Abstract
Background
With implementation of combination antiretroviral therapy (cART), changes to brain integrity in people with HIV (PWH) are subtle compared to those observed in the pre-cART era. T1-weighted/T2-weighted (T1w/T2w) ratio has been proposed as a measure of cortical myelin. This study examines T1w/T2w values between virologically controlled PWH and persons without HIV (PWoH).
Methods
Virologically well-controlled PWH (n = 164) and PWoH (n = 120) were compared on global and regional T1w/T2w values. T1w/T2w values were associated with HIV disease variables (nadir and current CD4 T-cell count, and CNS penetration effectiveness of cART regimen) in PWH, and as a function of age for both PWoH and PWH.
Results
PWH had reduced global and regional T1w/T2w values compared to PWoH in the posterior cingulate cortex, caudal anterior cingulate cortex, and insula. T1w/T2w values did not correlate with HIV variables except for a negative relationship with CNS penetration effectiveness. Greater cardiovascular disease risk and older age were associated with lower T1w/T2w values only for PWH.
Conclusions
T1w/T2w values obtained from commonly acquired MRI protocols differentiates virologically well-controlled PWH from PWoH. Changes in T1w/T2w ratio do not correlate with typical HIV measures. Future studies are needed to determine the biological mechanisms underlying this measure.
Keywords: myelin, HIV, T1w/T2w
The T1w/T2w signature, calculated from commonly acquired MRI scans and proposed as a marker of cortical myelin, is significantly reduced in virologically well-controlled people with HIV compared to HIV-negative individuals.
Since the introduction of combination antiretroviral therapy (cART), the life expectancy of people with HIV (PWH) has significantly improved and is approaching that of people without HIV (PWoH). Although the severity of cognitive impairment associated due to human immunodeficiency virus (HIV) has continued to diminish, milder cognitive impairment and changes in brain integrity persist in PWH on stable cART. Methods that identify these subtle brain changes in PWH are vital for evaluating the effectiveness of adjunctive therapies for PWH.
Neuroimaging represents a noninvasive method to identify changes to brain structure and function. While widespread use of cART has reduced the magnitude of macrostructural brain changes observed in PWH [1], white matter changes persist. For example, white matter hyperintensity load is higher in well-controlled PWH compared to PWoH [2]. Additionally, robust reductions in microstructural white matter integrity have been observed using diffusion tensor imaging (DTI), despite virological control with cART [3]. DTI is currently considered the most sensitive method to capture subtle changes to myelin and brain white matter integrity in PWH. However, this technique has several disadvantages, including a lack of specificity to myelin integrity, low sensitivity to cortical white matter, and it cannot account for the influence of crossing fibers on generating regional white matter values. Additionally, DTI acquisition is sometimes difficult and is not consistent. Consequently, novel techniques that utilize imaging modalities that are acquired in most standard imaging protocols to identify subtle white matter changes in PWH would be beneficial.
The T1-weighted/T2-weighted (T1w/T2w) ratio was initially proposed as a method for quantifying cortical myelin concentration [4]. This metric relies on the relative signal intensity of T1-weighted and T2-weighted images that are typically acquired. High T1w/T2w signals are seen in areas with increased cortical myelination, with change across the lifespan. This ratio is derived from the neuroimaging properties of the T1w image. The white matter and pial surface can be isolated from the T1w image by the suppression of signal from the dura and blood vessels, which is performed by dividing the T1 signal by the T2w image [5]. Prior work mapping this ratio across the human cortex has demonstrated a close resemblance to the distribution of myelin fibers seen with myeloarchitectonical studies [6]. In healthy individuals, the T1w/T2w ratio is sensitive to age-related changes in intracortical myelin and correlates with cognitive performance [7]. This technique has also successfully identified white matter changes in several neurodegenerative and neuroinflammatory diseases. For example, a decrease in the T1w/T2w signal has been repeatedly observed in multiple sclerosis [8–11] and schizophrenia [12]. Conversely, an increase in the T1w/T2w ratio has been observed in Parkinson disease [13], Huntington disease [14], and Alzheimer disease [15]. Overall conclusions from these studies indicate that both very low T1w/T2w ratio and very high T1w/T2w ratio, particularly in conjunction with the presence of other disease pathology, may indicate abnormal changes to the microstructural white matter and myelin content of the brain.
