Abstract
Polyhydroxy flavonols readily degraded during thermal processing. In this study, the UPLC-Q-tof-MS/MS was applied to explore the stability of dietary polyhydroxy flavonols, myricetin, kaempferol, galangin, fisetin, myricitrin, quercitrin and rutin, in boiling water. The decomposition of flavonols was mainly caused by the heterocyclic ring C opening to form simpler aromatic compounds. The degradation products mainly included 1,3,5-benzenetriol, 3,4,5-trihydroxybenzoic acid, 2,4,6-trihydroxybenzoic acid and 2,4,6-trihydroxybenzaldehyde, etc. Compared with myricetin with a pyrogallol-type structure on the ring B, the glycoside in myricitrin slightly affects the stability. However, the glycosides in rutin and quercitrin dramatically improved the stability in water. During the boiling process, flavonols underwent a series of chemical reactions, such as hydroxylation, dehydroxylation, deglycosidation, deprotonation, and C-ring cleavage.
Keywords: Stability, Flavonols, Degradation, Oxidation
Graphical abstract
Highlights
-
•
The stability of dietary polyhydroxy flavonols have been studied in thermal processing by UPLC-Q-tof-MS/MS.
-
•
The decomposition of flavonols was mainly caused by the heterocyclic ring C opening.
-
•
Flavonols underwent a series of chemical reactions in thermal processing.
1. Introduction
Over the last few decades, the epidemiological and nutritional data have shown that consuming vegetables and fruits rich in flavonoids leads to a general well-being of consumers (Liu, Fu, Ma, Yi & Cai, 2021; Higbee et al., 2022; Ozdal et al., 2016; Xiao, 2017; Xiao et al., 2016; Yang et al., 2022; Zhang et al., 2021; Zhao et al., 2020). It is mainly attributed to various dietary bioactive flavonoids, which showed numerous functions such as free radical scavenging (Rodriguez-Mateos et al., 2014), anti-inflammatory (Chen et al., 2018), anti-cancer (Kim et al., 2019), hypoglycemic (Bolouki, Zal & Bordbar, 2020; Kim et al., 2019) and weight loss (Lyu et al., 2022), etc., which played a crucial role in promoting health (Dong et al., 2021; Zhong et al., 2022). However, the bioactivities of dietary flavonoids depend largely on their stability in the food matrix (Xiao and Högger, 2015; Cao et al., 2016; Zhang et al., 2022; Chandra, Prihastyanti & Lukitasari, 2021).
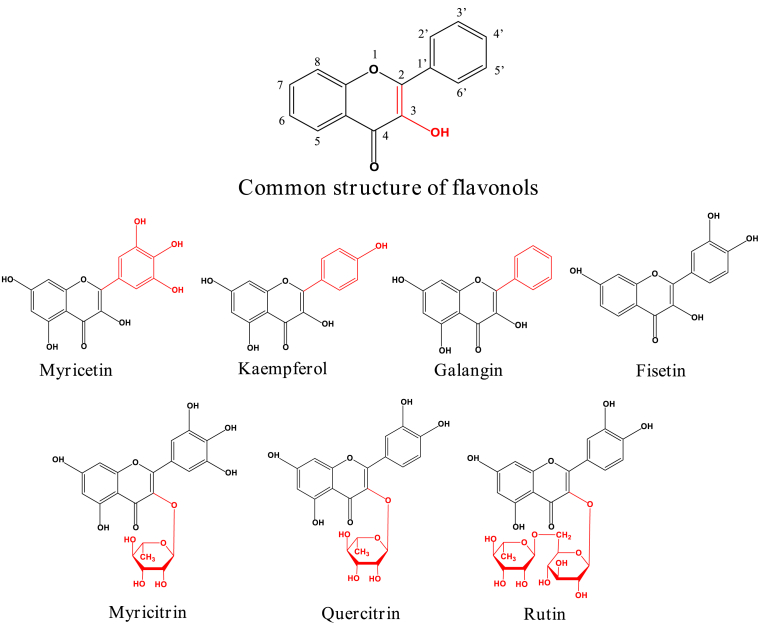
There have been a lot of studies on the effects of thermal treatment on flavonoids (Ganesan, Sukalingam & Xu, 2019; Hidalgo and Zamora, 2017; Wong, Chai & xiao, 2019; Chandra et al., 2021); however, the thermal degradation products and mechanisms of polyhydroxy flavonols in boiling water are still not clear. Herein, we selected the stability of seven polyhydroxy flavonols (Fig. 1) in boiling water were investigated for simulating common home cooking conditions via UPLC-Q-tof-MS-MS analysis.
Fig. 1.
Chemical structures of flavonols studied.
We further identified the thermal degradation products structures and revealed their thermal degradation mechanisms. The flow diagram is shown in Fig. 2.
Fig. 2.
Flow diagram demonstrating the stability of flavonols during heating.
2. Materials and methods
2.1. Chemicals
Myricetin, kaempferol, galangin, fisetin, myricitrin, quercitrin and rutin (>95%) were purchased from Yuanye Biotechnology Co. Ltd. (Shanghai, China). Methanol (LC-MS grade) was purchased from Merck Co. Ltd. (Darmstadt, Germany). Formic acid (HPLC grade) and DMSO were obtained from Aladdin Co. Ltd. (Shanghai, China). Deionized water was prepared using a MilliQ Integral water purification system (Millipore, Bedford, MA, USA).
2.2. Stability of flavonols in boiling water
Flavonol was dissolved in DMSO to obtain a standard stock solution (5 × 10−3 mol/L). One hundred microliters of flavonol standard stock solution were added to 900 μL MiliQ water in an autosampler vial. The vial was incubated in a boiling water bath, and the sample was taken out from the vial every 15 min, and was immediately cooled in an ice bath for further UPLC-Q-tof-MS/MS analysis.
