Abstract
Background:
A recent subanalysis of the EAST-AFNET 4 trial suggests a stronger benefit of early rhythm-control (ERC) in patients with atrial fibrillation (AF) and a high comorbidity burden when compared to patients with a lower comorbidity burden.
Methods:
We identified 109,739 patients with newly diagnosed AF in a large US de-identified administrative claims database (OptumLabsⓇ) and 11,625 patients in the population-based UK Biobank (UKB). ERC was defined as AF ablation and/or antiarrhythmic drug therapy within the first year after AF diagnosis. Patients were classified as 1) ERC & high comorbidity burden (CHA2DS2-VASc score ≥4); 2) ERC & lower comorbidity burden (CHA2DS2-VASc score 2–3); 3) no ERC & high comorbidity burden; and 4) no ERC & lower comorbidity burden. Patients without an elevated comorbidity burden (CHA2DS2-VASc score 0–1) were excluded. Propensity score overlap weighting and cox proportional hazards regression were used to balance patients and compare groups for the primary composite outcome of all-cause mortality, stroke, or hospitalization with the diagnoses heart failure or myocardial infarction as well as for a primary composite safety outcome of death, stroke, and serious adverse events related to ERC.
Results:
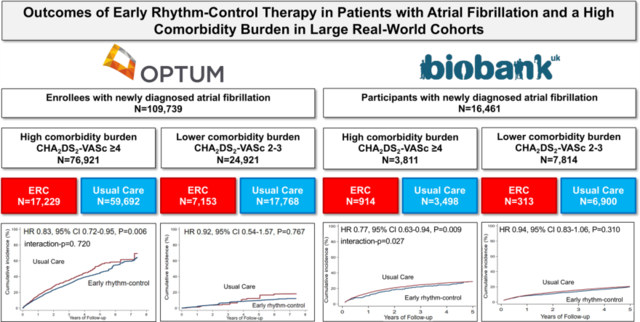
In both cohorts, ERC was associated with a reduced risk for the primary composite outcome in patients with a high comorbidity burden (OptumLabs: HR 0.83, CI 0.72–0.95, p=0.006; UKB: HR 0.77, CI 0.63–0.94, p=0.009). In patients with a lower comorbidity burden, the difference in outcomes was not significant (OptumLabs: HR 0.92, CI 0.54–1.57, p=0.767; UKB: HR 0.94, CI 0.83–1.06, p=0.310). The comorbidity burden interacted with ERC in the UKB (interaction-p=0.027) but not in OptumLabs (interaction-p=0.720). ERC was not associated with an increased risk for the primary safety outcome.
Conclusions:
ERC is safe and may be more favorable in a population-based sample of patients with high a comorbidity burden (CHA2DS2-VASc score ≥4).
Keywords: Atrial Fibrillation, Cather Ablation, Early Rhythm Control Therapy, Comorbidity, CHA2DS2-Vasc score, EAST, stroke
Graphical Abstract
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and poses a major burden on health care systems.1 Treatment domains include oral anticoagulation for prevention of stroke, treatment of concomitant and underlying cardiovascular conditions, rate control, and rhythm control.1,2
In the past decades, several large randomized clinical trials failed to show a prognostic benefit of rhythm-control therapy compared with rate-control.3–5 Recently, however, paradigms in the therapy of AF have shifted following the demonstration that early and systematic initiation of rhythm control therapy reduces outcomes compared to usual care:6,7 In the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4), early rhythm-control therapy (ERC) led to reduced cardiovascular complications, including fewer strokes and cardiovascular deaths, in patients with recently diagnosed AF and concomitant cardiovascular conditions compared to usual care consisting of rate control and symptom-limited rhythm-control therapy.6 Most patients seen in routine clinical care or included in population-based projects are eligible for ERC, and modern rhythm-control therapy appears safe in unselected patients treated in the United Kingdom, United States, and South Korea.8,9,10
A recent subgroup analysis of the EAST-AFNET 4 trial suggests a stronger benefit of ERC in patients with a high comorbidity burden, defined by a CHA2DS2-VASc score ≥4, challenging the current practice to mainly offer rhythm-control therapy to healthier patients with fewer comorbidities.11 The aim of this study was to evaluate the safety and efficacy of ERC in patients with a high comorbidity burden treated in routine care by interrogating a large US health records data set and the UK biobank.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to OptumLabs.
Data Sources
In this study, we analyzed two different patient cohorts: a large US de-identified administrative claims database (OptumLabs Data WarehouseⓇ) and the population-based UK Biobank:
OptumLabs, de-identified administrative claims data containing medical and pharmacy claims and enrollment records for more than 130 million private insurance and Medicare Advantage enrollees of all ages and races throughout the United States.8,12,13
The UK Biobank, a prospective, population-based cohort of more than 500,000 randomly selected participants aged 40 to 69 enrolled between 2006 and 2010 in the United Kingdom containing comprehensive medical information with additional data on incidence of disease and mortality obtained from UK death registers and inpatient records.10,14
This study used preexisting, deidentified data. No informed consent was required. For both data sources, at least one author had full access to all the data in this study and takes responsibility for its integrity and the data analysis.
Study Population
The study population in the OptumLabs cohort included adult patients (aged ≥18 years) who had newly diagnosed AF between July 28, 2011, and December 30, 2016, the enrollment period of EAST-AFNET 4.6,8 The UK Biobank cohort included all adult patients (aged ≥18 years) with incident AF during the entire study and follow up period up to March 2021.10 Patients without relevant comorbidity burden (CHA2DS2-VASc score 0–1) were excluded from the analysis, reflecting recent subgroup analysis of the EAST-AFNET 4 trial.11 Patients were devided into two treatment groups based on treatment as either patients who received ERC, i.e., AF ablation or antiarrhythmic drug (AAD) therapy, within the first year after AF diagnosis, or patients not receiving ERC within the first year after AF diagnosis.8,10 AF ablation and cardiovascular diseases were identified using ICD-10 codes.8,10,15,16 Furthermore, patients were classified based on their comorbidities and age as 1) ERC & high comorbidity burden (CHA2DS2-VASc score ≥4); 2) ERC & lower comorbidity burden (CHA2DS2-VASc score 2–3); 3) no ERC & high comorbidity burden; and 4) no ERC & lower comorbidity burden.11
Outcomes
The primary composite outcome was a composite of all-cause mortality, stroke, or hospitalization with the diagnoses heart failure or acute coronary syndrome. This replicated the primary outcome of the EAST-AFNET 4 trial with one difference due to the available mortality information, i.e., all-cause mortality instead of cardiovascular mortality.6,8,10 The primary safety outcome was a composite of death, stroke, and serious adverse events related to ERC such as non-fatal cardiac arrest, drug-induced bradycardia, atrioventricular block, torsade de pointes tachycardia, pericardial tamponade, and periprocedural bleeding, or blood pressure events.6,10,11 Patients in the OptumLabs cohort were followed until death, disenrollment or study end date (December 31st, 2019), patients in the UK Biobank cohort until March 2021.8,10
Sensitivity Analysis
In a sensitivity analysis replicating the EAST-AFNET 4 subgroup analysis, the inclusion and exclusion criteria of EAST-AFNET 4 were applied and only patients who were eligible for EAST-AFNET 4 trial inclusion were included in the analysis, i.e., patients who were either aged >75 years or had a previous transient ischemic attack or stroke, or met 2 of the following criteria: age >65 years, female sex, heart failure, hypertension, diabetes, severe coronary artery disease, chronic kidney disease (stage III or IV), and left ventricular hypertrophy.6 Patients were excluded if one of the following criteria was present: female sex and age <45 years, drug abuse, previous AF ablation, severe mitral stenosis, prosthetic mitral valve, hepatic dysfunction, thyroid dysfunction without treatment, and severe renal dysfunction (stage V or dialysis).6
Statistical Analysis
The propensity score was estimated using logistic regression based on patient characteristics listed in Supplemental Table I. Propensity score overlap weighting method was used to balance differences in baseline characteristics between patients who received ERC and patients who did not receive ERC.8 Standardized mean difference was used to assess the balance of covariates after weighting and a difference less than 0.1 was considered acceptable.8,17 The overlap weight was calculated as 1 minus the propensity score for patients in the ERC group and propensity score for patients not treated with ERC.18 Cox proportional hazards regression was used to compare outcomes of patients who received ERC and patients who did not receive ERC in the propensity score weighted cohort, with a robust sandwich estimator for variance estimation.8 Interaction was tested using a Cox model. A 2-sided P-value less than 0.05 was considered statistically significant for all tests. To exclude residual confounding, we tested falsification outcomes that are unlikely to be a result of undergoing ERC therapy and that might be related to unmeasured confounders.
