Abstract
Background
Global changes in amino acid levels have been described in severe malaria (SM), but the relationship between amino acids and long-term outcomes in SM has not been evaluated.
Methods
We measured enrollment plasma concentrations of 20 amino acids using high-performance liquid chromatography in 500 Ugandan children aged 18 months to 12 years, including 122 community children and 378 children with SM. The Kidney Disease: Improving Global Outcomes criteria were used to define acute kidney injury (AKI) at enrollment and chronic kidney disease (CKD) at 1-year follow-up. Cognition was assessed over 2 years of follow-up.
Results
Compared to laboratory-defined, age-specific reference ranges, there were deficiencies in sulfur-containing amino acids (methionine, cysteine) in both community children and children with SM. Among children with SM, global changes in amino acid concentrations were observed in the context of metabolic complications including acidosis and AKI. Increases in threonine, leucine, and valine were associated with in-hospital mortality, while increases in methionine, tyrosine, lysine, and phenylalanine were associated with postdischarge mortality and CKD. Increases in glycine and asparagine were associated with worse attention in children <5 years of age.
Conclusions
Among children with SM, unique amino acid profiles are associated with mortality, CKD, and worse attention.
Keywords: acute kidney injury, amino acids, cerebral malaria, chronic kidney disease, cognition
Children with severe malaria have widespread changes in amino acid levels at hospital admission associated with metabolic complications at presentation, predicting in-hospital and postdischarge mortality, chronic kidney disease at 1-year follow-up, and worse attention over 2 years of follow-up.
Severe malaria (SM) remains an important cause of child morbidity and mortality [1]. SM is a multisystem disease associated with neurocognitive and behavioral problems in survivors as well as chronic kidney disease (CKD) [2–9]. Children with severe anemia have high postdischarge morbidity and mortality [2, 10, 11].
The pathogenesis of SM centers on the cytoadherence of parasitized erythrocytes to the microvasculature. Metabolic abnormalities are common in SM, with hypoglycemia and hyperlactatemia among the most well-recognized metabolic changes. Acute kidney injury (AKI) is gaining broader recognition as a clinical complication in SM associated with metabolic abnormalities, mortality, and cognitive deficits [12, 13].
Amino acids perform a myriad of functions acting as key metabolites for gene expression, cell signaling, vascular function, and neurotransmission (reviewed in [14]) in addition to protein synthesis. Free amino acids in the plasma (ie, the amino acid pool) reflect a fraction of the total free amino acid pool, and can be affected by dietary input, protein turnover, protein biosynthesis, interorgan exchange of amino acids, transamination reactions, and oxidation [15]. Free amino acids are taken up and exchanged by multiple organ systems, with the intestine, liver, muscles, and kidney playing substantial roles in amino acid metabolism [16]. Amino acids are dynamically regulated through tightly controlled processes [15] affected in physiologic states of stress and infection [17, 18]. Most studies evaluating amino acid changes in SM focused on populations with neurologic complications [19–24], with phenylalanine consistently elevated in SM [20, 25, 26]. Differences in global patterns of plasma amino acid concentrations have been reported in adults [26] and children [19] with SM, but it is unclear whether this reflects limitations in sample size, study design, or age-related differences in SM pathogenesis.
The objective of this study was to evaluate amino acid concentrations in relation to clinical complications during hospitalization, mortality, and long-term neurologic and kidney function over follow-up. To this end, we quantified plasma amino acids at the time of hospital admission in a large cohort of children presenting with either cerebral malaria (CM) or severe malarial anemia (SMA).
METHODS
Study Participants
The study was performed in Kampala, Uganda from 2008 to 2015, enrolling children 18 months to 12 years of age [2]. All children with SM had Plasmodium falciparum on blood smear. Children with CM had a Blantyre Coma Score <3 or a Glasgow Coma Score ≤8 in children aged ≥5 years with no other identifiable cause: ruling out meningitis, a prolonged postictal state, or hypoglycemia-associated coma reversed by a glucose infusion. Children with SMA had a hemoglobin level ≤5 g/dL. Community children (CC) within 1 year of age of a recently enrolled child with SM were recruited from the nuclear family, extended family, or neighborhood households of children with SM. Exclusion criteria included prior coma, head trauma, hospitalization for malnutrition, cerebral palsy, or known chronic illness requiring medical care or causing developmental delay. Children were managed as described previously [27].
Laboratory Assessments
Ethylenediaminetetraacetic acid–anticoagulated plasma was collected at admission and stored at −80°C until testing. Plasma P. falciparum histidine-rich protein 2 (PfHRP2) levels were measured to assess parasite biomass (Cellabs, Australia) [28]. Amino acids were measured in plasma samples by high-performance liquid chromatography (HPLC) using a Hitachi Amino Acid Analyzer at Fairview Diagnostic Laboratories (Minneapolis, Minnesota). The amino acid analyzer is equipped with a refrigerated autosampler, lithium buffer system, an ion exchange column, a reaction coil for postcolumn derivatization with ninhydrin, and a data collection system. Samples were deproteinized, acidified, and injected into an HPLC system. Amino acids were eluted based on their pKa and cation exchange resin, and multiple buffer and temperature gradients were used to resolve the different compounds. The column effluent was reacted at high temperature with ninhydrin and the wavelength absorbance monitored. Each amino acid was quantitated relative to standards of known concentrations. Age-appropriate reference ranges for children in the United States were provided by the performing laboratory.