2.3. UPLC-Q-tof-MS analysis
UPLC-Q-tof-MS/MS analysis was performed on an ultra-performance liquid chromatography system coupled to the time-of-flight mass spectrometry (Waters XEVO G2-XS QTof). Chromatographic separation on the system was achieved on a Waters ACQUITY UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm). The mobile phase was a gradient of 0.1% formic acid in MiliQ water (A) and methanol (B) at a flow rate of 0.3 mL/min. The injection volume was 1 μL. The elution gradient was set as follows: 5–15% B, 0–2 min; 15–25% B, 3–4 min; 25–40% B, 4–5 min; 40–50% B, 5–6 min; 50–60% B, 6–7 min; 60–70% B, 7–8 min; and 70-5% B, 8–13 min. Acquisition range was 100–1000 m/z. Source temperature was 120 °C. Desolvation temperature was 450 °C. Capillary voltage was 2 kV. Sampling cone voltage was 40 V. Source Offset was 80 V. Cone gas flow was 50 L/h. Desolvation gas flow was 700 L/h. MS Data was acquired in MSE mode under sensitivity mode and negative electrospray ionization (ESI-). The data was processed by MassLynx V 4.1 software.
2.4. Strategies for determination of the chemical structures of flavonols derivatives
MS and MS/MS data, fragment ions, chemical reaction properties of flavonols and chemical reaction properties of candidate compounds were combined to infer the chemical structures of the derivatives. Taking myricetin as an example, it was determined whether the derivative contained the characteristic fragment ions of myricetin or myricetin after chemical modification such as oxidation. Secondly, based on the accurate molecular weight provided by MS information and the consideration of likely chemical reactions, the possible chemical structures of the myricetin derivatives were inferred. The structures of the degradation products of the other six flavonols were also predicted according to the above method.
3. Results and discussion
3.1. Stability of polyhydroxy flavonols in boiling water
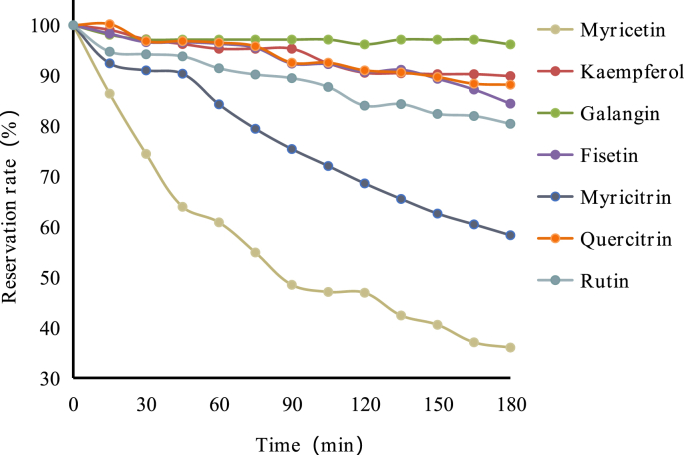
Fig. 3 and Table 1 showed the stability of myricetin, kaempferol, galangin, fisetin, myricitrin, quercitrin and rutin in boiling water. All these polyhydroxy flavonols were rapidly degraded when heated with in boiling water. The thermal stability in descending order was: galangin > rutin > fisetin > kaempferol > quercitrin > myricitrin > myricetin. Among them, galangin (no hydroxyl group in the B-ring) (500T10 > 180 min) was the most stable one in boiling water and less than 10% of galangin was degraded after 3 h heating process. The hydroxylation at the ring B of flavonols obviously influenced the thermal stability. Myricetin (3′,4′,5′-OH) (500T10 = 7.75 min) was significantly unstable in boiling water and they were degraded by more than 63.97% of myricetin was disappeared after 3-h heating. Combined with previous experiments on the stability of quercetin (3′,4′-OH) (500T10 = 17.57 min) in boiling water (Lin et al., 2022), it was shown that B-ring hydroxylation reduces the stability of flavonols and the thermal stability was resorcinol-type > catechol-type > pyrogallol-type. These results were in good agreement with observations of the stability of flavonoids in DMEM medium (Xiao and Högger, 2015). Compared quercetin (3,5,7,3′,4′-OH) and fisetin (3,7,3′,4′-OH) which both have 2 hydroxyl groups on ring B but different number of hydroxyl groups on ring A. During heating in boiling water, fisetin (500T10 = 131.24 min) exhibited higher thermal stability than quercetin (500T10 = 17.57 min) (Lin et al., 2022), which suggested that the hydroxylation in ring A of flavonols improved its stability. Moreover, the glycosylation on the hydroxyl group of flavonols significantly enhanced their stability. Compared with quercetin (500T10 = 17.57 min), quercitrin (500T10 = 74.08 min) and rutin (500T10 = 135.64 min) showed much higher stability during 3-h heating treatment. Myricitrin (500T10 = 34.43 min) was also more stable than myricetin (500T10 = 7.75 min). However, althrough myricitrin has a glycoside on C-3 position, it was still unstable due to the pyrogallol moiety on ring B, which is the key part affecting the stability.
Fig. 3.
Degradation curve of flavonols in aqueous solution at 100 °C.
Table 1.