All analyses except those related to the primary outcome were considered to be exploratory and conducted using SAS Enterprise Guide 7.1 (SAS Institute Inc.) and Stata 16.0 (Stata Corp) for the OptumLabs cohort and using R 4.0.3 for the UK Biobank cohort, respectively.8,10
Results
Patient Characteristics
In the OptumLabs cohort, 720,516 patients with AF were identified during the study period of EAST-AFNET 4. Of these, 109,739 patients had newly diagnosed AF and were eligible for analysis, 7,897 patients (7.2%) with a CHA2DS2-VASc score 0–1 were excluded. Most patients did not receive ERC within the first year after AF diagnosis (ERC: N=24,382 [23.9%]; no ERC: N=77,460 [76.1%]; Figure 1). In the UK Biobank cohort, patients without primary care data were excluded. 16,461 patients were identified with incident AF during the entire observation period, 4,034 patients (24.5%) with a CHA2DS2-VASc score 0–1 were excluded, and 11,625 patients were included in the analysis. The remaining patients were excluded due to missing or incomplete follow up data. Patients in the UK Biobank had a lower comorbidity burden than in OptumLabs (OptumLabs: CHA2DS2-VASc score ≥4: N=76,921 [75.5%], CHA2DS2-VASc score 2–3: N=24,921 [24.5%]; UK Biobank: CHA2DS2-VASc score ≥4: N=3,811 [32.8%], CHA2DS2-VASc score 2–3: N=7,814 [67.2%]).
Figure 1.
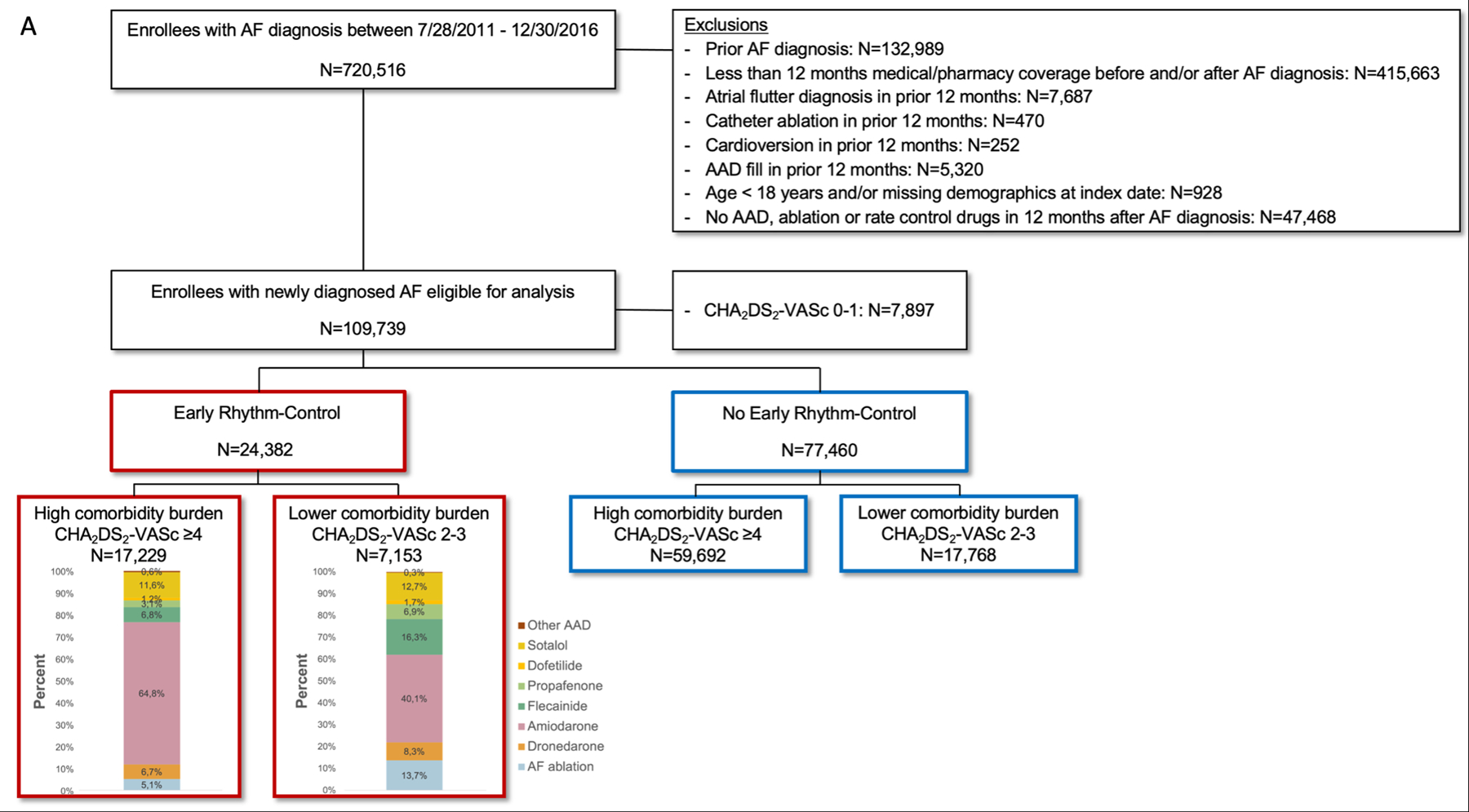
Patient Selection Flow Chart. Patient selection in the OptumLabs cohort (A) and in the UK Biobank cohort (B).
Patients who received ERC were on average three years younger than patients who did not receive ERC (OptumLabs: 70.7 ± 10.2 years vs. 73.0 ± 10.4 years; UK Biobank: 64.6 ± 6.0 years vs. 67.4 ± 7.7 years) and were less often female (OptumLabs: 43.9% vs. 52.4%; UK Biobank: 43.8% vs. 44.8%). Although patients in the UK Biobank were younger and healthier than in OptumLabs, the rates of oral anticoagulation, especially in the ERC group, were higher (OptumLabs: ERC: 45.2% vs. no ERC: 30.4%; UK Biobank: ERC: 69.3% vs. 33.1%). After propensity score weighting no significant baseline characteristics were present. For more details, see Table 1, Supplemental Table I, and Supplemental Table II.
Table 1.
