Abstract
Background
Pharmacokinetic data are lacking for progestin-releasing subdermal contraceptive implants when used with either rilpivirine- or darunavir/ritonavir-based ART.
Objectives
To characterize the pharmacokinetics of etonogestrel or levonorgestrel implants when administered with these ART regimens over 48 weeks.
Patients and methods
Two separate, parallel, three-group, non-randomized, pharmacokinetic studies evaluated either etonogestrel or levonorgestrel in women receiving rilpivirine- or darunavir-based ART compared with women without HIV (control group). Participants on ART were switched to rilpivirine-based ART with a run-in period of 6 weeks or darunavir-based ART with a run-in of 2 weeks prior to implant insertion. Plasma was collected on Day 0, and 1, 4, 12, 24, 36 and 48 weeks post-insertion. Plasma progestin concentrations were compared between ART and control groups by geometric mean ratio (GMR) and 90% CI.
Results
At the primary endpoint of Week 24, progestin concentrations were similar between the rilpivirine and control groups [etonogestrel: 1.18 (0.99–1.37); levonorgestrel: 1.16 (0.97–1.33)]. At Week 24, progestin exposure was higher in the darunavir groups compared with the control group [etonogestrel: 2.56 (1.69–3.28); levonorgestrel: 1.89 (1.38–2.29)]. Results remained consistent through to Week 48. No differences in etonogestrel-related adverse events were observed, but both ART groups experienced more menstrual abnormalities versus the control group with levonorgestrel.
Conclusions
Etonogestrel and levonorgestrel concentrations were not altered by rilpivirine-based ART. Although progestin concentrations were higher in the ART groups containing ritonavir-boosted darunavir, no implant-related serious adverse events were observed. Both progestin-releasing implants are an appropriate contraceptive option with either rilpivirine- or darunavir/ritonavir-based ART.
Introduction
In 2020, 36 million adults were living with HIV, 53% of whom were women and girls.1 Lifelong ART suppresses replication of HIV, delays disease progression and improves survival in women with HIV.2 In non-pregnant women, effective family planning reduces the risk of mother-to-child HIV transmission by averting unintended pregnancies.3,4 In Uganda and other countries, family planning services are integrated with ART services to improve coverage of contraception services for women living with HIV.5 However, antiretroviral drugs have potential for drug–drug interactions that could lead to altered pharmacokinetics and reduced efficacy of co-administered drugs, including contraceptives.6
Subdermal progestin-releasing contraceptive implants are the most widely used reversible long-acting contraceptive method among Ugandan women.7 In Uganda, etonogestrel implants are licensed for 3 years of continuous use, while levonorgestrel implants are licensed for 5 years.8,9 Pharmacokinetics of progestins released from implants are characterized by reaching a peak in concentrations within 1 week following insertion, then decreasing most rapidly within the first 3 months after insertion, then reaching a steady state with only slow decline over the duration of product use. Levonorgestrel is released from the 150 mg implant at an initial rate of 100 μg/day, decreasing to 40 μg/day in the first year and 30 μg/day by the third year of use, with a peak levonorgestrel concentration of 772 ± 414 pg/mL at 2 days, then decreasing slowly to 357 ± 155 pg/mL at 6 months, 340 ± 159 pg/mL at 12 months and 279 ± 123 pg/mL at 60 months.8 The etonogestrel 68 mg implant release rate is 50–60 μg/day after 5–6 weeks, decreasing to 35–45 μg/day after the first year, and 25–30 μg/day by the end of the third year of use, with peak etonogestrel concentrations of 1200 ± 604 pg/mL in the first 2 weeks, slowly declining to 297 ± 104 pg/mL at 6 months, 202 ± 55 at 12 months and then 138 ± 43 pg/mL at 36 months.9
Both levonorgestrel and etonogestrel are metabolized via the cytochrome P450 (CYP) 3A4 enzyme and are prone to drug–drug interactions when co-administered with inhibitors or inducers of this enzyme system.8,9 Indeed, significant drug–drug interactions between each of these progestins and efavirenz-based ART have been demonstrated in prior studies in Uganda, resulting in 84% lower exposure for etonogestrel and 47% lower exposure for levonorgestrel, with evidence of loss of contraceptive efficacy in the levonorgestrel study.10,11 However, data are lacking for newer antiretrovirals used for treatment of HIV. Rilpivirine is an NNRTI used in combination with other ART to treat HIV-1 infection in treatment-naive patients with a viral load of ≤100 000 copies/mL.12 Darunavir is an HIV-1 PI recommended by the WHO in combination with ritonavir as part of second- or third-line ART regimens.13 Ritonavir is a potent inhibitor of CYP3A4, but also an inducer of other CYP enzymes, both characteristics resulting in clinically significant drug–drug interactions. There are limited data available for the combined use of either the levonorgestrel or the etonogestrel contraceptive implant with rilpivirine- or darunavir/ritonavir-based ART. Therefore, we aimed to characterize the pharmacokinetics of etonogestrel and levonorgestrel in women with HIV when administered with either rilpivirine-based ART or darunavir/ritonavir-based ART compared with a control group of HIV-negative women over 48 weeks. We also aimed to describe the safety and tolerability of etonogestrel or levonorgestrel implants in participants using rilpivirine- or darunavir/ritonavir-based ART.
