Abstract
The interactions of coxsackievirus B3 (CVB3), CVB4E2 (diabetogenic), and CVB4JBV (nondiabetogenic) strains with human pancreatic islets from eight adult brain-dead donors were investigated. Persistent replication of viruses in human islets was proved by detection of viral RNA by in situ hybridization, VP1 capsid protein by immunofluorescence (IF) staining, negative-strand viral RNA by reverse transcription-PCR in extracted RNA from islets, and release of infectious particles up to 30 days after infection without obvious cytolysis. By double IF staining, glucagon-containing α cells and insulin-containing β cells were shown to be susceptible to CVB. The persistence of CVB3 and CVB4 in islet cells was associated with the chronic synthesis of alpha interferon (IFN-α), as evidenced by the detection of IFN-α mRNA and immunoreactive IFN-α with antiviral activity. By double IF staining, IFN-α was detected in insulin-producing β cells only. Experiments with neutralizing anti-coxsackievirus and adenovirus receptor (CAR) antibodies provided evidence that CAR was expressed by α and β cells and that it played a role in the infection of these cells with CVB and the consecutive IFN-α expression in β cells. The viral replication and the expression of IFN-α in islets were not restricted to the CVB4E2 diabetogenic strain and did not depend on the genetic background of the host. The neutralization of endogenous IFN-α significantly enhanced the CVB replication in islet cells and resulted in rapid destruction of islets. Thus, human β cells can harbor a persistent CVB infection, and CVB-induced IFN-α plays a role in the initiation and/or maintenance of chronic CVB infection in human islets.
Insulin-dependent diabetes mellitus (IDDM) results from a chronic autoimmune destruction of the pancreatic insulin-producing β cell that is probably initiated by exposure of a genetically susceptible host to environmental factors, including enteroviruses (EVs) (20). Epidemiological data showed an increased incidence of IDDM after epidemics caused by EVs (14), and it appeared that coxsackievirus B (CVB) infection was a possible etiological factor in initiating the destruction of β cells. Indeed, CVB4 was isolated at autopsy from the pancreas of a 10-year-old boy with diabetic ketoacidosis (51). It has also been shown that CVB4 strain E2 isolated from children with IDDM induced β-cell autoimmunity and hyperglycemia in some strains of mice (19). Using reverse transcription (RT)-PCR, we and other authors have reported the presence of the EV genome in the peripheral blood of 27 to 64% of diabetic patients during the clinical manifestation of the disease (1, 10, 32). Recently, we have detected increased levels in plasma of IFN-α associated with CVB infection in 75% of IDDM patients at different stages of the disease (9).
The mechanism by which CVB may induce diabetes is unknown. It has been shown that variants of CVB circulating in the natural population are tropic for the mouse β cells (45). The prototype CVB4, which had been serially passaged through mouse pancreas and then mouse β cells, could replicate in mouse islets and was able to damage β cells in vitro and to induce IDDM in vivo (46). CVB4 can latently infect a rat insulinoma cell line, and a strong correlation between the persistence of viral RNA in the pancreases of mice infected with CVB4 strain E2 and the development of diabetes at 6 months after infection has been observed (16, 41).
In human in vitro systems, Yoon et al. showed the capacity of the prototype CVB3 (Nancy strain) to replicate in β and non-β cells and to destroy human islets 72 h after infection (52). Recently, Roivainen et al. demonstrated that in addition to the diabetogenic strain E2 of CVB4, the prototype strains of CVB3, CVB4, and CVB5 were able to infect human β cells and to cause cell death (39). A clear finding in the pancreases of both recent-onset and previously diagnosed diabetic patients was that β cells in many islets expressed IFN-α detected by immunohistochemistry (15). An increase in IFN-α mRNA expression has also been reported in the pancreases and/or islets of IDDM patients (24, 42). The local production of IFN-α may play an important role in the pathogenesis of IDDM; indeed, transgenic mice in which the β cells express IFN-α develop insulitis, β-cell loss, and diabetes (43). IFN-α is known to be induced by viruses; therefore, the expression of IFN-α in islets of patients with type 1 diabetes may result from the presence of a persistent virus in islet cells.
CVBs have been reported to cause persistent infections of various organs—heart, skeletal muscle, and the central nervous system (13, 27, 44)—and a persistent CVB replication has been observed in different types of cultured cells: i.e., myocardial fibroblasts, lymphoid cells, vascular endothelial cells, and glomerular mesangial cells (11, 12, 21, 31). In some of these models, CVBs appeared to stimulate the synthesis of IFN-β (12, 21).
Proof that CVB can cause a persistent infection of human β cells has not yet been established. The aim of this study was to investigate whether the CVB4 E2 strain compared to other CVB3 and CVB4 strains was able to persist in human islet β cells and whether a CVB infection could induce IFN-α synthesis by these cells.
MATERIALS AND METHODS
Human islet isolation.
Human pancreases (n = 8) were procured from adult brain-dead donors in accord with the local ethical committee and were cold stored (4°C, 10 ± 2 h) in University of Wisconsin solution (Viaspan; Dupont Pharma, Paris, France). Islets were isolated by the semiautomated method of Ricordi et al. (37), with modifications (26), and purified with Euroficoll (Ficoll 400 DL; Sigma, Saint Quentin Fallavier, France) discontinuous density gradients following a multilayer test gradient (26). To increase the purity of preparations, handpicking of islets was performed after 24 h of culture by staining with dithizone (Sigma) under an ocular loop to reduce contaminating exocrine tissue and ductal fragments. The purity of the resulting preparation was visually estimated at >80% after islet handpicking. The number of cells per islet after dissociation was between 200 and 1,000 cells. Immunocytochemical characterization of islet preparations demonstrated that 10 to 20% of the cells were positive for glucagon (α cells), 70 to 80% were positive for insulin (β cells), and 5 to 10% were positive for somatostatin (δ cells).
Human islet culture.
Islet cultures in noncoated membrane inserts have been shown to prevent fibroblast growth and to maintain normal islet function (22). In our islet culture system, we used Nunc tissue culture 8-well strip inserts (A/S Nunc, Roskilde, Denmark) and 96-well plate inserts to study IFN-α production and Millicell-CM (12 mm in diameter; Millipore, Bedford, Mass.) 24-well tissue culture plate inserts to study virus infection. Purified islets collected from each pancreatic preparation were cultured in RPMI 1640 medium (Gibco BRL, Eragny, France) enriched with 1 g of glucose per liter, 10% inactivated fetal calf serum (FCS) (Eurobio, Les Ulis, France), 1% l-glutamine (Eurobio), and antibiotics at 37°C in the presence of 5% CO2. The medium was changed three times per week. By using membrane inserts, the islets remained three dimensional and maintained a free-floating form. The loss of viability of islets was determined by the trypan blue exclusion assay.
Viruses.
CVB3 (American Type Culture Collection [ATCC], Manassas, Va.), the CVB4 JBV strain (provided by J. W. Almond, Whiteknights, United Kingdom), and the CVB4 E2 diabetogenic strain (provided by Ji-Won Yoon, Julia McFarlane Diabetes Research Center, Calgary, Alberta, Canada) were grown in HEp-2 cells (BioWhittaker, Verviers, Belgium) in Eagle's minimum essential medium (MEM; Gibco BRL) supplemented with 10% FCS and 1% l-glutamine. Supernatants were collected 3 days postinfection (p.i.) and then clarified at 1,000 rpm for 10 min. Virus titers were determined by plaque formation assay on HEp-2 cells, and aliquots of virus preparations were then stored frozen at −80°C.
Sendai virus (SV) was provided by D. Garcin (Department of Genetics and Microbiology, University of Geneva, Geneva, Switzerland). The virus was cultivated as previously described (18). Briefly, SV was propagated in eggs and purified by first clarifying chorioallantoic fluid containing SV by centrifugation for 10 min at 1,500 × g at +4°C and then pelleting the SV at 15,000 × g for 16 h. Virus titers were assayed by plaque formation on HeLa cells, and the virus preparation was stored frozen at −80°C until use.
Herpes simplex virus type 1 (HSV-1 [laboratory strain]) was cultivated in a Vero cell line (ATCC) in MEM supplemented with 5% FCS and 1% l-glutamine. After 48 h of incubation at 37°C in a 5% humidified CO2 atmosphere, supernatants were collected and clarified at 1,000 rpm for 10 min. HSV-1 titers were assayed by plaque formation on Vero cells, and virus preparations were stored at −80°C.
Infection of islets.
Pancreatic islets in a final concentration of 100 islets were plated out in Millicell-CM 24-well plate inserts before infection with 1 ml of CVB3, CVB4 JBV, and CVB4 E2 at 2 × 106 PFU/ml (2 × 104 PFU/islet). SV and HSV-1 were used as control viruses at 108 and 106 PFU/ml, respectively. Islet cultures were then incubated in a humidified incubator at 37°C with 5% CO2. At 2 h p.i., islets were washed three times with phosphate-buffered saline (PBS) buffer and then resuspended in fresh growth medium and incubated at 37°C with 5% CO2. For virus titration assay, samples (islets plus supernatants) were collected on days 1, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, and 40 p.i., and then freeze-thawed three times, and after clarification, the supernatants were stored at −80°C until the titer for infectivity was determined with HEp-2 cells. Islet cultures mock infected with medium alone and the last washing solution recovered served as controls. For in situ EV detection, the infected islets were removed from the wells, washed extensively to remove residual viruses, and treated appropriately for EV VP1 immunostaining and EV RNA detection by in situ hybridization (ISH). Positive- and negative-strand RNA detection were also carried out by RT-PCR as described below.
