Abstract
Background
Post–kala-azar dermal leishmaniasis (PKDL) is a dermal complication of visceral leishmaniasis. Oral miltefosine (MF) is the first-line treatment for PKDL patients in South Asia. This study assessed the safety and effectiveness of MF therapy after 12 months of follow-up to explore more precise data.
Methods
In this observational study, 300 confirmed PKDL patients were enrolled. MF with the usual dose was administered to all patients for 12 weeks and followed up for 1 year. Clinical evolution was recorded systematically by photographs at screening and at 12 weeks, 6 months, and 12 months after treatment onset. Definitive cure consisted of disappearance of skin lesions with a negative PCR at 12 weeks or with >70% of lesions, disappearing or fading at 12-month follow-up. Patients with reappearing clinical features and any positive diagnostics of PKDL during the follow-up were considered as nonresponsive.
Results
Among 300 patients, 286 (95.3%) completed 12 weeks of treatment. The per-protocol cure rate at 12 months was 97%, but 7 patients relapsed and 51 (17%) were lost to 12-month follow-up, resulting in a final cure rate of only 76%. Eye-related adverse events were noted in 11 (3.7%) patients and resolved in most (72.7%) within 12 months. Unfortunately, 3 patients had persistent partial vision loss. Mild to moderate gastrointestinal side effects were seen in 28% patients.
Conclusions
Moderate effectiveness of MF was observed in the present study. A significant number of patients developed ocular complications, and thus MF for treatment for PKDL should be suspended and replaced with a safer alternative regimen.
Keywords: amphotericin B deoxycholate, miltefosine, post–kala-azar dermal leishmaniasis, visceral leishmaniasis
Oral miltefosine is the first-line treatment for post–kala-azar dermal leishmaniasis in South Asia. This study assessed safety and effectiveness of miltefosine. The cure rate was 76%; however, a significant number of patients developed ocular complications.
Post–kala-azar dermal leishmaniasis (PKDL) is a dermal complication of visceral leishmaniasis (VL), which is caused by the Leishmania donovani parasite and transmitted by phlebotomine sandflies [1]. The prevalence and severity of PKDL vary in several aspects between geographical regions. In the Indian subcontinent, only 2%–17% of treated VL patients develop PKDL. It is generally reported between 6 months to 3 years after cured VL and presents with hypopigmented macules, papules, or nodules on the face and other body parts. Macular lesions are most common, followed by polymorphic lesions [2]. Around 20% of patients manifest with more severe papular or nodular skin lesions, and spontaneous cure does not occur [1]. Considering the fact that the parasites within PKDL lesions are an important reservoir for the transmission of infection that sustains VL [3, 4], it is of paramount importance for public health reasons to achieve control of VL by eliminating these PKDL reservoirs with early detection and treatment to prevent future VL epidemic resurgence. Traditionally Indian PKDL was treated with pentavalent antimonials for up to 120 days or amphotericin B deoxycholate for up to 60 infusions [5]. These long and arduous regimens resulted in poor compliance and incomplete treatment. Furthermore, the amphotericin B regimen required hospitalization of patients for 3–4 months. Drug-induced serious adverse reactions were common [2].
Miltefosine (MF; hexadecylphosphocholine), the phospholipid derivative initially developed as an anti-cancer drug, is the leading orally administrable anti-leishmanial drug available for the treatment of VL, first licensed in India in 2002 [6]. It was initially introduced as the drug for VL elimination program in India; however, due to safety concerns and its teratogenic potential, it was replaced by single-dose liposomal amphotericin B (LAmB) as the treatment of choice in 2013 [7]. MF was used for the treatment of PKDL for the first time in 2006 [8]. Besides this, moderate efficacy has also been reported for VL in East Africa [9]. Currently, MF for 12 weeks, in the usual daily doses, is the recommended first-line treatment for PKDL in India, Nepal, and Bangladesh [10]. The major side effects are related to the gastrointestinal tract. Some recent reports suggest a substantial increase in relapse rate by up to 15% in MF monotherapy [11]. However, standard treatment and duration for Indian PKDL are needed to achieve more therapeutic effectiveness and safety. Therefore, our objective was to evaluate the effectiveness and safety of this 12-week-long MF treatment of PKDL after 12 months of follow-up, employing the currently recommended national treatment protocol, and routine outcomes from patients being treated with the approved regimen, with more in-depth safety follow-up and analysis.
