Summary
Background
After an initial stroke, current clinical practice is aimed at preventing recurrent stroke. Thus far, population-based estimates on the risk of recurrent stroke remain scarce. Here we describe the risk of recurrent stroke in a population-based cohort study.
Methods
We included Rotterdam Study participants who developed a first-ever incident stroke during follow-up between 1990 until 2020. During further follow-up, these participants were monitored for the occurrence of a recurrent stroke. We determined stroke subtypes based on clinical and imaging information. We calculated ten-year overall and sex-specific cumulative incidences of first recurrent stroke. To reflect changing secondary preventive strategies employed in recent decades, we then calculated the risk of recurrent stroke within ten-year epochs based on first-ever stroke date (1990–2000, 2000–2010 and 2010–2020).
Findings
In total, 1701 participants (mean age 80.3 years, 59.8% women) from 14,163 community-living individuals suffered a first stroke between 1990 and 2020. Of these strokes, 1111 (65.3%) were ischaemic, 141 (8.3%) haemorrhagic, and 449 (26.4%) unspecified. During 6585.3 person-years of follow-up, 331 (19.5%) suffered a recurrent stroke, of which 178 (53.8%) were ischaemic, 34 (10.3%) haemorrhagic and 119 (36.0%) unspecified. Median time between first and recurrent stroke was 1.8 (interquartile range 0.5–4.6) years. Overall ten-year recurrence risk following first-ever stroke was 18.0% (95% CI 16.2%–19.8%), 19.3% (16.3%–22.3%) in men and 17.1% (14.8%–19.4%) in women. Recurrent stroke risk declined over time, with a ten-year risk of 21.4% (17.9%–24.9%) between 1990 and 2000 and 11.0% (8.3%–13.8%) between 2010 and 2020.
Interpretation
In this population-based study, almost one in five people with first-ever stroke suffered a recurrence within ten years of the initial stroke. Furthermore, recurrence risk declined between 2010 and 2020.
Funding
Netherlands Organization for Health Research and Development, EU’s Horizon 2020 research programme and the Erasmus Medical Centre MRACE grant.
Keywords: Stroke, Recurrence, Incidence studies
Research in context.
Evidence before this study
We searched PubMed for literature on cohort studies, published in English until January 11 2023 that reported on the risk of recurrent stroke among community-living individuals that had developed a stroke. The search terms included “recurr∗” “stroke” “risk” “population-based”. A systematic review and meta-analysis published in 2021 on the subject of recurrence risk after ischaemic stroke, with stratifications for TOAST etiologies of ischaemic stroke, retrieved 26 studies published between 1993 (when the TOAST framework was introduced) and 2020. This review reported stroke recurrence rates from 5.7% to 17.7% in the first year to 14.0%–26.0% in the first five years after the initial stroke. The authors suggested that the risk of recurrent stroke has remained stable in the last 20 years, despite the advances in stroke healthcare that have been introduced since then. Most of these articles described registry studies from single hospitals. As such, these studies may have missed patients with first-ever stroke who never presented to the hospital. Additionally, these studies may have missed those patients with recurrent stroke that, due to their initial stroke related impairments, are not sent in for another stroke workup. These two biases may have led to an underestimation of the risk of recurrent stroke.
Added value of this study
This study reports the risk of recurrent stroke within a large sample of first-ever stroke patients from a population-based cohort study, with a specific focus on short- and longterm follow-up. We additionally stratify these estimates for sex and for epoch during which the first-ever stroke occurred to detect potential changes in the risk of recurrent stroke following important developments in secondary preventative strategies.
Implications of all the available evidence
In our study, one in five stroke patients suffered a recurrent stroke in the ten years after their initial stroke. This result is in line with the first report on the subject published in 1982, 40 years prior to this study, and confirms the findings of a recent systematic review on the risk of recurrent stroke that suggest the risk remained stable for 20 years. However, strikingly, we observe a reduction in the risk of recurrent stroke among first-ever strokes between 2010 and 2020, suggesting that advances in secondary prevention have only recently began to have an effect on the risk of recurrent stroke.
Introduction
The lifetime risk of first-ever stroke has increased globally from 22.8% in 1990 to 25.0% in 20161 and stroke is currently estimated to be the second leading cause of death and disability.2 To combat the devastating consequences of stroke, several advances in clinical neurological practice have been introduced in the past thirty years such as the implementation of stroke units,3 optimisation of secondary prevention protocols,4 intravenous thrombolysis, and intra-arterial treatment of ischaemic strokes.5 These advances have led to better survival after stroke compared to before the turn of the millennium.6 This also means that more people are now at risk for recurrent stroke, highlighting the increasing importance of secondary prevention.
Current data on the risk of recurrent stroke predominantly originate from hospital or administrative registry-based studies7, 8, 9, 10, 11, 12 with five-year risk estimates ranging from 9.0%8 to 12.6%.7 These offer valuable insights into the short-term risk of recurrent stroke during the period when patients are still under neurovascular specialists’ care, ensuring optimal adherence to secondary prevention protocols. In the long-term, we know that adherence to secondary prevention protocols decreases, in particular for those patients with less severe post-stroke disabilities.13 As such, the long-term risk of recurrent stroke remains largely unknown. Additionally, patients that move to a nursing home environment after their stroke may no longer be sent in for periodic control visits with their neurovascular specialist, leading to underestimation in assessing recurrent stroke risk. Alternatively, population-based cohort studies can offer more robust estimates on the risk of recurrent stroke over time. However, data from such studies are rare and the most recent estimates from a Western population are now almost fifteen years old.14,15 An updated population-based perspective is needed to assess the effects of new clinical advances on the risk of recurrent stroke. Against this background, we determined the risk of recurrent stroke in a general population-based cohort study. Additionally, we provide a comprehensive overview of how the underlying stroke subtypes relate from the first-ever stroke to the recurrent stroke, of sex-specific differences in recurrent stroke risk, and use ten-year epoch-specific risk estimates to assess whether the risk of recurrent stroke has changed over the past three decades.