Stability of seven flavonols (initial concentration 500 μM) in boiling water at 100 °C.
| Name | Substitutions |
500T10 |
500T50 |
||
|---|---|---|---|---|---|
| OH | others | min | min | ||
 |
Myricetin | 3,5,7,3′,4′,5′ | 7.75 | 107.24 | |
| Kaempferol | 3,5,7,4′ | 101.20 | >180 | ||
| Galangin | 3,5,7 | >180 | >180 | ||
| Fisetin | 3,7,3′,4′ | 131.24 | >180 | ||
| Myricitrin | 5,7,3′,4′,5′ | 3-O-β-D-glucopyranose | 34.43 | >180 | |
| Quercitrin | 5,7,3′,4′ | 3-O-β-D-glucopyranose | 74.08 | >180 | |
| Rutin | 5,7,3′,4′ | 3-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranose | 135.64 | >180 | |
3.2. Identification of new products of myricetin in boiling water
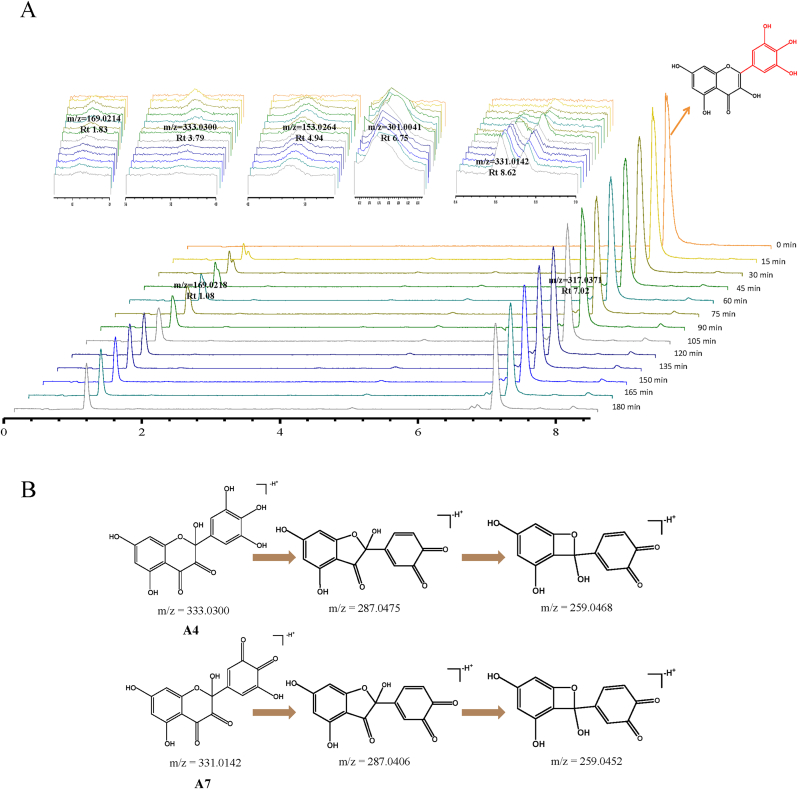
In order to characterize the thermal degradedation products of flavonols in boiling water, the UPLC-Q-tof-MS/MS analysis was applied. The main results were shown in Fig. 4A and Table 2. Myricetin was degraded rapidly in boiling water and formed several new products with peaks at m/z 169.0218 (A1), m/z 169.0214 (A2), m/z 333.0300 (A3), m/z 153.0264 (A4), m/z 301.0041 (unidentified product), m/z 331.0142 (A5) and m/z 343.0146 (unidentified product). Compound A3 was identified as the characteristic degradation product of myricetin, which reached its maximum intensity after 15-min incubation and then gradually decreased. Compound A3 was the hydroxylated product of myricetin, while compound A5 was an o-quinone further deprotonated from A3. The oxidation of flavonols easily opens the heterocyclic ring C, but the introduction of a hydroxyl group from H2O is not stable and continues to undergo intramolecular rearrangements or tautomerization to forming chalcone and ring B quinones (Zhou et al., 2007). Compared with standards, compound A1 (3,4,5-trihydroxybenzoic acid), A2 (2,4,6-trihydroxybenzoic acid) and A4 (2,4,6-trihydroxybenzaldehyde) were also identified ( see Table 3). The degradation of myricetin was based on the formation of simpler aromatic compounds by opening heterocyclic ring C. Unfortunately, there were some unidentified compounds, such as m/z 301.0041 and m/z 343.0146.
Fig. 4.
(A) TIC chromatograms of myricetin in boiling water. (B) Fragmentation scheme of myricetin degradation products.
Table 2.
Retention time (Rt), MS-MS fragments of important ions present in the mass spectra of seven flavonols degraded products in boiling water via UPLC-Q-tof-MS.