Patients and methods
Two similar non-randomized, open-label, parallel, three-group studies evaluating either etonogestrel (etonogestrel study) or levonorgestrel (levonorgestrel study) pharmacokinetics when administered with either rilpivirine- or darunavir/ritonavir-based ART compared with HIV-negative women (control group) were conducted concurrently at the Infectious Diseases Institute (IDI), Makerere University, Kampala, Uganda. Both studies were conducted in accordance with the Declaration of Helsinki and received ethics approval from the Joint Clinical Research Centre Research Ethics Committee and studies were registered with the Uganda National Council for Science and Technology (HS145ES, HS146ES) and ClinicalTrials.gov (NCT03589027, NCT03589040). All participants provided written informed consent prior to study procedures.
Study participants
Women living with HIV seeking family planning services were identified at the IDI Clinic, while women without HIV were referred from IDI-affiliated clinics in the Kampala area. Potential participants received information on available contraceptive methods, including implants, intrauterine devices, oral contraceptive pills, depo-medroxyprogesterone, and male and female condoms. Women were considered eligible for enrolment if they were aged 18 years or above, desired a contraceptive implant and were clinically eligible for the implant.14 If not abstinent, study participants in the ART arms were required to use an effective non-hormonal method of contraception for the study duration, including male or female condoms or the copper intrauterine device. Participants in the control group were counselled on HIV prevention strategies and offered male and female condoms. Women with HIV were eligible for the rilpivirine groups (n = 30 per study) if they were on efavirenz-based ART, or for the darunavir/ritonavir groups (n = 30 per study) if they were on lopinavir- or atazanavir-based ART. Participants had to be on eligible ART for at least 1 year with an HIV-1 RNA of <50 copies/mL and a CD4 count of >200 cells/mL at study entry. Participants in the control group (n = 20 per study) were excluded if HIV positive at screening and referred to HIV care services. Pregnant or breastfeeding women were excluded. Medications with potential for interaction with the study drugs were to be avoided prior to and throughout the study period.15
Procedures
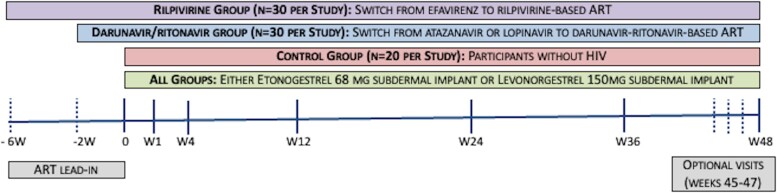
The study schema is described in Figure 1. At enrolment, participants on efavirenz were switched to rilpivirine (rilpivirine group) while those on either lopinavir or atazanavir were switched to darunavir/ritonavir-based ART (darunavir/ritonavir group). Participants in the rilpivirine groups took 25 mg rilpivirine tablets orally with food once daily. Based on the local guidelines, patients in the darunavir/ritonavir group took darunavir 600 mg twice daily plus ritonavir 100 mg twice daily, both with food. NRTIs (either tenofovir plus lamivudine or zidovudine plus lamivudine) in the pre-entry regimen were continued during the study. To ensure elimination of prior antiretrovirals before the pharmacokinetic evaluation, a washout/run-in period was required prior to implant insertion (2 weeks post-switch for darunavir/ritonavir groups and 6 weeks post-switch for the rilpivirine groups). Temporal relationship to rilpivirine or darunavir was assessed for any adverse events that occurred after initiation of study ART drugs but before implant insertion. Participants in the control groups progressed directly to implant insertion. For all groups, the date of implant insertion was assigned as Day 0.
Figure 1.
Study schema for the etonogestrel and levonorgestrel studies. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
After implant insertion, participants returned after 1, 4, 12, 24, 36 and 48 weeks. On these visits, we documented occurrence of all adverse events and determined their temporal relationship to the implant. The use of concomitant medications and herbal remedies was also assessed. A urine pregnancy test was performed, and endogenous progesterone was assessed at every visit. Safety laboratory assessments were conducted throughout the study period, including a complete blood count, HIV-1 RNA, serum creatinine, ALT, lipid profiles and haemoglobin A1c. Optional visits occurred at Weeks 45, 46 and 47 to collect blood samples for endogenous progesterone. Endogenous progesterone concentrations greater than 3 ng/mL were considered consistent with ovulatory activity.16
Pharmacokinetic analysis
For levonorgestrel and etonogestrel pharmacokinetic analysis, whole blood samples were collected on Day 0, and at 1, 4, 12, 24, 36 and 48 weeks post-insertion of implant. Week 24 was the primary endpoint, and participants were excluded if they did not meet this endpoint. Plasma was obtained by centrifugation and samples were batched and stored at −80°C until sample shipment. Etonogestrel and levonorgestrel concentrations in plasma were quantified by validated, quality-controlled LC-MS/MS methods.17 The lower limit of quantitation for both progestins was 25 pg/mL, and the coefficient of variation was less than 15% for both assays. We assessed suppression of ovulation based on a 350 and 90 pg/mL threshold for levonorgestrel and etonogestrel, respectively.
