Abstract
A 1.5-stage exchange total knee arthroplasty for periprosthetic joint infection has been described; however, achieving a balanced and well-aligned construct can sometimes be difficult given the bony defects often encountered in these cases. The use of robotic navigation technologies allows for accurate and precise implant placement. This technique report details the utilization of robotic navigation in a 1.5-stage exchange total knee arthroplasty for periprosthetic joint infection and describes the outcome of 6 patients. This technique guide highlights how robotic technology can account for many commonly encountered bone voids, joint line identification, and component orientation, while achieving a balanced and well-aligned knee.
Keywords: Total knee arthroplasty, Robotic-assisted, Revision total knee arthroplasty, Periprosthetic joint infection
Introduction
As the number of primary total knee arthroplasties (TKA) increases, a 78% to 182% increase in revision TKAs is projected by 2030 [1]. Periprosthetic joint infection (PJI) has been associated with the most common reason for performing a revision TKA, closely followed by aseptic loosening [2,3]. Traditionally, revision TKA for PJI has been performed as two-staged procedures with manual instrumentation with an 80% infection eradication rate [4]. Through strict patient selection and improved surgical techniques, 1.5-stage exchange TKA can result in similar success rates, postoperative clinical scores, and knee range of motion compared to two-stage exchange revision TKA [3,[5], [6], [7]]. The 1.5-stage exchange TKA also offers advantages of only 1 operation, reduced hospitalization, and reduced cost compared to two-stage revision TKAs [3,8,9]. Eliminating a second surgery can decrease associated surgical morbidity and potentially decrease the complexity of the operation as increased number of surgeries are often linked with increased bone loss requiring augmentation and loss of motion.
Robotic-assisted TKA (RATKA) can assist the surgeon, facilitating the management of bone loss in revision TKA, typically associated with loss of anatomic landmarks, difficulty in creating femoral and tibia bone cuts, implant placement, and alignment. Robotic-assisted primary TKA cases have shown improved accuracy and precision of component implantation and achievement of neutral mechanical axis [[10], [11], [12], [13]]. This technology also allows for improved soft-tissue protection with visual, auditory, and tactile feedback [[13], [14], [15]]. Studies have shown that patients have increased satisfaction with RATKA through improved outcomes and decreased number of outliers [14,16,17]. Published case reports have shown that RATKA can be used to convert unicondylar knee arthroplasty to TKA and for the second stage of a two-stage revision TKA; however, there is currently no literature highlighting the merit of this technology in 1.5-stage exchange RATKAs for PJI [[18], [19], [20], [21]].
Indications for use of a 1.5-stage exchange RATKA included the presence of a PJI, an adequate soft-tissue envelope without a sinus tract, adequate bone stock on the femur and tibia that would not be anticipated to require major augmentation or use of stems or cones for fixation, and intact collateral ligaments. Contraindications for 1.5-stage exchange RATKA included a previously failed 1-stage, 1.5-stage, or 2-stage exchange TKA; incompetent collateral ligaments; the presence of a sinus tract or soft-tissue compromise; extensive bone loss requiring stem augmentation for bone fixation; extensor mechanism disruption; and the presence of highly resistant bacterial or fungal organisms.
The purpose of this technique guide is to describe a novel use of robotic-assisted 1.5-stage exchange TKA for PJI. A small case series of 6 patients who were diagnosed with chronic PJI using the 2018 Musculoskeletal Infection Society criteria and treated using this novel technique is described as well [22].
Surgical technique
Planning for a robotic-assisted 1.5-stage exchange TKA begins prior to the operating room, starting with preoperative radiographs (Fig. 1). The patient undergoes a standard Mako-protocol computed tomography (CT) scan using a markerball reference. The surgeon collaborates with the Mako product specialist to confirm the quality of the scan and for segmentation to identify bone beneath the implant, femoral epicondyles, and the distal aspect of the metal femoral component on the medial and lateral sides. This is a critical landmark as it will be later utilized during the registration process. The curved portion of the existing implant and anterior flange are unreliable as landmarks, as various implants can have varied shapes in these regions, which can appear further distorted by metal refraction on the image. The tibia baseplate and bony prominences can be identified for later use to facilitate anatomic registration. Other traditional landmarks are similarly registered including medial/lateral malleoli and femoral head center. Digital implants are planned to be placed on a base of bone support, and identification of existing implant and bone cement is critical in this process.