| Compounds | Rt (min) | Molecular [M-H]- | MS-MS fragment |
|---|---|---|---|
| Myricetin in 100 °C water | |||
| A1 | 1.08 | 169.0218 | 169.0335, 151.0199, 125.0394, 107.0280, 97.0427 |
| A2 | 1.83 | 169.0214 | 169.0312, 151.0196, 125.0365, 107.0277, 83.0263 |
| A3 | 3.79 | 333.0300 | 287.0475, 259.0468, 253.0421, 207.0115, 125.0385 |
| A4 | 4.94 | 153.0264 | 153.0350, 151.0196, 107.0270 |
| Not identified | 6.75 | 301.0041 | 301.0213, 273.0239, 245.0290, 229.0335, 201.0374, |
| Myricetin | 7.02 | 317.0371 | 317.0535, 289.0568, 271.0453, 227.0546, 193.0314, |
| Not identified | 8.13 | 343.0146 | 343.0325, 325.0217, 315.0358, 299.0407, 287.0403, 269.0296, 229.0333, 151.0194 |
| A5 | 8.62 | 331.0142 | 287.0406, 259.0452, 215.0531, 187.0581 |
| Kaempferol in 100 °C water | |||
| B1 | 0.85 | 125.0328 | 97.0553, 83.0464, 77.0204 |
| B2 | 1.09 | 169.0222 | 153.0353, 151.0208, 125.0423, 107.0289, 83.0283 |
| B3 | 1.79 | 169.0223 | 169.0316, 151.0206, 125.0396, 107.0285, 83.0278 |
| B4 | 3.44 | 137.0329 | 111.0259, 93.0484, 75.0389 |
| B5 | 5.10 | 153.0275 | 153.0222, 151.0083, 125.0318 |
| B6 | 6.61 | 409.0604 | 393.0736, 363.0708, 319.0810, 298.0355, 257.0653, 243.0527, 277.0541, 211.0621, 151.0200, 107.0279 |
| B7 | 6.96 | 467.0638 | 449.0705, 405.0871, 377.0896, 337.0966, 297.0615, 285.0610, 269.0682, 253.0714, 181.0209, 137.0403, |
| Kaempferol | 8.33 | 285.0504 | 285.0622, 255.0509, 239.0547, 227.0545, 211.0589, 187.0580, 159.0617, 143.0661, 117.0497 |
| Not identified | 9.23 | 569.0735 | 551.0902, 499.0782, 405.0862, 311.0419, 257.0656, 239.0547, 151.0201 |
| Galangin in 100 °C water | |||
| C1 | 0.87 | 125.0322 | 117.0021, 83.0279, 57.0460 |
| C2 | 1.09 | 169.0200 | 151.0169, 125.0362, 107.0261, 83.0253 |
| C3 | 1.83 | 169.0168 | 169.0279, 151.0172, 125.0372, 107.0266, 83.0258 |
| C4 | 2.37 | 121.0372 | No data obtained |
| C5 | 5.05 | 153.0269 | 151.0169, 125.0506, 107.0236 |
| C6 | 9.21 | 393.0533 | 377.0792, 321.0912, 269.0609, 169.0789, 123.0214 |
| Galangin | 9.57 | 269.055 | 269.0657, 252.0582, 227.0502, 197.0753, 169.0799, |
| C7 | 10.29 | 451.0570 | 435.0387, 385.0263, 269.0604, 255.0695, 169.0276, 163.0170, 151.0165, 137.0376 |
| Fisetin in 100 °C water | |||
| D1 | 0.70 | 183.0386 | 137.0413, 121.0454, 109.0447, 93.0491 |
| D2 | 1.12 | 181.0232 | 135.0257, 109.0451, 91.0334 |
| D3 | 2.16 | 153.0288 | 153.0326, 109.0424, 91.0208, 81.0466 |
| D4 | 3.31 | 269.0526 | 269.0691, 241.0730, 225.0770, 197.0805, 183.0640 |
| D5 | 4.30 | 153.0288 | 153.0334, 109.0420, 67.0287 |
| D6 | 5.31 | 137.0336 | 137.0412, 135.0259, 109.0449, 91.0339 |
| D7 | 6.16 | 301.0190 | 301.0515, 285.0951, 271.0404, 255.0466, 277.0501, 211.0566, 201.0689, 183.0592, 151.0163 |
| D8 | 6.85 | 273.0477 | 257.0535, 197.0442, 163.0207, 135.0261, 109.0454, |
| Fisetin | 7.35 | 285.0588 | 285.0652, 257.0687, 229.0728, 163.0225, 135.0263, 121.0461, 91.0337 |
| D9 | 7.60 | 283.0232 | 283.0491, 255.0527, 277.0566, 211.0607, 182.0646, 171.0639, 147.0265, 135.026, 119.0303, 91.0337 |
| Myricitrin in 100 °C water | |||
| E1 | 0.88 | 125.0312 | 83.0230 |
| E2 | 1.12 | 169.0219 | 151.0188, 107.0223, 83.0281 |
| E3 | 1.90 | 169.0221 | 151.0114, 125.0369, 107.0236, 83.0259 |
| Not identified | 3.66 | 355.0718 | 355.0827, 309.0769, 153.0246, 83.0295 |
| E4 | 5.29 | 469.0733 | 453.0736, 421.0406, 381.0408, 327.0260, 319.0400, 267.0326, 179.0078, 151.0188, 125.0405 |
| E5 | 6.04 | 455.0768 | 39.0960, 423.0494, 407.0341, 393.0391, 287.0299 |
| Myricitrin | 6.70 | 463.0931 | 445.0908, 317.0420, 271.0381, 179.0105, 151.0151 |
| Not identified | 6.94 | 509.0780 | 417.0436, 333.0132, 317.0474, 301.0462, 271.0330, |
| Not identified | 8.18 | 733.0981 | 587.0588, 475.1033, 393.0396, 307.0349 |
| Quercitrin in 100 °C water | |||
| F1 | 5.28 | 445.0815 | 445.0968, 415.0592, 299.0359, 221.0310 |
| F2 | 6.03 | 445.0823 | 299.0346, 271.0384, 227.0472, 197.0065 |
| Quercitrin | 7.17 | 447.1078 | 447.0257, 373.0764, 301.0518, 271.0414, 255.0460, |
| F3 | 8.22 | 453.0981 | 375.0719, 301.0504, 285.0567, 271.0390, 223.0270 |
| Rutin in 100 °C water | |||
| G1 | 4.72 | 607.1331 | 581.0595, 453.1035, 299.0438, 171.9053 |
| G2 | 6.13 | 607.1141 | 601.0953, 583.0806, 419.0679, 301.0570, 151.0197 |
| G3 | 6.36 | 607.1290 | 607.1645, 315.0381, 301.0576, 287.0422, 151.0198 |
| Rutin | 6.71 | 609.1500 | 609.1802, 373.0816, 343.0704, 301.0581, 271.0464, |
| G4 | 7.13 | 607.1299 | 607.1656, 443.0902, 317.0535, 259.0462, 151.0201 |
Table 3.
