Abstract
Objectives
Critical illness reduces β-lactam pharmacokinetic/pharmacodynamic (PK/PD) attainment. We sought to quantify PK/PD attainment in patients with hospital-acquired pneumonia.
Methods
Meropenem plasma PK data (n = 70 patients) were modelled, PK/PD attainment rates were calculated for empirical and definitive targets, and between-patient variability was quantified [as a coefficient of variation (CV%)].
Results
Attainment of 100% T>4×MIC was variable for both empirical (CV% = 92) and directed (CV% = 33%) treatment.
Conclusions
Individualization is required to achieve suggested PK/PD targets in critically ill patients.
Introduction
Broad-spectrum β-lactam antibiotics such as meropenem are frequently used in hospital-acquired pneumonia (HAP) treatment.1 Pharmacokinetic (PK) variability caused by augmented renal function, renal replacement therapy and/or expanded intravascular volume can lead to insufficient pharmacodynamic (PD) exposures.2–4 Importantly, pathogens infecting critically ill patients in the ICU are more likely to have higher meropenem MICs versus non-ICU patients.5,6
A suggested target for plasma concentrations of β-lactams is 100% T>1×MIC in ICU patients.7 However, 100% T>4×MIC is considered to be an optimal PK/PD target,8 and attainment of T>4×MIC has been associated with improved clinical outcomes.9 However, whether population-based β-lactam dosing consistently yields sufficient PK/PD for ICU patients is unclear.10–14
We evaluated meropenem PK/PD attainment in plasma among HAP patients admitted to our ICU and characterized the inter-individual variability in target PK/PD attainment.
Materials and methods
We analysed meropenem concentrations within plasma samples collected as part of the Successful Clinical Response In Pneumonia Therapy (SCRIPT) study (https://script.northwestern.edu). Critically ill patients with HAP admitted to the Medical ICU at Northwestern Memorial Hospital (Chicago, IL, USA) were enrolled between 30 June 2018 and 1 March 2021. Plasma samples salvaged from clinical care were stored per protocol prior to −80°C. Pathogens were cultured from deep (i.e. bronchoalveolar lavage) respiratory sampling and identified using standard microbiological methods.
Total meropenem was quantified using the Agilent 1260 Infinity Binary LC system paired with the Agilent 6420 Triple Quadrupole MS system. A reversed phase Poroshell (Agilent) C18 column (100 mm × 3.0 mm × 2.7 μm) was used. Mobile phases A and B were 0.1% formic acid in water and LC/MS-grade acetonitrile, respectively. Transitions (m/z) for meropenem were: quantitation 384.1 → 68.2, qualification 384.1 → 141.0; ceftazidime (m/z: 274.1 → 80.2) served as an internal standard. The assay was linear from 0.125 to 100 μg/mL (R2 = 0.999). Accuracy (inter-day: 93.95%; intra-day: 96.45%) and precision [inter-day coefficient of variation (CV%): 3.9%; intra-day CV%: 0.9%] met FDA requirements for bioanalytical method validation.15
A non-parametric population PK model was developed with the non-parametric adaptive grid (NPAG) algorithm using the Pmetrics package (version 1.9.7) for R (version 4.0.0).16 Given the sparse nature of sampling, only a one-compartment model was evaluated. Calculated CLCR,17 serum creatinine, total body weight, height, age, gender and renal replacement therapy were evaluated as covariates. Model selection was performed using visual inspection of goodness-of-fit plots and minimization of the Akaike information criterion (AIC) among competing models. Patients with meropenem concentrations below the limit of quantification (BLQ) were excluded from the PK/PD analysis.
Posterior meropenem PK profiles (predictions generated every 0.2 h) were generated for the first 24 h. PK/PD target attainment was evaluated for 100% T>1×MIC and 100% T>4×MIC for empirical (i.e. pathogen-specific MICs determined by the microbiology laboratory) and directed [i.e. the breakpoint MIC for Pseudomonas aeruginosa of 2 mg/L18] treatment. Between-patient variability in target attainment was quantified using CV%.
Ethics
Institutional Review Boards (IRBs) approved the study (IRB ID# STU00204868 at Northwestern University and IRB ID# 20058 at Midwestern University).
