Abstract
The QT35 cell line was established from a methylcholanthrene-induced tumor in Japanese quail (Coturnix coturnix japonica) (C. Moscovici, M. G. Moscovici, H. Jimenez, M. M. Lai, M. J. Hayman, and P. K. Vogt, Cell 11:95–103, 1977). Two independently maintained sublines of QT35 were found to be positive for Marek's disease virus (MDV)-like genes by Southern blotting and PCR assays. Sequence analysis of fragments of the ICP4, ICP22, ICP27, VP16, meq, pp14, pp38, open reading frame (ORF) L1, and glycoprotein B (gB) genes showed a strong homology with the corresponding fragments of MDV genes. Subsequently, a serotype 1 MDV-like herpesvirus, tentatively name QMDV, was rescued from QT35 cells in chicken kidney cell (CKC) cultures established from 6- to 9-day-old chicks inoculated at 8 days of embryonation with QT35 cells. Transmission electron microscopy failed to show herpesvirus particles in QT35 cells, but typical intranuclear herpesvirus particles were detected in CKCs. Reverse transcription-PCR analysis showed that the following QMDV transcripts were present in QT35 cells: sense and antisense meq, ORF L1, ICP4, and latency-associated transcripts, which are antisense to ICP4. A transcript of approximately 4.5 kb was detected by Northern blotting using total RNA from QT35 cells. Inoculation of QT35 cells with herpesvirus of turkeys (HVT)-infected chicken embryo fibroblasts (CEF) but not with uninfected CEF resulted in the activation of ICP22, ICP27, VP16, pp38, and gB. In addition, the level of ICP4 mRNA was increased compared to that in QT35 cells. The activation by HVT resulted in the production of pp38 protein. It was not possible to detect if the other activated genes were translated due to the lack of serotype 1-specific monoclonal antibodies.
Marek's disease (MD) is a highly contagious lymphoproliferative disease of chickens caused by the MD herpesvirus (MDV). Infection with MDV results in a latent infection of mostly CD4+ T lymphocytes, which may become transformed, leading to neoplastic disease (50). Serotype 1 (oncogenic) and serotype 2 (nononcogenic) strains of MDV are grouped within the Alphaherpesvirinae subfamily, along with serotype 3 herpesvirus of turkeys (HVT) (4). The viral DNA consists of unique long (UL) and unique short (US) sequences, each flanked by a pair of inverted repeats designated terminal repeats (TRL and TRS) and internal repeats (IRL and IRS). Although the complete sequence of MDV has not yet been published, many of the genes have been mapped and sequenced (reviewed in references 46 and 69). Replication of MDV, like that of all herpesviruses, follows a well-defined pattern of gene expression in which immediate-early (IE) genes are expressed first. These IE genes transactivate early and late genes. For example, during replication of herpes simplex virus (HSV), the ICP4 IE gene transactivates several early and late genes (14, 15). The MDV homologue of the ICP4 gene of HSV has been located in the IRS (2). Pratt et al. (41) demonstrated that MDV ICP4 transactivates the expression of MDV pp38. Based on the similarities of MDV to HSV and the cascade pattern of MDV gene expression (55), it seems likely that the MDV genome and the related HVT genome encode proteins capable of activating MDV and HVT promoters. Tieber et al. (62) showed that MDV and HVT can transactivate the promoter region in the long terminal repeat (LTR) of Rous sarcoma virus. Furthermore, they reported that HVT can efficiently transactivate the ICP4 and beta-thymidine kinase gene promoters of HSV as well as the promoter of the IE gene of human cytomegalovirus.
More than 200 lymphoblastoid cell lines have been established from MD tumors (1, 7–9, 32, 35, 39). Most of these cell lines are CD4+ CD8− T cells (56). These lymphoblastoid cell lines are often considered the equivalent of latently infected cells, based on the limited expression of viral genes. In addition to the lymphoblastoid cell lines, an MDV-transformed chicken embryo fibroblast (CEF) cell line has been established, but this cell line also expresses late MDV genes, such as that for glycoprotein B (gB) (5).
Three groups of transcripts located in the repeat regions of MDV in MD lymphoblastoid cell lines have been described. The first group consists of the latency-associated transcripts (LATs), which are antisense to ICP4 (see Fig. 1). Several groups have reported the presence of LATs in MD lymphomas and MD-derived lymphoblastoid cell lines (10, 11, 21, 22, 27, 33); some of these (the MD small RNAs [MSRs]) are poly(A) negative and are retained in the nuclei (11), similar to the 1.5- and 2.0-kb LATs described for HSV (64). Interestingly, the MSRs are also found in nuclei of CEF productively infected with MDV.
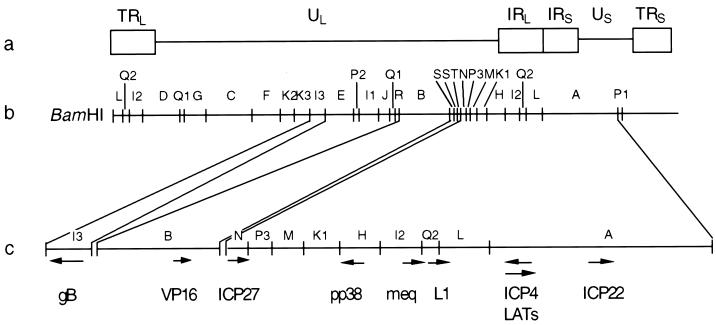
FIG. 1.
(a) Structure of the MDV genome. UL, US, and the flanking repeats (TRL, IRL, IRS, and TRS) are indicated. (b) BamHI restriction enzyme linkage map (16). (c) Locations and directions transcription of the genes of interest.
The second and third group of transcripts described for MD lymphomas and lymphoblastoid cell lines are located in the I2, Q2, and L regions of the repeats flanking the UL sequence (see Fig. 1). The major transcript, referred to as meq, produces the Meq protein, which is expressed in all MDV tumors. The protein has in the N-terminal portion a basic leucine zipper structure closely resembling proteins encoded by the jun/fos oncogene family (18). Meq is an intranuclear protein that binds to the nucleolus and coiled bodies (23). Overexpression of this protein in RAT-2 cells but not CEF leads to growth and/or transformation (24), probably by interaction with the cell cycle regulator CDK2 (25). Two spliced products have been identified in the meq region. The first one (38) produces a protein (Meq-sp) that still has the binding characteristics of Meq but lacks the transactivator domain. The function of this protein remains unclear (36). The second one shares the same carboxy terminus as Meq-sp and has been named vIL-8 (26). vIL-8 may play an important role during the early pathogenesis of MDV (52). Interestingly, two open reading frames (ORFs) antisense to meq have also been identified. The importance of these ORFs is also unknown at the present time (37). ORF L1 (34) is the third transcript, but its function remains unclear. ORF L1 deletion mutants were generated using the CVI988 strain of MDV. These mutants were able to establish latency, and virus could be rescued from latent infections, suggesting that ORF L1 is not important for these functions (57).
In 1977, Moscovici et al. (30) established several continuous cell lines from 20-methylcholanthrene (MCA)-induced tumors in Japanese quail (Coturnix coturnix japonica). These cells lines are free of avian retroviruses, but the presence of MDV in these cell lines has not been examined. QT35 cells are often used for virus propagation and are susceptible to infection with a wide range of avian viruses, including avian leukosis virus (ALV) subgroups C, E, and F and HVT (13, 30) but not serotype 1 MDV (12). In this paper, the presence of MDV genes in QT35 cells, the subsequent rescue of a herpesvirus from these cells, and the expression of transcripts of MDV genes associated with latency and/or transformation in these cells are reported. In addition, infection of QT35 cells with HVT resulted in the transactivation of several IE, early, and late genes of MDV. Subsequently, a herpesvirus was rescued from QT35 cells and was named QMDV solely for the purpose of differentiating it from chicken MDV isolates. Moreover, the QT35 cell line provides a new in vitro model allowing the study of the maintenance of and reactivation from latency of a truly latent alphaherpesvirus.
MATERIALS AND METHODS
Cells and viruses.
Two independently maintained sublines (A and B) of QT35 cells were used. Subline A was received in the Department of Avian and Aquatic Animal Medicine (currently part of the Department of Microbiology and Immunology) as passage 47 (p47) in 1982. Cells from this passage were recovered from the liquid nitrogen for this study and used at between p48 and p55. The second subline cannot be traced completely but was obtained from the laboratory of K. Skeeles (deceased in 1999) of the University of Arkansas and had never been propagated in a laboratory working with MDV. In addition, CEF and chicken kidney cells (CKC) were used. The CEF and CKC cultures were established from embryos and 2-week-old chicks, respectively, derived from departmental specific-pathogen-free (SPF) flocks of chickens. CEF, CKC, and QT35 cells were propagated in an M199-based medium (51) supplemented with 2, 5, and 4% fetal bovine serum, respectively, in a humidified CO2 incubator at 38.5°C. The percentage of serum was reduced to 0.2% after CEF and CKC cultures became confluent. QT35 cell cultures were subcultured at approximately weekly intervals. The MDV vaccine strain CVI988 (p47), which is a serotype 1 MDV (44), and HVT strain FC-126 (67) were propagated in CEF, while MDV strains JM-16 (p19) (54) and RB-1B (p14) (53) were propagated in CKC. The latter two serotype 1 MDV strains are classified as virulent and very virulent, respectively.