Identification of flavonols oxidation products (only simple aromatic compounds are shown).
| Compound | Structure | Myricetin | Kaempferol | Galangin | Fisetin | Myricitrin | Quercitrin | Rutin |
|---|---|---|---|---|---|---|---|---|
| 1,3,5-Benzenetriol |  |
– | B1 | C1 | – | E1 | – | – |
| 3,4,5-Trihydroxybenzoic acid |  |
A1 | B2 | C2 | – | E2 | – | – |
| 2,4,6-Trihydroxybenzoic acid |  |
A2 | B3 | C3 | – | E3 | – | – |
| 2,4,6-Trihydroxybenzaldehyde |  |
A3 | B5 | C5 | – | – | – | – |
| 4-Hydroxybenzoic acid | 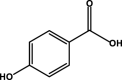 |
– | B4 | – | – | – | – | – |
| Benzoic acid | 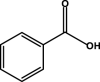 |
– | – | C4 | – | – | – | – |
| 3,4-Dihydroxymandelic acid | 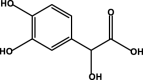 |
– | – | – | D1 | – | – | – |
| 3,4 -Dihydroxyphenylglyoxylate | 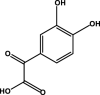 |
– | – | – | D2 | – | – | – |
| Protocatechuic acid | 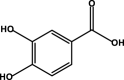 |
– | – | – | D3 | – | – | – |
| 2,4-Dihydroxybenzoic acid | 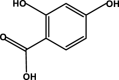 |
– | – | – | D5 | – | – | – |
| 3-Hydroxybenzoic acid | 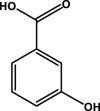 |
– | – | – | D6 | – | – | – |
3.3. Identification of new products of kaempferol in boiling water
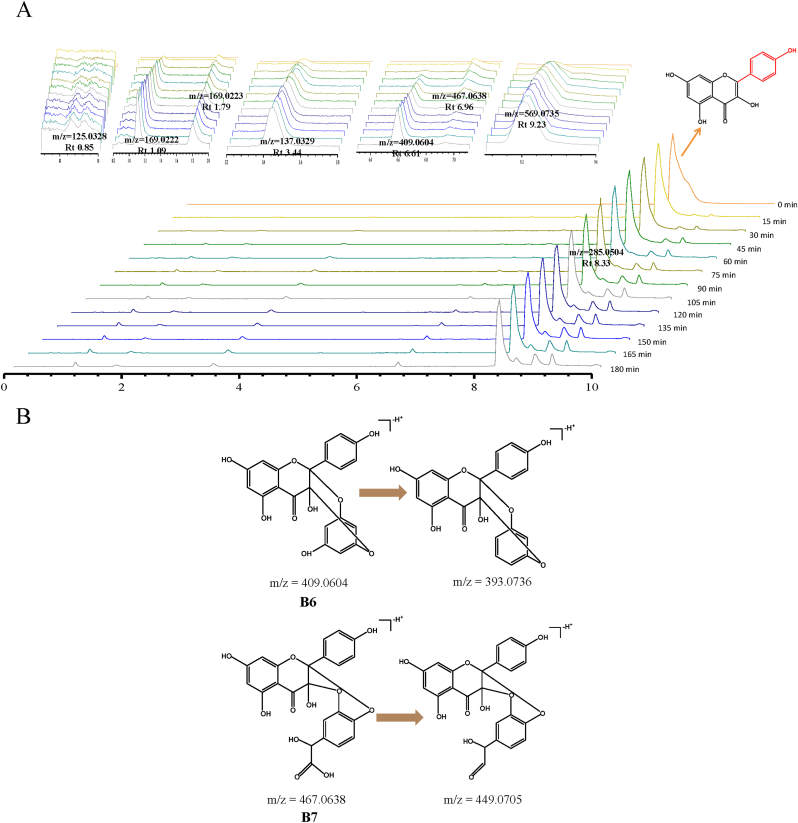
As shown in Fig. 5A, and the new products of kaempferol in boiling water included m/z 125.0328 (B1), m/z 169.0222 (B2), m/z 169.0223 (B3), m/z 137.0329 (B4), m/z 153.0275 (B5), m/z 409.0604 (B6), m/z 467.0638 (B7) and m/z 569.0735 (B8). Compared with the standards, compound B1 (1,3,5-benzenetriol), B2 (3,4,5-trihydroxybenzoic acid), B3 (2,4,6-trihydroxybenzoic acid), B4 (4-hydroxybenzoic acid) and B5 (2,4,6-trihydroxybenzaldehyde) were identified. According to the characteristic fragment ions, combined with the chemical properties of kaempferol and the verification of Mass frontier 8.0 software, compound B6 (m/z 409.0604) was speculated that it may contain kaempferol and an phloroglacinol molecule. The presumed structural of compound B6 is shown in Fig. 5B. Compound B7 was found to be kaempferol-3,4-dihydroxymandelic acid. The characteristic fragment ions of Compound B8 with m/z 569.0735 included m/z 551.0902, m/z 499.0782, m/z 311.0419, m/z 257.0656, m/z 239.0547 and m/z 151.0201, which indicated the existence of kaempferol core structure. Compound B8 maybe a kaempferol dimer formed by opening the carbon-carbon double bond of the ring C of kaempferol and oxidative polymerization with another kaempferol. Interestingly, kaempferol did not undergo hydroxylation on the ring C as for myricetin in water. The presence of a catechol unit on ring B and a free C-3 hydroxyl groups appear to be a prerequisite for the formation of ring C carbocation in the oxidative decomposition of flavonols (Krishnamachari et al., 2002). A poorly substituted flavonols on ring B skeleton are not able to form o-quinone (Krishnamachari et al., 2004). It may explain why no hydroxylation products and quinones of kaempferol were observed in boiling water.
Fig. 5.
(A) TIC chromatograms of kaempferol in boiling water. (B) Fragmentation scheme of kaempferol degradation products.