Plaque infectivity assay.
The titers of infectious virus particles in islet cultures were determined by the standard plaque formation assay as the cytopathic effect (CPE) on confluent 48-well plate cultures of HEp-2 cells by using 100 μl of a 10-fold dilution per well. Before inoculation, the cell suspensions were frozen and thawed three times to release the virus and clarified by low-speed centrifugation. The CPE was read on day 7 postinfection, and the results were expressed as end point titers.
IFN-α production in CVB-stimulated islet cultures.
Twenty islets were cultured in eight-well strip inserts placed in a microwell plate for 2 days before infection with 200 μl of CVB3, CVB4 JBV, and CVB4 E2 at 2 × 106 PFU/ml (2 × 104 PFU/islet). Islet cultures were then incubated in a humidified incubator at 37°C with 5% CO2. Supernatants of cultures were collected at days 1, 2, 3, 4, 5, 6, and 7 p.i., cleared from cells, and irradiated with UV light to inactivate residual viruses. They were stored at −20°C until assayed for the presence of IFN-α. To investigate the possibility of continuous IFN-α production, 48 h postinfection, supernatants of cultures were recovered for IFN-α detection, and then islets were washed with PBS buffer and cultured in fresh culture medium for an additional 48 h at 37°C; continuous IFN-α production was monitored for 2 weeks. Cultures not treated with viruses served as controls. The islets were removed from the wells, washed extensively to remove residual viruses, and treated appropriately for IFN-α staining and IFN-α RNA detection by RT-PCR.
Immunoassay for IFN-α.
The concentration of IFN-α in islet cultures was determined by a specific and sensitive dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) based on the direct sandwich technique by using a mixture of two murine monoclonal antibodies to human IFN-α (LT27:273 and LT27:293), which bind more than 90% of natural IFN-α subtypes used to coat microtiter plate wells (LKB WALLAC, Turku, Finland), and a europium-labeled murine anti-human IFN-α, as previously described (5). Samples (100 μl) diluted with an equal volume of dilution buffer containing irrelevant mouse monoclonal immunoglobulin G1 (IgG1) (B1:2) at 50 μg/ml were then added to the plate. After 2 h, plates were washed three times, and 0.2 ml of the europium-conjugated antibody at a 1/800 dilution was added per well in dilution buffer. After 1 h, the plates were washed six times, and 0.2 ml of enhancement solution (LKB WALLAC) was added per well to promote the dissociation of Eu3+ cations from the labeled antibody into solution, where they form fluorescent chelates with components of the Enhancement solution. After 20 min, the fluorescence in the microtitration strips wells was measured in a time-resolved fluorometer (1230 Arcus fluorometer; LKB WALLAC). The National Institutes of Health leukocyte reference IFN G-23-902-530 was used as a standard. The assay detection limit was 0.5 IU of IFN-α/ml.
Bioassay for IFN-α.
Samples from CVB-stimulated islet cultures were assayed for antiviral activity by protection of MDBK (BioWhittaker, Verviers, Belgium) cells against vesicular stomatitis virus (VSV)-induced CPEs as previously described (28). In brief, 0.5 × 105 cells were seeded into each well of 96-well plates and incubated with twofold serial dilutions of supernatant samples for 18 h at 37°C. After incubation, the cells were challenged with VSV, and the plates were incubated at 37°C for 18 h. Virus-induced CPEs were assessed by microscopic examination, and results were expressed as the inverse dilution that provided 50% protection of the cells from virus-induced CPEs. IFN-α concentrations in international units per milliliter were inferred from natural human IFN-α standard (Alferon N Injection; Interferon Sciences, Inc., New Brunswick, N.J.), kindly provided by M. S. Liao. The assay detection limit was 2 IU of IFN-α/ml.
Typing of IFN by neutralizing antibodies.
The antiviral activity of supernatant samples was neutralized with a rabbit antiserum to IFN-α (Biosource, Nivelles, Belgium) at a final concentration of 1,000 neutralizing units (NU)/ml: 1 NU of antibodies per ml neutralizes 1 IU of IFN-α activity per ml. Samples were preincubated for 60 min at 37°C with the relevant antibody before being assayed for antiviral activity by protection of MDBK cells against VSV.
RNA extraction.
Native RNA was extracted from islet specimens by the acid guanidinium thiocyanate-phenol chloroform extraction procedure by using a commercial system (RNAgents Total RNA Isolation System; Promega, Madison, Wis.). Extracted RNA was then dissolved in 50 μl of diethylpyrocarbonate-treated water (Sigma-Aldrich, Saint Quentin Fallavier, France) and used in the RT-PCR assays. Two positive extraction controls were used. The first was used for IFN-α RNA detection and consisted of RNA extracted from whole blood cultivated as previously described (8) and activated by SV for 5 h to induce IFN-α mRNA synthesis. The second positive control consisted of RNA extracted from HEp-2 cells infected with CVB3 for 4 h and was used for EV RNA detection.
Oligonucleotide primers.
Human IFN-α1 primers were purchased from Clontech, Palo Alto, Calif. The upstream primer 5′-TGATGGCAACCAGTTCCAGAAGGCTCAAG-3′ and the downstream primer 5′-ACAACCTCCCAGGCACAAGGGCTGTA-TTT-3′ generate a 303-bp PCR product.
The sequences of the EV-specific primers were selected in the 5′ untranslated region of the viral genome, since this region is highly conserved among all EV strains. The upstream primer 5′-CAAGCACTTCTGTTTCCCCGG-3′ and downstream primer 5′-ATTGTCACCATAAGCAGCCA-3′ generate a 435-bp fragment. These primers were synthesized by Eurogentec (Seraing, Belgium) and were previously described by Leparc et al. (29).
β-Actin primers were synthesized by Eurogentec (upstream primer, 5′-ATCATGTTTGAGACCTCCAA-3′; downstream primer, 5′-CATCTCTTGCTCGAAGTCCA-3′). The amplified PCR fragment size is 318 bp.
One-step RT-PCR for IFN-α RNA detection.
The cDNA synthesis and the cDNA amplification were performed in a single tube with the Sigma Enhanced avian RT-PCR kit according to the manufacturer's instructions. The one-step RT-PCR was performed in a total volume of 50 μl containing 1 μM (each) upstream and downstream primers, 0.1 μg of extracted RNA, 20 U of enhanced avian reverse transcriptase, 2.5 U of Accu Taq LA DNA polymerase, 40 U of RNase inhibitor, 0.5 mM (each) deoxynucleoside triphosphate (dNTP), 3 mM MgCl2, 10 mM Tris-HCl (pH 8.3), and 50 mM KCl. The RT reaction was performed at 50°C for 30 min, followed by RT inactivation at 94°C for 2 min; the mixture was then subjected to 35 cycles of amplification, consisting of denaturation for 30 s at 94°C, annealing for 30 s at 60°C, and extension for 1 min at 70°C. The RT-PCR was carried out with a Perkin-Elmer Applied Biosystems GeneAmp 9700 thermocycler.
For each RNA sample, β-actin mRNA was retranscribed in cDNA, amplified by RT-PCR, and used as a positive control to prove the absence of RT-PCR inhibitors. A negative control (no RNA) was also included in each PCR. The absence of introns within IFN-α genes precludes the distinction between amplification products resulting from reverse-transcribed IFN-α1 mRNA and those arising from residual genomic DNA. Therefore, the absence of contaminating genomic DNA was verified for each RNA sample by carrying out the RT-PCR reaction without adding the reverse transcriptase. Only samples without contaminating genomic DNA have been considered.
Two-step RT-PCR for positive- and negative-strand EV RNA detection.
A downstream or upstream EV primer at 0.4 μM was used as a template in synthesis of complementary DNA (cDNA) for 0.1 μg of positive- or negative-strand EV RNA, respectively, in a total volume of 20 μl containing 20 U of Enhanced avian reverse transcriptase, 0.5 mM each dNTP, 20 U of RNase inhibitor, 50 mM Tris-HCl (pH 8), 40 mM KCl, 8 mM MgCl2, and 1 mM dithiothreitol by using the Enhanced avian RT-PCR kit according to the manufacturer's instructions. The RT reaction was performed at 50°C for 50 min and was stopped by heating the samples for 5 min at 95°C. The PCR was carried out in a Perkin-Elmer Applied Biosystems GeneAmp 9700 thermocycler, with 5 μl of cDNA samples and 0.4 μM each primer, in a total volume of 50 μl containing 2.5 U of Accu Taq LA DNA polymerase, 0.2 mM each dNTP, 2.5 mM MgCl2, 50 mM Tris-HCl, and 15 mM ammonium sulfate (pH 9.3). The PCR mixture was subjected to 35 cycles of amplification, consisting of denaturation for 30 s at 94°C, annealing for 45 s at 55°C, and extension for 45 s at 68°C. RNA extracted from HEp-2 cells infected with CVB3 for 4 h was retrotranscribed and then amplified in the same way for the detection of positive- and negative-strand EV RNA. For all specimens, β-actin mRNA was amplified by using specific primers in an RT-PCR.
Detection of PCR products.
The amplified RT-PCR products were analyzed on a 2% agarose gel containing 0.5 μg of ethidium bromide per ml (Sigma) and visualized by using the Gel Doc 2000 system (Bio-Rad SA, Ivry-sur-seine, France). Image processing and analysis of DNA bands were performed with Quantity One software (Bio-Rad SA). A 100-bp DNA Ladder (Gibco BRL, Paris, France) was used as a molecular weight marker.