PATIENTS AND METHODS
Study Design and Duration
This was an observational study conducted at the Kala-Azar Medical Research Center, Muzaffarpur, the field site of the Institute of Medical Sciences, Banaras Hindu University. Patients with PKDL were recruited from June 2016 to September 2021 and followed up for 1 year.
Methodology
Patients with suspected PKDL having skin lesions were examined and investigated for diagnosis. A detailed medical history and demography were noted. All patients were tested for antibodies against rK39 antigen using an immunochromatographic rapid strip test (InBios, Seattle, Washington). Slit-skin smear was done to demonstrate amastigotes of Leishmania infection. Polymerase chain reaction (PCR) with skin tissues and blood was carried out in patients with negative skin smears to confirm the diagnosis. DNA isolation from skin and blood was performed using QIAamp tissue and blood DNA extraction Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The parasite detection by PCR was done using L donovani species-specific primers, and the methodology was performed as described elsewhere [12]. Moreover, along with clinical assessment, relevant investigations were performed to evaluate treatment efficacy and safety at baseline and follow-up. Parasitological examination was done at the end of treatment. The sample size for evaluating the anticipated cure rate of 95% was computed at 5% level of significance with an absolute precision of 2.5%; 292 subjects were needed [13] and a sample size of 300 was selected.
Inclusion and Exclusion Criteria
All confirmed PKDL patients of either sex and any age, with or without a history of VL, were included in the study. Informed written consent was taken from each adult participant and parents of patients aged <18 years. The following patients were excluded from the study: (1) those with anemia (hemoglobin <5.0 g/dL), leukopenia (total leukocyte count <2.5 × 109/L), or thrombocytopenia (platelet count <50 × 109/L); (2) those with hepatic dysfunction (serum bilirubin ≥2 times the upper limit of normal [ULN], liver enzymes ≥3 times the ULN, or chronic liver disease; (3) those with renal dysfunction (serum creatinine ≥1.5 times the ULN); (4) those with cardiac dysfunction (heart failure, past or recent myocardial infarction, or cardiomyopathy); (5) those with evidence of any active tuberculosis or major infections, or history of any anti-leishmanial drug within the last 12 weeks; (6) those with any immunocompromised state (eg, human immunodeficiency virus, solid or blood malignancy, organ transplant recipients); (7) pregnant women, lactating mothers, or women refusing contraception for the duration of treatment and 3 months after the end of treatment; and (8) any participant refusing consent.
Main and Alternative Treatment
As per hospital policy and government recommendations, oral MF was given to patients after meals with a target dose of 2.5 mg/kg/day for children aged 2–11 years; 50 mg/day for patients aged ≥12 years and >25 kg body weight; and 100 mg/day (in divided doses) for patients aged ≥12 years and >25 kg body weight. One patient received MF 150 mg/day as per instruction of the Control Program [14]; subsequently, this policy was withdrawn.
Most patients of childbearing potential readily accepted to practice contraception for the desired period. Contraception in women was ensured by using injectable long-acting contraceptives.
MF was administered after meals for 12 weeks. As most gastrointestinal adverse events (AEs) due to MF commonly occur at the beginning of the treatment, patients were hospitalized for the first 4 weeks for close observation and AEs were monitored. Patients were then discharged on medication for the rest of the treatment period. After discharge from the hospital, they were assessed at every 2 weeks for consumption of MF pills by return of empty strips for drug compliance. The number of pills taken was calculated by subtracting the count of the number of pills remaining from the total number of pills dispensed. Patients were monitored closely for AEs by telephonic contacts supplemented by visits to their residences. For AEs, the Common Toxicity Criteria of the National Cancer Institute were applied [15]. In patients with toxicity of grade ≥3, the treatment was discontinued, and alternative treatment was offered to these patients. The alternative treatment was amphotericin B deoxycholate with 1 mg/kg for 3 courses of 20 days, each with a drug-free interval of 20 days between the treatment courses [5].
