Abstract
Coronavirus disease 2019 (COVID-19) has speedily increased mortality globally. Although they are risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), less is known about the common molecular mechanisms behind COVID-19, influenza virus A (IAV), and chronic obstructive pulmonary disease (COPD). This research used bioinformatics and systems biology to find possible medications for treating COVID-19, IAV, and COPD via identifying differentially expressed genes (DEGs) from gene expression datasets (GSE171110, GSE76925, GSE106986, and GSE185576). A total of 78 DEGs were subjected to functional enrichment, pathway analysis, protein-protein interaction (PPI) network construct, hub gene extraction, and other potentially relevant disorders. Then, DEGs were discovered in networks including transcription factor (TF)-gene connections, protein-drug interactions, and DEG-microRNA (miRNA) coregulatory networks by using NetworkAnalyst. The top 12 hub genes were MPO, MMP9, CD8A, HP, ELANE, CD5, CR2, PLA2G7, PIK3R1, SLAMF1, PEX3, and TNFRSF17. We found that 44 TFs-genes, as well as 118 miRNAs, are directly linked to hub genes. Additionally, we searched the Drug Signatures Database (DSigDB) and identified 10 drugs that could potentially treat COVID-19, IAV, and COPD. Therefore, we evaluated the top 12 hub genes that could be promising DEGs for targeted therapy for SARS-CoV-2 and identified several prospective medications that may benefit COPD patients with COVID-19 and IAV co-infection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10142-023-01091-3.
Keywords: COVID-19, Influenza viruses, COPD, Differentially expressed genes, Hub genes, Drug molecule
Introduction
The pathogen responsible for the emerging pandemic coronavirus disease 2019 (COVID-19) is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Wang et al. 2021). When a sick person coughs or sneezes, droplets and aerosols are released into the air, facilitating the fast dissemination of the SARS-CoV-2 virus (Klompas et al. 2020). The most common symptoms of COVID-19, which resemble seasonal influenza, are fever, cough, sore throat, headache, muscle discomfort, diarrhea, and dyspnea. Additionally, COVID-19 might cause respiratory distress. In numerous countries, COVID-19 and influenza virus co-infections have been reported (Dadashi et al. 2021; Cong et al. 2022; Pawlowski et al. 2022). This co-infection raises the likelihood of invasive mechanical ventilation and hospital death (Swets et al. 2022). The simultaneous emergence of COVID-19 and the influenza virus could pose a substantial risk to public health.
Long-term exposure to harmful particles, notably cigarette smoking, enhances the risk of developing chronic obstructive pulmonary disease (COPD) in those over 50 years (Vogelmeier et al. 2017). Pulmonary disease is characterized by inflammation of the airways, remodeling of the airways, and variable destruction of the alveoli (Singh et al. 2020). COPD patients typically experience dyspnea, coughing, sputum production, and rapid deterioration (exacerbations) of their illness, frequently prompted by respiratory infections. Additionally, COPD is characterized by a high incidence of comorbid conditions like diabetes and cardiovascular disorders, which is not strange in a community of elderly smokers (Singh et al. 2022a). In more severe cases, numerous potentially hazardous interrelationships between COVID-19 and influenza can lead to worse outcomes, such as pneumonia, systemic inflammation, or acute respiratory distress syndrome (ARDS). Many of these potentially hazardous interrelationships also exist among COPD patients.
As a result, it was crucial to comprehend how COVID-19 and influenza co-infection affects COPD and discover potential effective drugs for COPD patients with COVID-19 and influenza co-infection, aiming to decrease the risk of hospitalization or fatality in COPD patients with COVID-19 and influenza co-infection. This study used biological data and a bioinformatic method to examine possible molecular pathways and determine viable medicines for treating COVID-19, influenza co-infection, and COPD.
Methods
Genetic dataset acquisition
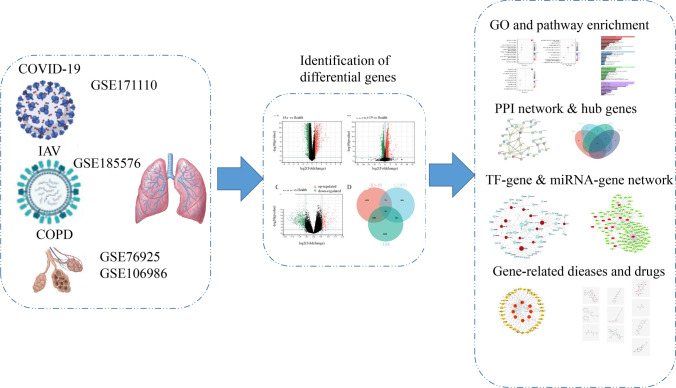
We used the Gene Expression Omnibus (GEO) database from the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) to investigate the genetic link between SARS-CoV-2, influenza virus A (IAV), and COPD. This database was available through the National Institutes of Health. We used a dataset relating to COVID-19 called GSE171110 (Levy et al. 2021), which includes blood RNA-seq data from 10 healthy people and 44 patients with COVID-19. GSE76925 (Morrow et al. 2017) and GSE106986 are COPD-related gene datasets. GSE76925 is based on GPL10558 platform, comparing 111 COPD cases and 40 control smokers. GSE106986 is based on GPL10558 platform and contains 19 samples, 5 health samples of non-smoking patients and 14 tumor-free lung tissue COPD samples (6 smokers and 8 ex-smokers). In order to remove the effect of smoking or not, we merge the two datasets. Together, they contain 210 COPD patients and 45 controls. GSE185576 contains12 normal people, 39 patients with respiratory tract bacterial infection, 56 samples of IAV infection, and 32 samples of mixed bacteria and virus infection. We selected 12 healthy persons and 56 persons with respiratory tract infection only infected with IAV for analysis.
Data aggregation and de-batching effects
To investigate the impacts of COPD in greater detail, we combined the datasets GSE76925 and GSE106986 to guarantee that smokers and non-smokers were included in the healthy control population. The datasets were merged using the R package inSilicoMerging (Taminau et al. 2012). We utilized Bioconductor’s “sva” tool to eliminate the batch effect and generate a single, unified GEO dataset (Chakraborty et al. 2012).
Identification of differentially expressed genes (DEGs)
In order to screen out DEGs, we utilized Rstudio software (version 4.1.2) in the “limma” to deal with the data, set the threshold to | log2FoldChange | > 0.585, and the false discover rate (FDR) was adjusted to < 0.05. We use Jveen, an online veen processing tool (http://jvenn.toulouse.inra.fr/app/example.html), to access common differential genes (Bardou et al. 2014).
Gene Ontology (GO) and pathway enrichment analysis
We employ Metascape (https://metascape.org/) (Zhou et al. 2019b), a combined web platform for functional enrichment, interactome analysis, gene annotation, and member search, to investigate the gene ontology associated with shared DEGs and pathway-enrichment analysis. GO analysis consists of three major sections: biological processes (BP), cellular components (CC), and molecular functions (MF). The route enrichment analysis revealed KEGG, WikiPathways, Reactome, and BioCarta as the most prominent resources. The P-value of 0.05 was chosen as the standard metric for quantifying the critical functional components and pathways.
Protein-protein interaction (PPI) network construct and analysis
The common DEGs of SARS-CoV-2, IAV, and COPD were analyzed by the PPI network utilizing STRING (https://string-db.org) in order to investigate the associated cellular activities and protein mechanisms. Moreover, to ensure the reliability of the PPI network, we set the minimum confidence score for network building at 0.40. Nodes that were disconnected were deleted from the network.
Hub gene extraction and submodule analysis
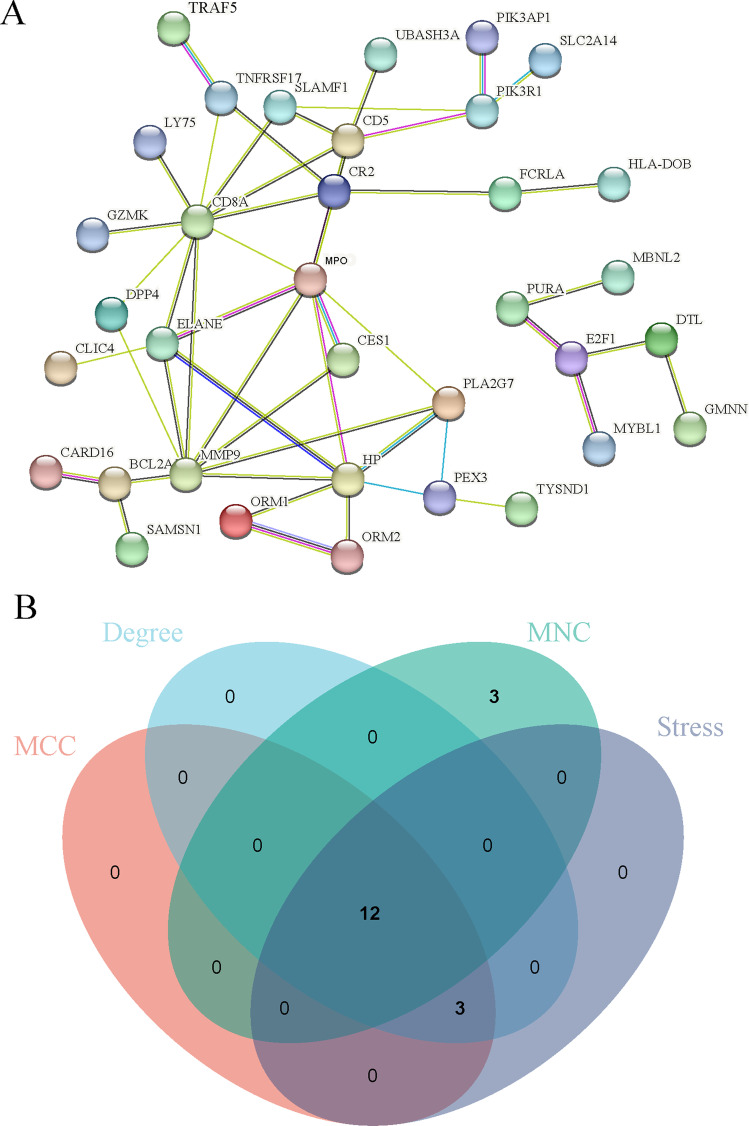
We imported the information from the String database into Cytoscape (version 3.9.1) to recreate the PPI network using this software. CytoHubba was invoked in Cytoscape to determine which genes are hubs in the PPI network. After that, a tally of the top 15 DEGs was performed using maximal clique centrality (MCC), stress, maximum neighborhood component (MNC), and node connect degree (degree) to determine the intersection of the various approaches.
