Abstract
Objective
The opioid crisis in the USA remains severe during the COVID-19 pandemic, which has reduced access to evidence-based interventions. This Stage 1 randomized controlled trial (RCT) assessed the preliminary efficacy of Zoom-based Mindfulness-Oriented Recovery Enhancement (MORE) plus Just-in-Time Adaptive Intervention (JITAI) prompts to practice mindfulness triggered by wearable sensors (MORE + JITAI).
Method
Opioid-treated chronic pain patients (n = 63) were randomized to MORE + JITAI or a Zoom-based supportive group (SG) psychotherapy control. Participants completed ecological momentary assessments (EMA) of craving and pain (co-primary outcomes), as well as positive affect, and stress at one random probe per day for 90 days. EMA probes were also triggered when a wearable sensor detected the presence of physiological stress, as indicated by changes in heart rate variability (HRV), at which time participants in MORE + JITAI were prompted by an app to engage in audio-guided mindfulness practice.
Results
EMA showed significantly greater reductions in craving, pain, and stress, and increased positive affect over time for participants in MORE + JITAI than for participants in SG. JITAI-initiated mindfulness practice was associated with significant improvements in these variables, as well as increases in HRV. Machine learning predicted JITAI-initiated mindfulness practice effectiveness with reasonable sensitivity and specificity.
Conclusions
In this pilot trial, MORE + JITAI demonstrated preliminary efficacy for reducing opioid craving and pain, two factors implicated in opioid misuse. MORE + JITAI is a promising intervention that warrants investigation in a fully powered RCT.
Preregistration
This study is registered on ClinicalTrials.gov (NCT04567043).
Keyword: Chronic pain, Craving, JITAI, Machine learning, mHealth, Opioid misuse
Patients with chronic pain are commonly treated with long-term opioid therapy, defined as ≥ 90 days of opioid use (Chou et al., 2009). Although many patients take opioids as prescribed by their physicians, approximately 25% of individuals receiving long-term opioid therapy (LTOT) for pain engage in opioid misusing behaviors (Vowles et al., 2015) such as unauthorized dose escalation or using opioids to alleviate negative emotions (Butler et al., 2007). Opioid misuse is linked with craving in patients on LTOT (Frimerman et al., 2021; Martel et al., 2014; Parisi et al., 2022a), and pain, stress, and withdrawal may elicit moment-by-moment increases in craving in this population (Bruneau et al., 2021; Frimerman et al., 2021; Martel et al., 2016). Opioids are now among the most commonly misused drugs in the USA. In 2020, 9.3 million people misused prescription opioids (McCance-Katz, 2021). Opioid misuse among people receiving LTOT contributed to the opioid crisis (Compton & Jones, 2019), which has only worsened during COVID-19 (Becker & Fiellin, 2020). To halt the opioid crisis during the COVID pandemic, there is an urgent need to increase the reach of evidence-based interventions.
Mindfulness-Oriented Recovery Enhancement (MORE) is an evidence-based intervention for opioid misuse and chronic pain. MORE has demonstrated efficacy across multiple RCTs including chronic pain patients on LTOT at risk for opioid misuse (Garland et al., 2019a, 2019b, 2019c), chronic pain patients on LTOT who misuse opioids (Garland et al., 2014a, 2014b), and patients with chronic pain and opioid use disorder (OUD) (Cooperman et al., 2021). A meta-analysis demonstrated that MORE reduces addictive behaviors (e.g., opioid misuse, days of drug use; standardized mean change (SMC) = 0.54) and chronic pain (SMC = 0.60) relative to control groups (Parisi et al., 2022a, 2022b). Recently, MORE was tested in a full-scale RCT of opioid misusing chronic pain patients (n = 250), which demonstrated the sustained efficacy of the treatment—with MORE reducing opioid misuse by 45% (OR = 2.94) and pain functional interference by 25% at a 9-month follow-up (Garland et al., 2022).
Face-to-face implementation of efficacious treatments like MORE has been limited by barriers to access—particularly in rural and frontier areas where many practitioners are less likely to have been trained to deliver evidence-based interventions with adequate fidelity (Dan et al., 2020; Dotson et al., 2014). Access in rural areas has been further limited by the emergence of COVID-19, which resulted in provider shortages, and caused severe stress among the currently uninfected, resulting in more patients pursuing limited mental health resources. Among its many adverse consequences, the COVID-19 pandemic has simultaneously exacerbated opioid misuse while reducing access to efficacious treatments like MORE.
Technology-based interventions may be able to overcome these barriers, improving treatment access for chronic pain patients receiving LTOT at risk for opioid misuse. Web-based cognitive behavioral therapy (CBT) interventions have been shown to be efficacious for treating symptoms of chronic back pain, irritable bowel syndrome, tinnitus, and headache (Beatty & Lambert, 2013; Cuijpers et al., 2008; Garg et al., 2016; Ziadni et al., 2021). HIPAA-compliant platforms like Zoom now allow for behavioral interventions to be delivered live by clinicians to patients in remote locations. However, telehealth may reduce treatment engagement in psychosocial interventions (Valentine et al., 2020; Young, 2012) such as mindfulness which often has poor engagement in home practice. A systematic review of mindfulness-based interventions found that fewer than 50% of participants completed the prescribed mindfulness training regimen (Cavanagh et al., 2014). Enhancing real-time, real-world engagement in mindfulness practice has the potential to improve the efficacy of telehealth MORE.
Just-in-Time Adaptive Interventions (JITAIs) may be useful for prompting engagement in mindfulness practice. JITAIs adapt over time to an individual’s changing status and are designed to provide appropriate intervention strategies based on real-time, real-world context (Nahum-Shani et al., 2015; Spruijt-Metz & Nilsen, 2014). These interventions have the potential to trigger an intervention prompt at moments in which they have been probabilistically assessed as more likely to be needed. JITAIs may serve as a promising means of enhancing mindfulness practice engagement by delivering a prompt to practice mindfulness when individuals are most vulnerable for opioid misuse, including when they are stressed, or experience elevations in pain or opioid craving. Advances in wearable technology now allow JITAIs to be triggered by real-time monitoring of physiological signals including heart rate variability (HRV) (Clarke et al., 2017). HRV is the beat-to-beat modulation of heart rate by the vagus nerve (Berntson et al., 1997). A meta-analysis demonstrates that phasic decreases in HRV reliably index stress (Kim et al., 2018). Continuous ambulatory monitoring of HRV may alert chronic pain patients on LTOT to practice mindfulness during times of stress, and thereby might prevent opioid misuse. In support of this contention, momentary ratings of stress, positive affect, and craving have been associated with ambulatory measures of HRV (Alinia et al., 2021; Bertz et al., 2018; Gullapalli et al., 2019; Määttänen et al., 2021; Murray et al., 2022).
Furthermore, wearable technology might identify digital biomarkers of mindfulness treatment response. Currently, no methods exist for objectively determining whether a given patient is responding to mindfulness. Wearable technology could relay real-time data to patients and healthcare providers to give feedback on the patient’s status. Such feedback could be used to encourage patients to engage in additional mindfulness practice or guide the selection of mindfulness practices that are most effective for a given patient in a given context. In addition, healthcare providers would benefit from being able to monitor their patients’ progress in mindfulness-based treatment, to ascertain whether a given patient was receiving the help that he or she needs, or whether additional intervention is required. Paralleling the precision medicine approach in healthcare, such an innovation in digital medicine would represent a precision mindfulness approach tailored to the needs of a particular patient. Machine learning, which analyzes patterns in large datasets to build predictive models, may be useful in this pursuit. Prior work has already demonstrated that modeling wearable sensor data with a temporal convolutional neural network can detect opioid use (Gullapalli et al., 2021). Hypothetically, a machine learning model can analyze underlying patterns in physiological signals passively recorded during a specific mindfulness session to predict the effectiveness of the session.
To our knowledge, no studies have examined the impact of remote mindfulness-based interventions for people receiving LTOT for chronic pain. We conducted a stage 1 RCT comparing Zoom-based MORE plus a stress-triggered JITAI (MORE + JITAI) to Zoom-based supportive group (SG) psychotherapy to pilot test and examine the preliminary efficacy of this remote, technology-based mindfulness intervention. Here, we present ecological momentary assessment (EMA) data from this trial to elucidate effects on opioid craving, pain, stress, and positive affect during the course of the MORE + JITAI intervention. We hypothesized that MORE + JITAI would result in greater improvements in these EMA outcomes over time than the SG. As an exploratory aim, we present results from machine learning analyses to test whether change in outcomes following the JITAI-initiated mindfulness practice sessions can be predicted from wearable sensor data.
Method
Participants
Participants were recruited from electronic health record (EHR), physician referral, community advertisements, and online advertisements placed in the Intermountain West. During recruitment, the study was framed as a trial comparing two behavioral treatments, and no indication was given that MORE was the experimental arm. Eligible patients were age ≥ 18 with daily prescribed opioid use for ≥ 3 months and reported a chronic pain condition as determined by physician assessment. We excluded patients experiencing active suicidality or psychosis (as assessed by the Mini-International Neuropsychiatric Interview); those who had clinically unstable systematic illness that might interfere with study treatment (e.g., active cancer); and those who had prior exposure to mindfulness interventions.