3.4. Identification of new products of galangin in boiling water
Galangin was the most stable one among the seven flavonols, and its degradation trend in boiling water was shown in Fig. 6A. The degradation products of galangin in boiling water were identified as 1,3,5-benzenetriol (C1, m/z 125.0322), 3,4,5-trihydroxybenzoic acid (C2, m/z 169.0222), 2,4,6-trihydroxybenzoic acid (C3, m/z 169.0168), benzoic acid (C4, m/z 121.0372) and 2,4,6-trihydroxybenzaldehyde (C5, m/z 153.0269). Galangin showed the similar oxidative degradation with kaempferol to form the compound C6 (m/z 393.0533) and compound C7 (m/z 451.0570), which were 124 and 181 Da more than the original substance, respectively. Compound C6 may contain an phloroglacinol molecule, and compound C7 may be that galangin bound the 3,4-dihydroxymandelic acid at the C2, C3 positions of the ring C.
Fig. 6.
(A) TIC chromatograms of galangin in boiling water. (B) Fragmentation scheme of galangin degradation products.
3.5. Identification of new products of fisetin in boiling water
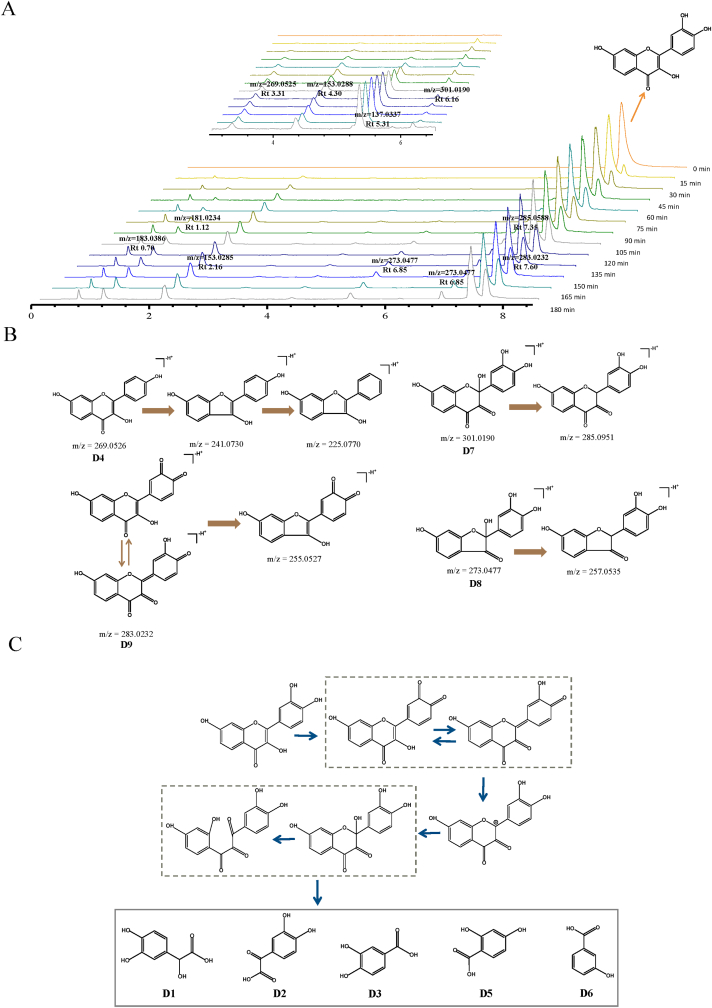
When fisetin was heated in boiling water, the new degradation products were observed as 3,4-dihydroxymandelic acid (D1, m/z 183.0386), 3,4 -dihydroxyphenylglyoxylate (D2, m/z 181.0232), protocatechuic acid (D3, m/z 153.0288), 2,4-dihydroxybenzoic acid (D5, m/z 153.0288) and 3-hydroxybenzoic acid (D6, m/z 137.0336) (Fig. 7A). 3,4-dihydroxymandelic acid and protocatechuic acid were also found in case of quercetin in boiling water (Lin et al., 2022). Comparing the chemical structures of fisetin and quercetin, the number of hydroxyl groups in ring A of fisetin and quercetin were different, but the structure of ring B was the same. In addition, were also observed. For compound D4 (m/z 269.0526), which was 16 Da less than fisetin, suggesting that it might undergone a dehydroxylation. The mass spectra of compound D7 (301.0190 m/z) was 16 Da more than that of fisetin, it may be the product of the hydroxylation of fisetin. But it was worth noting that the water adducts with the introduction of a hydroxyl group was not stable and continues to undergo intramolecular rearrangements or tautomerization (Zhou et al., 2007). Compound D8 (m/z 273.0477) had 28 Da less than D7, which may be a product of decarbonylation of the unstable intermediate D7. As for compound D9 (m/z 283.0232), based on the main oxidation pathway of flavonols, it was fisetin deprotonated to form quinones. According to the above new product characterization (Cao et al., 2020), the possible degradation mechanism of fisetin in boiling water was shown in (Fig. 7C).
Fig. 7.
(A) TIC chromatograms of fisetin in boiling water. (B) Fragmentation scheme of fisetin products. (C) Oxidative reaction pathway for degradation of fisetin.