Recognition of transcription factors and miRNAs in DEGs
The DEG-microRNA (miRNA)-interaction network and the DEG-transcription factor (TF)-interaction network were discovered by NetworkAnalyst (https://www.networkanalyst.ca/) (Xia et al. 2013; Xia et al. 2014; Xia et al. 2015; Zhou et al. 2019a). TF proteins can bind to specific DNA sequences and create DNA-protein complexes, thereby regulating the transcription of associated genes and influencing biological activity. We analyzed the transcription factor network of the common hub gene of COVID-19 and COPD using JASPAR (Khan et al. 2018) (http://jaspar.genereg.net). miRNAs are tiny single-stranded RNAs of 22 nucleotides in eukaryotes. miRNAs inhibit the expression of target genes by attaching to target mRNAs and degrading or inhibiting their translation. TarBase contains saved experimentally validated miRNA target gene data (Karagkouni et al. 2018). We analyzed the miRNAs related to the COVID-19, IAV, and COPD hub genes using TarBase, and we constructed the miRNA-gene network using Cytoscape.
Gene-targeted drug analysis
Under the disease/drug function in enrichr (https://maayanlab.cloud/Enrich/enrich), we utilized the Drug Signatures Database (DSigDB) to identify the medications that act on these targets. We employed a screening condition with a p-value of less than 0.5 to identify possible COVID-19, IAV, and COPD therapeutic targets.
Other gene-related diseases
The most comprehensive collection of publicly available genes and variations related to human disease can be found on the public data platform DisGeNET (Pinero et al. 2015; Pinero et al. 2017; Pinero et al. 2020). There are 1,134,942 gene-disease associations (GDAs) in the DisGeNET database. These relationships exist between 21,671 genes and 30,170 human illnesses, disorders, characteristics, and clinical or aberrant phenotypes. In addition, the database contains 369,554 variant-disease associations (VDAs). There are 194,515 variations associated with 14,155 illnesses, characteristics, and phenotypes. We explored the DisGeNET database for other illnesses to see whether COVID-19, IAV, and COPD share the same hub genes.
Results
Identification of DEGs common among COPD, IAV, and COVID-19
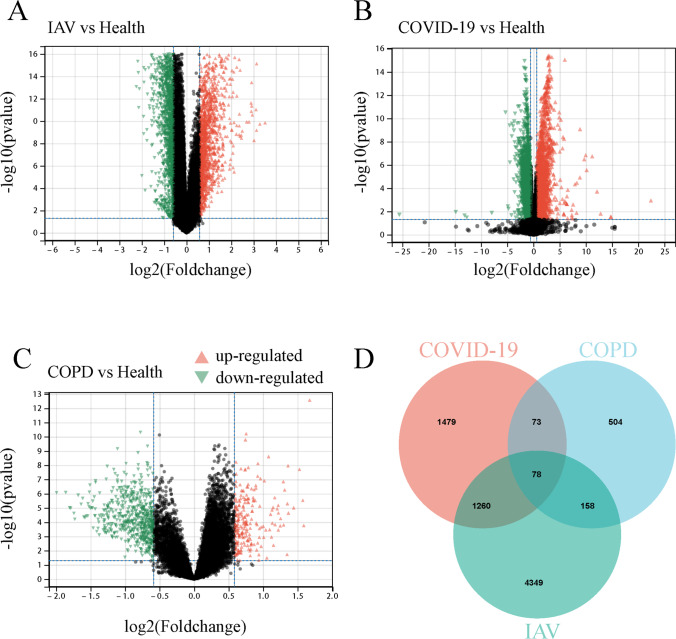
Figure 1 depicts our fundamental experimental procedure. NCBI RNA-seq and microarray datasets of human blood samples were used to investigate the relationship and impact of IAV and COVID-19 on COPD. Supplementary Fig. 1 depicts a box plot illustrating COPD gene expression before and after normalization. Our analysis revealed 5844 DEGs in the COVID-19 dataset, comprising 2716 up-regulated genes and 3128 down-regulated genes. Similarly, we identified 787 DEGs in the COPD sample, 210 up-regulated and 577 down-regulated. There were 3160 DEGs in the IAV dataset, with 1456 up-regulated and 1704 down-regulated genes. Sample information on the gene datasets is listed in Table 1. The DEGs for COPD, IAV, and COVID-19 are represented as volcano plots (Fig. 2A–C, Supplementary File S1). To evaluate the regulatory genes shared by the three illnesses, 78 DEGs were discovered using Jveen (Fig. 2D, Supplementary File S2).
Fig. 1.
A schematic overview of the study workflow
Table 1.
Information related to the dataset in this analysis and DEGs statistics
Fig. 2.
Visualization of the number of common differentially expressed genes (DEGs) among three diseases. A The volcano plot of differentially expressed genes in IAV datasets. B The volcano plot of differentially expressed genes in COVID-19 datasets. C The volcano plot of differentially expressed genes in COPD datasets. D Venn diagram showing the overlap of differentially expressed genes among COVID-19, IAV, and COPD
Analysis of GO and pathway enrichment
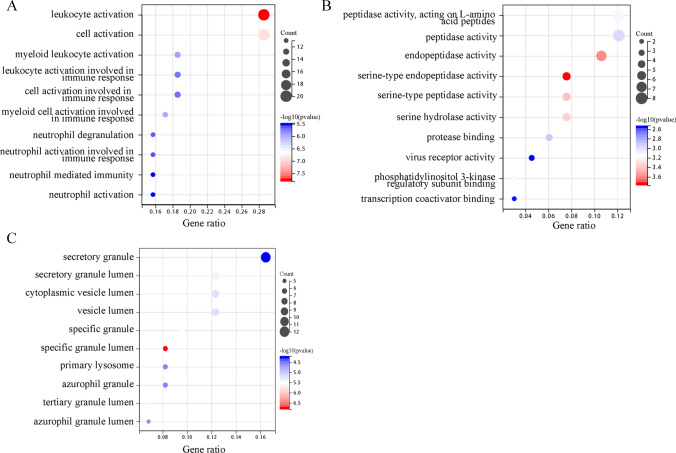
The most comprehensive and widely used repository of information about gene functions is the Gene Ontology Resource (GO; http://geneontology.org). Gene function is discussed in terms of its molecular aspect (the activity of the gene product at the molecular level), its cellular component (where the activity of the gene product is located concerning the biological structure), and its biological process (the molecular function of a more significant biological program gene is utilized) (The Gene Ontology 2019). Using the R package clusterProfiler (version 3.14.3) for GO enrichment analysis, we discovered that DEGs predominantly influence lymphocyte activation and cell activation in BP (Yu et al. 2012). DEGs were notably abundant in secretory granules in the category of CC. In contrast, DEGs were more active in regulating peptidase activity, acting on L-amino acid peptides, peptidase activity, and endopeptidase activity; bubble charts were used to illustrate these relationships in MF (Fig. 3, Supplementary File S3).
Fig. 3.