Patient Characteristics Before Propensity Score Weighting
| CHA2DS2-VASc score 2–3 |
CHA2DS2-VASc score ≥4 |
|||||
|---|---|---|---|---|---|---|
| |
No Early Rhythm Control |
Early Rhythm Control |
Total |
No Early Rhythm Control |
Early Rhythm Control |
Total |
| OptumLabs | N=17,768 | N=7,153 | N=24,921 | N=59,692 | N=17,229 | N=76,921 |
| Age, y | 63.4 (10.5) | 62.2 (9.2) | 63.1 (10.2) | 75.9 (8.4) | 74.2 (8.3) | 75.5 (8.4) |
| Female | 6034 (34.0%) | 1887 (26.4%) | 7921 (31.8%) | 34521 (57.8%) | 8816 (51.2%) | 43337 (56.3%) |
| Comorbidities | ||||||
| Systolic heart failure | 1023 (5.8%) | 787 (11.0%) | 1810 (7.3%) | 12924 (21.7%) | 5280 (30.6%) | 18204 (23.7%) |
| Hypertension | 16053 (90.3%) | 6369 (89.0%) | 22422 (90.0%) | 59044 (98.9%) | 16984 (98.6%) | 76028 (98.8%) |
| Diabetes mellitus | 3575 (20.1%) | 1338 (18.7%) | 4913 (19.7%) | 31676 (53.1%) | 8598 (49.9%) | 40274 (52.4%) |
| Stroke | 246 (1.4%) | 110 (1.5%) | 356 (1.4%) | 17103 (28.7%) | 4123 (23.9%) | 21226 (27.6%) |
| Coronary artery disease | 6880 (38.7%) | 3615 (50.5%) | 10495 (42.1%) | 44159 (74.0%) | 13958 (81.0%) | 58117 (75.6%) |
| Myocardial infarction | 2365 (13.3%) | 1116 (15.6%) | 3481 (14.0%) | 18018 (30.2%) | 5933 (34.4%) | 23951 (31.1%) |
| Hypertrophic cardiomyopathy | 182 (1.0%) | 101 (1.4%) | 283 (1.1%) | 826 (1.4%) | 323 (1.9%) | 1149 (1.5%) |
| Dilated cardiomyopathy | 1715 (9.7%) | 1081 (15.1%) | 2796 (11.2%) | 9264 (15.5%) | 3425 (19.9%) | 12689 (16.5%) |
| Peripheral vascular disease | 866 (4.9%) | 357 (5.0%) | 1223 (4.9%) | 15795 (26.5%) | 3716 (21.6%) | 19511 (25.4%) |
| Dyslipidemia | 14510 (81.7%) | 5871 (82.1%) | 20381 (81.8%) | 55235 (92.5%) | 16145 (93.7%) | 71380 (92.8%) |
| Chronic kidney disease | 1217 (6.8%) | 431 (6.0%) | 1648 (6.6%) | 15204 (25.5%) | 4220 (24.5%) | 19424 (25.3%) |
| Liver disease | 2978 (16.8%) | 1159 (16.2%) | 4137 (16.6%) | 11071 (18.5%) | 3242 (18.8%) | 14313 (18.6%) |
| Alcoholism | 1590 (8.9%) | 583 (8.2%) | 2173 (8.7%) | 3524 (5.9%) | 979 (5.7%) | 4503 (5.9%) |
| COPD | 2519 (14.2%) | 943 (13.2%) | 3462 (13.9%) | 17489 (29.3%) | 5126 (29.8%) | 22615 (29.4%) |
| Obstructive sleep apnea | 4483 (25.2%) | 2428 (33.9%) | 6911 (27.7%) | 12190 (20.4%) | 4438 (25.8%) | 16628 (21.6%) |
| Oral anticoagulation | 4633 (26.1%) | 3083 (43.1%) | 7716 (31.0%) | 18878 (31.6%) | 7940 (46.1%) | 26818 (34.9%) |
| CHA 2 DS 2 -VASc score | ||||||
| Mean (SD) | 2.6 (0.5) | 2.6 (0.5) | 2.6 (0.5) | 5.7 (1.4) | 5.5 (1.3) | 5.6 (1.4) |
| Median (IQR) | 3 (2–3) | 3 (2–3) | 5 (4–6) | 5 (5–7) | 5 (4–6) | 5 (3–6) |
| UK Biobank | N=6,900 | N=914 | N=7,814 | N=3,498 | N=313 | N=3,811 |
| Age, y | 65.3 (7.7) | 63.2 (6.9) | 65.0 (7.7) | 71.7 (5.4) | 68.8 (5.0) | 71.4 (5.4) |
| Female | 2664 (38.6%) | 344 (37.5%) | 3008 (38.5%) | 1991 (56%) | 193 (61.7%) | 2184 (57.3%) |
| Comorbidities | ||||||
| Systolic heart failure | 2436 (35.3%) | 444 (48.6%) | 2880 (36.9%) | 2778 (79.4%) | 280 (89.5%) | 3058 (80.2%) |
| Hypertension | 6225 (90.2%) | 838 (91.7%) | 7063 (90.4%) | 3400 (97.2%) | 307 (98.1%) | 3707 (97.3%) |
| Diabetes mellitus | 728 (10.6%) | 80 (8.8%) | 808 (10.3%) | 1232 (35.2%) | 102 (32.6%) | 1334 (35.0%) |
| Stroke | 92 (1.3%) | 11 (1.2%) | 103 (1.3%) | 796 (22.8%) | 62 (19.8%) | 858 (22.5%) |
| Coronary artery disease | 1436 (20.8%) | 234 (25.6%) | 1670 (21.4%) | 1221 (34.9%) | 108 (34.5%) | 1329 (34.9%) |
| Myocardial infarction | 657 (9.5%) | 95 (10.4%) | 752 (9.6%) | 609 (17.4%) | 48 (15.3%) | 657 (17.2%) |
| Hypertrophic cardiomyopathy | 25 (0.4%) | 3 (0.3%) | 28 (0.4%) | 12 (0.3%) | 3 (1.0%) | 15 (0.4%) |
| Dilated cardiomyopathy | 42 (0.6%) | 11 (1.2%) | 53 (0.7%) | 39 (1.1%) | 9 (2.9%) | 48 (1.3%) |
| Peripheral vascular disease | 164 (2.4%) | 27 (3.0%) | 191 (2.4%) | 196 (5.6%) | 14 (4.5%) | 210 (5.5%) |
| Chronic kidney disease | 487 (7.1%) | 63 (6.9%) | 550 (7.0%) | 530 (15.2%) | 38 (12.1%) | 568 (14.9%) |
| Liver disease | 74 (1.1%) | 2 (0.2%) | 76 (1.0%) | 61 (1.7%) | 2 (0.6%) | 63 (1.7%) |
| Alcoholism | 205 (3.0%) | 13 (1.4%) | 218 (2.8%) | 133 (3.8%) | 8 (2.6%) | 141 (3.7%) |
| COPD | 448 (6.5%) | 35 (3.8%) | 483 (6.2%) | 419 (12.0%) | 17 (5.4%) | 436 (11.4%) |
| Obstructive sleep apnea | 197 (2.9%) | 17 (1.9%) | 214 (2.7%) | 158 (4.5%) | 6 (1.9%) | 164 (4.3%) |
| Oral anticoagulation | 2543 (36.9%) | 635 (69.5%) | 3178 (40.7%) | 900 (25.7%) | 215 (68.7%) | 1115 (29.3%) |
| CHA 2 DS 2 -VASc score | ||||||
| Mean (SD) | 2.5 (0.5) | 2.4 (0.5) | 2.5 (0.5) | 4.5 (0.8) | 4.4 (0.7) | 4.5 (0.8) |
| Median (IQR) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 4 (4–5) | 4 (4–5) | 4 (4–5) |
Values are presented as mean (SD) or as absolute numbers (%). COPD indicates chronic obstructive pulmonary disease. For extended baseline characteristics and for baseline characteristics of the overall cohort, see Supplemental Table I and Supplemental Table II.
Early Rhythm-Control Therapy
In the OptumLabs cohort, patients who received ERC mainly had a high comorbidity burden (CHA2DS2-VASc score ≥4: N=17,229 vs. CHA2DS2-VASc score 2–3: N=7,153; Figure 1). AF ablation, with or without AAD treatment, was more frequent in patients with a lower comorbidity burden (CHA2DS2-VASc score ≥4: N=880 [5.1%] vs. CHA2DS2-VASc score 2–3: N=979 [13.7%]). Independent of the comorbidity burden, amiodarone was the most frequently used AAD (CHA2DS2-VASc score ≥4: N=11,171 [64.8%] vs. CHA2DS2-VASc score 2–3: N=2,867 [40.1%]).
Patients who received ERC in the UK Biobank cohort had a lower comorbidity burden (CHA2DS2-VASc score ≥4: N= 313 [25.5%] vs. CHA2DS2-VASc score 2–3: N= 914 [74.5%]). The type of ERC was comparable to OptumLabs. AF ablation rates were similar (CHA2DS2-VASc score ≥4: N=40 [12.8%] vs. CHA2DS2-VASc score 2–3: N=104 [11.4%]) and amiodarone was the most common AAD (CHA2DS2-VASc score ≥4: N=146 [46.6%] vs. CHA2DS2-VASc score 2–3: N=377 [41.2%]) followed by sotalol (CHA2DS2-VASc score ≥4: N=91 [29.1%] vs. CHA2DS2-VASc score 2–3: N=268 [29.3%]).