Figure 1.
Preoperative radiographs. (a) Radiographs of the right knee showing the anteroposterior view, (b) lateral view, and (c) patella sunrise view.
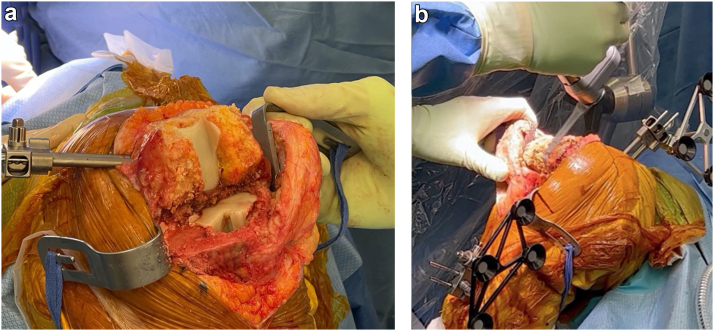
After the induction of anesthesia, antibiotics are administered, a time-out is performed, and then the previous midline incision is utilized dissecting down to capsule. Adipose flaps are elevated, the joint is aspirated for culture, followed by a medial parapatellar arthrotomy. An extensive synovectomy is then performed, with 2 samples sent for culture (Fig. 2). A quadriceps snip was not required in any case but can be utilized as needed. A standard medial release is performed deep to the medial collateral ligament allowing the tibia to be dislocated during later knee flexion. The implants are kept in place and not removed at this point, in order to allow for dynamic balancing of the knee.
Figure 2.
(a) The infected synovium is debrided from the medial and lateral gutters as well as from the suprapatellar and infrapatellar regions and posterior capsule. (b) Image showing the knee after debridement is completed.
The femoral and tibial arrays are then placed, and we recommend using the 3.2-mm threaded pins to minimize the size of additional bone violations being created in these revision cases. The surgeon has the option to place the arrays in a traditional extraarticular fashion with the femoral and tibial pins outside the joint or to have the femoral array intraarticular at the anteromedial aspect of the metaphyseal-diaphyseal junction at approximately the 45° angle with respect to the epicondylar axis. This location avoids the existing implant, while minimizing any canal compromise should the surgeon wish to enter the canal with femoral stems at a later point in the procedure. On the tibial side, 2 pins are placed through separate stab incisions at least 150 mm from the joint line (Fig. 3) to avoid interfering with access for debridement of the tibia canal and bone preparation and implant placement [19]. Additionally, checkpoints can be placed about the femur and tibia.
Figure 3.
The tibia array should be placed at least 150 mm from the joint line to avoid interference with the tibia canal debridement and stem preparation.
Registration proceeds in standard fashion. The operative leg is manipulated to find the center of the femoral head, and then the medial and lateral malleoli are identified along with the checkpoints. Forty points are then identified about the femur, which is complicated by the existing metal implant. We have determined that a unique series of landmarks can be used in lieu of the traditional femoral landmarks including 12 bony points on the anterior aspect of the femur proximal to the anterior flange, 12 points about the medial portion of the medial femoral condyle (Fig. 4) and about the epicondyle, 8 points about the lateral femoral condyle and epicondyle, and 8 points on the metal femoral component including 4 points on the distal lateral and 4 points on the distal medial femoral condyles (Fig. 5, Video 1). Similarly, on the tibial side, registration commences about the anterior aspect of the tibia baseplate (Fig. 6). Twelve points can be captured along the anterior margin on the metal tibia baseplate, followed by an additional 8 points just below this on the bone surface. Next, the surgeon can choose another 12 points about any anteromedial and anterior bone landmarks that can be achieved, typically using a v-shaped pattern for this, and finally 8 points about the tibial tubercle (Video 1).
Figure 4.
Registration of the medial femoral condyle. (a) Intraoperative medial femoral condyle registration, which can be correlated to the model as shown in (b) with the probe pointing to the area of interest.
Figure 5.