Case Definitions
During follow-up, all patients were examined clinically and investigated accordingly for any AEs and changes in lesions or emergence of new lesions from day 1 of the study till a follow-up of 1 year. Clinical evolution was recorded systematically by the investigators via comparison of photographs, captured at standardized conditions (light, distance, exposure, etc) at screening and at 12 weeks, 6 months, and 12 months after treatment onset. Characterization of skin lesions as macular, nodular, and polymorphic was based on physical examination by the investigators.
Patients were considered to be cured if skin lesions disappeared completely with flattening of nodules and disappearance of macules with a negative PCR at 12 weeks after the onset of the treatment or if there was an improvement, with >70% of lesions disappearing or fading at the end of the 12-month follow-up. Patients were labeled nonresponsive if there were persistent signs and symptoms or the appearance of new lesions at 30 days of MF therapy. Relapse was defined as a patient with an initial cure but reappearing clinical features and any positive diagnostics of PKDL during the follow-up period [11]. In nonresponsive patients, patients with AEs requiring discontinuation of MF, or relapsed patients, alternative amphotericin B deoxycholate treatment was offered to them. Analysis of final cure was calculated using intention-to-treat (ITT) analysis considering all enrolled participants in the analysis, whether they dropped out or not. Conversely, in a per-protocol analysis, only those patients who strictly adhered to the study protocol were included.
Statistical Analysis
The statistical analysis was performed using SPSS software, version 23.0 (IBM Corporation Armonk, New York). Simple descriptive statistics were used. Mean ± standard deviation was used to summarize quantitative variables, and frequency (with percentage distribution) for categorized variables. The statistical analysis was carried out for various categorical parameters using the χ2 test and Fisher exact test. Student t test and Mann-Whitney U test were performed to compare 2 groups of mean or median. For paired samples, Student paired t test was used. P value <.05 is considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
A total of 300 patients with confirmed PKDL were included in this study. Patients were treated with MF and followed up for 1 year. One hundred sixty-eight (56%) patients were male. Skin lesions were characterized as macular (212 [71%] patients), nodular (38 [13%]), or polymorphic (50 [17%]) (Table 1).
Table 1.
Clinical and Laboratory Data at Baseline and Follow-up
| Characteristic | Baseline | EOT | At 12-mo Follow-up | Baseline vs EOT, P Value | Baseline vs 12 mo, P Value |
|---|---|---|---|---|---|
| Age, y | 28.03 ± 17.66 | … | … | … | … |
| Male sex, No. (%) | 168 (56) | … | … | … | … |
| Type of lesions, No. (%) | |||||
| Macular | 212 (70.7) | … | … | … | … |
| Nodular | 38 (12.7) | … | … | … | … |
| Polymorphic | 50 (16.6) | … | … | … | … |
| Weight, kg | 45.85 ± 15.86 | 45.73 ± 15.51 | 48.01 ± 15.61 | 0.274 | <0.001 |
| BUN, mg/dL | 8.63 ± 2.81 | 9.42 ± 2.34 | 9.35 ± 1.82 | <0.001 | 0.002 |
| Hb, g/dL | 12.65 ± 1.90 | 12.72 ± 1.64 | 13.13 ± 1.16 | 0.462 | <0.001 |
| WBC count, ×109 cells/L | 8.7 ± 2.9 | 9.3 ± 2.82 | 8.7 ± 1.7 | <0.001 | 0.211 |
| Creatinine, mg/dL | 0.82 ± 0.20 | 0.88 ± 0.21 | 0.87 ± 0.59 | <0.001 | 0.163 |
| SGPT, IU/L | 30.53 ± 16.07 | 30.78 ± 18.52 | 27.94 ± 10.11 | 0.829 | 0.008 |
Data are presented as mean ± standard deviation unless otherwise indicated.