Transactivation of QMDV genes by HVT.
QT35 cells and CEF, cultured in 25-cm2 flasks (Falcon, Franklin Lakes, N.J.), were inoculated with 0.5 × 106 CEF containing 1,000 to 2,000 focus-forming units of HVT. QT35 cell cultures were also inoculated with an equal number of uninfected CEF. Uninfected CEF and MDV CVI988-infected CEF were used as negative and positive controls, respectively. RNA was prepared from these cultures after incubation at 38.5°C for 3 to 4 days and analyzed for the presence of MDV transcripts by reverse transcription (RT)-PCR using primers specific for MDV serotype 1.
DNA and RNA extraction.
Cellular DNA was extracted from cultured cells as described by Morgan et al. (29). Briefly, 5 × 106 QT35 cells, CKC, CKC infected with the RB-1B (p19) strain of MDV, or CEF infected with CVI988 (p53) were washed with phosphate-buffered saline (PBS) and resuspended in 0.25% Triton X-100–10 mM Tris-HCl (pH 7.9)–10 mM EDTA. One-tenth volume of 2 M NaCl was added, and the cells were centrifuged at 14,000 rpm for 10 min. The pellet was resuspended in digestion buffer (0.5% sodium dodecyl sulfate, 100 mM NaCl, 10 mM Tris-HCl [pH 8.0], 1 mM EDTA) and incubated for 3 h at 50°C. The solution was extracted once with phenol and twice with chloroform-isoamyl alcohol (24:1). Total viral an cellular DNAs were precipitated by the addition of 2 volumes of absolute ethanol, recovered by centrifugation, dissolved in 100 μl of 10 mM Tris-HCl (pH 7.4)–1 mM EDTA, and stored at 4°C until use. Cellular RNA was extracted from 5 × 106 QT35 cells or CEF infected with CVI988 (p53) using a Micro RNA preparation kit (Stratagene, San Diego, Calif.). RNA pellets were washed twice with 75% ethanol and dissolved in 60 μl of RNase-free H2O. Subsequently, the following components were added for DNase treatment prior to RT-PCR: 2 U of RNase-free RQ1 DNase (Promega, Madison, Wis.) and 8 μl of RQ1 RNase-free DNase 10× reaction buffer to a final volume of 80 μl. The reaction mixture was incubated at 37°C for 30 min, incubated for 10 min at 65°C to inactivate the DNase, and stored at −20°C until use.
Southern and Northern blotting.
One microgram (per lane) of total DNA extracted from QT35 cells (equivalent to 50,000 cells), CKC, and RB-1B-infected CKC was digested with BamHI; the fragments were separated in a 1.5% Tris-acetate-EDTA–agarose gel at 100 V and transferred to a MagnaGraph nylon transfer membrane (MSI, Westboro, Mass.) by standard techniques. Probes were prepared by labeling the BamHI-H, and -I2 fragments (16) with 32P using RediprimeII (Amersham Pharmacia, Piscataway, N.J.). Hybridization was carried out using Rapid-hyb buffer (Amersham Pharmacia) in a hybridization oven (Hybaid Instruments, Holbrook, N.Y.), and membranes were exposed to BioMax MS film (Eastman Kodak Co., Rochester, N.Y.).
Total RNA from QT35 cells and CEF infected with the CVI988 strain of MDV was used to determine the presence of MDV-specific transcripts. RNA obtained from 106 cells was loaded in each well of a nondenaturing Northern Max-Gly gel (Ambion, Austin, Tex.) following the manufacturer's protocol. Gels were transferred to BrightStar plus Nylon membranes (Ambion) and hybridized to 32P-labeled probes using RediprimeII as described above. Membranes were exposed to BioMax MS film for up to 10 days. Probes for LATs meq, ORF L1, and pp38 were prepared by PCR using the primers shown in Table 1, and plasmid DNAs for LATs, meq, and pp38 or RB-1B DNA was used as a template. The primers for the ICP4 probe consisted of 5′CCCGCCGATGCTGCCCTAAAC3′ and 3′TCCGCCAGACACCTACTCAAG5′, and plasmid DNA was used as a template.
TABLE 1.
Primer pairs used for amplification of QMDV fragments from QT35 cells by PCR and RT-PCR
| MDV gene | GenBank data
|
Primer sequences (5′ to 3′) | Size (bp) | Primer | |
|---|---|---|---|---|---|
| Accession no. | Nucleotides | ||||
| ICP4 | U17705 | 6978–6953 | CTTTATGCGACTGGCGTGCTGCCGTA | 674 | ICP4-5′a |
| 6305–6328 | TCCTCCTGCACGCCTGTTTGCGGT | ICP4-3°a | |||
| LAT | U17705 | 6242–6261 | TGTCACCTGAATATCATTGC | 329 | LATs2-5 |
| 6570–6551 | CCTCTTCATCTTCCTCCTCT | LATs2-3 | |||
| ICP22 | L22174 | 1282–1302 | ATGAGAGCGACGACGAAGATT | 331 | 5′RT ICP22 |
| 1612–1595 | AGAGTCCTGCGCAGATGTGGA | 3′RT ICP22 | |||
| ICP27 | U10040 | 2167–2188 | CAAGAAGGGCATCACCGAAGAA | 31 | 5′RT ICP27 |
| 2482–2461 | GACCCGCATGCAATAAATACTC | 3′RT ICP27 | |||
| meq | M89471 | 431–450 | GGTCGACTTCGAGACGGAAA | 337 | 5meq/meqsRT |
| 767–748 | GTAAGCAGTCCAAGGGTCAC | 3meqRTanti | |||
| pp38 | M73484 | 1303–1320 | CGAGGACGGCGAGAAATG | 404 | 5′pp38 |
| 1706–1686 | TTAATGCGCGAACGGAATGTA | 3′pp38 | |||
| VP16 | L10283 | 2069–2091 | GGCCGATTTACCTTCTTATGTAG | 451 | 5′VP16 |
| 2519–2500 | CCGGAGTTCAGGAGCAGTC | 3′VP16 | |||
| ORF L1 | L19763 | 869–892 | TTGTTCACTGTGCGGCATTATTAC | 345 | 5′RT ORF L1 |
| 1213–1192 | TACCGGCACCGACAGTTCTTTA8 | 3′RT ORF L1 | |||
| pp14 | D13389 | 5804–5823 | GAGGTTCTGGCAGAGATTCC | 542 | pp14F |
| 6345–6325 | CTGCTTGTATGCTACAACGGC | pp14R | |||
| gB | D13713 | 1292–1315 | GTGCAACAGTTAATGGGAGATACA | 457 | 5′gB |
| 1748–1725 | AGCATGGCGAATTGAACAGACGAT | 3′gB | |||
Data are from Xing et al. (68).
PCR and RT-PCR.
PCR assays were carried out to detect the presence of ICP4, ICP22, ICP27, ORF L1, pp145, VP16, gB, pp38, and meq sequences. The location and direction of these genes are shown in Fig. 1 using the BamHI restriction map of MDV (16). The primes for the PCR assays and the expected fragment sizes are listed in Table 1. The nucleotide numbers are based on the accession numbers in GenBank. The following components were used in the PCR assays, to a final volume of 50 μl: 2 μl of QT35 cell DNA, which is equivalent to 105 cells; 2.5 U of Taq Gold DNA polymerase (Perkin-Elmer, Foster City, Calif.); 0.2 mM each deoxynucleoside triphosphate; PCR buffer II (10 mM Tris-HCl [pH 8.3], 50 mM KCl); 2 mM MgCl2; and 1 μM each pair of primers specific for the respective genes. The following conditions were used for the PCR: denaturation at 94°C for 5 min, annealing at 50°C for 30 s, and extension at 72°C for 45 s, followed by 30 cycles of denaturation at 94°C for 45 s, annealing at 50°C for 30 s, and extension at 72°C for 45 s; in the last cycle, the extension was done at 70°C for 7 min. The amplified fragments were resolved by electrophoresis in 1.5% agarose gels, stained with ethidium bromide, and visualized on the Eagle Eye II still-video system (Stratagene).
RT-PCR assays were carried out for the detection of LATs, ICP4, ICP22, ICP27, VP16, pp38, meq, ORF L1, and gB transcripts. cDNA synthesis and PCR amplification were carried out using a GeneAmp RNA PCR kit (Perkin-Elmer) according to the manufacturer's instructions. Briefly, RT of RNA was performed with a 10-μl final volume containing 1 mM deoxynucleoside triphosphate, PCR buffer II, 1.25 U of murine leukemia virus reverse transcriptase, 2.5 μM random hexamer or 2.5 μM specific primer and 2 μl of total RNA, which is equivalent to 1.25 × 105 cells. Random hexamers were used as primers for cDNA synthesis for the detection of gB, VP16, ICP27, pp38, meq, ORF L1, and ICP22 transcripts. For the detection of ICP4 transcripts and LATs, primers LATs2-5 and LATs2-3 (Table 1), respectively, were used for cDNA synthesis. The reaction mixture was incubated at 42°C for 15 min and heated at 95°C for 5 min. The synthesized cDNA was amplified by PCR as described for DNA. β-Actin was used in each assay to ensure that relatively comparable amounts of cDNA were used and that a uniform amplification process was obtained.