GO analysis bubble diagram of the common DEGs among COVID-19, IAV, and COPD. A Biological processes. B Molecular function. C Cellular component
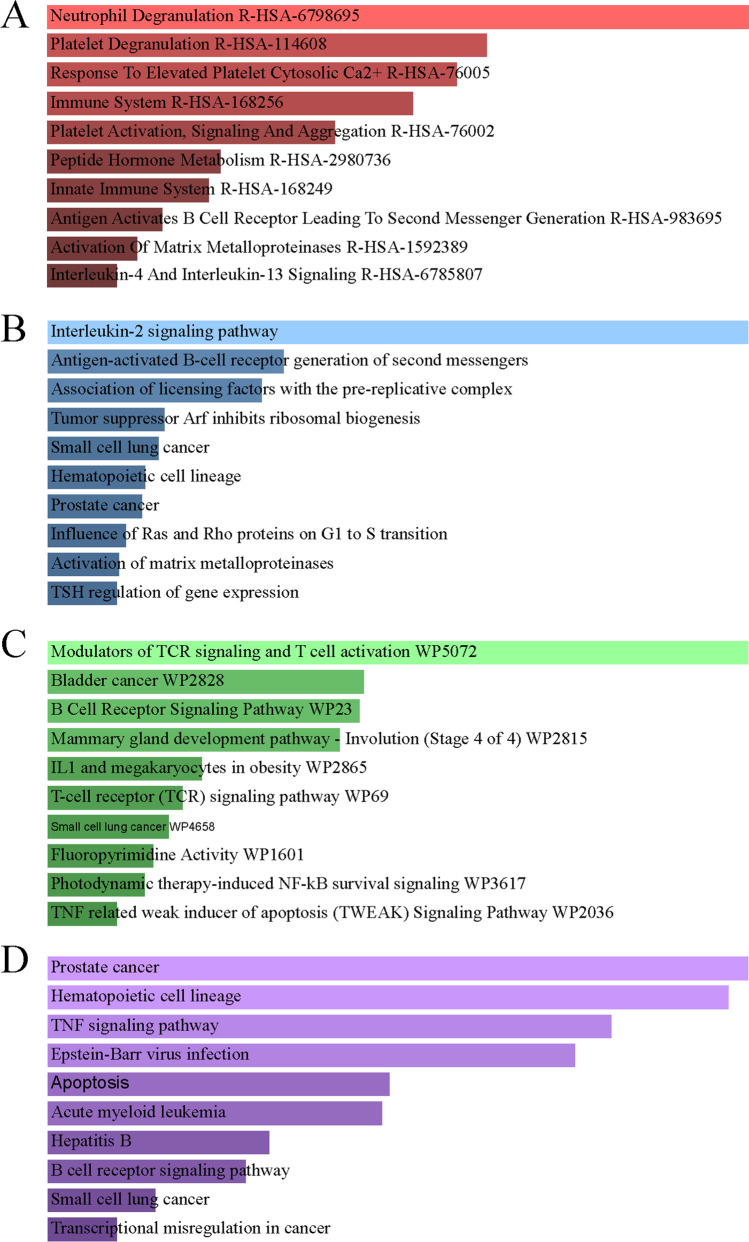
Through pathway analysis, researchers have established correlations between complex illness characteristics and their associated pathways and functions (Maghsoudi et al. 2022). From four databases, KEGG, WikiPathways, Reactome, and BioCarta, the most affected pathways of DEGs shared by IAV, COPD, and COVID-19 were studied and presented using bar graphs (Fig. 4, Supplementary File S4). Neutrophil degranulation, regulation of TCR signaling and T cells, activation of prostate cancer, blood cell lines, TNF signaling pathway, and interleukin-2 signaling pathway were the primary signaling pathways enriched by DEGs in the four datasets.
Fig. 4.
Pathway enrichment analysis of the common DEGs among COVID-19, IAV, and COPD. A Reactome. (B) KEGG 2021. C BioPlanet. D WikiPathway 2021
Construction of PPI network and extraction of hub genes
A detailed investigation of these shared DEGs between COVID-19, IAV, and COPD was performed to establish a PPI network using the String database. The network of PPI has 75 nodes and 51 edges (Fig. 5A). The data were imported into Cytoscape, and the Cytohubba plugin was used to calculate MCC, MNC, stress, and degree. The top 15 DEGs were picked by intersecting sets, and 12 DEGs were identified as hub genes (Fig. 5B, Supplementary File S5). MPO, MMP9, CD8A, HP, ELANE, CD5, CR2, PLA2G7, PIK3R1, SLAMF1, PEX3, and TNFRSF17 are these genes. They could represent biomarkers shared by all three disorders.
Fig. 5.
Protein-protein interaction (PPI) networks and hub genes for DEGs common to COVID-19, IAV, and COPD. A COVID-19, IAV, and COPD common DEGs in the PPI network. B The intersection of pivotal genes of different algorithms
Construction of TF-gene and miRNA-gene regulatory networks
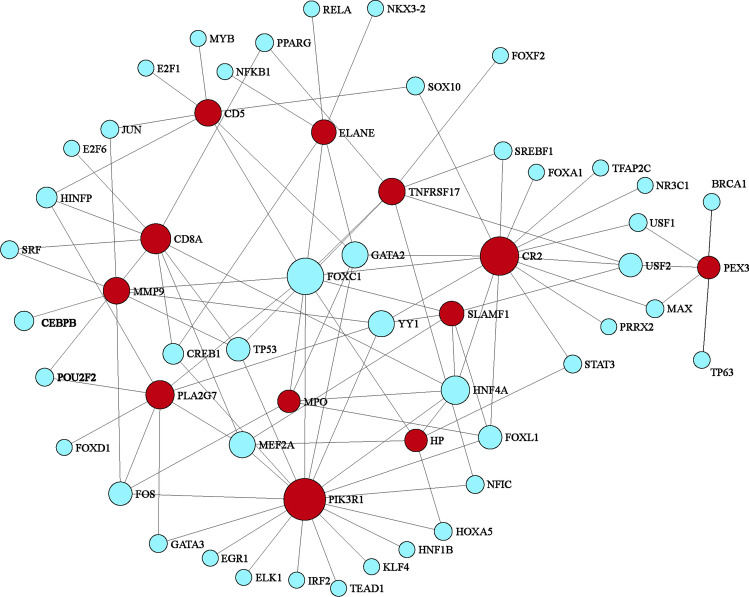
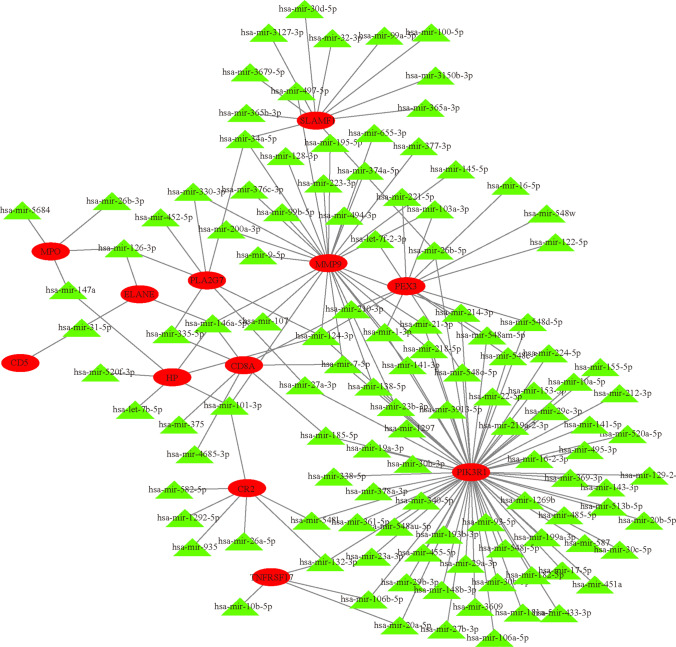
Transcription factors (TFs) and microRNAs (miRNAs) are the primary transcriptional control mechanisms. We utilized NetworkAnalyst to investigate the transcriptional regulatory effects of hub genes through TF-gene and miRNA-gene networks. Our analysis revealed that 44 transcription factors and 118 miRNAs are directly associated with these hub genes. The TF-gene network comprises 57 nodes and 98 edges (Fig. 6, Supplementary File S6). Ranking by node degree, RELA, NFKB1, HDAC1, TP53, BRCA1, E2F1, ESR1, ETS1, NR3C1, and RUNX1 are the top 10 TFs. A total of 130 nodes and 162 edges constitute the miRNA-gene network (Fig. 7, Supplementary File S7). The top 8 miRNA are hsa-mir-8485, hsa-mir-143-3p, hsa-mir-15b-5p, hsa-mir-21-5p, hsa-mir-29b-3p, hsa-mir-302a-5p, hsa-mir-335-5p, and hsa-mir-4789-3p.
Fig. 6.
Hub gene-transcription factor regulatory interaction network. Blue circles indicate transcription factors, and red circles are hub genes
Fig. 7.