Methods
Setting
This study was performed as part of the on-going Rotterdam Study, a population-based, observational cohort study among residents of the Ommoord district in Rotterdam, the Netherlands, aged ≥45 years. The study began in 1990 with 7983 participants and was further expanded in 2000 and 2006 with 3011, and 3932 participants, respectively. Participants undergo follow-up examination visits to the study centre every 3–6 years and their medical records are continuously monitored through electronic linkage with the study database as part of the follow-up for clinical endpoints. Further details of the study are described elsewhere.16 The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. The Rotterdam Study has been entered into the Netherlands National Trial Register and into the WHO International Clinical Trials Registry Platform under shared catalogue number NTR6831. All participants provided written informed consent for participation in the study and for researchers to access medical information from their personal physicians.
Study population
For this study, we selected those participants without prevalent stroke at baseline who developed a first-ever stroke during follow-up. These participants were then followed from their first-ever stroke date until a combined endpoint consisting of date of death, last health status update when they were known to be free of recurrent stroke, or January 1, 2020, whichever came first. Those participants who died on the date of first-ever stroke were not at-risk of recurrent stroke and were excluded from this study. Follow-up was complete for 99.1% of potential person-years.
Stroke assessment
The definition of stroke was based on the World Health Organization criteria as a ‘syndrome of rapidly developing clinical signs of focal or global disturbance of cerebral function, where symptoms last 24 h or longer or lead to death, with no apparent cause other than of vascular origin’.16 Subjects with prevalent stroke at baseline were identified during an interview with trained physicians and verified with their medical records. After enrollment participants were continuously monitored for any incident stroke through automatic digital linkage of the study database with files from their general practitioners or files from their nursing homes. Additional information (e.g., clinical notes and neuroimaging reports) were obtained from hospital records and used to classify stroke events as ischaemic or haemorrhagic. If this information was insufficient to determine the subtype, then the stroke was classified as unspecified. Subarachnoid haemorrhages due to ruptured aneurysms were not considered stroke events since the onset of our study, as these were considered too rare to systematically track in a population-based study. Potential stroke events were reviewed by research physicians and verified in consensus by an experienced vascular neurologist. As first-ever strokes can occur anywhere between research centre examination rounds, we lack systematic information on cardiovascular risk factors through our medical records-based assessment and therefore describe relevant self-reported medication use and smoking behaviour assessed at their most recent visit prior to the first-ever stroke.
Statistical methods
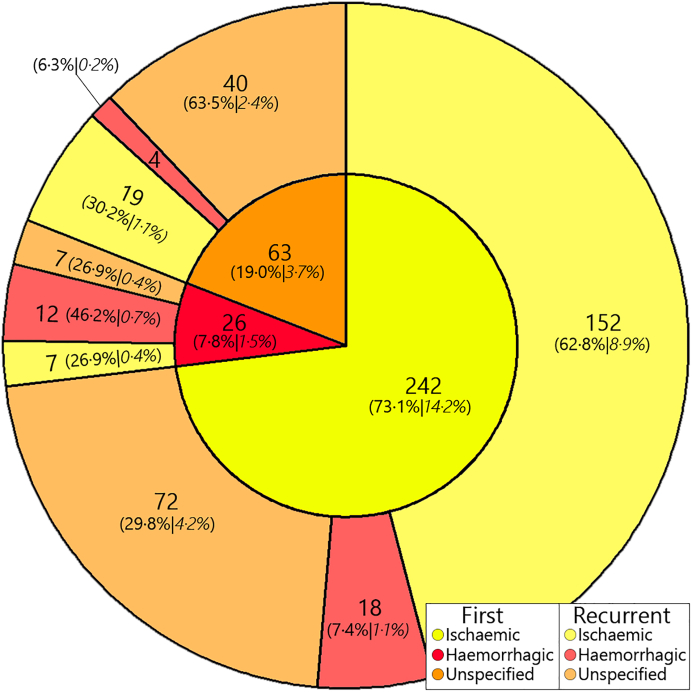
The summary characteristics from the study population of age at first-ever and recurrent stroke, sex, ancestry,17 medication use for diabetes mellitus, lipid control, hypertension and any anti-thrombotics, smoking behaviour and stroke subtypes at first-ever and recurrent stroke are presented in frequencies and percentages, or mean and standard deviation. Next, we describe subtype patterns of first-ever and recurrent strokes and provide an overview of these patterns using a pie-donut chart where the center pie denotes the first-ever subtype and the outer donut denotes the corresponding recurrent stroke subtype. To assess risk of recurrent stroke we first calculated cumulative incidences of first recurrent stroke using the following method:
Next, we calculated time period specific incidence rates of the first stroke recurrence following first-ever stroke to reflect changes in the rate of recurrence during follow-up over time:
Both cumulative incidences and incidence rates are presented numerically and graphically for the first ten years following first-ever stroke. We also assessed the incidence rate of total recurrences during follow-up. Next, we stratified cumulative incidence and incidence rate estimates based on 5-year age categories of age at first-ever stroke, and also stratified the cumulative incidence on sex to assess recurrence risk differences for men and women. Finally, we divided the study population in three separate epochs based on when their first-ever event occurred. The first epoch ranged from start of the study until 1999, whereafter statins were introduced into Dutch healthcare.18 The second was between 2000 and 2009. The last epoch starts after 2009 when new oral anticoagulants were registered for use in Dutch healthcare.19 We stratified on stroke epoch to illustrate how the risk of recurrent stroke has changed over time for the first ten years following first-ever stroke. Stratified cumulative incidence curves were compared using a log-rank test with an α of 0.05 to assume a statistically significant difference. In a sensitivity analyses, we estimated risk of stroke recurrence while considering all-cause mortality as a competing risk, by modelling the cause-specific hazard of stroke recurrence according to the methods described elsewhere.20 In this analysis, the probability for the rate of stroke recurrence is estimated through Kaplan–Meier method, where all-cause mortality is seen as an additional outcome. This assesses the potential interference of possible reasons for censoring (i.e. moving abroad or declining further follow-up) on the risk of recurrent stroke, separately from all-cause mortality. All analyses were performed using R (version 4.2.2) with packages ‘survival’ (3.5.2), ‘survminer’ (0.4.9) and ‘cmprsk’ (2.2–11).