Cognitive Outcomes
Cognitive assessments were performed on enrolled children by neuropsychology testers who were blinded to the study group 1 week postdischarge for children with SM or at enrollment for the CC and then 6, 12, and 24 months after enrollment. All tests were adapted for use in Uganda [3, 29]. In children <5 years of age, the Mullen Scales of Early Learning [30], Color Object Association Test [31], and Early Childhood Vigilance Test [32] were used to assess cognition, associative memory, and attention, respectively, as previously described [2]. In children ≥5 years of age, the Kaufman Assessment Battery for Children (second edition) was used to measure overall cognitive ability and working memory [33] while the Test of Variables of Attention was used to assess attention [34]. Age-adjusted z-scores were created using the scores of the CC. Age-adjusted z-scores were created using the scores of the CC and generated separately for children stratified by age (<5 or ≥5 years for cognition, attention, memory) [35]. Additional sensitivity analyses were conducted to evaluate the impact of age at SM exposure on cognitive outcomes. Cognitive deficit was defined as a z-score < −2 in any of the 3 primary cognitive domains of overall cognition, attention, and memory.
Statistical Analyses
Analyses were done using Stata version 17.0 (StataCorp, 2021) and GraphPad Prism version 9.0 software. Differences in continuous variables between study groups were assessed using Student t test or Wilcoxon rank-sum test, as appropriate. Differences in proportions were compared using Pearson χ2 or Fisher exact test. To evaluate the global change in patterns of amino acids, we standardized amino acids to have a mean of zero and a standard deviation of 1 in the population. To examine the effect of admission amino acid levels (log10-transformed) on longitudinal changes in cognitive z-scores in children with SM, linear mixed-effects (LME) models were fit where observations within subject were correlated using a subject-specific intercept and time points were treated as categorical variables. The LME models were analyzed according to the following age groups: (1) children who were <5 years of age at time of malaria episode and received the preschool tests, (2) children who were <5 years of age at time of malaria episode but turned 5 during study follow-up and received school-age tests, and (3) children who were ≥5 years of age at time of malaria episode and received school-age tests. All models adjusted for age, sex, baseline weight-for-age z-score, socioeconomic status, child education, home z-score, maternal and paternal education, number of seizures during hospitalization, AKI, and disease group. To adjust for 20 amino acids measured, we used a fixed α of .0025.
Ethics Approval and Consent to Participate
Written informed consent was obtained from parents/guardians of study participants. Ethical approval was granted by the institutional review boards at Makerere University School of Medicine and the University of Minnesota, and additional regulatory approval was obtained by the Uganda National Council for Science and Technology.
RESULTS
We assessed plasma amino acid profiles in 378 Ugandan children with SM (172 children with SMA, 206 children with CM) and 122 CC (Figure 1). The mean age of the study population was 3.9 years (standard deviation, 1.9 years) and 221 of 500 (44.2%) were female. The demographic characteristics of study participants are presented in Table 1. Children with SM were younger than the CC and had lower height-for-age, weight-for-height, and weight-for-age z-scores compared to the CC (Table 1).
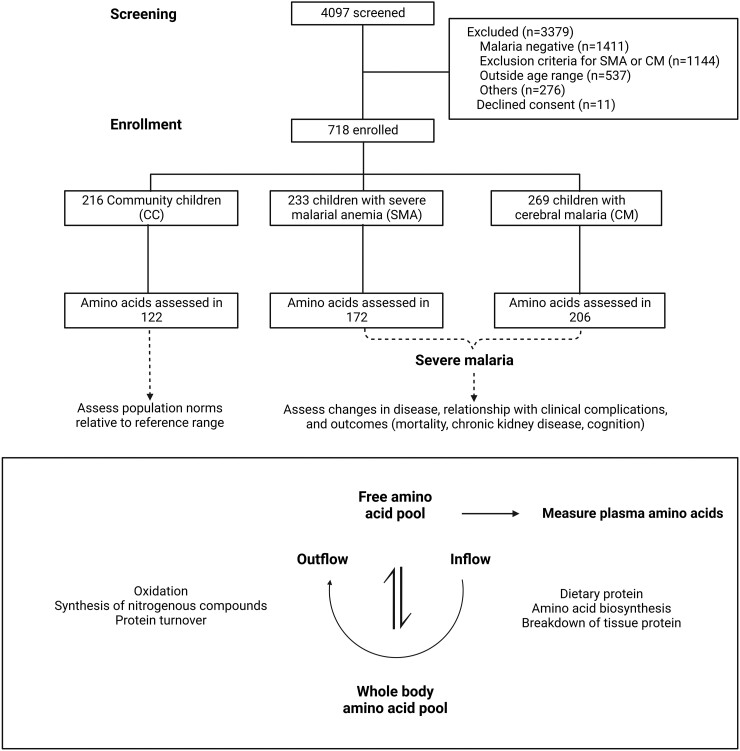
Figure 1.
Flowchart of the study population. A flow diagram depicting the study population in which amino acids were measured (top) and a description of the inflow and outflow of amino acids related to the plasma amino acid pool. Abbreviations: CM, cerebral malaria; SMA, severe malarial anemia.
Table 1.