Results
A total of 83 blood samples were available from 70 (35.7% female) patients, as shown in Figure S1, available as Supplementary data at JAC Online. A stability analysis indicated that degradation under study protocol conditions would be <<15% (Figure S2). Twelve patients had BLQ concentrations. Table 1 summarizes demographic and clinical characteristics. The median first 24 h meropenem dose was 4 g/day (IQR 3–4 g/day).
Table 1.
Demographic data and clinical characteristics
| Demographics | N | Median | Range |
|---|---|---|---|
| Age (years) | 70 | 65.5 | 25–83 |
| TBW (kg) | 70 | 75.2 | 46.7–166.4 |
| HT (cm) | 70 | 168 | 147–188 |
| BMI (kg/m2) | 70 | 25.9 | 16.6–51.2 |
| SCr (mg/dL) | 70 | 0.92 | 0.20–4.92 |
| CLCR (mL/min) | 70 | 82.6 | 14.3–418.5 |
| RRT | 6 | ||
| Sex | |||
| Male | 45 | ||
| Female | 25 |
N, number of patients; TBW, total body weight; HT, height; BSA, body surface area; SCr, serum creatinine; RRT, renal replacement therapy (includes intermittent haemodialysis and continuous renal replacement).
A one-compartment multiplicative residual error model best fitted the data. The final model included 22 support points (Table S1). The mean estimates for volume of distribution (V) and CL were 34.07 L and 5.3 L/h, respectively. Population and individual predicted versus observed goodness-of-fit plots are shown in Figure S3.
Within our cohort, PK/PD data were available (i.e. non-missing) for 15 patients who were treated empirically and 43 patients who were treated definitively. For those treated empirically, pathogen MICs ranged from 0.25 to 32 mg/L. Detected pathogens, their MICs and meropenem doses are summarized in Table S2.
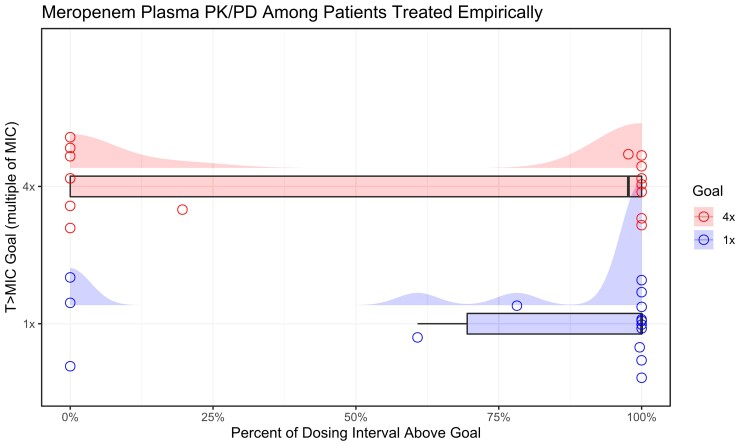
Individual target attainments for those treated empirically are illustrated in Figure 1. PK/PD variation, quantified as CV%, was 54%–92% with empirical treatment and 26%–33% for directed treatment considering targets of 100% T>1×MIC and T>4×MIC, respectively.
Figure 1.
Individual meropenem plasma PK/PD attainment among patients treated for HAP. PK/PD target attainment stratified by target (blue = T>1×MIC, red = T>4×MIC) among patients treated empirically. Individual observations (open circles) are jittered to increase visual clarity where values overlap. The distribution of observed PK/PD ratios is shown in box plots, with overlaid density (half-eye) plots demonstrating the range and density of PK/PD ratios in the sample.
Discussion
We found considerable between-patient variability in meropenem PK/PD target attainment. Most patients received our institutionally recommended dosing of 1 g every 8 h infused over 3 h (or adjusted for kidney function). Considering a PK/PD goal of 100% T>1×MIC, we found that 47% of patients treated empirically and 35% of patients receiving directed therapy failed to achieve this goal. If the more stringent target of 100% T>4×MIC is applied, 53% of patients treated empirically and 70% of patients receiving directed therapy would fail to achieve this goal. While population approaches increase the likelihood of target attainment, individualization is clearly required for many critically ill patients.