3.6. Identification of new products of myricitrin, quercitrin and rutin in boiling water
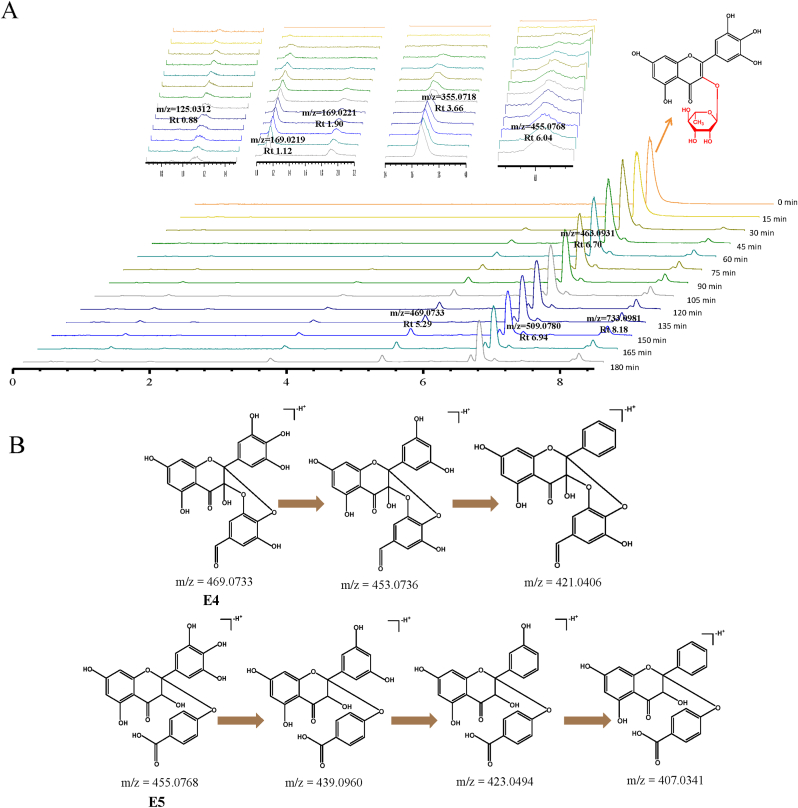
The degradation process of myricitrin, quercitrin and rutin in boiling water were shown in Fig. 8, Fig. 9, Fig. 10A. Firstly, 1,3,5-benzenetriol (E1, m/z 125.0288), 3,4,5-trihydroxybenzoic acid (E2, m/z 169.0218) and 2,4,6-trihydroxybenzoic acid (E3, m/z 169.0214) were observed in myricitrin, which were also found in myricetin. In addition, E4 (m/z 469.0733) and E5 (m/z 455.0768) were observed, their m/z values were 152 Da and 138 Da more than myricetin, which may be myricitrin lost the rhamnose group, and then linked with 3,4,5-trihydroxybenzaldehyde and 4-hydroxybenzoic acid (Fig. 8B). Quercitrin and rutin were much more stable in boiling water than that of quercetin, and the glycosylation of 3-OH significantly improved the oxidability of flavonols in boiling water. Therefore, in boiling water, the deprotonation process of quercitrin and rutin was mainly observed, and the corresponding quinones were generated. Due to its more complex structure, rutin with a two-part glycoside on the C-3 position has more isomers. During the heating process, rutin was oxidized to form four quinones, compound G1 (m/z 607.1331), G2 (m/z 607.1141), G3 (m/z 607.1290) and G4 (m/z 607.1299), which were similar to that observed by Olivier Dangles et al. (Dangles et al., 1999). However, because these compounds have many possible deprotonation sites to form various isomers.
Fig. 8.
(A) TIC chromatograms of myricitrin in boiling water. (B) Fragmentation scheme of myricitrin products.
Fig. 9.
(A) TIC chromatograms of quercitrin in boiling water. (B) Fragmentation scheme of quercitrin products.
Fig. 10.
(A) TIC chromatograms of rutin in boiling water. (B) Degradation product of rutin.
4. Conclusion
In this study, the stability of seven polyhydroxy flavonols in boiling water was analyzed by UPLC-MS/MS. According to their structure, flavonols were more or less sensitive to heat treatment. Hydroxylation of flavonols at the ring B usually weakened their stability, but hydroxylation at the ring A will increase its stability. Glycosylated flavonols were more resistant to heat treatment than their aglycon form. During the boiling process, flavonols underwent a series of chemical reactions, such as hydroxylation, dehydroxylation, deglycosidation, deprotonation, and C-ring cleavage. The major products were found to be 1,3,5-benzenetriol, 2,4,6-trihydroxybenzoic acid and 2,4,6-trihydroxybenzaldehyde, dimers and quinones, etc. It was worth noting that most of these degradation products were active substances with excellent antioxidant properties. Our results may provide deeper insights into their subsequent complex biological activities.
CRediT authorship contribution statement
Shiye Lin: Investigation, Data curation, Writing – original draft. Jesus Simal-Gandara: Formal analysis, Data curation. Hui Cao: Writing – review & editing, Supervision. Jianbo Xiao: Funding acquisition, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by National Natural Science Foundation of China (31972072).
Handling Editor: Professor A.G. Marangoni
Contributor Information
Shiye Lin, Email: linshiye33@163.com.
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
Hui Cao, Email: hui_cao0830@yahoo.com, mprieto@uvigo.es.
Jianbo Xiao, Email: jianboxiao@yahoo.com.
Data availability
Data will be made available on request.