Characteristics of Study Participants
| Characteristic | CC (n = 122) | SMA (n = 172) | CM (n = 206) | P Value |
|---|---|---|---|---|
| Age, y | 3.7 (2.8–4.9) | 2.9 (2.1–4.5) | 3.5 (2.6–5.1) | .0001 |
| Female sex, No. (%) | 69 (56.6) | 68 (39.5) | 84 (40.8) | .007 |
| Weight-for-age z-score | −0.7 (−1.2 to −0.1), n = 118 | −1.3 (−1.9 to −0.5), n = 170 | −1.0 (−1.7 to −0.4), n = 204 | .0001 |
| Height-for-age z-score | −1.3 (−1.7 to −0.4) | −1.4 (−2.3 to −0.5), n = 171 | −1.0 (−1.8 to −0.2) | .02 |
| Weight-for-height z-score | −0.1 (−0.8 to 0.5), n = 97 | −0.6 (−1.7 to 0.4), n = 147 | −0.7 (−1.6 to 0.0), n = 155 | .001 |
| Socioeconomic status scorea | 9 (8–11) | 9 (7–11) | 9 (8–11) | .775 |
| Home environment z-scorea | −0.02 (0.59 to 0.85) | −0.06 (−0.66 to 0.70) n = 165 |
−0.06 (−0.72 to 0.70) n = 178 |
.516 |
| HIV positive, no./No. (%) | 2 (1.6) | 4/170 (2.4) | 5/192 (2.6) | .929 |
| No. of seizures during hospitalization | … | 0 (0–0) | 1 (0–2) | .0001 |
| AKI, no./No. (%) | … | 36/161 (22.4) | 81/199 (40.7) | <.0001 |
| Mother's highest education levela, no./No. (%) | ||||
| Primary 6 or lower | 38 (31.2) | 65/166 (39.2) | 68/178 (38.2) | .305 |
| Primary 7 | 27 (22.1) | 35/166 (21.1) | 39/178 (21.9) | |
| Secondary or higher | 51 (41.8) | 65/166 (39.2) | 66/178 (37.1) | |
| Unknown or missing | 6 (4.9) | 1/166 (0.60) | 5/178 (2.8) | |
| Father's highest education levela, no./No. (%) | ||||
| Primary 6 or lower | 18 (14.8) | 37/166 (22.3) | 34/178 (19.1) | .410 |
| Primary 7 | 23 (18.9) | 24/166 (14.5) | 30/178 (16.9) | |
| Secondary or higher | 63 (51.6) | 77/166 (46.4) | 76/178 (42.7) | |
| Unknown or missing | 18 (14.8) | 28/166 (16.9) | 38/178 (21.4) | |
| Child has preschool educationa, no./No. (%) | 58 (47.5) | 43/164 (26.2) | 69/177 (39.0) | .001 |
| Neurologic deficit at discharge, no./No. (%) | … | … | 67/179 (37.4) | |
| Cognitive deficitsb, preschool tests, no./No. (%) | ||||
| 1-wk follow-up | 4/94 (4.3) | 28/134 (20.9) | 36/133 (27.1) | <.0001 |
| 24-mo follow-up | 1/35 (2.9) | 24/76 (31.6) | 17/56 (30.4) | .001 |
| Cognitive deficitsb, school-age tests, no./No. (%) | ||||
| 1-wk follow-up | 1/28 (3.6) | 8/32 (25.0) | 16/47 (34.0) | .005 |
| 24-mo follow-up | 4/82 (4.9) | 16/79 (20.3) | 38/116 (32.8) | <.0001 |
| Completed 24-mo cognitive testing | 117 | 155 | 172 | |
Data are presented as median (interquartile range) unless otherwise indicated. Data were analyzed using Pearson χ2 or Fisher exact test for categorical measures and Kruskal–Wallis test for continuous measures. Total number is shown if fewer than column number.
Abbreviations: AKI, acute kidney injury; CC, community children; CM, cerebral malaria; HIV, human immunodeficiency virus; SMA, severe malarial anemia.
Assessed in severe malaria survivors during home visit (SMA, n = 164–166; CM, n = 177–178).
Defined as the presence of a deficit (age-adjusted z-score < −2) in any of the primary cognitive domains of cognition, attention, memory).
Cysteine and Methionine Concentrations Are Low in Community Children Compared to Reference Ranges
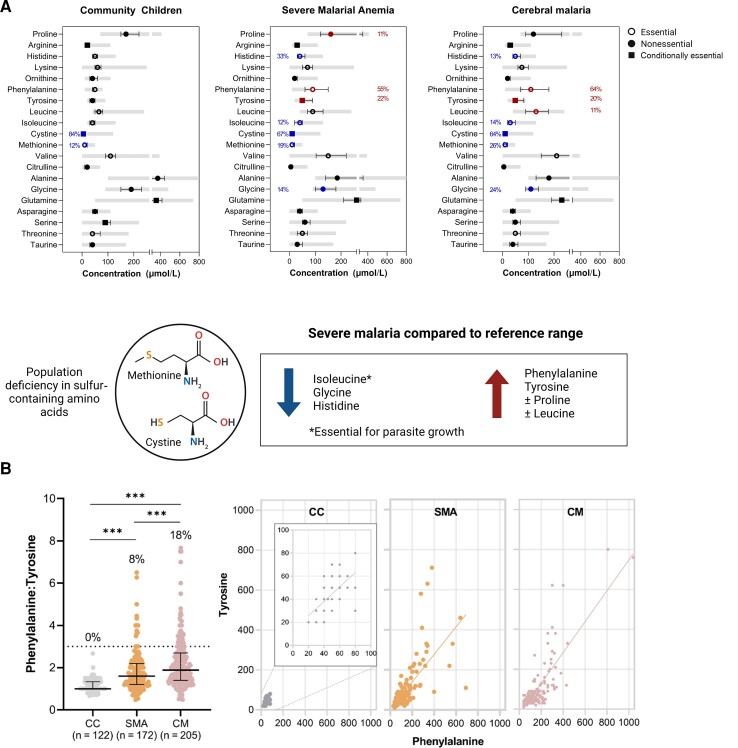
To understand amino acid concentrations in the study population, we evaluated the frequency of children with amino acid concentrations outside pediatric reference ranges (Figure 2). Among the CC, all amino acid concentrations were within the expected concentrations except for the sulfur-containing amino acids cysteine and methionine, where 84% and 12% of CC, respectively, had levels below the reference standard. Among children with SM (SMA or CM), cysteine and methionine remained deficient in a significant proportion of children (≥19% methionine, ≥64% cysteine). Elevations or reductions in amino acid concentrations were generally similar in children with SMA and children with CM. Among children with SM (SMA or CM), levels of histidine, isoleucine, and glycine were below the reference range in >5% of children whereas phenylalanine and tyrosine were elevated in >50% and ≥20% of children with SM, respectively (Figure 2A). Proline was elevated in 11% of children with SMA and leucine was elevated in 11% of children with CM. As there were striking elevations in phenylalanine, we evaluated whether there was evidence of reduced phenylalanine metabolism to tyrosine through phenylalanine hydroxylase. Among the CC, the median phenylalanine:tyrosine ratio was 1 and increased in children with SMA and CM (Figure 2B). Abnormal phenylalanine metabolism (phenylalanine:tyrosine ratio >3) was only observed in children with severe malaria and occurred in 8% of children with SMA and 18% of children with CM.