Despite known white matter changes in PWH, the T1w/T2w ratio has not been well studied in PWH. One recent study has examined differences in the T1w and T2w signals between PWH who were either taking or not taking cART and healthy controls [16]. However, the number of participants included was very small (n = 12). An established pattern of T1w/T2w ratio in a well-characterized sample of PWH who are virologically well controlled would allow clinicians and researchers to generate a more complete picture of brain integrity using simple scans that are already typically acquired during a magnetic resonance imaging (MRI) scan. This study evaluated both the global and regional T1w/T2w ratio in PWH and PWoH. Additionally, T1w/T2w ratios were examined with regards to HIV disease variables (CD4 T-cell count, nadir CD4 T-cell count, CD8 T-cell count, CD4/CD8 ratio, duration of infection, and cART regimen). Finally, the relationship between age and the T1w/T2w ratio was evaluated across the lifespan for PWH and PWoH.
METHODS
Study Participants
All data was acquired between 2010 and 2017 and PWH were recruited (n = 164) from the Infectious Disease Clinic and AIDS Clinical Trial Unit at Washington University in St Louis. PWoH (n = 120) were recruited from the St Louis community or research participant registry.
Study inclusion criteria included more than 8 years of education, at least 18 years of age, English as the primary language, and ability to provide informed written consent. Exclusion criteria included a history of opportunistic central nervous system infection, traumatic brain injury (loss of consciousness for 30 minutes or longer), confounding neurological or psychiatric disorders, current severe depressive symptoms (Beck depression inventory-II ≥ 29 [17]), self-reported substance use disorder, or contraindications for MRI scanning.
Clinical Characteristics of PWH Participants
For the PWH group, all individuals were on a stable cART regimen for at least 6 months had had a recent viral load <200 copies/mL. Viral load was obtained from either blood samples collected at the research study visit or from the medical evaluations that occurred within 1 month of assessment. Duration of HIV infection and nadir CD4 T-cell counts were obtained from either medical records or self-report; CD4 and CD8 T-cell counts were obtained from medical records within 3 months of the study visit. The central nervous system penetrating effectiveness score, a measure that quantifies how well an antiretroviral drug crosses the blood-brain barrier [18], was calculated for each participant's current cART regimen [19, 20].
Cardiovascular Risk Score
For a subset of participants (PWoH n = 28; PWH n = 85) with available data, we calculated the Framingham 10-year cardiovascular disease risk score [21, 22]. This score incorporates age, sex, systolic blood pressure, hypertension diagnosis and treatment, self-reported smoking status, diagnosis of diabetes, high-density lipoprotein, and total cholesterol.
MRI Acquisition and Processing
The structural/anatomical 3-dimensional T1w and T2w images were acquired sagittally on a high-resolution 3T MR Siemens Tim Trio (Siemens AG) with a 12-channel head coil. For the T1w images, scanning was performed with magnetization-prepared rapid gradient echo (MP-RAGE) sequence with repetition time = 2400 ms, echo time = 3.16 ms, flip angle = 8°, and inversion time = 1000 ms. The T2w scan sequence had repetition time = 3200 ms, echo time = 450 ms, and variable flip angle. Both sequences were configured with matching resolution = 1 × 1 × 1 mm3 voxels, 256 × 256 × 176 acquisition matrix, and generalized autocalibrating partial parallel acquisition acceleration of 2.
T1w/T2w ratio maps were generated by 3 sequentially executed Human Connectome Project structural pipelines. These pipelines [23] were containerized using the Quantitative Neuroimaging Environment and Toolbox (Qu|Nex) Suite version 0.45.07, which included the Human Connectome Project processing pipelines [23], FreeSurfer version 6.0 [24], FSL version 6.0.1 [25], and Connectome Workbench version 1.3.2 [26]. Briefly, the Human Connectome Project pipeline applied preparatory transformations to the T1w and T2w scans—reduction of the field of view, removal of oblique orientation, brain extraction, and radio frequency field homogeneity correction. This pipeline constructed the white matter and pial surfaces as well as segmented the cortical structures according to the Desikan-Killiany standard atlas in FreeSurfer [27]. A trained technician (AB) inspected the volume and surface images generated by FreeSurfer. The individual midthickness surface was calculated between the white matter and pial surfaces with FreeSurfer default 164 k vertices and 0.9 mm average spacing between them [28]. T1w/T2w ratios from the cortical ribbon were projected onto the midthickness surface to generate a T1w/T2w ratio map for each participant. A myelin field map was applied to compensate for overestimation in lightly myelinated areas. The T1w/T2w ratio was calculated both globally (average of the whole-cortex T1w/T2w ratio values) and regionally for the 34 cortical regions of interest (ROIs). For the regional analysis, we combined results from both hemispheres for the 34 cortical ROIs.