Registration of the distal medial femoral condyle. (a) Intraoperative distal medial femoral condyle registration, which can be correlated to the model as shown in (b) with the arrow pointing to the area of interest.
Figure 6.
Registration of the tibia baseplate. (a) Intraoperative tibia baseplate registration, which can be correlated to the model as shown in (b) with the arrow pointing to the area of interest.
The balancing portion of the procedure can identify any existing malalignment that can be corrected with the revision surgery. Extension and flexion of the joint replacement can be confirmed, as well as varus-valgus stability in extension and flexion. The surgeon can work with the Mako product specialist to plan for new implants to be placed at supporting bone surfaces deep to the existing implant. One must remember that bone cannot be added, and thus, either the surgeon must plan for augmentation of any bone defects to correct for malrotation or the implant must be rotated in such a way as to remove only existing bone. The surgeon can use the probe to identify the existing implant or cement mantle base to allow the new implants to be placed at this level. This allows for minimizing bone resection as well as ensuring all the existing cement mantle is removed in the surgery.
The existing implants (femur, tibia, patella) are removed in standard fashion, taking care to avoid additional bone loss as this described technique uses primary implants that do not have the advantage of intramedullary stems for additional fixation (Fig. 7a). With the implants removed, the surgeon has access to the posterior capsule for debridement. The robotic-assisted saw can then be used to perform all bone cuts to receive the new implant, while simultaneously debriding any bone in previous contact with prior infection (Fig. 7b). The senior surgeon’s preference is to make bone cuts in the following order: proximal tibia, posterior femur, anterior femur, anterior chamfer, change blade to 90° cutting tool, then distal femur and posterior chamfer. A burr can then be used to remove any remaining cement. At the surgeon’s discretion, the femoral and tibial canals can be opened and debrided with reverse curettes. One advantage of this technique is that it does not mandate opening of the femoral canal if a violation does not already exist. Samples of femoral and tibial canal tissue are additionally sent for culture in these infection cases.
Figure 7.
Debridement of bone surfaces with robotic cuts. (a) Rough bony surfaces after implants were removed. (b) The process of recutting bony surfaces.
If bone defects are encountered, be it from the debridement process or bone loss during implant removal, these defects can be identified with the blunt (green) probe and seen on the planning screen. These defects can be prepared for a defined-size augment to fill the space, as the virtual computer implants can be moved as needed by a measured amount to plan for a new resection depth for that portion of the implant. For example, if a 3- to 4-mm distal medial defect is noted, the virtual implant can be moved on the screen proximal to the base of the defect by 5 mm, and then only this portion of the distal medial femur was recut to prepare for a measured 5-mm distal medial femur augment (Fig. 8).
Figure 8.
Defect on the distal medial femoral condyle measuring 5 mm.
The knee is now prepared for trial components. The femoral box cut is made if not previously present. The tibia baseplate is pinned in position and femoral component placed followed by a trial polyethylene, and the knee joint is relocated. Magnetic augments can be used as needed. The patella can be resurfaced at the surgeon's discretion if there is sufficient patella bone stock, which was performed in all 6 example cases. Knee range of motion and joint stability are confirmed at 0° and 90°, with the robotic tracking technology. Laxity in extension or flexion can be addressed through additional augments, as needed. With the robotic portion of the procedure complete, all trials, arrays, and checkpoints are removed.
The standard protocol for the senior surgeon was used for wound irrigation including 3 liters of normal saline followed by 200 mL of a 50:50 mixture of hydrogen peroxide and sterile water soaked for 3 minutes. Then 3 liters of normal saline is irrigated in the wound, and the wound is soaked in 500 mL of dilute betadine for 3 minutes, followed by another 3 liters of normal saline irrigation. Then the wound is temporarily closed over betadine-soaked sponges, and a new set of drapes and surgical equipment are utilized for the second portion of the procedure.