Figure 2.
Plasma amino acid concentrations in study population with reference ranges. A, Graphs depicting the median and interquartile range (IQR) for amino acids by study group (left, community children; center, severe malarial anemia; right, cerebral malaria) with the laboratory reference ranges shaded in gray. In instances where the frequency of abnormal levels was >10%, the amino acid is colored in blue for decreased levels or red for increased levels. The frequency of amino acid concentrations outside the reference range for flagged amino acids is indicated to the left (decreased) or right (increased) of the amino acid symbol. Amino acids were classified according to whether they were essential (open circle), nonessential (closed circle), or conditionally essential (closed square). B, Dot plot depicting median (IQR) for the phenylalanine/tyrosine concentration ratio and scatterplots between tyrosine (y-axis) and phenylalanine (x-axis) in each disease group. The percentages above each group represent % with a phenylalanine/tyrosine ratio >3. ***P < .001, Dunn test with Bonferroni correction for multiple comparisons. Created with BioRender.com. Abbreviations: CC, community children; CM, cerebral malaria; SMA, severe malarial anemia.
Amino Acid Concentrations Differ in Children With SM Compared to Community Children
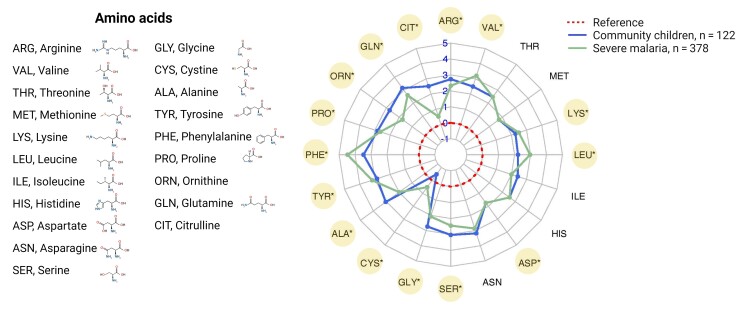
To further understand how amino acids changed in malaria relative to the CC, and to facilitate comparisons of amino acid profiles previously described in adults with SM [26], we calculated the mean fold change in log2 amino acid concentrations relative to the median reference level in a radar plot (Figure 3). Among the 20 amino acids measured in the study population, there were differences in 15 amino acids in children with SM relative to the CC, with increases in 6 amino acids (lysine, leucine, cysteine, tyrosine, phenylalanine, and valine) and decreases in 9 amino acids (aspartate, serine, glycine, alanine, proline, ornithine, glutamine, citrulline, and arginine).
Figure 3.
Radar plot showing global changes in amino acid concentrations in severe malaria. Amino acid concentrations were normalized to the median reference provided by the performing laboratory. Each concentric line from the center representing a log2 fold change in amino acid concentrations. The radar plot depicts global changes in patterns of amino acids in children with severe malaria compared to the community children. Amino acid concentrations that were different between children with severe malaria and the community children after adjusting for multiple comparisons are depicted with an * and highlighted in yellow. Differences were compared using the Wilcoxon rank-sum test with Bonferroni correction. Created with BioRender.com.
Amino Acid Concentrations Differ in Complications of SM
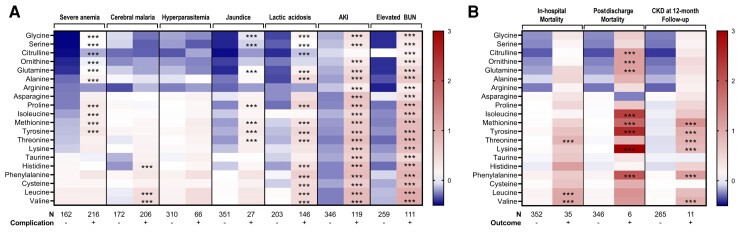
While children were selected for participation in the study according to selected SM criteria—severe anemia and coma—multiorgan dysfunction and metabolic complications were common. To understand how host and parasite factors impacted global expression patterns of plasma amino acid concentrations, we compared differences in standardized amino acid concentrations across clinical complications (Figure 4). While severe anemia and coma were selection criteria for enrollment and were associated with changes in the profiles of amino acids, differences in children with coma were less pronounced than in other complications, despite CM being one of the most severe manifestations of malaria. When examining patterns in amino acid levels and several clinical and metabolic complications, the most notable changes were observed in children with metabolic complications, with widespread changes in amino acids in children with lactic acidosis, AKI, and elevated blood urea nitrogen (BUN). While there were reduced levels of sulfur-containing amino acids in the population (methionine, cysteine) and some amino acids in SM (isoleucine, glycine, histidine) (Figure 2), the mean standardized amino acid concentration increased—without exception—in the context of metabolic complications.
Figure 4.