Statistical Analyses
Demographic variables including age, sex, race, and education were compared between PWH and PWoH using Mann-Whitney U tests for continuous variables and χ2 tests for categorical variables. Prior to all analyses, the global T1w/T2w value was adjusted for cortical thickness using linear regression. For each ROI, similar regressions were performed for the T1w/T2w ratio by adjusting for the corresponding ROI cortical thickness in the regression. Standardized residuals from these analyses were used as the dependent variables in subsequent analyses.
The global T1w/T2w ratio was compared between PWH and PWoH using an analysis of covariance (ANCOVA) test, with age and sex included as covariates. Similar ANCOVAs were performed for each of the cortical ROIs.
Partial correlation analyses were performed to examine relationships between global T1w/T2w ratios and duration of infection, CNS penetration effectiveness score, nadir CD4 T-cell count, recent CD4 T-cell count, recent CD8 T-cell count, and the CD4/CD8 ratio, after adjusting for age. Linear regression models analyzed relationships between global or ROI T1w/T2w ratios and the Framingham cardiovascular risk score in PWoH and PWH. Finally, regression analyses examined the pattern of change in global T1w/T2w ratios for PWoH and PWH over the lifespan. A false discovery rate method was used to adjust for multiple comparisons for all analyses [29].
RESULTS
Demographics
Demographic and clinical variables are presented for PWoH and PWH participants in Table 1. The groups were well matched with regards to race. However, the PWH were significantly older (P = .01), reported a lower degree of education (P = .01), and a greater percentage were male (P < .001) compared to PWoH. No difference was observed between PWH and PWoH in the Framingham risk score for cardiovascular risk (P = .17). Age, education, and sex were included as covariates in subsequent analyses when appropriate.
Table 1.
Demographic, Clinical, and Cognitive Characteristics of Persons Without HIV and Persons With HIV
| Characteristics | PWoH (n = 120) | PWH (n = 164) | P Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y, mean (SD) | 41.3 (17.1) | 47.9 (14.7) | .01 |
| Sex, % male | 48 | 79 | <.001 |
| Race, % African American | 59 | 66 | .14 |
| Education, y, mean (SD) | 13.8 (2.2) | 13.1 (2.7) | .015 |
| Framingham 10-y cardiovascular risk score, mean (SD) | 13.2 (5.1) | 19.2 (13.6) | .17 |
| Current smoker, % yes | 27 | 47 | <.001 |
| HIV characteristics | |||
| Duration of infection, mo, median (IQR) | NA | 196 (114–288) | NA |
| Duration of cART, mo, median (IQR) | NA | 170 (63–237) | NA |
| Recent CD4 T-cell count, median (IQR) | NA | 628 (438–907) | NA |
| Nadir CD4 T-cell count, median (IQR) | NA | 200 (33–333) | NA |
| Recent CD8 T-cell count, median (IQR) | NA | 820 (597–1340) | NA |
| CD4/CD8 ratio, mean (SD) | NA | 0.70 (0.5) | NA |
| CNSPE, mean (SD) | NA | 7.3 (1.3) | NA |
Bolded P values are significant at P < .05.
Abbreviations: cART, combination antiretroviral therapy; CNSPE,central nervous system penetrating effectiveness score; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; PWH, persons with HIV; PWoH, persons without HIV.
Global and ROI T1w/T2w Ratios Significantly Differ Between PWoH and PWH
The global T1w/T2w ratio, adjusted for total cortical thickness and demographic variables, significantly differed between PWoH and PWH (P < .001; Cohen d = 0.64). PWH had significantly lower T1w/T2w values compared to PWoH (Figure 1).
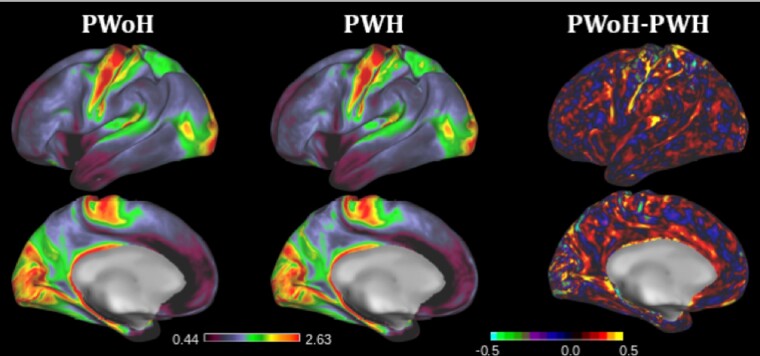
Figure 1.
