Summary
Cholangiocarcinoma (CCA) is a rare primary liver cancer associated with high mortality and few systemic treatment options. The behaviour of the immune system has come into focus as a potential treatment modality for many cancer types, but immunotherapy has yet to dramatically alter the treatment paradigm for CCA as it has for other diseases. Herein, we review recent studies describing the relevance of the tumour immune microenvironment (TIME) in CCA. Various non-parenchymal cell types are critically important in controlling CCA progression, prognosis, and response to systemic therapy. Knowledge of the behaviour of these leukocytes could help generate hypotheses to guide the development of potential immune-directed therapies. Recently, an immunotherapy-containing combination was approved for the treatment of advanced-stage CCA. However, despite level 1 evidence demonstrating the improved efficacy of this therapy, survival remained suboptimal. In the current manuscript, we provide a comprehensive review of the TIME in CCA, preclinical studies of immunotherapies against CCA, as well as ongoing clinical trials applying immunotherapies for the treatment of CCA. Particular emphasis is placed on microsatellite unstable tumours, a rare CCA subtype that demonstrates heightened sensitivity to approved immune checkpoint inhibitors. We also discuss the challenges involved in applying immunotherapies to the treatment of CCA and the importance of understanding the TIME.
Keywords: Cholangiocarcinoma, Tumour immune microenvironment, Immune checkpoint inhibitors, Immunotherapy, Combination therapy
Key points.
-
•
Accumulating data from clinical studies has shown that immunotherapy is associated with manageable toxicity and safety in patients with CCA.
-
•
Currently the overall therapeutic benefit of immunotherapy for CCA is still very limited.
-
•
Profiling the immune microenvironment of CCA will provide new insights that could guide the development of novel immune-targeting therapy or combination therapy for CCA treatment.
-
•
There remain major challenges to the effective application of immunotherapies for CCA, including disease heterogeneity, difficulties conducting clinical trials, and a lack of adequate experimental models for basic and translational research.
Introduction
Cholangiocarcinoma (CCA) is the second most common primary liver cancer type and an aggressive malignancy associated with poor prognosis.1,2 Most patients with CCA are diagnosed at an advanced stage, at which point there are limited therapeutic options. Hence, curative surgical treatment is limited to a small subset of patients with early-stage tumours. The first-line therapy for unresectable CCA is either gemcitabine plus platin-based chemotherapy3 or the recently approved durvalumab in combination with chemotherapy,4 though both regimens are associated with suboptimal efficacy and response rates. Several targeted therapeutic agents have been approved for a minority of cases in the second-line setting, including pemigatinib and futibatinib for FGFR2 (fibroblast growth factor receptor 2)-rearranged CCA as well as ivosidenib for IDH1 (isocitrate dehydrogenase 1) mutated CCA.[5], [6], [7] Despite these advances, the overall prognosis for patients with CCA is very poor, with a median survival of less than 1 year,3 hence, novel treatment strategies are urgently needed.
Immunotherapy has been a major breakthrough in cancer research in the last decade, with many promising applications still being discovered. The ability of the immune system to recognise non-self tumour components is often inhibited by a variety of cancer intrinsic mechanisms that promote immune evasion. One prominent reason is the exhaustion of activated lymphocytes typified by upregulation of inhibitory markers, including programmed cell death protein 1 (PD1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and T-cell immunoglobulin domain and mucin domain-3 (TIM3). Tumour cells, as well as the surrounding stromal cells, often express or secrete the ligands of these inhibitory proteins, including programmed cell death 1 ligand 1 (PD-L1). Secreted inhibitory cytokines such as vascular endothelial growth factor (VEGF) or transforming growth factor beta (TGF-β) further inhibit the activation of lymphocytes. The principle of first-generation immune checkpoint inhibitors (ICIs) is to reinvigorate the potential of the host immune system to target and eradicate malignant cells. Checkpoint inhibitors have proven to be effective when used as monotherapies or in combination for multiple common epithelial tumour types, including non-small cell lung cancer, colorectal adenocarcinoma, and, despite a generally immunosuppressed microenvironment, advanced hepatocellular carcinoma (HCC).
Numerous efforts have been made to profile the immune microenvironment of CCA to identify potential targets for traditional immunotherapy. Additionally, accumulating evidence from promising preclinical studies and preliminary clinical data suggest that “second-generation” checkpoint inhibition or cellular-based immunotherapies might be effective against CCA.8,9 Herein, we review our current understanding of the tumour immune microenvironment (TIME) of CCA and discuss the recent and emerging developments in immunotherapy for CCA.
The tumour immune microenvironment of CCA
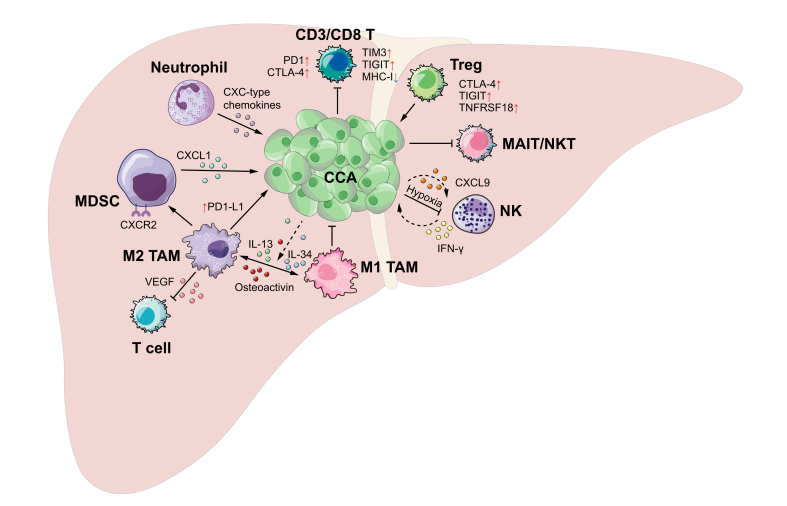
CCAs are adenocarcinomas arising from biliary cells, although it has been reported that the tumours may also originate from hepatic stem cells or mature hepatocytes.10 A key histological feature of CCA is that tumour cells are often surrounded with dense desmoplasia populated by cancer-associated fibroblasts. It has been reported that the fibrotic tumour microenvironment, plus the infiltrated innate immune cells, such as tumour-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), facilitate the immunosuppressive TIME of CCA (Fig. 1 and Table 1).11 Recent high-throughput genomic and transcriptomic analyses, as well as single-cell RNA-sequencing (scRNAseq) studies, have helped to define a comprehensive genetic and immunological landscape of CCA.[12], [13], [14]
Fig. 1.
Graphic illustration of immune processes in cholangiocarcinoma.
CCA, Cholangiocarcinoma; Treg, regulatory T cells; MAIT, Mucosal-associated invariant T cells; MDSC, Myeloid-derived suppressor cells; NK, Natural killer cell; TAM, tumour-associated macrophages.
Table 1.
Summary of immune cells in CCA tumours.
| Cell types | Anti-tumour/tumour-promoting | Comments | Ref. |
|---|---|---|---|
| CD3+/CD8+ T | Anti-tumour | Associated with favourable survival and lower recurrence risk | 21 |
| Tregs | Tumour-promoting | A poor prognostic marker in patients with resected CCA Treg-specific MEOX1 expression causes enhanced suppression and reduced survival |
21,48 |
| CD8+ T | Anti-tumour | Significantly reduced in the CCAs | 22 |
| MAIT cells | Anti-tumour | MAIT cells are cytotoxic innate-like T cells whose infiltration into tumours positively correlates with favourable anti-tumour immune response and long-term survival | 24 |
| NKT cells | Anti-tumour | NKT cells have potent cytotoxic and immunomodulatory effects | 25 |
| CD68+CD163+ macrophages | Tumour-promoting | Positively correlated with the infiltration of Tregs and neovascularisation in tumours, as well as poor survival outcome | 34 |
| CD68+ macrophages | Tumour-promoting | Related to the increased microvascular density within the primary tumours | 35 |
| Macrophages | Tumour-promoting | Inflammatory macrophages required for WNT pathway activation in CCA tumours | 36 |
| PD-L1+ macrophages | Tumour-promoting | Positively correlated with high PD1-expressing CTLs and a risk factor for survival outcome | 101 |
| MDSCs | Tumour-promoting | Blockade of TAM leads to a compensatory infiltration of MDSCs in CCA models, resulting in impaired T-cell response and immune escape | 37 |
| MDSCs | Tumour-promoting | Depletion of MDSCs abrogated tumour progression in the subcutaneous CCA model | 38 |
| CXCR2+ PMN-MDSCs | Tumour-promoting | Its recruitment within the liver depends on CXCL1-secreting hepatocytes driven by gut microbial products | 39 |
| NK cells | Anti-tumour | Prolongs survival outcomes | 40 |
| Neutrophils | Tumour-promoting | Associated with poor prognosis and high tumour recurrence rate | 42,43 |
| Neutrophils | Tumour-promoting | Neutrophils recruited into CCA tumours by chemokine CXCL5 via PI3K-Akt and extracellular signal-regulated kinase 1/2 signalling pathways | 44 |
CCA, cholangiocarcinoma; MAIT, mucosal-associated invariant T; MDSC, myeloid-derived suppressor cell; NK, natural killer; NKT, natural killer T; TAM, tumour-associated macrophage; Tregs, regulatory T cells.
