Abstract
Background
Measles virus infection induces acute immunosuppression for weeks following infection, and also impairs preexisting immunological memory, resulting in “immune amnesia” that can last for years. Both mechanisms predispose the host to severe outcomes of subsequent infections. Therefore, measles dynamics could potentially affect the epidemiology of other infectious diseases.
Methods
To examine this hypothesis, we analyzed the annual mortality rates of children aged 1–9 years in Brazil from 1980 to 1995. We calculated the correlation between nonmeasles infectious disease mortality rates and measles mortality rates using linear and negative-binomial models, with 3 methods to control the confounding effects of time. We also estimated the duration of measles-induced immunomodulation.
Results
The mortality rates of nonmeasles infectious diseases and measles virus infection were highly correlated. This positive correlation remained significant after removing the time trends. We found no evidence of long-term measles immunomodulation beyond 1 year.
Conclusions
These results support that measles virus infection could increase the mortality of other infectious diseases. The short lag identified for measles effects (<1 year) implies that acute immunosuppression was potentially driving this effect in Brazil. Overall, our study indicates disproportionate contributions of measles to childhood infectious disease mortality, highlighting the importance of measles vaccination.
Keywords: measles virus infection, childhood infectious disease mortality, Brazil, correlation analysis, immunomodulation
This study found a significantly positive association between measles and nonmeasles infectious disease mortality rates in children aged 1–9 years in Brazil, from 1980 to 1995, supporting the hypothesis that measles virus infection facilitates other childhood infectious diseases through immunomodulation.
As one of the most transmissible human pathogens, the measles virus used to infect nearly every child, causing periodic outbreaks and heavy mortality tolls globally. The measles burden was reduced substantially following the development of effective measles vaccines that induce life-long immunity [1]. However, measles still causes more than 100 000 deaths per year globally [2], and measles incidence has been resurging in the last few years, likely due to stagnant or even declining vaccine coverage [3–5]. Furthermore, vaccine coverage and measles burden are highly uneven worldwide, with low- and middle-income countries (LMIC) and rural locations generally having a lower vaccine coverage and, therefore, being at a higher risk of measles virus infections [6]. The public health threat posed by the measles virus could be further exacerbated by the disruptions of routine childhood immunization programs due to the ongoing coronavirus disease 2019 (COVID-19) pandemic [7, 8].
Why are measles virus infections so dangerous? In addition to its high transmissibility, the measles virus has been shown to increase the host’s susceptibility to other infectious diseases, leading to an increased risk of secondary infections, particularly respiratory and gastrointestinal diseases [9–11]. Such effects were observed in clinical investigations possibly as early as the 18th century [12], and recent studies have uncovered the likely immunological mechanisms. Measles virus infection first elicits transient immunosuppression that lasts for weeks, featuring depleted lymphocytes, altered cytokine responses, etc. [13, 14]. However, the damage from measles can last much longer. Recent immunological studies have found that the virus infects memory B, T, and plasma cells. As a result, it depletes a large proportion of preexisting immunological memory [15–17]. This measles-induced “immune amnesia” can lead to more severe outcomes of secondary infections and can last for months to years [18–20]. Consistent with this result, matched cohort studies have associated measles virus infections with an elevated incidence and severity of other infectious diseases up to 3 to 5 years following measles [21, 22]. Lastly, following measles vaccination programs, the reduction of all-cause childhood mortality usually exceeded the number of averted measles deaths alone [23, 24], suggesting nonspecific effects of the measles vaccine. As the vaccine does not induce immune amnesia but effectively prevents measles virus infections, it likely reduces secondary infections [15]. Collectively, these studies suggested a disproportionate impact of measles virus infections on the overall childhood infectious disease burden.