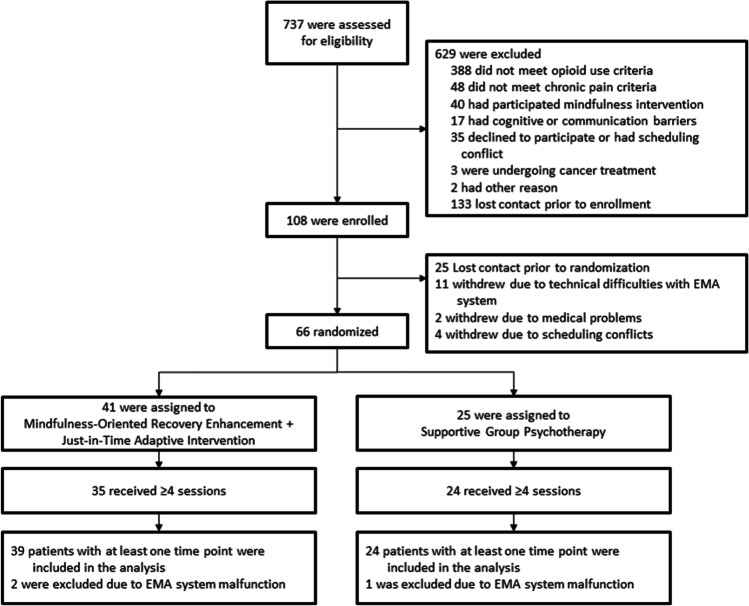
Of the sample, 41.3% were women, with a mean age of 53.6 years (SD = 12.8). The majority of the sample were white (92.1%), but 11.1% were of a Hispanic or Latino ethnicity. Low back pain (66.6%) and joint pain (36.5%) were the most commonly reported pain conditions, with a mean pain severity of 5.5 on a 0–10 scale (SD = 1.4). Patients had been in pain an average of 18.4 (SD = 10.3). Oxycodone, hydrocodone, and tramadol were the most prevalent opioids used, and the mean morphine equivalent daily dose was 130.3 mg (SD = 219.9)—a high dose according to the Centers for Disease Control (Dowell et al., 2016). See Fig. 1 for the study CONSORT flowchart. There were no significant between-groups differences on any demographic or baseline clinical variable aside from pain duration (Table 1).
Fig. 1.
CONSORT study flowchart
Table 1.
Demographic and clinical characteristics of the sample of opioid-treated chronic pain patients (n = 63)
| Measure | MORE (n = 39) | SG (n = 24) |
|---|---|---|
| Age | 52.56 ± 12.4 | 55.2 ± 12.30 |
| Female, n (%) | 15 (38%) | 11 (46%) |
| Race | ||
| Black | 2 (5%) | - |
| Other | 1 (3%) | 1 (4%) |
| White | 36 | 22 |
| Not reported | - | 1 (4%) |
| Hispanic or Latino | 3 (8%) | 4 (17%) |
| Estimated household income, N (%) | ||
| Less than $25,000 | 11 (28%) | 7 (29%) |
| $25,000–49,999 | 16 (41%) | 12 (50%) |
| $50,000–99,999 | 7 (18%) | 1 (4%) |
| $100,000 or greater | 5 (13%) | 4 (17%) |
| Pain condition/location, N (%)* | ||
| Back pain | 25 (64%) | 17 (71%) |
| Joint pain | 18 (46%) | 5 (21%) |
| Fibromyalgia | 4 (10%) | 4 (17%) |
| Neuropathic pain | 7 (18%) | 5 (21%) |
| Cervical pain | 10 (26%) | 6 (25%) |
| Extremity pain | 11 (28%) | 6 (25%) |
| Migraine or tension headache pain | 5 (13%) | 1 (4%) |
| Pelvic pain | 6 (15%) | 3 (13%) |
| Other | 6 (15%) | 3 (13%) |
| Pain severity (0–10) | 5.7 ± 1.3 | 5.2 ± 1.6 |
| Pain duration in years | 15.2 ± 7.53a | 23.5 ± 12.1a |
| Opioid prescription, n (%)* | ||
| Oxycodone | 12 (31%) | 9 (38%) |
| Hydrocodone | 7 (18%) | 1 (4%) |
| Tramadol | 15 (39%) | 7 (29%) |
| Morphine | 2 (5%) | 5 (21%) |
| Buprenorphine | 5 (13%) | 6 (25%) |
| Methadone | 1 (3%) | 1 (4%) |
| Other | 2 (5%) | - |
| Morphine equivalent daily dose, mg | 111.7 ± 216.32 | 160.9 ± 227.6 |
| Duration of opioid use in years | 6.34 (6.8) | 6.4 (7.6) |
Data are mean (SD) or n (%)
*Because patients could report multiple pain conditions/locations and opioid prescriptions, these percentages could sum to greater than 100%
aSignificant between-groups differences were observed for this variable at p < 0.05
Procedure
In a Zoom-based research visit, after obtaining written informed consent, research staff collected demographics and then scheduled 90 consecutive days of EMAs to be delivered through the mEMA app (Illumivu) to participants’ smartphones, beginning on the first day of treatment. EMA is a method of data collection that overcomes retrospective biases by gathering data via real-time reports of momentary experiences in the context of everyday life (Csikszentmihalyi & Larson, 2014; Shiffman et al., 2008). After setting up the mEMA app, participants were mailed a Garmin Vivosmart smartwatch, which was then paired with the app. If participants did not own their own smartphone, they were supplied with a loaner phone for the duration of the study. An investigator uninvolved in assessments or analysis generated treatment allocations to MORE or SG with random assignment (1:1 ratio) in blocks of 2–4 via computerized random number generator. To prevent bias and maintain allocation concealment, participants were not allocated until the day of the first treatment session by the coordinator. To maintain blinding, the study key with allocations was inaccessible to staff involved in assessment or treatment, as well as the investigators until study completion. Finally, 66 participants were randomized to receive 8 weeks of MORE + JITAI or SG delivered via telehealth, and underwent treatment (Fig. 1).
During the course of treatment and for the month thereafter, the mEMA app was connected via Bluetooth to the Garmin Vivosmart smartwatch. Each day, participants were sent one random EMA probe between 7 am and 9 pm. We chose to send only one random probe per day to reduce participant burden, given that participants could also be sent multiple stress-triggered probes each day. Participants responded to a mean of 60.0 (SD = 59.2) random probes with EMA ratings over the 90 days. In addition, from 7 am to 9 pm, when the Garmin Vivosmart stress metric exceeded 1 SD of a participant’s moving average value, the participant was sent a stress-triggered probe. After one stress-triggered probe was sent, another could not be sent for 90 min to prevent participant burden. However, due to excessive Garmin Vivosmart signal artifacts, a system malfunction resulted in 3 participants receiving several hundred more probes than the sample average (> 2.5 SD of the mean number of probes), and as such, they were excluded from outcome analyses. In the final study sample, 3904 probes were completed in total (mean probes per day 1.96, SD = 1.69). At each EMA probe, participants were asked to rate their craving, pain, positive affect, and stress.
Intervention
MORE
The telehealth MORE intervention followed the validated MORE treatment manual (Garland, 2013). The MORE arm participated in eight weekly, 2-hr telehealth group sessions (comprising 6–12 participants) via a HIPAA-compliant Zoom platform led by doctoral-level therapists. MORE therapists had 5 years of experience providing mindfulness-based therapy to treat chronic pain and addiction, completed didactic and experiential training in MORE, and then received weekly clinical supervision by the developer of MORE until achieving full fidelity on the MORE Fidelity Measure (Hanley and Garland, 2021). MORE sessions involved training in mindfulness and reappraisal to reduce craving, pain, and stress, and training in savoring to augment natural reward processing and positive emotion. MORE participants were asked to engage in 15 min of mindfulness, reappraisal, and savoring practice at home each day.
Supportive Group (SG) Psychotherapy
The SG arm participated in eight weekly, 2-hr telehealth SG sessions (comprising 6–12 participants) via a HIPAA-compliant Zoom platform led by the same doctoral-level therapists who delivered the MORE arm (to prevent therapist effects), who guided discussions on themes pertinent to chronic pain and opioid misuse selected to match topics in the MORE intervention. SG participants were encouraged to disclose feelings and thoughts about session topics, as well as to provide advice and support to their peers. During the SG, the therapist employed empathic responding, elicited emotional expression, and promoted a positive group climate, but did not provide any specific therapeutic skill training. The SG intervention typifies a widely available form of conventional, process-oriented, Rogerian client-centered psychotherapy (Rogers, 2003). To match the MORE homework requirement, SG participants were asked to journal for 15 min a day on the weekly session topics.
Participants in MORE completed 6.6 (SD = 1.7) sessions, whereas those in SG completed 7.4 (SD = 0.8) sessions. Treatment session attendance, but not any other clinical (baseline or EMA rating) or demographic variable, was significantly associated with responding to random EMA probes, B = 10.85, SE = 4.94, p = 0.032.