PCR products were analyzed by Southern blotting using standard methods (48). Probes were prepared from cloned and sequenced PCR products obtained from CVI988 DNA. The cloned fragments were excised by EcoRI digestion and labeled with digoxigenin (DIG)-11-dUTP using a random-primer labeling kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). Hybridization was carried out at 68°C for 12 to 16 h, and hybridized DNA bands were detected using a DIG luminescent detection kit (Boehringer) according to the manufacturer's instructions.
PCR assays to examine QT35 cells for the presence of ALV pol sequences and the reticuloendotheliosis virus LTR were conducted as described by Wade et al. (63).
Nucleotide sequencing.
The PCR products for ICP4, ICP22, ICP27, VP16, pp38, meq, ORF L1, and gB amplified from QT35 cell DNA, DNA from CKC infected with QMDV, and CVI988-infected CEF DNA were cloned using a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.) and sequenced at the BioResource Center of Cornell University. The forward universal and M13 reverse primers were used for the sequencing reactions to obtain the nucleotide sequence in both directions. At least two different clones originating from the same amplified reaction were sequenced for QT35 cell DNA, CVI988 DNA obtained from CEF, and QMDV DNA obtained from CKC.
Computer analysis of the nucleic acid sequence data was accomplished using LASERGENE software (DNASTAR Inc., Madison, Wis.). The nucleotide sequences and the deduced amino acid sequences were aligned with and compared to published MDV sequences, using the multiple-sequence alignment program CLUSTAL W (D. Higgins, Heidelberg, Germany).
IFA.
For indirect immunofluorescence assays (IFA), uninfected QT35 cells, QT35 cells infected with HVT, and CKC infected with the rescued QMDV were harvested onto coverslips after 72 h and fixed in cold acetone for 10 min. CKC infected with JM-16 and uninfected CKC were used as positive and negative controls, respectively, for the expression of pp38. The coverslips were incubated for 1 h at 37°C with monoclonal antibody (MAb) H19, kindly provided by L. F. Lee, Avian Disease and Oncology Laboratory, East Lansing, Mich.; this MAb detects pp38 of serotype 1 MDV (20). The coverslips were washed three times with PBS and incubated for 1 h at 37°C with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin (heavy and light chains) (Southern Biotechnology Associates, Inc., Birmingham, Ala.). The coverslips were washed three times with PBS, mounted on microscope slides with glycerol-PBS (9:1), and examined using a Leitz fluorescence microscope with epiillumination.
The dual expression of pp38 of type 1 MDV and the HVT glycoprotein complex consisting of gp100, gp60, and gp49 (61) in QT35 cells infected with HVT was examined using a double-staining technique (65). Coverslips with acetone-fixed cells were incubated in PBS containing 0.1% Tween 20 (PBS-T) and 3% bovine serum albumin for 1 h at room temperature (RTe) to block nonspecific binding sites, washed in PBS for 5 min, incubated with MAb H19 for 1 h at RTe, and washed three times in PBS-T. Subsequently, the coverslips were incubated for 1 h at RTe with FITC-conjugated goat Fab anti-mouse IgG (ICN Biomedicals, Inc., Costa Mesa, Calif.) diluted in PBS-T, washed three times in PBS-T, and incubated for 1 h at RTe with MAb L78, kindly provided L. F. Lee; this MAb is specific for the HVT gp100-gp60-gp49 complex (61). The coverslips were washed three times in PBS-T, incubated for 1 h at RTe with tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat affinity-purified anti-mouse IgG (ICN Pharmaceuticals), washed three times in PBS-T, and mounted on microscope slides with glycerol-PBS (9:1). Cells were examined for the expression of pp38 and HVT glycoproteins by switching filters appropriate for exciting FITC and TRITC, respectively.
In vitro and in vivo rescue of QMDV from QT35 cells.
Duplicate cultures of primary CEF and CKC were inoculated with 102 to 106 QT35 cells when the cultures were 70 to 90% confluent. These cultures were incubated at 38.5°C in 5% CO2 and were examined daily for cytopathic effects (CPE) for 10 days. One blind passage of the inoculated CEF and CKC cultures were made, and the cultures were incubated and examined for an additional 10 days. In order to increase the possibility of virus rescue, 8-day-old chicken embryos from the departmental SPF flock were inoculated in the allantoic cavity with 107 QT35 cells, and the eggs were transferred into isolation units and hatched. This approach had previously been used to enhance the oncogenic potential of MDV isolate with log oncogenicity (6). Chicks were removed from the isolators at 6 days of age, and the kidneys were harvested aseptically and used for cell cultures. Cultures were examined for the presence or MDV-like foci for 7 days.
TEM.
For transmission electron microscopy (TEM), QT35 cells and CKC infected with QMDV (p8) were pelleted, fixed in fixation buffer (2% glutaraldehyde, 1% formaldehyde, 0.1% sodium cacodylate), embedded in Epon-Araldite, sectioned at 50 μm, and stained with uranyl acetate and lead citrate. Sections were examined using a Zeiss 10C transmission electron microscope. A total of 1,000 QT35 cells were examined for the presence of intranuclear herpesvirus particles and C-type virus particles.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the QMDV genes described here are as follows: AF193004 for gB, AF193005 for pp38, AF193003 for ORF L1, AF193002 for meq, AF193001 for VP16, AF193000 for ICP22, AF192998 for ICP27, and AF192997 for ICP4. The accession numbers for gene fragments of MDV strain CVI988 are AF193012 for ORF L1, AF193011 for meq, AF193010 for VP16, AF193009 for ICP22, AF193008 for ICP27, and AF193006 for ICP4.
RESULTS
Characterization of QT35 cells.
Herpesvirus particles and C-type virus particles were not observed in thin sections of 1,000 cells by TEM. The results of PCR assays for detection of reticuloendotheliosis virus LTR fragments and the ALV pol gene were negative (data not shown).
Detection of MDV genes in QT35 cells by PCR and Southern blotting.
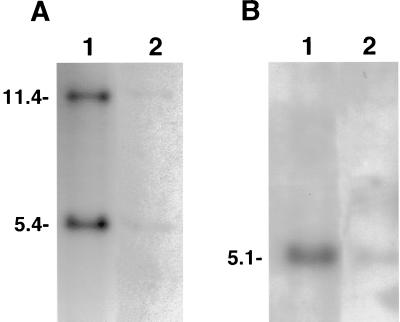
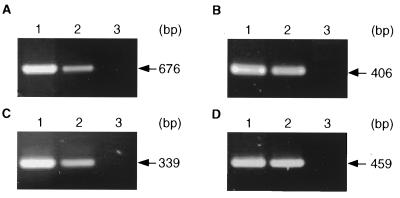
Total DNA extracted from QT35 cells was found positive for MDV by Southern blotting with probes representing several regions of the MDV genome (Fig. 2). Further studies to characterize the MDV genome in QT35 cells were done by PCR. Gene fragments were amplified from QT35 DNA prepared from two independently maintained sublines of QT35 cells with primers for the MDV ICP4, gB, pp38, and meq genes. These fragments were identical in size to the corresponding fragments obtained from CVI988 (Fig. 3). Subsequently, fragments of ICP22, ICP27, VP16, ORF L1, and pp14 were amplified by PCR. All PCR products obtained from QT35 cells with MDV primers were of the expected sizes. These products, referred to as QMDV, were cloned and sequenced. The corresponding CVI988 gene fragments were also amplified, cloned, and sequenced when the sequences were not available from GenBank.
FIG. 2.
Southern blot hybridization of QT35 cell DNA (lanes 2) and DNA from CKC infected with the RB-1B strain of MDV (lanes 1), probed with 32P-labeled BamHI-H (A) and BamHI-I2(B) probes. Each lane was loaded with the equivalent of 50,000 cells. DNA from control CKC was negative (data not shown). The sizes of the fragments (in kilobases) are indicated.
FIG. 3.
Amplification by PCR of ICP4 (A), pp38 (B), meq (C), and gB (D) using DNA from CEF infected with MDV strain CVI988 (lanes 1), QT35 cells (lanes 2), or uninfected CEF (lanes 3). The PCR products were separated by electrophoresis in 1.5% agarose gels, stained with ethidium bromide, and visualized with an Eagle Eye II still-video system. The sizes of the amplified fragments are indicated.
The comparison of QMDV sequences with MDV sequences in GenBank or with CVI988 sequences showed that the sequence of QMDV gB is identical to that of four MDV strains (RB-1B [GenBank accession no. D13713] [45], JM [GenBank accession no. X91985] [M. A. Sousloparov, unpublished data], the virulent strain Woodlands no. 1 [GenBank accession no. U39846] [70], and CVI988 [our data]). The sequence of ICP22 is identical to that of two strains (the virulent GA strain [GenBank accession no. M80595 {47} and GenBank accession no. L22174 {3}] and CVI988 [our data]), and that of ORF L1 is identical to that of three strains (GA [GenBank accession no. U34965] [38], RB-1B [GenBank accession no. L19763] [34], and CVI988 [our data]). Minor sequence differences were found between the QMDV and the MDV meq, ICP27, VP16, pp38, and pp14 genes. These differences are summarized in Tables 2 and 3. Based on the differences in nucleotides, several differences are predicted for the meq-encoded, VP16, and pp38 amino acid sequences between QMDV and MDV (Table 4).