Building on these findings, a comprehensive evaluation of measles’ threats and vaccine importance could benefit from more epidemiological analysis in distinct settings. If measles-induced immunosuppression and immune amnesia indeed facilitate the transmission of other infectious diseases, or increase their burden, one would expect a positive association between measles incidence and nonmeasles infectious disease burden, particularly mortality. Such associations have been described in the United Kingdom, the United States, and Denmark [18, 25]. However, such evidence remains scarce in LMICs, which suffer the heaviest measles burden. Previous epidemiological studies in individual countries have yielded contrasting conclusions [20, 26, 27], although a recent cross-country analysis by Sato and Haraguchi reported a strong association between measles incidence and the prevalence and mortality of 5 other infectious diseases cross 46 African countries [28]. Another question that remains open is the duration of the measles-induced immune amnesia. Mina et al found prolonged measles effects in 3 developed countries (the United Kingdom, United States, and Denmark) using nationwide epidemiological records [18]. Gadroen et al also reported an impact of measles on host resistance to other infectious diseases for up to 5 years in a cohort study [21]. In contrast, cohort-based studies in Bangladesh [24, 29] and Guinea-Bissau [30] found no evidence of long-term measles effects. However, studies using large epidemiological databases in LMICs were still scarce.
To fill these knowledge gaps, in this study, we analyzed the correlation of annual mortality rates between measles and nonmeasles infectious diseases in Brazil between 1980 and 1995, focusing on children aged 1–9 years [31]. In addition, we estimated the duration of measles virus infection-induced immunomodulation. Measles was a leading cause of childhood mortality in Brazil before vaccination [32, 33], which was first introduced in the late 1960s. Nationwide measles vaccination programs intensified in the early 1980s and substantially reduced measles prevalence to near elimination in the 1990s [33]. Examining this period of measles eradication thus allowed us to estimate the benefits of measles vaccination programs in reducing overall childhood infectious disease mortality.
METHODS
Data Sources
We downloaded the annual mortality numbers of measles and nonmeasles infectious diseases (Supplementary Table 1) in children aged 1–9 years between 1980 and 1995 from the Brazilian Ministry of Health DATASUS—Vital Statistics. We also acquired annual population size estimates for the same age group, which were used to calculate mortality rates. In addition to the national level data, we analyzed mortality data from São Paulo and Rio de Janeiro, 2 major metropolitan regions in Brazil. The data source is further described in the Supplementary Material.
Correlations Between Measles Mortality and Nonmeasles Infectious Disease Mortality
The primary goal of this study is to assess the correlations between annual measles mortality and nonmeasles infectious disease mortality. To achieve this, we regressed the latter against the former using linear models and calculated the R2 to quantify the correlation. The regression models also allowed us to test if measles variation is a statistically significant predictor of nonmeasles disease mortality. Mortality of most disease categories decreased over the years, likely reflecting overall improvements in the health care quality in Brazil. This time trend could generate spurious correlation signals in our regression models. To control for this, we applied 3 methods, including (1) adding year as a covariate in the regression models; (2) estimating the general time trends by change-point analysis and using the residuals from the time trends in the regression models (detrending; see details in the Supplementary Material and Supplementary Figure 1); and (3) calculating the difference of mortality rates between 2 consecutive years as inputs for the regression models (difference; Supplementary Figure 1). Because mortality data (death counts) was overdispersed in our dataset, we also constructed negative-binomial models for regressing nonmeasles disease mortality counts against measles mortality, with and without year as a covariate. We performed all analyses in the 3 datasets (Brazil national, São Paulo, and Rio de Janeiro). Lastly, as a negative control, we examined the correlation between measles mortality rate and circulatory disease mortality rates at the national level. Circulatory diseases should not be affected by measles-induced immunomodulation, so we expect no correlations between them.
The substantial reduction of measles mortality during our study period allowed us to perform another analysis, in which we contrasted the periods with high and low measles prevalence. If measles virus infections were strongly associated with mortality from other infectious diseases, we would expect a high synchronization between different disease categories (eg, between flu and diarrhea) when measles was prevalent. On the other hand, when the measles virus was nearly eradicated, such synchronization between other diseases would break down. To test these predictions, using Brazilian national data, we first determined the time point of measles’ near eradication using change-point analysis [34] (see Supplementary Material and Supplementary Table 2 for more details). We then calculated correlations of mortality rates between each pair of infectious disease categories before and after that change point. This analysis was performed with the original mortality rates, as well as the detrended mortality rate and mortality rate differences between years.