Sequence analysis of IFN-α-amplified products.
Prior to sequencing, the IFN-α PCR products were purified with the Wizard PCR Preps DNA Purification System (Promega). Both strands of the DNA fragments were sequenced. The nucleotide sequence of fragments was determined by double-strand DNA cycle sequencing with an AmpliTaq FS Big Dye Terminators dichlororhodamine cycle sequencing kit (Applied Biosystems, Foster City, Calif.) according to the manufacturer's instructions. Electrophoresis and analysis of DNA sequence reactions were carried out with an automated DNA sequencer (model 377 XL; Applied Biosystems). The sequencing primers for IFN-α were the same as those used in RT-PCR. The derived sequences were then analyzed and compared to each other and to sequences available from GenBank database by using the Usedit and Sequence Navigator programs (Applied Biosystems).
Antibodies for immunofluorescence.
Monoclonal antibodies to human IFN-α LT27:273 and LT27:293 (see “Immunoassay for IFN-α”) were used for IFN-α immunostaining. Monoclonal anti-EV VP1 peptide, polyclonal rabbit anti-human glucagon, and polyclonal rabbit anti-human somatostatin antibodies were purchased from DAKO (Carpinteria, Calif.). Polyclonal guinea pig anti-human insulin was purchased from Biogenex (San Ramon, Calif.). Fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse immunoglobulin G (IgG), tetramethyl rhodamine isothiocyanate (TRITC)-conjugated sheep anti-mouse IgG, TRITC-conjugated rabbit anti-guinea pig IgG, and TRITC-conjugated goat anti-rabbit IgG were purchased from Sigma.
IF.
CVB-infected islets and mock-infected islets were carefully washed in RPMI medium after different time intervals p.i. The islets were then gently removed from the wells and dissociated as previously described (34), and then 2 × 105 cells were cytocentrifuged onto clean glass slides. The slides were air dried and fixed in a solution of 4% paraformaldehyde–PFA (Sigma) for 20 min at 4°C. The slides were washed twice in PBS and permeabilized with cold acetone-methanol (1 vol/2 vol) for 10 min at −20°C. Free aldehyde groups were reduced by immersing the slides in solution containing 50 mM NH4Cl (Merck) and 20 mM glycine (Sigma) for 30 min at room temperature. Unspecific protein binding sites were blocked by incubating the slides for 30 min at room temperature in PBS supplemented with 1% human serum albumin (Sigma) and 1% FCS. After rinsing in PBS, cells were stained for IFN-α, EV VP1 peptide, insulin, glucagon, or somatostatin with the antibodies described above for 1 h at room temperature. Following three washes in PBS, incubation with an FITC- or TRITC-conjugated second antibody was performed for 1 h at RT. For double staining, the respective antibodies were applied sequentially. When only FITC-conjugated second antibody was applied, slides were counterstained with Evans blue (Sigma). The slides were then mounted with Permafluor (Coulter Immunotech), and positive cells were enumerated in a fluorescence microscope (Leitz Diaplan, Wetzlar, Germany).
ISH.
EV RNA-specific ISH was performed with cytospin preparations with nuclease-free polylysine-coated glass slides (Poly Labo, Paris, France). Cells of dissociated islets (2 × 105 per spot) were fixed in acetone for 20 min at room temperature and then treated for 5 min at room temperature with proteinase K (DAKO) diluted at 1:5,000 in 50 mM Tris-HCl buffer (pH 7.6) (E. Merck). Slides were washed and immersed in 0.3% H2O2 in methanol at room temperature for 1 h. Afterwards, secondary structures in RNA template were denatured by immersing the slides in Tris-buffered saline at 75°C for 10 min. After washing, slides were hybridized in mRNA ISH solution (DAKO) containing 1 ng of biotinylated probe [5′-(biotin)AAC-ACG-GAC-ACC-CAA-AGT-A-3′] per μl. The probe was synthesized and labeled by Eurogentec. For each slide, 15 μl of hybridization mixture was applied before nuclease-free Hybrislips (Poly Labo) were mounted, and hybridization was performed at 50°C for 60 min in a humidified chamber. Slides were then washed in Tris-buffered saline solution containing Tween (DAKO) for 20 min at room temperature. The hybridized probe was revealed by using the Genpoint System (DAKO). The Genpoint System consists of an amplification of biotin signal by initial binding of peroxidase-conjugated streptavidin to the biotinylated probe, followed by application of biotinyl-tyramide. The additional biotin is then used to capture more peroxidase-conjugated streptavidin. The signal is finally developed by adding diaminobenzidine dye to produce a dark brown precipitate at the site of hybridization. The sensitivity of the ISH test was determined on HEp-2 cells infected with CVB3. Highly significant labeling was observed in 20% of HEp-2 cells when infected at a multiplicity of infection (MOI) of 1 PFU and then hybridized 4 h after infection, whereas 90 to 100% of HEp-2 cells showed a positive signal when infected at an MOI of 100 PFU (data not shown). To determine the specificity of the signal, dissociated islet cells were subjected to RNAse A (Boehringer Mannheim) treatment at 100 μg/ml for 2 h at 37°C before hybridization. RNAse was heated at 100°C for 10 min and then allowed to cool to room temperature before use, to abolish residual DNase activity.
Blocking and control antibodies.
Monoclonal IgG1 anti-human coxsackievirus and adenovirus receptor (CAR) antibody (RmcB) was kindly provided by J. Bergelson (Division of Infectious Disease, Children's Hospital of Philadelphia, Philadelphia, Pa.). Polyclonal rabbit neutralizing anti-CVB3 and anti-CVB4 antibodies were purchased from Eurobio. Murine IgG1 (Coulter Immunotech) and normal rabbit serum (Sigma) were used as antibody controls.
Statistical analysis.