Abbreviations: BUN, blood urea nitrogen; EOT, end of treatment (12 weeks); Hb, hemoglobin; SGPT, serum glutamic pyruvic transaminase; WBC, white blood cell.
Laboratory Characteristics
Among diagnostics, anti-rK-39 antibody was positive in 296 (98.7%) patients. On microscopy, 33 (11%) patients were skin smear positive. In smear-negative patients, PCR was done in skin and blood, with positive results in 207 (69%) and 179 (59.7%) patients, respectively. Laboratory parameters of all patients at baseline and follow-up are summarized in Table 1.
Drug Safety and Efficacy Data
In total, 91 of 300 (30.3%) patients experienced AEs. The most common AEs were gastrointestinal in 84 (28%), and these included vomiting in 66 (22%) patients, loose stool and abdominal discomfort in 13 (4.3%) patients each, loss of appetite in 6 (2.0%) patients, or a combination of these events. Eleven (3.7%) patients experienced eye-related AEs, among which blurred vision was most common (10 of 11 [91%]) patients. These complications were more frequent in females (7/11 [63.6%]). The mean duration of developing vision problems was 64 days from the start of the treatment (range, 13–97 days). In most patients (8/11 [72.7%]), the visual deficiencies resolved completely within 30–180 days, with a mean of 94 days. In the remaining 3 patients, 2 recovered partially and 1 had partial vision loss in both eyes. MF treatment was stopped in 7 patients, and the remaining 4 had completed 12 weeks of treatment before developing the visual problem. In August 2018, before the MF ocular toxicity was known, 1 patient, after complete recovery of vision loss, was rechallenged with MF treatment, leading to the recurrence of vision loss. Stoppage of the drug resulted in vision recovery. Other AEs were noted in 26 of the 300 (8.7%) patients (Table 2).
Table 2.
Adverse Events (N = 300)
| Adverse Event | No. (%) |
|---|---|
| Eye related | 11 (3.7) |
| Blurred vision | 8 (2.7) |
| Red eye | 2 (0.66) |
| Eye pain | 2 (0.66) |
| Watery eye | 1 (0.33) |
| Gastrointestinal related | 84 (28) |
| Nausea/vomiting | 66 (22) |
| Loose stool | 13 (4.3) |
| Abdominal discomfort | 13 (4.3) |
| Loss of appetite | 6 (2) |
| Other | 26 (8.7) |
| Body pain | 10 (3.3) |
| Weakness | 7 (2.3) |
| Giddiness | 4 (1.3) |
| Headache | 3 (1) |
| Abnormal behavior | 2 (0.66) |
| Burning sensation of body | 2 (0.66) |
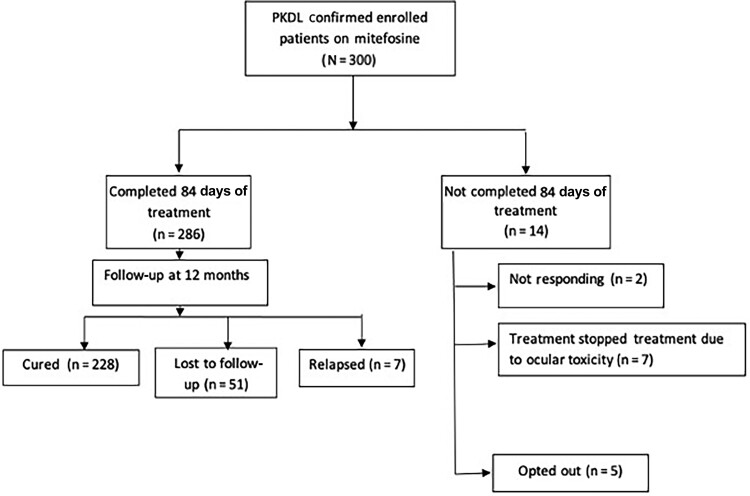
Among the 300 patients, 286 (95.3%) completed 84 days of MF treatment. Of the remaining 14 (4.7%) patients, 9 were switched to amphotericin B deoxycholate treatment due to 2 patients (0.7%) not responding to MF and 7 (2.3%) for ocular toxicity of MF. By per-protocol calculations, the cure rate at 12 months of follow-up was 97% (228/235 patients; 95% CI, .94–.99). Relapse was reported in 7 (2.9%) patients and 51 (17%) were lost to 12-month follow-up (Figure 1). Thus, by ITT analysis, the cure rate was only 76% (228/300 patients; 95% CI, .71–.81).