Role of the funding sources
This work was supported by the European Union's Horizon 2020 research and innovation programme (grant number 667375; CoSTREAM); the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO; grant numbers 948-00-010, 918-46-615; the Netherlands Organization for Health Research and Development (ZonMw); The Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. BPB, DB and MKI were supported by the Erasmus Medical Centre MRACE grant (grant number 386070). The funding organizations and sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Results
Participant characteristics
Of the 14,926 participants, 461 with prevalent strokes and another 302 participants, who did not consent to stroke follow-up, were excluded. After 195,788.8 person-years of follow-up, 1701 participants suffered a first-ever stroke (8.7 per 1000 person-years). Table 1 shows an overview of baseline characteristics of study participants at first-ever stroke. Mean age at first-ever stroke was 80.3 years (9.0 SD) and 1017 (59.8%) were female, and 1362 (80.1%) were of Caucasian ancestry. Regarding first-ever stroke subtypes, 1111 (65.3%) were ischaemic, 141 (8.3%) were haemorrhagic and 449 (26.4%) were unspecified. Next, after a total of 6585.3 person-years of follow-up, 331 (19.5%) participants developed recurrent stroke (50.3 per 1000 person-years). Among these, in 1209.0 additional person-years of follow-up until 01-01-2020, 56 (3.3% of 1701 first-ever incident strokes) people developed a third stroke, 14 (0.8%) developed a fourth stroke, and 2 (0.1%) developed a fifth stroke, leading to an incidence rate of total recurrences of 54.0 per 1000 person-years (421 in 7794.3 person-years). In this study, we focused solely on the first recurrence of stroke. The median time between first-ever and recurrent stroke was 1.8 (0.5–4.6 interquartile range) years. Mean age at recurrent stroke was 80.7 years (7.9 SD), 186 (56.2%) were female, and 275 (83.1%) were of Caucasian ancestry. Of the recurrent strokes, 178 (53.8%) were ischaemic, 34 (10.3%) were haemorrhagic and 119 (36.0%) were unspecified. Fig. 1 shows an overview of how first-ever stroke subtypes relate to those at recurrence.
Table 1.
Baseline characteristics at first-ever stroke of N = 1701 participants, total sample and stratified per epoch.
| Baseline characteristics of study population, total and per epoch | ||||
|---|---|---|---|---|
| Totala (N = 1701) | Epoch 1 (N = 529) | Epoch 2 (N = 674) | Epoch 3 (N = 498) | |
| Categorical characteristic | n (%) | |||
| Sex, female | 1017 (59.8) | 311 (58.8) | 417 (61.9) | 289 (58.0) |
| Cohort | ||||
| RS-I | 1285 (75.5) | 529 (100) | 507 (75.2) | 249 (50.0) |
| RS-II | 295 (17.3) | 0 (0) | 143 (21.2) | 152 (30.5) |
| RS-III | 121 (7.1) | 0 (0) | 24 (3.6) | 97 (19.5) |
| Ancestry | ||||
| Caucasian | 1362 (80.2) | 398 (75.2) | 562 (83.4) | 402 (80.7) |
| Non-Caucasian | 18 (1.1) | 2 (0.4) | 7 (1.0) | 9 (1.8) |
| Stroke type, first event | ||||
| Haemorrhagic | 141 (8.3) | 25 (4.7) | 64 (9.4) | 52 (10.4) |
| Unspecified | 449 (26.4) | 210 (39.9) | 180 (26.7) | 59 (11.8) |
| Ischaemic | 1111 (65.3) | 294 (55.6) | 430 (63.8) | 387 (77.7) |
| Large-artery atherosclerosis | 87 (7.8) | 27 (9.2) | 28 (6.5) | 32 (8.3) |
| Cardioembolism | 216 (19.4) | 45 (15.3) | 88 (20.5) | 83 (21.4) |
| Small-vessel occlusion | 80 (7.2) | 40 (13.6) | 28 (6.5) | 12 (3.1) |
| Stroke of other determined etiology | 28 (2.5) | 9 (3.1) | 14 (3.3) | 5 (1.3) |
| Stroke of undetermined etiology | 700 (63.0) | 173 (58.8) | 272 (63.3) | 255 (65.9) |
| Stroke type, second event | 331 (19.5) | 130 (39.3) | 146 (44.1) | 55 (16.6) |
| Haemorrhagic | 34 (10.3) | 15 (11.5) | 12 (8.2) | 7 (12.7) |
| Unspecified | 119 (36.0) | 63 (48.6) | 47 (32.2) | 9 (16.4) |
| Ischaemic | 178 (53.8) | 52 (40.0) | 87 (59.6) | 39 (70.9) |
| Large-artery atherosclerosis | 13 (7.3) | 3 (5.8) | 7 (8.0) | 3 (7.7) |
| Cardioembolism | 42 (23.6) | 15 (28.8) | 16 (18.4) | 11 (28.2) |
| Small-vessel occlusion | 10 (5.6) | 7 (13.5) | 3 (3.4) | 0 (0) |
| Stroke of other determined etiology | 2 (1.1) | 1 (1.9) | 1 (1.1) | 0 (0) |
| Stroke of undetermined etiology | 111 (62.4) | 26 (50.0) | 60 (69.0) | 25 (64.1) |
| Medication use prior to first stroke | ||||
| Diabetes medication | 156 (9.2) | 30 (5.7) | 69 (10.2) | 11.4 (11.4) |
| Anti-hypertensives | 818 (48.1) | 214 (40.5) | 320 (47.5) | 284 (57.0) |
| Statins & other lipid control | 218 (12.8) | 9 (1.7) | 69 (10.2) | 140 (28.1) |
| Anti-thrombotic agents | 446 (26.2) | 32 (6.6) | 230 (34.1) | 181 (36.3) |
| Smoking prior to first stroke | ||||
| Never | 410 (24.1) | 106 (20.0) | 179 (26.6) | 125 (25.1) |
| Past | 618 (36.3) | 125 (23.6) | 254 (37.7) | 239 (48.0) |
| Current | 417 (24.5) | 165 (31.2) | 173 (25.7) | 79 (15.9) |
| Continuous, normally distributed, characteristic | Mean (SD) | |||
| Age at first event, years | 80.3 (9.0) | 79.7 (8.8) | 80.3 (9.2) | 80.9 (8.9) |
| Continuous, not-normally distributed, characteristic | Median (IQR) | |||
| Time between first-ever and first recurrent stroke, years | 1.8 (0.5–4.6) | 2.5 (0.8–6.0) | 1.5 (0.4–3.6) | 1.5 (0.5–3.6) |
| Time between last control visit and first-ever stroke, years | 2.9 (1.4–4.9) | 1.6 (0.9–3.0) | 3.5 (1.9–5.2) | 3.9 (2.2–7.0) |
Data and percentages represent original values including missing data.