Stressed-Triggered, JITAI-Initiated Mindfulness Practice
In cooperation with Ilumivu, using the mEMA app, we built a JITAI system that prompts mindfulness practice when a physiological stress metric obtained from the Garmin Vivosmart smartwatch is > 1 SD of the individual participant’s moving average level. The Garmin Vivosmart uses continuous physiological data from photoplethysmogram (PPG), accelerometer, and pulse oximeter sensors to detect physiological stress via Firstbeat PRO’s heartbeat analysis software (Firstbeat Technologies, Ltd, 2014). Based on the participant age, gender, height, weight, and physical activity level, the Firstbeat PRO software estimates the individual’s maximal HR (HRmax), maximum respiration rate, and maximum oxygen consumption (VO2max). RR-interval data obtained by the Garmin Vivosmart are processed with an artifact detection filter to correctly classify heartbeats, after which linear interpolation is used to re-sample artifact-corrected RR intervals at 5 Hz. The software removes low-frequency trends and variances below and above the frequency bands of interest from the re-sampled RR-interval data with a polynomial filter and digital FIR band-pass (0.03–1.2 Hz) filter. The software calculates time domain and frequency domain HRV variables including high-frequency HRV (0.15–0.40 Hz). The software uses neural network modeling for segmentation of second-by-second RR-interval data into stationary segments. Prior to detection of stress, the software distinguishes movement artifacts, physical activity, and postural changes from other non-metabolic factors that influence cardiac activity (e.g., stress and other psychological states). Respiration rate is estimated from RR intervals and HRV spectral frequency data (Charlton et al., 2016), with respiration rate determined as the frequency of peak power (amplitude) in the respiratory spectrum. Segments associated with physical activity were detected via accelerometer data and oxygen consumption. If the VO2 is > 20% of the participant’s VO2max, the segment was labeled as physical activity and excluded from the stress metric. Accelerometer data further refines the detection of postural changes and other body movements. For non-physical activity, the segments when the body is in a stress state are determined. Stress is defined as a state of sympathetic predominance, indicated by decreases in HRV from the individual’s typical resting HRV level and elevated HR. The Firstbeat PRO algorithm-derived physiological stress metric has been validated in multiple other studies (Föhr et al., 2015; Madison et al., 2021; Myllymäki et al., 2011, 2012; Palesh et al., 2019; Rusko et al., 2006; Teisala et al., 2014; Uusitalo et al., 2011). We performed our own validation study by examining whether chronic pain patients on LTOT (n = 68) were more likely to give affirmative responses on the dichotomous item “Are you stressed?” delivered by EMA probes when the physiological stress metric was > 1 SD of their moving average individual level vs. when EMA probes are delivered randomly. Across 2098 sampling moments, participants had 64% greater odds of reporting being stressed at EMA probes triggered by physiological stress metric than at random EMA probes (B = 0.49, SE = 0.09, p < 0.001, OR = 1.64, 95% CI 1.36, 1.97), and stress ratings were significantly higher at EMA probes triggered by the physiological stress metric than at random EMA probes (M = 4.34 ± 0.08 vs. M = 3.13 ± 0.08; F1,2084 = 90.56, p < 0.001).
When the physiological stress metric was > 1 SD of the individual’s moving average, the mEMA app triggered an EMA probe asking the participant to rate their craving, pain, positive affect, and stress. Next, MORE + JITAI participants were asked by the app whether they wished to practice mindfulness. If the participant responded affirmatively, the mEMA app launched the JITAI-initiated mindfulness practice session by playing an audio-recorded mindfulness practice. One minute after each audio-recorded mindfulness practice, participants in MORE were asked to rate their craving, pain, positive affect, and stress to assess the impact of the audio-recorded mindfulness practice on acute symptoms. Participants in the SG only completed the first set of EMA questions and were not asked to practice mindfulness.
EMA Measures
The primary outcome was opioid craving, measured with two items on a 0–10 numeric rating scale (NRS) to more broadly capture the construct. First, we asked “How much do you want to take opioids right now?” (Garland et al., 2019a, 2019b, 2019c; Garland and Howard, 2014) based on Robinson and Berridge’s (2001) concept of craving as mesolimbic dopamine-mediated wanting. Second, we asked, “How strong of an urge do you have to take more opioid medication than prescribed?” to assess craving for opioid dose escalation (Wasan et al., 2012). Because these items were so highly correlated (r = 0.92, p < 0.001), we treated them as two indicators of the same construct and used their average in outcome analysis.
The co-primary outcome was pain, assessed with two 0–10 numeric rating scale (NRS) items “How intense is your pain right now?” and “How unpleasant is your pain right now?” (Farrar et al., 2001). Because these items were so highly correlated (r = 0.85, p < 0.001), we treated them as two indicators of the same construct and used their average in pain outcome analysis.
Secondary outcomes were positive affect—“How positive are you feeling right now?” (Lindsay et al., 2018)—and stress—“How stressed are you right now?”—both rated on 0–10 NRS (Garland et al., 2019a, 2019b, 2019c).
HRV Measures
HRV was analyzed offline using the raw inter-beat interval data (IBI) streamed from the Garmin Vivosmart. A fast Fourier transform was applied separately to IBI data to extract HF-HRV from a de-trended, end-tapered inter-beat interval time series. The spectrum for the selected R-R interval segment was calculated via Welch’s periodogram method, in which R-R interval data were reduced using a window width of 300 s applied every 60 s with a window overlap of 240 s (80%). High-frequency heart rate variability (HF-HRV, 0.15–0.40 Hz) was computed for each minute of the 5 min before and 5 min after each mindfulness practice session in 1-min epochs to assess the impact of audio-guided JITAI mindfulness practice.
Data Analyses
Analyses were performed using the R statistical platform, version 4.2.2. We estimated several multilevel models using the Generalized Additive Mixed Models for Location, Scale, and Shape package (“gamlss”). “Gamlss” is unique in its ability to model a wide range of distribution families, parameters, and both linear and non-linear multilevel models (Stasinopoulos et al., 2017).
Analyses first examined the distributional form of the outcomes craving, pain, stress, and positive affect using the “fitdistrplus” package, distribution selection features of “gamlss,” and null models. All methods indicated craving, stress, and positive affect were best modeled using a distribution from the beta family owing to considerable positive skew. Pain was relatively well-described by a normal distribution but, because of thick tails, a t-distribution was a better fit by Generalized Akaike Information Criteria (GAIC). Because the beta-family requires data bound 0 to 1, craving, stress, and positivity were rescaled as a proportion from the original 0 to 10 metric, and a beta-inflated distribution was adopted. Beta-inflated models utilized a “logit” link function. When exponentiated, the coefficients provide mean ratios, similar to odds ratios, that represent how much less or greater the mean of one group is compared to another. Results from the t-family model of pain are interpreted just as in Gaussian regression.
For each outcome, we tested two- (time within person) and three-level (time within day within person) linear, quadratic, and generalized additive mixed models (GAMMs) both with and without a random slope for time. Three-level models with day at level 2 either failed to converge, or were overparameterized according to GAIC. In all cases, two-level random slope additive models were indicated. Because the fixed effect for time was non-linear, the random slope was also entered as a non-linear term, specifically a cubic spline. In all cases, native diagnostics of “gamlss” revealed excellent model fit and residual diagnostics. To provide a meaningful test of the main effect for condition (and to avoid convergence issues), time was standardized and rescaled such that 0 represented the end of observation. This scaling tests whether the MORE and SG groups differ on outcomes at the end of the study period.
It is worth noting that, in models that are non-linear or curvilinear, polynomials (a special case of a linear model) can provide very inaccurate predictions, including those outside the bounds of the scale, because they provide a global fit. Smooth functions are fit locally, and, therefore, avoid impossible predictions and are also not easily influenced by extreme observations or sparse regions of data (Stasinopoulos et al., 2017).
Because “gamlss” estimates models that are considerably more complicated than linear mixed models, several caveats are worth noting. “Gamlss” does not produce variances for random effects because the estimator is not maximum likelihood. Instead, the significance of random effects is tested using a native “gamlss” function that provides a likelihood ratio test (LRT); if the result is significant, the random effect should be retained. Fixed effects are tested similarly. As noted by Stasinopoulos et al., (2017, p. 18), p-values for fixed effects estimates do not consider the uncertainty of the smoothing terms, and an LRT is again used to test their significance by fixing the penalized degrees of freedom of the smooth terms; this provides a more accurate p-value. For both random and fixed effects, only LRTs of the highest order term are returned given that models cannot drop lower-level terms in a significant interaction. In order to provide a test of the main effect of Condition at the end of observation, sub-models removed the interaction and again utilized an LRT to test the term. Just as ICCs cannot be computed accurately for models with random slopes, they also cannot be computed for models with smooth terms and smoothed random slopes. Therefore, ICCs in the tables presented in the results reflect a linear model with no random slope, and are for reference only. Finally, because groups differed at baseline on EMA outcomes by random chance, we conducted a sensitivity analysis where the baseline EMA rating was included as a covariate.
GAMMs are typically interpreted graphically because of the complications interpreting smooth terms. In all cases, figures present the predictions from the final models. Time in the figures is transformed back to the scale of days for easier interpretation. Figures for beta-inflated models also rescale the predictions back from a proportion to the original metrics, 0–10. Random effects in figures are fixed at the average value of the outcome.
Participants were included in the analysis if they had at least one timepoint of data. Power analysis via Optimal Design software (Spybrook et al., 2011) assuming a medium–large standardized effect size and low variability of the level-1 residual and coefficient (power calculation inputs derived from prior EMA studies of MORE (Garland et al., 2017, 2019a, 2019b, 2019c)) indicated that power > 0.80 with 90 measurement points per participant and sample size of 60 participants. The Group (MORE vs. SG) × Time interaction was the primary fixed effect of interest. To control for false discovery in co-primary outcomes, we compared the unadjusted p-values against Bonferroni-adjusted alpha = 0.05/2 = 0.025. Regarding analysis of changes in acute symptoms and HF-HRV from before to after JITAI-initiated mindfulness practice among participants in the MORE arm, MLM was also employed. The MLM comprised a random intercept per subject ID, a random intercept for JITAI instance (representing the day and time of the instance), and single fixed effect, the main effect of Practice (before mindfulness practice, after mindfulness practice), which tested whether EMA ratings and HF-HRV values before and after mindfulness practice significantly differed.
We sought to explore if the biometric data collected by the wearable sensor during a JITAI-initiated mindfulness practice session could be directly used to train a machine learning algorithm to automatically predict the impact of mindfulness practice on reducing stress, craving, and pain ratings. While training such a predictive machine learning algorithm is a significantly more challenging problem than frequentist statistical analyses, a moderately accurate predictive machine learning algorithm can fundamentally enable personalized mindfulness therapeutics by providing an objective metric of the impact of mindfulness practice sessions.