Group average T1w/T2w values in PWoH (left) and PWH (middle), and the group difference (PWoH − PWH) are displayed based on the ratio intensity (right). Higher group averages of T1w/T2w ratios (red in the left and middle images), such as those observed in the motor cortex, are hypothesized to correspond to regions of higher myelination. Overall, PWH exhibit reduced T1w/T2w ratios compared to PWoH (right). Abbreviations: PWH, people with HIV; PWoH, people without HIV; T1w/T2w, T1-weighted/T2-weighted.
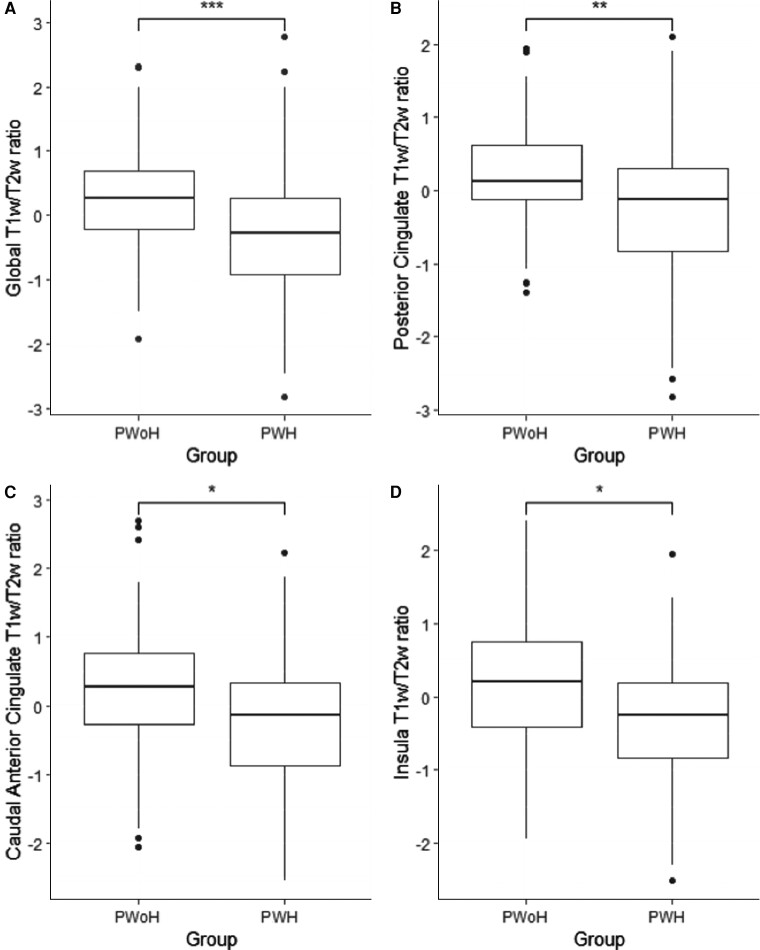
Analyses examining differences in T1w/T2w values for the 34 ROIs identified significantly lower T1w/T2w values within the posterior cingulate cortex (P < .001; adjusted P value = .003; Cohen d = 0.58), caudal anterior cingulate cortex (P = .002; adjusted P value = .02; Cohen d = 0.52), and insula (P = .002; adjusted P value = .02; Cohen d = 0.61) for PWH compared to PWoH (Figure 2). There were no ROIs where PWH had a higher T1w/T2w ratio compared to PWoH.
Figure 2.
Box plots representing the quartiles (box and whiskers), outliers (circles), the median and 95% confidence interval for the global (A) and regional posterior cingulate (B), caudate anterior cingulate (C), and insula (D) T1w/T2w values for PWoH compared to PWH. Significance was determined using the analysis of covariance general linear model. PWH demonstrated significantly lower global and regional T1w/T2w values compared to PWoH. Abbreviations: PWH, people with HIV; PWoH, people without HIV; T1w/T2w, T1-weighted/T2-weighted. *P < .05, **P < .01, ***P < .001.
Relationships Between T1w/T2w Values and HIV Disease Variables in PWH
After adjusting for age, T1w/T2w values did not significantly correlate with duration of HIV infection in PWH. T1w/T2w ratios also was not significantly associated with current or nadir CD4 T-cell counts, current CD8 T-cell count, or CD4/CD8 ratio. Regression analyses examining relationships between T1w/T2w ratios and cardiovascular disease risk scores in a subset of participants with available data (PWoH n = 28; PWH n = 85) identified a significant effect of a higher risk score and lower global (r = −0.25; P = .01) and insula (r = −0.4; P < .001) T1w/T2w ratios within PWH but not PWoH.