Primary Composite Outcome
As expected, event rates per 100 person-years were higher in patients with a higher comorbidity burden (OptumLabs: no ERC 15.37% vs. ERC 12.69%; UK Biobank: no ERC 10.81% vs. ERC 8.40%; Table 2) than in patients with a lower comorbidity burden (OptumLabs: no ERC 2.39% vs. 2.18%; UK Biobank: no ERC 5.05% vs. ERC 4.66%; Table 2). In both cohorts, ERC was associated with a reduced risk for the primary composite outcome of all-cause mortality, stroke, or hospitalization with the diagnoses heart failure or acute coronary syndrome in patients with a high comorbidity burden (OptumLabs: hazard ratio [HR] 0.83, 95% confidence interval [CI] 0.72–0.95, p=0.006; UK Biobank: HR 0.77, CI 0.63–0.94, p=0.009; Table 2, Figure 2, and Supplemental Figure S1). In patients with a lower comorbidity burden, the difference in outcomes was not significant (OptumLabs: HR 0.92, CI 0.54–1.57, p=0.767; UK Biobank: HR 0.94, CI 0.83–1.06, p=0.310). The comorbidity burden interacted with ERC in the UK Biobank (interaction-p=0.027) but not in OptumLabs (interaction-p=0.720). Crude event numbers and event rates are listed in Supplemental Table III. The risk reduction for the components of the primary composite outcome differed slightly between the cohorts (Table 2 and Figure 2).
Table 2.
Primary Composite Outcome
| |
No Early Rhythm Control |
Early Rhythm Control |
Absolute Rate Difference (95% CI) |
Hazard Ratio (95% CI) |
P-value |
P-value for interaction |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events |
Person Years |
Event Rate per 100 person-years |
No. of Events |
Person Years |
Event Rate per 100 person-years |
|||||
| OptumLabs | N=77,460 | N=24,382 | ||||||||
| Composite | 0.720 | |||||||||
| 2–3 | 11 | 454 | 2.39 | 10 | 439 | 2.18 | −0.20 (−1.44, 1.03) | 0.92 (0.54– 1.57) | 0.767 | |
| ≥4 | 218 | 1,419 | 15.37 | 183 | 1,441 | 12.69 | −2.69 (−4.70, −0.67) | 0.83 (0.72, 0.95) | 0.006 | |
| Stroke | 0.497 | |||||||||
| 2–3 | 2 | 460 | 0.36 | 2 | 447 | 0.37 | 0.00 (−0.48, 0.50) | 1.02 (0.27, 3.85) | 0.979 | |
| ≥4 | 34 | 1,554 | 2.21 | 22 | 1,558 | 1.40 | −0.81 (−1.52, −0.10) | 0.65 (0.45, 0.93) | 0.019 | |
| Heart failure | 0.969 | |||||||||
| 2–3 | 3 | 460 | 0.59 | 2 | 445 | 0.54 | −0.05 (−0.63, 0.53) | 0.91 (0.34, 2.47) | 0.859 | |
| ≥4 | 80 | 1,493 | 5.36 | 75 | 1,493 | 5.02 | −0.34 (−1.54, 0.86) | 0.95 (0.76, 1.19) | 0.646 | |
| ACS | 0.029 | |||||||||
| 2–3 | 1 | 461 | 0.32 | 3 | 444 | 0.68 | 0.36 (−0.14, 0.88) | 2.14 (0.77, 5.97) | 0.144 | |
| ≥4 | 35 | 1,562 | 2.25 | 23 | 1,554 | 1.45 | −0.80 (−1.51, 0.09) | 0.65 (0.46, 0.92) | 0.015 | |
| All-cause mortality | 0.454 | |||||||||
| 2–3 | 6 | 463 | 1.35 | 4 | 449 | 0.90 | −0.45 (−1.38, 0.47) | 0.68 (0.32, 1.44) | 0.312 | |
| ≥4 | 132 | 1,606 | 8.22 | 116 | 1,587 | 7.30 | −0.91 (−2.28, 0.45) | 0.89 (0.75, 1.06) | 0.187 | |
|
| ||||||||||
| UK Biobank | N=10,398 | N=1,227 | ||||||||
| Composite | 0.027 | |||||||||
| 2–3 | 262 | 5,179 | 5.05 | 238 | 5,101 | 4.66 | −0.39 (−0.41, −0.37) | 0.94 (0.83, 1.06) | 0.310 | |
| ≥4 | 97 | 893 | 10.81 | 98 | 1,170 | 8.40 | −2.4 (−2.46, −2.36) | 0.77 (0.63, 0.94) | 0.009 | |
| Stroke | 0.231 | |||||||||
| 2–3 | 58 | 5,774 | 1.00 | 45 | 5,675 | 0.79 | −0.21 (−0.22, −0.2) | 0.82 (0.61, 1.10) | 0.180 | |
| ≥4 | 17 | 1,035 | 1.66 | 21 | 1,270 | 1.65 | −0.01 (−0.03, 0.02) | 0.99 (0.63, 1.53) | 0.947 | |
| Heart failure | 0.088 | |||||||||
| 2–3 | 154 | 5,446 | 2.82 | 149 | 5,274 | 2.83 | 0.01 (−0.01, 0.02) | 1.02 (0.87, 1.20) | 0.812 | |
| ≥4 | 63 | 940 | 6.65 | 50 | 1,230 | 4.08 | −2.58 (−2.62, −2.54) | 0.60 (0.46, 0.79) | <0.001 | |
| ACS | 0.077 | |||||||||
| 2–3 | 35 | 5,862 | 0.59 | 31 | 5,695 | 0.54 | −0.05 (−0.06, −0.05) | 0.91 (0.64, 1.29) | 0.603 | |
| ≥4 | 15 | 1,048 | 1.46 | 8 | 1,302 | 0.65 | −0.8 (−0.82, −0.79) | 0.43 (0.23, 0.83) | 0.012 | |
| All-cause mortality | 0.580 | |||||||||
| 2–3 | 119 | 5,997 | 1.98 | 117 | 5,809 | 2.01 | 0.04 (0.03, 0.05) | 1.07 (0.90, 1.29) | 0.444 | |
| ≥4 | 45 | 1,083 | 4.20 | 55 | 1,327 | 4.15 | −0.05 (−0.09, −0.03) | 0.97 (0.74, 1.27) | 0.810 | |
Primary composite outcome in the weighted OptumLabs (upper lines) and UK biobank (lower lines) data sets. Event rate was calculated as the number of events per 100 person-years. Propensity score weight was applied when calculating number of events, person-years, event rates, absolute reduction, and hazard ratios. ACS indicates acute coronary syndrome; CI, confidence interval. For crude numbers and event rates, see Supplemental Table III.
Figure 2.