Missing values were present for ancestry (18.9%), diabetes medication (24.3%), anti-hypertensive (7.0%), statins & other lipid control (24.3%), anti-thrombotic agents (24.3%) and smoking status (15.0%).
Fig. 1.
Stroke subtype distribution at first-ever and subsequent recurrence in n = 331 participants with recurrent stroke. Central pie denotes first-ever stroke subtype and the outer donut denotes the corresponding recurrent stroke subtype. In parentheses, first the subtype percentages of n = 331 recurrent strokes and in cursive the percentages under N = 1701 first-ever strokes.
Risk of recurrent stroke
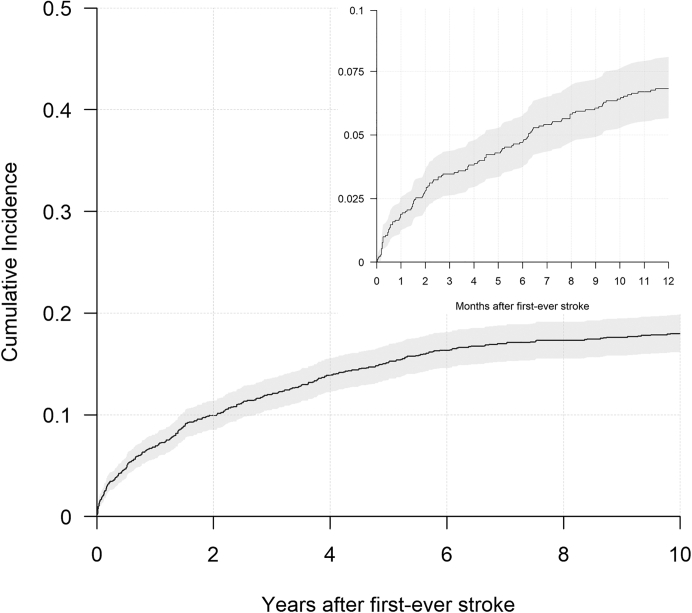
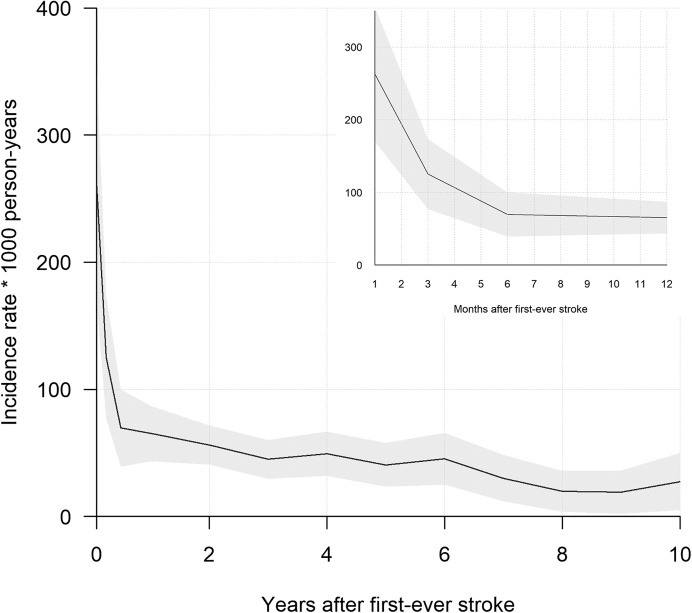
Table 2 shows an overview of events, deaths and censoring due to other reasons per year for the first ten years post first-ever stroke. The one-year cumulative incidence of recurrent stroke was 6.8% (95% Confidence Interval (CI) [5.6%–8.0%]) with a 10-year cumulative incidence of 18.0% (95% CI [16.2%–19.8%]). Fig. 2 shows risk of recurrent stroke for the first ten years following first-ever stroke, where we see that almost half of the recurrences occurred after the second year post first-ever stroke. Fig. 3 shows the incidence rate of first recurrent stroke per 1000 person-years in each of the first ten years after the first-ever stroke. The incidence rate decreased from 262.7 (95% CI [169.8–355.6]) per 1000 person-years one month after first-ever stroke, to 65.3 (95% CI [43.5–87.1]) per 1000 person-years one year after first-ever stroke and to 27.6 (95% CI [5.1–50.0]) per 1000 person-years ten years after first-ever stroke. Table 3 shows the cumulative incidence and incidence rates stratified per five year category of age at first-ever stroke.
Table 2.