To this end, we trained three machine learning-based classifiers with varying complexities (i.e., logistic regression, decision tree, and random-forest) to automatically predict if a JITAI-initiated mindfulness practice session would occasion decreased craving, pain, and stress ratings using biometric data (e.g., heart rate, HRV, pulse oximeter, accelerometer, calorie, and step count) captured from the Garmin wristwatch during the session. Each participant was asked to self-report their craving, pain, and stress ratings with EMA prompts before and after the JITAI-initiated mindfulness practice. Mindfulness practice sessions were designed to decrease craving, pain, and stress. A specific session was classified as effective if the participant’s EMA rating was lower after the session compared to the rating immediately prior to the session. Consequently, we trained the predictive machine learning algorithm to solve this binary classification problem (i.e., the post-EMA rating decreased from pre-EMA rating vs. an increase or no-change in rating between pre- and post-EMA). We trained and tested all these classifiers using their implementation in sklearn (Pedregosa et al., 2011) with different parameter combinations. For logistic regression, we considered different regularization strengths {0.1,1,10}, and penalty norms {L2 and L1}. Similarly, for both the decision tree and random forest, we considered split criterion of {‘gini’, ‘entropy’, ‘log_loss’}, and maximum depth of the tree {20, 50, 100}. To maximize the information from the features, we selected the best features using a recursive feature selection method where we iteratively removed the feature of least importance (based on the weight of the parameter) until we reached an optimal subset. Every minute, the mEMA sense app and Garmin API provided a total of 35 features from the biometric data that include features from heart rate (minimum R-R interval, average R-R, NN50, etc.), HRV (spectral frequency activity, e.g., LF, HF), pulse oximeter value, calorie count, and step count information. These features, collected over a time window of 5 min before and 5 min after a mindfulness session, were used as inputs for our machine learning model. We z-normalized the features of each subject to have a mean of 0 and standard deviation of 1 to remove any subject-related information. We used leave-one-person-out cross validation (LOOCV), where each subject’s data is iteratively held out for testing while remaining subjects’ data are used for training to predict the JITAI-initiated mindfulness practice effectiveness.
Results
Effects of Treatment on Momentary Craving, Pain, and Affective State
Craving
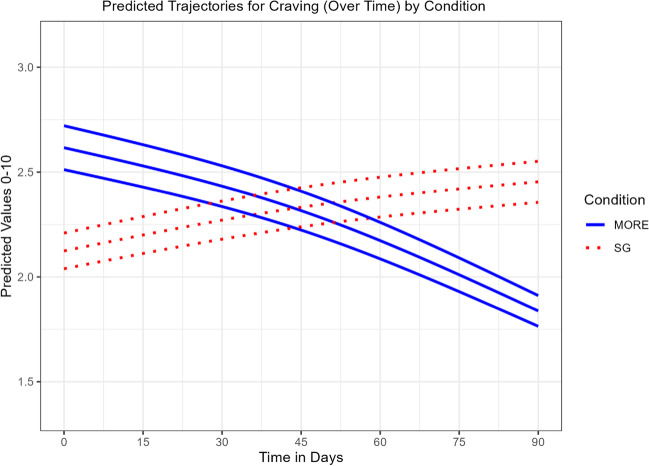
Compared to SG participants, participants in MORE + JITAI reported significantly greater decreases in opioid craving over time, Group × Time MR = 1.19 (SE = 0.05), p < 0.001 (Table 2). The modified likelihood test of the main effect of condition indicates the MORE + JITAI group had significantly less craving at the end of observation. GAMMs are also usually interpreted visually. Figure 2 shows the SG group increased in craving over time, while the MORE + JITAI group declined in craving over time, both in a non-linear fashion. Because the MORE + group began the study with higher craving levels than the SG, we controlled for baseline craving as a covariate in a sensitivity analysis. The LRT test of the interaction in the presence of the baseline craving covariate again found a significant Group × Time interaction favoring MORE, χ2(0.70) = 5.2, p = 0.013.
Table 2.
Parameter estimates and likelihood ratio tests for the two-level GAMM assessing craving
| Parameter | Mean ratio (MR) | SE | 95% CI | t(3699.5) | p |
|---|---|---|---|---|---|
| Fixed effects | |||||
| (intercept) | 0.43 | 0.06 | (0.33, 0.57) | -6.04 | < 0.001 |
| cs(Time) | 0.94 | 0.03 | (0.88, 1.00) | -1.88 | 0.060 |
| Condition (SG) | 1.46 | 0.22 | (1.08, 1.96) | 2.48 | 0.013 |
| cs(Time × Condition (SG)) | 1.19 | 0.05 | (1.10, 1.28) | 4.48 | < 0.001 |
| Deviance statistics and ICC | |||||
| Global deviance | 3174.9 | ||||
| AIC | 3342.0 | ||||
| ICC | 0.49 | ||||
| Likelihood ratio tests | df | AIC | LRT | p | |
| re(cs(Time × Condition (SG)) | 73.5 | 4988.1 | 1803.2 | < 0.001 | |
| cs(Time × Condition (SG)) | 0.8 | 3348.8 | 8.4 | 0.002 | |
| Condition (SG) (modified) | 1 | 5052.4 | 17.5 | < 0.001 |
SG is the reference group for condition. “re” stands for the random effect term and “cs” stands for cubic spline. Likelihood ratio tests (LRTs) utilize the drop1() function in “gamlss” to test the significance of the terms given the uncertainty of the smooth terms. Only the highest order term is tested. The LRT of Condition is modified by removing the interaction term to test the main effect of Condition at the end of observation. ICC was tested for a simplified, linear model with no smoothed random slope
Fig. 2.
Predicted trajectories for craving (over time) by condition. Effects of Mindfulness-Oriented Recovery Enhancement + Just-in-Time Adaptive Intervention (MORE) vs. supportive group (SG) psychotherapy on ecological momentary assessments of craving (mean with 95% C.I.)
Pain
Compared to SG participants, participants in MORE + JITAI reported significantly greater decreases in pain over time, Group × Time b = 0.47 (SE = 0.03), p < 0.001 (Table 3). The modified likelihood test of the main effect of condition indicates the MORE + JITAI group had significantly less pain at the end of observation. Figure 3 indicates the SG group showed an increase in pain over time, while the MORE + JITAI group decreased pain over time, both in a non-linear fashion. After an initial decline, the MORE + JITAI group hit a lower bound about 70 days into observation. In our sensitivity analysis, the LRT test of the interaction and in the presence of the baseline pain covariate again found a significant Group × Time interaction favoring MORE, χ2 (0.32) = 55.9, p < 0.001.
Table 3.
Parameter estimates and likelihood ratio tests for the two-level GAMM assessing pain
| Parameter | b | SE | 95% CI | t(3693.6) | p |
|---|---|---|---|---|---|
| Fixed effects | |||||
| (intercept) | 4.28 | 0.07 | (4.14, 4.43) | 58.83 | < 0.001 |
| cs(Time) | -0.16 | 0.02 | (-0.21. -0.12) | -7.50 | < 0.001 |
| Condition (SG) | 2.01 | 0.11 | (1.78, 2.23) | 17.53 | < 0.001 |
| cs(Time × Condition (SG)) | 0.47 | 0.03 | (0.41, 0.54) | 14.26 | < 0.001 |
| Deviance statistics and ICC | |||||
| Global deviance | 13,319.1 | ||||
| AIC | 13,489.4 | ||||
| ICC | 0.77 | ||||
| Likelihood ratio tests | df | AIC | LRT | p | |
| re(cs(Time × Condition (SG)) | 70.1 | 16,708 | 3358.9 | < 0.001 | |
| cs(Time × Condition (SG)) | 1.7 | 13,602 | 115.5 | < 0.001 | |
| Condition (SG) (modified) | 1 | 16,942 | 23.6 | < 0.001 |
SG is the reference group for condition. “re” stands for the random effect term and “cs” stands for cubic spline. Likelihood ratio tests (LRTs) utilize the drop1() function in “gamlss” to test the significance of the terms given the uncertainty of the smooth terms. Only the highest order term is tested. The LRT of Condition is modified by removing the interaction term to test the main effect of Condition at the end of observation. ICC was tested for a simplified, linear model with no smoothed random slope
Fig. 3.
Predicted trajectories for pain (over time) by condition. Effects of Mindfulness-Oriented Recovery Enhancement + Just-in-Time Adaptive Intervention (MORE) vs. supportive group (SG) psychotherapy on ecological momentary assessments of pain (mean with 95% C.I.)
Stress
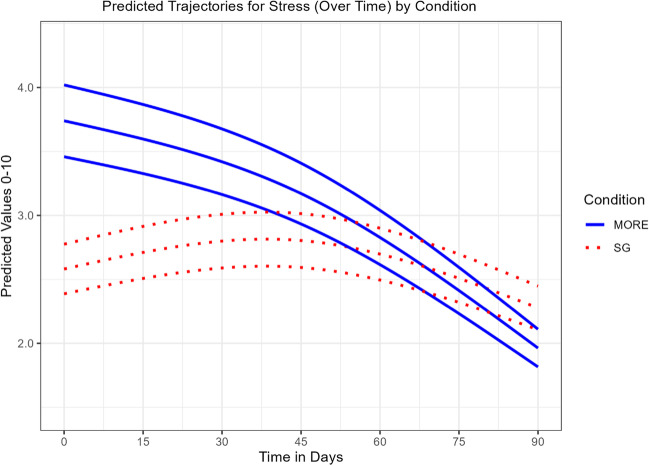
Compared to SG participants, participants in MORE + JITAI reported significantly less stress over time, Group × Time MR = 1.20 (SE = 0.04), p < 0.001 (Table 4). The modified likelihood test of the main effect of condition indicated the MORE + JITAI did not have significantly less stress at the end of observation. Figure 4 indicates the SG group showed a small decrease in stress over time in a non-linear fashion. The MORE + JITAI group showed a much greater decline in stress, but this group started with a higher initial level of stress. In our sensitivity analysis, the LRT test of the interaction in the presence of the baseline stress covariate again found a significant Group × Time interaction favoring MORE, χ2 (1.04) = 6.9, p = 0.009.