Statistical analysis
Based on our previously observed etonogestrel and levonorgestrel intrapatient variability (30%–50%),10,11 25 participants in each ART group and 17 participants in each control group were expected to provide 87% power to test our primary hypotheses of null effect [geometric mean ratio (GMR) 90% CIs falling between 0.7 and 1.43] at the study-defined primary endpoint (Week 24).18 Etonogestrel and levonorgestrel concentrations were summarized as medians and IQRs at each study visit within each group. We compared concentrations between each ART group with the respective control group by calculating GMRs for the control group versus the rilpivirine group or the darunavir/ritonavir group with 90% CIs obtained using a non-parametric bootstrap performed with R software, v.4.0.2 and the boot package.19–21 We compared each ART arm with the control group using Pearson’s chi-squared or Fisher’s exact test for categorical baseline characteristics and adverse events. We used Wilcoxon rank-sum test to compare continuous baseline characteristics (age, weight, BMI and parity). All adverse events were coded using MedDRA (https://www.meddra.org/). Severity of adverse events was graded according to the National Institute of Allergy and Infectious Diseases Division of AIDS (DAIDS) classification system for reporting adverse experiences in adults.22
Results
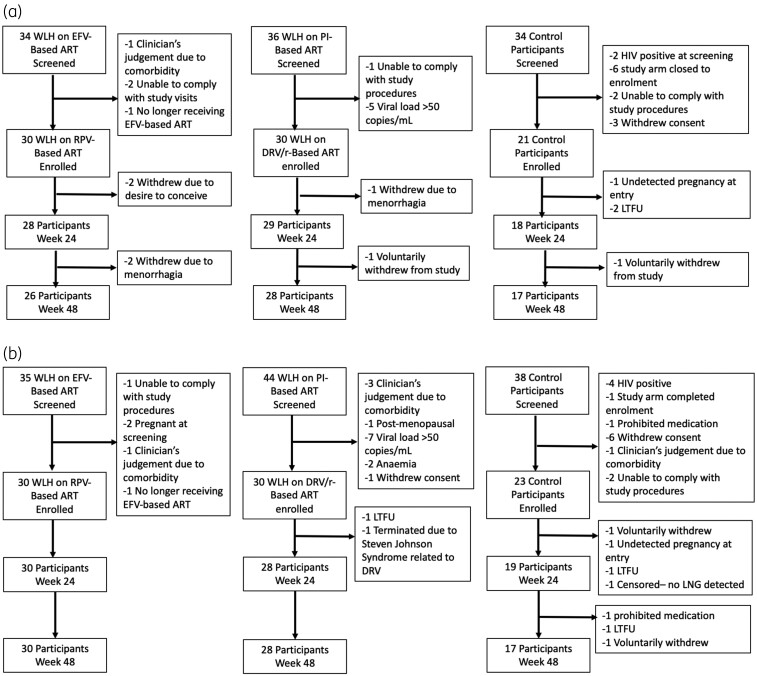
Participants were recruited between July 2018 and December 2019. In the etonogestrel study (Figure 2a), 81 participants were enrolled (30 each in darunavir/ritonavir and rilpivirine groups; 21 in the control group). Seventy-five participants, (rilpivirine = 28; darunavir/ritonavir = 29; control = 18) were included in the Week 24 analysis and 71 participants (rilpivirine = 26; darunavir = 28; control = 17) were included in the Week 48 analysis. In the levonorgestrel study (Figure 2b), 83 participants were enrolled (30 each in darunavir/ritonavir and rilpivirine groups; 23 in the control group). A total of 77 participants (rilpivirine = 30; darunavir/ritonavir = 28; control = 19) were included in Week 24 analysis and 74 participants (rilpivirine = 30; darunavir = 28; control = 16) were included in Week 48 analysis.
Figure 2.
(a) Participant disposition in the etonogestrel study through the primary endpoint at Week 24 and secondary endpoint at Week 48. EFV, efavirenz; DRV/r, darunavir/ritonavir; RPV, rilpivirine; LTFU, lost to follow-up; WLH, women living with HIV. (b) Participant disposition in the levonorgestrel study through the primary endpoint at Week 24 and secondary endpoint at Week 48. DRV/r, darunavir/ritonavir; EFV, efavirenz; RPV, rilpivirine; LTFU, lost to follow-up; LNG, levonorgestrel; WLH, women living with HIV.
Table 1 describes participants’ baseline characteristics for each study. In the etonogestrel study, demographic characteristics were similar between the ART groups and the control group, except the median age, which was higher in the darunavir arm (37 versus 32 years; P = 0.003) and the rilpivirine arm (36 versus 32 years; P = 0.048) compared with the control group. In the levonorgestrel study, demographic characteristics were also similar between the ART groups and the control group, except the median age, which was higher in the darunavir arm [38 versus 34 (rilpivirine) and 33 years (control); P = 0.002]. The weight was higher in the control group compared with both ART groups [65 versus 56 kg (rilpivirine), P = 0.008; 65 versus 58 kg (darunavir), P = 0.034], but the BMI was only higher in the control group compared with the rilpivirine group (26.6 versus 21.9 kg/m2; P = 0.007).
Table 1.