Overview of recurrences, deaths and censoring due to other reasons for N = 1701 participants with first-ever stroke.
| Event and censoring information of study population (N = 1701) | |||||||
|---|---|---|---|---|---|---|---|
| Time period | N at risk period start | N recurrent strokes during period | N deaths during period | N other censoring during period | Total follow-up time at period enda | Incidence rate of recurrent stroke within period | Cumulative incidence of recurrent stroke at the end of period |
| 0–1 year | 1701 | 116 | 515 | 25 | 1190.2 | 65.3 | 6.9% |
| 1–2 year | 1045 | 53 | 116 | 27 | 2133.2 | 56.2 | 10.1% |
| 2–3 year | 849 | 35 | 94 | 20 | 2909.4 | 45.1 | 12.2% |
| 3–4 year | 700 | 32 | 59 | 17 | 3556.5 | 49.5 | 14.1% |
| 4–5 year | 593 | 22 | 70 | 11 | 4097.2 | 40.7 | 15.4% |
| 5–6 year | 489 | 20 | 51 | 17 | 4538.1 | 45.4 | 16.6% |
| 6–7 year | 401 | 11 | 45 | 12 | 4902.7 | 30.2 | 17.3% |
| 7–8 year | 333 | 6 | 36 | 11 | 5206.0 | 19.8 | 17.7% |
| 8–9 year | 280 | 5 | 30 | 3 | 5467.6 | 19.1 | 18.0% |
| 9–10 year | 242 | 6 | 34 | 7 | 5685.3 | 27.6 | 18.3% |
Person-years of follow-up from first-ever stroke until end of time period.
Fig. 2.
Plot of overall risk of recurrent stroke over time following first-ever stroke, grey area denotes 95% confidence interval for proportions.
Fig. 3.
Graph of period specific incidence rates of first recurrent stroke per 1000 person-years over time following first-ever stroke, grey area denotes 95% confidence interval for rates.
Table 3.
Cumulative incidence and incidence rates stratified per 5-year age category at first-ever stroke.
| Cumulative incidences and incidence rates (per 1000 person-years) of first recurrent stroke, stratified across 5 year age categories at first-ever stroke | ||||||
|---|---|---|---|---|---|---|
| Age at first-ever stroke, years | N | n recurrences | Cumulative incidence |
Incidence rate |
||
| 1-year | 10-year | Total follow-up time, years | Rate per 1000 person-yearsa | |||
| <55 | 9 | 1 | 0.0% | 11.1% | 70.9 | 14.1 |
| 55–60 | 22 | 6 | 0.0% | 18.2% | 176.3 | 34.0 |
| 60–65 | 67 | 21 | 9.0% | 26.9% | 577.0 | 36.4 |
| 65–70 | 139 | 32 | 6.5% | 16.5% | 1052.6 | 30.4 |
| 70–75 | 224 | 62 | 6.7% | 25.0% | 1302.3 | 47.6 |
| 75–80 | 326 | 85 | 9.2% | 25.5% | 1400.1 | 60.7 |
| 80–85 | 364 | 64 | 7.4% | 17.0% | 1160.7 | 55.1 |
| 85–90 | 299 | 39 | 5.7% | 12.7% | 520.1 | 75.0 |
| >90 | 251 | 21 | 4.8% | 8.4% | 325.4 | 64.5 |
N, participants at risk for recurrent stroke; n, count of participants with recurrent stroke.
Incidence rate derived by dividing count of recurrences by total follow-up time and multiplying by 1000 person-years.
Sex differences in recurrence risk
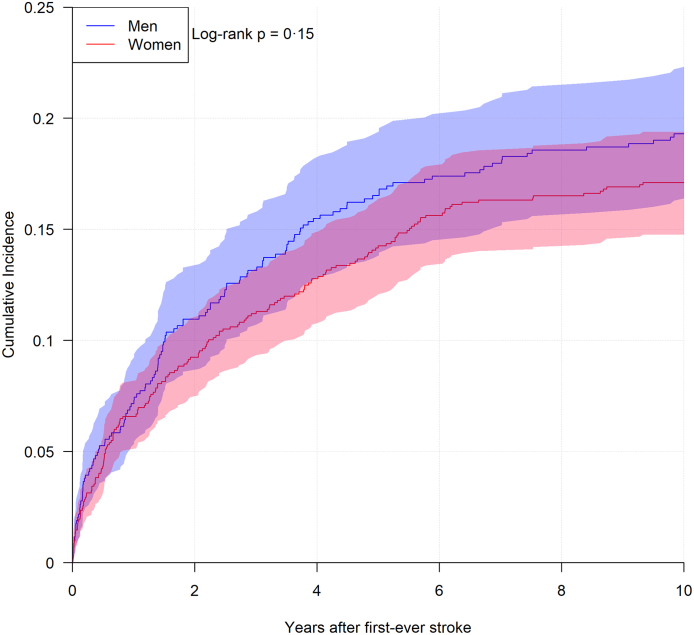
Men were younger than women at their first-ever event, mean 77.8 years (SD 8.6) versus mean 82.0 (SD 8.9). This distribution was similar at the recurrent event, mean 77.8 years (SD 7.6) versus mean 83.0 (SD 7.5). The time between first-ever and recurrent stroke did not significantly differ between men and women, with a median 1.7 (0.5–4.4 interquartile range) years for men and a median 1.9 (0.5–4.7 interquartile range) years for women. Fig. 4 shows the risk of recurrent stroke for the first ten years following first-ever stroke, stratified for men and women. After one and ten years, the absolute risk for recurrent stroke for men was 7.2% (95% CI [5.2%–9.1%]) and 19.3% (95% CI [16.3%–22.3%]) whereas this was 6.6% (95% CI [5.1%–8.1%]) and 17.1% (95% CI [14.8%–19.4%]) for women. No statistically significant difference between men and women was observed when comparing the risk of recurrent stroke over time.
Fig. 4.
Plots of risk of recurrent stroke over time, stratified for men and women separately, coloured areas denote 95% confidence interval for proportions.