Hub gene-miRNA regulatory interaction network. Green triangle nodes indicate miRNAs, and red ovals are hub genes
Screening of drug candidates
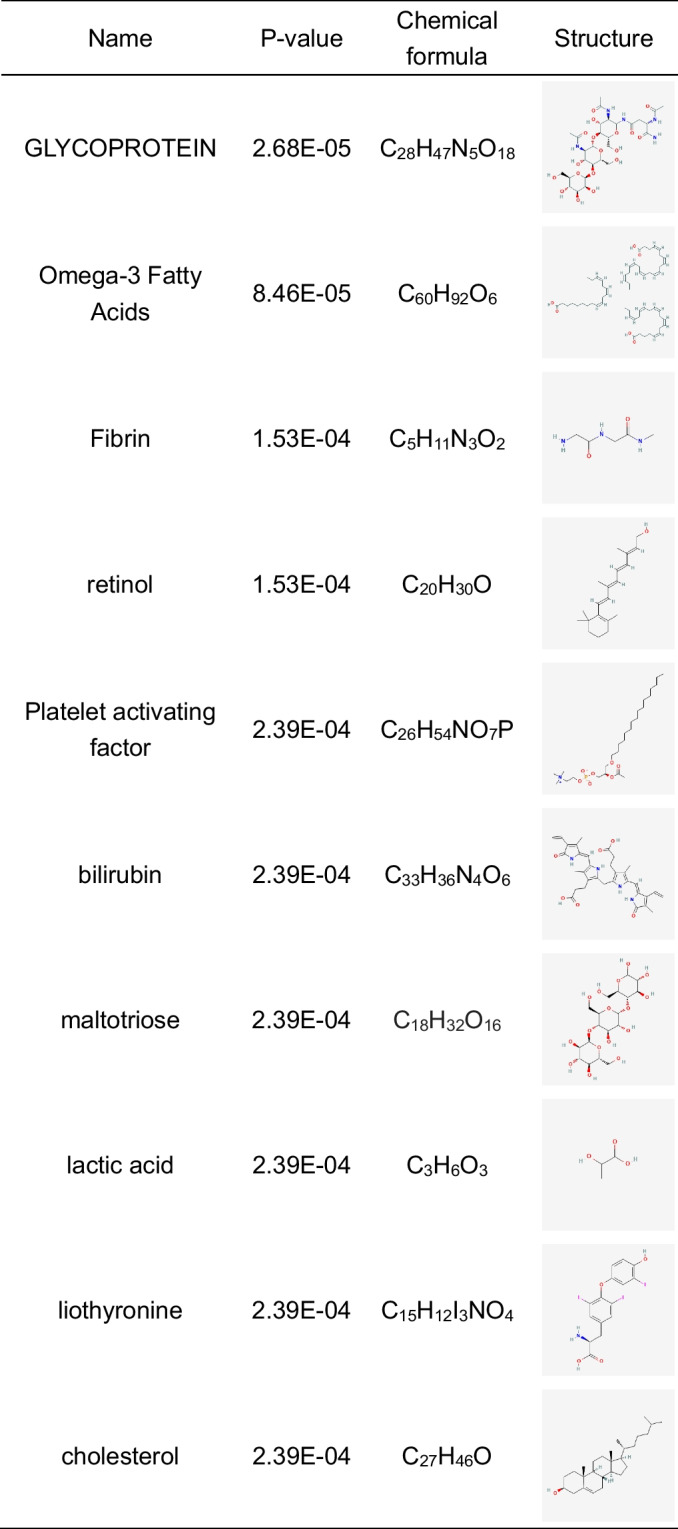
To find possible treatment agents for COVID-19, IAV, and COPD, we scoured the DSigDB database for potential pharmacological candidates. The PubChem database was searched to eliminate more hazardous chemicals from this molecular medication study. According to the data, the chemicals with the lowest P-values were glycoprotein, omega-3 fatty acids, fibrin, retinol, platelet-activating factor, bilirubin, maltotriose, lactic acid, liothyronine, and cholesterol (Table 2).
Table 2.
List of potential drugs for COVID-19, IAV, and COPD
Identification of other potentially relevant diseases
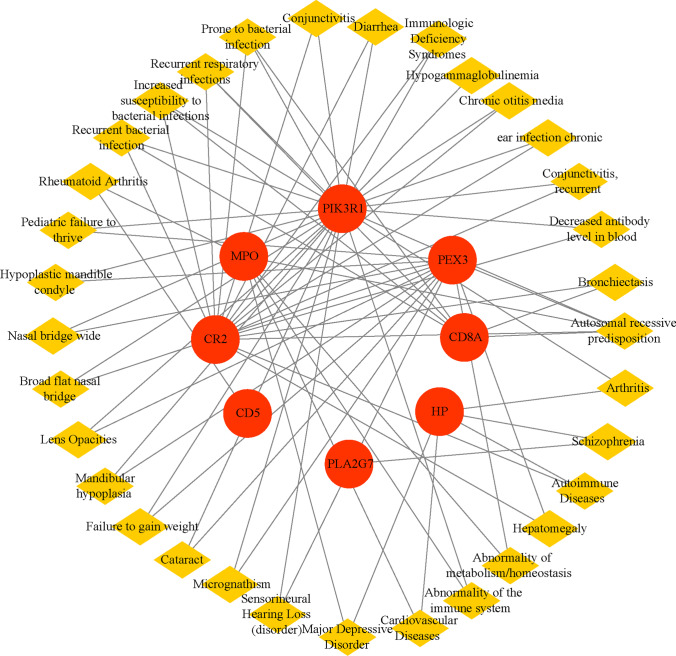
Utilizing the DisGeNET library in Enrichr, we screened for illnesses that shared hub genes (Fig. 8, Supplementary File S8). Autosomal recessive genetic susceptibility, recurrent bacterial infections, higher susceptibility to bacterial infections, recurrent respiratory infections, and susceptibility to bacterial infections were the top five disorders sorted by nodal degree.
Fig. 8.
Gene-disease association network. The yellow diamond node represents the disease, and the red circle node represents the gene
Discussion
SARS-CoV-2 co-infections with other respiratory viruses, including influenza virus, respiratory syncytial virus (RSV), and adenovirus, have been reported in several studies (Arguni et al. 2022). The lower influenza rates for the 2020–2021 flu season have been considered to be due to social distancing policies for SARS-CoV-2 (Dhanasekaran et al. 2022; Zheng et al. 2022). However, as COVID-19 pathogenicity decreased and transmission rates increased, more co-infection cases were observed during the Omicron era. In a recent report in Lancet, tests for respiratory viral co-infections were performed on 6965 COVID-19 patients. Viral co-infection was found in 583 (84%) patients (227 with influenza viruses, 220 with RSV, and 136 with adenoviruses) (Swets et al. 2022). Compared to SARS-CoV-2 mono-infection, co-infection with influenza viruses was linked to a severe chance of invasive mechanical ventilation. Mice co-infected with IAV showed higher COVID-19 virus load and more extensive lung injury in vivo (Bai et al. 2021). It was indicated that COVID-19 co-infection with influenza viruses had increased disease severity and deaths.
COPD is a complicated, accelerated lung disease that causes reduced airflow and difficulty breathing (Barnes et al. 2015b). The primary pathologic features of COPD are pulmonary emphysema caused by alveolar wall destruction and alveolar enlargement and obstructive bronchiolitis caused by the chronic inflammatory response of the peripheral airways and pulmonary parenchyma. Previous studies have found that influenza is a severe and frequent viral infection in COPD patients (Chen et al. 2021). Patients with COVID-19 had 5.9-fold increased risk of disease progression compared to non-COVID-19 patients (Wang et al. 2020). Mono-infection with COVID-19 or influenza increases the chances of acute respiratory failure and cardiovascular events (Barnes et al. 2015a). Therefore, COVID-19 co-infection with influenza virus was a risk factor for aggravation of COPD. In addition, clinical studies have found that nearly half of the recovered patients with COVID-19 have abnormal pulmonary function tests during the follow-up process. Among them, the proportion of those with abnormal diffusion capacity for carbon monoxide (DLCO) is more prominent. Clinicians should pay attention to the future progression of these patients to pulmonary fibrosis and obstructive pneumonia.
In this research, we established a network-based method to investigate RNA-seq data from patients with COVID-19, IAV, and COPD, and distinguished molecular targets that could serve as possible biomarkers for these diseases. In biomedical systems research, expression profiling analysis from high-throughput sequencing datasets has emerged as an essential source of biomarkers for discovering various diseases (Rahman et al. 2020; Mousavi et al. 2022; Liu et al. 2023). We investigated gene pool enrichment by observing the biological processes of cellular signaling pathways and GO to determine the relationship between IAV, COVID-19, and COPD from the standpoint of overlapping DEGs. Transcriptomics analysis revealed 78 shared DEGs with similar expression patterns in the three diseases, as performed by GO pathway analysis based on P-value. As a result, leukocyte activation (20 genes) and cell activation (20 genes) were essential GO terms in the biological process. Leukocyte activation and lymphocyte activation remarkably increased their expression in COPD or COVID-19 patients (Fu et al. 2021; Zhao et al. 2022). In thecellular component experiment, peptidase activity acting on L-amino acid peptides (8 genes) and endopeptidase activity (7 genes) were two major GO pathways. Many peptidases such as endopeptidases and L-amino acid peptides have immunological functions. For example, endopeptidases can be protective against COVID-19 or influenza infection (Tan et al. 2017; Abdel-Aziz et al. 2021). Meanwhile, the top GO term for the molecular function was secretory granule (12 genes). The secretion of leukocyte peptidase is the response of the organism to invasion by foreign pathogens, and peptidase may exert a bactericidal effect. Although peptidase secretion is necessary for normal physiological functioning, excessive release of peptidase into the airways can lead to detrimental effects such as restructuring of the airways and lung parenchyma, chronic inflammation, and impaired innate immunity (Niedzwiedzka-Rystwej et al. 2021; Voynow and Shinbashi 2021).