TABLE 2.
Sequence differences between MDV meq, ICP27, and VP16 genes in QMDV and MDV strains CVI988, GA, and RPL
| MDV strain | GenBank accession no. | Sequence at the indicated nucleotide positiona in the following MDV gene:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
meq
|
ICP27, 2436 | VP16
|
||||||||
| 565 | 583 | 584 | 592 | 698 | 2234 | 2312 | 2313 | |||
| QMDVb (QT35) | G | G | A | T | T | T | T | G | C | |
| QMDVb(CKC) | + | + | + | + | + | + | + | + | + | |
| CVI988b | T | + | + | G | + | C | + | + | + | |
| GA | M898481 | + | A | + | G | + | ||||
| U55025 | T | + | C | G | G | |||||
| U10040 | C | |||||||||
| GA | L10283 | + | + | + | ||||||
| RPL | X73370 | C | C | G | ||||||
Positions of nucleotides for QMDV genes are based on the numbers corresponding to the sequence shown in Table 1. Letters indicate nucleotides different from those in the sequence of the QMDV (QT35) gene. Plus signs indicate nucleotides identical to those in the sequence of the QMDV (QT35) gene.
Nucleotide sequences were determined in this study.
TABLE 3.
Sequence differences between MDV pp38 and pp14 genes in QMDV and MDV strains CVI988, GA, HPRS16, MD5, and RB-1B
| MDV strain | GenBank accession no. | Sequence at the indicated nucleotide positiona in the following MDV gene:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pp38
|
pp14
|
||||||||||
| 1488 | 1494 | 1515 | 1516 | 279 | 314 | 315 | 316 | 425 | 436 | ||
| QMDVb(QT35) | A | A | G | G | A | T | T | − | T | C | |
| QMDVb(CKC) | + | + | + | + | |||||||
| CVI988b | + | − | − | − | C | + | |||||
| GA | M74036 | + | + | + | + | ||||||
| M73484 | + | + | + | + | |||||||
| HPRS16 | S58431 | + | G | + | + | ||||||
| MD5 | D13389 | + | + | C | C | ||||||
| CVI988 | S76060 | G | G | + | + | ||||||
| MD5 | L13389 | + | + | + | T | C | + | ||||
| L26395 | − | + | + | T | C | + | |||||
| L26394 | − | + | + | T | C | + | |||||
| M77343 | + | + | + | T | C | − | |||||
| RB-1B | L01618 | + | + | + | T | C | − | ||||
| M77342 | + | + | + | T | C | − | |||||
TABLE 4.
Predicted differences in amino acid sequences for Meq, VP16, and pp38 between QMDV and MDV strains
| MDV strain | GenBank accession no. | Sequence at the indicated amino acid positionsa in the following MDV protein:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meq
|
VP216
|
pp38
|
||||||||
| 71 | 77 | 80 | 115 | 246 | 247 | 107 | 109 | 116 | ||
| QMDVb | Ala | Glu | Tyr | Val | Lys | Leu | Gln | Glu | Arg | |
| CVI988b | Ser | + | Asp | + | + | + | ||||
| GA | M89471 | + | Lys | Asp | + | |||||
| U55025 | Ser | Ala | Asp | Ala | ||||||
| RPL | X73370 | Asn | Val | |||||||
| HPRS16 | S58431 | + | Gly | + | ||||||
| MD5 | D13389 | + | + | Pro | ||||||
| CVI988 | S76060 | Arg | Gly | + | ||||||
Plus signs indicate amino acids identical to those in the sequence of the QMDV protein.
Amino acid sequences were determined in this study.
QMDV transcripts in QT35 cells and QT35 cells infected with HVT.
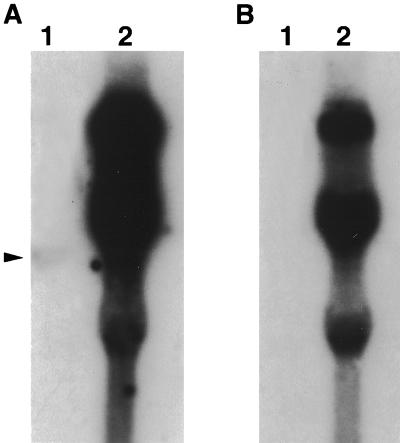
Examination of QT35 cells for the presence of MDV-specific transcripts was done by Northern blotting and RT-PCR. Northern blots were positive when hybridized with the fragment covering ICP4 transcripts (Fig. 4A, lane 1). This fragment detected a 4.5-kb transcript in QT35 cell RNA when the double-stranded probe made to detect ICP4 was used. The LAT probe did not detect any transcripts in QT35 cell DNA (Fig. 4B, lane 1). Several transcripts were detected with both of those probes and RNA from CVI988-infected CEF (Fig. 4A and B, lanes 2). Transcripts were not detected in QT35 cell RNA when ORF L1, meq, and pp38 probes were used but were present in RNA from CVI988-infected CEF (data not shown).
FIG. 4.
Northern blot hybridization of total RNA from QT35 cells (lane 1) and CEF infected with the CVI988 strain of MDV (lanes 2), probed with 32P-labeled PCR fragments for ICP4 (A) and LATs (B). The equivalent of 2.5 × 106 cells was loaded in each lane. Note the transcript for ICP4 in lane 1 (arrowhead).
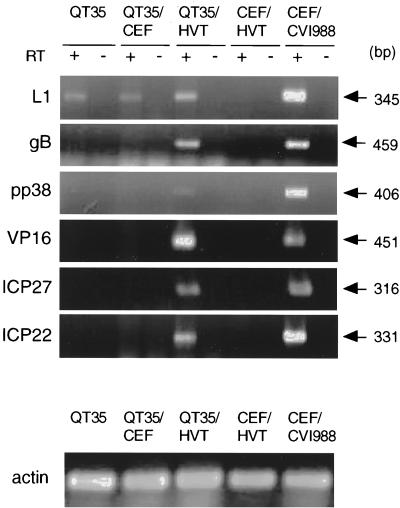
The following QMDV transcriptase were detected in QT35 cells by RT-PCR using random hexamers: ORF L1 (Fig. 5), ICP4 and/or LAT (Fig. 6), and meq (Fig. 7). All amplified products were of the predicted sizes and were comparable to the fragments amplified from RNA from CEF infected with MDV CVI988. Transcripts for ICP22, ICP27, VP16, pp38, or gB were not detected in QT35 cells, while the presence of transcripts for pp14 was not examined.
FIG. 5.
Detection by RT-PCR of transcripts for ORF L1 (L1), gB, pp38, VP16, ICP27, and ICP22 in QT35 cells, QT35 cells cocultivated with CEF (QT35/CEF), QT35 cells cocultivated with HVT-infected CEF (QT35/HVT), CEF infected with HVT (CEF/HVT), and CEF infected with CVI988 (CEF/CVI988). CEF/HVT and CEF/CVI988 were used as negative and positive controls, respectively. RT-PCR for actin was used as a positive control for RNA isolation. cDNA was prepared using reverse transcriptase (RT); a PCR assay in the absence of RT was used to determine if contaminating DNA was present. The PCR products were separated by electrophoresis in 1.5% agarose gels, stained with ethidium bromide, and visualized with an Eagle Eye II still-video system. The sizes of the amplified fragments are indicated.
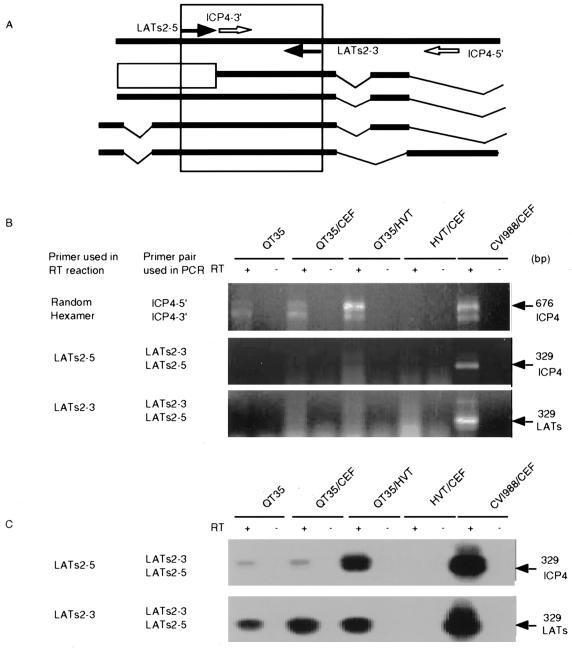
FIG. 6.