Herein, to analyse the potential of treatments that modulate certain components of the TIME, we review different preclinical studies on each immune cell type in the liver, both within the context of CCA and beyond. In general, the liver is traditionally considered to be an immune-privileged organ.15,16 The immunosuppressive microenvironment in the liver is regulated by innate lymphoid cells, regulatory T cells (Tregs), dendritic cells (DCs), macrophages/Kupffer cells, and MDSCs, and pro-/anti-inflammatory cytokines, to prevent excessive immune responses to pathogen- and damage-associated molecular patterns derived from microorganisms absorbed via the intestine.15 Although this immunosuppressive microenvironment is essential to maintain the dynamic balance of physiological functions in the liver, the implications of this intrinsic tolerogenic state on the effectiveness of immunotherapy during the initiation and progression of CCA must be fully considered, as evidenced by liver metastasis-specific acquired resistance of otherwise sensitive tumour subtypes.17
Immunological landscape and immune cell composition in CCA (low-resolution data)
According to the immune cell composition and function in CCA tumours,18 the TIME can be divided into four distinct subtypes. In one study, 46% of CCAs belonged to the immune desert group, which presents very weak immune signature expression, while 13% of CCA tumours showed high infiltration of lymphocytes and strong activation of inflammatory cells and fibroblasts. The other two types were characterised by their low expression of lymphoid signatures (19%) and mesenchymal features of activated fibroblasts (22%). Notably, the inflamed subtype was associated with the longest survival, suggesting that the TIME plays an important role in tumour control.
T cells in CCA
T cells are a highly heterogeneous population of cells including CD8 cytotoxic T lymphocytes (CTLs), CD4 helper T cells, and CD4+CD25+FOXP3+ Tregs. Both CD8 and CD4 helper T cells exhibit anti-tumour effects through a number of mechanisms and can be further divided into several sub-populations.19,20 The infiltration of CD3+ and CD8+ T cells into CCA tumours is associated with favourable survival and lower recurrence risk, while the infiltration of Tregs is a poor prognostic marker in patients with resected CCA.21 In one study, Tregs were found in comparable quantities in HCC and CCA, but the prevalence of CTLs, which represent the anti-tumour response, was significantly reduced in CCA compared to HCC.22
Other T cells function primarily through innate-like mechanisms, including mucosal-associated invariant T cells (MAITs) and natural killer T (NKT) cells. MAITs are highly enriched in the liver tissue and respond to MR1-restricted epitopes.23 The infiltration of MAITs into tumours positively correlates with favourable anti-tumour immune responses and predicts long-term survival.24 NKT cells recognise CD1d-restricted epitopes and can have potent cytotoxic and immunomodulatory effects. Some CCA cell lines have been found to express CD1d and can stimulate NKT cells in vitro,25 a property that has not yet been explored in detail but could potentially serve as a biomarker for CCAs with NKT immunoreactivity.
T-cell penetration and expression of surface markers in CCA have particular mechanistic and therapeutic importance for ICI. Cancer cells have been found to express PD-L1 to escape attack from T cells via the PD-L1/PD1 axis by promoting tumour-infiltrating lymphocyte (TIL) apoptosis.26 Elevated PD-L1 expression is correlated with tumour pTNM stage and poor overall survival (OS), and is inversely correlated with CD8+ TILs in CCAs.27,28 In addition to PD-L1, the expression of HLA-I molecules may be associated with the infiltration of CTLs, and a positive correlation between HLA-I and CD8+ cells has been demonstrated in CCA.29 Positive HLA-I expression combined with negative PD-L1 expression, as well as high CD8+ T-cell frequencies at the tumour border area, have both been associated with a favourable clinical outcome in patients with CCA.29,30 The latter point may be underappreciated, as infiltration of CTLs and CD4 helper cells appears to be blocked spatially at the tumour margins. Finally, PD1 and CTLA-4 expression on the surface of T cells were increased in lymphocytes within the CCA lesions, suggesting increased T-cell exhaustion that may be amenable to targeting by ICI therapies.31
Macrophages in CCA
Macrophages are another promising target in CCA that may influence the TIME both directly and indirectly.32 Like tumour cells, TAMs found in CCA may contribute to the immunosuppressive TIME via antigen presentation and expression of ligands for T-cell exhaustion markers. In fact, TAMs are identified to be the main source of PD-L1 both in human and murine CCA tumours 37. The level of PD-L1 expression on macrophages positively correlated with the quantity of high-PD1-expressing CTLs and was a negative prognostic factor 101.
In addition, TAMs can polarise to promote either tumour progression (M2) or pro-inflammatory processes (M1). TAM polarisation may be influenced by the cytokines IL-13, IL-34, and osteoactivin secreted by tumour cells, which are strong differentiation factors for macrophage shaping toward TAM-like features, contributing to tumour invasion both in vitro and in vivo.33 The M2 CD68+CD163+ macrophages may mediate their immunosuppressive effects indirectly through mechanisms such as the infiltration of Tregs and neovascularisation in CCA tumours, correlating with poor survival.34 However, targeting TAMs in advanced CCA may not be straightforward. The infiltration of CD68+ macrophages appears to be significantly increased in locally advanced primary tumours compared to metastatic sites, possibly related to increased microvascular density within the primary tumours.35 Also paradoxically, inflammatory macrophages appear to be required for WNT pathway activation in CCA tumours, as macrophage depletion or WNT signalling inhibition resulted in CCA tumour regression.36 Thus, the decision to investigate macrophage depletion in CCA using newer targeted therapies or biologics may be complicated by discrepancies between their phenotype and function in the TIME. Further research is needed to understand their intricate biology and predict the effects of TAM modulation.
MDSCs in CCA
Separate from TAMs, MDSCs are characterised by their immunosuppressive characteristics, which have been observed in numerous malignancies. MDSCs appear to have a tumour-promoting function that overlaps with that of TAMs, as suggested by the observation of a compensatory infiltration of MDSCs after the blockade of TAMs in CCA models, resulting in impaired T-cell responses and immune escape.37 Studies have uncovered the tumour-promoting activities of MDSCs in CCA, as depletion of MDSCs in the subcutaneous CCA model abrogated tumour progression.38 However, factors that cause MDSC recruitment may be dependent on the organ-specific context of a tumour – a quality that subcutaneous models of CCA do not capture. In an orthotopic mouse model of CCA established in the context of colitis, CXCR2+ polymorphonuclear MDSC (PMN-MDSC) recruitment within the liver was demonstrated to be dependent on CXCL1-secreting hepatocytes driven by gut microbial products.39 Such an indirect mechanism of tumour promotion by the compromised gut barrier is particularly relevant in Western patients for whom inflammatory bowel disease plays a causative role in carcinogenesis and points to an underappreciated role of the gut microbiome in the TIME of CCA. Thus, MDSCs represent a promising target for immunomodulation-based therapeutics for CCA.
NK cells in CCA
NK cells are potently cytotoxic lymphocytes with established roles in other tumour types, yet studies on the role of NK cells in CCA pathogenesis are quite limited. It has been shown that the high expression of CXCL9, induced by IFN-γ, is correlated with abundant NK cell infiltration into CCA tumours and improved survival outcomes.40 Furthermore, an antibody neutralizing MICA/B, the soluble NKG2D decoy shed from tumour cells, can increase IFN-γ secretion and degranulation of NK cells co-cultured with CCA tumour cells ex vivo.41 While NK cells could have promising anti-tumour functions, high-dimensional analysis suggests that their viability may be compromised in CCA (see below), making their relevance questionable.
Neutrophils in CCA
Neutrophils are a subtype of polymorphonuclear cells that act as first-responders in inflammatory processes through direct cytotoxicity and release of chromatin into the extracellular space. Multiple studies have demonstrated that neutrophils within CCA lesions are associated with poor prognosis and a high rate of tumour recurrence.42,43 It has been shown that neutrophils can traffic into CCA lesions via the overexpressed chemokine CXCL5, a member of the CXC-type chemokine family, through the PI3K-Akt and extracellular signal-regulated kinase 1/2 signalling pathways.44 However, the precise roles of neutrophils during CCA pathogenesis remain to be determined.