Participant demographics at baseline for participants who met the primary endpoint at Week 24
| Characteristics | ENG study (n = 75) | LNG study (n = 78) | ||||
|---|---|---|---|---|---|---|
| RPV (n = 28) | DRV/r (n = 29) | Control (n = 18) | RPV (n = 30) | DRV/r (n = 28) | Control (n = 19) | |
| Age (years) | 36a (30–39) | 37b (32–41) | 32 (26–35) | 34 (29–37) | 38c (35–42) | 33 (31–36) |
| Weight (kg) | 60 (54–72) | 59 (47–65) | 60 (50–69) | 56d (51–64) | 58e (50–68) | 65 (59–74) |
| BMI (kg/m2) | 24.1 (21.3–29.5) | 23.2 (20.9–25.3) | 23.7 (20.5–28.3) | 21.9f (19.6–24.4) | 24.0 (21.2–28.4) | 26.6 (23.2–28.7) |
| Marital status, n (%) | ||||||
| Single | 18 (64.3) | 18 (62.1) | 11 (61.1) | 12 (40.0) | 10 (35.7) | 8 (40.0) |
| Married | 10 (35.7) | 11 (37.9) | 7 (38.9) | 18 (60.0) | 18 (64.3) | 12 (60.0) |
| Parity | 3 (2–5) | 3 (3–4) | 3 (3–5) | 3 (2–4) | 4 (3–5) | 3 (2–4) |
| Highest educational level, n (%) | ||||||
| None | 2 (7.1) | 4 (13.8) | 1 (5.6) | 3 (10.0) | 1 (3.6) | 0 |
| Primary | 15 (53.6) | 16 (55.2) | 10 (55.6) | 8 (26.7) | 14 (50.0) | 10 (50.0) |
| Secondary+ | 11 (39.3) | 9 (31.0) | 7 (38.8) | 19 (63.3) | 13 (46.4) | 10 (50.0) |
| CD4 count (cells/mL) | 738 (609–1019) | 730 (480–801) | N/A | 762 (564–1045) | 719 (407–917) | N/A |
Unless indicated, results are presented as median (IQR) and not statistically different between groups. Control, women without HIV; DRV/r, darunavir/ritonavir-based ART group; ENG, etonogestrel; LNG, levonorgestrel; N/A, not applicable; RPV, rilpivirine-based ART group.
RPV versus control; P = 0.048; Wilcoxon rank-sum.
DRV versus control; P = 0.003; Wilcoxon rank-sum.
DRV versus control; P = 0.002; Wilcoxon rank-sum.
RPV versus control; P = 0.008; Wilcoxon rank-sum.
DRV versus control; P = 0.034; Wilcoxon rank-sum.
RPV versus control; P = 0.007; Wilcoxon rank-sum.
Implant progestin concentrations
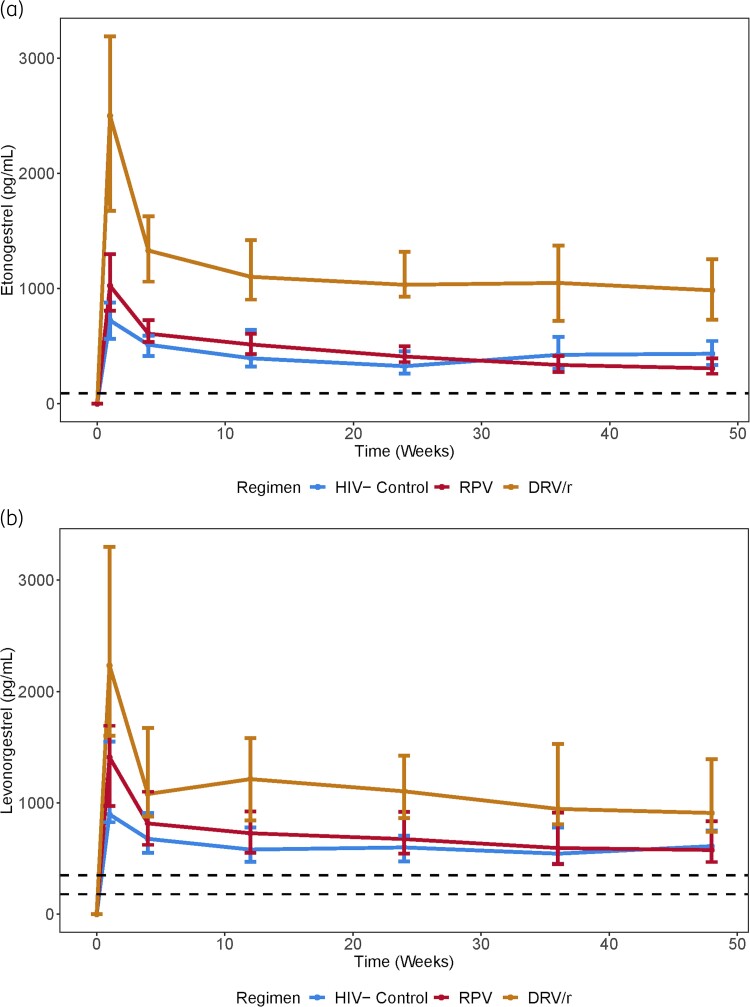
Pharmacokinetic parameters for etonogestrel and levonorgestrel are presented in Table 2 and plasma concentrations are shown in Figure 3. At both the primary endpoint (Week 24) and Week 48, both etonogestrel and levonorgestrel concentrations in the rilpivirine groups were not statistically different compared with the respective control groups. In the darunavir/ritonavir groups, etonogestrel was 156% and 120% higher and levonorgestrel was 89% and 74% higher compared with the control groups at Weeks 24 and 48, respectively.
Table 2.