Heatmap of amino acid concentrations by severe malaria complication and outcome. Amino acid concentrations were standardized for the cohort to have a mean of zero and standard deviation of 1 so the relative differences in amino acids could be compared. Within each complication (A) and outcome (B), relationships significant by Student t test with a P < .0025 are indicated with *** in the column where the complication or outcome is present. The presence of a complication or outcome is indicated as − or + with the numbers in each group indicated. Amino acid levels lower than the population mean are indicated in blue and amino acid levels higher than the mean are represented in red. Abbreviations: AKI, acute kidney injury; BUN, blood urea nitrogen; CKD, chronic kidney disease.
Specific Amino Acid Concentrations Correlate With Total Parasite Biomass but Not Circulating Parasite Density
Amino acids are metabolized by both host and parasite. There were no differences in amino acid levels in the context of hyperparasitemia. However, as there were reductions in isoleucine in children with SM but not CC, and isoleucine is an essential amino acid for malaria parasite growth [36], we further evaluated changes in amino acid levels by parasite biomass, to account for sequestered mature-stage parasites with higher metabolic demands. Several amino acids were positively correlated with sequestered, but not circulating, parasite biomass (phenylalanine, tyrosine, threonine, histidine, lysine, leucine, and valine) (Table 2).
Table 2.
Amino Acid Correlations With Parasite Measures
| Amino Acid | Spearman Correlation | ||
|---|---|---|---|
| Total Biomass | Sequestered Biomass | Circulating Biomass | |
| Glycine | −0.0604 | −0.0577 | −0.0817 |
| Serine | −0.0651 | −0.0510 | −0.1186 |
| Citrulline | −0.0304 | −0.0545 | 0.0443 |
| Ornithine | −0.0640 | −0.0458 | −0.0734 |
| Glutamine | −0.0246 | −0.0190 | −0.0717 |
| Alanine | 0.1527 | 0.1211 | 0.1094 |
| Arginine | 0.1712 | 0.1688 | −0.0106 |
| Asparagine | 0.0126 | 0.0082 | −0.0024 |
| Proline | 0.1484 | 0.1275 | 0.1145 |
| Isoleucine | −0.1190 | −0.1083 | −0.0262 |
| Methionine | 0.0872 | 0.0818 | −0.0504 |
| Tyrosine | 0.2201a | 0.2080a | 0.0226 |
| Threonine | 0.2729a | 0.2673a | −0.0350 |
| Lysine | 0.2134a | 0.2173a | −0.0460 |
| Taurine | −0.0666 | −0.0857 | 0.0722 |
| Histidine | 0.4172a | 0.3935a | 0.0459 |
| Phenylalanine | 0.3161a | 0.2751a | 0.1516 |
| Cysteine | 0.1796 | 0.1712 | 0.0661 |
| Leucine | 0.4453a | 0.4161a | 0.1008 |
| Valine | 0.4459a | 0.4098a | 0.1122 |
Significant following adjustment for 60 comparisons.
Unique Amino Acid Profiles Correlate With Acute Mortality and Long-term Outcomes
While there were widespread changes in amino acids in children with metabolic complications, 3 essential amino acids were higher among children who died in-hospital—threonine, leucine, and valine (Figure 4). Children who died over follow-up had higher levels of citrulline, ornithine, glutamine, isoleucine, methionine, tyrosine, lysine, and phenylalanine on hospital admission (adjusted P < .05; Figure 4). At 12 months of follow-up, kidney function was assessed in survivors and CKD, defined as an estimated glomerular filtration rate <90 mL/minute/1.73 m2. Children with CKD at 12-month follow-up had increases in methionine, tyrosine, threonine, lysine, phenylalanine, and valine (adjusted P < .05).
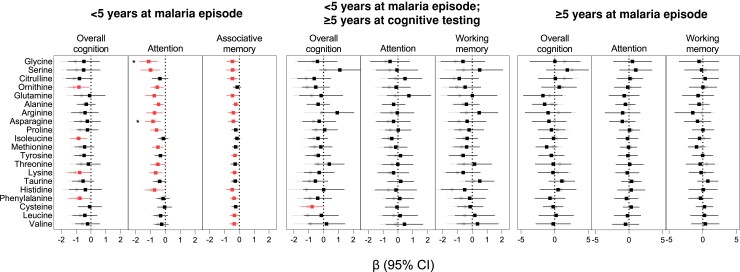
Finally, we evaluated whether acute perturbations in amino acids during SM were associated with cognitive differences over 2 years of follow-up. As cognitive assessment tools differ based on child age, results are presented stratified based on age at SM exposure and age at testing. Population characteristics based on age at enrollment are presented in Supplementary Table 1. Using longitudinal modeling, increases in several amino acids were associated with cognitive, attention, or memory outcomes in children <5 years of age (Figure 5). However, after adjustment for multiple comparisons, only glycine or asparagine at enrollment was associated with reduced attention in children <5 years of age at SM exposure and cognitive evaluation. This relationship was consistent in CM and SMA (Supplementary Figure 1). There were no differences in amino acid levels and cognition in children >5 years of age.
Figure 5.
Relationship between admission amino acid levels and cognitive outcomes over 24-months of follow-up in children with severe malaria. Beta coefficients and 95% confidence intervals (CIs) are from linear mixed-effects models with estimates in gray (circles) representing unadjusted models and adjusted models in black or red (squares) adjusting for age, sex, weight-for-age z-score at baseline, socioeconomic status, child education, home z-score, maternal and paternal education, number of seizures during hospitalization, acute kidney injury at baseline, and disease group. The number of observations and participants for each estimate are presented in Supplementary Tables 2–4. * indicates significant following adjustment for 20 comparisons.