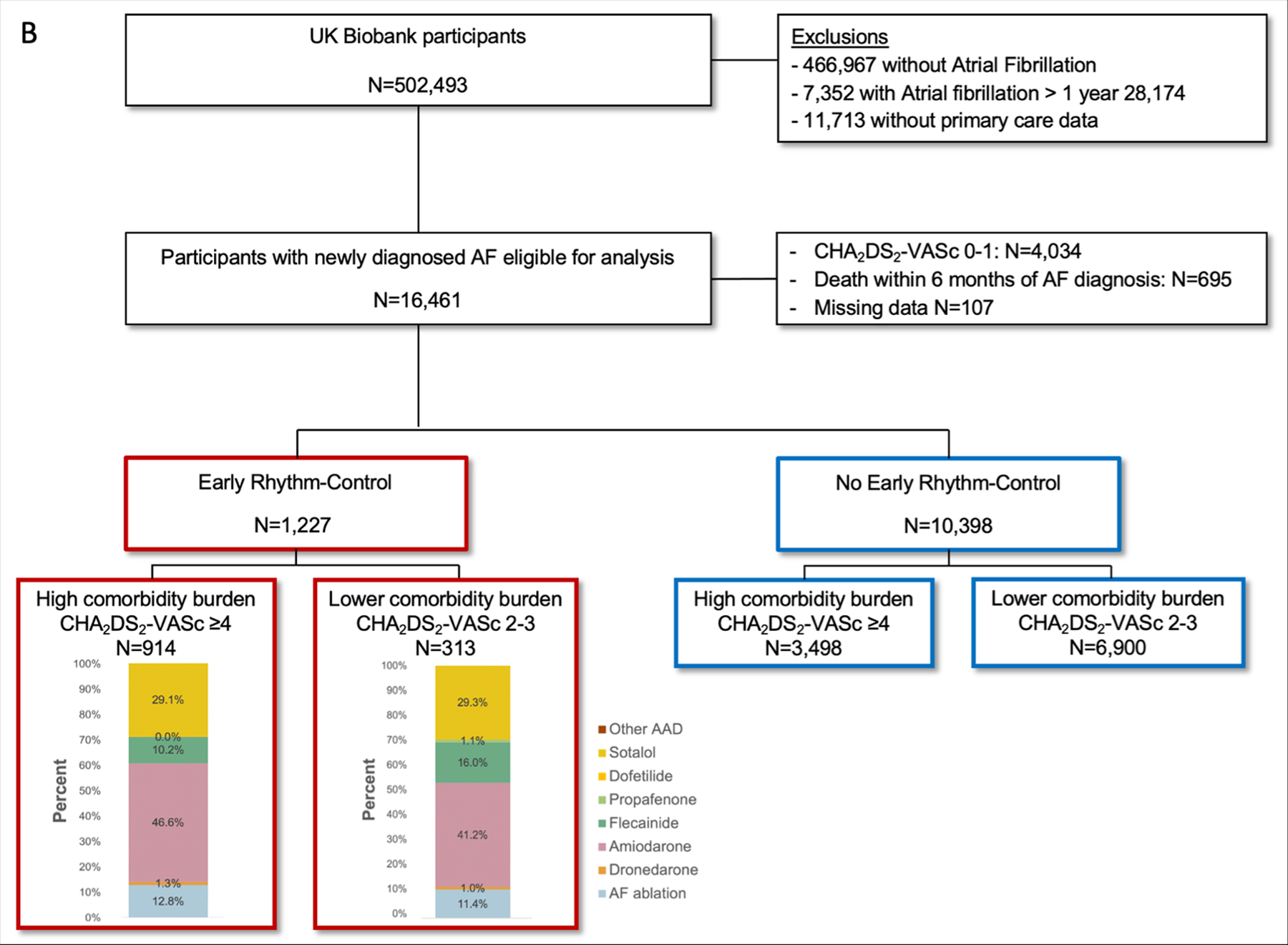
Outcomes. Forest plot of the primary composite outcome and the safety outcome in the OptumLabs cohort (A) and in the UK Biobank cohort (D) and cumulative incidence curves of the primary composite outcome for patients with a high comorbidity burden (C: OptumLabs, F: UK Biobank) and for patients with a lower comorbidity burden (B: OptumLabs, E: UK Biobank).
Safety Outcome
The primary safety outcome did not occur more often in patients treated with ERC in comparison to patients not treated with ERC, independent of their comorbidity burden. In the OptumLabs cohort, in patients with a high comorbidity burden, ERC was associated with a reduced risk for the primary safety outcome (OptumLabs: CHA2DS2-VASc score ≥4: HR 0.87, CI 0.77–1.00, p=0.045; CHA2DS2-VASc score 2–3: HR 0.78, CI 0.49–1.22, p=0.277; interaction-p=0.612; UK Biobank: CHA2DS2-VASc score ≥4: HR 0.99, CI 0.79–1.24, p=0.947; CHA2DS2-VASc score 2–3: HR 1.05, CI 0.92–1.21, p=0.464; interaction-p=0.552; Table 3 and Figure 2) and a reduced risk for drug-induced bradycardia (CHA2DS2-VASc score ≥4: HR 0.61, CI 0.32–1.20, p=0.152; CHA2DS2-VASc score 2–3: HR 9.91, CI 1.53–64.39, p=0.016; interaction-p=0.006; Table 3). For crude event numbers and event rates, see Supplemental Table IV.
Table 3.
Safety Outcomes
| |
No Early Rhythm Control |
Early Rhythm Control |
Absolute Rate Difference (95% CI) |
Hazard Ratio (95% CI) |
P-value |
P-value for interaction |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events |
Person Years |
Event Rate per 100 person-years |
No. of Events |
Person Years |
Event Rate per 100 person-years |
|||||
| OptumLabs | N=77,460 | N=24,382 | ||||||||
| Composite | 0.612 | |||||||||
| 2–3 | 16 | 444 | 3.59 | 12 | 435 | 2.78 | −0.81 (−2.29, 0.68) | 0.78 (0.49, 1.22) | 0.277 | |
| ≥4 | 222 | 1,398 | 15.90 | 197 | 1,416 | 13.88 | −2.02 (−4.08, 0.04) | 0.87 (0.77, 1.00) | 0.045 | |
| Onset stroke | 0.191 | |||||||||
| 2–3 | 1 | 462 | 0.27 | 2 | 447 | 0.39 | 0.12 (−0.20, 0.44) | 1.44 (0.54, 3.82) | 0.467 | |
| ≥4 | 37 | 1,549 | 2.41 | 27 | 1,552 | 1.75 | 0.66 (−1.42, 0.10) | 0.74 (0.53, 1.05) | 0.092 | |
| Mortality | 0.454 | |||||||||
| 2–3 | 6 | 463 | 1.35 | 4 | 449 | 0.90 | −0.45 (−1.38, 0.47) | 0.68 (0.32, 1.44) | 0.312 | |
| ≥4 | 132 | 1,606 | 8.22 | 116 | 1,587 | 7.30 | −0.91 (−2.28, 0.45) | 0.89 (0.75, 1.06) | 0.187 | |
| Non-fatal cardiac arrest | 0.373 | |||||||||
| 2–3 | 1 | 463 | 0.20 | 1 | 449 | 0.12 | −0.09 (−0.44, 0.27) | 0.58 (0.09, 3.73) | 0.562 | |
| ≥4 | 6 | 1,604 | 0.37 | 9 | 1,586 | 0.54 | 0.17 (−0.14, 0.48) | 1.48 (0.68, 3.22) | 0.322 | |
| Bradycardia | 0.006 | |||||||||
| 2–3 | 0 | 463 | 0.03 | 1 | 449 | 0.26 | 0.23 (−0.19, 0.65) | 9.91 (1.53, 64.39) | 0.016 | |
| ≥4 | 8 | 1,594 | 0.50 | 5 | 1,580 | 0.30 | −0.20 (−0.50, 0.11) | 0.61 (0.32, 1.20) | 0.152 | |
| Atrioventricular block | - | |||||||||
| 2–3 | 0 | 463 | 0.00 | 0 | 449 | 0.04 | - | - | - | |
| ≥4 | 4 | 1,601 | 0.24 | 4 | 1,583 | 0.23 | - | - | - | |
| Torsades de pointes tachycardia | - | |||||||||
| 2–3 | 0 | 463 | 0.00 | 0 | 449 | 0.00 | - | - | - | |
| ≥4 | 0 | 1,606 | 0.00 | 0 | 1,587 | 0.00 | - | - | - | |
| Pericardial tamponade | - | |||||||||
| 2–3 | 0 | 463 | 0.00 | 0 | 449 | 0.00 | - | - | - | |
| ≥4 | 0 | 1,606 | 0.00 | 0 | 1,587 | 0.00 | - | - | - | |
| Major bleed | 0.397 | |||||||||
| 2–3 | 3 | 458 | 0.64 | 3 | 443 | 0.79 | 0.14 (−0.36, 0.65) | 1.23 (0.58, 2.62) | 0.587 | |
| ≥4 | 57 | 1,510 | 3.75 | 50 | 1,518 | 3.27 | −0.49 (−1.46, 0.49) | 0.89 (0.68, 1.16) | 0.371 | |
| Blood pressure-related | - | |||||||||
| 2–3 | 0 | 463 | 0.00 | 0 | 449 | 0.00 | - | - | - | |
| ≥4 | 2 | 1,605 | 0.11 | 1 | 1,586 | 0.04 | - | - | - | |
| Syncope | 0.216 | |||||||||
| 2–3 | 6 | 452 | 1.35 | 3 | 444 | 0.76 | −0.60 (−1.47, 0.28) | 0.56 (0.25, 1.25) | 0.159 | |
| ≥4 | 38 | 1,547 | 2.48 | 36 | 1,527 | 2.37 | −0.10 (−0.88, 0.67) | 0.97 (0.71, 1.32) | 0.834 | |
| Cardiac device implantation | 0.578 | |||||||||
| 2–3 | 2 | 460 | 0.48 | 2 | 446 | 0.34 | −0.14 (−0.69, −0.40) | 0.70 (0.21, 2.32) | 0.560 | |
| ≥4 | 19 | 1,576 | 1.21 | 19 | 1,559 | 1.23 | 0.01 (−0.55, 0.59) | 1.03 (0.64, 1.64) | 0.912 | |
| UK Biobank | N=10,495 | N=1,237 | ||||||||
| Composite | 0.552 | |||||||||
| 2–3 | 190 | 5,597 | 3.40 | 191 | 5,456 | 3.49 | 0.10 (0.08, 0.11) | 1.05 (0.92, 1.21) | 0.464 | |
| ≥4 | 65 | 1,008 | 6.48 | 79 | 1,223 | 6.47 | −0.02 (−0.06, 0.03) | 0.99 (0.79, 1.24) | 0.947 | |
| Onset stroke | 0.296 | |||||||||
| 2–3 | 48 | 5,834 | 0.82 | 38 | 5,696 | 0.66 | −0.17 (−0.17, −0.16) | 0.84 (0.61, 1.15) | 0.267 | |
| ≥4 | 15 | 1,045 | 1.42 | 17 | 1,275 | 1.37 | −0.05 (−0.08, −0.04) | 0.96 (0.59, 1.54) | 0.855 | |
| Mortality | 0.580 | |||||||||
| 2–3 | 119 | 5,997 | 1.98 | 117 | 5,809 | 2.01 | 0.04 (0.03, 0.05) | 1.07 (0.9, 1.29) | 0.444 | |
| ≥4 | 45 | 1,083 | 4.20 | 55 | 1,327 | 4.15 | −0.05 (−0.09, −0.03) | 0.97 (0.74, 1.27) | 0.810 | |
| Non-fatal cardiac arrest | 0.022 | |||||||||
| 2–3 | 3 | 5,994 | 0.04 | 6 | 5,797 | 0.10 | 0.06 (0.05, 0.06) | 2.41 (0.94, 6.22) | 0.068 | |