Table 4.
Parameter estimates and likelihood ratio tests for the two-level GAMM assessing stress
| Parameter | Mean ratio (MR) | SE | 95% CI | t(3703.8) | p |
|---|---|---|---|---|---|
| Fixed effects | |||||
| (intercept) | 0.19 | 0.02 | (0.15, 0.24) | -14.32 | < 0.001 |
| cs(Time) | 0.86 | 0.03 | (0.81, 0.91) | -4.94 | < 0.001 |
| Condition (SG) | 1.20 | 0.17 | (0.91, 1.58) | 1.30 | 0.194 |
| cs(Time × Condition (SG)) | 1.20 | 0.04 | (1.11, 1.29) | 4.83 | < 0.001 |
| Deviance statistics and ICC | |||||
| Global deviance | -7842.3 | ||||
| AIC | -7667.8 | ||||
| ICC | 0.47 | ||||
| Likelihood ratio tests | df | AIC | LRT | p | |
| re(cs(Time × Condition (SG)) | 73.2 | -5603.5 | 2210.8 | < 0.001 | |
| cs(Time × Condition (SG)) | 1.67 | -7639.4 | 31.8 | < 0.001 | |
| Condition (SG) (modified) | 0.75 | -5428.8 | -0.63 | 1.000 |
SG is the reference group for condition. “re” stands for the random effect term and “cs” stands for cubic spline. Likelihood ratio tests (LRTs) utilize the drop1() function in “gamlss” to test the significance of the terms given the uncertainty of the smooth terms. Only the highest order term is tested. The LRT of Condition is modified by removing the interaction term to test the main effect of Condition at the end of observation. ICC was tested for a simplified, linear model with no smoothed random slope
Fig. 4.
Predicted trajectories for stress (over time) by condition. Effects of Mindfulness-Oriented Recovery Enhancement + Just-in-Time Adaptive Intervention (MORE) vs. supportive group (SG) psychotherapy on ecological momentary assessments of stress (mean with 95% C.I.)
Positive Affect
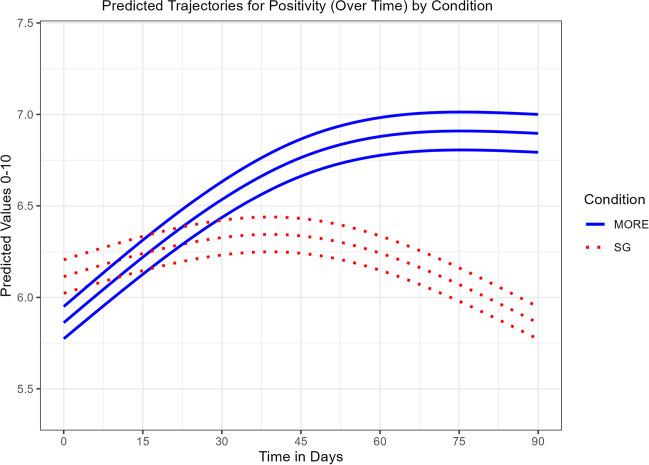
Compared to SG participants, participants in MORE + JITAI reported significantly greater increases in positive affect over time, Group × Time MR = 0.87 (SE = 0.02), p < 0.001 (Table 5). The modified likelihood test of the main effect of condition indicates the MORE + JITAI showed significantly more positive affect at the end of observation. Figure 5 indicates the SG group had a small increase in positivity initially, but then showed a decline below baseline by the end of observation, while the MORE + JITAI group showed increased positive affect until hitting an upper bound at about 70 days into observation. In our sensitivity analysis, the LRT test of the interaction in the presence of the baseline positive affect covariate again found a significant Group × Time interaction favoring MORE, χ2 (1.35) = 27.0, p < 0.001.
Table 5.
Parameter estimates and likelihood ratio tests for the two-level GAMM assessing positive affect
| Parameter | Mean ratio (MR) | SE | 95% CI | t(3697.3) | p |
|---|---|---|---|---|---|
| Fixed effects | |||||
| (intercept) | 2.31 | 0.15 | (2.03, 2.63) | 12.70 | < 0.001 |
| cs(Time) | 1.17 | 0.02 | (1.14, 1.21) | 9.43 | < 0.001 |
| Condition (SG) | 0.63 | 0.05 | (0.54, 0.74) | -5.68 | < 0.001 |
| cs(Time × Condition (SG)) | 0.87 | 0.02 | (0.83, 0.91) | -6.50 | < 0.001 |
| Deviance statistics and ICC | |||||
| Global deviance | -1325.7 | ||||
| AIC | -1150.3 | ||||
| ICC | 0.39 | ||||
| Likelihood ratio tests | df | AIC | LRT | p | |
| re(cs(Time × Condition (SG)) | 77.7 | 2209.8 | 3515.5 | < 0.001 | |
| cs(Time × Condition (SG)) | 1.42 | -1129.7 | 23.4 | < 0.001 | |
| Condition (SG) (modified) | 3.04 | -878.2 | 23.5 | < 0.001 |
SG is the reference group for condition. “re” stands for the random effect term and “cs” stands for cubic spline. Likelihood ratio tests (LRTs) utilize the drop1() function in “gamlss” to test the significance of the terms given the uncertainty of the smooth terms. Only the highest order term is tested. The LRT of Condition is modified by removing the interaction term to test the main effect of Condition at the end of observation. ICC was tested for a simplified, linear model with no smoothed random slope
Fig. 5.
Predicted trajectories for positive affect (over time) by condition. Effects of Mindfulness-Oriented Recovery Enhancement + Just-in-Time Adaptive Intervention (MORE) vs. supportive group (SG) psychotherapy on ecological momentary assessments of positive affect (mean with 95% C.I.)
Additional Sensitivity Analyses
Sensitivity analyses controlling for between-groups differences in pain duration did not change the valence or significance of the observed effects on any outcome.
JITAI-Initiated Mindfulness Practice Impact on Craving, Pain, and Affective State
When MORE + JITAI participants were asked by the app whether they wished to practice mindfulness, participants responded affirmatively 20.5% of the time. Across the study, participants in MORE + JITAI engaged in 395 audio-guided, JITAI-initiated mindfulness practice sessions. Stress (B = 0.49, SE = 0.19, p = 0.009), craving (B = 0.54, SE = 0.16, p < 0.001), and pain (B = 0.37, SE = 0.14, p = 0.011) ratings were higher, and positive affect lower (B = − 0.32, SE = 0.15, p = 0.037) when participants chose to practice mindfulness following stress-triggered probes.
From before to after the audio-guided, JITAI-initiated mindfulness practice sessions, participants reported significant decreases in craving (B = 0.76, SE = 0.16, p < 0.00001), pain (B = 0.61, SE = 0.12, p < 0.00001), and stress (B = 0.85, SE = 0.15, p < 0.00001), and significant increases in positive affect (B = 1.32, SE = 0.20, p < 0.00001).
Mindfulness JITAI Impact on HRV
We observed significant increases in HF-HRV (B = 205.15, SE = 25.91, p < 0.0000001) from before (M = 647.31, SE = 72.79) to after the audio-guided, JITAI-initiated mindfulness practice sessions (M = 852.46, SE = 72.14).
Machine Learning Prediction of JITAI-Initiated Mindfulness Practice Impact
The best machine learning models detected mindfulness JITAI session impact on stress with a sensitivity of 0.76 and specificity of 0.64, on craving with a sensitivity of 0.60 and specificity of 0.73, and on pain with a sensitivity of 0.68 and specificity of 0.67.
Discussion
In this pilot RCT, we found evidence for the preliminary efficacy of a Zoom-based MORE + JITAI intervention for people with chronic pain on LTOT. Relative to a Zoom-based supportive group (SG) psychotherapy control, participants in the MORE + JITAI intervention reported greater reductions in EMA ratings of craving and pain symptoms over time. JITAI-prompted mindfulness practice sessions were associated with robust improvements in stress and positive affect, as well as reductions in craving and pain. Furthermore, we observed significant increases in HF-HRV following JITAI-initiated mindfulness practice, suggesting that the acute practice of mindfulness enhances autonomic regulation in everyday life.
The observed effects on craving and pain indicate that MORE may be efficacious when delivered via telehealth. A meta-analysis demonstrates MORE’s efficacy for reducing craving and pain (Parisi et al., 2022b), with effects evident on EMA measures of these constructs (Garland et al., 2017, 2019b). Although this study was not powered to detect changes in opioid use and misuse, craving and pain are both proximal outcomes linked with opioid consumption. In that regard, pain severity is associated with opioid use and misuse (Goesling et al., 2016; Griffin et al., 2016; Rogers et al., 2020). Similarly, craving has been shown to predict future risk of opioid misuse (Garland & Howard, 2014a; Griffin et al., 2016; Messina & Worley, 2019; Wasan et al., 2012). Given the observed effects of MORE + JITAI on craving and pain, future fully powered RCTs should test whether this digital intervention format, like the face-to-face intervention, might also reduce opioid use and misuse.