Plasma progestin concentrations per visit over 48 weeks for participants reaching the primary endpoint at Week 24
| Week | Median (IQR) concentrations (pg/mL) | GMR (90% CI) | |||
|---|---|---|---|---|---|
| RPV group | DRV/r group | Control group | RPV:control | DRV/r:control | |
| Etonogestrel | n = 28 | n = 29 | n = 18 | ||
| 1 | 1025 (807–1299) | 2500 (1675–3190) | 724 (563–878) | 1.38 (1.16–1.57) | 3.22 (2.54–3.80) |
| 4 | 609 (536–727) | 1330 (1060–1628) | 513 (413–591) | 1.24 (1.03–1.41) | 2.61 (2.14–3.02) |
| 12 | 515 (431–609) | 1102 (904–1422) | 395 (324–643) | 1.19 (0.98–1.36) | 2.58 (2.08–3.03) |
| 24 | 410 (362–501) | 1033 (930–1320) | 326 (263–455) | 1.18 (0.99–1.37) | 2.56 (1.69–3.28) |
| 36 | 337 (277–414) | 1049 (720–1374) | 425 (303–580) | 0.91 (0.66–1.10) | 2.34 (1.56–2.94) |
| 48 | 308 (260–396) | 985 (729–1256) | 434 (338–545) | 0.85 (0.69–0.98) | 2.20 (1.52–2.77) |
| Levonorgestrel | n = 30 | n = 28 | n = 19 | ||
| 1 | 1410 (972–1693) | 2234 (1602–3300) | 897 (827–1550) | 1.33 (1.03–1.58) | 2.17 (1.62–2.61) |
| 4 | 816 (623–1100) | 1080 (877–1674) | 677 (552–910) | 1.18 (0.94–1.38) | 1.63 (1.21–1.96) |
| 12 | 728 (550–922) | 1214 (841–1583) | 581 (472–778) | 1.25 (1.02–1.44) | 2.06 (1.61–2.44) |
| 24 | 675 (545–921) | 1104 (863–1425) | 600 (474–707) | 1.16 (0.97–1.33) | 1.89 (1.38–2.29) |
| 36 | 595 (449–913) | 946 (809–1528) | 544 (452–780) | 1.07 (0.84–1.26) | 1.76 (1.36–2.08) |
| 48 | 577 (469–835) | 910 (741–1394) | 612 (469–753) | 1.06 (0.86–1.22) | 1.74 (1.36–2.04) |
Control, women without HIV; DRV/r, darunavir/ritonavir-based ART group; RPV, rilpivirine-based ART group.
Figure 3.
(a) Median etonogestrel concentration–time curve over 48 weeks. Error bars represent the IQR. Dashed line is 90 pg/mL. RPV, rilpivirine-based ART; DRV/r, darunavir/ritonavir-based ART. (b) Median levonorgestrel concentration–time curve over 48 weeks. Error bars represent IQR. Dashed lines are 180 and 350 pg/mL thresholds. RPV, rilpivirine-based ART; DRV/r, darunavir/ritonavir-based ART. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Endogenous progesterone
In the etonogestrel study, 71 participants had at least one optional visit, while 54 participants completed all optional visits from Weeks 45 to 47 in addition to the standard visit at Week 48. No ovulatory activity was detected among participants in the etonogestrel study. In the levonorgestrel study, 71 participants had at least one optional visit, while 59 completed all optional visits plus Week 48. Two participants in the levonorgestrel study, one in the rilpivirine group and the other in the darunavir/ritonavir group, had endogenous progesterone values (>3 ng/mL) consistent with ovulatory activity at Weeks 46 and 47, respectively.
Adverse events
Implant-related adverse events are presented in Tables S1 and S2, available as Supplementary data at JAC Online. Six out of 60 participants in the etonogestrel study and 10 out of 60 in the levonorgestrel study reported an ART drug-related adverse event before implant insertion. In the levonorgestrel study, one serious adverse event occurred related to darunavir, requiring study discontinuation. The participant developed a generalized maculopapular rash 4 weeks after initiating darunavir-based ART and symptoms resolved upon discontinuation. All other adverse events were either Grade 1 or 2 and they included headache, rash, nausea and diarrhoea.
Implant-related adverse events are listed in Tables S1 and S2. In the etonogestrel study, the number of participants with any adverse events was similar when ART groups were compared with the control group. The majority of adverse events were either Grade 1 or 2. Two participants in the rilpivirine group and one in the darunavir group reported Grade 3 weight gain (10%–19% increase from baseline), with weight increases of 16 and 10.5 kg in the rilpivirine group and 11 kg in the darunavir group. Bleeding irregularities were less common in the control groups; however, there was no statistically significant difference when compared between the ART groups and the control group. Two participants in the etonogestrel study experienced persistent menorrhagia (one each from darunavir and rilpivirine groups) leading to study discontinuation. Eleven participants (36.7%) in the rilpivirine arm reported nausea, compared with two participants (9.5%) from the control group (P = 0.048).
In the levonorgestrel study, the number of participants with any adverse events was similar when ART groups were compared with the control group. The majority of adverse events were Grade 1 (80.8%); the remainder were Grade 2 (19.2%). No Grade 3 or higher adverse events were reported in this study. Menstrual changes were more common in the ART groups compared with the control group (rilpivirine versus control, P = 0.001; and darunavir versus control, P = 0.025).
No pregnancies or ART treatment failures were observed in either study. No significant increases in haemoglobin A1c levels or serum lipids were noted in either study.
Discussion
In these studies, median etonogestrel and levonorgestrel concentrations were higher than ovulation thresholds across all study.23 Throughout the study period, the levonorgestrel and etonogestrel concentrations were similar between the rilpivirine group and the control group of healthy volunteers in each study. In contrast, progestin concentrations were higher in the darunavir/ritonavir groups versus the control groups throughout the study. Higher concentrations in the darunavir/ritonavir group may be explained by inhibition of CYP3A4 by ritonavir resulting in slower metabolism of etonogestrel and levonorgestrel, both of which are substrates of CYP3A4.24 No excess implant-related adverse events occurred in the darunavir groups of the etonogestrel study, suggesting that these increases may not be clinically significant. Although there were more menstrual changes reported in the darunavir/ritonavir group compared with the control group of the levonorgestrel study, there were also more menstrual changes in the rilpivirine group despite similar total concentrations to the control group, suggesting this was unrelated to levonorgestrel exposure with darunavir/ritonavir.