Neutrophil degranulation, TCR signaling and T cell regulation, blood cell lines, TNF signaling pathway, and interleukin-2 signaling pathway were the main DEG-enriched signaling pathways. TNF-α was positively associated with pulmonary fibrosis after COVID-19 infection. Additionally, IL-2 was identified as a potential biomarker after COVID-19 infection, although it was not found to be associated with pulmonary fibrosis (Maranatha et al. 2022; Pandey et al. 2022). Studies suggest that enhanced IL-2 production by T helper 1 (Th1) cells in COPD patients may increase inflammatory amplification in infection-induced COPD exacerbations and exacerbate chronic inflammation in stable COPD (Knobloch et al. 2016).
Neutrophils are innate immune cells that primarily work against infection by using phagocytosis, degranulation, the formation of neutrophil extracellular traps (NETs), and the release of cytokines (Hoenderdos et al. 2016). Neutrophils generate intracellular particles in a specified order, such as azurophilic, specific, gelatinase, and secretory (Borregaard et al. 2007; Legebeke et al. 2022). However, in COVID-19 and COPD patients, it has been found that excessive neutrophil degranulation is an important cause of tissue damage and inflammatory storm (Bankar et al. 2021; Lodge et al. 2022). Neutrophils can act as antigen-presenting cells (APCs), presenting influenza virus antigens to CD8+T cells (Borges et al. 2020). In addition, neutrophils can secrete TNF-α and be regulated by IL-2. Meanwhile, TNF-α plays a role in the infiltration, degranulation, and survival of neutrophils throughout inflammation and is a potent stimulator of neutrophil activity (Bawa et al. 2020). Therefore, we speculate that the excessive proliferation and activation of neutrophils are the key factors leading to COVID-19-combined IAV infection and the development of obstructive pneumonia after infection.
Hub genes can act as critical chemical targets or biological markers for COVID-19, IAV, and COPD, and are linked to various pathologies and biological mechanisms. A total of 12 hub-proteins (MPO, MMP9, CD8A, HP, ELANE, CD5, CR2, PLA2G7, PIK3R1, SLAMF1, PEX3, and TNFRSF17) are involved in these diseases. The myeloperoxidase (MPO) found in neutrophils has strong oxidative and inflammatory properties. It is crucial in the formation of NET through lipid peroxidation (Tokuhiro et al. 2021). MPO activation plays a critical role in the development of COPD, and inhibiting its action can slow down the progression of the disease and reduce inflammation (Singh et al. 2022b). Similarly, excessive neutrophil recruitment during influenza produces toxic enzymes such as MPO, exacerbating alveolar-capillary injury (Narasaraju et al. 2011). Additionally, matrix metalloproteinase 9 (MMP9) is a primary elastolytic enzyme generated from alveolar macrophages of COPD patients (Baker et al. 2022). Neutrophil elastase (ELANE) is a protease of serine liberated during the maturation of neutrophils and has been associated with the progression of COPD (Doherty et al. 2019; Chu et al. 2021). Thus, MPO, MPP9, and ELANE contribute to SARS-CoV-2 severity and cause ARDS among COVID-19 patients (Bhargava et al. 2020; Middleton et al. 2020; Ueland et al. 2020; Gueant et al. 2021; Petito et al. 2021). Also, phospholipase A2 group VII(PLA2G7) is a secretase that catalyzes the breakdown of platelet-activating factors into biologically non-active products and is mainly expressed by pulmonary pro-inflammatory macrophages that emerge with the development of SARS-CoV-2 (Zhu et al. 2021).
CD8+T cells are essential in controlling viral infection by killing virus-infected cells and contributing to protective immune responses against COVID-19 and influenza (Koutsakos et al. 2019; Rha and Shin 2021). Lung CD8+T lymphocytes are elevated in COPD patients and produce pro-inflammatory cytokines (Williams et al. 2021). However, CD5 is a surface receptor that inhibits the activation of T cells. T cells that express even more CD5 survive longer in the effector/memory phase after pathogenic insult (Matson et al. 2020). Moreover, complement receptor 2 (CR2) is developmentally restricted to late-pre and mature human B lymphocytes. B cell activation following C3d binding of antigen is associated with CR2, which stimulates the generation of specialized antibodies (Santiesteban-Lores et al. 2021). Furthermore, heparin (HP) binding with B cells is regulated by complement and CR2 interactions (Khandelwal et al. 2016). TNFRSF17 encodes a TNF receptor superfamily member that participates in the MAPK8/JNK and NF-κB pathways and facilitates the survival and growth of B cells to support humoral immune response (Gomez-Carballa et al. 2022). Therefore, these 12 hub genes could be regarded as prospective biomarkers or, if the biology of COVID-19 co-infection with IAV is proven, could be used as new pharmaceutical targets.
Subsequently, we analyzed the relationships between COVID-19, IAV, and COPD regarding TF-gene and miRNAs. The identified TFs such as RELA, NFKB1, HDAC1, TP53, BRCA1, E2F1, ESR1, ETS1, NR3C1, and RUNX1 are the top 10 TFs associated with these diseases. The specificity of transcription factors largely determines gene expression or transcription. miRNAs play a critical function in the gene modulation and silencing of RNA at the post-transcriptional level. In visualization of DEG-miRNAs, hsa-mir-15b-5p, hsa-mir-335-5p, hsa-mir-21-5p, hsa-mir-302a-5p, and hsa-mir-29b-3p are related to the pathogenesis of COVID-19 (Centa et al. 2020; Nersisyan et al. 2020; Teodori et al. 2020; Mukhopadhyay et al. 2021; Sato et al. 2021). In addition, the inhibition of hsa-miR-335p results in the activation of inflammation and angiogenesis-related pathways in patients with severe emphysema (Esquinas et al. 2017). Hsa-mir-15b-5p and hsa-mir-143-3p strongly correlated with monocyte chemoattractant protein-1 (MCP-1), IL-1β, IL-6, IL-15, and IL-17A of influenza viruses (Othumpangat et al. 2021).
Various chemicals and drugs are used as possible treatments for COVID-19, IAV, and COPD. For example, omega-3 fatty acids are correlated with a better prediction among COVID-19 patients by initiating an immune response against viral infection and promoting an inflammatory resolution to avoid tissue damage (Sun et al. 2022). Omega-3 fatty acids are thought to be anti-inflammatory and associated with IL-6 reduction in COPD patients (Yu et al. 2021). Vitamin A (retinol) is also required to convert naive T cells to regulatory T cells. It was suggested that retinol deficiency might contribute to the progression of severe COVID-19 (Sarohan et al. 2021; Sarohan et al. 2022). Retinol is also required for the integrity of the epithelium and is negatively linked to COPD (Caram et al. 2015). Conversely, platelet-activating factor (PAF) enhances leukocyte chemotaxis and is a potent inflammatory mediator, especially in bacterial or viral infection reactions. PAF may contribute to the morbidity and mortality linked to severe SARS-CoV-2 or IAV (Garcia et al. 2010; Klein et al. 2021).
Lactic acid is the major anaerobic metabolic byproduct. Serum lactic acid predicts the mortality of patients with acute respiratory failure with COVID-19 (Carpene et al. 2022). Lactic acid promotes IAV replication in human airway epithelium by impairing MAVS-dependent induction of type I IFN (Thyrsted et al. 2021). Furthermore, higher bilirubin or cholesterol levels increase the risk of developing COVID-19 (Paliogiannis and Zinellu 2020; Zinellu et al. 2021). Thyroid dysfunction could be linked to poorer COVID-19 outcomes (Duntas and Jonklaas 2021). Liothyronine is a synthetic form of the thyroid hormone triiodothyronine (T3) that may help COVID-19 patients recover (Pantos et al. 2020). Hence, these drugs can treat COPD patients with COVID-19 and IAV co-infection.
We used the gene-disease (GD) analysis to estimate the relationship between essential DEGs and various diseases in COVID-19, IAV, and COPD. According to the results, the top 5 diseases ranked according to node degree were autosomal recessive genetic susceptibility, recurrent bacterial infections, increased susceptibility to bacterial infections, recurrent respiratory infections, and susceptibility to bacterial infections. In COPD patients, viral and bacterial infections contribute to worsening lung function and, as a result, disease progression (D’Anna et al. 2021). Bacterial co-infections and subsequent bacterial infections are becoming more common in SARS-CoV-2 or IAV patients suffering from serious diseases (Morris et al. 2017; Cox et al. 2020; Vaillancourt and Jorth 2020).
The current study implemented a study framework to reveal interaction networks and treatment implications for COPD patients with COVID-19 co-infection with IAV based on thorough bioinformatic and systemic biological analysis. The present study did have certain shortcomings. Nevertheless, it is possible that the model size of some illness studies is insufficient to find these common DEGs by including all of the essential disease-related genes. Second, the conclusions may be less reliable and precise due to the limitations of the computational methodologies and the incompleteness of the available interactome data. The current findings were derived from various computational methods, and more in vivo or in vitro tests would be essential to evaluate the relevance of biological candidates thoroughly.
In this research, we analyzed DEGs to identify common DEGs and ascertain the illness reaction of COPD and COVID-19 co-infection with IAV based on transcriptome datasets. We get 78 general DEGs and identify the top 12 hub genes for further analysis. These hub genes retrieved indicated multiple signaling pathways and drug-target interactions. Future experimental and clinical studies should be conducted with predicted agents to explore pharmacological mechanisms and inform possible interventions for COVID-19, IAV, and COPD.