DISCUSSION
In the present study we show widespread changes in amino acid concentrations in children with SM (CM or SMA) compared to CC. Among children with SM, unique amino acid profiles were associated with in-hospital mortality, postdischarge mortality, and CKD over follow-up, and worsened long-term attention. On a population level, children had a high frequency of deficiencies in the sulfur-containing amino acids methionine and cysteine. Together these findings provide evidence that metabolic complications associated with amino acid derangements on admission portend adverse clinical outcomes.
Changes in amino acid concentrations are widely recognized in SM, with high phenylalanine levels well described in adults and children with SM [20, 26]. As phenylalanine is associated with the biosynthesis of neurotransmitters, several studies have evaluated whether phenylalanine is related to coma and neurologic complications in SM [19, 20, 26]. This study included a large group of children with coma, and neither phenylalanine nor tyrosine, the immediate byproduct of phenylalanine metabolism, were elevated in children with coma. Rather, phenylalanine and tyrosine were elevated in children with metabolic complications, suggesting global changes in amino acid metabolism in SM. In the present study, a significant proportion of children had phenylalanine and tyrosine levels outside the reference range, with children with SM having an elevated phenylalanine:tyrosine ratio suggestive of impaired phenylalanine hydroxylase activity, which is expressed in the liver and kidney. While levels of phenylalanine and tyrosine were not associated with in-hospital mortality, they were higher in children who died during follow-up or had CKD at 1-year follow-up.
The kidney is instrumental in the interorgan exchange of amino acids and nitrogen excretion. As this process is sensitive to pH [16], it may be disturbed in the context of acidosis [16]. In patients with kidney disease, kidney uptake of phenylalanine is reduced [37], which may explain, in part, increases in phenylalanine in SM in association with AKI, but not severe anemia or coma. The kidney is responsible for amino acid metabolism including the uptake of glutamine, proline, citrulline, and phenylalanine and release of serine, arginine, taurine, threonine, tyrosine, ornithine, and lysine [37]. Over the past decade, uremia and AKI have been recognized as important clinical complications in children with SM [12, 38–40]. While elevations in BUN occur in the context of AKI, elevated BUN and AKI represent independent risk factors for mortality in pediatric SM. Altered amino acid metabolism in SM, and its relationship with acidosis, AKI, and elevated BUN, highlight for additional studies to understand the interplay of multiorgan dysfunction on amino acid levels in the short-term and long-term differences that may relate to persistent organ injury including CKD.
In the present study, elevated levels of glycine and asparagine were associated with worse attention at 2 years of follow-up in children <5 years of age adjusting for child age, sex, nutritional status, socioeconomic status, enrichment in the home environment, education status, and disease severity at admission. These findings remained significant following adjustment for multiple comparisons and was seen in both CM and SMA (Supplementary Figure 1). Both asparagine and glycine have roles in neurotransmission and are reportedly upregulated in the cerebrospinal fluid (CSF) of children with tuberculous meningitis alongside proline, alanine, and lysine [41]. While proline, alanine, and lysine did not remain significantly associated with attention following adjustment for multiple comparisons, they showed similar trends as glycine and asparagine. Asparagine and glycine are metabolized in the kidney, and in the present study asparagine was selectively elevated in children with AKI and elevated BUN, suggestive of uremia. AKI was identified as a risk factor for neurocognitive deficits and behavioral problems in SM survivors [9, 42] and is implicated in systemic endothelial activation and blood-brain-barrier impairment [9, 43] as well as markers of neuronal injury in both the CSF and plasma [44, 45]. Given the strong relationship between plasma amino acid changes, AKI, and metabolic changes in this study, additional metabolomic investigations of the CSF are needed to understand the relationship between brain and plasma amino acid levels and long-term neurocognitive function.
Compared to CC, children with SM had decreases in isoleucine, glycine, and histidine. Isoleucine is an essential amino acid for parasite metabolism, and 12%–14% of children with SM had isoleucine levels below the reference range. However, there was no relationship between isoleucine and parasite biomass. There was a positive relationship between sequestered biomass and concentrations of several amino acids (tyrosine, threonine, lysine, histidine, phenylalanine, leucine, valine), which may reflect byproducts of parasite metabolism from late-stage sequestered trophozoites, red blood cell release of amino acids associated with hemolysis (tyrosine), or severe disease. Three amino acids associated with sequestered parasite biomass were also elevated in fatal malaria (threonine, leucine, and valine). Leucine and valine are essential amino acids that bypass hepatic clearance and are taken up by peripheral tissues. Our results are consistent with elevated valine in fatal cases of malaria, but not sepsis, in adults from Bangladesh [26]. We speculate that peripheral tissue uptake of essential amino acids may be impaired in children with more sequestered biomass reflecting a malaria-specific process, rather than a stress responses associated with severe infection.
A notable finding was the high prevalence of cysteine and methionine deficiencies in CC and children with SM. Cysteine and methionine are sulfur-containing amino acids with important roles in mitigating oxidative stress through glutathione synthesis as well as homeostatic control of micronutrient metabolism including zinc, Vitamin E and C, and other antioxidants involved in the antioxidant cascade [46]. Population deficiencies in sulfur-containing amino acids are associated with severe malnutrition [47] and may lead to reduced capacity to handle oxidative stress. Methionine is an essential amino acid that can only be obtained from the diet, with the richest food sources being animal-sourced proteins. The Ugandan diet is relatively low in protein and dominated by plant-based proteins, which may be insufficient to sustain normal levels of methionine. Reduced glutathione from dietary deficiencies of sulfur-containing amino acids may exacerbate the body’s capacity to handle oxidative stress, particularly in children with depleted glutathione from repeated infections, or G6PD deficiency, which occurs in 15%–30% of African children [48]. In the present study, sulfur-containing amino acids were depleted on a population level but elevated in children with metabolic complications and in children who died postdischarge. We speculate this may reflect a transient reduction in renal excretion of sulfate associated with AKI. In the context of sulfur-containing amino acid deficiencies, the body preferentially diverts sulfur to the synthesis of cysteine to support vital metabolic processes [46]. The brain is one of the first organs affected by cysteine deficiencies, resulting in reduced glutathione in the brain [46]. In models of sulfur-containing amino acid deficiencies, brain glutathione is depleted and there is reduced capacity to cope with an insult to the brain [49]. Additional studies are needed to understand how chronic deficiencies in sulfur-containing amino acids may impact antioxidant capacity and long-term health and neurodevelopment.