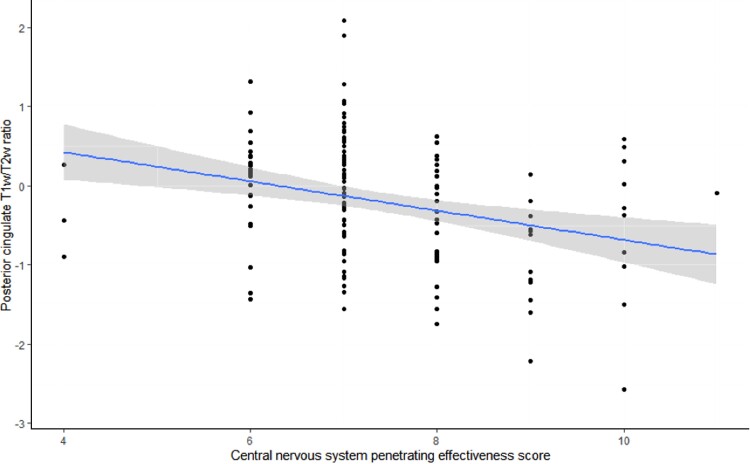
No significant relationship was observed between the global or regional T1w/T2w ratios and duration of ART, current ART regimen, or class of current antiretrovirals. Higher CNS penetration effectiveness scores were correlated with lower T1w/T2w ratios for the caudal anterior cingulate cortex (r = 0.20, P = .008), posterior cingulate cortex (r = −0.29, P < .001) (Figure 3), precuneus (r = −0.25, P < .001), and insula cortex (r = −0.20, P = .01).
Figure 3.
A linear regression was conducted with central nervous system penetrating effectiveness score as a function of the T1w/T2w ratio within the posterior cingulate cortex in people with HIV (r = −0.29, P < .001). The shaded areas represent the 95% confidence interval. Abbreviation: T1w/T2w, T1-weighted/T2-weighted.
Change in Global T1w/T2w Ratios Across the Lifespan for PWoH and PWH
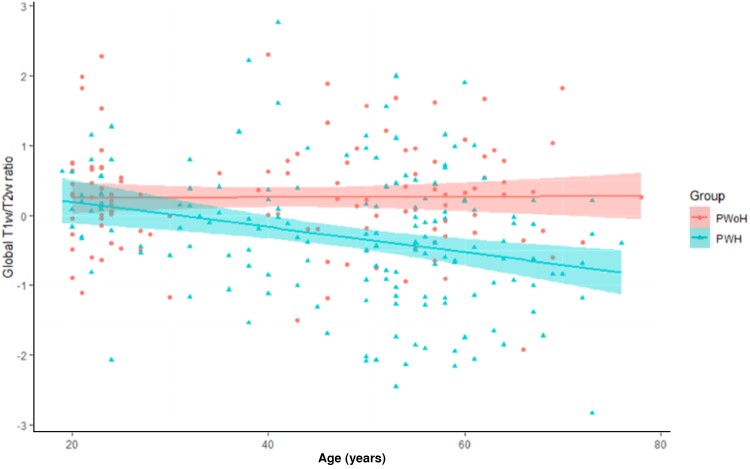
Regression analyses assessed linear and nonlinear relationships between age and T1w/T2w values for PWoH and PWH. Age was not significantly associated with global T1w/T2w ratios for PWoH (P = .92). In contrast, age was linearly associated with the global T1w/T2w ratio (P < .001) for PWH, with increasing age associating with lower global T1w/T2w values (Figure 4).
Figure 4.
A linear regression was conducted with age as a function of global T1w/T2w ratio for PWoH and PWH. The shaded areas represent the 95% confidence intervals for each group. A significant effect of age was seen for PWH (P < .001) but not PWoH (P = .92). Abbreviations: PWH, people with HIV; PWoH, people without HIV; T1w/T2w, T1-weighted/T2-weighted.
DISCUSSION
The focus of the present study was to evaluate global and regional T1w/T2w ratios between PWoH and PWH. We observed a significant reduction in T1w/T2w values both globally and regionally in PWH compared to PWoH. Anatomical regions with reduced T1w/T2w ratios for PWH included frontal, cingulate, and insular regions, which have previously been shown to be affected by HIV [30]. Although the T1w/T2w ratio was not significantly associated with any known HIV disease metrics, an association was observed with a measure of cardiovascular disease risk and a measure of cART penetration into the CNS. Finally, increasing age was associated with reductions in the T1w/T2w ratio for PWH but not PWoH.