References
- Bolouki A., Zal F., Bordbar H. Ameliorative effects of quercetin on folliculogenesis in diabetic mice: a stereological study. Gynecol. Endocrinol. 2020;36(10):864–868. doi: 10.1080/09513590.2019.1707796. [DOI] [PubMed] [Google Scholar]
- Cao H., Jia X., Shi J., Xiao J., Chen X. Non-covalent interaction between dietary stilbenoids and human serum albumin: structure-affinity relationship, and its influence on the stability, free radical scavenging activity and cell uptake of stilbenoids. Food Chem. 2016;202:383–388. doi: 10.1016/j.foodchem.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Cao H., Yi L., Zhong J., Högger P., Wang M., Prieto M.A., Simal Gandara J., Xiao J. Investigation of new products and reaction kinetics for myricetin in DMEM via an in situ UPLC–MS–MS analysis. Food Front. 2020;1(3):243–252. [Google Scholar]
- Chandra R.D., Prihastyanti M.N.U., Lukitasari D.M. Effects of pH, high pressure processing, and ultraviolet light on carotenoids, chlorophylls, and anthocyanins of fresh fruit and vegetable juices. eFood. 2021;2(3):113–124. [Google Scholar]
- Chen L., Teng H., Jia Z., Battino M., Miron A., Yu Z., Cao H., Xiao J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: the most recent evidence. Crit. Rev. Food Sci. Nutr. 2018;58(17):2908–2924. doi: 10.1080/10408398.2017.1345853. [DOI] [PubMed] [Google Scholar]
- Dangles O., Fargeix G., Dufour C. One-electron oxidation of quercetin and quercetin derivatives in protic and non protic media. J. Chem. Soci., Perkin Transact. 1999;2(7):1387–1396. [Google Scholar]
- Dong A., Huang Y.W., Yearsley M., Oshima K., Chen X., Yu J., Wang L.S. Dietary supplementation with black raspberries prolongs survival in ApcMin/+ mice. Food Front. 2021;2(3):324–328. doi: 10.1002/fft2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan K., Sukalingam K., Xu B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers- A critical review. Crit. Rev. Food Sci. Nutr. 2019;59(3):488–505. doi: 10.1080/10408398.2017.1379470. [DOI] [PubMed] [Google Scholar]
- Hidalgo F.J., Zamora R. Food processing antioxidants. Adv. Food Nutr. Res. 2017;81:31–64. doi: 10.1016/bs.afnr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Higbee J., Solverson P., Zhu M., Carbonero F. The emerging role of dark berry polyphenols in human health and nutrition. Food Front. 2022;3(1):3–27. [Google Scholar]
- Kim S., Yoo E., Woo J., Han S., Lee J., Jung S., Kim H., Jung J. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019;860 doi: 10.1016/j.ejphar.2019.172568. [DOI] [PubMed] [Google Scholar]
- Krishnamachari V., Levine L.H., Paré P.W. Flavonoid oxidation by the radical generator aibn: a unified mechanism for quercetin radical scavenging. J. Agric. Food Chem. 2002;50(15):4357–4363. doi: 10.1021/jf020045e. [DOI] [PubMed] [Google Scholar]
- Krishnamachari V., Levine L.H., Zhou C., Paré P.W. In vitro flavon-3-ol oxidation mediated by a B ring hydroxylation pattern. Chem. Res. Toxicol. 2004;17(6):795–804. doi: 10.1021/tx034242z. [DOI] [PubMed] [Google Scholar]
- Lin S., Zhang H., Simal-Gandara J., Cheng K., Wang M., Cao H., Xiao J. Investigation of new products of quercetin formed in boiling water via UPLC-Q-TOF-MS-MS analysis. Food Chem. 2022;386 doi: 10.1016/j.foodchem.2022.132747. [DOI] [PubMed] [Google Scholar]
- Liu X., Fu Y., Ma Q., Yi J., Cai S. Anti‐Diabetic effects of different phenolic‐rich fractions fromRhus chinensis mill. Fruitsin vitro. eFood. 2021;2(1):37–46. [Google Scholar]
- Lyu Q., Chen L., Lin S., Cao H., Teng H. A designed self-microemulsion delivery system for dihydromyricetin and its dietary intervention effect on high-fat-diet fed mice. Food Chem. 2022;390 doi: 10.1016/j.foodchem.2022.132954. [DOI] [PubMed] [Google Scholar]
- Ozdal T., Sela D.A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8(2):78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mateos A., Vauzour D., Krueger C.G., Shanmuganayagam D., Reed J., Calani L., Mena P., Del Rio D., Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch. Toxicol. 2014;88(10):1803–1853. doi: 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- Wong F.C., Chai T.T., Xiao J. The influences of thermal processing on phytochemicals and possible routes to the discovery of new phytochemical conjugates. Crit. Rev. Food Sci. Nutr. 2019;59(6):947–952. doi: 10.1080/10408398.2018.1479681. [DOI] [PubMed] [Google Scholar]
- Xiao J. Dietary flavonoid aglycones and their glycosides: which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017;57(9):1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- Xiao J., Högger P. Stability of dietary polyphenols under the cell culture conditions: avoiding erroneous conclusions. J. Agric. Food Chem. 2015;63(5):1547–1557. doi: 10.1021/jf505514d. [DOI] [PubMed] [Google Scholar]
- Xiao J., Capanoglu E., Jassbi A.R., Miron A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016;56(sup1):S29–S45. doi: 10.1080/10408398.2015.1067595. [DOI] [PubMed] [Google Scholar]
- Yang L., Gao Y., Gong J., Wang H., Farag M.A., Simal Gandara J., Zhao Y., Nie S., Xiao J. Myricetin ameliorated prediabetes via immunomodulation and gut microbiota interaction. Food Front. 2022 [Google Scholar]
- Zhang H., Caprioli G., Hussain H., Le N.P.K., Farag M.A., Xiao J. A multifaceted review on dihydromyricetin resources, extraction, bioavailability, biotransformation, bioactivities, and food applications with future perspectives to maximize its value. eFood. 2021;2(4):164–184. [Google Scholar]
- Zhang H.L., Wang M.L., Yi L.Z., Högger P., Arroo R., Bajpai V.K., Prieto M.A., Chen X.J., Simal-Gandara J., Cao H. Stability profiling and degradation products of dihydromyricetin in Dulbecco's modified eagle's medium. Food Chem. 2022;378 doi: 10.1016/j.foodchem.2021.132033. [DOI] [PubMed] [Google Scholar]
- Zhao C., Wan X., Zhou S., Cao H. Natural polyphenols: a potential therapeutic approach to hypoglycemia. eFood. 2020;1(2):107–118. [Google Scholar]
- Zhong R., Farag M.A., Chen M., He C., Xiao J. Recent advances in the biosynthesis, structure–activity relationships, formulations, pharmacology, and clinical trials of fisetin. eFood. 2022;3(1–2) [Google Scholar]
- Zhou A., Kikandi S., Sadik O.A. Electrochemical degradation of quercetin: isolation and structural elucidation of the degradation products. Electrochem. Commun. 2007;9(9):2246–2255. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.