;51:254. [Google Scholar]
- 4. Yassin A, Abdelkader MA, Mohammed RM, et al. CT pulmonary angiography in COVID-19 pneumonia: relationship between pulmonary embolism and disease severity. Egypt J Radiol Nucl Med 2021;52:10. [Google Scholar]
- 5. Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 2020;56:2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korevaar DA, van Es J. Pulmonary embolism in COVID-19: D-dimer threshold selection should not be based on maximising Youden’s index. Eur Respir J 2021;57:2004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arachchillage DR, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:1233–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Planquette B, Le Berre A, Khider L, et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID-19 patients with respiratory symptoms: a nested case–control study. Thromb Res 2021;197:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grimnes G, Isaksen T, Tichelaar YV, et al. Acute infection as a trigger for incident venous thromboembolism: results from a population‐based case‐crossover study. Res Pract Thromb Haemost 2018;2:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 2020;296:E186–E188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020;296:E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID-19. Radiology 2020;297:E335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaminetzky M, Moore W, Fansiwala K, et al. Pulmonary embolism at CT pulmonary angiography in patients with COVID-19. Radiol Cardiothorac Imaging 2020;2:e200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost 2020;18:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mestre-Gómez B, Lorente-Ramos RM, Rogado J, et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis 2021;51:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ooi MW, Rajai A, Patel R, et al. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography – prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol 2020;132:109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol 2020;30:6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallace A, Albadawi H, Hoang P, et al. Statins as a preventative therapy for venous thromboembolism. Cardiovasc Diagn Ther 2017;7(suppl 3):S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biere-Rafi S, Hutten BA, Squizzato A, et al. Statin treatment and the risk of recurrent pulmonary embolism. Eur Heart J 2013;34:1800–1806. [DOI] [PubMed] [Google Scholar]
- 22. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kollias A, Kyriakoulis KG, Dimakakos E, et al. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol 2020;189:846–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia-Olivé I, Sintes H, Radua J, et al. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med 2020;169:106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Meng G, Li W, et al. Relationship of chest CT score with clinical characteristics of 108 patients hospitalized with COVID-19 in Wuhan, China. Respir Res 2020;21:180. [DOI] [PMC free article] [PubMed] [Google Scholar]