| ≥4 | 1 | 1,081 | 0.11 | 4 | 1,324 | 0.26 | 0.16 (0.14, 0.16) | 2.47 (0.73, 8.37) | 0.145 | |
| Bradycardia | 0.825 | |||||||||
| 2–3 | 8 | 5,956 | 0.13 | 7 | 5,787 | 0.12 | −0.01 (−0.01, −0.01) | 0.88 (0.42, 1.82) | 0.728 | |
| ≥4 | 2 | 1,078 | 0.18 | 4 | 1,314 | 0.32 | 0.14 (0.14, 0.16) | 1.88 (0.65, 5.47) | 0.244 | |
| Atrioventricular block | 0.640 | |||||||||
| 2–3 | 9 | 5,962 | 0.16 | 9 | 5,773 | 0.16 | 0.01 (0, 0.01) | 1.11 (0.58, 2.12) | 0.753 | |
| ≥4 | 3 | 1,078 | 0.25 | 4 | 1,316 | 0.31 | 0.06 (0.04, 0.06) | 1.24 (0.43, 3.53) | 0.690 | |
| Torsades de pointes tachycardia | - | |||||||||
| 2–3 | 0 | 5,997 | 0.00 | 0 | 5,809 | 0.00 | - | - | - | |
| ≥4 | 0 | 1,083 | 0.00 | 0 | 1,327 | 0.00 | - | - | - | |
| Pericardial tamponade | - | |||||||||
| 2–3 | 0 | 5,997 | 0.00 | 0 | 5,809 | 0.00 | - | - | - | |
| ≥4 | 0 | 1,083 | 0.00 | 0 | 1,327 | 0.00 | - | - | - | |
| Major bleed | 0.800 | |||||||||
| 2–3 | 12 | 5,949 | 0.19 | 11 | 5,748 | 0.20 | 0 (0, 0) | 1 (0.56, 1.79) | 0.993 | |
| ≥4 | 2 | 1,077 | 0.19 | 2 | 1,321 | 0.12 | −0.07 (−0.08, −0.06) | 0.63 (0.14, 2.80) | 0.541 | |
| Blood pressure-related | 0.411 | |||||||||
| 2–3 | 1 | 5,993 | 0.01 | 1 | 5,803 | 0.02 | 0 (0, 0) | 1.22 (0.13, 11.24) | 0.864 | |
| ≥4 | 0 | 1,083 | 0.01 | 1 | 1,323 | 0.06 | 0.06 (0.06, 0.07) | 12.07 (1.03, 141.78) | 0.048 | |
| Syncope | 0.840 | |||||||||
| 2–3 | 33 | 5,853 | 0.57 | 37 | 5,687 | 0.65 | 0.08 (0.08, 0.09) | 1.15 (0.83, 1.59) | 0.409 | |
| ≥4 | 11 | 1,059 | 1.04 | 10 | 1,303 | 0.76 | −0.29 (−0.29, −0.26) | 0.72 (0.39, 1.35) | 0.304 | |
| Cardiac device implantation | 0.591 | |||||||||
| 2–3 | 69 | 5,667 | 1.22 | 63 | 5,496 | 1.15 | −0.07 (−0.08, −0.06) | 0.95 (0.74, 1.21) | 0.664 | |
| ≥4 | 18 | 1,039 | 1.72 | 21 | 1,276 | 1.61 | −0.11 (−0.14, −0.10) | 0.95 (0.61, 1.47) | 0.813 | |
Primary safety outcome and adverse events of special interest in the weighted OptumLabs (upper lines) and UK biobank (lower lines) data sets. Event rate was calculated as the number of events per 100 person-years. Propensity score weight was applied when calculating number of events, person-years, event rates, absolute reduction, and hazard ratios. CI indicates confidence interval. If event numbers were 0 no comparison was conducted. For crude numbers and even rates, see Supplemental Table IV.
Sensitivity Analysis
In the analysis replicating the EAST-AFNET 4 trial inclusion criteria, no risk reduction in the primary composite outcome was observed in both cohorts (Supplemental Table V). However, a significant interaction was observed for hospitalization with acute coronary syndrome in the OptumLabs cohort (CHA2DS2-VASc score ≥4: HR 0.67, CI 0.44–1.01, p=0.058; CHA2DS2-VASc score 2–3: HR 3.20, CI 1.15–8.93), p=0.027; interaction-p=0.005), and for hospitalization with heart failure in the UK Biobank (CHA2DS2-VASc score ≥4: HR 0.66, CI 0.49–0.89, p=0.006; CHA2DS2-VASc score 2–3: HR 0.95, CI 0.77–1.17, p=0.636; interaction-p=0.047).
The results of the falsification endpoint analysis showed that outcomes not related to AF or rhythm-control, e.g., lung cancer or major fracture, were not different between groups (Supplemental Table VI).
Discussion
Main Findings
Our analyses provide important confirmation that ERC is safe in patients with recently diagnosed AF and a high comorbidity burden, defined by a CHA2DS2-VASc score ≥4, in a large US claims data base and in the population-based UK biobank. Furthermore, ERC was associated with a 17% to 23% risk reduction in a composite outcome consisting of all-cause mortality, stroke, or hospitalization with the diagnoses heart failure or acute coronary syndrome in patients with a high comorbidity burden. Finally, although specific events related to rhythm-control therapy were different between groups, ERC was not associated with more safety events regarding the composite safety outcome. In context of the recent pre-specified subgroup analysis in the EAST-AFNET 4 population11, these results encourage the use of ERC in patients with recently diagnosed AF and a high comorbidity burden.
Outcome Reduction by Early Rhythm Control Therapy
Observational data has long suggested that AF is contributing to adverse cardiovascular outcomes and treatment may alleviate these events. This led to “rate versus rhythm” trials with neutral outcomes, driven by different factors, including discontinuation of anticoagulation and limited success of rhythm-control.5,19,20 The EAST-AFNET 4 trial, consistent with smaller trials comparing AF ablation to medical therapy in patients with heart failure, recently confirmed that ERC reduces cardiovascular outcomes in patients with recently diagnosed AF when added to anticoagulation and therapy of concomitant cardiovascular conditions.11,21,22 A recent observational study showed that patients with a CHA2DS2-VASc score of 3–4 are more susceptible to adverse events during AF than patients with lower score.23 This interplay between comorbidity and impact of AF burden may provide a possible explanation of our findings.