NGS data on TIME (high-resolution data)
scRNAseq illuminates the transcriptomes of individual cells with unparalleled granularity and has been revolutionary in our understanding of tumour cells and the TIME. In the first scRNAseq study of the human liver, MacParland et al. analysed the transcriptional profiles of 8,444 parenchymal and non-parenchymal cells.45 Two distinct CD68+ macrophage populations were identified. One population was characterised as inflammatory with enriched expression of LYZ, CSTA, CD74, and the second population of macrophages was characterised as tolerogenic. In addition, three clusters of effector T cells were identified as tissue-resident memory αβ T cells (CD8+CD69+), unconventional γδ T cells (T-bet+CD161+CD16+) and phosphoantigen-reactive γδ T cells in the liver. Furthermore, the heterogeneity of NK and NKT cells in the human liver was identified by clustering three populations – CD56+ NK cells, CD56-CD8A+ NKT, and CD56+CD8A+ NKT cells, which express different kinds of chemokine ligands, granzymes, and killer cell lectin-like receptors.46 This study provided a framework of the physiological subsets of liver-resident immune cells, allowing for analysis of their alterations in the context of CCA.
Ma et al. published the first scRNAseq analysis of human liver cancers for both HCC and CCA. It was found that VEGF may play an important role in TIME reprogramming.14 Except for malignant cells, VEGF was mainly expressed by TAMs within the tumour immune compartment. Furthermore, the infiltrated T cells showed significantly different expression profiles based on tumours’ transcriptomic diversity scores – an algorithm that estimates the correlation of gene expression and copy number variation in each tumour sample.47 It was found the top-ranking genes in T cells derived from high diversity (above median diversity value) tumours, which were associated with poor survival outcomes, were mainly enriched in the epithelial-mesenchymal transition and myogenesis process. However, T cells derived from low diversity (below median diversity value) tumours were associated with a better survival outcome than the highly diverse tumours and were mainly enriched in allograft rejection, oxidative phosphorylation, IFN-α/IFN-γ response, and proliferation pathways, indicating these cells may still have anti-tumour and/or cytotoxic activities.14 Although this study was not specific for CCA, it suggested an important link between the transcriptomic properties of primary tumour cells and T-cell function in the liver, which may have utility as a novel biomarker.
The major power of scRNAseq in cancer immunology lies in its ability to identify novel immune subsets and the factors/pathways on which they are dependent. In a subsequent scRNAseq study of eight human CCAs, it was found that proliferating CD8 T cells in CCAs express exhaustion markers, such as lymphocyte-activation gene 3 protein (LAG3), TIM3, and T-cell immunoreceptor with Ig and ITIM domains (TIGIT), suggesting they are hyporeactive.13 In addition, although NK cells in the tumour adjacent tissue appeared to be activated, based on high expression of cytotoxic markers, the intratumoral NK cells had a transcriptional profile reflecting hypoxia and apoptosis. Finally, Tregs in tumours were found to express inhibitory markers, including TIGIT, CTLA-4 and TNFR-related protein superfamily 18, indicating they could be highly immunosuppressive.13 Another study utilising scRNAseq showed that the transcription factor MEOX1 in Tregs caused immunosuppression and correlated with survival in patients with CCA.48 Other large studies using scRNAseq to examine the TIME of HCC and CCA identified LAYN as a novel activation marker in both CD8+ T cells and Tregs,49 as well as CCL4+ neutrophils, which are important immunosuppressive cells that are enriched in CCA.50 While the number of studies is still limited, the wealth of data generated from scRNAseq has revealed several new transcriptional states and subtypes of cells within the TIME that hold great promise for future investigations into novel targets specific for CCA.
Preclinical CCA immunotherapy
The immunosuppressive mechanisms of the TIME in CCA support the investigation of immunotherapies against CCA. Due to the lack of adequate animal models of CCA, early studies typically employed in vitro co-culture techniques or xenograft models. For example, it was reported that the cytokine-induced killer cells co-cultured with DCs suppressed the growth of human CCA cells in SCID mice.51 Another study showed that the combined treatment with cytokine-induced killer cells and cetuximab, an epidermal growth factor receptor inhibitor, demonstrated significant cytotoxicity to human CCA cells in vitro.52 Aspartate-β-hydroxylase is a type 2 transmembrane protein which is widely expressed in many cancer types, including CCA. Using a rat CCA model, Noda et al. showed that aspartate-β-hydroxylase-exposed DCs had significant cytotoxicity against CCA cells and increased tumour-infiltrating CD3+ T cells, leading to the inhibition of CCA growth and metastasis.53 A similar study found enhanced T-cell cytotoxicity in a model using monocyte-derived DCs loaded with PRKAR1A, another protein that is overexpressed in CCA tumour cells, compared with conventional DCs.54 Neutralizing IL-10 and TGF-β increased the production of IFN-γ and enhanced the DC-mediated cytotoxicity of CTLs against CCA tumour cells in vitro.55 While these studies are useful as proof-of-concept investigations into CCA antigens and antigen-presenting cells, their design may not accurately reflect the complex interactions that occur in an in vivo system.
Recently, multiple mouse models of CCA have been developed, including cell lines56 and in vivo delivery of certain oncogenic constructs.57,58 These tools significantly facilitate the preclinical studies of immunotherapies against CCAs in immune-competent mice, allowing for relevant in vivo examination of the TIME. For example, using a syngeneic orthotopic mouse model of CCA, Loeuillard E et al. reported that the TAMs recruited from the bone were the main source of PD-L1 in CCA and played key roles during tumour progression. However, blockade of TAMs led to a compensatory accumulation of an immunosuppressive signature subset of Ly6CloLy6Ghi PMN-MDSCs. This effect counteracted the anti-tumour effect of depleting TAMs in this CCA mouse model. Dual blockade of TAMs and PMN-MDSCs facilitated the anti-tumour effect of anti-PD1 in CCA.37 Such treatment combinations and multi-subtype depletions demonstrate an important application of these newer immunocompetent mouse models of CCA, especially their utility in predicting compensatory effects in a plastic cell type such as TAMs. As noted above, TAM polarisation oversimplifies the link between phenotype and function, which another group studied using an immunocompetent model of CCA. Establishing that TAMs were major immunosuppressive cells within CCA TIMEs, the authors showed that tumour cell-derived granulocyte macrophage colony-stimulating factor (GM-CSF) recruited and polarised TAMs, and blocking GM-CSF suppressed mouse CCA growth, leading to prolonged survival.59 GM-CSF canonically promotes M1 macrophage differentiation, which promotes tumour immune responses, while M-CSF promotes M2 macrophage differentiation, which promotes tumour growth and metastasis, and is correlated with poor outcomes.60
Immunocompetent mouse models of CCA are also being applied to investigations of combination therapies involving ICIs. It was reported that, although increased expression of PD-L1 is often observed in CCA tumours, CCA barely responds to anti-PD-L1 treatment,8,61 suggesting intrinsic resistance to ICIs. However, ICIs may be useful as part of combination therapies to overcome resistance. For example, Diggs L et al. reported that activation of antigen-presenting macrophages and DCs with an anti-CD40 antibody led to a moderate response in murine CCA models, but a combination of anti-CD40 and anti-PD1 exhibited a significant anti-tumour effect in vivo.8 A recent study showed that trametinib, a mitogen-activated kinase inhibitor, upregulated the expression of PD-L1 on CCA tumour cells. However, it also increased the immunogenicity of tumour cells by upregulating their MHC-I expression. The combination of trametinib and anti-PD-1 inhibited tumour growth in several CCA models by increasing the number of effector memory CD8+ and CD4+ T cells, as well as CTLs, in the liver.62
In summary, the recent preclinical studies support the possible usefulness of immunotherapy, especially in the setting of combination therapy, against CCA.