Detection by RT-PCR of transcripts for ICP4 and LATs in QT35, QT35 cocultivated with CEF (QT35/CEF), QT35 cocultivated with HVT-infected CEF (QT35/HVT), CEF infected with HVT (CEF/HVT), and CEF infected with CVI988 (CEF/CVI988). CEF/HVT and CEF/CVI988 were used as negative and positive controls, respectively. cDNA was prepared using reverse transcriptase (RT); a PCR assay in the absence of RT was used to determine if contaminating DNA was present. (A) Locations of primers used in the RT-PCR assay. The different transcripts, from top to bottom, are as follows: ICP4 ORF (2), MSRs (10, 11), the 2.2- and 1.8-kb RNAs (27), and the 2.7-kb RNA )21, 22). The open box indicates an unknown sequence within the MSRs. The amplified regions for the ICP4 transcript and each potential LAT predicted with the LATs2-5–LATs2-3 primer pair are boxed. (B) Detection of transcripts using random-hexamer primers, LATs2-5, and LATs2-3 to obtain c-DNA from total RNA. The sizes of the amplified fragments and the types of transcripts are indicated at the right. The primer used to obtain cDNA and the primer pair used for amplification are indicated at the left. The PCR products were separated by electrophoresis in 1.5% agarose gels, stained with ethidium bromide, and visualized with an Eagle Eye II still-video system. (C) Southern blot hybridization of amplified LATs and ICP4. The PCR products were separated by electrophoresis in 1.5% agarose gels, transferred to membranes by Southern blotting, and hybridized to a probe prepared from the cloned ICP4 gene from MDV strain CVI988.
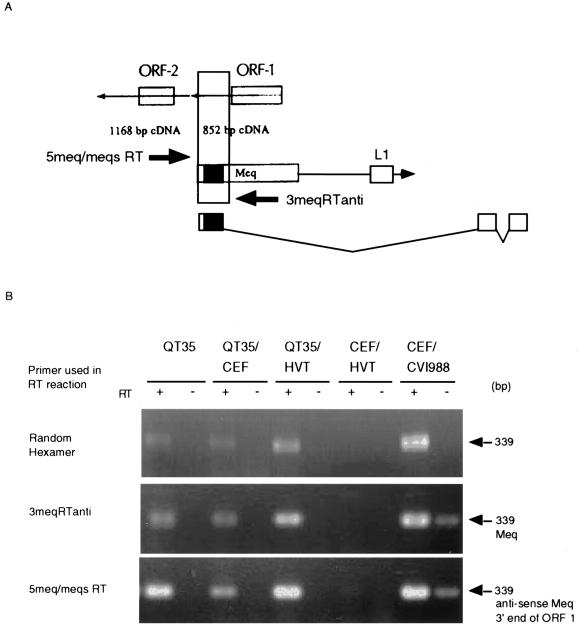
FIG. 7.
Detection by RT-PCR of meq and antisense meq transcripts in QT35 cells, QT35 cells cocultivated with CEF (QT35/CEF), QT35 cells cocultivated with HVT-infected CEF (QT35/HVT), CEF infected with HVT (CEF/HVT), and CEV infected with CVI988 (CEF/CVI988). CEF/HVT and CEF/CVI988 were used as negative and positive controls, respectively. cDNA was prepared using reverse transcriptase (RT); a PCR assay in the absence of RT was used to determine if contaminating DNA was present. (A) Locations of the 5meq/meqsRT and 3meqRTanti primers in relation to the meq and antisense meq ORFs and ORF L1. The predicted amplicon is indicated by a vertically oriented box and will detect nonspliced meq mRNA or antisense ORF-1, depending on the primer used to generate the cDNA. (B) Detection of transcripts by RT-PCR using random-hexamer primers, 3meqRTanti, or 5meq/meqsRT to obtain cDNA. The sizes of the amplified fragments and the types of transcripts are indicated at the right. The PCR products were separated by electrophoresis in 1.5% agarose gels, stained with ethidium bromide, and visualized with an Eagle Eye II still-video system.
Infection of QT35 with HVT-infected CEF but not with control CEF resulted in the activation of gB, pp38, VP16, ICP22, and ICP27 transcripts (Fig. 5 and Table 5) and an apparent enhancement in transcriptional activity for ICP4 (Fig. 6). The amplified products were of the predicted sizes and were comparable to the products amplified from RNA from CEF infected with CVI988. The primers used in the RT-PCR assays (Table 1) failed to amplify HVT-specific transcripts, demonstrating that these primers were specific for MDV serotype 1 (Fig. 5, 6, and 7). Amplification required the use of RT, demonstrating that the products were not the result of DNA contamination (Fig. 5, 6, and 7).
TABLE 5.
MDV transcripts in QT35 cells in the presence and absence of CEF infected with HVT
| Cells (treatment) | MDV transcripts for a:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ICP4b | ICP22 | ICP27 | VP16 | LATsc | ORF L1 | meq | pp38 | gB | |
| QT35 | + | − | − | − | + | + | + | − | − |
| QT35 (CEF) | + | − | − | − | + | + | + | − | − |
| QT35 (HVT) | ++ | + | + | + | + | + | + | + | + |
| CEF (HVT) | − | − | − | − | − | − | − | − | − |
| CEF (CVI988) | + | + | + | + | + | + | + | + | + |
++, increased transcription compared to QT35; +, transcripts present; −, no transcripts detected.
The strand-specific primer LATs2-3 was used to generate cDNA, and cDNAs were detected by Southern hybridization.
The strand-specific primer LATs2-5 was used to generate cDNA, and cDNAs were detected by Southern hybridization.
The location of the ICP4 ORF is overlapped by an antisense ORF coding for the LATs. The latter codes for a complex set of MSRs lacking a poly(A) tail (11) as well as a series of mRNAs (21, 22, 27) (Fig. 6A). RT-PCR products of the expected sizes were amplified from RNAs obtained from QT35 cells, QT35 cells inoculated with CEF, QT35 cells inoculated with HVT-infected CEF, and CEF infected with CVI988 when random hexamers were used in the RT reaction. Primers LATs2-5 and LATs2-3 were used in the RT reaction to obtain cDNAs coding for ICP4 and LATs, respectively, to determine if ICP4 transcripts and/or LATs were present in the RNA preparations. LATs and ICP4 transcripts were demonstrated when both primers were used in the RT reaction but only after Southern hybridization. The signals for LATs were stronger than those for ICP4 transcripts in RNAs obtained from QT35 cells and QT35 cells inoculated with CEF, but inoculation of QT35 cells with HVT-infected CEF resulted in a marked increase for ICP4 transcripts (Fig. 6B and C).
The meq region codes for ORF L1 (34, 38), the meq transcript, and a spliced meq transcript (38). In addition, two antisense ORFs have been identified (37) in this region (Fig. 7A). When random hexamers were used in the RT reaction, a PCR product of 339 bp was detected in the meq region for RNAs from QT35 cells, QT35 cells inoculated with CEF, QT35 cells inoculated with HVT-infected CEF, and CEF infected with CVI988 but not HVT-infected CEF (Fig. 7B). When strand-specific primers were used for the preparation of cDNAs, RT-PCR products for meq and antisense ORF1 were detected in all samples that were found positive when random hexamers were used. Unfortunately, the DNase treatment of the positive control (CEF infected with CVI988) was incomplete in this experiment, but all other RNA preparations were free of contaminating DNA (Fig. 7B).
Detection of serotype 1 MDV-specific pp38 protein in QT35 cells infected with HVT.
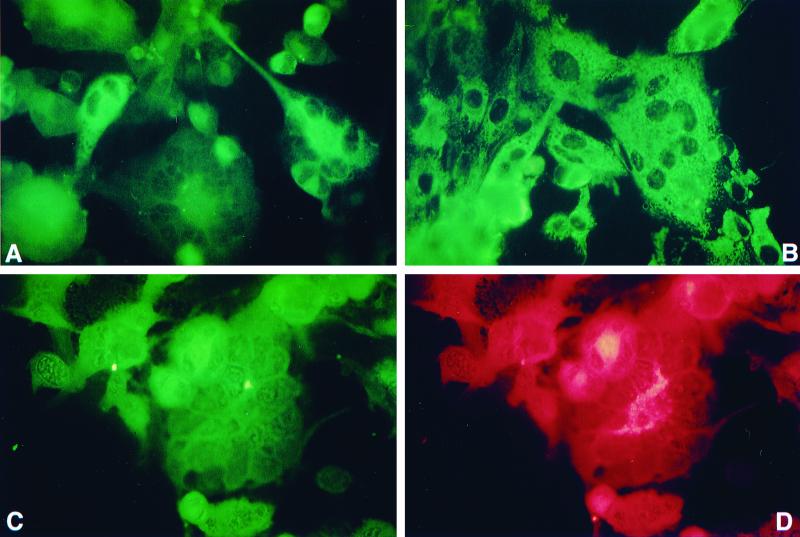
Inoculation of QT35 cells with HVT-infected CEF resulted in the development of CPE consisting of syncytia and rounded cells. CPE was not observed after inoculation of QT35 cells with CEF or in control QT35 cells. MDV pp38 was detected in the syncytia and rounded cells by IFA with MAb H19, which is specific for pp38 produced by serotype 1 MDV strains. Dual staining of the QT35 cells infected with HVT with MAb H19 and the HVT-specific MAb indicated that the syncytia and rounded cells were positive for HVT and pp38 (Fig. 8). QT35 cells and QT35 cells inoculated with CEF were negative for pp38.
FIG. 8.