Epoch differences in recurrence risk
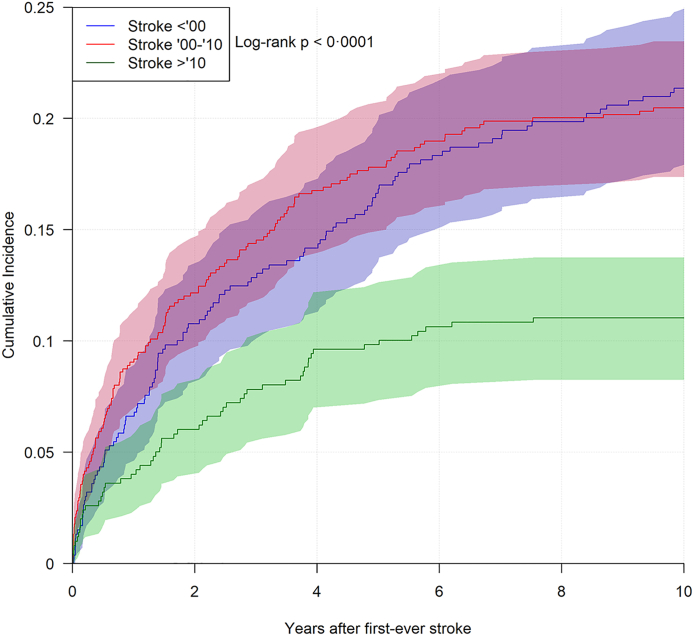
From 1989 until 2000, 529 first-ever strokes occurred and of these, 130 (24.6%) developed a recurrent event, with one-year and ten-year cumulative incidences for recurrent stroke of 6.6% (95% CI [4.5%–8.7%]) and 21.4% (95% CI [17.9%–24.9%]), respectively. Between 2000 and 2010, 674 first-ever strokes occurred and of these, 146 (22.0%) developed a recurrent event. The corresponding risks for this epoch were 9.1% (95% CI [6.9%–11.2%]) and 20.5% (95% CI [17.4%–23.5%]). After 2010 until end of study in 2020, 498 first-ever strokes occurred and of these, 55 (11.0%) developed a recurrent event. In this final epoch, the one-year and ten-year risk of recurrent stroke were 4.0% (95% CI [2.3%–5.7%]) and 11.0% (95% CI [8.3%–13.8%]), respectively. Fig. 5 shows the recurrence risk through epoch specific cumulative incidences over ten years. The most prominent difference in the risk of recurrent stroke over time was seen when comparing the first and third epochs, where there was an absolute risk reduction in one-year and ten-year risk of 2.6% and 10.3%.
Fig. 5.
Plots of risk of recurrent stroke over time, stratified for epoch where first-ever stroke occurred, coloured areas denote 95% confidence interval for proportions.
Sensitivity analyses
Appendix A shows the results of the ten-year risk of recurrent stroke estimation using a cause-specific hazard approach, with all-cause mortality as a competing risk for stroke recurrence, versus the risk found by calculating cumulative incidences. This approach estimates the recurrence risk higher, although not statistically significant, with a ten-year risk of 19.2% (95% CI [17.3%–21.2%]) as compared to the 10-year cumulative incidence of 18.0% (95% CI [16.2%–19.8%]) presented above. The absolute risk difference between the two methods was 1.2% (95% CI [−1.5% to 3.8%]).
Discussion
In this population-based cohort study, we observed that patients who suffered their first-ever stroke have a risk of 18.0% to develop a recurrent stroke in the ten years following their initial stroke. Men were on average younger at both their first-ever and recurrent stroke than women, but no difference in the risk of recurrent stroke was observed between men and women. Finally, we observed an overall reduction in recurrence risk between 2010 and 2020 compared to the previous two decades.
Our findings of a ten-year risk of recurrent stroke of 18.0% is in line with the sparse reports from previous similar population-based cohort studies. The earliest of these reports was published by the Framingham Study in 1982, where a risk of 21.3% for recurrent stroke was reported among 394 first-ever strokes after 26 years of follow-up.14 This report stems from a time where the only clinical option for secondary prevention was management of hypertension and thus predates the introduction of several secondary preventative strategies such as anticoagulative drugs, which were still only used in trial settings during the ‘70s,21 and statin therapy. An important observation, when comparing these data, is that after 40 years of clinical advances in recurrent stroke prevention, the risk of recurrent stroke in our study seems only slightly reduced compared to the estimate by the Framingham report. Taking into account our observation of a reduced recurrence risk in the last decade, it is possible that evolving secondary prevention strategies have only recently succeeded in reducing the risk of recurrent stroke.
In 2004, the Hisayama study reported data from a Japanese population with a ten-year risk of recurrent stroke of 26.3%.22 This estimate is higher than ours, perhaps again due to the study being performed at a time before effective secondary prevention strategies were introduced. Another potential reason for a higher estimate of the risk of recurrent stroke is due to the study being set in a predominantly Asian population, where the risk of first-ever stroke is also higher compared to European populations.23 The Hisayama study recently published updated estimates on five-year risks of recurrent stroke, demonstrating a trend over time where the risk has steadily decreased between 1961 and 1998, but has possibly increased in the final decade of follow-up for first-ever strokes occurring between 2002 and 2012.24 This finding contrasts with our epoch analysis where the risk of recurrent stroke initially remained stable but was lower in the last epoch of follow-up. A possible explanation for this difference is twofold: first, acute stroke management strategies have improved over the years, leading to improved stroke survival6 which subsequently leads to an increase in people at risk for a recurrent stroke. This is reflected in our epoch analysis where the risk of recurrent stroke increased, albeit not statistically significant, when comparing the second epoch with the first epoch. Second, a recent study described an improvement of over 50% in adequate management after transient ischaemic attack (TIA) when comparing incident TIA cases from 2006 to 2007 to those in 2015–2016.25 This supports the suggestion that optimal secondary prevention strategies for stroke have started to become widely implemented only in the last 15 years, the effects of which only partially overlaps with the final Hisayama cohort but fully overlaps our last epoch of follow-up.