Our findings align with previous studies of oral contraceptives used in combination with boosted PIs and rilpivirine-based ART. Crauwels et al.25 found that rilpivirine had no clinically relevant effect on the pharmacokinetics of orally administered hormonal contraceptive. That study, in combination with our findings, indicates that rilpivirine-based ART should have no impact on systemic exposure of hormonal contraception. Further, in both studies the median etonogestrel and levonorgestrel concentrations were higher than ovulation thresholds across all study visits. In a 3 year randomized controlled trial, Sivin et al.23 reported that no pregnancies occurred with levonorgestrel concentrations above 180 pg/mL. However, in a previous study conducted at the IDI, we reported two unintended pregnancies in women using the levonorgestrel implant with efavirenz-based ART at concentrations above 180 pg/mL (303 and 299 pg/mL).10 Therefore, we evaluated levonorgestrel concentrations with a higher threshold of 350 pg/mL and this was exceeded in all groups. Etonogestrel has a reported concentration threshold for suppression of ovulation of 90 pg/mL.26 Similarly, median etonogestrel concentrations across all study groups remained above the ovulation threshold throughout the study period.
Previous studies of lopinavir/ritonavir-based ART with the etonogestrel implants reported a 50% increase in etonogestrel exposure.27 Similar findings of increased progestin exposure with boosted PIs have also been reported with norgestimate and norethindrome combined oral contraceptives tablets, norethindrone-only oral contraceptive tablets, the transdermal combined contraceptive patch and the combined intravaginal ring.28–32 Previous to our study, there was one study published on darunavir/ritonavir combined with ethinyl oestradiol and norethindrone in healthy volunteers that found 30% lower minimum concentrations and 14% lower AUC of norethindrone when combined with darunavir/ritonavir in healthy volunteers. The authors concluded this decrease was not clinically significant.33 Thus the route of administration for the progestin as well as the type of PI must be considered when evaluating drug–drug interactions between ART and hormonal contraceptives. Our findings of increased etonogestrel and levonorgestrel exposure are consistent with the previous studies of other boosted PIs and hormonal contraceptives. Given the lack of increase in adverse events found in our study, this increased exposure is not likely to be clinically significant. However, our sample size was based on the primary outcome of pharmacokinetics and thus the sample might not be of sufficient size to show differences in adverse events.
Etonogestrel and levonorgestrel implants prevent pregnancy by blocking the release of luteinizing hormone, an important reproductive hormone for ovulation, as well as thickening cervical mucus.34 Ovulation leads to the production of endogenous progesterone by the corpus luteum, which therefore can serve as a surrogate biomarker for ovulation. We measured weekly serum progesterone concentrations over 4 weeks at the end of the study period (Weeks 45–48) to reflect one menstrual cycle, using 3 ng/mL as the threshold for ovulation.16 Two participants had a progesterone value above this threshold in the levonorgestrel study, and no participant had progesterone results above this threshold in the etonogestrel study. This is not unexpected as ovulations are more common with levonorgestrel use compared with etonogestrel use. Ovulation has been reported to occur in up to 50% of menstrual cycles over 5 years of levonorgestrel use.35 Two studies have demonstrated ovulation begins to occur within the first year of levonorgestrel implant use in the absence of drug interactions.36,37 Using progesterone as a surrogate marker, the studies compared the rate of ovulation among women on an levonorgestrel implant (Norplant®) versus those on a non-hormonal contraceptive method. In one of the studies, 3 out of 27 sampling periods (a set of blood samples were drawn every third or fourth day for five or six consecutive weeks) showed progesterone levels of >3 ng/mL in the treatment group.36 In the other study, 3 out of 20 sampling runs showed progesterone levels of >5 ng/mL in the treatment group.37
The etonogestrel and levonorgestrel implants were well tolerated when used in combination with either rilpivirine or darunavir/ritonavir. Adverse events related to the contraceptive implant were mostly of mild or moderate severity, and similar to those in published literature.38,39 Changes in bleeding patterns are common with progestin-only contraceptive methods and these menstrual changes are the most common reasons for contraceptive implant discontinuation in clinical practice.40 Menstrual changes were common in our study, reported more commonly among women in the ART groups, perhaps because they were more comfortable disclosing bleeding patterns with the study team, who they had a long-standing relationship with, or because menstrual changes are more common for women with HIV. Despite the high frequency of menstrual changes, only two participants discontinued the implant due to menorrhagia. Despite increased etonogestrel and levonorgestrel exposure in participants taking darunavir/ritonavir-based ART, no increase in progestin-related adverse events was noted, though our sample size was small. Dyslipidaemias have also been associated with the use of hormonal contraceptives, but we did not identify worsening of lipid profiles in these studies; however, follow-up was only 48 weeks in duration.41
Our study does have limitations that should be considered in interpreting the findings. First, study participants were not randomly assigned to treatment groups, therefore baseline differences did exist between study groups. Second, some adverse events overlap between the implants and the ART drugs, which made it hard to accurately determine the temporal relationship. Third, the period of follow-up was limited to 48 weeks for feasibility, recognizing that the implants are expected to be used for a longer duration. However, we do not expect findings to be significantly different given the stable results over 48 weeks. Further, these findings are consistent with other contraceptive implant–drug interaction studies. We used sparse sampling of the progestins to estimate the pharmacokinetics of the implants in combination with ART and not intensive sampling. Therefore, we cannot accurately describe the absorption phase or maximum concentration of progestins. However, 1 week post implant insertion is consistent with the observed peak of both levonorgestrel and etonogestrel implants, and concentrations later in implant use are more clinically relevant to prevent pregnancy. In addition, recent data suggest that sparse sampling of implants sufficiently describes the hormone pharmacokinetics.42
In conclusion, we found no clinically significant drug–drug interactions between rilpivirine- or darunavir/ritonavir-based ART and the etonogestrel or levonorgestrel implant. Importantly, we expect the contraceptive effectiveness of both implants to be maintained with these ART regimens. With ART containing darunavir/ritonavir, etonogestrel and levonorgestrel concentrations were increased, but the study drug combinations were well tolerated, and these pharmacokinetic findings are not considered clinically significant. These data offer support for the comprehensive care of women of childbearing potential living with HIV by ensuring effective contraceptive options.