Figure 1.
Treatment of post–kala-azar dermal leishmaniasis (PKDL) with miltefosine. Study flowchart, from patient enrollment to final 12-month follow-up.
DISCUSSION
PKDL is predominantly reported from East Africa and the Indian subcontinent. In India, the endemic areas are Bihar, Jharkhand, West Bengal, Assam, and Uttar Pradesh. PKDL cures spontaneously in the majority of patients in Sudan but rarely in India [2]. In the present study, there was no history of past VL in 19 of 300 (6.33%) patients with PKDL, which is similar to earlier reports [16].
It is well recognized that PKDL patients hold grave significance for the possibility of setting off a new epidemic of VL. In the absence of physical disability, PKDL patients are reluctant to seek treatment, despite having the condition for years. They want treatment only for cosmetic reasons. They continue to harbor the parasites and are infectious for a long duration [17]. There is evidence from several old and recent publications in which patients with PKDL have been identified as the origin of the resurgence of VL [18, 19]. In recent xenodiagnosis studies from India [4] and Bangladesh [20], large proportions of PKDL patients (54%–80%) transmitted L donovani infection to sandflies. These findings underscore the significance of early diagnosis and effective treatment in obliterating these infection reservoirs [17].
There was an immediate need to find a safer regimen for treating PKDL compared with existing toxic, expensive, drawn-out sodium stibogluconate or amphotericin B deoxycholate [5]. For the treatment of PKDL, 12 weeks of MF, recommended by the World Health Organization, was based on only 1 multicenter study, on the evidence generated from a small sample size (18 patients treated at 3 centers). In that study, patients with macular lesions, who constitute the majority of patients with PKDL, were excluded [21]. In the current study, a moderate efficacy of MF (76%) was observed with ITT calculations as a significant proportion (17%) of patients were lost to 12-month follow-up (Figure 1).
Miltefosine Monotherapy
In recent years, a greater focus on PKDL has led to many drug trials to find safe and short-duration treatments for PKDL in South Asia. Ramesh et al studied oral MF on 26 PKDL patients in doses of 50 mg thrice daily for 60–90 days with a 1-year follow-up. They concluded initially that there was no relapse, and good safety and high efficacy, with an initial and final (12-month follow-up) cure rate of 96% (95% CI, 79%–99%) [22]. However, a few years later, in another article by the same group, which included all patients reported in their earlier 2011 publication [22], a decline in the efficacy of MF for the treatment of PKDL was reported with a relapse rate of 15% (11/73) [11].
In another randomized trial from India, 100 PKDL patients were grouped into 2 arms, either to receive LAmB in a total dose of 30 mg/kg administered over 3 weeks or MF 2.5 mg/kg or 100 mg/day for 12 weeks. After 12 weeks of treatment, MF was found to be more efficacious (cure rate, 86.9%) than LAmB (cure rate, 74.5%) [23].
A single-arm open-label trial of MF on 27 PKDL patients with a treatment duration of 12 weeks versus 16 weeks showed efficacy of 57% and 100%, respectively. The study concluded that a 16-week course of MF may be reliable in curing PKDL; no ophthalmic toxicity was reported, possibly due to the small sample size [24].