In the absence of individualized dosing, a significant number of HAP patients are at risk of experiencing inadequate PK/PD based on our findings. Roberts et al.12 found that increasing T>MIC significantly improved the survival after adjusting for clinical covariates. Scharf et al.13 found that time to infection resolution was improved for patients who achieved 100% free-drug T>1×MIC versus those who did not. Gijsen et al.14 also found low attainment for 1× and 4× MIC (46% versus 11%, respectively). Penetration into the site of infection (i.e. alveolar fluid) is also important. Lodise et al.19 found high variability for meropenem epithelial lining fluid penetration and that even the highest approved doses of meropenem may not achieve PK/PD targets. Thus, individualized dosing appears to be necessary to ensure target attainment in ICU patients.
Sparse sampling constrained our ability to identify a more complex model. However, identification of a one- versus a two-compartment model does not impact the assessment of T>MIC greatly. While sample degradation is a potential concern, the results of our stability analysis indicate that drug loss should be minimal under the protocolized sample handling in SCRIPT. A limitation to our study was that we are not yet able to link PK/PD to clinical outcomes. Future studies will investigate the effect of suboptimal target attainment on clinical outcomes.
In conclusion, meropenem-treated HAP patients are at risk for inadequate PK/PD in spite of extended-infusion dosing due to patient-specific variability in PK. Thus, an individualized approach to treatment is needed to achieve suggested PK/PD targets.
Supplementary Material
Acknowledgements
We would like to thank Midwestern University Core Facility – Downers Grove campus for their support.
Contributor Information
Roxane Rohani, Midwestern University College of Pharmacy Downers Grove Campus, Downers Grove, IL, USA; Midwestern University College of Pharmacy Downers Grove Campus, Pharmacometrics Center of Excellence, Downers Grove, IL, USA; Department of Pharmacy, Northwestern Medicine, Chicago, IL, USA.
Marc H Scheetz, Midwestern University College of Pharmacy Downers Grove Campus, Downers Grove, IL, USA; Midwestern University College of Pharmacy Downers Grove Campus, Pharmacometrics Center of Excellence, Downers Grove, IL, USA; Department of Pharmacy, Northwestern Medicine, Chicago, IL, USA.
Helen K Donnelly, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Alvaro Donayre, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Mengjia Kang, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Estefani Diaz, Robert H. Lurie Comprehensive Cancer Research Center, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Kay Dedicatoria, Midwestern University College of Pharmacy Downers Grove Campus, Downers Grove, IL, USA.
Alan R Hauser, Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Division of Infectious Diseases, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Egon A Ozer, Division of Infectious Diseases, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Sophia Nozick, Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Chao Qi, Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Anna E Pawlowski, Clinical and Translational Sciences Institute, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Michael N Neely, Laboratory of Applied Pharmacokinetics and Bioinformatics, The Saban Research Institute, Children’s Hospital of Los Angeles, Los Angeles, CA, USA; Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Alexander V Misharin, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Richard G Wunderink, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Nathaniel J Rhodes, Midwestern University College of Pharmacy Downers Grove Campus, Downers Grove, IL, USA; Midwestern University College of Pharmacy Downers Grove Campus, Pharmacometrics Center of Excellence, Downers Grove, IL, USA; Department of Pharmacy, Northwestern Medicine, Chicago, IL, USA.