Data are summarized as means ± standard deviations. The significance of the differences of IFN-α levels was determined by Mann-Whitney U test. Correlations were evaluated with Spearman's test.
RESULTS
CVB infection of human pancreatic islets.
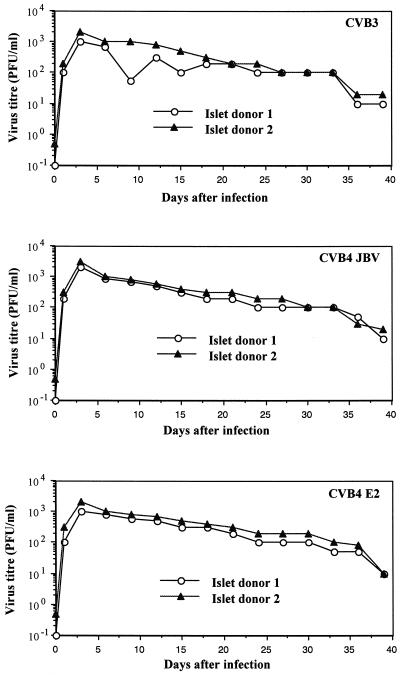
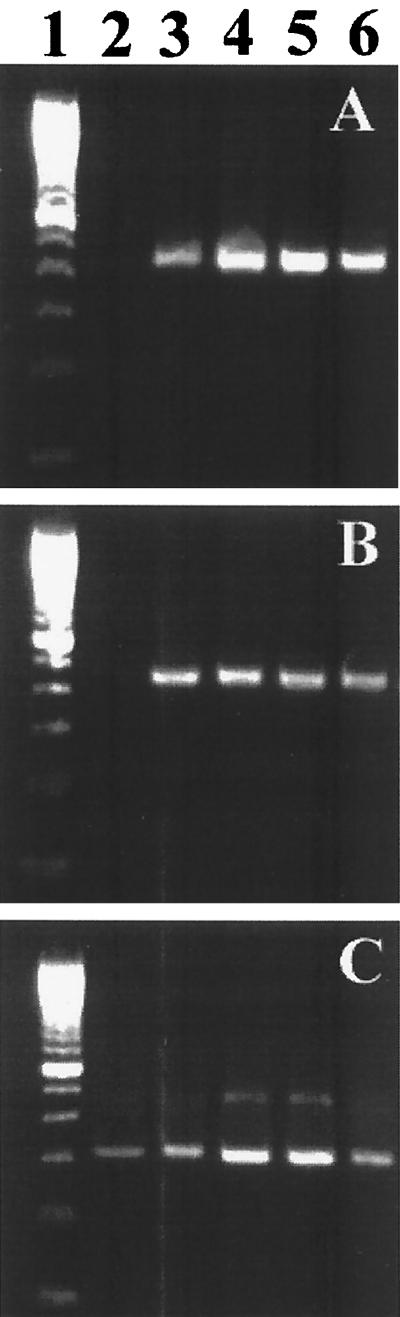
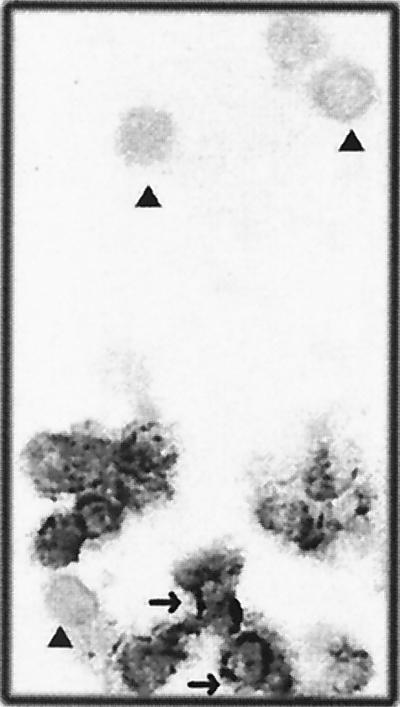
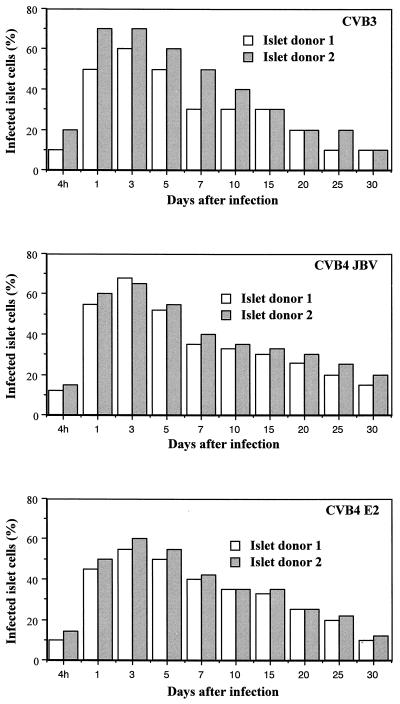
The islet viability in the 24-well plate inserts was 100% and remained constant for 4 weeks if the growth medium was changed three times per week, as determined by trypan blue exclusion assay. There was no obvious fibroblast growth. Islets in membrane inserts remained in a single form with a normal round three-dimensional structure. Only a few peripheral trypan blue-positive cells can be detected. Islet cultures from two donors consistently allowed replication of the three strains of CVB at 2 × 104 PFU/islet. As shown in Fig. 1, infectivity assays revealed a steady increase in viral titer of CVB3, CVB4 JBV, and CVB4 E2, peaking at 1 × 102 to 10 × 102 PFU/ml at 3 days after infection. Virus production decreased thereafter, but was not stopped by serial subculture for 30 days after infection, suggesting that CVB replicated persistently in pancreatic islet cells. By light microscopy, CVB-infected islets showed no evidence of cytopathology within 1 month of infection. The numbers of viable islets (trypan blue exclusion assay) did not change significantly in the infected cultures. The loss of islet viability appeared 30 days p.i. in both infected and mock-infected islets. No significant difference was seen in the time course of infection between CVB3, CVB4 JBV, and CVB4 E2. Positive- and negative-strand EV RNAs were detected by RT-PCR in CVB-infected islets at 4 h p.i. and during 30 days of infection (Fig. 2). By using ISH, we were able to detect positive-strand CVB RNA in dissociated infected islet cells (Fig. 3). The proportion of CVB RNA-positive cells was 10 to 20% at 4 h p.i., 50 to 70% in the acute phase of infection (1 to 5 days), and 10 to 20% in the chronic phase of infection (20 days p.i. and after) (Fig. 4). No specific signal was obtained in the absence of biotin-labeled probe, and staining was abolished by treating the cells with RNAse A for 2 h before hybridization.
FIG. 1.
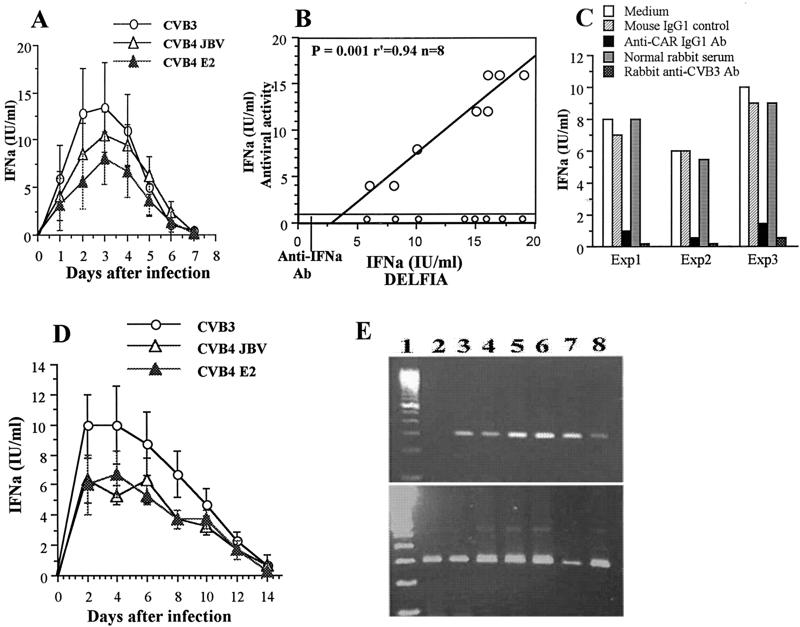
Release of infectious particles by human pancreatic islets infected with CVB3, CVB4 JBV, and CVB4 E2. The viral titer in culture supernatant was determined every 3 days of subculture by plaque infectivity assay on HEp-2 cell cultures.
FIG. 2.
Agarose gel electrophoresis of amplicons specific to the positive (A) and negative (B) strands of EV genomes. Strand-specific RT-PCR was carried out on total RNA taken from mock-infected islets (lane 2) and CVB4 E2-infected islets at 4 h, 7 days, 15 days, and 30 days p.i. (lanes 3 to 6, respectively). Lane 1 corresponds to a 100-bp DNA molecular size ladder. Equivalent expression of β-actin mRNA was detected in all samples (C). Similar results were obtained with CVB3 and CVB4 JBV and with islets from eight adult brain-dead donors.
FIG. 3.
In situ hybridization for sense-strand RNA of CVB4 E2 in human pancreatic islet cells. Islets were infected with CVB4E2 for 24 h and then dissociated and fixed before being hybridized with a biotinylated probe. ▴, islet cell without positive signal; ➞, islet cell with positive signal. Similar results were obtained with CVB3 and CVB4 JBV and with islets from eight adult brain-dead donors. Magnification, ×100.
FIG. 4.
Percentage of islet cells infected with CVB3, CVB4 JBV, and CVB4 E2 as determined by detection of sense-strand RNA by ISH at different intervals after infection.
Islets from eight donors infected in vitro with CVB3, CVB4 JBV, and CVB4 E2 were stained positively for EV VP1 by indirect FITC staining. A green color was seen in the cytoplasm of dissociated islet cells (Fig. 5A). The proportions of VP-1-positive cells were 30 to 60% 24 h p.i. and 5 to 10% in the chronic phase of infection (20 days p.i. and after). The preincubation of islet cells with antihuman CAR neutralizing antibody for 1 h before CVB infection resulted in a 20-fold decrease in the number of VP1-positive cells (data not shown).
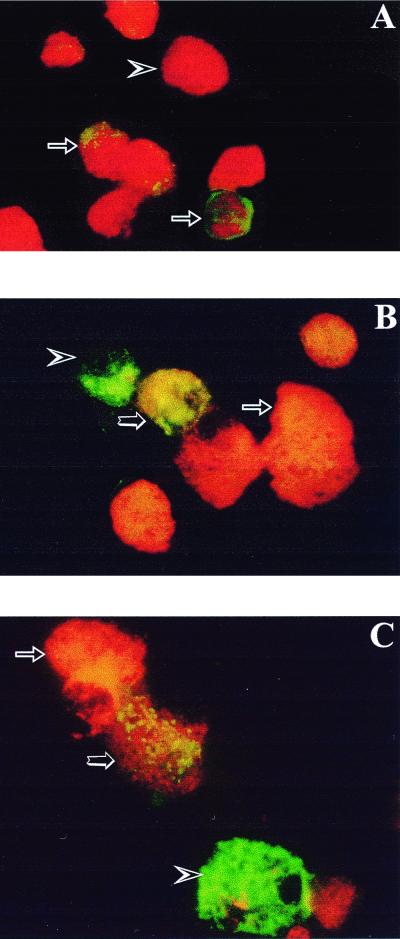
FIG. 5.
Detection of enterovirus VP1 by IF staining in dissociated human islet cells 24 h after infection with CVB4 E2. (A) Cells indirectly stained for VP1 with FITC-conjugated antibodies and counterstained with Evans blue. Arrows, VP1-positive cells (green); arrowhead, VP1-negative cells (red). (B) Cells double stained for VP1 with FITC-conjugated antibodies (arrowhead, green) and for insulin with TRITC-conjugated antibodies (arrow, orange). In double-stained cells (large arrow), yellow was observed. (C) Cells double stained for VP1 with FITC-conjugated antibody (arrowhead, green) and for glucagon with TRITC-conjugated antibody (arrow, orange). In double-stained cells (large arrow), yellow was observed. Original magnification, ×400.
To identify the human pancreatic cells infected with CVB, islet cultures were stained for EV VP1 with FITC-labeled antibody and for insulin, glucagon, or somatostatin with TRITC-labeled antibody. VP1 was detected in insulin-containing cells and in glucagon-containing cells (Fig. 5B and C). At 8 h p.i., EV VP1 was found in about 1 to 2% of the insulin-containing cells and in about 20 to 30% of the glucagon-containing cells. At 24 h p.i., 20 to 30% of the insulin-containing cells and 30 to 50% of the glucagon-containing cells were VP1 positive. After 20 days of infection, 2 to 5% of the insulin-containing cells and 10 to 20% of the glucagon-containing cells were VP1 positive. No evident positive VP1 signal could be detected in somatostatin-containing cells (data not shown).
IFN-α detection in CVB-stimulated islet cultures.