The T1w/T2w ratio was introduced as a measure of myelin concentration throughout the cortex [4]. The simplicity of this metric underlies its utility, as this measure can be easily calculated using T1w and T2w images that are commonly acquired during an MRI session, avoiding the need for long, costly MRI sequences. Additional reported benefits of the T1w/T2w ratio include test-retest reliability, high spatial resolution, and high signal-to-noise ratio that reduces bias of the MR signal, allowing comparison of T1w/T2w values across different sites, scanners, and sequences [4]. The T1w/T2w ratio has a demonstrated ability to differentiate between high-myelinated and low-myelinated cortex regions [31], in addition to differentiating between healthy versus clinical populations [8, 9, 13, 15], and associates with cognitive performance [6]. Importantly, this measure may provide researchers and clinicians with more-specific information about microstructural brain integrity compared to brain volumetrics, cortical thickness, or DTI, which demonstrates low sensitivity to cortical myelin. These features highlight the potential application of this measure to HIV, a disease that continues to be associated with microstructural changes within the white matter despite cART and viral suppression [1, 3].
The results of the current study add to this literature and support the sensitivity of the T1w/T2w ratio in detecting differences between PWoH and PWH who are virologically well controlled. We observed significantly lower global T1w/T2w values in PWH compared to PWoH, as well as regional reductions in cingulate cortices and the insula. Prior work has shown that impairment of the cingulate cortices and their connections are associated with worse cognitive performance and reduced quality of life in HIV-positive populations [32, 33]. Previous studies have also reported volumetric changes in both the cingulate and insula between PWH and PWoH [25]. Our findings accounted for cortical atrophy at each of these regions, suggesting microstructural neurodegenerative changes may still be present in PWH despite virological control. In contrast to our results, one previous study that evaluated the T1w/T2w metric in PWH observed an elevated signal, which was associated with positron emission tomography (PET) inflammatory markers. However, this study was based on a smaller sample of PWH and included individuals who were and were not on cART. Additionally, we observed significant correlations between increasing age and lower T1w/T2w values in PWH that were not observed in PWoH. Prior literature has shown a reduction in the T1w/T2w metric was associated with increased aging [7], and PWH often demonstrate accentuated aging even in virologically suppressed individuals [34, 35].
We did not observe significant relationships between HIV disease metrics and the T1w/T2w ratio. However, lower T1w/T2w values were associated with a higher Framingham risk score and higher medication penetration, quantified by the central nervous system (CNS) penetration effectiveness score. PWH have been reported to be at higher risk for cardiovascular disease that associates with worse neuroimaging metrics of brain integrity [36]. There is some debate as to whether high CNS penetration effectiveness medications are beneficial or deleterious to brain integrity. Several previous studies have observed no significant effect of CNS penetration effectiveness score on cognition and brain volumes [37, 38]. However, cART medications have been associated with mitochondrial toxicity and oligodendrocyte dysfunction, which may result in loss of myelin and reduced white matter microstructural integrity in PWH [39]. The T1w/T2w ratio may be more sensitive to these subtle changes than more conventional neuroimaging measures such as brain volumes and DTI. Further research with more detailed medication regimen information is needed to clarify this potential relationship.
Our study complements previous results of reduced T1w/T2w signal in inflammatory diseases, such as multiple sclerosis, compared to healthy individuals [8–10]. However, studies demonstrating higher values of T1w/T2w ratios in neurodegenerative diseases such as Alzheimer disease [15] and Parkinson disease [13] suggest that other disease processes, including increased iron accumulation and decreased neuronal density, may alter the T1w/T2w signal [40, 41]. Interestingly, a recent, large study of the T1w/T2w value in multiple clinical phenotypes of multiple sclerosis found both reduced cortical T1w/T2w ratios and increased subcortical T1w/T2w ratios that associated with the differing clinical phenotypes and disease phase or severity [11]. From these data, the authors concluded that the T1w/T2w value may reflect the combination of multiple etiologies, such as demyelination, loss of neuronal density, and iron accumulation. More specifically, at milder stages of disease pathology in the brain, changes in the T1w/T2w ratio may primarily reflect subtle white matter changes while high levels of neurodegeneration and brain pathology may result in other factors affecting this signal in a manner that increases the T1w/T2w ratio. Our sample of PWH that are on cART and virologically well controlled demonstrated a reduction in the T1w/T2w ratio that corresponded with CNS penetration effectiveness. Taken together, these results indicate that the T1w/T2w ratio is likely capturing subtle differences in white matter microstructural integrity that may be due to medications or legacy effects of HIV rather than the virus itself.