NU SCRIPT Study investigators:
Hiam Abdala-Valencia, Michael J Alexander, Jason M Arnold, Joseph Isaac Bailey, Elizabeth T Bartom, Ankit Bharat, Thomas Bolig, Nicole Borkowski, G R Scott Budinger, Navdeep S Chandel, Rebecca K Clepp, John Coleman, Michael J Cuttica, Thaddeus R Cybulski, Jane E Dematte, Joseph S Deters, Justin A Fiala, Gaurav T Gadhvi, Catherine A Gao, Khalilah L Gates, Samuel W M Gatesy, Ritika Giri, Pearl D Go, Cara J Gottardi, Rogan A Grant, Stefan J Green, Elen Gusman, Estefany R Guzman, SeungHye Han, Erica Marie Hartmann, Curt M Horvath, Mishaal Hukamdad, Sydney M Hyder, Manu Jain, Anthony M Joudi, Rachel B Kadar, Ravi Kalhan, David W Kamp, Manoj Kandpal, David A Kidd, Hermon Kihshen, Zasu M Klug, Erin A Korth, Jacqueline M Kruser, Romy Lawrence, Emily M Leibenguth, Anne R Levenson, Lindsey D Gradone, Gabrielle Y Liu, Jon W Lomasney, Theresa A Lombardo, Ziyan Lu, Amy Ludwig, Ali Mahmoud, Elizabeth S Malsin, Nikolay S Markov, Alexandra C McQuattie-Pimentel, Daniel Meza, Felix Leonardo Morales, Luisa Morales-Nebreda, Richard I Morimoto, Ruben J Mylvaganam, Prasanth Nannapaneni, Luís A Nunes Amaral, Radhika Patel, Lorenzo L Pesce, Chiagozie O Pickens, Yuliya Politanska, Taylor A Poor, Michelle Hinsch Prickett, Melissa Querrey, Luke V Rasmussen, Ziyou Ren, Karen M Ridge, Madeline L Rosenbaum, Sharon R Rosenberg, Timothy Rowe, Susan R Russell, Marc A Sala, Daniel Schneider, Clara J Schroedl, Katharine Secunda, Patrick C Seed, Karolina J Senkow, Todd Shamaly, Elisheva D Shanes, Jiaxian Shen, Ali Shilatifard, Lango Sichizya, Benjamin D Singer, Sean Smith, Peter H S Sporn, Justin Starren, Thomas Stoeger, Jack Sumner, Suchitra Swaminathan, Jacob I Sznajder, Heliodoro Tejedor Navarro, Lindsey N Textor, Sanket Thakkar, Rade Tomic, Betty Tran, Kaitlyn Vitale, Ajay A Wagh, James M Walter, Firas Wehbe, Deborah R Winter, Alexis Rose Wolfe, Lisa F Wolfe, and Anjana V Yeldandi
Members of The NU SCRIPT Study Investigators Consortium
Hiam Abdala-Valencia, Michael J. Alexander, Jason M. Arnold, Joseph Isaac Bailey, Elizabeth T. Bartom, Ankit Bharat, Thomas Bolig, Nicole Borkowski, G. R. Scott Budinger, Navdeep S. Chandel, Rebecca K. Clepp, John Coleman, Michael J. Cuttica, Thaddeus R. Cybulski, Jane E. Dematte, Joseph S. Deters, Justin A. Fiala, Gaurav T. Gadhvi, Catherine A. Gao, Khalilah L. Gates, Samuel W. M. Gatesy, Ritika Giri, Pearl D. Go, Cara J. Gottardi, Rogan A. Grant, Stefan J. Green, Elen Gusman, Estefany R. Guzman, SeungHye Han, Erica Marie Hartmann, Curt M. Horvath, Mishaal Hukamdad, Sydney M. Hyder, Manu Jain, Anthony M. Joudi, Rachel B. Kadar, Ravi Kalhan, David W. Kamp, Manoj Kandpal, David A. Kidd, Hermon Kihshen, Zasu M. Klug, Erin A. Korth, Jacqueline M. Kruser, Romy Lawrence, Emily M. Leibenguth, Anne R. Levenson, Lindsey D. Gradone, Gabrielle Y. Liu, Jon W. Lomasney, Theresa A. Lombardo, Ziyan Lu, Amy Ludwig, Ali Mahmoud, Elizabeth S. Malsin, Nikolay S. Markov, Alexandra C. McQuattie-Pimentel, Daniel Meza, Felix Leonardo Morales, Luisa Morales-Nebreda, Richard I. Morimoto, Ruben J. Mylvaganam, Prasanth Nannapaneni, Luís A. Nunes Amaral, Radhika Patel, Lorenzo L. Pesce, Chiagozie O. Pickens, Yuliya Politanska, Taylor A. Poor, Michelle Hinsch Prickett, Melissa Querrey, Luke V. Rasmussen, Ziyou Ren, Karen M. Ridge, Madeline L Rosenbaum, Sharon R. Rosenberg, Timothy Rowe, Susan R. Russell, Marc A. Sala, Daniel Schneider, Clara J. Schroedl, Katharine Secunda, Patrick C. Seed, Karolina J. Senkow, Todd Shamaly, Elisheva D. Shanes, Jiaxian Shen, Ali Shilatifard, Lango Sichizya, Benjamin D. Singer, Sean Smith, Peter H. S. Sporn, Justin Starren, Thomas Stoeger, Jack Sumner, Suchitra Swaminathan, Jacob I. Sznajder, Heliodoro Tejedor Navarro, Lindsey N. Textor, Sanket Thakkar, Rade Tomic, Betty Tran, Kaitlyn Vitale, Ajay A. Wagh, James M. Walter, Firas Wehbe, Deborah R. Winter, Alexis Rose Wolfe, Lisa F. Wolfe and Anjana V. Yeldandi.