Overall, the findings of this study confirm findings of altered amino acid concentrations in a focused metabolomic study in a cohort of Malawian children with CM [19], and Tanzanian children and Indonesian adults with SM, and provide novel data on postdischarge outcomes. Limitations of the present study include a single snapshot of the plasma pool of amino acids on enrollment. Although the evaluation of amino acids is costly, evaluation of amino acids at multiple time points would provide valuable additional information to understand dynamic changes in the plasma amino acid pool in response to cellular stress, organ dysfunction, and treatment. Strengths of the study include the large sample size, which included CC to assess normal amino acid levels in the population, the evaluation of AKI at enrollment and CKD at follow-up, and detailed neurocognitive evaluations over 2 years of follow-up. This enabled us to extend previous studies that assessed relationships with plasma amino acids during acute illness and their relationship with clinical complications and mortality and to evaluate whether the acute perturbations related to long-term outcomes. Amino acid levels at presentation predicted postdischarge mortality and CKD with increased levels of phenylalanine, tyrosine, lysine, and methionine shared in participants with CKD and those who died over follow-up. This is the first investigation to identify risk factors for CKD in the context of SM.
In summary, we identified specific amino acid profiles in SM associated with acute mortality, postdischarge mortality, CKD, and cognitive impairment. Additional studies are needed to understand the relationship between altered amino acid metabolism and the broader metabolic milieu in SM and validate whether there are specific patterns of amino acid derangements that may point to supplementation as a strategy to improve recovery and survival in children following hospital discharge.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Andrea L Conroy, Ryan White Center for Pediatric Infectious Disease and Global Health, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Tuan M Tran, Ryan White Center for Pediatric Infectious Disease and Global Health, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Caitlin Bond, Ryan White Center for Pediatric Infectious Disease and Global Health, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Robert O Opoka, Department of Paediatrics and Child Health, Makerere University College of Health Sciences, Global Health Uganda, Kampala, Uganda.
Dibyadyuti Datta, Ryan White Center for Pediatric Infectious Disease and Global Health, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Edward A Liechty, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Paul Bangirana, Department of Psychiatry, Makerere University College of Health Sciences, Global Health Uganda, Kampala, Uganda.
Ruth Namazzi, Department of Paediatrics and Child Health, Makerere University College of Health Sciences, Global Health Uganda, Kampala, Uganda.
Richard Idro, Department of Paediatrics and Child Health, Makerere University College of Health Sciences, Global Health Uganda, Kampala, Uganda.
Sarah Cusick, Division of Pediatric Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, Minnesota, USA.
John M Ssenkusu, Department of Epidemiology and Biostatistics, Makerere University School of Public Health, Kampala, Uganda.
Chandy C John, Ryan White Center for Pediatric Infectious Disease and Global Health, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Notes
Acknowledgments. The authors thank Mulago Hospital, and Global Health Uganda for conducting the study, study participants and their caregivers, and our dedicated study teams for the excellent clinical care and follow-up provided to all children. We specifically acknowledge Gloria Kyarisima for data management.
Disclaimer. The funders had no role in the study design, analysis of the data, or the decision to submit the manuscript for publication.
Financial support. This work was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (R01NS055349 to C. C. J.) and the Fogarty International Center (D43 TW010928 to C. C. J.).
References
- 1. World Health Organization (WHO) . World malaria report 2021. Geneva, Switzerland: WHO, 2021. [Google Scholar]
- 2. Bangirana P, Opoka RO, Boivin MJ, et al. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 2014; 59:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 2008; 122:e92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child 2006; 91:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Idro R, Kakooza-Mwesige A, Balyejjussa S, et al. Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes 2010; 3:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Idro R, Marsh K, John CC, Newton CRJ. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 2010; 68:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birbeck GL, Molyneux ME, Kaplan PW, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 2010; 9:1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langfitt JT, McDermott MP, Brim R, et al. Neurodevelopmental impairments 1 year after cerebral malaria. Pediatrics 2019; 143:e20181026. [DOI] [PubMed] [Google Scholar]
- 9. Conroy AL, Opoka RO, Bangirana P, et al. Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med 2019; 17:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ssenkusu JM, Hodges JS, Opoka RO, et al. Long-term behavioral problems in children with severe malaria. Pediatrics 2016; 138:e20161965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opoka RO, Hamre KES, Brand N, Bangirana P, Idro R, John CC. High postdischarge morbidity in Ugandan children with severe malarial anemia or cerebral malaria. J Pediatric Infect Dis Soc 2017; 6:e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batte A, Berrens Z, Murphy K, et al. Malaria-associated acute kidney injury in African children: prevalence, pathophysiology, impact, and management challenges. Int J Nephrol Renovasc Dis 2021; 14:235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sriboonvorakul N, Ghose A, Hassan MMU, et al. Acidosis and acute kidney injury in severe malaria. Malar J 2018; 17:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalangin R, Kim A, Campbell RE. The role of amino acids in neurotransmission and fluorescent tools for their detection. Int J Mol Sci 2020; 21:6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bröer S, Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J 2017; 474:1935–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr 2004; 79:185–97. [DOI] [PubMed] [Google Scholar]