There are several limitations to consider with the present study. As noted above, there is some controversy over the anatomical correlate for changes in the T1w/T2w signal and interpretation of this metric in certain cohorts may be challenging due to the presence of other neuropathological causes. The T1w/T2w signal is useful for determining low and high areas of myelination that are affected by age. However, in the presence of pathology, a reduction in the signal can only be attributed to nonspecific myelin damage as multiple sources can influence the overall signal. Consequently, interpretation of the T1w/T2w ratio should be done with caution and with the understanding that other etiologies may influence this measure. Additionally, this study is cross-sectional, and longitudinal data is necessary to aid in understanding whether the relationships observed would be consistent or change over time, as well as to help clarify the observed correlation between age and T1w/T2w values in PWH but not PWoH individuals. AIDS diagnosis was not acquired for some of our PWH and therefore could not be analyzed with the T1w/T2w ratio. With regards to age, we had relatively few participants between the ages of 25 and 45 years, particularly in our PWoH sample, which may have impacted the linear relationship with age observed (vs a nonlinear relationship). To avoid committing a type I error, we were more stringent in our significance criteria for the regional analyses. These statistical strategies are common for neuroimaging analyses but can inflate the potential for committing a type II error. Finally, PWH recruited into the study had well-controlled virus (<200 copies/mL) and were on cART, limiting the ability to examine potential changes in the T1w/T2w ratio in more severe cases.
The T1w/T2w ratio is a relatively novel neuroimaging marker that can be calculated from commonly acquired T1 and T2 MRI scans and may be sensitive to subtle changes in white matter microstructural integrity. In a sample of PWH with well-controlled virus, the T1w/T2w ratio was significantly reduced compared to PWoH, both globally and regionally in the cingulate cortices and insula. Lower T1w/T2w ratios were also associated with increased age and higher cART penetration in the CNS in PWH, highlighting the need for future research to more fully understand these relationships. Overall, the T1w/T2w represents an easily acquired neuroimaging metric that has potential utility in examining subtle microstructural changes to brain integrity in PWH, although more studies are needed to fully determine the pattern of change in T1w/T2w values with neuropathology in diseases.
Notes
Financial support. This work was supported the National Institute for Nursing Research (grant numbers R01NR014449 and R01NR015738); the National Institute of Mental Health (grant number R01MH118031); and the National Center for Advancing Translational Sciences (grant number UL-TR000448 to the Washington University Institute of Clinical and Translational Sciences).
Contributor Information
Jeremy F Strain, Department of Neurology, Washington University, St Louis, Missouri, USA.
Sarah A Cooley, Department of Neurology, Washington University, St Louis, Missouri, USA.
Dimitre Tomov, Department of Neurology, Washington University, St Louis, Missouri, USA.
Anna Boerwinkle, Department of Neurology, Washington University, St Louis, Missouri, USA.
Beau M Ances, Department of Neurology, Washington University, St Louis, Missouri, USA.
References
- 1. O’Connor EE, Zeffiro TA, Zeffiro TA. Brain structural changes following HIV infection: meta-analysis. AJNR Am J Neuroradiol 2018; 39:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mina Y, Wu T, Hsieh HC, et al. Association of white matter hyperintensities with HIV status and vascular risk factors. Neurology 2021; 96:e1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strain JF, Burdo TH, Song SK, et al. Diffusion basis spectrum imaging detects ongoing brain inflammation in virologically well controlled HIV+ patients. J Acquir Immune Defic Syndr 2017; 76:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glasser MF, van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci 2011; 31:11597–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glasser MF, Smith SM, Marcus DS, et al. The human connectome project's neuroimaging approach. Nat Neurosci 2016; 19:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nieuwenhuys R, Broere CAJ. A map of the human neocortex showing the estimated overall myelin content of the individual architectonic areas based on the studies of Adolf Hopf. Brain Struct Funct 2017; 222:465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci 2013; 33:18618–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Righart R, Biberacher V, Jonkman LE, et al. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann Neurol 2017; 82:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper G, Finke C, Chien C, et al. Standardization of T1W/T2W ratio improves detection of tissue damage in multiple sclerosis. Front Neurol 2019; 10:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beer A, Biberacher V, Schmidt P, et al. Tissue damage within normal appearing white matter in early multiple sclerosis: assessment by the ratio of T1- and T2-weighted MR image intensity. J Neurol 2016; 263:1495–502. [DOI] [PubMed] [Google Scholar]