LAmB Monotherapy
In patients with PKDL, LAmB, in a total cumulative dose of 30 mg/kg over 3 weeks, led to serious hypokalemia and rhabdomyolysis in a significant number of patients in Bangladesh [25].
Another study from Bangladesh included 280 PKDL patients and treated them with LAmB (15 mg/kg), given in 5 biweekly infusions of 3 mg/kg. It showed that this short-course LAmB regimen was safe and effective, and a complete or major improvement of lesions was seen in 245 patients (89.7%); 213 (78.0%) were considered completely cured for PKDL [26].
Multidrug Therapy
In a small comparative study from India, 16 PKDL patients each were treated either with MF (100 mg/day for 90 days) or MF (100 mg/day for 45 days) and LAmB (3 infusions of 5 mg/kg). Patients on combination therapy achieved 100% cure, with no relapse [27].
In another, just completed, multicenter trial from India and Bangladesh, a combination of LAmB (20 mg/kg) and MF (duration of 3 weeks) has been compared with LAmB alone (20 mg/kg over 15 days), and final results are likely to be published soon [28]. Results of this study might provide insight into an alternative safe and effective treatment for PKDL.
In the present study, safety was a matter of great concern; 11 of the 300 patients (3.7%) developed symptoms of ocular toxicity. Visual blurring and loss of vision were observed in these patients. After the stoppage of the drug, there was complete recovery of vision in all but 3 patients. Unfortunately, these 3 patients had persistent partial loss of vision. The mean duration of onset of ocular symptoms was 61 days after the start of MF treatment. Most (10/11 [91%]) patients developed ocular toxicity after 40–97 days after the start of treatment, barring 1 patient who developed eye symptoms only after 13 days of therapy; however, this patient received a higher daily dose (150 mg) [14]. Our first patient with MF ocular toxicity, after complete recovery of vision loss, was erroneously rechallenged as the causality between MF and ocular toxicity was not established then, and this led to the recurrence of vision loss. Fortunately, stoppage of the drug resulted in complete vision recovery. On reviewing the literature, ocular AEs related to MF were reported in 4 patients, 1 each from 4 treatment centers [29]. The ocular toxicity of MF is a serious issue. Though MF has been in use for the last 20 years and during this period several hundred thousand patients with VL have been treated with this drug, ocular toxicity has not been reported, but the duration of treatment was 28 days. MF treatment for a long duration appears to result in ocular toxicity in a significant proportion of patients, and it can occur even earlier with higher doses.
As expected with MF treatment, gastrointestinal AEs were the commonest AEs, but these were mild in most patients. Mild to moderate hepatic dysfunction was reported in 9% of patients. A mild increase in serum creatinine levels was also observed in 1% of patients. These AEs were mild and did not require any treatment. In the literature, there are reports of MF-related elevated serum aminotransferase and creatinine levels, which were mostly mild and reversible [30, 31].
Overall, the 12 weeks of treatment of oral MF was reasonably well tolerated, with the majority of AEs being gastrointestinal (eg, nausea, vomiting, and diarrhea). However, these symptoms were mild and subsided spontaneously or by the treatment of symptoms as described in previous studies [30, 31].
In conclusion, there is a need to seriously reconsider MF for the treatment of PKDL. Good results from LAmB monotherapy have been observed. Combination of 2 drugs with shorter treatment duration (eg, LAmB in combination with MF for 3–4 weeks) could be an alternative. Short duration of MF therapy should be safe while avoiding its serious toxicities, would improve compliance, and is likely to reduce the cost of treatment substantially. Until results of the clinical trials using such alternative ways of treatment of PKDL are available, use of the current MF regimen should be suspended and replaced with either LAmB monotherapy or a combination of LAmB and MF as soon as possible. Good treatment options for PKDL that are safer, shorter, and economical would prove important in the elimination initiative of VL/PKDL in the Indian subcontinent.