Duration of Measles-Induced Immunomodulation
To examine the duration of measles-induced immunomodulation, we performed similar analyses as Mina et al 2015 [18]. In brief, we accumulated the measles mortality for different numbers of years, either by simple addition (ie, the additive method) or by weighing the contribution of years with a gamma function (see Supplementary Table 3 and the Supplementary Material). We then searched for the duration that results in the strongest correlations (ie, R2 in regression models) with nonmeasles disease mortality. All analyses were performed in R version 4.0.2 [35], and the scripts can be found on Github (https://github.com/siyangxia419/Brazil_measles_mortality).
RESULTS
Nonmeasles Infectious Disease Annual Mortality Rates Correlated With Measles Mortality Rates
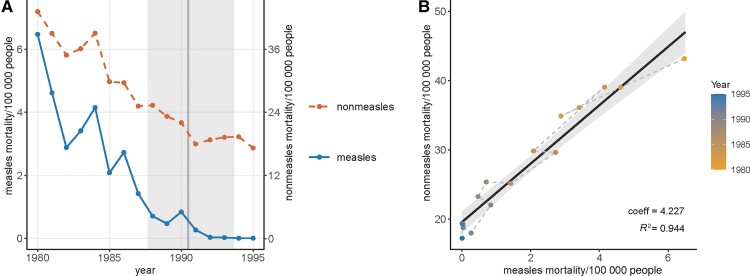
In Brazil, measles mortality rates decreased substantially in the 1980s and leveled off near zero from the beginning of the 1990s (Figure 1A). Accordingly, the change point of the measles time series was found between 1990 and 1991 (mean = 1990.5, 95% credible interval = 1987.7–1993.7; Supplementary Figure 2A). Mortality rates of most nonmeasles infectious diseases also reduced from 1980 to 1995 (Supplementary Figure 3), and their sum decreased by 53% (Figure 1A). The change point analysis identified decreasing total nonmeasles mortality rates from 1980 to 1991, followed by no temporal trend since 1992 (mean change point = 1991.6, 95% credible interval = 1989.6–1993.7; Supplementary Figure 2B).
Figure 1.
Mortality rates of measles and nonmeasles infectious diseases in children aged 1–9 years in Brazil between 1980 and 1995. A, Time series of the measles mortality rates (left y-axis, solid line) and nonmeasles mortality rates (right y-axis, dashed line) per 100 000 people. The vertical line and the shaded area indicate the point estimate and the 95% credible interval of the change point in the measles mortality time series. B, Correlation between measles and nonmeasles disease mortality rates. The colors of the points indicate the year, and dashed segments connect consecutive years. The solid line and the shade indicate the linear model and the 95% confidence interval. Coefficients (coeff) of measles mortality rates and the R2 of the model are shown on the bottom right of the panel.