The senior surgeon’s preferred implant construct is a standard metal femoral posterior-stabilized component with an all-polyethylene posterior-stabilized tibia. As described in the article by Gililland et al., a threaded Steinmann pin is drilled into the base of an all-polyethylene tibia component and then coated in antibiotic cement [9]. Similarly, an intramedullary dowel for the femoral side is fashioned over a threaded Steinmann pin and coated in antibiotic cement. If augmentation of the femoral or tibia components is needed (Fig. 8), additional antibiotic cement can be utilized to custom prepare antibiotic cement augments directly on the implants as needed (Fig. 9). The femoral stem is impacted into the femur, and then the implants are cemented using Palacos cement (Heraeus Medical, Concord, CA) mixed with 6 g of vancomycin, 7.2 g of tobramycin for sensitive organisms. The cocktail of antibiotics can be tailored to the particular infecting organism and known sensitivities as needed. The knee is reduced, and cement allowed to dry. At this point, the tourniquet is let down, and hemostasis achieved. The wound is irrigated and closed in standard fashion. Postoperative radiographs of a representative case can be seen in Figure 10.
Figure 9.
Tibia and femoral components with antibiotic cement augmentation. (a) The final tibia with attached tibia canal stem (1), femoral component with medial augment (2), and femoral canal cement stem (3). (b) The 5-mm cement augmentation for the distal medial femur defect.
Figure 10.
Postoperative anteroposterior (a) and lateral (b) radiographs.
Patients are placed in a knee immobilizer with bulky Jones wrap for 72 hours of soft-tissue rest and made weightbearing as tolerated. Drains are removed after 48 hours, and knee range of motion is initiated at approximately 72 hours, after which they are discharged on intravenous antibiotics as recommended in consultation with infectious disease consultants. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are monitored weekly for 6 weeks and then twice a month until resolution.
This technique was performed on a consecutive series of 6 patients without any patients requiring exclusion per the criteria defined earlier. All patients (Table 1) involved provided informed consent for a 1.5-stage exchange RATKA with intraoperative robotic assistance with Mako (Stryker, Mahwah, NJ) that included removal of existing implants, thorough irrigation and debridement, and cemented implantation of a metal femoral component with an all-polyethylene tibial component as well as for participation in this technique report. Each patient presented with at least 1 month of knee pain, and PJI was diagnosed utilizing the 2018 Musculoskeletal Infection Society criteria [22]. Pathogens diagnosed included Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mitis, and Streptococcus oralis. Each of these organisms were broadly sensitive to antibiotics, and there were no cases of multidrug-resistant pathogens. One case was culture negative (case 5), although this case met the 2018 Musculoskeletal Infection Society criteria satisfying 8 points based on ESR, CRP, white blood cell count, and synovial polymorphonuclear neutrophil percentage, and despite being equal in points value to white blood cell count, it was found to be additionally alpha-defensin positive [22]. A single fellowship-trained arthroplasty surgeon (J.R.D.) at a large academic institution performed the operations from May 2021 to May 2022. All patients underwent standardized postoperative pain management and physical rehabilitation. Patients achieved on average 0°-117.5° of knee range of motion after 6 weeks postoperatively, and after a minimum of 12 months in follow-up, there have been no signs of recurrence of infection based on clinical examination, resolution of the ESR/CRP, and restoration of lower limb function and ambulation without assistive devices. No patient has required second-stage revision TKA.
Table 1.
Case series of robotic-assisted 1.5-stage exchange TKA for PJI.
| Case | Age | Gender | Laterality |
ESR (mm/h)/CRP (mg/L) | Cell count (PMN%) | Alpha-defensin/crystal analysis | Culture | Six-week postop. knee ROM | Signs of infection at 90 d/1 y |
|---|---|---|---|---|---|---|---|---|---|
| Implant | |||||||||
| 1 | 62 | Male | Right | 53/157 | 157,000 (94%) | n/a/negative | Staphylococcus aureus (methicillin-sensitive) | 0-120° | No/no |
| UKA | |||||||||
| 2 | 83 | Female | Left | 88/79 | 74,560 (97%) | ++/negative | Streptococcus mitis | 0-120° | No/no |
| TKA | |||||||||
| 3 | 83 | Female | Right | 88/79 | 59,634 (95%) | ++/negative | Streptococcus mitis | 0-120° | No/no |
| TKA | |||||||||
| 4 | 89 | Male | Right | 71/224 | 42,027 (90%) | ++/negative | Streptococcus oralis | 0-110° | No/no |
| TKA | |||||||||
| 5 | 85 | Male | Left | 80/58 | 43,864 (93%) | ++/negative | Culture negative | 0-120° | No/no |
| TKA | |||||||||
| 6 | 66 | Female | Right | 92/12 | 58,380 (88%) | n/a/negative | Staphylococcus epidermidis | 0-115° | No/no |
| TKA |
n/a, not available; Postop., postoperative; ROM, range of motion; UKA, unicondylar knee arthroplasty; PMN, Polymorphonuclear leukocytes.