Detection of serotype 1 MDV-specific pp38 in QT35 cells inoculated with HVT-infected CEF (QT35/HVT). (A) QT35/HVT cells stained with pp38-specific MAB H19 and FITC-conjugated goat anti-mouse immunoglobulin (heavy and light chains) [Ig (H+L)]. Note the cytoplasmic fluorescence. QT35 cells or QT35 cells inoculated with CEF and treated in a similar way were negative (data not shown). (B) Positive control for pp38 staining. CKC infected with the JM-16 strain of MDV were stained as described for panel A. (C and D) QT35/HVT cells were examined for coexpression of serotype 1 MDV pp38 and an HVT glycoprotein using MAbs H19 and L78, respectively, FITC-conjugated goat anti-mouse Ig (H+L), and TRITC-conjugated goat affinity-purified anti-mouse IgG. Cells were examined for expression of pp38 and HVT glycoprotein by switching filters appropriate for exciting FITC and TRITC, respectively.
Virus isolation and characterization.
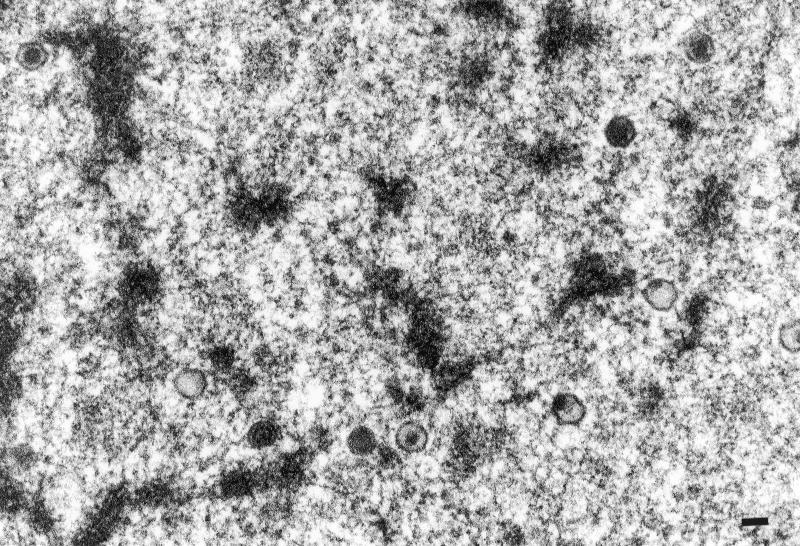
CKC and CEF inoculated with different numbers of QT35 cells did not develop CPE after one blind passage. In contrast, CPE developed within 4 days in parallel cultures inoculated with CVI988. Kidney cell cultures were prepared from two 6-day-old chicks and one 9-day-old chick that had been inoculated with QT35 cells at 8 days of embryonation. MDV-like CPE consisting of small clusters of round cells were detected in the culture from both chicks at 6 days postplating. QMDV (p3) stained positive for pp38 with MAb H19 in IFA (data not shown). TEM examination of thin sections revealed typical nonenveloped herpesvirus particles that were randomly scattered in the nuclei of CKC. Some of the capsids lacked DNA (Fig. 9).
FIG. 9.
Nonenveloped herpesvirus particles in the nucleus of a CKC infected with MDV recovered from QT35 cells. Bar, 100 nm.
Selected fragments of virus-infected CKC were amplified by PCR with the same primers as those used for the amplification of QMDV fragments from QT35 cells. All fragments were identical to the QT35 fragments (Tables 2 and 3).
DISCUSSION
The finding that two independently maintained sublines of QT35 cells are positive for an MDV-like virus, or QMDV, was quite unexpected. This result raises important questions concerning the origin of QMDV in QT35 cells, its potential role in the development of MCA-induced tumors in Japanese quail, and the development of cell lines from these tumors. Moreover, the experiments in which QT35 cells were inoculated with HVT suggest that QT35 cells can be used as a model to study reactivation of a truly latent alphaherpesvirus, especially because TEM examination failed to demonstrate herpesvirus particles in these cells.
Southern hybridization and PCR assays were used to examine QT35 cells for the presence of MDV-like sequences. Viral DNA representing parts of the TRL and IRL sequences and adjacent parts of the UL sequence was detected by Southern hybridization. Subsequent studies were done by PCR to facilitate cloning and sequencing of specific gene fragments. All primer combinations yielded fragments of the expected sizes, and sequence analysis indicated that the QMDV genes are identical or almost identical to the IE, early, and late genes of MDV. These genes are apparently functional, because infection of QT35 cells with HVT induced the transcription of these genes, and an MDV-like agent was recovered. Unfortunately, the paucity of serotype-specific MAbs made it impossible to demonstrate that these transcripts were also translated, with the exception of pp38, for which a serotype-specific MAb is available.
Attempts to isolate a herpesvirus from the QT35 cells by cocultivation with CKC or CEF failed. It was hypothesized that virus might be rescued after inoculation of chicken embryos, hatching of the chicks, and use of their kidneys in cell cultures for virus isolation attempts. This approach was used for the following reasons: (i) MDV is not transmitted vertically in chickens (66); (ii) cell cultures are not as sensitive to virus replication as chicks (49); and (iii) inoculation of immunologically incompetent chicken embryos had previously been used by Calnek et al. (6) to demonstrate that MDV strains with low oncogenic potential can result in increased tumor formation. The increased oncogenicity observed in their study was the consequence of enhanced viral replication, suggesting that this approach could work for virus isolation from QT35 cells. A virus with the typical morphology of nonenveloped herpesvirus particles was indeed isolated from CKC (Fig. 9), as has been described for the cell-associated replication of MDV in cell cultures (31). This virus probably belongs to serotype 1, based on staining with a serotype 1-specific MAb, and sequence information of selected fragments suggests that it is similar to QMDV present in QT35 cells. It is highly unlikely that this virus could be the result of contamination by horizontal infection of the chicks, because they were housed in thoroughly disinfected isolators with Hepa filters (Andersen 2000, Peachtree City, Ga.). Imai et al. (17) were also unable to isolate an MDV-like virus by inoculation of CKC and CEF cultures with blood from Japanese quail with lymphoproliferative lesions. However, virus was rescued by inoculation of quail blood cells into SPF chickens. This group suggested that the quail virus was different from but related to serotype 1 MDV.
The origin of QMDV sequences in QT35 cells is not clear. The fact that QMDV sequences were found in two independently maintained QT35 cell lines, one of which has to our knowledge never been in a laboratory with MDV, argues against laboratory contamination. In addition, MDV sequences have been detected recently in four other sublines of QT35 cells (D. Junker, personal communication; V. Zelnik, personal communication). If it was laboratory contamination, it must have occurred at least before 1982. Independently of the origin of MDV, the findings in this paper clearly demonstrate that QMDV is present as a latent virus that can be fully reactivated in vivo or partially in QT35 cells by superinfection with HVT. It is possible that QMDV came from an adventitious infection in the quail that were used to generate the MCA-induced tumors. Japanese quails can become infected with MDV when they are kept in close contact with chickens (40). Japanese quail can also be experimentally infected with chicken MDV strains causing lesions (19) or only establishing infection (28). A more intriguing explanation is that an MDV-like virus is present in Japanese quail either as a complete or as an incomplete virus. Shih and coworkers (42, 58–60) found that DNA extracted from atherosclerotic lesions in Japanese quail was positive for MDV DNA in Southern hybridization assays using nonspecified fragments from an EcoRI MDV DNA library as a probe. They were able to develop a quail line after four generations that was highly susceptible to atherosclerosis. DNA samples extracted from individual embryos from this highly susceptible line were all positive for MDV DNA in a dot blot hybridization assay, while DNA samples from embryos from a more resistant line were either positive or negative. However, an MDV-like virus could not be isolated from embryonal or adult tissues, a result which is certainly compatible with the failure to rescue QMDV from QT35 cells. The suggestion of vertical transmission of an MDV-like virus in quail is intriguing, especially because there is no evidence for vertical transmission in chickens (66).
As with other herpesviruses, the induction and maintenance of latency in MDV are poorly understood. Transcripts were not detected by Northern blot hybridization, with the exception of a 4.5-kb fragment detected by the ICP4 probe. However, this was a double-stranded probe and may have detected one of the LATs. The use of RT-PCR demonstrated the presence of additional transcripts. The ORF L 1, LATs, ICP4, meq, and antisense meq transcripts detected by RT-PCR in QT35 cells have been previously reported for lytic MDV infections and/or for lymphoblastoid cell lines, but the relative contributions to transformation and/or latency have not been fully elucidated for these transcripts. ORF L1 was originally isolated from a tumor cell line by use of a cDNA library, but this transcript could also be detected during lytic infection. Schat et al. (57) demonstrated that the deletion of ORF L1 did not interfere with the establishment of or reactivation from latency. The presence of this transcript in QT35 cells is certainly compatible with a role in the transformation process.
MDV ICP4 can transactivate other MDV genes, e.g., the pp38 gene (41), and activation of ICP4 is probably also a prerequisite for the reaction of MDV from latency. The findings that LATs are the major transcripts and ICP4 is the minor transcript in QT35 cells (Fig. 4), with no reactivation of other IE, early, or late gene transcripts, suggest that the balance between LATs and ICP4 is important in maintaining latency in QT35 cells. After infection with HVT, transcriptional activity for ICP4 increases, and downstream genes are activated. Studies to determine if the expression of HVT ICP4 alone is able to cause the transactivation of QMDV ICP4 and other genes are in progress.