CCA immunotherapy in clinical practice
Despite an increased understanding of the tumour microenvironment in CCA, the application of novel and repurposed immunotherapies has been challenging. The rarity and aggressiveness of CCA have caused progress to be slow and incremental, exemplified by the 12-year gap between the ABC-02 and TOPAZ-1 trials, demonstrating an improved survival in the order of weeks. Herein, we discuss select biologic-based immunotherapies in the treatment of CCA that are approved or show experimental promise. Cell-based immunotherapies for CCA are discussed elsewhere.[63], [64], [65], [66]
CCAs with MSI-H and TMB-H status
Two well-characterised molecular subtypes within various tumours, including CCAs, are tumour mutation burden high (TMB-H) and microsatellite instability high (MSI-H). Both TMB-H and MSI-H are associated with an increase of tumour-specific neoantigens,67,68 leading to robust recognition and activation of immune cells and, often, excellent response to ICI-based immunotherapy.69,70 A comprehensive genomic analysis of 260 biliary tract cancers found that 14 cases (5.9%) were classified as hypermutated, and only five of these harboured inactivating mutations in mismatch-repair genes.71 In a cohort study of 352 CCA samples analysed by next-generation sequencing, 2.0% of tumours were identified as MSI-H, while 4.0% were classified as TMB-H based on a cut-off of 17 somatic missense mutations per Mb.72
Despite their rarity, there are several reports that patients with CCA tumours harbouring TMB-H experienced significant ongoing anti-tumour responses to anti-PD-1 antibody immunotherapy.73,74 Two patients with CCA and high insertion-deletion ratios achieved complete response by combining PD1 blockade with chemotherapy.75 In another patient with advanced MSI-H CCA, although the expression of PD-L1 and the infiltrated CTLs were not elevated, there was a strong and durable response to pembrolizumab therapy.76 Recently, more studies have reported similar dramatic anti-tumour or even complete tumour responses.[77], [78], [79], [80]
Results from a phase II study (NCT01876511) evaluating anti-PD1 immunotherapy for progressive metastatic carcinomas included four patients with ampullary cancer or CCA. Surprisingly, the response rates of patients with MSI-H colorectal cancer were similar to those of patients with non-colorectal cancers, including CCA.81 Based on these promising results, the trial was expanded to further evaluate the efficacy of anti-PD1 immunotherapy in 12 different tumour types with advanced mismatch-repair deficiency. It was reported that three of the four enrolled patients experienced stable disease, while another experienced a complete response. These results suggest that neoantigens generated by cancer cells caused by MSI-H genomes lead to the enhanced sensitivity of CCA to PD1-blockade in a manner similar to other cancer types.68 These promising results accelerated the approval of anti-PD1 immunotherapy for adult and paediatric patients with unresectable or metastatic solid tumours, including CCA, that harbour MSI-H and have progressed following prior treatment.
KEYNOTE-158, a larger trial, evaluated the efficiency of pembrolizumab for 233 patients with MSI-H advanced non-colorectal cancer who failed on prior therapy, including 22 CCAs. The combined objective response rate (ORR) was 34.3%, median progression-free survival (mPFS) was 4.1 months, and median overall survival (mOS) was 23.5 months. Specifically, in the CCA cohort, two patients achieved a complete response and seven patients a partial response. The ORR of 40.9% for CCA was similar to other cancers, and a similar mPFS (4.2 months) and mOS (24.3 months) were observed.82 These results were remarkable but not unexpected based on previous smaller studies of single-agent nivolumab, in which all responders were found to have a MSI-H profile.83 Together, these promising results in the MSI-H/TMB-H subset of CCA have significantly altered the prognosis for this unique population that responds to ICI favourably, opening up the possibility for further application of immunotherapy to patients lacking these biomarkers.
PD-L1 as a biomarker for ICI immunotherapies
Unfortunately, the results from ICI monotherapy for TMB-L/MSI-L CCAs have been unencouraging, and there are no approved immunotherapy-alone regimens for CCA. Some investigations have focused on finding biomarkers in CCA that correlate with response to ICI (Table 2). PD-L1 expression within the tumour is such a marker for the prediction of anti-tumour responses to ICI therapy across multiple tumour types.84 It has been found the PD1/PD-L1 axis is both expressed in CCA tumour cells as well as its TILs,85 suggesting the potential for responses to anti-PD1 or anti-PD-L1 immunotherapy. In a phase Ib trial (Keynote 028) evaluating the anti-tumour efficacy of pembrolizumab in PD-L1-positive (≥1% on immunohistochemistry) CCA tumours, a 13% ORR was observed in 24 patients.86,87 In a larger trial of 104 enrolled patients with CCA (Keynote 158), a total ORR of 5.5% was reported, with ORRs of 6.6% and 2.9% in patients with PD-L1-positive (n = 61) and PD-L1-negative (n = 34) tumours, respectively.86 In a phase II multi-institutional trial of nivolumab, a PD-L1 antagonist, it was found that the positive expression of PD-L1 in tumours was associated with significantly prolonged PFS.83 Despite PD-L1 expression correlating with response, these results suggest that both pembrolizumab and nivolumab monotherapy showed only modest efficacy for patients with CCA, and intrinsic tolerance mechanisms need to be overcome in order to unlock the efficacy of ICI.
Table 2.
Summary of completed and ongoing clinical trials of ICI-based CCA immunotherapy∗.
| NCT number | Interventions | ICI general name | Phase | Status | Enrolment (estimated) | Ref. |
|---|---|---|---|---|---|---|
| ICI monotherapy | ||||||
| NCT01876511 | Pembrolizumab/MK-3475 | Anti-PD1 | II | Completed | 41 | 81 |
| NCT02829918 | Nivolumab | Anti-PD1 | II | Completed | 54 | 83 |
| NCT03695952 | Nivolumab or pembrolizumab | Anti-PD1 | Recruiting | 100 | ||
| NCT02054806 | Pembrolizumab | Anti-PD1 | I | Completed | 24 | 86 |
|
NCT02628067 |
Pembrolizumab |
Anti-PD1 |
II |
Recruiting |
104 |
82,86 |
|
Dual ICIs therapy | ||||||
| NCT04969887 | Nivolumab+ipilimumab | Anti-PD1+anti-CTLA4 | II | Recruiting | 240 | |
| NCT02443324 | Pembrolizumab+ramucirumab | Anti-PD1+anti-VEGFR2 | I | Completed | 155 | 102 |
| NCT03704480 | Durvalumab+tremelimumab | Anti-PD-L1+anti-CTLA4 | II | Completed | 106 | 95 |
| NCT04238637 | Durvalumab+tremelimumab | Anti-PD-L1+anti-CTLA4 | II | Recruiting | 50 | |
| NCT01938612 | MEDI4736+tremelimumab | Anti-PD-L1+anti-CTLA4 | I | Completed | 269 | |
| NCT03849469 | XmAb22841+pembrolizumab | Bispecific anti-CTLA4/LAG3 +Anti-PD1 | I | Recruiting | 242 | |
|
NCT03833661 |
M7824 |
Bispecific anti-PD-L1/TGF-β |
II |
Completed |
159 |
103 |
|
Combined ICI + chemotherapy | ||||||
| NCT03311789 | Nivolumab+GEMCIS | Anti-PD1 | I/II | Completed | 30 | 91 |
| NCT03111732 | Pembrolizumab+XELOX | Anti-PD1 | II | Completed | 11 | 104 |
| NCT03092895 | SHR-1210+apatinib or FOLFOX4/GEMOX | Anti-PD1 | II | Completed | 152 | 96,105 |
| NCT03486678 | SHR-1210+GEMOX | Anti-PD1 | II | Completed | 38 | 97 |
| NCT04782804 | Tislelizumab+capecitabine | Anti-PD1 | I/II | Recruiting | 30 | |
| NCT03796429 | Toripalimab+gemcitabine | Anti-PD1 | II | Recruiting | 40 | |
| NCT04961788 | Toripalimab+GEMOX | Anti-PD1 | II | Recruiting | 30 | |
| NCT04506281 | Toripalimab+lenvatinib+GEMOX | Anti-PD1 | II | Recruiting | 128 | |
| NCT04669496 | Toripalimab+lenvatinib+GEMOX | Anti-PD1 | II/3 | Recruiting | 178 | |
| NCT04413734 | Triprilumab+GEMCIS | Anti-PD1 | II | Recruiting | 120 | |
| NCT03101566 | Nivolumab+ipilimumab+GEMCIS | Anti-PD1+anti-CTLA4 | II | Recruiting | 75 | |
| NCT03058289 | Pembrolizumab+ipilimumab +INT230-6 | Anti-PD1+anti-CTLA4 | I/II | Recruiting | 180 | |
| NCT05007106 | MK-7684 A+Chemotherapy | Anti-PD1 and anti-TIGIT. Co-formulation | II | Recruiting | 480 | |
| NCT04217954 | Toripalimab+bevacizumab+HAIC | Anti-PD1+anti-VEGF | II | Recruiting | 32 | |
| NCT03046862 | Durvalumab+GEMCIS | Anti-PD-L1 | II | Completed | 128 | 106 |
| NCT04308174 | Durvalumab+GEMCIS | Anti-PD-L1 | II | Recruiting | 45 | |
| NCT03478488 | KN035+GEMOX | Anti-PD-L1 | 3 | Recruiting | 480 | |
|
NCT04066491 |
Bintrafusp alfa+GEMCIS |
Bispecific Anti-PD-L1/TGF-β |
II/3 |
Completed |
512 |
103 |
|
Combined ICI + targeted therapy | ||||||
| NCT04642664 | Camrelizumab+apatinib | Anti-PD1 | II | Completed | 22 | 107 |
| NCT04454905 | Camrelizumab+apatinib | Anti-PD1 | II | Recruiting | 50 | |