Currently, the CHA2DS2-VASc score is used to assess the need for oral anticoagulation therapy in patients with AF. However, the score stratifies comorbidity burden and has shown to be also prognostic for mortality in heart failure patients who are hospitalized or in need of cardiac resynchronization therapy.24,25 In a sub-study of the ENTRUST-AF PCI, an increased CHA2DS2-VASc score was associated with adverse events such as bleeding and stent thrombosis providing further evidence of a general risk marker in AF.26
Our analysis confirms that the score can be used to identify patients with AF at highest need for risk reduction. The data, collected in two independent data sets, also confirm that ERC is safe and effectively reduces outcomes in patients with AF and a high comorbidity burden. The interaction analysis suggests that comorbidity burden interacts with ERC in the UK Biobank but not in the OptumLabs dataset. Importantly, the falsification endpoints provide a measure of robustness for our findings.
Both, AAD treatment and catheter ablation of AF have improved significantly in the past decades,27–29 first-line therapy of catheter ablation approaches have been investigated,30–32 and new evidence is supporting the reduction of adverse cardiovascular events by implementing ERC in the management of new-onset AF.6,7 Several prespecified EAST-AFNET 4 subgroup analyses demonstrated the effectiveness and safety of ERC in subgroups of interest, including in patients with heart failure, in asymptomatic patients, and in patients with different AF patterns.11,21,22 These findings are in line with other randomized clinical trials and their subgroup analyses.33–35
In routine care, reservations to offer rhythm-control more widely to patients with other comorbidities and elderly patients persist, due to fear of adverse events and futility concerns, resulting in low use of rhythm-control therapy among patients with AF.36,37 A recently published subanalysis of the EAST-AFNET 4 trial challenges this practice by demonstrating that the outcome-reducing effect of ERC is much more pronounced in patients with recently diagnosed AF and a high comorbidity burden.11
Approximately 25% of the patients randomized to ERC underwent AF ablation in the EAST-AFNET 4 trial.6 A similar pattern of ERC was found in both data sets used in this analysis. AF ablation is more effective in maintaining sinus rhythm and in delaying progress of paroxysmal AF to persistent AF than AAD therapy.30–32,38 Hence, it could be speculated that early AF ablation could be a more effective means to deliver ERC than the intervention tested in EAST- AFNET 4 and in this analysis. The overall safety of AF ablation in the CABANA trial is a first signal that such a treatment strategy could be safe.39 Future research is needed to evaluate early AF ablation to deliver ERC.
Safety of Rhythm-Control Therapy in Patients With AF and Multiple Comorbidities
Current guidelines for the management of AF recommend rhythm-control therapy to control AF-related symptoms if rate-control is not sufficient.1 These recommendations are based on increased rates of adverse events associated with rhythm-control observed in earlier trials comparing rhythm-control and rate-control approaches.5 The safety profile of the EAST-AFNET 4 trial and the safety of ERC found in OptumLabs and the UK Biobank show that modern rhythm-control therapy is not associated with excess mortality or severe adverse events6,8,10, although rates of catheter ablation of AF were comparatively low. The neutrality of the primary safety outcome points towards an improved safety profile of the prescribed AADs and should reassure clinicians who aim to implement ERC in their practice. Our analysis and the EAST-AFNET 4 subanalysis looking at comorbidity burden identified areas where rhythm-control can be optimized, e.g. particular reduction of drug toxicity or side effects.11
To assess efficacy and safety of ERC in patients fulfilling EAST-AFNET 4 criteria, we limited a sensitivity analysis to only patients who would be eligible for ERC in the trial. Due to the applied exclusion criteria, less patients were included than in the main analysis (OptumLabs: N=79,143/109,739 patients and UK Biobank: N=9,604/16,461 patients, Supplemental Table V). Furthermore, excluding the cause of death in this analysis could be a potential source of bias. Although interaction effects were observed in components of the primary composite outcome, in both cohorts, we did not find a significantly reduced composite primary outcome in patients with a higher comorbidity burden, and no safety signals were observed.
Strengths and Limitations
Strengths of our analyses are the use of two large, independent datasets in different health care systems.
The observational nature and the lack of randomized treatment assignment is a limitation. Despite careful adjustment, residual confounding cannot be excluded. We provide a sensitivity analysis to assess the effects in a population similar to the EAST-AFNET 4 trial. Further falsification analyses using non-cardiovascular outcomes did not detect a systematic bias in our analysis. Outcome analyses in both cohorts rely on accurate coding of diagnoses and procedures by health care providers. By interrogating multiple data sources, incoherent coding and misclassification could be a potential source of bias. Additional limitations apply to each cohort. OptumLabs represents only insured U.S. patients. The UK Biobank recruits from the general population but has been reported to be healthier and more female than the true general population in the UK.40 Conclusions cannot be made with confidence with respect to the general population.
Conclusion
Early rhythm-control therapy is safe in patients with recently diagnosed AF and a high comorbidity burden. Furthermore, the reduction in cardiovascular outcomes (all-cause mortality, stroke, or hospitalization for heart failure or acute coronary syndrome) is predominantly found in patients with recently diagnosed AF and a high comorbidity burden. These results encourage the preferential use of ERC in patients with multiple cardiovascular comorbidities.
Supplementary Material
What is Known
In the EAST-AFNET 4 trial, early rhythm-control therapy (ERC) reduced the risk for adverse cardiovascular outcomes in patients with atrial fibrillation.
A recent subanalysis of the EAST-AFNET 4 trial suggests a stronger benefit of ERC in patients with a high comorbidity burden when compared to patients with a lower comorbidity burden.
What the Study Adds
Our analyses provide important confirmation that ERC is safe in routine care in patients with recently diagnosed atrial fibrillation and a high comorbidity burden, defined by a CHA2DS2-VASc score ≥4, in a large US claims data base and in the population-based UK biobank.
Furthermore, ERC was associated with a 17% to 23% risk reduction in a composite outcome consisting of all-cause mortality, stroke, or hospitalization with the diagnoses heart failure or acute coronary syndrome in patients with a high comorbidity burden.
Sources of Funding:
G.V.G. is funded by EU Horizon 2020 MAESTRIA (Grant agreement ID 965286). The Institute of Cardiovascular Research, University of Birmingham, has received an Accelerator Award by the British Heart Foundation AA/18/2/34218. P.K. was partially supported by European Union CATCH ME (grant agreement number 633196) BigData@Heart (grant agreement EU IMI 116074), British Heart Foundation (FS/13/43/30324; PG/17/30/32961; and PG/20/22/35093). P.K. is partially supported by German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, via a grant to AFNET), Leducq Foundation, and by EU Horizon 2020 MAESTRIA (Grant agreement ID 965286, to AFNET). P.T.E. is supported by grants from the National Institutes of Health (1RO1HL092577, 1R01HL157635, 1R01HL157635), by a grant from the American Heart Association Strategically Focused Research Networks (18SFRN34110082), and by a grant from the European Union Horizon 2020 MAESTRIA (965286). G.V.G and V.R.C. also acknowledge support from the NIHR Birmingham ECMC, NIHR Birmingham SRMRC, Nanocommons H2020-EU (731032) and the MRC Heath Data Research UK (HDRUK/CFC/01), an initiative funded by UK Research and Innovation, Department of Health and Social Care (England) and the devolved administrations, and leading medical research charities. P.A.N. receives research funding from National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI, R21AG 62580–1, R01HL 131535–4, R01HL 143070–2] the National Institute on Aging [NIA, R01AG 062436–1]), Agency for Healthcare Research and Quality (AHRQ, R01HS 25402–3), Food and Drug Administration (FDA, FD 06292), and the American Heart Association (18SFRN34230146, AHA). Over the past 36 months, X.Y. has received research support through Mayo Clinic from the National Institutes of Health (R21HL140205, R01AG062436), the Agency for Healthcare Research and Quality (R01HS025402), the Food and Drug Administration (U01FD005938), the University of Nebraska Medical Center, and the Medical Devices Innovation Consortium/National Evaluation System for health Technology.
Non-standard Abbreviations and Acronyms
- AF
atrial fibrillation
- AAD
antiarrhythmic drug
- CABANA
Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation
- EAST-AFNET 4
Early Treatment of Atrial Fibrillation for Stroke Prevention Trial
- ERC
early rhythm-control therapy
Footnotes
Presented in part at the European Society of Cardiology Congress 2022 in Barcelona, Spain, 8/26/2022 to 8/29/2022 at the late breaking clinical science session “latest science in atrial fibrillation”.