Contributor Information
Shyam Sundar, Department of General Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India; Department of Clinical Medicine, Kala-azar Medical Research Center, Muzaffarpur, India.
Jitendra Singh, Department of General Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
Anju Dinkar, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
Neha Agrawal, Department of Medicine, University of Florida, Jacksonville, Florida, USA.
Notes
Patient consent. The study was approved by the Ethics Committee of Kala-azar Medical Research Center. Informed and written consent was taken from each eligible patient or the patient’s parents if their age was <18 years.
References
- 1. Pijpers J, den Boer ML, Essink DR, Ritmeijer K. The safety and efficacy of miltefosine in the long-term treatment of post–kala-azar dermal leishmaniasis in South Asia—a review and meta-analysis. PLoS Negl Trop Dis 2019; 13:e0007173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundar S, et al. Leishmaniasis. In: Loscalzo J, Kasper DL, Longo DL, Fauci AS, Hauser SL, Jameson JL, eds. Harrison's principles of internal medicine. 21st ed. New York: McGraw-Hill, 2022:1741–48. [Google Scholar]
- 3. Ganguly S, Das NK, Barbhuiya JN, Chatterjee M. Post-kala-azar dermal leishmaniasis—an overview. Int J Dermatol 2010; 49:921–31. [DOI] [PubMed] [Google Scholar]
- 4. Singh OP, Tiwary P, Kushwaha AK, et al. Xenodiagnosis to evaluate the infectiousness of humans to sandflies in an area endemic for visceral leishmaniasis in Bihar, India: a transmission-dynamics study. Lancet Microbe 2021; 2:e23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thakur CP, Narain S, Kumar N, et al. Amphotericin B is superior to sodium antimony gluconate in the treatment of Indian post-kala-azar dermal leishmaniasis. Ann Trop Med Parasitol 1997; 91:611–16. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . New therapy for visceral leishmaniasis: India licenses miltefosine, the first oral drug for visceral leishmaniasis. Available at: https://www.who.int/publications/i/item/who-wer7725. Accessed 9 January 2022.
- 7. Directorate of National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare of India . National road map for kala-azar elimination August 2014. 2014. Available at: http://nvbdcp.gov.in/Doc/Road-map-KA_2014.pdf. Accessed 1 December 2022.
- 8. Sundar S, Kumar K, Chakravarty J, et al. Cure of antimony unresponsive Indian post kala-azar dermal leishmaniasis with oral miltefosine. Tran Roy Soc Trop Med Hyg 2006; 100:698–700. [DOI] [PubMed] [Google Scholar]
- 9. Monge-Maillo B, López-Vélez R. Miltefosine for visceral and cutaneous leishmaniasis: drug characteristics and evidence-based treatment recommendations. Clin Infect Dis 2015; 60:1398–404. [DOI] [PubMed] [Google Scholar]
- 10. National Vector Borne Disease Control Program, India . Guidelines for treatment of post-kala-azar dermal leishmaniasis (based on WHO Technical Report Series 949). Available at: https://ncvbdc.mohfw.gov.in/WriteReadData/l892s/PKDL-Guidelines-220512.pdf. Accessed 9 January 2022.
- 11. Ramesh V, Singh R, Avishek K, et al. Decline in clinical efficacy of oral miltefosine in treatment of post kala-azar dermal leishmaniasis (PKDL) in India. PLoS Negl Trop Dis 2015; 9:e0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurya R, Singh RK, Kumar B, Salotra P, Rai M, Sundar S. Evaluation of PCR for diagnosis of Indian kala-azar and assessment of cure. J Clin Microb 2005; 43:3038–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganapathy S, Deepthy MS, Mishra A. Contextual role of absolute and relative precision in estimation of sample size for single proportion in health research. Int J Health Sci Res 2023; 13:63–8. [Google Scholar]
- 14. Sharma MP; Vector Borne Disease Control Programme, Chief Malaria Office, Government of Bihar. Treatment guidelines for fresh VL, relapse VL and PKDL. Letter no. 184.2018.