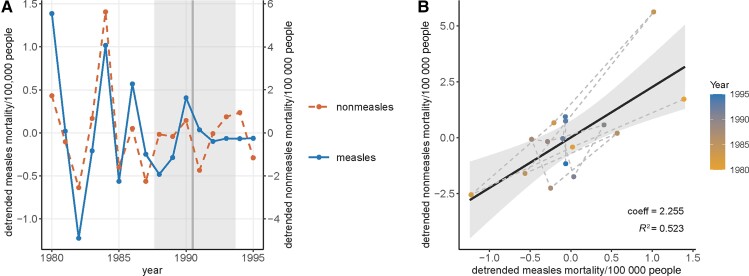
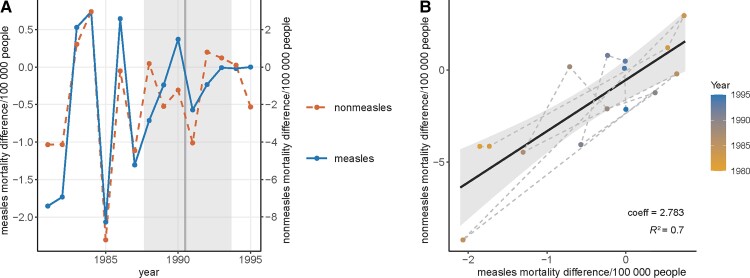
Using the original mortality rates, the linear models found a strong and significant correlation between measles and nonmeasles infectious disease mortality rates (R2 = 0.944; Figure 1B and Table 1). Measles remained a significant predictor of nonmeasles disease mortality after adding year as a covariate (Table 1). Applying the other 2 methods to control for time effects, that is, detrending the mortality rates (Figure 2) and using the mortality rate differences between consecutive years (Figure 3), resulted in lower R2, but the correlations were still statistically significant (Table 1). Lastly, the negative-binomial models generated similar conclusions as the linear models, except that the interaction between measles mortality rate and the year became a significant predictor.
Table 1.
Regression Analysis Between Nonmeasles Infectious Disease Mortality Rates and Measles Mortality Rates in Brazil
| Model | Nonmeasles ∼ Measles |
Nonmeasles ∼ Year + Measles |
Detrend Nonmeasles ∼ Detrend Measles |
Difference (Nonmeasles) ∼ Difference (Measles) |
Negative Binomial Modela |
|---|---|---|---|---|---|
| Measles mortality rate |
β = 4.227b (3.686–4.767) t = 15.33 P < .001 |
β = 2.573 (1.568–3.578) t = 5.02 P < .001 |
β = 2.255 (1.126–3.385) t = 3.91 P = .002 |
β = 2.783 (1.793–3.774) t = 5.51 P < .001 |
β = 0.059 (.021–.096) t = 3.05 P = .003 |
| Year | … | β = −0.751 (−1.169 to −.332) t = −3.52 P = .004 |
… | … | β = −0.041 (−.057 to −.025) t = −5.08 P < .001 |
| Intercept | 19.536b (18.083–20.989) |
29.033 (23.631–34.435) |
0.014 (−.669 to .689) |
−0.530 (−1.512 to .452) |
3.507 (3.304–3.710) |
| n | 16 | 16 | 16 | 15 | 16 |
| R 2 | 0.944 | 0.971 | 0.523 | 0.700 | NAc |
Nonmeasles ∼ year + measles + offset (population size/100 000).
Point estimate (95% confidence interval).
R 2 was not calculated for negative binomial model.
Figure 2.
Detrended mortality rates of measles and nonmeasles infectious diseases in children aged 1–9 years in Brazil between 1980 and 1995. A, Time series of the detrended measles mortality rates (left y-axis, solid line) and nonmeasles mortality rates (right y-axis, dashed line) per 100 000 people. The vertical line and the shaded area indicate the point estimate and the 95% credible interval of the change point in the original measles mortality time series. B, Correlation between detrended measles and nonmeasles disease mortality rates. The colors of the points indicate the year, and dashed segments connect consecutive years. The solid line and the shade indicate the linear model and the 95% confidence interval. Coefficients of detrended measles mortality rates and the R2 of the model are shown on the bottom right of the panel.
Figure 3.
Difference of mortality rate between consecutive years for measles and nonmeasles infectious diseases in children aged 1–9 years in Brazil between 1980 and 1995. A, Time series of the measles mortality rate difference (left y-axis, solid line) and nonmeasles mortality rate difference (right y-axis, dashed line) between consecutive years. The vertical line and the shaded area indicate the point estimate and the 95% credible interval of the change point in the original measles mortality time series. B, Correlation between measles and nonmeasles disease mortality rate differences. The colors of the points indicate the year, and dashed segments connect consecutive years. The solid line and the shade indicate the linear model and the 95% confidence interval. Coefficients of measles mortality rate differences and the R2 of the model are shown on the bottom right of the panel.