The finding that infection of QT35 cells with HVT causes increased transcription of ICP4 and the initiation of transcription of two other IE genes (ICP27 and ICP22) and of early and late genes is of considerable interest. It suggests that QT35 cells can be used as an in vitro model to study the molecular mechanisms of reactivation of an alphaherpesvirus from latency. For example, the roles of ICP4 and ICP27 in the activation of transcription of pp38 and pp14 are currently not clear. ICP4 and ICP27 can both promote the transcription of pp38 and pp14 by independent activation of the bidirectional promoter of pp38 and pp14, but apparently not in a cooperative fashion (43). Current studies are directed toward the elucidation of the role of both IE genes in the QMDV reactivation cascade in QT35 cells.
ACKNOWLEDGMENTS
We thank Robert Nordhausen (California Veterinary Diagnostic Laboratory System, University of California—Davis) for assistance with TEM; Carol Cardona, P. H. O'Connell, and S. Khan for technical assistance and valuable suggestions; and K. W. Jarosinski and C. J. Markowski for critical reading of the manuscript.
REFERENCES
- 1.Akiyama Y, Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974;17:105–116. [PubMed] [Google Scholar]
- 2.Anderson A S, Francesconi A, Morgan R W. Complete nucleotide sequence of the Marek's disease virus ICP4 gene. Virology. 1992;189:657–667. doi: 10.1016/0042-6822(92)90589-h. [DOI] [PubMed] [Google Scholar]
- 3.Brunovskis P, Velicer L F. The Marek's disease virus (MDV) unique short region: alphaherpesvirus-homologous, fowlpox virus-homologous, and MDV-specific genes. Virology. 1995;206:324–338. doi: 10.1016/s0042-6822(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 4.Buckmaster A E, Scott S D, Sanderson M J, Boursnell M E, Ross N L, Binns M M. Gene sequence and mapping data from Marek's disease virus and herpesvirus of turkeys: implications for herpesvirus classification. J Gen Virol. 1988;69:2033–2042. doi: 10.1099/0022-1317-69-8-2033. [DOI] [PubMed] [Google Scholar]
- 5.Buranathai C, Rodriguez J, Grose C. Transformation of primary chick embryo fibroblasts by Marek's disease virus. Virology. 1997;239:20–35. doi: 10.1006/viro.1997.8854. [DOI] [PubMed] [Google Scholar]
- 6.Calnek B W, Fabricant J, Schat K A, Murthy K K. Pathogenicity of low-virulence Marek's disease viruses in normal versus immunologically compromised chickens. Avian Dis. 1977;21:346–358. [PubMed] [Google Scholar]
- 7.Calnek B W, Murthy K K, Schat K A. Establishment of Marek's disease lymphoblastoid cell lines from transplantable versus primary lymphomas. Int J Cancer. 1978;21:100–107. doi: 10.1002/ijc.2910210117. [DOI] [PubMed] [Google Scholar]
- 8.Calnek B W, Shek W R, Schat K A. Spontaneous and induced herpesvirus genome expression in Marek's disease tumor cell lines. Infect Immun. 1981;34:483–491. doi: 10.1128/iai.34.2.483-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calnek B W, Lucio B, Schat K A, Lillehoj H S. Pathogenesis of Marek's disease virus-induced local lesions. 1. Lesion characterization and cell line establishment. Avian Dis. 1989;33:291–302. [PubMed] [Google Scholar]
- 10.Cantello J L, Anderson A S, Morgan R W. Identification of latency-associated transcripts that map antisense to the ICP4 homolog gene of Marek's disease virus. J Virol. 1994;68:6280–6290. doi: 10.1128/jvi.68.10.6280-6290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantello J L, Parcells M S, Anderson A S, Morgan R W. Marek's disease virus latency-associated transcripts belong to a family of spliced RNAs that are antisense to the ICP4 homolog gene. J Virol. 1997;71:1353–1361. doi: 10.1128/jvi.71.2.1353-1361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho B R. A simple in vitro differentiation between turkey herpesvirus and Marek's disease virus. Avian Dis. 1981;25:839–846. [PubMed] [Google Scholar]
- 13.Cowen B S, Braune M O. The propagation of avian viruses in a continuous cell line (QT35) of Japanese quail origin. Avian Dis. 1988;32:282–297. [PubMed] [Google Scholar]
- 14.DeLuca N A, Schaffer P A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985;5:1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon R A, Schaffer P A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein VP175. J Virol. 1980;36:189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuchi K, Sudo M, Lee Y S, Tanaka A, Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984;51:102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai K, Yuasa N, Kobayashi K, Nakamura K, Tsukamoti K, Hihara H. Isolation of Marek's disease virus from Japanese quail with lymphoproliferative disease. Avian Pathol. 1990;19:119–129. doi: 10.1080/03079459008418661. [DOI] [PubMed] [Google Scholar]
- 18.Jones D, Lee L, Liu J L, Kung H J, Tillotson J K. Proc. Natl Acad. Sci. USA 89:40432–4046. 1992. Marek's disease virus encodes a basic-leucine zipper gene resembling the fos/jun oncogenes that is highly expressed in lymphoblastoid tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khare M L, Grun J, Adams E V. Marek's disease in Japanese quail: a pathological, virological and serological study. Poultry Sci. 1975;54:2066–2068. doi: 10.3382/ps.0542066. [DOI] [PubMed] [Google Scholar]
- 20.Lee L F, Liu X, Witter R L. Monoclonal antibodies with specificity for three different serotypes of Marek's disease viruses in chickens. J Immunol. 1983;130:1003–1006. [PubMed] [Google Scholar]
- 21.Li D, O'Sullivan G, Greenall L, Smith G, Jiang C, Ross N. Further characterization of the latency-associated transcription of Marek's disease virus. Arch Virol. 1998;143:293–311. doi: 10.1007/s007050050287. [DOI] [PubMed] [Google Scholar]
- 22.Li D S, Pastorek J, Zelnik V, Smith G D, Ross L J. Identification of novel transcripts complementary to the Marek's disease virus homologue of the ICP4 gene of herpes simplex virus. J Gen Virol. 1994;75:1713–1722. doi: 10.1099/0022-1317-75-7-1713. [DOI] [PubMed] [Google Scholar]
- 23.Liu J L, Lee L F, Ye Y, Qian Z, Kung H J. Nucleolar and nuclear localization properties of a herpesvirus bZIP oncoprotein, Meq. J Virol. 1997;71:3188–3196. doi: 10.1128/jvi.71.4.3188-3196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J L, Ye Y, Lee L F, Kung H J. Transforming potential of the herpesvirus oncoprotein Meq: morphological transformation, serum-independent growth, and inhibition of apoptosis. J Virol. 1998;72:388–395. doi: 10.1128/jvi.72.1.388-395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J L, Ye Y, Qian Z, Qian Y, Templeton D J, Lee L F, Kung H J. Functional interactions between herpesvirus oncoprotein Meq and cell cycle regulator CDK2. J Virol. 1999;73:4208–4219. doi: 10.1128/jvi.73.5.4208-4219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J-L, Lin S-F, Xia L, Brunovskis P, Li D, Davidson I, Lee L F, Kung H-J. Meq and v-IL8: cellular genes in disguise? Acta Virol. 1999;43:94–101. [PubMed] [Google Scholar]
- 27.McKie E A, Ubukata E, Hasegawa S, Zhang S, Nonoyama M, Tanaka A. The transcripts from the sequences flanking the short components of Marek's disease virus during latent infection form a unique family of 3′-coterminal RNAs. J Virol. 1995;69:1310–1314. doi: 10.1128/jvi.69.2.1310-1314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikami T, Onuma M, Hayashi T T, Narita M, Okada K. Pathogenic and serologic studies of Japanese quail infected with JM strain of Marek's disease herpesvirus. J Natl Cancer Inst. 1975;54:607–614. [PubMed] [Google Scholar]
- 29.Morgan R W, Cantello J L, McDermott C H. Transfection of chicken embryo fibroblasts with Marek's disease virus DNA. Avian Dis. 1990;34:345–351. [PubMed] [Google Scholar]
- 30.Moscovici C, Moscovici M G, Jimenez H, Lai M M, Hayman M J, Vogt P K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977;11:95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- 31.Nazerian K, Purchase H G. Combined fluorescent-antibody and electron microscopy study of Marek's disease virus-infected cell culture. J Virol. 1970;5:79–90. doi: 10.1128/jvi.5.1.79-90.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazerian K, Witter R L. Properties of a chicken lymphoblastoid cell line from Marek's disease tumor. J Natl Cancer Inst. 1975;54:453–458. [PubMed] [Google Scholar]