Discussion
As 1.5-stage exchange TKA for PJI increases in popularity in the United States, the methods used to achieve a stable, well-aligned knee require improvement. RATKA has been associated with increased accuracy and precision of component placement compared to manual techniques [[10], [11], [12], [13]]. The senior authors' practice has evolved to perform this operation in lieu of traditional articulating cement spacers, except when contraindicated as listed in the introduction. Early outcomes from this technique, as demonstrated in the consecutive 6-case series, are promising with excellent ability to clear the infection, while restoring lower extremity function.
Traditionally, these cases have been treated with a two-stage TKA revision [3,7]. Two-stage revision TKAs require 2 surgeries, an extended course of antibiotics prior to the second surgery, longer recovery and rehabilitation, as well as decreased weightbearing and restricted knee range of motion while the spacer is in place. On the other hand, 1.5-stage exchange RATKA offers the advantages of only 1 operation, reduced hospitalization, and reduced overall cost [3,8]. The 1.5-stage exchange RATKA with cemented all-polyethylene tibial components places the patient at risk of the polyethylene to not fix as well as traditional cemented metal implants or hybrid constructs with intramedullary stems, resulting in potential midterm failure. Should instability or loosening result, however, the patient can undergo a second surgery later under controlled elective conditions, when infection is definitively ruled out and presumably the patient is healthier, which may not have been the case for a planned second stage of a two-stage TKA revision. Therefore, 1.5-stage exchange RATKA gives the patient the chance to avoid a second surgery, without substantially increasing the complication potential should a subsequent surgery be required. This technique further benefits patients as it creates a more stable knee that has a more precise fit to the patient’s bone and allows for full weightbearing immediately after surgery without restrictions on knee range of motion.
One limitation of 1.5-stage exchange TKA is the bone loss encountered during surgery, which can make it difficult to identify bony landmarks critical to identify the joint line, allow for implant rotation and gap balancing, and create a stable knee. An advantage of RATKA is the ability to utilize CT landmarks to identify implant position, prepare for augments as needed, and avoid canal instrumentation, which can decrease the need for metaphyseal augments such as cones and sleeves, bulk allografts, or high-constrained implants [23,24]. If a bone defect is identified, the navigation technology can simulate on the computer a new cut edge at the precise location of a stable bone base, allow the surgeon to precisely re-cut the bone at this depth, and prepare for an augment made of cement or metal (Fig. 7) [9]. Previous case reports by Kalavrytinos et al. (2020) and Yun et al. (2020) have shown the use of robotic conversion of failed unicompartmental knee arthroplasty to TKA secondary to aseptic loosening [18,20]. Yun et al. demonstrated that 29% of the knees manually revised to a TKA required an augment or stem, whereas 0% of the robotically converted knees required an augment as the robotic technology allows for minimization of bone resections during bone preparation [20]. Steelman et al. (2021) demonstrated the use of RATKA revision when treating a failed TKA due to aseptic loosening [21]. Both Kalavrytinos et al. and Steelman et al. used Stryker Mako robotic-assisted techniques and Stryker Triathlon implants and included the patient’s current implants as native bone in the operative plan. MacAskill et al. (2021) showed 2 cases of RATKA revision, one for instability and the other for a two-stage revision arthroplasty [19].