Results from São Paulo largely mimicked those from the whole of Brazil (Supplementary Table 4 and Supplementary Figure 4), except for an earlier change point in both measles and nonmeasles diseases mortality rates between 1987 and 1988 (Supplementary Figure 2C and 2D). The measles virus was largely eradicated in São Paulo in 1987. This epidemiological observation aligned with a state-wide mass measles vaccine campaign in May 1987 [33]. On the other hand, the decreases in measles and nonmeasles disease mortality in Rio de Janeiro were more sudden, featuring a sharp drop between 1984 and 1985 (Supplementary Figure 2E and 2F) that largely drove the correlation between measles and nonmeasles disease mortality rates. The correlation was significant when calculated with the original mortality rates and the difference between years, but was no longer significant when using the detrended data (Supplementary Table 5 and Supplementary Figure 5).
Measles Mortality Rate Showed No Correlation With Circulatory Disease Mortality Rates
As shown in Supplementary Table 6 and Supplementary Figure 6, the original mortality rates of measles virus infection and circulatory diseases in Brazil correlated significantly. However, this correlation resulted mainly from the decreasing trends in both disease categories. Models using detrended mortality data and mortality difference between consecutive years found no significant correlations, and the R2 was negligible under both methods (Supplementary Figure 6C–F). Contrasting this result with that from the analysis between measles and nonmeasles infectious diseases further increased our confidence that measles variations were strongly associated with the mortality of other infectious diseases.
Pairwise Correlations Between Individual Disease Categories Were Higher When Measles Was Present
Pearson correlation coefficients between mortality rates of each pair of infectious diseases were calculated separately in the period before (1980–1990) and after (1991–1995) the change point. In addition to the original data, we also performed this analysis using the detrended mortality rates and the mortality rate differences between consecutive years. Supplementary Figure 7 suggested that correlations between disease categories were generally higher before the change point when the measles virus was still circulating than after the change point when the measles burden was very low. This observation was consistent with our expectations that measles had strong effects on the dynamics of other infectious diseases’ mortality.
Measles-Induced Immunomodulation Was Mostly Short-term
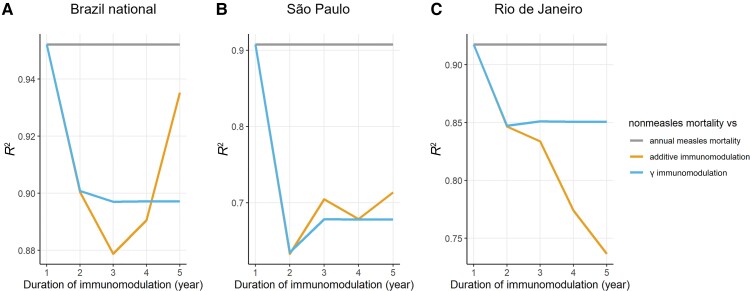
In all 3 Brazilian datasets (Brazil national, São Paulo, and Rio de Janeiro), the optimal duration of measles-induced immunomodulation was 1 year, regardless of the method of accumulating measles mortality (Figure 4). Therefore, we did not find evidence that measles had effects on other infectious disease mortality beyond 1 year.
Figure 4.
Changes of R2 with different duration of immunomodulation (1–5 years) in (A) the whole of Brazil; (B) São Paulo; and (C) Rio de Janeiro. Colors represent the different methods of accumulating measles mortality. The gray horizontal line indicates the R2 between nonmeasles mortality rate and annual measles mortality rate (ie, duration of immunomodulation = 1 year).