| NCT03250273 | Nivolumab+entinostat | Anti-PD1 | II | Completed | 44 | |
| NCT04704154 | Nivolumab+regorafenib | Anti-PD1 | II | Recruiting | 200 | |
| NCT03639935 | Nivolumab+rucaparib | Anti-PD1 | II | Recruiting | 35 | |
| NCT03895970 | Pembrolizumab+lenvatinib | Anti-PD1 | II | Recruiting | 50 | |
| NCT05010681 | Sintilimab+lenvatinib | Anti-PD1 | II | Recruiting | 25 | |
| NCT04010071 | Toripalimab+axitinib | Anti-PD1 | II | Recruiting | 60 | |
| NCT04211168 | Toripalimab+lenvatinib | Anti-PD1 | II | Recruiting | 44 | |
| NCT04641871 | Sym021+Sym023 +irinotecan hydrochloride | Anti-PD1+anti-TIM3 | I | Recruiting | 100 | |
| NCT03201458 | Atezolizumab+cobimetinib | Anti-PD-L1 | II | Completed | 77 | 108 |
| NCT04298008 | Durvalumab+AZD6738 | Anti-PD-L1 | II | Recruiting | 26 | |
| NCT03991832 | Durvalumab+olaparib | Anti-PD-L1 | II | Recruiting | 78 | |
|
NCT03996408 |
TQB2450+anlotinib| |
Anti-PD-L1 |
I/II |
Recruiting |
42 |
|
|
Combined ICI + targeted interventions | ||||||
| NCT01853618 | Tremelimumab+ablation | anti-CTLA4 | I/II | Completed | 61 | 109 |
| NCT04299581 | Camrelizumab+cyoablation | Anti-PD1 | II | Recruiting | 25 | |
| NCT03898895 | Camrelizumab+radiotherapy | Anti-PD1 | II | Recruiting | 184 | |
| NCT04295317 | SHR-1210+capecitabine+surgery | Anti-PD1 | II | Recruiting | 65 | |
| NCT04866836 | Tislelizumab+radiotherapy | Anti-PD1 | II | Recruiting | 20 | |
| NCT02866383 | Nivolumab+ipilimumab+radiotherapy | Anti-PD1+anti-CTLA4 | II | Recruiting | 160 | |
| NCT03482102 | Durvalumab+tremelimumab+radiotherapy | Anti-PD-L1+anti-CTLA4 | II | Recruiting | 70 | |
| NCT03937830 | Durvalumab+bevacizumab+tremelimumab+TACE | Anti-PD-L1+anti-VEGF + anti-CTLA4 | II | Recruiting | 22 | |
|
NCT04708067 |
Bintrafusp alfa+hypofractionated radiotherapy |
Bispecific Anti-PD-L1/TGF-β |
I |
Recruiting |
15 |
|
|
Other | ||||||
| NCT04278144 | Pembrolizumab+BDC-1001 | Anti-PD1 | I/II | Recruiting | 390 | |
| NCT04460456 | Pembrolizumab+SBT6050 | Anti-PD1 | I | Recruiting | 294 | |
| NCT04301778 | Durvalumab+SNDX-6352 | Anti-PD-L1+anti–CSF–1α | II | Recruiting | 30 | |
These clinical trials (https://www.clinicaltrials.gov/) were included from their first start date until March 20, 2022. A search strategy was developed in combination with the Medical Subject Headings, Emtree and text terms, include ‘liver cancer’, ‘liver tumor’, ‘biliary cancer’, ‘biliary tumor’, ‘biliary tract cancer’, ‘biliary carcinoma’, ‘cholangiocarcinoma’, ‘intrahepatic cholangiocarcinoma’, ‘ICC’, ‘iCCA’, ‘CCA’, ‘immunotherapy’, ‘immune checkpoint blockade’, ‘immune checkpoint inhibitor’, ‘anti-PD1’, ‘anti-PD-L1’, ‘anti-CTLA4’, ‘anti-TIM3’. According to the retrieved results, camrelizumab, cemiplimab, nivolumab, pembrolizumab, sintilimab, Sym021, tislelizumab and toripalimab, were classified as anti-PD1; atezolizumab, durvalumab, and envafolimab were classified as anti-PD-L1; tremelimumab and ipilimumab were classified as anti-CTLA4. CCA, cholangiocarcinoma; ICI, immune checkpoint inhibitor; GEMOX, gemcitabine and oxaliplatin; GEMCIS, gemcitabine and cisplatin; FOLFOX4, oxaliplatin, folinic acid and 5-fluorouracil; HAIC, hepatic artery infusion chemotherapy; TACE, transarterial chemoembolisation; XELOX, oxaliplatin and capecitabine.
ICI-based combination therapy for CCA
Based on both preclinical and clinical data showing that ICIs have limited efficacy in CCAs, many clinical trials have attempted to combine ICIs with other ICIs, chemotherapy, locoregional therapy, or targeted therapies to improve response rates (Table 2).
The ABC-02 trial demonstrated the superiority of gemcitabine plus platin-based chemotherapy to gemcitabine monotherapy.3 Interestingly, it was found that the chemotherapy regimen upregulates the expression of PD-L1 and MHC-I molecules in tumour cells,88,89 and stimulates the infiltrated immune cells by inhibiting the immunosuppressive cells,90 thus providing a rationale to combine ICIs with standard of care (gemcitabine and cisplatin) (Table 2). In a phase II study of nivolumab in combination with gemcitabine and cisplatin chemotherapy, 15 patients achieved an objective response in 27 response-evaluable patients, of whom five patients (18.6%) had a complete response, and the disease control rate was 92.6%. Meanwhile, an encouraging ORR of 61.9% was achieved in the 21 chemotherapy-naive patients. The mPFS in this study was 6.1 months and the mOS was 8.5 months, respectively, and the toxicity profile of nivolumab in combination with chemotherapy was acceptable.91
More recently, results from the phase III TOPAZ-1 trial demonstrated an improvement in overall survival for patients with CCA treated with durvalumab (an anti-PD-L1 antibody) in combination with gemcitabine and cisplatin,4 the first since the ABC-02 trial. mOS was 12.8 months in the durvalumab combination group and 11.5 months in the placebo treatment group, and rates of grade 3/4 adverse events were comparable. On post hoc analysis, only modest survival effects were seen in subgroups defined by PD-L1 expression, and over 50% of patients had an unknown MSI status. Nonetheless, this big achievement emphasised the promise of combining CCA immunotherapy with chemotherapy and led to the recent approval of this combination therapy for CCA in the US.4 A similar phase I study was performed in Japan, where relatively favourable results have already been achieved by combining nivolumab with cisplatin plus gemcitabine chemotherapy; in this study, the combination was associated with a reported mOS of 15.4 months and a mPFS of 4.2 months.92 In the combination group, 11 of 30 patients had an objective response compared with only 1 of 30 patients in the nivolumab monotherapy group, in whom mOS and mPFS were 5.2 and 1.4 months, respectively.92
Currently, there are over 25 ICI combination-based clinical trials for CCA treatment (Table 2). For example, a phase II study combining nivolumab with ipilimumab for advanced biliary tract cancer enrolled 39 patients (20 men and 19 women) who all received prior chemotherapy and had no MSI. The mPFS and mOS were 2.9 months and 5.7 months, respectively. This combination therapy showed improved efficacy when compared with results from a separate trial using anti-PD1 monotherapy.83,93 In a phase I study evaluating durvalumab (anti-PD-L1) combined with tremelimumab (anti-CTLA-4) in Asian patients with CCA, the durvalumab monotherapy group (n = 42) had a median OS of 8.1 months, and the combination group (n = 65) had a median OS of 10.1 months.94 While the treatment-related adverse events were comparable between the two groups, the combination group had one treatment-related death, pointing to the difficulty of combining immunotherapy regimens. Another promising phase II trial was terminated before reaching the study endpoint due to an unexpected increase of anaphylactic adverse events from combining durvalumab, tremelimumab, and paclitaxel. The dose-limiting toxicities were observed in five patients in the combination group (n = 10).95
Further studies are testing enhanced ICI blockade of established targets. A phase II trial evaluating first-line combination camrelizumab, a humanized high-affinity PD-1 IgG4 monoclonal antibody, plus oxaliplatin-based chemotherapy for advanced biliary tract cancer, enrolled 92 patients: 29 received camrelizumab plus FOLFOX (5-fluorouracil, leucovorin and oxaliplatin) while 63 received GEMOX (camrelizumab plus gemcitabine and oxaliplatin). The authors reported a combined objective response rate of 16.3%, a mPFS of 5.3 months, and an mOS of 12.4 months.96 In a similar study, 37 patients with advanced biliary tract cancer were recruited to evaluate the efficacy and safety of camrelizumab plus gemcitabine and oxaliplatin as the first-line treatment. Fifty-four percent of patients (20/37) experienced an objective response, and a mPFS of 6.1 months and an mOS of 11.8 months, with a manageable safety profile, were reported for the combination therapy.97 In a cohort study comparing the efficacy and safety of PD-1 inhibitors plus chemotherapy (n = 75) and chemotherapy alone (n = 59) as first-line treatments for patients with advanced CCA, though no significant differences were found in the ORR and disease control rate between the two groups, a significantly longer mPFS was observed in the combination group (5.8 months vs. 3.2 months, p = 0.004).98
In summary, multiple clinical trials are currently examining the therapeutic efficacy of ICI-based combination therapy against CCA. Most of the trials are still in early phases. Nevertheless, we expect that during the next few years, the results from these ongoing clinical trials may provide novel therapeutic options for the treatment of this deadly malignancy.