The last report from a similar designed population-based cohort study by the ARIC study was published in 2013, reporting 145 recurrences among 946 first-ever strokes, leading to an overall risk of recurrent stroke of 15.3% in a median of 5.3 years of follow-up after first-ever stroke. This study uses follow-up data until 31-12-2008 and thus also precedes the change in secondary prevention strategies.15
Half of the stroke recurrences in the present study occurred in the period between the first two years and ten years following first-ever stroke. This suggests that long follow-up times are necessary to capture the total picture of the risk of recurrent stroke. Previous population-based registry studies that used shorter follow-up times, such as the Evros Stroke registry with a maximum follow-up timepoint of two years following first-ever stroke,9 may therefore have underestimated the risk of recurrent stroke. Another study that potentially underestimated the risk of recurrent stroke due to shorter follow-up times is the TIAregistry.org study,8 where patients with transient ischaemic attack and minor stroke were followed-up for a maximum of five years and reported a lower five-year recurrent stroke risk estimate of 9.0% versus our estimate of 15.4%. Although this difference could also be explained by their study assessing stroke patients with little to no disabilities, who are therefore more prone to attend control visits at neurovascular specialists initially after their stroke and are thus more likely to adhere to secondary stroke prevention compared to more severely disabled stroke patients.
Another possible explanation for the differences in risk reported in previous studies and ours is their use of Kaplan–Meier estimation of the risk of recurrent stroke,8,10,15,22,24 as native Kaplan–Meier estimation assumes no interference from competing risks such as overall mortality risk. This is rarely the case when assessing recurrence risk after a potentially lethal event such as a stroke6 and studies using this method will therefore produce results that are biased upwards, leading to overestimations of risk of recurrent stroke.20 Cause-specific hazard estimation can offer more appropriate estimations of the risk of recurrent stroke, as it estimates risk separately from specific forms of censoring.20 We used cause-specific hazard estimation to assess recurrence risk separately from all-cause mortality, however the recurrence risk increased non-significantly compared to our estimates with the cumulative incidence function. This suggests that those participants that are lost to follow-up after first-ever stroke, for reasons other than all-cause mortality, do not contribute significantly to the risk of recurrent stroke.
No statistically significant difference in recurrence risk was observed in this study for men and women, in contrast to sex differences that have previously been described for other cardiovascular events such as coronary heart disease. Previous literature on coronary heart disease has shown that men more frequently suffer another heart attack compared to women,26,27 but this does not seem to be the case for recurrent stroke in our study. We did find that men were younger than women at both first-ever and recurrent strokes, a finding largely driven by the age distribution at the initial stroke. And indeed, this is a concordant finding with what is known from the cardiovascular field where men are younger at their first heart attack than women.26
Important strengths of our study are the almost complete long-term follow-up of participants and its community-based setting, which avoids important selection bias that stems from hospital-based studies and is therefore more appropriate to determine recurrence risk in the general population. Furthermore, we used a robust adjudication method to identify strokes based on health records from GP offices, hospitals and nursing homes after consensus with experienced vascular neurologists. However, this method is also a limitation in some facets, as health records from nursing homes are usually less detailed compared to those from hospitals. As stroke patients are often referred to nursing homes after their initial recovery, it is possible that recurrent strokes in these care settings are more often missed by our method due to less precise documentation. This could have led to a conservative estimation of the risk of recurrent stroke and precludes estimation of what subtype of stroke has occurred in these facilities, which has contributed to a relatively large share of unspecified first-ever strokes in our study of 26.4%. A previous study from our group28 demonstrated that the share of unspecified strokes has decreased over the last three decades, from 75% in 1990 to 16% since 2016, indicating that patients are more often referred to a hospital for a stroke diagnostic workup than at the onset of the study. And secondly, that this group represents a different patient category that more often suffers from dementia and multimorbidities who usually don't get hospitalised. A further limitation of this records-based method is lack of documentation of cardiovascular risk factors at the time of first-ever stroke, meaning we could only offer insights into their cardiovascular risk profiles from their most recent visit prior to the first-ever stroke. Nevertheless, our use of nursing home records is an added benefit over studies with selection bias stemming from the use of records from a single type of medical setting, at the cost of less precise estimation of the risk of recurrent stroke among the stroke subtypes. Next, this study was performed among subjects aged ≥45 years. Young stroke patients more often have different stroke etiologies29 and higher post-stroke mortality rates30 compared to older stroke patients, so it is possible that our results do not entirely translate to younger stroke patients. Another limitation of note is that our study was performed among predominately white participants and since the lifetime risk of stroke varies between ethnicities,31 the results of our study may not be fully generalisable to other ethnic populations.23 Finally, we did not include sub-arachnoid haemorrhages in our stroke definition and were therefore unable to assess risk of recurrent stroke that incorporates this cerebrovascular disease, because it was deemed too rare to appropriately investigate in a population-based study at the onset of the Rotterdam study.
Based on this study, we conclude that almost one in five people with first-ever stroke will have a recurrent stroke in the first ten years after their initial stroke. Despite clinical advances over the years, this finding reaffirms previous population-based cohort studies’ estimates, the earliest of which was published 40 years ago.14 This demonstrates that there is still much work to be done to prevent recurrent stroke, although our study does describe the favorable finding of a reduced stroke recurrence risk in the last decade. It is important to validate this finding by replicating it in other population-based cohort studies. Subsequently, further studies can be designed to investigate the underlying cause of this decline and determine whether this effect can be enhanced to further reduce the risk of recurrent stroke.
Contributors
BPB, DB, MAI and MKI contributed to study design. BPB and MKI did the data assessment and verification. BPB performed the data analyses and drafted the manuscript. DB, PJK, MAI and MKI all critically reviewed the manuscript. All authors had access to the data reported in the study. MKI had full access to all of the data and had final responsibility for the decision to submit for publication.
Data sharing statement
The data underlying this article will be shared upon reasonable request to the corresponding author. All requests will be directed towards the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, the data underlying this article cannot be made freely available in a public repository.
Declaration of interests
None.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100651.
Contributor Information
Brian, Email: b.p.berghout@erasmusmc.nl.
Daniel Bos, Email: d.bos@erasmusmc.nl.
Peter J. Koudstaal, Email: p.j.koudstaal@erasmusmc.nl.