- 17. Vente JP, von Meyenfeldt MF, van Eijk HM, et al. Plasma-amino acid profiles in sepsis and stress. Ann Surg 1989; 209:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deutz NEP, Singer P, Wierzchowska-McNew RA, et al. Comprehensive metabolic amino acid flux analysis in critically ill patients. Clin Nutr 2021; 40:2876–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta S, Seydel K, Miranda-Roman MA, et al. Extensive alterations of blood metabolites in pediatric cerebral malaria. PLoS One 2017; 12:e0175686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopansri BK, Anstey NM, Stoddard GJ, et al. Elevated plasma phenylalanine in severe malaria and implications for pathophysiology of neurological complications. Infect Immun 2006; 74:3355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopansri BK, Anstey NM, Weinberg JB, et al. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 2003; 361:676–8. [DOI] [PubMed] [Google Scholar]
- 22. Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 2007; 204:2693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeo TW, Lampah DA, Tjitra E, et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog 2010; 6:e1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinberg JB, Volkheimer AD, Rubach MP, et al. Monocyte polarization in children with falciparum malaria: relationship to nitric oxide insufficiency and disease severity. Sci Rep 2016; 6:29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enwonwu CO, Afolabi BM, Salako LA, Idigbe EO, al-Hassan H, Rabiu RA. Hyperphenylalaninaemia in children with falciparum malaria. QJM 1999; 92:495–503. [DOI] [PubMed] [Google Scholar]
- 26. Leopold SJ, Apinan S, Ghose A, et al. Amino acid derangements in adults with severe falciparum malaria. Sci Rep 2019; 9:6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conroy AL, Opoka RO, Bangirana P, et al. Parenteral artemisinins are associated with reduced mortality and neurologic deficits and improved long-term behavioral outcomes in children with severe malaria. BMC Med 2021; 19:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park GS, Opoka RO, Shabani E, Wypyszynski A, Hanisch B, John CC. Plasmodium falciparum histidine-rich protein-2 plasma concentrations are higher in retinopathy-negative cerebral malaria than in severe malarial anemia. Open Forum Infect Dis 2017; 4:ofx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boivin MJ, Bangirana P, Byarugaba J, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 2007; 119:e360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service, 1995. [Google Scholar]
- 31. Jordan CM, Johnson AL, Hughes SJ, Shapiro EG. The Color Object Association Test (COAT): the development of a new measure of declarative memory for 18- to 36-month-old toddlers. Child Neuropsychol 2008; 14:21–41. [DOI] [PubMed] [Google Scholar]
- 32. Goldman DZ, Shapiro EG, Nelson CA. Measurement of vigilance in 2-year-old children. Dev Neuropsychol 2004; 25:227–50. [DOI] [PubMed] [Google Scholar]
- 33. Kaufman AS, Kaufman NL. Kaufman assessment battery for children manual, 2nd ed. Circle Pines, MN: American Guidance Service, 2004. [Google Scholar]
- 34. Greenberg LM, Waldman ID. Developmental normative data on the test of variables of attention (T.O.V.A.). J Child Psychol Psychiatry 1993; 34:1019–30. [DOI] [PubMed] [Google Scholar]
- 35. Mburu W, Conroy AL, Cusick SE, et al. The impact of undernutrition on cognition in children with severe malaria and community children: a prospective 2-year cohort study. J Trop Pediatr 2021; 67:fmab091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnan A, Soldati-Favre D. Amino acid metabolism in apicomplexan parasites. Metabolites 2021; 11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest 1980; 65:1162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis 2012; 54:1080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sypniewska P, Duda JF, Locatelli I, Althaus CR, Althaus F, Genton B. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med 2017; 15:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Namazzi R, Opoka R, Datta D, et al. Acute kidney injury interacts with coma, acidosis, and impaired perfusion to significantly increase risk of death in children with severe malaria. Clin Infect Dis 2022; 75:1511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mason S, Reinecke CJ, Solomons R. Cerebrospinal fluid amino acid profiling of pediatric cases with tuberculous meningitis. Front Neurosci 2017; 11:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hickson MR, Conroy AL, Bangirana P, et al. Acute kidney injury in Ugandan children with severe malaria is associated with long-term behavioral problems. PLoS One 2019; 14:e0226405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ouma BJ, Ssenkusu JM, Shabani E, et al. Endothelial activation, acute kidney injury, and cognitive impairment in pediatric severe malaria. Crit Care Med 2020; 48:e734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Datta D, Conroy AL, Castelluccio PF, et al. Elevated cerebrospinal fluid tau protein concentrations on admission are associated with long-term neurologic and cognitive impairment in Ugandan children with cerebral malaria. Clin Infect Dis 2019; 70:1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Datta D, Bangirana P, Opoka RO, et al. Association of plasma tau with mortality and long-term neurocognitive impairment in survivors of pediatric cerebral malaria and severe malarial anemia. JAMA Netw Open 2021; 4:e2138515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nimni ME, Han B, Cordoba F. Are we getting enough sulfur in our diet? Nutr Metab 2007; 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fitzpatrick MC, Kurpad AV, Duggan CP, Ghosh S, Maxwell DG. Dietary intake of sulfur amino acids and risk of kwashiorkor malnutrition in eastern Democratic Republic of the Congo. Am J Clin Nutr 2021; 114:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Howes RE, Piel FB, Patil AP, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med 2012; 9:e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bobyn PJ, Franklin JL, Wall CM, Thornhill JA, Juurlink BH, Paterson PG. The effects of dietary sulfur amino acid deficiency on rat brain glutathione concentration and neural damage in global hemispheric hypoxia-ischemia. Nutr Neurosci 2002; 5:407–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.