Supplementary information
(TIF 9008 kb)
(XLSX 4452 kb)
(CSV 79 kb)
(XLSX 109 kb)
(XLSX 99 kb)
(XLSX 13 kb)
(XLSX 11 kb)
(XLSX 15 kb)
(DOCX 12 kb)
(XLSX 16 kb)
Author contributions
KC and YG conceived and designed the study. XZ and ZL performed data analysis and data interpretation. ZL and KC conducted the bioinformatic and statistical analyses. ZL and YW plotted the figures. ZL and YG prepared the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kai Chu, Email: chukai19812007@163.com.
Yanan Gao, Email: gyn3737@gmail.com.
References
- Abdel-Aziz MI, Kermani NZ, Neerincx AH, Vijverberg SJH, Guo Y, Howarth P, Dahlen SE, Djukanovic R, Sterk PJ, Kraneveld AD, Maitland-van der Zee AH, Chung KF, Adcock IM, On behalf the UBC Association of endopeptidases, involved in SARS-CoV-2 infection, with microbial aggravation in sputum of severe asthma. Allergy. 2021;76:1917–1921. doi: 10.1111/all.14731. [DOI] [PubMed] [Google Scholar]
- Arguni E, Supriyati E, Hakim MS, Daniwijaya EW, Makrufardi F, Rahayu A, Rovik A, Saraswati U, Oktoviani FN, Prastiwi N, Nuryastuti T, Wibawa T, Haryana SM. Co-infection of SARS-CoV-2 with other viral respiratory pathogens in Yogyakarta, Indonesia: a cross-sectional study. Ann Med Surg (Lond) 2022;77:103676. doi: 10.1016/j.amsu.2022.103676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Zhao Y, Dong J, Liang S, Guo M, Liu X, Wang X, Huang Z, Sun X, Zhang Z, Dong L, Liu Q, Zheng Y, Niu D, Xiang M, Song K, Ye J, Zheng W, Tang Z, Tang M, Zhou Y, Shen C, Dai M, Zhou L, Chen Y, Yan H, Lan K, Xu K. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021;31:395–403. doi: 10.1038/s41422-021-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JR, Fenwick PS, Koss CK, Owles HB, Elkin SL, Fine J, Thomas M, El Kasmi KC, Barnes PJ, Donnelly LE (2022) IL-36 receptor agonist and antagonist imbalance drives neutrophilic inflammation in COPD. JCI Insight 7. 10.1172/jci.insight.155581 [DOI] [PMC free article] [PubMed]
- Bankar R, Suvarna K, Ghantasala S, Banerjee A, Biswas D, Choudhury M, Palanivel V, Salkar A, Verma A, Singh A, Mukherjee A, Pai MGJ, Roy J, Srivastava A, Badaya A, Agrawal S, Shrivastav O, Shastri J, Srivastava S. Proteomic investigation reveals dominant alterations of neutrophil degranulation and mRNA translation pathways in patients with COVID-19. iScience. 2021;24:102135. doi: 10.1016/j.isci.2021.102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101:1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, Wouters EF. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- Bawa KK, Krance SH, Herrmann N, Cogo-Moreira H, Ouk M, Yu D, Wu CY, Black SE, Lanctot KL, Swardfager W, Alzheimer's Disease Neuroimaging I. A peripheral neutrophil-related inflammatory factor predicts a decline in executive function in mild Alzheimer's disease. J Neuroinflammation. 2020;17:84. doi: 10.1186/s12974-020-01750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P, Panda P, Ostwal V, Ramaswamy A (2020) Repurposing valproate to prevent acute respiratory distress syndrome/acute lung injury in COVID-19: a review of immunomodulatory action. 3:S65-S70. DOI 10.4103/crst.Crst_156_20
- Borges L, Pithon-Curi TC, Curi R, Hatanaka E. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:8829674. doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, Sorensen OE, Theilgaard-Monch K (2007) Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28:340-345. 10.1016/j.it.2007.06.002 [DOI] [PubMed]
- Caram LM, Amaral RA, Ferrari R, Tanni SE, Correa CR, Paiva SA, Godoy I. Serum vitamin A and inflammatory markers in individuals with and without chronic obstructive pulmonary disease. Mediators Inflamm. 2015;2015:862086. doi: 10.1155/2015/862086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpene G, Onorato D, Nocini R, Fortunato G, Rizk JG, Henry BM, Lippi G. Blood lactate concentration in COVID-19: a systematic literature review. Clin Chem Lab Med. 2022;60:332–337. doi: 10.1515/cclm-2021-1115. [DOI] [PubMed] [Google Scholar]
- Centa A, Fonseca AS, Ferreira S, Azevedo MLV, Vaz de Paula CB, Nagashima S, Machado-Souza C, Miggiolaro A, Baena CP, de Noronha L, Cavalli LR. Deregulated miRNA expression is associated with endothelial dysfunction in post-mortem lung biopsies of COVID-19 patients. Am J Physiol Lung Cell Mol Physiol. 2020;320:L405–L412. doi: 10.1152/ajplung.00457.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Datta S, Datta S. Surrogate variable analysis using partial least squares (SVA-PLS) in gene expression studies. Bioinformatics. 2012;28:799–806. doi: 10.1093/bioinformatics/bts022. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lin CH, Hao WR, Yeh JS, Chiang KH, Fang YA, Chiu CC, Yang TY, Wu YW, Liu JC. Influenza vaccination and the risk of ventricular arrhythmias in patients with chronic obstructive pulmonary disease: a population-based longitudinal study. Front Cardiovasc Med. 2021;8:731844. doi: 10.3389/fcvm.2021.731844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Sun Z, Baek DS, Li W, Mellors JW, Shapiro SD, Dimitrov DS (2021) Human antibody domains and fragments targeting neutrophil elastase as candidate therapeutics for cancer and inflammation-related diseases. Int J Mol Sci 22. 10.3390/ijms222011136 [DOI] [PMC free article] [PubMed]
- Cong B, Deng S, Wang X, Li Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: a systematic review and meta-analysis. J Glob Health. 2022;12:05040. doi: 10.7189/jogh.12.05040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Loman N, Bogaert D, O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1:e11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna SE, Maniscalco M, Cappello F, Carone M, Motta A, Balbi B, Ricciardolo FLM, Caramori G, Stefano AD. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann Med. 2021;53:135–150. doi: 10.1080/07853890.2020.1831050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadashi M, Khaleghnejad S, Abedi Elkhichi P, Goudarzi M, Goudarzi H, Taghavi A, Vaezjalali M, Hajikhani B. COVID-19 and influenza co-infection: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:681469. doi: 10.3389/fmed.2021.681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran V, Sullivan S, Edwards KM, Xie R, Khvorov A, Valkenburg SA, Cowling BJ, Barr IG. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat Commun. 2022;13:1721. doi: 10.1038/s41467-022-29402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty DF, Nath S, Poon J, Foronjy RF, Ohlmeyer M, Dabo AJ, Salathe M, Birrell M, Belvisi M, Baumlin N, Kim MD, Weldon S, Taggart C, Geraghty P. Protein phosphatase 2A reduces cigarette smoke-induced cathepsin S and loss of lung function. Am J Respir Crit Care Med. 2019;200:51–62. doi: 10.1164/rccm.201808-1518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntas LH, Jonklaas J. COVID-19 and thyroid diseases: a bidirectional impact. J Endocr Soc. 2021;5:bvab076. doi: 10.1210/jendso/bvab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquinas C, Janciauskiene S, Gonzalo R, Mas de Xaxars G, Olejnicka B, Belmonte I, Barrecheguren M, Rodriguez E, Nunez A, Rodriguez-Frias F, Miravitlles M. Gene and miRNA expression profiles in PBMCs from patients with severe and mild emphysema and PiZZ alpha1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2017;12:3381–3390. doi: 10.2147/COPD.S145445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Fei J, Tan ZX, Chen YH, Hu B, Xiang HX, Zhao H, Xu DX. Low vitamin D status is associated with inflammation in patients with chronic obstructive pulmonary disease. J Immunol. 2021;206:515–523. doi: 10.4049/jimmunol.2000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Russo RC, Guabiraba R, Fagundes CT, Polidoro RB, Tavares LP, Salgado AP, Cassali GD, Sousa LP, Machado AV, Teixeira MM. Platelet-activating factor receptor plays a role in lung injury and death caused by influenza A in mice. PLoS Pathog. 2010;6:e1001171. doi: 10.1371/journal.ppat.1001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Carballa A, Rivero-Calle I, Pardo-Seco J, Gomez-Rial J, Rivero-Velasco C, Rodriguez-Nunez N, Barbeito-Castineiras G, Perez-Freixo H, Cebey-Lopez M, Barral-Arca R, Rodriguez-Tenreiro C, Dacosta-Urbieta A, Bello X, Pischedda S, Curras-Tuala MJ, Viz-Lasheras S, Martinon-Torres F, Salas A, group G-Cs (2022) A multi-tissue study of immune gene expression profiling highlights the key role of the nasal epithelium in COVID-19 severity. Environ Res 210:112890. 10.1016/j.envres.2022.112890 [DOI] [PMC free article] [PubMed]