By using 24-well plate inserts which contained 100 islets in total volume of 1 ml, no IFN-α production could be detected in supernatants of infected-islet cultures, whereas by using 96-well plate inserts which contained 20 islets in a total volume of 0.2 ml, significant IFN-α production was detected in supernatants of islet cultures 24 h after challenge with CVB3, CVB4 JBV, and CVB4 E2 at 2 × 104 PFU/islet by the DELFIA method. Concentrations peaked at 72 h (p.i.) and remained high for 24 h before falling at 5 days p.i. (Fig. 6A). The mean levels of IFN-α produced 72 h p.i. by pancreatic islets isolated from eight donors were 13.4 ± 4.7 IU/ml (range, 8 to 19 IU/ml) for CVB3, 10.6 ± 2.4 IU/ml (range, 6 to 13 IU/ml) for CVB4 JBV, and 8.1 ± 2.7 IU/ml (range, 4 to 12 IU/ml) for CVB4 E2. No IFN-α production was detected in mock-infected islets, and no significant difference in IFN-α production levels was observed between the three strains of CVB. To further examine the biological activity of supernatants of CVB-stimulated islet cultures, we determined its antiviral activity by protection of MDBK cells against VSV-induced CPEs. The supernatant samples were absorbed with anti-IFN-α neutralizing antibodies (vol/vol). This treatment resulted in a complete removal of the antiviral effect. Therefore the results were expressed as IFN-α concentrations in international units per milliliter, inferred from the human IFN-α standard. A significant correlation was obtained between the IFN-α levels detected by using DELFIA and those detected by the antiviral assay (P < 0.0001; n = 8) (Fig. 6B). The preincubation of CVB3, CVB4 JBV, and CVB4 E2 with anti-CVB3 or anti-CVB4 neutralizing antibodies before infecting the islets resulted in no IFN-α detection in supernatants of islet cultures (Fig. 6C). To further examine the role of CAR on IFN-α production, islet cultures were preincubated for 1 h with monoclonal anti-CAR antibody before challenging the islets with CVB3 or CVB4. As shown in Fig. 6C, the levels of IFN-α were decreased significantly in supernatants of islet cultures.
FIG. 6.
Synthesis of IFN-α in CVB-infected human islets cultured in 96-well plate inserts. (A) Kinetics of production of IFN-α (IFNα) in supernatants of CVB-infected human islet cultures as determined by DELFIA. Means and standard deviations of IFN-α values obtained from eight different islet donors are shown. (B) Relationship between the values of IFN-α assayed by the DELFIA method and antiviral titers in supernatants of CVB3-infected islet cultures at 3 days p.i. Ab, antibodies. (C) Effect of anti-CAR and anti-CVB3 neutralizing antibodies on the levels of IFN-α measured by DELFIA in supernatants of CVB3-infected human islets at 3 days p.i. Human islets were preincubated with monoclonal anti-CAR IgG1 antibodies for 1 h before challenge with CVB3. In parallel experiments, CVB3 was preincubated with polyclonal rabbit anti-CVB3 antibodies for 1 h before infecting human islets. Mouse IgG1 and normal rabbit serum were used as immune serum controls. These experiments were performed with islets from three brain-dead donors. (D) Continuous release of IFN-α in supernatants of human islet cultures infected with CVB3, CVB4, JBV, and CVB4 E2 for 2 h and submitted to serial subculture. Means and standard deviations of IFN-α detected by DELFIA are shown. (E) (Top) Agarose gel electrophoresis of amplicons specific to the IFN-α mRNA. Strand-specific RT-PCR was carried out on total RNA taken from mock-infected islets (lane 2) and CVB4 E2-infected islets at 8 h and 1, 2, 6, 8, and 12 days p.i. (lanes 3 to 8, respectively). Lane 1 correspond to a 100-bp DNA molecular size ladder. (Bottom) The expression of β-actin mRNA in each sample was investigated.
Continuous IFN-α production was detected when islets were challenged for 2 h with CVB and then washed and refed with complete medium every 48 h. The mean levels of IFN-α released every 48 h as determined from three experiments were 8.8 ± 2.3 IU/ml for CVB3, 5.4 ± 1.5 IU/ml for CVB4 JBV, and 5.4 ± 1.6 IU/ml for CVB4 E2. Due to the difficulty of subculturing the islets for more than 2 weeks in 96-well plate inserts, continuous release of IFN-α in islet cultures was stopped 10 to 12 days after challenge with CVB (Fig. 6D). This continuous release of IFN-α was due to de novo synthesis of IFN-α mRNA as detected in extracts of CVB-stimulated islets at the time course of infection, whereas no IFN-α transcripts were detected in mock-infected islets (Fig. 6E). The continuous synthesis of IFN-α mRNA was also detected in extracts of CVB-stimulated islets cultured in 24-well plate inserts (data not shown). Sequence analysis of amplified products demonstrated 99% homology with the IFN-α1 sequence (data not shown).
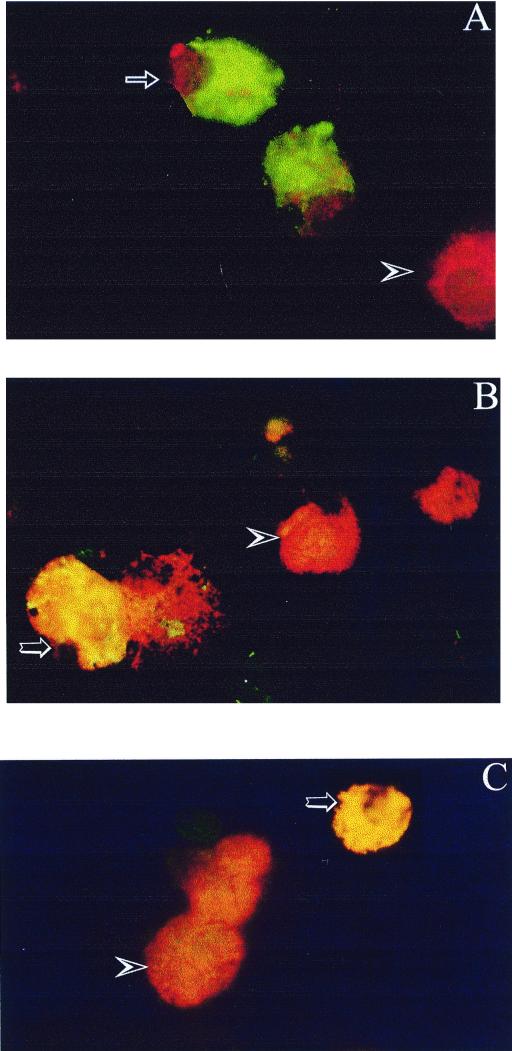
Islet cells cultured in 96-well plate inserts and infected with CVB3, CVB4 JBV, or CVB4 E2 were stained positively for IFN-α by indirect FITC staining (Fig. 7A). At 8 h p.i., IFN-α was found in 0.1 to 1% of dissociated islet cells, whereas at 24 h p.i., IFN-α was found in 50 to 75% of islet cells. No staining was detected in mock-infected islet cells. IFN-α was detected by indirect staining in CVB-infected islets cultured in 24-well plate inserts as well; in the chronic phase of infection (20 to 30 days p.i.), 5% of islet cells were IFN-α positive (data not shown). To identify the islet cells that produce IFN-α, CVB4 E2-infected islets were stained 8 h p.i. for IFN-α with FITC-labeled antibody and for insulin with TRITC-labeled antibody. Yellow spots were seen in the cytoplasm of double-stained cells (Fig. 7B). At 8 and 24 h p.i., IFN-α was found only in insulin-containing cells, whereas no positive signal was found in the other cells. The islet cells were double stained for IFN-α and VP1 (Fig. 7C). At 24 h p.i., all IFN-α-positive cells were VP1 positive, whereas almost 30% of VP1-positive cells were IFN-α positive.
FIG. 7.
Immunofluorescence staining of IFN-α protein in dissociated human islet cells 24 h after infection with CVB4 E2. (A) Cells indirectly stained for IFN-α with FITC-conjugated antibody and counterstained with Evans blue. Arrow, IFN-α-positive cell (green); arrowhead, IFN-α-negative cell (red). (B) Cells stained for IFN-α with FITC-conjugated antibody and for insulin with TRITC-conjugated antibody. The insulin-positive cell is orange (arrowhead), and the double-stained cell is yellow (large arrow). (C) Cells stained for IFN-α with FITC-conjugated antibody and for EV VP1 peptide with TRITC-conjugated antibody. The VP1-positive cell is orange (arrowhead), and the double-stained cell is yellow (large arrow).