A limitation of this study is the lack of long-term follow-up for the patients. We have found that 1.5-stage exchange RATKA allows for more precise cuts and implant placement, with excellent 90-day and 1-year outcomes; however, long-term follow-up is needed to determine if this technique is superior to conventional 1.5-stage exchange RATKA or 2-stage revision TKA. Another limitation is the initial cost of acquiring the robot technology and associated software and CT scans, yet previous studies have shown that robotic primary arthroplasty is more cost-effective than conventional hip and knee arthroplasty especially as the case volume increases [25,26]. The final limitation is that this surgical technique using the Stryker Mako Robotic-Arm Assisted device is not Food and Drug Administration approved for this use and is considered off-label use of this technology for RATKA. All patients provided consented preoperatively for off-label use of the implants and surgical technique. Previous case reports and this study have shown success with RATKA for revision surgery and the steps it takes to replicate outcomes. As the robotic-assisted arthroplasty technology improves, hopefully the indications for robotic assistance will be broadened to include revision TKAs.
Summary
We describe the use of robotic-assisted 1.5-stage exchange TKA for PJI. We have found excellent early success with this technique, which provides several advantages to the patient including 1 surgery, immediate full weightbearing, unrestricted knee range of motion, and a balanced and well-aligned knee. While further studies are needed to investigate the medium-term and long-term impact of robotic-assisted single-stage revision TKAs, this case series serves as a guide to surgeons looking to implement this 1.5-stage exchange TKA technique for PJI.
Conflicts of interest
Dr. J. R. Danoff is a paid presenter or speaker for Flexion Therapeutics; is a paid consultant for Acelrx and Surgical Specialties Corp; is in the editorial or governing board of Arthroplasty Today; and a committee member of the American Association of Hip and Knee Surgeons. All other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2023.101126.
Appendix A. Supplementary Data
References
- 1.Schwartz A.M., Farley K.X., Guild G.N., Bradbury T.L. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. 2020;35:S79–S85. doi: 10.1016/j.arth.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delanois R.E., Mistry J.B., Gwam C.U., Mohamed N.S., Choksi U.S., Mont M.A. Current epidemiology of revision total knee arthroplasty in the United States. J Arthroplasty. 2017;32:2663–2668. doi: 10.1016/j.arth.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 3.Gehrke T., Alijanipour P., Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J. 2015;97-B:20–29. doi: 10.1302/0301-620X.97B10.36475. [DOI] [PubMed] [Google Scholar]
- 4.Pangaud C., Ollivier M., Argenson J.-N. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019;4:495–502. doi: 10.1302/2058-5241.4.190003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunutsor S.K., Whitehouse M.R., Lenguerrand E., Blom A.W., Beswick A.D., INFORM Team Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One. 2016;11:e0151537. doi: 10.1371/journal.pone.0151537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaghmour K.M., Chisari E., Khan W.S. Single-stage revision surgery in infected total knee arthroplasty: a PRISMA systematic review. J Clin Med. 2019;8:1–13. doi: 10.3390/jcm8020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakrar R.R., Horriat S., Kayani B., Haddad F.S. Indications for a single-stage exchange arthroplasty for chronic prosthetic joint infection: a systematic review. Bone Joint J. 2019;101-B:19–24. doi: 10.1302/0301-620X.101B1.BJJ-2018-0374.R1. [DOI] [PubMed] [Google Scholar]
- 8.Gehrke T., Zahar A., Kendoff D. One-stage exchange: it all began here. Bone Joint J. 2013;95-B:77–83. doi: 10.1302/0301-620X.95B11.32646. [DOI] [PubMed] [Google Scholar]