Future directions and challenges
Patients suffering from CCA are in urgent need of new systemic therapies. Despite the established efficacy of ICI monotherapy for the minority of patients whose CCAs carry TMB-H or MSI-H genotypes, the introduction of immunotherapy into treatment regimens for CCA broadly has been slow for several reasons. First, unlike HCC, clinical trials for CCA are challenging to perform due to its low incidence, making it difficult to demonstrate or disprove the efficacy of any new therapy prospectively without the coordination of an international clinical trial. Second, cholangiocarcinoma cells and the overall liver microenvironment demonstrate particularly strong resistance to immunotherapies that are otherwise effective in other cancer types/sites, making treatment combinations necessary. Third, the lack of identifiable biomarkers means that the majority of CCAs are treated the same way, despite divergent driver mutations and anatomic sites.
Fortunately, the diverse molecular landscape of CCA is being actively addressed. In addition to approved targeted therapies for known driver mutations of intrahepatic CCA, the preclinical studies reviewed above demonstrate unique mechanistic attributes that may explain the relative resistance of CCA to therapy. Some of these molecular features are being addressed by second-generation ICIs (Table 2), including TIGIT-, LAG3-, or TGF-β-targeting therapies; however, further identification of biomarkers will be critical to this effort. The introduction of large scRNAseq studies in patients with CCA have already identified various different immune cell types and tumour cell states that may serve as suitable biomarkers for future therapeutics. The results should be combined with other omics studies, including whole-exome sequencing, copy number variations, proteomics and metabolomics. These integrated studies will provide a comprehensive picture of CCAs and their immune microenvironments. The results will also be critical for the development of novel immunotherapies or combined immunotherapies and targeted therapies for CCA treatment.
However, significant challenges remain. One of the major challenges is that CCA is a heterogenous disease on multiple levels. Anatomically, CCAs consist of three subsets that have distinct driver mutations, histological features1,2,99 and possibly distinct responsiveness to immunotherapies. Indeed, based on the TOPAZ-1 clinical trial, it appears that durvalumab/gemcitabine/cisplatin combination therapy is much more effective against intrahepatic CCA than extrahepatic CCA.4 This issue has not been adequately addressed in clinical and preclinical studies.
In addition, the success of these future approaches will depend on access to preclinical testing in CCA, and until recent years, mouse models for CCA have been lacking. For CCA cell lines, few of them are commercially available. In most cases, intrahepatic CCA, distal CCA, and gallbladder cancer cell lines are used interchangeably.100 Mouse CCA models include chemically induced CCA, such as thiocetamide-induced CCA, as well as genetically engineered mouse CCA models. The latter includes transgenic/knockout mouse models, as well as mouse CCAs produced by hydrodynamic injections. All of these models have been used to investigate the therapeutic efficacy of immunotherapies. Most of these murine CCA models are intrahepatic CCA models and few perihilar or distal CCA models exist. Clearly, additional efforts are required to develop clinically relevant mouse CCA models that harbour the various genetic alterations seen in human CCAs, especially for perihilar or distal CCAs.
Although immunotherapy has been used for advanced CCA treatment in combination with chemotherapy in the first-line setting, response rates and clinical outcomes are still suboptimal. It would be of great significance to identify biomarkers of predictive or prognostic value. Clinical biospecimens, including blood, urine, tumour samples and radiographs, collected during the trials will be valuable for this purpose by enabling researchers to dissect tumoral responses and the dynamic immune landscape of CCA using current cutting-edge omics technologies. These findings will help to guide the design of different immunotherapy/chemotherapy combinations, with the ultimate aim of improving outcomes. Additionally, drug resistance has been linked to failure of targeted and immunological therapies, and elucidation of drug resistance mechanisms will be helpful for the study of next-generation immunotherapies or combination therapies. As multiple modalities are on the table for the treatment of advanced CCA, selection and sequencing of therapies for individual patients will become an important consideration.
In summary, immunotherapy against CCA presents exciting opportunities as well as unique challenges. The combined efforts of basic scientists, translational researchers and clinicians will be required to advance this field. In the future, we must improve our understanding of the molecular mechanisms underlying CCA pathogenesis, develop better and representative small animal models for CCA, and identify biomarkers for patient selection and international collaborative clinical trials.
Financial support
This work is supported by NIH grant R01CA239251 to XC, the National Natural Science Foundation of China (82202981) and the fellowship of China Postdoctoral Science Foundation (2022TQ0386) to XL.
Authors’ contributions
XL and BG contributed to this paper with conception, literature review and writing. CX participated in literature review and revision. CL and XC participated in drafting, critical revision and editing. All the authors approved the final version of this manuscript.
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100723
Contributor Information
Chao Liu, Email: Liuchao3@mail.sysu.edu.cn.
Xin Chen, Email: xinchen3@hawaii.edu.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizvi S., Khan S.A., Hallemeier C.L., Kelley R.K., Gores G.J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 4.Oh D.-Y., He A.R., Qin S., Chen L.-T., Okusaka T., Vogel A., et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou-Alfa G.K., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA grants accelerated approval to futibatinib for cholangiocarcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma. Accessed 30 Sep 2022.