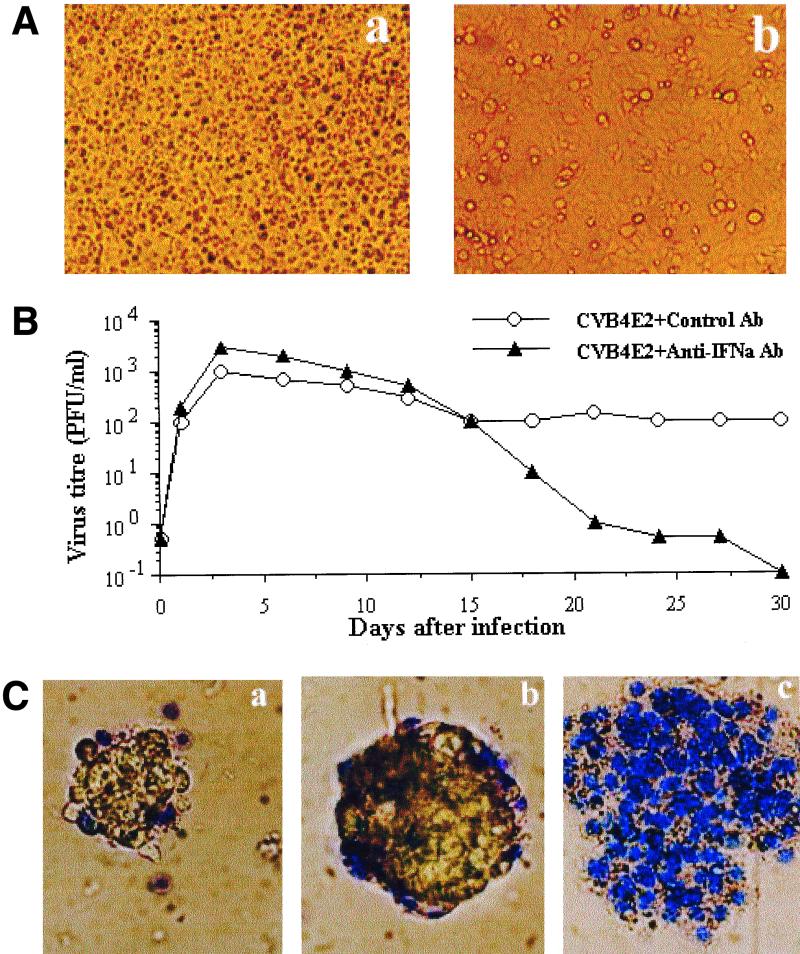
The anti-CVB activity of IFN-α produced in pancreatic islet cultures was analyzed on HEp-2 cells. The preincubation of HEp-2 cells with supernatant of islet culture irradiated with UV light to inactivate residual viruses protected these cells against CVB4-induced CPE (Fig. 8A). This anti-CVB activity was abolished when the supernatant of islet culture was pretreated with neutralizing anti-IFN-α antibody (data not shown).
FIG. 8.
Antiviral activity of IFN-α produced by CVB4 E2-infected islets. (A) Antiviral activity of culture supernatant of CVB4 E2-infected islets. (a) Mock-infected islets. (b) CVB4 E2-infected islets. Human islets were cultured in 96-well plate inserts. Supernatants of islet cultures were UV irradiated before being added to HEp-2 cells and incubated for 24 h. HEp-2 cells were then washed and challenged with CVB4 E2 for 2 h and incubated for 24 h at 37°C. (B) Effect of anti-IFN-α neutralizing antibodies (Ab) on the susceptibility of human pancreatic islets to CVB4 E2. Islets cultured in 24-well plate inserts were infected with CVB4 E2 in the presence of anti-IFN-α neutralizing antibodies for 2 h. Islets were then washed and refed each 3 days with fresh medium supplemented with anti-IFN-α antibodies. The viral titers in culture supernatants were determined every 3 days of subculture by plaque infectivity assay on HEp-2 cell cultures. (C) Islet viability. (a) Mock-infected islets. (b) CVB4 E2-infected islets (c) CVB4 E2-infected islets cultured in the presence of anti-IFN-α neutralizing antibodies. The viability was assessed at 12 days after infection by using the trypan blue exclusion assay. These experiments were performed with islets from six brain-dead donors. Magnification, ×100.
The influence of endogenous IFN-α on islet susceptibility to the CVB4 E2 strain was analyzed in islets cultured in 24-well plate inserts and infected in the presence of growth medium containing anti-IFN-α neutralizing antibody at 1,000 NU/ml. After 2 h of infection, islets were washed and refed each 3 days with fresh medium supplemented with anti-IFN-α antibody. As shown in Fig. 8B, the neutralization of IFN-α increased two- to threefold the titer of infectious viruses released 2 days p.i. In the absence of anti-IFN-α antibody, infected islets showed no evidence of cytopathology. Viable islets in the membrane insert remained in a single islet form with a normal three-dimensional structure for several weeks. Only a few peripheral trypan blue-positive cells could be detected (Fig. 8Ca and b). A loss of viability of the CVB-inoculated islets was detected in the presence of anti-IFN-α neutralizing antibody, almost 50% of islets were destroyed 7 days after infection, and no viable islet was seen at 12 days postinfection. Dead islets appeared as a disturbed flat structures with every cell positive for trypan blue dye (Fig. 8Cc). The survival of islets cultured with the anti-IFN-α neutralizing antibody without being infected by virus was not different from the one in control islets (data not shown).
DISCUSSION
In other studies, the infection of human islets with CVB has been described (39, 49, 52); however, our study is different in many respects from those of other investigators. First, the infection of long-term islet cultures with CVB has been studied; second, the viral replication at the molecular level was investigated; third, the relationship between CVB infection and IFN-α synthesis was determined; and fourth, the nature of the infected cells and of the IFN-α-producing cells was studied.
Studies of the interaction of virus with insulin-containing cells have been restricted by the difficulty in preparing cultures enriched in β cells. In addition, long-term maintenance of human pancreatic islets in culture is difficult because of rapid fibroblast proliferation. In these cultures, the purity of the endocrine component decreases and islet viability is impaired. In the current study, the purity of islet culture was increased (>80%) by reducing contaminating exocrine tissue and ductal fragments by islet handpicking, and to avoid fibroblast contamination, pancreatic islets were cultured with noncoated transparent membrane inserts. Since the membrane does not allow cell attachment or fibroblast growth, islets cultured in the insert maintained their original structure and remained in a free-floating form after long-term culture (22). Virus infection was carried out with intact islets rather than dissociated cells, because tissues that are resistant to virus infections in vivo can artificially be made susceptible by dispersing the cells into culture (40).
The detection of infectious particles released in the supernatants of islet cultures, as well as the presence of VP1 and viral RNA in islet cells and the presence of viral negative-strand RNA in cultures up to 30 days p.i., shows that continuous CVB replication can occur in pancreas islets. α and β cells are susceptible to CVB, as displayed by IF and ISH. This is in agreement with previous studies from Yoon et al., who showed by double staining that human insulin- and noninsulin-containing cells can be infected with CVB3 (52). CAR is a 46-kDa protein recently purified from HeLa cells and identified as a receptor responsible for CVB and adenovirus infection of human cells, and the expression of human CAR mRNA in various organs, including the pancreas, has been reported (2, 3). The strong reduction in the number of VP1-positive cells obtained by preincubating the cells with anti-CAR neutralizing antibodies in our experiments suggests for the first time that CAR is expressed by human α and β cells and plays a major role in the infection of these cells with CVB3 and CVB4.
This is the first study of the synthesis of IFN-α by human pancreatic islets after challenge with CVB. A factor present in the supernatant of CVB-infected islets was able to protect MDBK and HEp-2 cells from CPEs induced by VSV and CVB, respectively. This antiviral factor has been identified as IFN-α, since absorption with anti-IFN-α antibodies could eliminate its antiviral activity. IFN-α mRNA was detected in extracts of CVB-stimulated islets cultured in 24- and 96-well inserts, whereas no IFN-α transcripts were detected in mock-infected islets. IFN-α was detected in supernatants of CVB-infected islets cultured in 96-well plate inserts only. It can be due to the larger dilution of IFN-α in 24-well inserts (1-ml total volume) than in smaller inserts (0.2-ml total volume); in addition, since fivefold more islets were cultured in 24-well plate inserts, IFN-α may be released and consumed locally at the site where the reaction occurred. Indeed, IFN type 1 receptor is present at the surface of islet cells, since IFN-α induces TAP-1 and HLA class I hyperexpression in human β-cells, as previously reported (48). The absence or low level (under the limit of the assay) of IFN-α in culture supernatants of islets cultured in 24-well inserts explains the successful detection of infectious particles in HEp-2 cells in our experiments; otherwise, significant amounts of IFN-α in culture supernatants of islets would have inhibited the infection of HEp-2 cells and hence would have prevented the detection of infectious particles. In mock-infected islet cultures, immunoreactive IFN-α and IFN-α RNA were not detected, which is in disagreement with studies from other authors who reported increased levels of mRNA encoding IFN-α in human islets (6, 24). These discrepancies may be due to the conditions of isolation and culture of islet cultures; indeed in these studies, mRNA IFN-α was detected in short-term cultures (6 h), probably as a result of hypoxia, whereas in our experiments, islets were obtained 24 h after the isolation processing. Moreover, a method for detecting mRNA IFN-α1 was used in our studies, whereas constitutive expression of mRNA IFN-α subtypes 5 and 21 in pancreas tissue has been reported, which can explain the negative detection of IFN-α in control cultures in our experiments. Together, our results strongly indicate that the IFN-α system is activated in CVB-infected islet cells.
Thirteen different IFN-α proteins have been recognized (33). The consensus primers used in this study were capable of detecting IFN-α1 sequences in the islets of eight donors infected with CVB in vitro. Interestingly, IFN-α1 was one of the different IFN-α subtypes detected by RT-PCR and sequencing in islets from a diabetic patient in previous studies (24). Together these results suggest that CVB can be responsible for the expression of IFN-α detected in the pancreases of diabetic patients (15, 24, 42).