Disclosures: P.K. is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). P.K. receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past, but not in the last 3 years. A.R. received consultant fees from Medtronic, KODEX-EPD, Biosense Webster and travel grants and lecture fees from Medtronic, Cardiofocus, Biosense Webster, Abbott, Boehringer Ingelheim, Philips KODEX-EPD, Ablamap, Bayer and Novartis. P.A.N. is a study investigator in an ablation trial sponsored by Medtronic. P.A.N. and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. P.A.N. has served on an expert advisory panel for OptumLabs. P.A.N. and Mayo Clinic have filed patents related to the application of AI to the ECG for diagnosis and risk stratification. P.T.E. has received sponsored research support from Bayer AG and IBM Research and he has served on advisory boards or consulted for Bayer AG, MyoKardia, and Novartis. All other authors declared no conflict of interest.
Supplemental Material:
References
- 1.Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GA, Fauchier L, Kalman JM, Lane DA, Lettino M, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal 2021;42(5):373–498. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140(2):e125–e151. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJM, Tijssen JGP, et al. A Comparison of Rate Control and Rhythm Control in Patients with Recurrent Persistent Atrial Fibrillation. New England Journal of Medicine 2002;347(23):1834–1840. [DOI] [PubMed] [Google Scholar]
- 4.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. The Lancet 2000;356(9244):1789–1794. [DOI] [PubMed] [Google Scholar]
- 5.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. New England Journal of Medicine 2002;347(23):1825–1833. [DOI] [PubMed] [Google Scholar]
- 6.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. New England Journal of Medicine 2020;383(14):1305–1316. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Naccarelli GV., Mittal S, Crijns HJGM, Hohnloser SH, Ma CS, Natale A, Turakhia MP, Kirchhof P. The Increasing Role of Rhythm Control in Patients With Atrial Fibrillation: JACC State-of-the-Art Review. Journal of the American College of Cardiology 2022;79(19):1932–1948. [DOI] [PubMed] [Google Scholar]
- 8.Dickow J, Kirchhof P, Van Houten HK, Sangaralingham LR, Dinshaw LHW, Friedman PA, Packer DL, Noseworthy PA, Yao X. Generalizability of the EAST-AFNET 4 Trial: Assessing Outcomes of Early Rhythm-Control Therapy in Patients With Atrial Fibrillation. Journal of the American Heart Association 2022;11(11):e024214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HT, Kim TH, Pak HN, Lee MH, Lip GYH, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: Nationwide cohort study. The BMJ 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kany S, Cardoso VR, Bravo L, Williams JA, Schnabel R, Fabritz L, Gkoutos GV, Kirchhof P. Eligibility for early rhythm control in patients with atrial fibrillation in the UK Biobank. Heart 2022;108(23):1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rillig A, Breithardt G, Camm AJ, Crijns H, Goette A, Kuck KH, Metzner A, Vardas P, Vettorazzi E, Wegscheider K, et al. Early Rhythm Control in Patients With Atrial Fibrillation and High Comorbidity Burden. Circulation 2022;146(11):836–847. [DOI] [PubMed] [Google Scholar]
- 12.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: Building a novel node in the learning health care system. Health Affairs 2014;33(7):1187–1194. [DOI] [PubMed] [Google Scholar]
- 13.Optum Research Data Assets https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed October 17, 2020.
- 14.Littlejohns TJ, Sudlow C, Allen NE, Collins R. UK Biobank: opportunities for cardiovascular research. European Heart Journal 2019;40(14):1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noseworthy PA, Gersh BJ, Kent DM, Piccini JP, Packer DL, Shah ND, Yao X. Atrial fibrillation ablation in practice: Assessing CABANA generalizability. European Heart Journal 2019;40(16):1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah ND, Dunlay SM, Siontis KC, Piccini JP, Yao X. Generalizability of the CASTLE-AF trial: Catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm 2020;17(7):1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Morgan KL, Zaslavsky AM. Balancing Covariates via Propensity Score Weighting. Journal of the American Statistical Association 2018;113(521):390–400. [Google Scholar]
- 19.Hagens VE, Ranchor AV, Van Sonderen E, Bosker HA, Kamp O, Tijssen JGP, Kingma JH, Crijns HJGM, Van Gelder IC. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation: Results from the Rate Control Versus Electrical Cardioversion (RACE) study. Journal of the American College of Cardiology 2004;43(2):241–247. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AE. Relationships Between Sinus Rhythm, Treatment, and Survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109(12):1509–1513. [DOI] [PubMed] [Google Scholar]
- 21.Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, et al. Early Rhythm Control Therapy in Patients with Atrial Fibrillation and Heart Failure. Circulation 2021;144(11):845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. European Heart Journal 2022;43(12):1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke Risk as a Function of Atrial Fibrillation Duration and CHA2DS2-VASc Score. Circulation 2019;140(20):1639–1646. [DOI] [PubMed] [Google Scholar]
- 24.Paoletti Perini A, Bartolini S, Pieragnoli P, Ricciardi G, Perrotta L, Valleggi A, Vergaro G, Michelotti F, Boggian G, Sassone B, et al. CHADS2 and CHA2DS2-VASc scores to predict morbidity and mortality in heart failure patients candidates to cardiac resynchronization therapy. EP Europace 2014;16(1):71–80. [DOI] [PubMed] [Google Scholar]
- 25.Yoshihisa A, Watanabe S, Kanno Y, Takiguchi M, Sato A, Yokokawa T, Miura S, Shimizu T, Abe S, Sato T, S, et al. The CHA2DS2-VASc score as a predictor of high mortality in hospitalized heart failure patients. ESC Heart Failure 2016;3(4):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goette A, Eckardt L, Valgimigli M, Lewalter T, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Tijssen JG, Vranckx P. Clinical risk predictors in atrial fibrillation patients following successful coronary stenting: ENTRUST-AF PCI sub-analysis. Clinical Research in Cardiology 2021;110(6):831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hohnloser SH, Crijns HJGM, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ. Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation. New England Journal of Medicine 2009;360(7):668–678. [DOI] [PubMed] [Google Scholar]
- 28.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, et al. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-line Treatment of Symptomatic Atrial Fibrillation: A Randomized Trial. JAMA 2005;293(21):2634–2640. [DOI] [PubMed] [Google Scholar]
- 29.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2): A Randomized Trial. JAMA 2014;311(7):692–699. [DOI] [PubMed] [Google Scholar]
- 30.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, et al. Cryoballoon Ablation of Pulmonary Veins for Paroxysmal Atrial Fibrillation: First Results of the North American Arctic Front (STOP AF) Pivotal Trial. Journal of the American College of Cardiology 2013;61(16):1713–1723. [DOI] [PubMed] [Google Scholar]
- 31.Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, Roux J-F, Yung D, Skanes A, Khaykin Y, et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. New England Journal of Medicine 2021;384(4):305–315. [DOI] [PubMed] [Google Scholar]
- 32.Kuniss M, Pavlovic N, Velagic V, Hermida JS, Healey S, Arena G, Badenco N, Meyer C, Chen J, Iacopino S, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. EP Europace 2021;23(7):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagens VE, Crijns HJGM, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, Bosker HA, Kamp O, Tijssen JGP, Veeger NJGM, et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: Results from the RAte Control versus Electrical cardioversion (RACE) study. American Heart Journal 2005;149(6):1106–1111. [DOI] [PubMed] [Google Scholar]
- 34.Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L, Merkely B, Pokushalov E, Sanders P, Schunkert H, et al. Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure: The CASTLE-AF Trial. JACC: Clinical Electrophysiology 2021;7(5):594–603. [DOI] [PubMed] [Google Scholar]
- 35.Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, Poole JE, Bahnson TD, Lee KL, Mark DB. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation 2021;143(14):1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fosbol EL, Holmes DJN, Piccini JP, Thomas L, Reiffel JA, Mills RM, Kowey P, Mahaffey K, Gersh BJ, Peterson ED, ORBIT-AF Investigators. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. Journal of the American Heart Association 2013;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events—European Registry in Atrial Fibrillation (PREFER in AF). EP Europace 2014;16(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Geller L, Kalejs O, Neumann T, Davtyan K, On YK, Popov S, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). EP Europace 2021;23(3):362–369a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321(13):1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. American Journal of Epidemiology 2017;186(9):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