- 8.Diggs L.P., Ruf B., Ma C., Heinrich B., Cui L., Zhang Q., et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:1145–1154. doi: 10.1016/j.jhep.2020.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan B., Malato Y., Calvisi D.F., Naqvi S., Razumilava N., Ribback S., et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijgen S., Terris B., Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:22–34. doi: 10.21037/hbsn.2016.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farshidfar F., Zheng S., Gingras M.C., Newton Y., Shih J., Robertson A.G., et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M., Yang H., Wan L., Wang Z., Wang H., Ge C., et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118–1130. doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Ma L., Hernandez M.O., Zhao Y., Mehta M., Tran B., Kelly M., et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer cell. 2019;36:418–430 e6. doi: 10.1016/j.ccell.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 16.Ringelhan M., Pfister D., O'Connor T., Pikarsky E., Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 17.Yu J., Green M.D., Li S., Sun Y., Journey S.N., Choi J.E., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Job S., Rapoud D., Dos Santos A., Gonzalez P., Desterke C., Pascal G., et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raskov H., Orhan A., Christensen J.P., Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donnell J.S., Teng M.W.L., Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 21.Vigano L., Soldani C., Franceschini B., Cimino M., Lleo A., Donadon M., et al. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. Impact on prognosis after complete Surgery. J Gastrointest Surg : official J Soc Surg Aliment Tract. 2019;23:2216–2224. doi: 10.1007/s11605-019-04111-5. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N., Hiraoka N., Yamagami W., Ojima H., Kanai Y., Kosuge T., et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res : official J Am Assoc Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 23.Greene J.M., Dash P., Roy S., McMurtrey C., Awad W., Reed J.S., et al. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 2017;10:802–813. doi: 10.1038/mi.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmer C.L., Filipovic I., Cornillet M., O'Rourke C.J., Berglin L., Jansson H., et al. Mucosal-associated invariant T-cell tumor infiltration predicts long-term survival in cholangiocarcinoma. Hepatology. 2022;75:1154–1168. doi: 10.1002/hep.32222. [DOI] [PubMed] [Google Scholar]
- 25.Schrumpf E., Tan C., Karlsen T.H., Sponheim J., Bjorkstrom N.K., Sundnes O., et al. The biliary epithelium presents antigens to and activates natural killer T cells. Hepatology. 2015;62:1249–1259. doi: 10.1002/hep.27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye Y., Zhou L., Xie X., Jiang G., Xie H., Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–504. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- 28.Deng M., Li S.H., Fu X., Yan X.P., Chen J., Qiu Y.D., et al. Relationship between PD-L1 expression, CD8+ T-cell infiltration and prognosis in intrahepatic cholangiocarcinoma patients. Cancer Cell Int. 2021;21:371. doi: 10.1186/s12935-021-02081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asahi Y., Hatanaka K.C., Hatanaka Y., Kamiyama T., Orimo T., Shimada S., et al. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg Today. 2020;50:931–940. doi: 10.1007/s00595-020-01967-y. [DOI] [PubMed] [Google Scholar]
- 30.Sabbatino F., Villani V., Yearley J.H., Deshpande V., Cai L., Konstantinidis I.T., et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res : official J Am Assoc Cancer Res. 2016;22:470–478. doi: 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou G., Sprengers D., Mancham S., Erkens R., Boor P.P.C., van Beek A.A., et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol. 2019;71:753–762. doi: 10.1016/j.jhep.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M., Wang C., Lu S., Xu Y., Li Z., Jiang H., et al. Tumor-associated macrophages in cholangiocarcinoma: complex interplay and potential therapeutic target. EBioMedicine. 2021;67 doi: 10.1016/j.ebiom.2021.103375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raggi C., Correnti M., Sica A., Andersen J.B., Cardinale V., Alvaro D., et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasita H., Komohara Y., Okabe H., Masuda T., Ohnishi K., Lei X.F., et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamma R., Annese T., Ruggieri S., Brunetti O., Longo V., Cascardi E., et al. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur J Clin Invest. 2019;49 doi: 10.1111/eci.13087. [DOI] [PubMed] [Google Scholar]
- 36.Boulter L., Guest R.V., Kendall T.J., Wilson D.H., Wojtacha D., Robson A.J., et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loeuillard E., Yang J., Buckarma E., Wang J., Liu Y., Conboy C., et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380–5396. doi: 10.1172/JCI137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y., Li B., Yang X., Cai Q., Liu W., Tian M., et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019;21:1133–1142. doi: 10.1016/j.neo.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q., Ma C., Duan Y., Heinrich B., Rosato U., Diggs L.P., et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11:1248–1267. doi: 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda Y., Asaoka T., Eguchi H., Yokota Y., Kubo M., Kinoshita M., et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020;111:323–333. doi: 10.1111/cas.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliviero B., Varchetta S., Mele D., Pessino G., Maiello R., Falleni M., et al. MICA/B-targeted antibody promotes NK cell-driven tumor immunity in patients with intrahepatic cholangiocarcinoma. Oncoimmunology. 2022;11 doi: 10.1080/2162402X.2022.2035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu F.M., Gao Q., Shi G.M., Zhang X., Wang J., Jiang J.H., et al. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506–2514. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Bo X., Suo T., Liu H., Ni X., Shen S., et al. Tumor-infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Sci. 2018;109:2266–2274. doi: 10.1111/cas.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou S.L., Dai Z., Zhou Z.J., Chen Q., Wang Z., Xiao Y.S., et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35:597–605. doi: 10.1093/carcin/bgt397. [DOI] [PubMed] [Google Scholar]
- 45.MacParland S.A., Liu J.C., Ma X.Z., Innes B.T., Bartczak A.M., Gage B.K., et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aizarani N., Saviano A., Sagar, Mailly L., Durand S., Herman J.S., et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572:199–204. doi: 10.1038/s41586-019-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon S.M., Budhu A., Woo H.G., Chaisaingmongkol J., Dang H., Forgues M., et al. Functional genomic complexity defines intratumor heterogeneity and tumor aggressiveness in liver cancer. Scientific Rep. 2019;9 doi: 10.1038/s41598-019-52578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvisi G., Termanini A., Soldani C., Portale F., Carriero R., Pilipow K., et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J Hepatol. 2022;77:1359–1372. doi: 10.1016/j.jhep.2022.05.043. [DOI] [PubMed] [Google Scholar]
- 49.Zheng C., Zheng L., Yoo J.K., Guo H., Zhang Y., Guo X., et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–1356 e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 50.Xue R., Zhang Q., Cao Q., Kong R., Xiang X., Liu H., et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612:141–147. doi: 10.1038/s41586-022-05400-x. [DOI] [PubMed] [Google Scholar]
- 51.Wongkajornsilp A., Somchitprasert T., Butraporn R., Wamanuttajinda V., Kasetsinsombat K., Huabprasert S., et al. Human cytokine-induced killer cells specifically infiltrated and retarded the growth of the inoculated human cholangiocarcinoma cells in SCID mice. Cancer Invest. 2009;27:140–148. doi: 10.1080/07357900802189832. [DOI] [PubMed] [Google Scholar]
- 52.Morisaki T., Umebayashi M., Kiyota A., Koya N., Tanaka H., Onishi H., et al. Combining cetuximab with killer lymphocytes synergistically inhibits human cholangiocarcinoma cells in vitro. Anticancer Res. 2012;32:2249–2256. [PubMed] [Google Scholar]
- 53.Noda T., Shimoda M., Ortiz V., Sirica A.E., Wands J.R. Immunization with aspartate-beta-hydroxylase-loaded dendritic cells produces antitumor effects in a rat model of intrahepatic cholangiocarcinoma. Hepatology. 2012;55:86–97. doi: 10.1002/hep.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panya A., Thepmalee C., Sawasdee N., Sujjitjoon J., Phanthaphol N., Junking M., et al. Cytotoxic activity of effector T cells against cholangiocarcinoma is enhanced by self-differentiated monocyte-derived dendritic cells. Cancer Immunol Immunother : CII. 2018;67:1579–1588. doi: 10.1007/s00262-018-2212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thepmalee C., Panya A., Junking M., Chieochansin T., Yenchitsomanus P.T. Inhibition of IL-10 and TGF-beta receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14:1423–1431. doi: 10.1080/21645515.2018.1431598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada D., Rizvi S., Razumilava N., Bronk S.F., Davila J.I., Champion M.D., et al. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology. 2015;61:1627–1642. doi: 10.1002/hep.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Wang H., Peters M., Ding N., Ribback S., Utpatel K., et al. Loss of Fbxw7 synergizes with activated Akt signaling to promote c-Myc dependent cholangiocarcinogenesis. J Hepatol. 2019;71:742–752. doi: 10.1016/j.jhep.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu X., Peng B., Chen G., Pes M.G., Ribback S., Ament C., et al. YAP accelerates notch-driven cholangiocarcinogenesis via mTORC1 in mice. Am J Pathol. 2021;191:1651–1667. doi: 10.1016/j.ajpath.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruffolo L.I., Jackson K.M., Kuhlers P.C., Dale B.S., Figueroa Guilliani N.M., Ullman N.A., et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut. 2022;71:1386–1398. doi: 10.1136/gutjnl-2021-324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ushach I., Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100:481–489. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kam A.E., Masood A., Shroff R.T. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol. 2021;6:956–969. doi: 10.1016/S2468-1253(21)00171-0. [DOI] [PubMed] [Google Scholar]