According to the double-staining IF of CVB-infected islets, and within the limits of the assay, IFN-α was present in insulin-containing cells, whereas IFN-α was not detected in glucagon-containing cells. The significant decrease in IFN-α levels in culture supernatants when CVB was preincubated with anti-CVB neutralizing antibodies and when islets were preincubated with anti-CAR neutralizing antibodies provides evidence that the expression of IFN-α in our experiments was induced by CVB as a result of an interaction with CAR. Moreover, a continuous IFN-α synthesis was observed in the course of the culture of CVB-infected islets, and IFN-α was detected in cells stained for VP1 only. In contrast to the results obtained with human islets, it has been reported that CVB viruses are poor IFN-α inducers in vitro in the presence of peripheral blood mononuclear cells and that they do not replicate in these cells (36; Chehadeh et al., unpublished data). No IFN-α production was detected in SV- and HSV-1-infected islets, whereas high IFN-α levels (>500 IU/ml) were detected in culture supernatant of peripheral blood mononuclear cells incubated in the presence of these viruses (data not shown), which argues against the role of immune cells contained in islets in the CVB-induced IFN-α synthesis in our experiments. Taken together, these data show that CVB replicated in α and β cells which resulted in the synthesis of IFN-α by β cells only.
A relatively high proportion (50 to 70%) of cells containing viral proteins was detected in the earlier stages of infection by IF and ISH, which can be explained by the susceptibility of both α and β cells to CVBs and by the widespread expression of CAR on the cell membranes of both cell types. By contrast, a small proportion of islet cells (10 to 20%) appeared to be involved in viral replication during chronic infection, as evaluated by ISH. The relative quantities of positive and negative RNA strands, compared by serial end point dilution, indicated that the amount of positive RNA strands was in a large excess over negative RNA strands—almost 100- and 50-fold in lytic CVB4-infected HEp-2 cells and in CVB4-infected human islets, respectively (data not shown). These results suggest that CVB replicates in human islets and that virus persistence in islets is probably established through the mechanism of the carrier-state culture as reported for other cell types (11, 21, 31). Several factors (e.g., modulation of virus receptors, production of antiviral mediators, and selection of viral variants) may be responsible for the initiation and maintenance of CVB persistence. The neutralization of endogenous IFN-α significantly enhanced the CVB replication in islet cells cultured in 24-well plate inserts and resulted in a rapid destruction of islets; these results suggest that IFN-α is released by islet cells, even though it was not detected in the culture supernatant and that, through short autocrine or paracrine loops, IFN-α plays a role in the initiation and/or the maintenance of chronic CVB infection in human islets. This is in agreement with the role of endogenous type I IFN (IFN-β) in the persistence of CVB in human endothelial cells, myocardial fibroblasts, and glomerular mesangial cells as previously described (11, 12, 21).
There is heterogeneity in the susceptibility of β cells to the CVB infection. This can be due to the presence of subpopulations of β cells in islets or to the metabolic heterogeneity of these cells (35). In addition, a heterogeneous susceptibility of β cells to the effects of IFN-α could play a role; indeed, it has been reported that there is a differential response to IFN-α in islet cells in a rat model (4). Further experiments are needed to clarify the mechanisms of the susceptibility of human islet cells to CVB.
The decrease in the proportion of infected cells in the course of the culture may be due in part to the lysis of some cells, since recently, it has been reported that after 2 days of infection, CVB-infected human islets had undergone morphological changes characteristic of pyknosis (39). However, our results are not consistent with those of this team, since in their experiments, at 4.5 days after infection, the whole CVB-infected islets were covered by dead cells (39), whereas in ours, the viability of islets was not different in CVB-infected cultures from that of controls within 1 month, as evaluated by trypan blue exclusion assay, which indicates that a significant lysis of infected islets did not occur. The discrepancy in the results obtained by us and Roivainen et al. can be explained in part by the conditions of the islet cultures and infection, since we used membrane inserts and lower virus titers (39).
CVB3 and CVB4 JBV strains were capable of infecting and replicating in human islets from different donors (eight individuals) with the same intensity as the CVB4 diabetogenic E2 strain did, and no significant difference was detected in levels of IFN-α induced by these virus strains. Thus, the viral replication and expression of IFN-α were not restricted to the so-called CVB4 E2 diabetogenic strain and did not depend on the genetic background of the host. In addition to CVB3 and CVB4, the role of other EV serotypes like CVB2, CVB5, coxsackievirus A, and echoviruses in the pathogenesis of type 1 diabetes has been suggested (17, 25, 30, 32, 38, 50). Thus other EVs are candidate viruses to be included in our assay, and the detection of IFN-α (mRNA and/or protein) in primary islet cultures in response to EV is an alternative screening process for detecting infection of islet cells.
Our experiments demonstrate that the expression of IFN-α by β cells in pancreases from IDDM patients reported by Foulis et al. may be due to CVB (15). Together, these results support the notion that the β cells of IDDM patients can harbor a persistent viral infection, therefore, further works starting with appropriated fresh tissue are needed to explore this hypothesis. The persistent CVB infection of human islets and the resulting IFN-α synthesis displayed in our studies can result in β-cell damage and can generate autoimmune reactions to the β cells, which may play a role in the expression of IDDM. In mouse models, it has been demonstrated that the persistence of viral RNA in pancreases of animals infected with CVB4 E2 is correlated with the development of diabetes (41) and that a CVB4-induced IDDM is dependent on the pancreatic tropism of the virus, release of sequestered antigen, and nonspecific activation of preexisting autoreactive T lymphocytes (23). Interestingly, it has been reported that IFN-α can play a role in the viral expansion of nonspecific T-cell responses (47). The local production of IFN-α may result in HLA class I and ICAM-1 expression that characterize the islets of patients with type 1 diabetes (6). IFN-α may be an initiator of autoimmunity against β cells; indeed, it has been demonstrated that the expression of IFN-α in β cells may lead to the development of diabetes in transgenic mice (43) through the activation of autoimmune (islet-reactive) CD4+ Th1 cells (7). Thus, together the CVB infection of islet cells and the local IFN-α production can work toward promoting the expression of the disease. The detection of IFN-α was negative in α cells in CVB-infected islets, whereas IFN-α was found in β cells, even though CVB replicated in both cell types. The mechanism of the restricted expression of IFN-α in β cells in CVB infected islets is unknown, but it could explain why only the β cells are destroyed in the diabetogenic process.
ACKNOWLEDGMENTS
We thank Valery Gmyr for helpful discussions.
This work was supported by “Fondation de France,” “Conseil Régional Nord Pas-De-Calais,” “CHRU Lille AOCHU 98/1911,” “Université Lille II,” and “Ministère de l'Education Nationale de la Recherche et de la Technologie, UPRES EA 1048, Université Lille II: Nouvelles Thérapeutiques du Diabète de Type 1 et Pathogenèse Virale de la Maladie,” which encompasses the “Laboratoire de Virology” and the “Laboratoire de Recherche sur les Ilots de Pancréas.”