- 9.Gililland J.M., Carlson V.R., Fehring K., Springer B.D., Griffin W.L., Anderson L.A. Balanced, stemmed, and augmented articulating total knee spacer technique. Arthroplast Today. 2020;6:981–986. doi: 10.1016/j.artd.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liow M.H.L., Xia Z., Wong M.K., Tay K.J., Yeo S.J., Chin P.L. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29:2373–2377. doi: 10.1016/j.arth.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal V.O., Gadekar A.P., Vaidya N. Does robotic technology successfully restore the joint line after total knee arthroplasty? A retrospective analysis. Arthroplasty. 2022;4:6. doi: 10.1186/s42836-021-00103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampp E.L., Chughtai M., Scholl L.Y., Sodhi N., Bhowmik-Stoker M., Jacofsky D.J., et al. Robotic-Arm assisted total knee arthroplasty demonstrated greater accuracy and precision to plan compared with manual techniques. J Knee Surg. 2019;32:239–250. doi: 10.1055/s-0038-1641729. [DOI] [PubMed] [Google Scholar]
- 13.Marchand R.C., Sodhi N., Khlopas A., Sultan A.A., Higuera C.A., Stearns K.L., et al. Coronal correction for severe deformity using robotic-assisted total knee arthroplasty. J Knee Surg. 2018;31:2–5. doi: 10.1055/s-0037-1608840. [DOI] [PubMed] [Google Scholar]
- 14.Khlopas A., Sodhi N., Sultan A.A., Chughtai M., Molloy R.M., Mont M.A. Robotic arm-assisted total knee arthroplasty. J Arthroplasty. 2018;33:2002–2006. doi: 10.1016/j.arth.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 15.Sultan A.A., Piuzzi N., Khlopas A., Chughtai M., Sodhi N., Mont M.A. Utilization of robotic-arm assisted total knee arthroplasty for soft tissue protection. Expert Rev Med Devices. 2017;14:925–927. doi: 10.1080/17434440.2017.1392237. [DOI] [PubMed] [Google Scholar]
- 16.Liow M.H.L., Goh G.S.-H., Wong M.K., Chin P.L., Tay D.K.-J., Yeo S.-J. Robotic-assisted total knee arthroplasty may lead to improvement in quality-of-life measures: a 2-year follow-up of a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:2942–2951. doi: 10.1007/s00167-016-4076-3. [DOI] [PubMed] [Google Scholar]
- 17.Marchand R.C., Sodhi N., Khlopas A., Sultan A.A., Harwin S.F., Malkani A.L., et al. Patient satisfaction outcomes after robotic arm-assisted total knee arthroplasty: a short-term evaluation. J Knee Surg. 2017;30:849–853. doi: 10.1055/s-0037-1607450. [DOI] [PubMed] [Google Scholar]
- 18.Kalavrytinos D., Koutserimpas C., Kalavrytinos I., Dretakis K. Expanding robotic arm-assisted knee surgery: the first attempt to use the system for knee revision arthroplasty. Case Rep Orthop. 2020;2020:4806987. doi: 10.1155/2020/4806987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacAskill M., Blickenstaff B., Caughran A., Bullock M. Revision total knee arthroplasty using robotic arm technology. Arthroplast Today. 2022;13:35–42. doi: 10.1016/j.artd.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun A.G., Qutami M., Chen C.-H.M., Pasko K.B.D. Management of failed UKA to TKA: conventional versus robotic-assisted conversion technique. Knee Surg Relat Res. 2020;32:38. doi: 10.1186/s43019-020-00056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steelman K., Carlson K., Ketner A. Utilization of robotic arm assistance for revision of primary total knee arthroplasty: a case report. J Orthop Case Rep. 2021;11:50–54. doi: 10.13107/jocr.2021.v11.i08.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parvizi J., Tan T.L., Goswami K., Higuera C., della Valle C., Chen A.F., et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309–1314.e2. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 23.Sires J.D., Craik J.D., Wilson C.J. Accuracy of bone resection in MAKO total knee robotic-assisted surgery. J Knee Surg. 2021;34:745–748. doi: 10.1055/s-0039-1700570. [DOI] [PubMed] [Google Scholar]
- 24.Sheth N.P., Bonadio M.B., Demange M.K. Bone loss in revision total knee arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2017;25:348–357. doi: 10.5435/JAAOS-D-15-00660. [DOI] [PubMed] [Google Scholar]
- 25.Rajan P v, Khlopas A., Klika A., Molloy R., Krebs V., Piuzzi N.S. The cost-effectiveness of robotic-assisted versus manual total knee arthroplasty: a markov model-based evaluation. J Am Acad Orthop Surg. 2022;30:168–176. doi: 10.5435/JAAOS-D-21-00309. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado D.R., Go C.C., Kyin C., Rosinsky P.J., Shapira J., Lall A.C., et al. Robotic arm-assisted total hip arthroplasty is more cost-effective than manual total hip arthroplasty: a markov model analysis. J Am Acad Orthop Surg. 2021;29:e168–e177. doi: 10.5435/JAAOS-D-20-00498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.