- 11. Margoni M, Pagani E, Meani A, et al. Exploring in vivo multiple sclerosis brain microstructural damage through T1w/T2w ratio: a multicentre study. J Neurol Neurosurg Psychiatry 2022; 93:741–52. [DOI] [PubMed] [Google Scholar]
- 12. Iwatani J, Ishida T, Donishi T, et al. Use of T1-weighted/T2-weighted magnetic resonance ratio images to elucidate changes in the schizophrenic brain. Brain Behav 2015; 5:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du G, Lewis MM, Sica C, Kong L, Huang X. Magnetic resonance T1w/T2w ratio: a parsimonious marker for Parkinson's disease. Ann Neurol 2019; 85:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowley CD, Tabrizi SJ, Scahill RI, et al. Altered intracortical T1-weighted/T2-weighted ratio signal in Huntington's disease. Front Neurosci 2018; 12:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pelkmans W, Dicks E, Barkhof F, et al. Gray matter T1-w/T2-w ratios are higher in Alzheimer's disease. Hum Brain Mapp 2019; 40:3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sari H, Galbusera R, Bonnier G, et al. Multimodal investigation of neuroinflammation in aviremic patients with HIV on antiretroviral therapy and HIV elite controllers. Neurol Neuroimmunol Neuroinflamm 2022; 9:e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck depression inventories-IA and -II in psychiatric outpatients. J Pers Assess 1996; 67:588–97. [DOI] [PubMed] [Google Scholar]
- 18. Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med 2010; 18:45. [PMC free article] [PubMed] [Google Scholar]
- 19. Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santos GMA, Locatelli I, Métral M, et al. Cross-sectional and cumulative longitudinal central nervous system penetration effectiveness scores are not associated with neurocognitive impairment in a well treated aging human immunodeficiency virus-positive population in Switzerland. Open Forum Infect Dis 2019; 6:ofz277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008; 117:743–53. [DOI] [PubMed] [Google Scholar]
- 22. Framingham Heart Study . Cardiovascular disease (10-year risk). https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/. Accessed 17 July 2022.
- 23. Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage 2013; 80:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fischl B. FreeSurfer. Neuroimage 2012; 62:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62:782–90. [DOI] [PubMed] [Google Scholar]
- 26. Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral Cortex. J Am Med Inform Assoc 2001; 8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–80. [DOI] [PubMed] [Google Scholar]
- 28. Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex 2012; 22:2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B (Statistical Methodol) 2002; 64:479–98. [Google Scholar]
- 30. Pfefferbaum A, Rogosa DA, Rosenbloom MJ, et al. Accelerated aging of selective brain structures in HIV infection: a controlled, longitudinal MRI study. Neurobiol Aging 2014; 35:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura K, Chen JT, Ontaneda D, Fox RJ, Trapp BD. T1-/T2-weighted ratio differs in demyelinated cortex in multiple sclerosis. Ann Neurol 2017; 82:635–9. [DOI] [PubMed] [Google Scholar]
- 32. Wang B, Liu Z, Liu J, Tang Z, Li H, Tian J. Gray and white matter alterations in early HIV-infected patients: combined voxel-based morphometry and tract-based spatial statistics. J Magn Reson Imaging 2016; 43:1474–83. [DOI] [PubMed] [Google Scholar]
- 33. Strain JF, Cooley S, Kilgore C, et al. The structural and functional correlates of frailty in persons living with human immunodeficiency virus. Clin Infect Dis 2022; 75:1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petersen KJ, Metcalf N, Cooley S, et al. Accelerated brain aging and cerebral blood flow reduction in persons with human immunodeficiency virus. Clin Infect Dis 2021; 73:1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seider TR, Gongvatana A, Woods AJ, et al. Age exacerbates HIV-associated white matter abnormalities. J Neurovirol 2016; 22:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glans M, Cooley SA, Florin V, et al. Effects of Framingham 10-year cardiovascular risk score and viral load on brain integrity in persons with HIV. J Acquir Immune Defic Syndr 2022: 90:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker LM, Paul RH, Heaps-Woodruff JM, et al. The effect of central nervous system penetration effectiveness of highly active antiretroviral therapy on neuropsychological performance and neuroimaging in HIV infected individuals. J Neuroimmune Pharmacol 2015; 10:487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanford R, Fellows LK, Ances BM, Collins DL. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA Neurol 2018; 75:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen BK, Roth LM, Grinspan JB, Jordan-Sciutto KL. White matter loss and oligodendrocyte dysfunction in HIV: a consequence of the infection, the antiretroviral therapy or both? Brain Res 2019; 1724:146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arshad M, Stanley JA, Raz N. Test–retest reliability and concurrent validity of in vivo myelin content indices: myelin water fraction and calibrated T1w/T2w image ratio. Hum Brain Mapp 2017; 38:1780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uddin MN, Figley TD, Solar KG, Shatil AS, Figley CR. Comparisons between multi-component myelin water fraction, T1w/T2w ratio, and diffusion tensor imaging measures in healthy human brain structures. Sci Rep 2019; 9:2500. [DOI] [PMC free article] [PubMed] [Google Scholar]