REFERENCES
- 1.Andreoletti L, Hober D, Hober-Vandenberghe C, Belaich S, Vantyghem M-C, Lefebvre J, Wattré P. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol. 1997;52:121–127. doi: 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Kirthivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnevie-Nielsen V, Buschard K, Dyrberg T. Differential responsiveness to interferon-α in β-cells and non-β-cells. Diabetes. 1996;45:818–821. doi: 10.2337/diab.45.6.818. [DOI] [PubMed] [Google Scholar]
- 5.Cederblad B, Blomberg S, Vallin H, Perers A, Alm G V, Ronnblom L. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha-producing cells. J Autoimmun. 1998;11:465–470. doi: 10.1006/jaut.1998.0215. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti D, Huang X, Beck J, Henrich J, McFarland N, James R F L, Stewart T A. Control of islet intercellular adhesion molecule-1 expression by interferon-α and hypoxia. Diabetes. 1996;45:1336–1343. doi: 10.2337/diab.45.10.1336. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti D, Hultgren B, Stewart T A. IFN-α induces autoimmune T cells through the induction of intracellular adhesion molecule-1 and B7.2. J Immunol. 1996;157:522–528. [PubMed] [Google Scholar]
- 8.Chehadeh W, Hober D, Chieux V, Alm G, Harvey J, Lion G, Mouton Y, Wattré P. Biological properties of IFN-α produced ex-vivo by whole blood of HIV-1 infected patients. Scand J Immunol. 1999;49:660–666. doi: 10.1046/j.1365-3083.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 9.Chehadeh W, Weill J, Vantyghem M-C, Alm G, Lefèbvre J, Wattré P, Hober D. Increased level of interferon alpha in blood of insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis. 2000;181:1929–1939. doi: 10.1086/315516. [DOI] [PubMed] [Google Scholar]
- 10.Clements G B, Galbraith D N, Taylor K W. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 11.Conaldi P G, Biancone L, Bottelli A, De Martino A, Camussi G, Toniolo A. Distinct pathogenic effects of group B coxsackieviruses on human glomerular and tubular kidney cells. J Virol. 1997;71:9180–9187. doi: 10.1128/jvi.71.12.9180-9187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conaldi P G, Serra C, Mossa A, Falcone V, Basolo F, Camussi G, Dolei A, Toniolo A. Persistent infection of human vascular endothelial cells by group B coxsackieviruses. J Infect Dis. 1997;175:693–966. doi: 10.1093/infdis/175.3.693. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham L, Bowles N E, Lane R J M, Dubowitz V, Archard L C. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol. 1990;71:1399–1402. doi: 10.1099/0022-1317-71-6-1399. [DOI] [PubMed] [Google Scholar]
- 14.Fohlman J, Friman G. Is juvenile diabetes a viral disease? Ann Med. 1993;25:569–574. [PubMed] [Google Scholar]
- 15.Foulis A K, Farquharson M A, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;ii:1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- 16.Franck J A, Jr, Schmidt E V, Smith R E, Wilfert C M. Persistent infections of rat insulinoma cells with coxsackie B4 virus. Arch Virol. 1986;87:143–150. doi: 10.1007/BF01310551. [DOI] [PubMed] [Google Scholar]
- 17.Frisk G, Nilsson E, Tuvemo T, Friman G, Diderholm H. The possible role of coxsackie A and echo viruses in the pathogenesis of type I diabetes mellitus studied by IgM analysis. J Infect. 1992;24:13–22. doi: 10.1016/0163-4453(92)90814-m. [DOI] [PubMed] [Google Scholar]
- 18.Garcin D, Pelet T, Calain P, Roux L, Curran J, Kolakofsky D. A high recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA. Generation of a novel copy-back non-defective interfering virus. EMBO J. 1995;14:6087–6094. doi: 10.1002/j.1460-2075.1995.tb00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerling I, Chatterjee N K, Nejman C. Coxsackievirus B4-induced development of antibodies to 64,000-Mr islet autoantigen and hyperglycemia in mice. Autoimmunity. 1991;10:49–56. doi: 10.3109/08916939108997147. [DOI] [PubMed] [Google Scholar]
- 20.Graves P M, Norris J M, Pallansch M A, Gerling I C, Rewers M. Perspectives in diabetes. The role of enteroviral infections in the development of IDDM. Limitations of current approaches. Diabetes. 1997;46:161–168. doi: 10.2337/diab.46.2.161. [DOI] [PubMed] [Google Scholar]
- 21.Heim A, Canu A, Kirschner P, Simon T, Mall G, Hofschneider P H, Kandolf R. Synergistic interaction of interferon-β and interferon-γ in coxsackievirus B3 infected carrier cultures of human myocardial fibroblasts. J Infect Dis. 1992;166:958–965. doi: 10.1093/infdis/166.5.985. [DOI] [PubMed] [Google Scholar]
- 22.Hober C, Benhamou P Y, Watt P C, Watanabe Y, Nomura Y, Stein E, Brunicardi F C, Mullen Y. A new culture method for human pancreatic islets using a biopore membrane insert. Pancreas. 1997;14:199–204. doi: 10.1097/00006676-199703000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz M S, Bradeley L M, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Yuan J, Goddard A, Foulis A, James R F L, Lernmark A, Pujol-Borell R, Rabinovitch A, Somoza N, Stewart T A. Interferon expression in the pancreases of patients with type 1 diabetes. Diabetes. 1995;44:658–664. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- 25.Hyöty H, Hiltunen M, Knip M, Laakkonen M, Vähäsalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T, Akerblom H K The Childhood Diabetes in Finland Study Group. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM: Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 26.Kerr-Conte J, Pattou F, Xia Y, Proye C, Lefebvre J. Simple dithizone-stained multilayer test for selection of density gradient before human islet mass purification. Transplant Proc. 1994;26:4013–4015. [PubMed] [Google Scholar]
- 27.Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analyses of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA. 1992;89:314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebon P, Commoy-Chevalier M J, Robertgalliot B, Morin A, Chany C. Production d'interferon humain de type 1 par des lymphocytes au contact de cellules infectées par le virus herpes et fixées par le glutaraldehyde. C R Acad Sci Ser D. 1980;290:37–40. [PubMed] [Google Scholar]
- 29.Leparc I, Aymard M, Fuchs F. Acute, chronic and persistent enterovirus and poliovirus infections: detection of viral genome by seminested PCR amplification in culture-negative samples. Mol Cell Probes. 1994;8:487–495. doi: 10.1006/mcpr.1994.1070. [DOI] [PubMed] [Google Scholar]
- 30.Lönnrot M, Knip M, Roivainen M, Koskela P, Akerblom H K, Hyöty H. Onset of type 1 diabetes mellitus in infancy after enterovirus infections. Diabetes Med. 1998;15:431–434. doi: 10.1002/(SICI)1096-9136(199805)15:5<431::AID-DIA598>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Matteucci D, Toniolo A, Conaldi P G, Basolo F, Gori Z, Bendinelli M. Systemic lymphoid atrophy in coxsackie B3 infected mice: effects of virus and immunopotentiating agents. J Infect Dis. 1985;151:1100–1108. doi: 10.1093/infdis/151.6.1100. [DOI] [PubMed] [Google Scholar]
- 32.Nairn C, Galbraith D N, Taylor K W, Clements G B. Enterovirus variants in the serum of children at the onset of type 1 diabetes mellitus. Diabetes Med. 1999;16:509–513. doi: 10.1046/j.1464-5491.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 33.Pestka S, Langer J A, Zoon K C, Samuel C E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 34.Pipeleers D G, in't Veld P A, Van de Winkel M, Maes E, Schuit F C, Gepts W. A new in vitro model for the study of pancreatic A and B cells. Endocrinology. 1985;117:806–816. doi: 10.1210/endo-117-3-806. [DOI] [PubMed] [Google Scholar]
- 35.Pipeleers D G. Heterogeneity in pancreas β-cell population. Diabetes. 1992;41:777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- 36.Pitkäranta A, Linnavuori K, Hovi T. Virus-induced interferon production in human leukocytes: a low responder to one virus can be a high responder to another virus. J Interferon Res. 1991;11:17–23. doi: 10.1089/jir.1991.11.17. [DOI] [PubMed] [Google Scholar]
- 37.Ricordi C, Lacy P E, Finke E H, Olack B J, Scharp D W. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 38.Roivainen M, Knip M, Hyöty H, Kulmala P, Hiltunen M, Vähäsalo P, Hovi T, Akerblom H K The Childhood Diabetes in Finland (DiMe) Study Group. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. J Med Virol. 1998;56:74–78. doi: 10.1002/(sici)1096-9071(199809)56:1<74::aid-jmv12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Roivainen M, Rasilainen S, Ylipaasto P, Nissinen R, Ustinov J, Bouwens L, Eizirik D L, Hovi T, Otcnkoski T. Mechanisms of coxsackievirus-induced damage to human pancreatic β-cells. J Clin Endocrinol Metab. 2000;85:432–440. doi: 10.1210/jcem.85.1.6306. [DOI] [PubMed] [Google Scholar]
- 40.Rotbart H A, Kirkegaard K. Picornavirus pathogenesis: viral access, attachment and entry into susceptible cells. Semin Virol. 1992;3:483–499. [Google Scholar]
- 41.See D M, Tilles J G. Pathogenesis of virus-induced diabetes in mice. J Infect Dis. 1995;171:1131–1138. doi: 10.1093/infdis/171.5.1131. [DOI] [PubMed] [Google Scholar]
- 42.Somoza N, Vargas F, Roura-Mir C, Vives-Pi M, Fernandez-Figueras M T, Ariza A, Gomis R, Bragado R, Marti M, Jaraquemada D, Pujol-Borrell R. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor Vβ usage, and cytokine profile. J Immunol. 1994;153:1360–1377. [PubMed] [Google Scholar]
- 43.Stewart T A, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan N J. Induction of type 1 diabetes by interferon-α in transgenic mice. Science. 1993;260:1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- 44.Stone R. Post-polio syndrome—remembrance of virus past. Science. 1994;264:909. doi: 10.1126/science.8178150. [DOI] [PubMed] [Google Scholar]
- 45.Szopa T M, Ward T, Dronfield N D, Portwood N D, Taylor K W. Coxsackie B4 viruses with the potential to damage β-cells of the islets are present in clinical isolates. Diabetologia. 1990;33:325–328. doi: 10.1007/BF00404634. [DOI] [PubMed] [Google Scholar]
- 46.Szopa T M, Titchener P A, Portwood N D, Taylor K W. Diabetes mellitus due to viruses—some recent developments. Diabetologia. 1993;36:687–695. doi: 10.1007/BF00401138. [DOI] [PubMed] [Google Scholar]
- 47.Tough D F, Srent J. Viruses and T cell turnover: evidence for bystander proliferation. Immunol Rev. 1996;150:129–142. doi: 10.1111/j.1600-065x.1996.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 48.Vives-Pi M, Armengol M P, Alcalde L, Costa M, Somoza N, Vargas F, Jaraquemada D, Pujol-Borrell R. Expression of transporter associated with antigen processing-1 in the endocrine cells of human pancreatic islets. Diabetes. 1996;45:779–788. doi: 10.2337/diab.45.6.779. [DOI] [PubMed] [Google Scholar]
- 49.Vuorinen T, Nikolakaros G, Simell O, Hyypiä T, Vainionpää R. Coxsackievirus B3 and mumps virus infection in human fetal islet cell cultures. Pancreas. 1992;7:460–464. doi: 10.1097/00006676-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Wagenknecht L E, Roseman J M, Herman W H. Increased incidence of insulin-dependent diabetes mellitus following an epidemic of coxsackievirus B5. Am J Epidemiol. 1991;133:1024–1031. doi: 10.1093/oxfordjournals.aje.a115811. [DOI] [PubMed] [Google Scholar]
- 51.Yoon J W, Austin M, Onodera T, Notkins A L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 52.Yoon J-W, Onodera T, Jenson A B, Notkins A L. Virus-induced diabetes mellitus. XI. Replication of coxsackie B3 virus in human pancreatic beta cell cultures. Diabetes. 1978;27:778–781. doi: 10.2337/diab.27.7.778. [DOI] [PubMed] [Google Scholar]