- Gueant JL, Gueant-Rodriguez RM, Fromonot J, Oussalah A, Louis H, Chery C, Gette M, Gleye S, Callet J, Raso J, Blanchecotte F, Lacolley P, Guieu R, Regnault V. Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19. Allergy. 2021;76:1846–1858. doi: 10.1111/all.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderdos K, Lodge KM, Hirst RA, Chen C, Palazzo SG, Emerenciana A, Summers C, Angyal A, Porter L, Juss JK, O’Callaghan C, Chilvers ER, Condliffe AM. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax. 2016;71:1030–1038. doi: 10.1136/thoraxjnl-2015-207604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagkouni D, Paraskevopoulou MD, Chatzopoulos S, Vlachos IS, Tastsoglou S, Kanellos I, Papadimitriou D, Kavakiotis I, Maniou S, Skoufos G, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018;46:D239–D245. doi: 10.1093/nar/gkx1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Fornes O, Stigliani A, Gheorghe M, Castro-Mondragon JA, van der Lee R, Bessy A, Cheneby J, Kulkarni SR, Tan G, Baranasic D, Arenillas DJ, Sandelin A, Vandepoele K, Lenhard B, Ballester B, Wasserman WW, Parcy F, Mathelier A. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018;46:D1284. doi: 10.1093/nar/gkx1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal S, Lee GM, Hester CG, Poncz M, McKenzie SE, Sachais BS, Rauova L, Kelsoe G, Cines DB, Frank M, Arepally GM. The antigenic complex in HIT binds to B cells via complement and complement receptor 2 (CD21) Blood. 2016;128:1789–1799. doi: 10.1182/blood-2016-04-709634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Dao V, Khan F (2021) A review of platelet-activating factor as a potential contributor to morbidity and mortality associated with severe COVID-19. Clin Appl Thromb Hemost 27:10760296211051764. DOI 10.1177/10760296211051764 [DOI] [PMC free article] [PubMed]
- Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- Knobloch J, Chikosi SJ, Yanik S, Rupp J, Jungck D, Koch A. A systemic defect in toll-like receptor 4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;310:L24–L39. doi: 10.1152/ajplung.00367.2014. [DOI] [PubMed] [Google Scholar]
- Koutsakos M, Illing PT, Nguyen THO, Mifsud NA, Crawford JC, Rizzetto S, Eltahla AA, Clemens EB, Sant S, Chua BY, Wong CY, Allen EK, Teng D, Dash P, Boyd DF, Grzelak L, Zeng W, Hurt AC, Barr I, Rockman S, Jackson DC, Kotsimbos TC, Cheng AC, Richards M, Westall GP, Loudovaris T, Mannering SI, Elliott M, Tangye SG, Wakim LM, Rossjohn J, Vijaykrishna D, Luciani F, Thomas PG, Gras S, Purcell AW, Kedzierska K. Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses. Nat Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- Legebeke J, Lord J, Penrice-Randal R, Vallejo AF, Poole S, Brendish NJ, Dong X, Hartley C, Holloway JW, Lucas JS, Williams AP, Wheway G, Strazzeri F, Gardner A, JPR S, Skipp PJ, Hiscox JA, Polak ME, Clark TW, Baralle D (2022) Evaluating the immune response in treatment-naive hospitalised patients with influenza and COVID-19. Front Immunol. 13:853265. 10.3389/fimmu.2022.853265 [DOI] [PMC free article] [PubMed]
- Levy Y, Wiedemann A, Hejblum BP, Durand M, Lefebvre C, Surenaud M, Lacabaratz C, Perreau M, Foucat E, Dechenaud M, Tisserand P, Blengio F, Hivert B, Gauthier M, Cervantes-Gonzalez M, Bachelet D, Laouenan C, Bouadma L, Timsit JF, Yazdanpanah Y, Pantaleo G, Hocini H, Thiebaut R, French Ccsg CD177, a specific marker of neutrophil activation, is associated with coronavirus disease 2019 severity and death. iScience. 2021;24:102711. doi: 10.1016/j.isci.2021.102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Long J, Liang T, Lv M, Huang X, Liang X, Su L, Zhou L. Bioinformatics analysis based on high-throughput sequencing data to identify hub genes related to different clinical types of COVID-19. Funct Integr Genomics. 2023;23:71. doi: 10.1007/s10142-023-00998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge KM, Vassallo A, Liu B, Long M, Tong Z, Newby PR, Agha-Jaffar D, Paschalaki K, Green CE, Belchamber KBR, Ridger VC, Stockley RA, Sapey E, Summers C, Cowburn AS, Chilvers ER, Li W, Condliffe AM. Hypoxia increases the potential for neutrophil-mediated endothelial damage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2022;205:903–916. doi: 10.1164/rccm.202006-2467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghsoudi Z, Nguyen H, Tavakkoli A, Nguyen T (2022) A comprehensive survey of the approaches for pathway analysis using multi-omics data integration. Brief Bioinform 23. 10.1093/bib/bbac435 [DOI] [PMC free article] [PubMed]
- Maranatha D, Hasan H, Bakhtiar A, Widyoningroem A, Aryati (2022) Association of TNF-alpha, TGF-beta1, amphiregulin, IL-2, and EGFR with pulmonary fibrosis in COVID-19. J Infect Public Health 15:1072-1075. DOI 10.1016/j.jiph.2022.08.007 [DOI] [PMC free article] [PubMed]
- Matson CA, Choi S, Livak F, Zhao B, Mitra A, Love PE, Singh NJ. CD5 dynamically calibrates basal NF-kappaB signaling in T cells during thymic development and peripheral activation. Proc Natl Acad Sci U S A. 2020;117:14342–14353. doi: 10.1073/pnas.1922525117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD, Yost CC. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Zhou X, Lao T, Jiang Z, DeMeo DL, Cho MH, Qiu W, Cloonan S, Pinto-Plata V, Celli B, Marchetti N, Criner GJ, Bueno R, Washko GR, Glass K, Quackenbush J, Choi AM, Silverman EK, Hersh CP. Functional interactors of three genome-wide association study genes are differentially expressed in severe chronic obstructive pulmonary disease lung tissue. Sci Rep. 2017;7:44232. doi: 10.1038/srep44232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SZ, Rahmanian M, Sami A. Organ-specific or personalized treatment for COVID-19: rationale, evidence, and potential candidates. Funct Integr Genomics. 2022;22:429–433. doi: 10.1007/s10142-022-00841-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Al Sawaftah N, Husseini GA. Identification of novel microRNAs as promising therapeutics for SARS-CoV-2 by regulating the EGFR-ADAM17 axis: an in silico analysis. ACS Pharmacol Transl Sci. 2021;4:396–399. doi: 10.1021/acsptsci.0c00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersisyan S, Shkurnikov M, Turchinovich A, Knyazev E, Tonevitsky A. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS One. 2020;15:e0235987. doi: 10.1371/journal.pone.0235987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiedzka-Rystwej P, Grywalska E, Hrynkiewicz R, Bebnowska D, Wolacewicz M, Majchrzak A, Parczewski M (2021) Interplay between neutrophils, NETs and T-cells in SARS-CoV-2 infection-a missing piece of the puzzle in the COVID-19 pathogenesis? Cells 10. 10.3390/cells10071817 [DOI] [PMC free article] [PubMed]
- Othumpangat S, Lindsley WG, Beezhold DH, Kashon ML, Burrell CN, Mubareka S, Noti JD (2021) Differential expression of serum exosome microRNAs and cytokines in influenza A and B patients collected in the 2016 and 2017 influenza seasons. Pathogens 10. 10.3390/pathogens10020149 [DOI] [PMC free article] [PubMed]
- Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: a pooled analysis. Liver Int. 2020;40:1787–1788. doi: 10.1111/liv.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SP, Bender MJ, McPherson AC, Phelps CM, Sanchez LM, Rana M, Hedden L, Sangani KA, Chen L, Shapira JH, Siller M, Goel C, Verdu EF, Jabri B, Chang A, Chandran UR, Mullett SJ, Wendell SG, Singhi AD, Tilstra JS, Pierre JF, Arteel GE, Hinterleitner R, Meisel M. Tet2 deficiency drives liver microbiome dysbiosis triggering Tc1 cell autoimmune hepatitis. Cell Host Microbe. 2022;30(1003-1019):e1010. doi: 10.1016/j.chom.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos C, Tseti I, Mourouzis I. Use of triiodothyronine to treat critically ill COVID-19 patients: a new clinical trial. Crit Care. 2020;24:209. doi: 10.1186/s13054-020-02934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski C, Silvert E, O’Horo JC, Lenehan PJ, Challener D, Gnass E, Murugadoss K, Ross J, Speicher L, Geyer H, Venkatakrishnan AJ, Badley AD, Soundararajan V. SARS-CoV-2 and influenza coinfection throughout the COVID-19 pandemic: an assessment of coinfection rates, cohort characteristics, and clinical outcomes. PNAS Nexus. 2022;1:pgac071. doi: 10.1093/pnasnexus/pgac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito E, Falcinelli E, Paliani U, Cesari E, Vaudo G, Sebastiano M, Cerotto V, Guglielmini G, Gori F, Malvestiti M, Becattini C, Paciullo F, De Robertis E, Bury L, Lazzarini T, Gresele P, investigators Cs Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis. 2021;223:933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero J, Bravo A, Queralt-Rosinach N, Gutierrez-Sacristan A, Deu-Pons J, Centeno E, Garcia-Garcia J, Sanz F, Furlong LI. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:D833–D839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero J, Queralt-Rosinach N, Bravo A, Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F, Furlong LI. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database (Oxford) 2015;2015:bav028. doi: 10.1093/database/bav028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero J, Ramirez-Anguita JM, Sauch-Pitarch J, Ronzano F, Centeno E, Sanz F, Furlong LI. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–D855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MR, Islam T, Zaman T, Shahjaman M, Karim MR, Huq F, Quinn JMW, Holsinger RMD, Gov E, Moni MA. Identification of molecular signatures and pathways to identify novel therapeutic targets in Alzheimer’s disease: insights from a systems biomedicine perspective. Genomics. 2020;112:1290–1299. doi: 10.1016/j.ygeno.2019.07.018. [DOI] [PubMed] [Google Scholar]