M. Arfan Ikram, Email: m.a.ikram@erasmusmc.nl.
M. Kamran Ikram, Email: m.ikram@erasmusmc.nl.
Appendix A. Supplementary data
References
- 1.Collaborators GBDLRoS, Feigin V.L., Nguyen G., et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379(25):2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDS Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhorne P., Ramachandra S., Stroke Unit Trialists Collaboration Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. 2020;4:CD000197. doi: 10.1002/14651858.CD000197.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleindorfer D.O., Towfighi A., Chaturvedi S., et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. 2021;52(7):e364–e467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer O.A., Fransen P.S., Beumer D., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 6.Waziry R., Heshmatollah A., Bos D., et al. Time trends in survival following first hemorrhagic or ischemic stroke between 1991 and 2015 in the Rotterdam study. Stroke. 2020;51(3) doi: 10.1161/STROKEAHA.119.027198. [DOI] [PubMed] [Google Scholar]
- 7.Flach C., Muruet W., Wolfe C.D.A., Bhalla A., Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. 2020;51(8):2435–2444. doi: 10.1161/STROKEAHA.120.028992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarenco P., Lavallee P.C., Monteiro Tavares L., et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378(23):2182–2190. doi: 10.1056/NEJMoa1802712. [DOI] [PubMed] [Google Scholar]
- 9.Tsivgoulis G., Katsanos A.H., Patousi A., et al. Stroke recurrence and mortality in northeastern Greece: the Evros stroke registry. J Neurol. 2018;265(10):2379–2387. doi: 10.1007/s00415-018-9005-6. [DOI] [PubMed] [Google Scholar]
- 10.Kolmos M., Christoffersen L., Kruuse C. Recurrent ischemic stroke - a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2021;30(8) doi: 10.1016/j.jstrokecerebrovasdis.2021.105935. [DOI] [PubMed] [Google Scholar]
- 11.Akpalu A., Sarfo F.S., Akinyemi J., et al. Frequency & factors associated with recurrent stroke in Ghana and Nigeria. J Neurol Sci. 2022;439 doi: 10.1016/j.jns.2022.120303. [DOI] [PubMed] [Google Scholar]
- 12.Xu J., Zhang X., Jin A., et al. Trends and risk factors associated with stroke recurrence in China, 2007-2018. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.16341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendlebury S.T., Rothwell P.M. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc Dis. 2009;27(Suppl 3):1–11. doi: 10.1159/000209260. [DOI] [PubMed] [Google Scholar]
- 14.Sacco R.L., Wolf P.A., Kannel W.B., McNamara P.M. Survival and recurrence following stroke. The Framingham study. Stroke. 1982;13(3):290–295. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 15.Jones S.B., Sen S., Lakshminarayan K., Rosamond W.D. Poststroke outcomes vary by pathogenic stroke subtype in the Atherosclerosis Risk in Communities Study. Stroke. 2013;44(8):2307–2310. doi: 10.1161/STROKEAHA.113.000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieberdink R.G., Ikram M.A., Hofman A., Koudstaal P.J., Breteler M.M. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27(4):287–295. doi: 10.1007/s10654-012-9673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verlouw J.A.M., Clemens E., de Vries J.H., et al. A comparison of genotyping arrays. Eur J Hum Genet. 2021;29(11):1611–1624. doi: 10.1038/s41431-021-00917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centraal Begeleidingsorgaan voor de Intercollegiale Toetsing. Nederlandse Hartstichting . tweede herziening; Utrecht: 1998. Behandeling en preventie van coronaire hartziekten door verlaging van de plasmacholesterolconcentratie: consensus cholesterol. [Google Scholar]
- 19.Schalij M., Dubois E., Boersma L., et al. Dutch Ministry of Health, Welfare and Sport; 2012. Leidraad begeleide introductie nieuwe orale antistollingsmiddelen. [Google Scholar]
- 20.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller R.L., Scheidt S. History of drugs for thrombotic disease. Discovery, development, and directions for the future. Circulation. 1994;89(1):432–449. doi: 10.1161/01.cir.89.1.432. [DOI] [PubMed] [Google Scholar]
- 22.Hata J., Tanizaki Y., Kiyohara Y., et al. Ten year recurrence after first ever stroke in a Japanese community: the Hisayama study. J Neurol Neurosurg Psychiatry. 2005;76(3):368–372. doi: 10.1136/jnnp.2004.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaborators GBDS Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi Y., Furuta Y., Hata J., et al. Long-term trends in the 5-year risk of recurrent stroke over A half century in A Japanese community: the Hisayama study. J Atheroscler Thromb. 2022;29(12):1759. doi: 10.5551/jat.63344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isnard F., Termoz A., Haesebaert J., et al. Temporal trend of transient ischemic attack management over a 10-year period: data from the rhone county, France. Cerebrovasc Dis. 2022;51(4):517–524. doi: 10.1159/000520840. [DOI] [PubMed] [Google Scholar]
- 26.Leening M.J., Ferket B.S., Steyerberg E.W., et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. doi: 10.1136/bmj.g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters S.A.E., Colantonio L.D., Dai Y., et al. Trends in recurrent coronary heart disease after myocardial infarction among US women and men between 2008 and 2017. Circulation. 2021;143(7):650–660. doi: 10.1161/CIRCULATIONAHA.120.047065. [DOI] [PubMed] [Google Scholar]
- 28.Heshmatollah A., Mutlu U., Rojas-Saunero L.P., et al. Unspecified strokes: time trends, determinants, and long-term prognosis in the general population. Neuroepidemiology. 2020;54(4):334–342. doi: 10.1159/000506130. [DOI] [PubMed] [Google Scholar]
- 29.George M.G. Risk factors for ischemic stroke in younger adults: a focused update. Stroke. 2020;51(3):729–735. doi: 10.1161/STROKEAHA.119.024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutten-Jacobs L.C., Arntz R.M., Maaijwee N.A., et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309(11):1136–1144. doi: 10.1001/jama.2013.842. [DOI] [PubMed] [Google Scholar]
- 31.Feinstein M., Ning H., Kang J., Bertoni A., Carnethon M., Lloyd-Jones D.M. Racial differences in risks for first cardiovascular events and noncardiovascular death: the atherosclerosis risk in communities study, the cardiovascular health study, and the multi-ethnic study of atherosclerosis. Circulation. 2012;126(1):50–59. doi: 10.1161/CIRCULATIONAHA.111.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.