- 62.Wabitsch S., Tandon M., Ruf B., Zhang Q., McCallen J.D., McVey J.C., et al. Anti-PD-1 in combination with trametinib suppresses tumor growth and improves survival of intrahepatic cholangiocarcinoma in mice. Cell Mol Gastroenterol Hepatol. 2021;12:1166–1178. doi: 10.1016/j.jcmgh.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi M., Sakabe T., Abe H., Tanii M., Takahashi H., Chiba A., et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg : official J Soc Surg Aliment Tract. 2013;17:1609–1617. doi: 10.1007/s11605-013-2286-2. [DOI] [PubMed] [Google Scholar]
- 64.Feng K.C., Guo Y.L., Liu Y., Dai H.R., Wang Y., Lv H.Y., et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. doi: 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alnaggar M., Xu Y., Li J., He J., Chen J., Li M., et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer. 2019;7:36. doi: 10.1186/s40425-019-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimizu K., Kotera Y., Aruga A., Takeshita N., Takasaki K., Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J hepato-biliary-pancreatic Sci. 2012;19:171–178. doi: 10.1007/s00534-011-0437-y. [DOI] [PubMed] [Google Scholar]
- 67.Wells D.K., van Buuren M.M., Dang K.K., Hubbard-Lucey V.M., Sheehan K.C.F., Campbell K.M., et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell. 2020;183:818–834 e13. doi: 10.1016/j.cell.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riviere P., Goodman A.M., Okamura R., Barkauskas D.A., Whitchurch T.J., Lee S., et al. High tumor mutational burden correlates with longer survival in immunotherapy-naive patients with diverse cancers. Mol Cancer Ther. 2020;19:2139–2145. doi: 10.1158/1535-7163.MCT-20-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu C., Guan J., Lu S., Jin Q., Rousseau B., Lu T., et al. DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell. 2021;39:96–108 e6. doi: 10.1016/j.ccell.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura H., Arai Y., Totoki Y., Shirota T., Elzawahry A., Kato M., et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 72.Weinberg B.A., Xiu J., Lindberg M.R., Shields A.F., Hwang J.J., Poorman K., et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J Gastrointest Oncol. 2019;10:652–662. doi: 10.21037/jgo.2018.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gbolahan O., Hashemi-Sadraei N., O'Neil B. Prolonged response to anti-PD-1 antibody therapy in chemotherapy-refractory cholangiocarcinoma with high tumor mutational burden. J Natl Compr Canc Netw. 2019;17:644–648. doi: 10.6004/jnccn.2019.7304. [DOI] [PubMed] [Google Scholar]
- 74.Mou H., Yu L., Liao Q., Hou X., Wu Y., Cui Q., et al. Successful response to the combination of immunotherapy and chemotherapy in cholangiocarcinoma with high tumour mutational burden and PD-L1 expression: a case report. BMC cancer. 2018;18:1105. doi: 10.1186/s12885-018-5021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sui M., Li Y., Wang H., Luo Y., Wan T., Wang X., et al. Two cases of intrahepatic cholangiocellular carcinoma with high insertion-deletion ratios that achieved a complete response following chemotherapy combined with PD-1 blockade. J Immunother Cancer. 2019;7:125. doi: 10.1186/s40425-019-0596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Czink E., Kloor M., Goeppert B., Fröhling S., Uhrig S., Weber T.F., et al. Successful immune checkpoint blockade in a patient with advanced stage microsatellite-unstable biliary tract cancer. Cold Spring Harbor Mol case Stud. 2017;3 doi: 10.1101/mcs.a001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eguchi S., Shinkawa H., Sato Y., Nakai K., Takemura S., Tanaka S., et al. Durable response after discontinuation of pembrolizumab therapy for intrahepatic cholangiocarcinoma: a case report. Clin J Gastroenterol. 2021;14:858–865. doi: 10.1007/s12328-021-01396-5. [DOI] [PubMed] [Google Scholar]
- 78.Ikeda Y., Ono M., Ohmori G., Ameda S., Yamada M., Abe T., et al. Successful pembrolizumab treatment of microsatellite instability-high intrahepatic cholangiocarcinoma: a case report. Clin case Rep. 2021;9:2259–2263. doi: 10.1002/ccr3.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kai Y., Ikezawa K., Takada R., Daiku K., Maeda S., Abe Y., et al. Success rate of microsatellite instability examination and complete response with pembrolizumab in biliary tract cancer. JGH open : open access J Gastroenterol Hepatol. 2021;5:712–716. doi: 10.1002/jgh3.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toshida K., Itoh S., Yoshizumi T., Shimagaki T., Wang H., Kurihara T., et al. Efficacy of pembrolizumab in microsatellite instability-high locally advanced cholangiocarcinoma: a case report. Clin J Gastroenterol. 2021;14:1459–1463. doi: 10.1007/s12328-021-01458-8. [DOI] [PubMed] [Google Scholar]
- 81.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.P., et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim R.D., Chung V., Alese O.B., El-Rayes B.F., Li D., Al-Toubah T.E., et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6:888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gibney G.T., Weiner L.M., Atkins M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fontugne J., Augustin J., Pujals A., Compagnon P., Rousseau B., Luciani A., et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:24644–24651. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piha-Paul S.A., Oh D.Y., Ueno M., Malka D., Chung H.C., Nagrial A., et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 87.Ott P.A., Bang Y.J., Piha-Paul S.A., Razak A.R.A., Bennouna J., Soria J.C., et al. T-Cell-Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: keynote-028. J Clin Oncol. 2019;37:318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 88.Koido S., Kan S., Yoshida K., Yoshizaki S., Takakura K., Namiki Y., et al. Immunogenic modulation of cholangiocarcinoma cells by chemoimmunotherapy. Anticancer Res. 2014;34:6353–6361. [PubMed] [Google Scholar]
- 89.Liu W.M., Fowler D.W., Smith P., Dalgleish A.G. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–123. doi: 10.1038/sj.bjc.6605465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Homma Y., Taniguchi K., Nakazawa M., Matsuyama R., Mori R., Takeda K., et al. Changes in the immune cell population and cell proliferation in peripheral blood after gemcitabine-based chemotherapy for pancreatic cancer. Clin Transl Oncol. 2014;16:330–335. doi: 10.1007/s12094-013-1079-0. [DOI] [PubMed] [Google Scholar]
- 91.Feng K., Liu Y., Zhao Y., Yang Q., Dong L., Liu J., et al. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: results from a phase II study. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ueno M., Ikeda M., Morizane C., Kobayashi S., Ohno I., Kondo S., et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:611–621. doi: 10.1016/S2468-1253(19)30086-X. [DOI] [PubMed] [Google Scholar]
- 93.Klein O., Kee D., Nagrial A., Markman B., Underhill C., Michael M., et al. Evaluation of combination nivolumab and ipilimumab immunotherapy in patients with advanced biliary tract cancers: subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA Oncol. 2020;6:1405–1409. doi: 10.1001/jamaoncol.2020.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ioka T., Ueno M., Oh D.-Y., Fujiwara Y., Chen J.-S., Doki Y., et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC) J Clin Oncol. 2019;37:387. [Google Scholar]
- 95.Boileve A., Hilmi M., Gougis P., Cohen R., Rousseau B., Blanc J.F., et al. Triplet combination of durvalumab, tremelimumab, and paclitaxel in biliary tract carcinomas: safety run-in results of the randomized IMMUNOBIL PRODIGE 57 phase II trial. Eur J Cancer. 2021;143:55–63. doi: 10.1016/j.ejca.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 96.Chen X., Qin S., Gu S., Ren Z., Chen Z., Xiong J., et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: a multicenter, phase 2 trial. Int J Cancer. 2021;149:1944–1954. doi: 10.1002/ijc.33751. [DOI] [PubMed] [Google Scholar]
- 97.Chen X., Wu X., Wu H., Gu Y., Shao Y., Shao Q., et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gou M., Zhang Y., Liu T., Si H., Wang Z., Yan H., et al. PD-1 inhibitors could improve the efficacy of chemotherapy as first-line treatment in biliary tract cancers: a propensity score matching based analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.648068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Massa A., Varamo C., Vita F., Tavolari S., Peraldo-Neia C., Brandi G., et al. Evolution of the experimental models of cholangiocarcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12082308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian L., Ma J., Ma L., Zheng B., Liu L., Song D., et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J Surg Oncol. 2020;18:303. doi: 10.1186/s12957-020-02082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arkenau H.T., Martin-Liberal J., Calvo E., Penel N., Krebs M.G., Herbst R.S., et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF) Oncologist. 2018;23:1407-e136. doi: 10.1634/theoncologist.2018-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoo C., Oh D.Y., Choi H.J., Kudo M., Ueno M., Kondo S., et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monge C., Pehrsson E.C., Xie C., Duffy A.G., Mabry D., Wood B.J., et al. A phase II study of pembrolizumab in combination with capecitabine and oxaliplatin with molecular profiling in patients with advanced biliary tract carcinoma. The oncologist. 2022;27:e273–e285. doi: 10.1093/oncolo/oyab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mei K., Qin S., Chen Z., Liu Y., Wang L., Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort A report in a multicenter phase Ib/II trial. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oh D.Y., Lee K.H., Lee D.W., Yoon J., Kim T.Y., Bang J.H., et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7:522–532. doi: 10.1016/S2468-1253(22)00043-7. [DOI] [PubMed] [Google Scholar]
- 107.Wang D., Yang X., Long J., Lin J., Mao J., Xie F., et al. The efficacy and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: a prospective clinical study. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.646979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yarchoan M., Cope L., Ruggieri A.N., Anders R.A., Noonan A.M., Goff L.W., et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J Clin Invest. 2021;131 doi: 10.1172/JCI152670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie C., Duffy A.G., Mabry-Hrones D., Wood B., Levy E., Krishnasamy V., et al. Tremelimumab in combination with microwave ablation in patients with refractory biliary tract cancer. Hepatology. 2019;69:2048–2060. doi: 10.1002/hep.30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.