- Rha MS, Shin EC. Activation or exhaustion of CD8(+) T cells in patients with COVID-19. Cell Mol Immunol. 2021;18:2325–2333. doi: 10.1038/s41423-021-00750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban-Lores LE, Amamura TA, da Silva TF, Midon LM, Carneiro MC, Isaac L, Bavia L. A double edged-sword - the complement system during SARS-CoV-2 infection. Life Sci. 2021;272:119245. doi: 10.1016/j.lfs.2021.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarohan AR, Akelma H, Arac E, Aslan O, Cen O. Retinol depletion in COVID-19. Clin Nutr Open Sci. 2022;43:85–94. doi: 10.1016/j.nutos.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarohan AR, Kizil M, Inkaya AC, Mahmud S, Akram M, Cen O. A novel hypothesis for COVID-19 pathogenesis: retinol depletion and retinoid signaling disorder. Cell Signal. 2021;87:110121. doi: 10.1016/j.cellsig.2021.110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Ogino Y, Tanuma SI, Uchiumi F. Human microRNA hsa-miR-15b-5p targets the RNA template component of the RNA-dependent RNA polymerase structure in severe acute respiratory syndrome coronavirus 2. Nucleosides Nucleotides Nucleic Acids. 2021;40:790–797. doi: 10.1080/15257770.2021.1950759. [DOI] [PubMed] [Google Scholar]
- Singh D, Long G, Cancado JED, Higham A. Small airway disease in chronic obstructive pulmonary disease: insights and implications for the clinician. Curr Opin Pulm Med. 2020;26:162–168. doi: 10.1097/MCP.0000000000000637. [DOI] [PubMed] [Google Scholar]
- Singh D, Mathioudakis AG, Higham A. Chronic obstructive pulmonary disease and COVID-19: interrelationships. Curr Opin Pulm Med. 2022;28:76–83. doi: 10.1097/MCP.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Salman KA, Shameem M, Warsi MS. Withania somnifera (L.) dunal as add-on therapy for COPD patients: a randomized, placebo-controlled, double-blind study. Front Pharmacol. 2022;13:901710. doi: 10.3389/fphar.2022.901710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chatterjee R, Ronanki A, Ye K. Circulating polyunsaturated fatty acids and COVID-19: a prospective cohort study and Mendelian randomization analysis. Front Med (Lausanne) 2022;9:923746. doi: 10.3389/fmed.2022.923746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan M, Hardwick HE, Investigators IC, Visser LG, Openshaw PJM, Groeneveld GH, Semple MG, Baillie JK. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;399:1463–1464. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taminau J, Meganck S, Lazar C, Steenhoff D, Coletta A, Molter C, Duque R, de Schaetzen V, Weiss Solis DY, Bersini H, Nowe A. Unlocking the potential of publicly available microarray data using inSilicoDb and inSilicoMerging R/Bioconductor packages. BMC Bioinformatics. 2012;13:335. doi: 10.1186/1471-2105-13-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SY, Chowdhury S, Polak N, Gorrell MD, Weninger W. Fibroblast activation protein is dispensable in the anti-influenza immune response in mice. PLoS One. 2017;12:e0171194. doi: 10.1371/journal.pone.0171194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodori L, Sestili P, Madiai V, Coppari S, Fraternale D, Rocchi MBL, Ramakrishna S, Albertini MC. MicroRNAs bioinformatics analyses identifying HDAC pathway as a putative target for existing anti-COVID-19 therapeutics. Front Pharmacol. 2020;11:582003. doi: 10.3389/fphar.2020.582003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Gene Ontology C. The Gene Ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyrsted J, Storgaard J, Blay-Cadanet J, Heinz A, Thielke AL, Crotta S, de Paoli F, Olagnier D, Wack A, Hiller K, Hansen AL, Holm CK. Influenza A induces lactate formation to inhibit type I IFN in primary human airway epithelium. iScience. 2021;24:103300. doi: 10.1016/j.isci.2021.103300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhiro T, Ishikawa A, Sato H, Takita S, Yoshikawa A, Anzai R, Sato S, Aoyagi R, Arita M, Shibuya T, Aratani Y, Shimizu S, Tanaka M, Yotsumoto S. Oxidized phospholipids and neutrophil elastase coordinately play critical roles in NET formation. Front Cell Dev Biol. 2021;9:718586. doi: 10.3389/fcell.2021.718586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland T, Holter JC, Holten AR, Muller KE, Lind A, Bekken GK, Dudman S, Aukrust P, Dyrhol-Riise AM, Heggelund L. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect. 2020;81:e41–e43. doi: 10.1016/j.jinf.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt M, Jorth P (2020) The unrecognized threat of secondary bacterial infections with COVID-19. mBio 11. 10.1128/mBio.01806-20 [DOI] [PMC free article] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, Lopez Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R et al (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J 49. 10.1183/13993003.00214-2017 [DOI] [PubMed]
- Voynow JA, Shinbashi M (2021) Neutrophil elastase and chronic lung disease. Biomolecules 11. 10.3390/biom11081065 [DOI] [PMC free article] [PubMed]
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu C, Wang Y, Hong Q, Zhang C, Li Z, Xu S, Zuo Q, Liu C, Huang Z, Cong Y. Conformational dynamics of the beta and kappa SARS-CoV-2 spike proteins and their complexes with ACE2 receptor revealed by cryo-EM. Nat Commun. 2021;12:7345. doi: 10.1038/s41467-021-27350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Todd I, Fairclough LC. The role of CD8 + T lymphocytes in chronic obstructive pulmonary disease: a systematic review. Inflamm Res. 2021;70:11–18. doi: 10.1007/s00011-020-01408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Benner MJ, Hancock RE. NetworkAnalyst--integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014;42:W167–W174. doi: 10.1093/nar/gku443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Fjell CD, Mayer ML, Pena OM, Wishart DS, Hancock RE. INMEX--a web-based tool for integrative meta-analysis of expression data. Nucleic Acids Res. 2013;41:W63–W70. doi: 10.1093/nar/gkt338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10:823–844. doi: 10.1038/nprot.2015.052. [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Su X, Lei T, Zhang C, Zhang M, Wang Y, Zhu L, Liu J. Effect of omega-3 fatty acids on chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2021;16:2677–2686. doi: 10.2147/COPD.S331154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Zou J, Zhou F, Zhu Y, Song Q, Yu D, Li X. Immune features of COVID-19 convalescent individuals revealed by a single-cell RNA sequencing. Int Immunopharmacol. 2022;108:108767. doi: 10.1016/j.intimp.2022.108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Qi J, Wu J, Zheng M. Changes in influenza activity and circulating subtypes during the COVID-19 outbreak in China. Front Med (Lausanne) 2022;9:829799. doi: 10.3389/fmed.2022.829799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–W241. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Wu Y, Huang S, Zhang R, Son YM, Li C, Cheon IS, Gao X, Wang M, Chen Y, Zhou X, Nguyen Q, Phan AT, Behl S, Taketo MM, Mack M, Shapiro VS, Zeng H, Ebihara H, Mullon JJ, Edell ES, Reisenauer JS, Demirel N, Kern RM, Chakraborty R, Cui W, Kaplan MH, Zhou X, Goldrath AW, Sun J. Uncoupling of macrophage inflammation from self-renewal modulates host recovery from respiratory viral infection. Immunity. 2021;54(1200-1218):e1209. doi: 10.1016/j.immuni.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinellu A, Paliogiannis P, Fois AG, Solidoro P, Carru C, Mangoni AA. Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: a systematic review and meta-analysis with meta-regression. Front Public Health. 2021;9:705916. doi: 10.3389/fpubh.2021.705916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF 9008 kb)
(XLSX 4452 kb)
(CSV 79 kb)
(XLSX 109 kb)
(XLSX 99 kb)
(XLSX 13 kb)
(XLSX 11 kb)
(XLSX 15 kb)
(DOCX 12 kb)
(XLSX 16 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.