- 33.Ohashi K, O'Connell P H, Schat K A. Characterization of Marek's disease virus BamHI-A-specific cDNA clones obtained from a Marek's disease lymphoblastoid cell line. Virology. 1994;199:275–283. doi: 10.1006/viro.1994.1125. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi K, Zhou W, O'Connell P H, Schat K A. Characterization of a Marek's disease virus BamHI-L-specific cDNA clone obtained from a Marek's disease lymphoblastoid cell line. J Virol. 1994;68:1191–1195. doi: 10.1128/jvi.68.2.1191-1195.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne L N, Howes K, Rennie M, Bumstead J M, Kidd A W. Use of an agar culture technique for establishing lymphoid cell lines from Marek's disease lymphomas. Int J Cancer. 1981;28:757–766. doi: 10.1002/ijc.2910280615. [DOI] [PubMed] [Google Scholar]
- 36.Peng Q, Shirazi Y. Characterization of the protein product encoded by a splicing variant of the Marek's disease virus Eco-Q gene Meq. Virology. 1996;226:77–82. doi: 10.1006/viro.1996.0629. [DOI] [PubMed] [Google Scholar]
- 37.Peng Q, Shirazi Y. Isolation and characterization of Marek's disease virus (MDV) cDNAs from a MDV-transformed lymphoblastoid cell line: identification of an open reading frame antisense to the MDV Eco-Q protein (Meq) Virology. 1996;221:368–374. doi: 10.1006/viro.1996.0388. [DOI] [PubMed] [Google Scholar]
- 38.Peng Q, Zeng M, Bhuiyan Z A, Ubukata E, Tanaka A, Nonoyama M, Shirazi Y. Isolation and characterization of Marek's disease virus (MDV) cDNAs mapping to the BamHI-I2, BamHI-Q2, and BamHI-L fragments of the MDV genome from lymphoblastoid cells transformed and persistently infected with MDV. Virology. 1995;213:590–599. doi: 10.1006/viro.1995.0031. [DOI] [PubMed] [Google Scholar]
- 39.Powell P C, Payne L N, Frazier J A, Rennie M. T lymphoblastoid cell lines from Marek's disease lymphomas. Nature. 1974;251:79–80. doi: 10.1038/251079a0. [DOI] [PubMed] [Google Scholar]
- 40.Pradham H K, Mohanty G C, Mukit A. Marek's disease in Japanese quails (Coturnix coturnix japonica): a study of natural cases. Avian Dis. 1985;29:575–582. [PubMed] [Google Scholar]
- 41.Pratt W D, Cantello J L, Morgan R W, Schat K A. Enhanced expression of the Marek's disease virus-specific phosphoproteins after stable transfection of MSB-1 cells with the Marek's disease virus homolog of ICP4. Virology. 1994;201:132–136. doi: 10.1006/viro.1994.1273. [DOI] [PubMed] [Google Scholar]
- 42.Pyrzak R, Shih J C. Detection of specific DNA segments of Marek's disease herpes virus in Japanese quail susceptible to atherosclerosis. Atherosclerosis. 1987;68:77–85. doi: 10.1016/0021-9150(87)90096-7. [DOI] [PubMed] [Google Scholar]
- 43.Ren D, Lee L F, Coussens P M. Regulatory function of the Marek's disease virus ICP27 gene product. In: Silva R F, Cheng H H, Coussens P M, Lee L F, Velicer L F, editors. Current research on Marek's disease. Proceedings of the 5th International Symposium on Marek's disease. Kennett Square, Pa: American Association of Avian Pathologists; 1996. pp. 170–175. [Google Scholar]
- 44.Rispens B H, Vloten J V, Mastenbroek N, Maas H J L, Schat K A. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI988) and its use in laboratory vaccination trials. Avian Dis. 1972;16:108–125. [PubMed] [Google Scholar]
- 45.Ross L J, Sanderson M, Scott S D, Binns M M, Doel T, Milne B. Nucleotide sequence and characterization of the Marek's disease virus homologue of glycoprotein B of herpes simplex virus. J Gen Virol. 1989;70:1789–1804. doi: 10.1099/0022-1317-70-7-1789. [DOI] [PubMed] [Google Scholar]
- 46.Ross L J N. T-cell transformation by Marek's disease virus. Trends Microbiol. 1999;7:22–29. doi: 10.1016/s0966-842x(98)01427-9. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi M, Urakawa T, Hirayama Y, Miki N, Yamamoto M, Hirai K. Sequence determination and genetic content of an 8.9-kb restriction fragment in the short unique region and the internal inverted repeat of Marek's disease virus type 1 DNA. Virus Gene. 1992;6:365–378. doi: 10.1007/BF01703085. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 49.Schat K A. Characteristics of the virus. In: Payne L N, editor. Marek's disease. Developments in veterinary virology. Boston, Mass: Martinus Nijhoff Publishers; 1984. pp. 77–112. [Google Scholar]
- 50.Schat K A. Marek's disease—a model for protection against herpesvirus-induced tumors. Cancer Surv. 1987;6:1–37. [PubMed] [Google Scholar]
- 51.Schat K A, Purchase H G. Cell-culture methods. In: Swayne D E, Glisson J R, Jackwood M W, Pearson J E, Reed W M, editors. A laboratory manual for the isolation and identification of avian pathogens. 4th ed. Kennett Square, Pa: American Association of Avian Pathologists; 1998. pp. 223–234. [Google Scholar]
- 52.Schat K A, Xing Z. Specific and nonspecific immune responses to Marek's disease virus. Dev Comp Immunol. 2000;24:201–221. doi: 10.1016/s0145-305x(99)00073-7. [DOI] [PubMed] [Google Scholar]
- 53.Schat K A, Calnek B W, Fabricant J. Characterization of two highly oncogenic strains of Marek's disease virus. Avian Pathol. 1982;11:593–605. doi: 10.1080/03079458208436134. [DOI] [PubMed] [Google Scholar]
- 54.Schat K A, Calnek B W, Fabricant J, Graham D L. Pathogenesis of infection with attenuated Marek's disease virus strains. Avian Pathol. 1985;14:127–146. doi: 10.1080/03079458508436213. [DOI] [PubMed] [Google Scholar]
- 55.Schat K A, Buckmaster A, Ross L J N. Partial transcription map of Marek's disease herpesvirus in lytically infected cells and lymphoblastoid cell lines. Int J Cancer. 1989;44:101–109. doi: 10.1002/ijc.2910440119. [DOI] [PubMed] [Google Scholar]
- 56.Schat K A, Chen C L, Calnek V W, Char D. Transformation of T-lymphocyte subsets by Marek's disease herpesviruses. J Virol. 1991;65:1408–1413. doi: 10.1128/jvi.65.3.1408-1413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schat K A, Hooft van Iddekinge B J L, Boerrigter H, O'Connell P H, Koch G. Open reading frame L1 of Marek's disease herpesvirus is not essential for in vitro and in vivo replication and establishment of latency. J Gen Virol. 1998;79:841–849. doi: 10.1099/0022-1317-79-4-841. [DOI] [PubMed] [Google Scholar]
- 58.Shih J C, Kelemen D W. Possible role of viruses in atherosclerosis. Adv Exp Med Biol. 1995;369:89–98. doi: 10.1007/978-1-4615-1957-7_9. [DOI] [PubMed] [Google Scholar]
- 59.Shih J C, Kelemen D W, Landers S C. Culture characterization and viral infection of aortic smooth muscle cells from Japanese quail susceptible to atherosclerosis. Exp Mol Pathol. 1994;61:191–202. doi: 10.1006/exmp.1994.1036. [DOI] [PubMed] [Google Scholar]
- 60.Shih J C, Pyrzak R, Guy J S. Discovery of noninfections viral genes complementary to Marek's disease herpes virus in quail susceptible to cholesterol-induced atherosclerosis. J Nutr. 1989;119:294–298. doi: 10.1093/jn/119.2.294. [DOI] [PubMed] [Google Scholar]
- 61.Silva R F, Lee L F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease or turkey herpesvirus. Virology. 1984;136:307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- 62.Tieber V L, Zalinskis L L, Silva R F, Finkelstein A, Coussens P M. Transactivation of the Rous sarcoma virus long terminal repeat promoter by Marek's disease virus. Virology. 1990;179:719–727. doi: 10.1016/0042-6822(90)90139-i. [DOI] [PubMed] [Google Scholar]
- 63.Wade L L, Polack E W, O'Connell P H, Starrak G S, Abou-Madi N, Schat K A. Multicentric lymphoma in a European starling (Sturnus vulgaris) J Avian Med Surg. 1999;13:108–115. [Google Scholar]
- 64.Wagner E K, Bloom D C. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wessel G M, McClay D R. Two embryonic, tissue-specific molecules identified by a double-label immunofluorescence technique for monoclonal antibodies. J Histochem Cytochem. 1986;34:703–706. doi: 10.1177/34.6.3084626. [DOI] [PubMed] [Google Scholar]
- 66.Witter R L, Solomon J J. Prospects for the control of Marek's disease through isolation rearing. Prog Immunobiol Stand. 1972;5:163–168. [PubMed] [Google Scholar]
- 67.Witter R L, Nazerian K, Purchase H G, Burgoyne G H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am J Vet Res. 1970;31:525–538. [PubMed] [Google Scholar]
- 68.Xing Z, Xie Q, Morgan R W, Schat K A. A monoclonal antibody to ICP4 of MDV recognizing ICP4 of serotype 1 and 3 MDV strains. Acta Virol. 1999;43:113–120. [PubMed] [Google Scholar]
- 69.Zelnik V. Marek's disease and new approaches to its control. Acta Virol. 1995;39:53–63. [PubMed] [Google Scholar]
- 70.Zerbes M, Tannock G A, Jenner R J, Young P L. Some characteristics of a recent virulent isolate of Marek's disease virus. Aust Vet J. 1994;71:21–22. doi: 10.1111/j.1751-0813.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]