Funding
This study was supported by internal funding. Roxane Rohani received Midwestern University’s Research Pilot Grant. Alexander V. Misharin was supported by NIH grants U19AI135964, P01AG049665, R56HL135124, R01HL153312 and NUCATS COVID-19 Rapid Response Grant. Helen K. Donnelly, Alvaro Donayre, Mengjia Kang, Estefani Diaz, Alan R. Hauser, Egon A. Ozer, Sophia Nozick, Chao Qi, Anna E. Pawlowski and Richard G. Wunderink were supported by NIH grant U19AI135964. Nathaniel J. Rhodes reports research support from Paratek and the American Association of Colleges of Pharmacy outside the present study.
Transparency declarations
None to declare.
Supplementary data
Figures S1 to S3 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Kalil AC, Metersky ML, Klompas Met al. . Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts JA, Lipman J. Optimal doripenem dosing simulations in critically ill nosocomial pneumonia patients with obesity, augmented renal clearance, and decreased bacterial susceptibility. Crit Care Med 2013; 41: 489–95. [DOI] [PubMed] [Google Scholar]
- 3. Udy AA, De Waele JJ, Lipman J. Augmented renal clearance and therapeutic monitoring of β-lactams. Int J Antimicrob Agents 2015; 45: 331–3. [DOI] [PubMed] [Google Scholar]
- 4. Ulldemolins M, Soy D, Llaurado-Serra Met al. . Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother 2015; 59: 5520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiffer CR, Mendes C, Kuti JLet al. . Pharmacodynamic comparisons of antimicrobials against nosocomial isolates of Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa from the MYSTIC surveillance program: the OPTAMA Program, South America 2002. Diagn Microbiol Infect Dis 2004; 49: 109–16. [DOI] [PubMed] [Google Scholar]
- 6. Valenza G, Seifert H, Decker-Burgard Set al. . Comparative activity of carbapenem testing (COMPACT) study in Germany. Int J Antimicrob Agents 2012; 39: 255–8. [DOI] [PubMed] [Google Scholar]
- 7. Guilhaumou R, Benaboud S, Bennis Yet al. . Optimization of the treatment with β-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit Care 2019; 23: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Udy AA, Varghese JM, Altukroni Met al. . Subtherapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 2012; 142: 30–9. [DOI] [PubMed] [Google Scholar]
- 9. Tam VH, McKinnon PS, Akins RLet al. . Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother 2002; 50: 425–8. [DOI] [PubMed] [Google Scholar]
- 10. Kitzes-Cohen R, Farin D, Piva Get al. . Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents 2002; 19: 105–10. [DOI] [PubMed] [Google Scholar]
- 11. Taccone FS, Laterre PF, Dugernier Tet al. . Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 2010; 14: R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts JA, Paul SK, Akova Met al. . DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58: 1072–83. [DOI] [PubMed] [Google Scholar]
- 13. Scharf C, Liebchen U, Paal Met al. . The higher the better? Defining the optimal β-lactam target for critically ill patients to reach infection resolution and improve outcome. J Intensive Care 2020; 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gijsen M, Elkayal O, Annaert Pet al. . Meropenem target attainment and population pharmacokinetics in critically ill septic patients with preserved or increased renal function. Infect Drug Resist 2022; 15: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) . Bioanalytical Method Validation Guidance for Industry. 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
- 16. Neely MN, van Guilder MG, Yamada WMet al. . Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 2012; 34: 467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 18. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty second Edition: M100. 2022. [Google Scholar]
- 19. Lodise TP, Sorgel F, Melnick Det al. . Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 2011; 55: 1606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.