Supplementary Material
Acknowledgements
We thank the members of the clinical study team, Johnson Magoola and Emmanuel Ssempijja, and staff of the IDI Core Laboratory. We thank members of the Safety Monitoring Committee for oversight of this study.
Contributor Information
Shadia Nakalema, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Catherine A Chappell, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh, Pittsburgh, PA, USA.
Michelle Pham, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Pauline Byakika-Kibwika, Department of Medicine, Makerere University, Kampala, Uganda.
Julian Kaboggoza, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Stephen I Walimbwa, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Joseph Musaazi, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Ritah Nakijoba, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Leah Mbabazi, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Isabella Kyohairwe, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Sylvia Nassiwa, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Jeffrey Jeppson, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Lee Winchester, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Marco Siccardi, Department of Pharmacology, University of Liverpool, Liverpool, UK.
Courtney V Fletcher, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Kimberly K Scarsi, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA.
Mohammed Lamorde, Infectious Diseases Institute, Makerere University, Kampala, Uganda.
Funding
This work was supported by an investigator-initiated study grant from Janssen Pharmaceutica NV to M.L. and the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant to K.K.S. (R01 HD085887).
Transparency declarations
K.K.S., C.A.C. and M.L. have received research grant support paid to their institution from Organon, LLC. All other authors: none to declare.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. UNAIDS . Global HIV and AIDS statistics fact sheet. 2021. https://www.unaids.org/en/resources/fact-sheet.
- 2. Lathrop E, Jamieson DJ, Danel I. HIV and maternal mortality. Int J Gynaecol Obstet 2014; 127: 213–5. 10.1016/j.ijgo.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reynolds HW, Janowitz B, Homan Ret al. The value of contraception to prevent perinatal HIV transmission. Sex Transm Dis 2006; 33: 350–6. 10.1097/01.olq.0000194602.01058.e1 [DOI] [PubMed] [Google Scholar]
- 4. Reynolds HW, Janowitz B, Wilcher Ret al. Contraception to prevent HIV-positive births: current contribution and potential cost savings in PEPFAR countries. Sex Transm Infect 2008; 84: ii49–53. 10.1136/sti.2008.030049 [DOI] [PubMed] [Google Scholar]
- 5. USAID . DHS Analytical Studies 30. Integration of HIV and family planning health services in sub-Saharan Africa. 2012. https://dhsprogram.com/pubs/pdf/AS30/AS30.pdf.
- 6. Scarsi KK, Darin KM, Chappell CAet al. Drug–drug interactions, effectiveness, and safety of hormonal contraceptives in women living with HIV. Drug Saf 2016; 39: 1053–72. 10.1007/s40264-016-0452-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uganda Bureau of Statistics (UBOS) and ICF . Uganda 2016 Demographic and Health survey. https://dhsprogram.com/pubs/pdf/SR245/SR245.pdf.
- 8. JADELLE (Levonorgestrel Implants) for Subdermal Use. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020544s010lbl.pdf.
- 9. IMPLANON (Etonogestrel Implant) for Subdermal Use. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021529s008lblrev.pdf.
- 10. Scarsi KK, Darin KM, Lamorde Met al. Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz-based antiretroviral therapy: a three-arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 2016; 62: 675–82. 10.1093/cid/civ1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chappell CA, Lamorde M, Scarsi KKet al. Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS 2017; 31: 1965. 10.1097/QAD.0000000000001591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. EDURANT (Rilpivirine) Tablets for Oral Use. 2022. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/EDURANT-pi.pdf.
- 13. WHO . Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. 2021. https://apps.who.int/iris/handle/10665/342899.
- 14. WHO . Reproductive Health. Medical Eligibility Criteria for Contraceptive Use. 2015. https://www.who.int/publications/i/item/9789241549158
- 15. University of Liverpool . HIV Drug Interactions. https://www.hiv-druginteractions.org/.
- 16. Israel R, Mishell DR Jr, Stone SCet al. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol 1972; 112: 1043–6. 10.1016/0002-9378(72)90178-0 [DOI] [PubMed] [Google Scholar]
- 17. Cirrincione LR, Penchala SD, Scarsi KKet al. Development, validation and utilization of a highly sensitive LC-MS/MS method for quantification of levonorgestrel released from a subdermal implant in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2018; 1084: 106–12. 10.1016/j.jchromb.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diletti E, Hauschke D, Steinijans VW. Sample size determination: extended tables for the multiplicative model and bioequivalence ranges of 0.9 to 1.11 and 0.7 to 1.43. Int J Clin Pharmacol Ther Toxicol 1992; 8: 287–90. 10.1007/BF01061471 [DOI] [PubMed] [Google Scholar]
- 19. R Core Team . R: A Language and Environment for Statistical Computing. 2020. https://www.R-project.org/.