- 15. National Cancer Institute, Division of Cancer Treatment and Diagnosis . Cancer therapy evaluation program. Common toxicity criteria. Version 2.0.1999. https://ctep.cancer.gov › docs › ctcv20_4-30-992. Accessed 23 December 2022.
- 16. Islam S, Kenah E, Bhuiyan MA, et al. Clinical and immunological aspects of post-kala-azar dermal leishmaniasis in Bangladesh. Am J Trop Med Hyg 2013; 89:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: a threat to the South-East Asia region kala-azar elimination programme. PLoS Negl Trop Dis 2017; 11:e0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addy M, Nandy A. Ten years of kala-azar in West Bengal, part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ 1992; 70:341–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Napier LE, Das Gupta CR. An epidemiological investigation of kala-azar in a rural area in Bengal. Indian J Med Res 1931; 19:295–341. [Google Scholar]
- 20. Mondal D, Bern C, Ghosh D, et al. Quantifying the infectiousness of post-kala-azar dermal leishmaniasis toward sand flies. Clin Infect Dis 2019; 69:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundar S, Sinha P, Jha TK, et al. Oral miltefosine for Indian post–kala-azar dermal leishmaniasis: a randomized trial. Trop Med Int Health 2013; 18:96–100. [DOI] [PubMed] [Google Scholar]
- 22. Ramesh V, Katara GK, Verma S, Salotra P. Miltefosine as an effective choice in the treatment of post-kala-azar dermal leishmaniasis. Br J Dermatol 2011; 165:411–4. [DOI] [PubMed] [Google Scholar]
- 23. Pandey K, Pal B, Siddiqui NA, et al. A randomized, open-label study to evaluate the efficacy and safety of liposomal amphotericin B (AmBisome) versus miltefosine in patients with post–kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol 2021; 87:34–41. [DOI] [PubMed] [Google Scholar]
- 24. Ghosh S, Das NK, Mukherjee S, et al. Inadequacy of 12-week miltefosine treatment for Indian post–kala-azar dermal leishmaniasis. Am J Trop Med Hyg 2015; 93:767–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marking U, den Boer M, Das AK, et al. Hypokalaemia-induced rhabdomyolysis after treatment of post-kala-azar dermal leishmaniasis (PKDL) with high-dose AmBisome in Bangladesh—a case report. PLoS Negl Trop Dis 2014; 8:e2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Boer M, Das AK, Akhter F, et al. Safety and effectiveness of short-course AmBisome in the treatment of post-kala-azar dermal leishmaniasis: a prospective cohort study in Bangladesh. Clin Infect Dis 2018; 67:667–75. [DOI] [PubMed] [Google Scholar]
- 27. Ramesh V, Dixit KK, Sharma N, Singh R, Salotra P. Assessing the efficacy and safety of liposomal amphotericin B and miltefosine in combination for treatment of post kala-azar dermal leishmaniasis. J Infect Dis 2020; 221:608–17. [DOI] [PubMed] [Google Scholar]
- 28. Sundar S, Pandey K, Mondal Det al. Efficacy and safety of short course combination regimens of liposomal amphotericin B and miltefosine for treatment of PKDL in the Indian subcontinent [Abstract O15-05]. In: 7th World Congress on Leishmaniasis, Cartagena, Colombia, 1–6 August 2022.
- 29. Saurabh S, Mahabir M. Adverse ocular events on miltefosine treatment for post-kala-azar dermal leishmaniasis in India. Trop Doct 2020; 50:37–42. [DOI] [PubMed] [Google Scholar]
- 30. Sundar S, Jha TK, Thakur CP, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med 2002; 347:1739–46. [DOI] [PubMed] [Google Scholar]
- 31. Bhattacharya SK, Sinha PK, Sundar S, et al. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J Infect Dis 2007; 196:591–8. [DOI] [PubMed] [Google Scholar]