With regard to the secondary outcomes, MORE + JITAI improved momentary stress and positive affect to a significantly greater extent than the SG. The effects on stress, while robust, should be considered with the caveat that the MORE + JITAI group began the study with substantially higher stress levels; thus, the observed effects may be due to regression to the mean. Yet, treatment session attendance among participants in MORE + JITAI was inversely associated with stress ratings, suggesting that the observed stress-reducing effects result from the specific content in the MORE intervention and not merely due to the passage of time. Nonetheless, our findings converge with those of a recent meta-analysis indicating that MORE improves multifarious forms of psychiatric distress (Parisi et al., 2022b). Regarding positive affect, multiple trials have demonstrated MORE’s effects on enhancing positive emotional processes, including EMA measures of positive affect (Garland et al., 2017, 2019a, 2019b, 2019c). MORE may increase positive affect in chronic pain patients on LTOT by enhancing neurophysiological responsiveness to natural rewards, as previously observed (Garland et al., 2021, 2014a, 2019a).
Engagement with the Zoom-based interventions was quite good, with 95% of the sample surpassing the treatment completion threshold (≥ 4 sessions) set in other mindfulness trials (Garland et al., 2022; Kuyken et al., 2015), and most participants attending the majority of the sessions. We maximized attendance and engagement through a number of strategies. First, we had a research staff member serve as an “operator” who remained on the Zoom call while the rest of the group met in a Zoom breakout room. The operator helped solve participant technical difficulties without causing disruption to the group. Study therapists also increased engagement by requiring cameras to be turned on, regularly checking in with group members, and engaging them in processing their therapeutic experiences. We worked to increase engagement with the smartwatch and mEMA app by providing technical support as needed, including support in downloading and setting up the app as well as maintaining an ongoing connection. Although study participants wore the smartwatches throughout the protocol, the JITAI response rate was modest, with participants choosing to practice mindfulness only 20.5% of the time when prompted. Because stress prompts could be delivered when participants were harried and busy with activities of daily living, having a selection of ultra-brief mindfulness practices (< 5 min) in future studies might help increase JITAI-initiated mindfulness practice engagement.
The stress-triggered, JITAI-initiated mindfulness practice component of the remote MORE + JITAI intervention is one of its most novel features. Given the role of stress in escalating the downward spiral of opioid use towards opioid misuse and OUD, intervening at critical periods of high stress by prompting mindfulness practice engagement might significantly improve opioid and pain-related outcomes. Here, we offered patients timely assistance with stress coping by alerting them of the need to practice mindfulness with a wearable sensing system that provides quantified physiologic estimates of stress in the natural environment. Given that an individual’s pain level and desire for opioids can change rapidly and unexpectedly with the onset of stressors in the environment, the real-time information from our JITAI system provided a dynamic personalized treatment by providing mind–body interventions for chronic pain and opioid misuse at moments when they are more likely to be useful. In the present study, participants were more likely to choose to listen to the mindfulness audio when they experienced more severe momentary symptom profiles (higher pain, craving, and stress, and lower positive affect), and the JITAI-initiated mindfulness practice sessions were associated with significant reductions in momentary opioid craving and pain symptoms, as well as improvements in stress and positive affect. Yet, in the absence of a randomized control JITAI condition, we cannot make strong causal inferences regarding JITAI efficacy.
Wearable technologies are ubiquitous. Their rise has been accelerated during the COVID-19 pandemic as people are becoming increasingly interested in tracking their health while working from home. The current results show a promising direction in predicting the effectiveness of JITAI-initiated mindfulness practice in treating stress, pain, and opioid craving using a commercially available, low-cost wristwatch sensor. We show that machine learning models using attributes derived from optical heart rate, SpO2, and accelerometer sensors can detect symptom improvement following JITAI-initiated mindfulness practice sessions with some degree of sensitivity and specificity. Previous research has utilized biometric data to create machine learning models that predict stress (Carreiro et al., 2020; Chen et al., 2021; Sandulescu et al., 2015), pain (Chen et al., 2021; Pouromran et al., 2021), and craving (Carreiro et al., 2020; Gullapalli et al., 2019). Sensitivity and specificity metrics to detect stress and craving among populations who use substances have been observed to range between 0.65 and 0.76 (Carreiro et al., 2020) and are thus comparable to the values we obtained. Yet, no previous studies have examined the use of machine learning models to evaluate mindfulness-based or JITAI practices. Traditional methods often rely on self-reported answers from participants, which have limitations such as memory and response biases, under- or over-reporting, and a lack of standardization (Maisto et al., 1990). Our work aims at addressing these issues by developing machine learning models that demonstrate promising initial results in detecting the impact of mindfulness on stress, craving, and pain. These models can be used to compare the effectiveness of various mindfulness practices and determine the optimal time to prompt mindfulness sessions for maximum impact. In the future, we plan to improve the models’ performance by using raw physiological signals and incorporating standard features like heart rate, HRV, calorie count, and step information. The fact that such metrics are available in most commercial wristwatches attests to the scalability of our work, though our models should be optimized by being trained on larger datasets. Altogether, our findings demonstrate that a generalizable wearable computing platform can be developed that can passively and continuously monitor the effectiveness of mindfulness interventions in real time. Developing an objective, machine learning metric for mindfulness effectiveness can unlock new opportunities for a personalized mindfulness approach, using an intelligent recommendation system that can identify optimal mindfulness practices based on user-specific characteristics from a range of possible techniques. Wearable computing systems have also been proposed to recognize moments of craving (Gullapalli et al., 2019). By integrating such a craving detection system with the objective mindfulness effectiveness estimation algorithm, we envision a complete closed-loop system that will not only identify optimal moments (based on craving inference) to trigger mindfulness intervention but also continuously learn to update the recommendations based on person-specific traits, contexts, and outcomes.
Limitations and Future Directions
The primary limitation of this study relates to its research design. Although this was a RCT, the current design cannot disentangle the relative impact of the telehealth MORE intervention from the mindfulness JITAIs. Such intervention dismantling would have required a much larger study than was feasible for this pilot trial. Future full-scale trials should employ a 2 × 2 factorial design to determine the independent and interactive effects of the two intervention components. Such a design is critical for determining if using the wearable sensor to trigger JITAI-initiated mindfulness practice sessions actually increases mindfulness practice engagement above the unprompted base rate. Parallel to meta-analytic results indicating that wearable sensors do increase physical activity engagement in individuals with chronic disease (Kirk et al., 2019), we hypothesized that use of wearable sensors would increase mindfulness practice engagement. Nonetheless, this approach still requires participant engagement and motivation: the individual must choose to attach the sensor each morning, and must choose to practice when prompted. Gamification, contingency management, and motivational strategies to increase engagement and facilitate readiness to change might help to increase the uptake of the mindfulness JITAI intervention.
This study had several other limitations. First, by random chance, our simple randomization allocation schedule resulted in imbalanced group sizes, yet mixed modeling is robust to imbalances in group sizes. Nonetheless, future studies should employ adaptive randomization to ensure a more balanced allocation ratio. Second, the overall EMA response rate was suboptimal, but not unexpected, given the vulnerable nature of the study participants, many of whom were taking high opioid doses, suffered from severe levels of pain and disability, had comorbid psychiatric disorders, and were troubled by multiple social challenges (low income, family conflict) that made responding to EMA probes difficult. That said, treatment session attendance, but not clinical or demographic variables, predicted the EMA response rate, suggesting that participant conscientiousness or treatment engagement is a key factor in EMA responding. EMA response rates might be improved in future studies using best practices (Bertz et al., 2018). For instance, regular retraining of participants on EMA recording procedures, maximizing user friendliness of app interfaces, and contacting participants when response rates begin to lag below 75% could improve responding. Third, because we did not use a control JITAI probe, we cannot determine the efficacy of the JITAI-initiated mindfulness practice. The changes in symptoms from before to after the JITAI-initiated mindfulness practice sessions represent observational data, but no causal inferences can be drawn from these findings. It is possible that symptoms improved following JITAI-initiated mindfulness practice due to the passage of time or regression to the mean. Fourth, the study was not powered to assess for changes in actual opioid consumption; in the future, full-scale clinical trials are needed to test MORE’s effects on opioid use and misuse. Fifth, although the Garmin Vivosmart provided an estimate of ambulatory HRV that was sensitive to treatment, the autonomic effects of MORE + JITAI should be replicated with well-validated, laboratory-based psychophysiological protocols. Though multiple validation studies have been conducted to date, additional studies are needed to validate and optimize the Firstbeat PRO stress algorithm. Finally, the relative lack of racial diversity in the sample is a limitation to be improved in future studies.
In sum, the present stage 1 RCT suggests that implementing MORE via a Zoom platform is feasible for people with chronic pain receiving LTOT, and when combined with stress-triggered JITAI prompts to practice mindfulness skills, may result in improvements in symptoms of craving and pain in everyday life. Machine learning may further enhance this intervention approach by optimizing and personalizing the selection of mindfulness techniques based on their efficacy from moment-to-moment. Given these promising findings, a future, full-scale RCT of this fully remote, digital mind–body intervention approach is warranted.
Author Contribution
EG conceived and designed the study, and acquired funding. EG as principal investigator had overall responsibility for the management of the study, with support from AH. MS was responsible for data acquisition. MT wrote the code for the mEMA app and stress-triggered JITAI. KP, BG, EG, TR, and MT analyzed the data. EG and TR wrote the first draft of the report. All the authors contributed to, and approved, the final manuscript.