- 20. Canty A, Ripley B. boot: Bootstrap R (S-Plus) Functions. R package Version 1.3-25. 2020. https://cran.r-project.org/web/packages/boot/boot.pdf.
- 21. Davison AC, Hinkley DV. Bootstrap Methods and Their Applications. Cambridge University Press. ISBN 0-521-57391-2. 1997. https://www.cambridge.org/core/books/bootstrap-methods-and-their-application/ED2FD043579F27952363566DC09CBD6A. [Google Scholar]
- 22. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS . Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. July 2017. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf.
- 23. Sivin I, Viegas O, Campodonico Iet al. Clinical performance of a new two-rod levonorgestrel contraceptive implant: a three-year randomized study with Norplant® implants as controls. Contraception 1997; 55: 73–80. 10.1016/s0010-7824(96)00275-2 [DOI] [PubMed] [Google Scholar]
- 24. Aarnoutse RE, Kleinnijenhuis J, Koopmans PPet al. Effect of low-dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin Pharmacol Ther 2005; 78: 664–74. 10.1016/j.clpt.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 25. Crauwels HM, Van Heeswijk RP, Buelens Aet al. Lack of an effect of rilpivirine on the pharmacokinetics of ethinylestradiol and norethindrone in healthy volunteers. Int J Clin Pharmacol Ther 2014; 52: 118–28. 10.5414/CP201943 [DOI] [PubMed] [Google Scholar]
- 26. Diaz S, Pavez M, Moo-Young AJet al. Clinical trial with 3-keto-desogestrel subdermal implants. Contraception 1991; 44: 393–408. 10.1016/0010-7824(91)90030-J [DOI] [PubMed] [Google Scholar]
- 27. Vieira CS, Bahamondes MV, de Souza RMet al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. J Acquir Immune Defic Syndr 2014; 66: 378–85. 10.1097/QAI.0000000000000189 [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Chung E, Yones Cet al. The effect of atazanavir/ritonavir on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy women. Antivir Ther 2011; 16: 157–64. 10.3851/IMP1724 [DOI] [PubMed] [Google Scholar]
- 29. DuBois BN, Atrio J, Stanczyk FZet al. Increased exposure of norethindrone in HIV+ women treated with ritonavir-boosted atazanavir therapy. Contraception 2015; 91: 71–5. 10.1016/j.contraception.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vogler MA, Patterson K, Kamemoto Let al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of ACTG Trial A5188. J Acquir Immune Defic Syndr 2010; 55: 473. 10.1097/QAI.0b013e3181eb5ff5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atrio J, Stanczyk FZ, Neely Met al. Effect of protease inhibitors on steady-state pharmacokinetics of oral norethindrone contraception in HIV-infected women. J Acquir Immune Defic Syndr 2014; 65: 72–7. 10.1097/QAI.0b013e3182a9b3f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scarsi KK, Cramer YS, Rosenkranz SLet al. Antiretroviral therapy and vaginally administered contraceptive hormones: a three-arm, pharmacokinetic study. Lancet HIV 2019; 6: e601–12. 10.1016/S2352-3018(19)30155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sekar VJ, Lefebvre E, Guzman SSet al. Pharmacokinetic interaction between ethinyl estradiol, norethindrone and darunavir with low-dose ritonavir in healthy women. Antivir Ther 2008; 13: 563–9. 10.1177/135965350801300415 [DOI] [PubMed] [Google Scholar]
- 34. Maddox DD, Rahman Z. Etonogestrel (Implanon), another treatment option for contraception. Pharmacol Ther 2008; 33: 337. [Google Scholar]
- 35. Sivin I, Moo-Young A. Recent developments in contraceptive implants at the Population Council. Contraception. 2002; 65: 113–9. 10.1016/S0010-7824(01)00288-8 [DOI] [PubMed] [Google Scholar]
- 36. Croxatto HB, Diaz S, Pavez Met al. Plasma progesterone levels during long-term treatment with levonorgestrel silastic implants. Eur J Endocrinol 1982; 101: 307–11. 10.1530/acta.0.1010307 [DOI] [PubMed] [Google Scholar]
- 37. Croxatto HB, Diaz S, Brandeis Aet al. Plasma levonorgestrel and progesterone levels in women treated with silastic covered rods containing levonorgestrel. Contraception 1985; 31: 643–54. 10.1016/0010-7824(85)90064-2 [DOI] [PubMed] [Google Scholar]
- 38. Bhatia P, Nangia S, Aggarwal Set al. Implanon: subdermal single rod contraceptive implant. J Obstet Gynaecol India 2011; 61: 422–5. 10.1007/s13224-011-0066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adeyemi AS, Owonikoko KM, Adekanle DAet al. Subdermal contraceptive implants: experience at a tertiary health institution in Southwestern Nigeria. Sahel Med J 2018; 21: 137. 10.4103/smj.smj_7_18 [DOI] [Google Scholar]
- 40. NEXPLANON (etonogestrel implant) . https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021529s011lbl.pdf.
- 41. Bakesiima R, Byakika-Kibwika P, Tumwine JKet al. Dyslipidaemias in women using hormonal contraceptives: a cross sectional study in Mulago Hospital Family Planning Clinic, Kampala, Uganda. BMJ Open 2018; 8: e022338. 10.1136/bmjopen-2018-022338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lazorwitz A, Sheeder J, Teal S. Variability in repeat serum etonogestrel concentrations among contraceptive implant users during the steady-release pharmacokinetic period. Contraception 2022; 108: 65–8. 10.1016/j.contraception.2021.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.