Author Credit Statement
Eric Garland: conceptualization, supervision, writing-original, project administration, funding acquisition. Bhanu Gullapalli: software, formal analysis, writing-original draft. Kort Prince: formal analysis, writing-review and editing. AH: supervision, writing-review and editing. Mathias Sanyer: investigation, software, data curation, writing-review and editing. Mark Tuomenoksa: software, formal analysis, data curation, writing-review and editing. Tahidur Rahman: software, formal analysis, writing-original.
Funding
This research was supported by R01DA042033 (PI: Garland) and W81XWH-16–1-0522 (PI: Garland). ELG was also supported by R01AT011772 (PI: Garland), R01DA056537 (PI: Garland), and R01DA057631 (PI: Garland) during the preparation of this manuscript.
Data Availability
De-identified data (demographics, summary outcome variables) are available for meta-analysis upon reasonable request with a signed data access agreement.
Declarations
Ethics Approval
The University of Utah provided IRB approval for the study.
Informed Consent
Participants gave written, informed consent to participate in this study.
Conflict of Interest
Eric Garland, PhD, LCSW is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness-Oriented Recovery Enhancement (MORE), mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received payment for delivering lectures, and teaching engagements (related to training clinicians in mindfulness) sponsored by institutions of higher education, government agencies, and medical centers. Dr. Garland also receives royalties from the sale of books related to MORE. Dr. Garland is a licensor to BehaVR, LLC. Mark Tuomenoksa is the Chief Technology Officer of Illumivu. No other authors have any related conflicts of interest to disclose.
Disclaimer
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alinia P, Sah RK, McDonell M, Pendry P, Parent S, Ghasemzadeh H, Cleveland MJ. Associations between physiological signals captured using wearable sensors and self-reported outcomes among adults in alcohol use disorder recovery: development and usability study. JMIR Formative Research. 2021;5(7):e27891. doi: 10.2196/27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty L, Lambert S. A systematic review of internet-based self-help therapeutic interventions to improve distress and disease-control among adults with chronic health conditions. Clinical Psychology Review. 2013;33(4):609–622. doi: 10.1016/j.cpr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA. When epidemics collide: Coronavirus disease 2019 (COVID-19) and the opioid crisis. Annals of Internal Medicine. 2020;173(1):59–60. doi: 10.7326/M20-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufman PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bertz JW, Epstein DH, Preston KL. Combining ecological momentary assessment with objective, ambulatory measures of behavior and physiology in substance-use research. Addictive Behaviors. 2018;83:5–17. doi: 10.1016/j.addbeh.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau A, Frimerman L, Verner M, Sirois A, Fournier C, Scott K, Perez J, Shir Y, Martel MO. Day-to-day opioid withdrawal symptoms, psychological distress, and opioid craving in patients with chronic pain prescribed opioid therapy. Drug and Alcohol Dependence. 2021;225:108787. doi: 10.1016/j.drugalcdep.2021.108787. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiro S, Chintha KK, Shrestha S, Chapman B, Smelson D, Indic P. Wearable sensor-based detection of stress and craving in patients during treatment for substance use disorder: a mixed methods pilot study. Drug and Alcohol Dependence. 2020;209:107929. doi: 10.1016/j.drugalcdep.2020.107929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh K, Strauss C, Forder L, Jones F. Can mindfulness and acceptance be learnt by self-help?: A systematic review and meta-analysis of mindfulness and acceptance-based self-help interventions. Clinical Psychology Review. 2014;34(2):118–129. doi: 10.1016/j.cpr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Charlton PH, Bonnici T, Tarassenko L, Clifton DA, Beale R, Watkinson PJ. An assessment of algorithms to estimate respiratory rate from the electrocardiogram and photoplethysmogram. Physiological Measurement. 2016;37(4):610–626. doi: 10.1088/0967-3334/37/4/610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Abbod M, Shieh J-S. Pain and stress detection using wearable sensors and devices—a review. Sensors. 2021;21(4):1030. doi: 10.3390/s21041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, S., Jaimes, L. G., & Labrador, M. A. (2017). mstress: a mobile recommender system for just-in-time interventions for stress. 2017 14th IEEE Annual Consumer Communications & Networking Conference (CCNC), 1–5. 10.1109/CCNC.2017.8015367
- Compton WM, Jones CM. Epidemiology of the US opioid crisis: The importance of the vector. Annals of the New York Academy of Sciences. 2019;1451(1):130–143. doi: 10.1111/nyas.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperman, N. A., Hanley, A. W., Kline, A., & Garland, E. L. (2021). A pilot randomized clinical trial of mindfulness-oriented recovery enhancement as an adjunct to methadone treatment for people with opioid use disorder and chronic pain: impact on illicit drug use, health, and well-being. Journal of Substance Abuse Treatment, 127, 108468. 10.1016/j.jsat.2021.108468 [DOI] [PMC free article] [PubMed]
- Csikszentmihalyi, M., & Larson, R. (2014). Validity and reliability of the experience-sampling method. In Flow and the foundations of positive psychology (35–54). Springer. [DOI] [PubMed]
- Cuijpers P, Van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: A systematic review. Journal of Behavioral Medicine. 2008;31(2):169–177. doi: 10.1007/s10865-007-9144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan DK, Herschell AD, Bodea-Crisan T, Schake PL, Gavin JG. The Core 4 Clinical Model: Strengthening the rural behavioral health workforce through a focus on foundational clinical skills. Journal of Rural Mental Health. 2020;44(2):118. doi: 10.1037/rmh0000133. [DOI] [Google Scholar]
- Dotson JAW, Roll JM, Packer RR, Lewis JM, McPherson S, Howell D. Urban and rural utilization of evidence-based practices for substance use and mental health disorders. The Journal of Rural Health. 2014;30(3):292–299. doi: 10.1111/jrh.12068. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- Firstbeat Technologies, Ltd. (2014). Stress and recovery analysis method based on 24-hour heart rate variability. https://assets.firstbeat.com/firstbeat/uploads/2015/11/Stress-and-recovery_white-paper_20145.pdf
- Föhr T, Tolvanen A, Myllymäki T, Järvelä-Reijonen E, Rantala S, Korpela R, Peuhkuri K, Kolehmainen M, Puttonen S, Lappalainen R. Subjective stress, objective heart rate variability-based stress, and recovery on workdays among overweight and psychologically distressed individuals: A cross-sectional study. Journal of Occupational Medicine and Toxicology. 2015;10(1):39. doi: 10.1186/s12995-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimerman L, Verner M, Sirois A, Scott K, Bruneau A, Perez J, Shir Y, Martel MO. Day-to-day hedonic and calming effects of opioids, opioid craving, and opioid misuse among patients with chronic pain prescribed long-term opioid therapy. Pain. 2021;160(8):2214–2224. doi: 10.1097/j.pain.0000000000002220. [DOI] [PubMed] [Google Scholar]
- Garg S, Garg D, Turin TC, Chowdhury MFU. Web-based interventions for chronic back pain: a systematic review. Journal of Medical Internet Research. 2016;18(7):e4932. doi: 10.2196/jmir.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Howard MO. Opioid attentional bias and cue-elicited craving predict future risk of prescription opioid misuse among chronic pain patients. Drug and Alcohol Dependence. 2014;144:283–287. doi: 10.1016/j.drugalcdep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (berl) 2014;231(16):3229–3238. doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology. 2014;82(3):448. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Finan PH, Thomas EA, Priddy SE, Riquino MR, Howard MO. Pain, hedonic regulation, and opioid misuse: Modulation of momentary experience by Mindfulness-Oriented Recovery Enhancement in opioid-treated chronic pain patients. Drug and Alcohol Dependence. 2017;173:S65–S72. doi: 10.1016/j.drugalcdep.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Garland EL, Atchley RM, Hanley AW, Zubieta J-K, Froeliger B. Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: neural and affective evidence of target engagement. Science Advances. 2019;5(10):eaax1569. doi: 10.1126/sciadv.aax1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Kline A, Cooperman NA. Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessmens from a stage 1 randomized controlled trial. Drug and Alcohol Dependence. 2019;203(1):61–65. doi: 10.1016/j.drugalcdep.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, Howard MO. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2019;87(10):927–940. doi: 10.1037/ccp0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fix S, Hudak J, Bernat EM, Nakamura Y, Froeliger B, Hanley AW, Donaldson GW, Marchand WR. Mindfulness-Oriented Recovery Enhancement remediates anhedonia in chronic opioid use by enhancing neurophysiological indices of natural reward responsiveness. Psychological Medicine. 2021 doi: 10.1017/S0033291721003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Nakamura Y, Barrett JW, Baker AK, Reese SE, Riquino MR, Froeliger B, Donaldson GW. Mindfulness-Oriented Recovery Enhancement vs supportive group therapy for co-occurring opioid misuse and chronic pain in primary care: A randomized clinical trial. JAMA Internal Medicine. 2022;182(4):407–417. doi: 10.1001/jamainternmed.2022.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland, E. L. (2013). Mindfulness-oriented recovery enhancement for addiction, stress, and pain. NASW Press.
- Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ, Brummett CM. Trends and predictors of opioid use following total knee and total hip arthroplasty. Pain. 2016;157(6):1259. doi: 10.1097/j.pain.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, McDermott KA, McHugh RK, Fitzmaurice GM, Jamison RN, Weiss RD. Longitudinal association between pain severity and subsequent opioid use in prescription opioid dependent patients with chronic pain. Drug and Alcohol Dependence. 2016;163:216–221. doi: 10.1016/j.drugalcdep.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullapalli BT, Natarajan A, Angarita GA, Malison RT, Ganesan D, Rahman T. On-body sensing of cocaine craving, euphoria and drug-seeking behavior using cardiac and respiratory signals. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies. 2019;3(2):1–31. doi: 10.1145/3328917. [DOI] [Google Scholar]
- Gullapalli BT, Carreiro S, Chapman BP, Ganesan D, Sjoquist J, Rahman T. OpiTrack: A wearable-based clinical opioid use tracker with temporal convolutional attention networks. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies. 2021;5(3):1–29. doi: 10.1145/3478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley AW, Garland EL. The Mindfulness-Oriented Recovery Enhancement Fidelity Measure (MORE-FM): Development and validation of a new tool to assess therapist adherence and competence. Journal of Evidence-Based Social Work. 2021;19(3):308–322. doi: 10.1080/26408066.2020.1833803. [DOI] [PubMed] [Google Scholar]
- Kim H-G, Cheon E-J, Bai D-S, Lee YH, Koo B-H. Stress and heart rate variability a meta-analysis and review of the literature. Psychiatry Investigation. 2018;15(3):235–245. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk MA, Amiri M, Pirbaglou M, Ritvo P. Wearable technology and physical activity behavior change in adults with chronic cardiometabolic disease: A systematic review and meta-analysis. American Journal of Health Promotion. 2019;33(5):778–791. doi: 10.1177/0890117118816278. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): A randomised controlled trial. The Lancet. 2015;386(9988):63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- Lindsay EK, Chin B, Greco CM, Young S, Brown KW, Wright AG, Smyth JM, Burkett D, Creswell JD. How mindfulness training promotes positive emotions: Dismantling acceptance skills training in two randomized controlled trials. Journal of Personality and Social Psychology. 2018;115(6):944. doi: 10.1037/pspa0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Määttänen I, Henttonen P, Väliaho J, Palomäki J, Thibault M, Kallio J, Mäntyjärvi J, Harviainen T, Jokela M. Positive affect state is a good predictor of movement and stress: Combining data from ESM/EMA, mobile HRV measurements and trait questionnaires. Heliyon. 2021;7(2):e06243. doi: 10.1016/j.heliyon.2021.e06243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison, A., Vasey, M., Emery, C. F., & Kiecolt-Glaser, J. K. (2021). Social anxiety symptoms, heart rate variability, and vocal emotion recognition in women: evidence for parasympathetically-mediated positivity bias. Anxiety, Stress, & Coping, 34(3), 243–257. 10.1080/10615806.2020.1839733 [DOI] [PubMed]
- Maisto, S. A., McKay, J. R., & Connors, G. J. (1990). Self-report issues in substance abuse: state of the art and future directions. Behavioral Assessment, 12(1), 117–134.
- Martel MO, Dolman AJ, Edwards RR, Jamison RN, Wasan AD. The association between negative affect and prescription opioid misuse in patients with chronic pain: The mediating role of opioid craving. The Journal of Pain. 2014;15(1):90–100. doi: 10.1016/j.jpain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MO, Finan PH, McHugh RK, Issa M, Edwards RR, Jamison RN, Wasan AD. Day-to-day pain symptoms are only weakly associated with opioid craving among patients with chronic pain prescribed opioid therapy. Drug and Alcohol Dependence. 2016;162:130–136. doi: 10.1016/j.drugalcdep.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz, E. F. (2021). The National Survey on Drug Use and Health (NSDUH): 2020. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases. Accessed May 7.
- Messina BG, Worley MJ. Effects of craving on opioid use are attenuated after pain coping counseling in adults with chronic pain and prescription opioid addiction. Journal of Consulting and Clinical Psychology. 2019;87(10):918–926. doi: 10.1037/ccp0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, D. W., Ridenour, T. A., Swingler, M. M., Morgan, A., & Hegarty-Craver, M. (2022). Feasibility of combining biosensor and ecological momentary assessment to measure stress experiences among economically disadvantaged adolescents. Stress and Health. 10.1002/smi.3211 [DOI] [PubMed]
- Myllymäki T, Kyröläinen H, Savolainen K, Hokka L, Jakonen R, Juuti T, Martinmäki K, Kaartinen J, Kinnunen M-L, Rusko H. Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. Journal of Sleep Research. 2011;20(1pt2):146–153. doi: 10.1111/j.1365-2869.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- Myllymäki T, Rusko H, Syväoja H, Juuti T, Kinnunen M-L, Kyröläinen H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. European Journal of Applied Physiology. 2012;112(3):801–809. doi: 10.1111/j.1365-2869.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- Nahum-Shani I, Hekler EB, Spruijt-Metz D. Building health behavior models to guide the development of just-in-time adaptive interventions: a pragmatic framework. Health Psychology. 2015;34(S):1209. doi: 10.1037/hea0000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesh O, Scheiber C, Kesler S, Gevirtz R, Heckler C, Guido JJ, Janelsins M, Cases MG, Tong B, Miller JM. Secondary outcomes of a behavioral sleep intervention: A randomized clinical trial. Health Psychology. 2019;38(3):196. doi: 10.1037/hea0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi A, Landicho HL, Hudak J, Leknes S, Froeliger B, Garland EL. Emotional distress and pain catastrophizing predict cue-elicited opioid craving among chronic pain patients on long-term opioid therapy. Drug and Alcohol Dependence. 2022;233:109361. doi: 10.1016/j.drugalcdep.2022.109361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi A, Roberts RL, Hanley AW, Garland EL. Mindfulness-Oriented Recovery Enhancement for addictive behavior, psychiatric distress, and chronic pain: A multilevel meta-analysis of randomized controlled trials. Mindfulness. 2022;13:2396–2412. doi: 10.1007/s12671-022-01964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V. Scikit-learn: Machine learning in Python. The Journal of Machine Learning Research. 2011;12:2825–2830. [Google Scholar]
- Pouromran F, Radhakrishnan S, Kamarthi S. Exploration of physiological sensors, features, and machine learning models for pain intensity estimation. Plos One. 2021;16(7):e0254108. doi: 10.1371/journal.pone.0254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rogers C. Client-centered therapy: Its current practice, implications, and theory. Constable; 2003. [Google Scholar]
- Rogers AH, Bakhshaie J, Zvolensky MJ, Vowles KE. Pain anxiety as a mechanism linking pain severity and opioid misuse and disability among individuals with chronic pain. Journal of Addiction Medicine. 2020;14(1):26–31. doi: 10.1097/ADM.0000000000000538. [DOI] [PubMed] [Google Scholar]
- Rusko, H., Rönkä, T., Uusitalo, A., Kinnunen, U., Mauno, S., Feldt, T., Kinnunen, M. L., Martinmäki, K., Hirvonen, A., & Hyttinen, S. (2006). Stress and relaxation during sleep and awake time, and their associations with free salivary cortisol after awakening. Nordic Ergonomics Society Congress.
- Sandulescu, V., Andrews, S., Ellis, D., Bellotto, N., & Mozos, O. M. (2015). Stress detection using wearable physiological sensors. Artificial Computation in Biology and Medicine: International Work-Conference on the Interplay Between Natural and Artificial Computation, IWINAC 2015, Elche, Spain, June 1–5, 2015, Proceedings, Part I 6, 526–532.
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Spruijt-Metz D, Nilsen W. Dynamic models of behavior for just-in-time adaptive interventions. IEEE Pervasive Computing. 2014;13(3):13–17. doi: 10.1109/MPRV.2014.46. [DOI] [Google Scholar]
- Spybrook, J., Bloom, H., Congdon, R., Hill, C., Martinez, A., Raudenbush, S., & To, A. (2011). Optimal design plus empirical evidence: documentation for the “optimal design” software. Retrieved on November, 5, 2012: https://www.stat.cmu.edu/~brian/463-663/week14/od/od-manual-20111016-v300.pdf.
- Stasinopoulos MD, Rigby RA, Heller GZ, Voudouris V, De Bastiani F. Flexible regression and smoothing: Using GAMLSS in R. CRC Press; 2017. [Google Scholar]
- Teisala T, Mutikainen S, Tolvanen A, Rottensteiner M, Leskinen T, Kaprio J, Kolehmainen M, Rusko H, Kujala UM. Associations of physical activity, fitness, and body composition with heart rate variability–based indicators of stress and recovery on workdays: A cross-sectional study. Journal of Occupational Medicine and Toxicology. 2014;9(1):16. doi: 10.1186/1745-6673-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo A, Mets T, Martinmäki K, Mauno S, Kinnunen U, Rusko H. Heart rate variability related to effort at work. Applied Ergonomics. 2011;42(6):830–838. doi: 10.1016/j.apergo.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Valentine LM, Donofry SD, Broman RB, Smith ER, Rauch SA, Sexton MB. Comparing PTSD treatment retention among survivors of military sexual trauma utilizing clinical video technology and in-person approaches. Journal of Telemedicine and Telecare. 2020;26(7–8):443–451. doi: 10.1177/1357633X19832419. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain. 2015;156(4):569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving of prescription opioids in patients with chronic pain: A longitudinal outcomes trial. The Journal of Pain. 2012;13(2):146–154. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LB. Telemedicine interventions for substance-use disorder: A literature review. Journal of Telemedicine and Telecare. 2012;18(1):47–53. doi: 10.1258/jtt.2011.110608. [DOI] [PubMed] [Google Scholar]
- Ziadni MS, Gonzalez-Castro L, Anderson S, Krishnamurthy P, Darnall BD. Efficacy of a single-session “Empowered Relief” Zoom-delivered group intervention for chronic pain: randomized controlled trial conducted during the COVID-19 pandemic. Journal of Medical Internet Research. 2021;23(9):e29672. doi: 10.2196/29672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data (demographics, summary outcome variables) are available for meta-analysis upon reasonable request with a signed data access agreement.