DISCUSSION
Taking advantage of the well-curated mortality records in Brazil, this study demonstrated a significant positive correlation between measles mortality and other infectious disease mortality in young children, even after controlling for confounding effects of time. In addition, we observed higher synchronizations between different infectious diseases when the measles virus was more prevalent, suggesting a driving effect of measles dynamics on other infections. These findings reconciled with previous reports of increased susceptibility to infections in measles patients [19, 21, 22] and that secondary infections were the main cause of measles-related childhood deaths [10, 36, 37]. Similar epidemiological correlations between measles and other infectious diseases were also reported in 46 African countries [27]. A strong correlation was expected from the immunological consequences of measles virus infection. The virus suppresses the immune system for weeks and also impairs existing immunological memories, causing long-term immune amnesia that could last for years [13–16]. Both processes predispose the host to more severe outcomes of following infections and, therefore, influence the mortality of other infectious diseases in epidemiological records. The interactions between measles and other infectious disease epidemiology can be further studied in depth using mathematical models, such as a recent study by Morales and Muñoz [38]. Such models will provide valuable information for fully understanding the contribution of measles to overall childhood infectious disease burden.
As a major public health measure to combat measles, the measles vaccine has been shown to produce strong and durable immunity and does not impair immune memories as do natural infections [1, 15, 39]. By preventing measles virus infections and hence averting immune amnesia, vaccination programs can disproportionately reduce childhood disease burdens [9, 23, 26]. In Brazil, we observed a more than 50% decrease in childhood infectious disease mortality from 1980 to 1995. Although the decrease likely resulted from multiple factors, including the improvement of the health care system and sanitation, the near eradication of measles viruses due to intensive vaccine programs likely had major contributions to this mortality decrease.
We found no evidence of measles-induced immunomodulation lasting more than 1 year in the Brazilian dataset. This finding contrasts with the study of Mina et al 2015, which showed 2–3 years of immunomodulation in the United Kingdom, United States, and Denmark [18]. This disagreement may result from Brazil’s higher infectious disease prevalence (17–43 deaths/100 000 people in Brazil vs 0–11 deaths/100 000 people in the United Kingdom, United States, and Denmark [18]). Such high prevalence could lead to many secondary infections occurring rapidly following a measles virus infection, which exposes the effects of acute immunosuppression. Furthermore, for children that survived, such secondary infections could rapidly restore the lost immunological memories [15, 18], thus negating the impacts of long-term amnesia. To test these hypotheses, future studies could explicitly model the reinfection process during immunosuppression and immune amnesia. Mortality data with finer temporal resolutions would also help deconvolute the effects of acute immunosuppression versus long-term immune amnesia. Lastly, various demographic and epidemiological factors, such as age distribution and vaccine coverage, could affect the duration of measles-induced immunomodulation.
Despite our efforts to control confounding factors, analyses presented in this study are still subject to some limitations. First of all, a more immunologically relevant correlation should be assessed between measles incidence, instead of mortality, and nonmeasles infection mortality. However, reliable incidence data were not available. Furthermore, because measles incidence surveillance may suffer from significant underreporting [40, 41], mortality may reflect the actual disease burden more accurately. A second potential issue of this study is the relatively small sample size (ie, 16 years). The time period used in this study (1980–1995) was restricted by data availability and comparability. Mortality data were not publicly available before 1980 and in 1996 the country adopted revised International Classification of Diseases (ICD) codes (from ICD-9 to ICD-10). The change in disease diagnostics prevented us from extending our analysis beyond 1995. However, since 1996, measles has caused fewer than 5 deaths in children aged 1–9 years per year nationwide, except in 1997 when Brazil experienced a resurgence of measles [31], which resulted in 10 deaths in children 1–9 years old (DATASUS). The low mortality numbers make it difficult to examine possible correlations with nonmeasles infection mortality. Overall, despite these limitations, within the time period examined in this study the results were coherent with the hypothesis that measles virus infections influence mortality rates of other infectious diseases.
This study adds to existing medical, immunological, and epidemiological research that demonstrated the unique and critical role of measles virus infection in childhood infectious disease burden and suggested the heterologous benefits of measles control [9, 13, 15, 21, 26]. These findings highlighted the importance of measles vaccination programs. In addition to public health benefits, the measles vaccine was also shown to yield substantial economic returns [42]. However, despite the substantial progress made in reducing the measles burden, the goal of global elimination is yet to be met. The ongoing COVID-19 pandemic has further interrupted routine measles vaccine programs in many countries, creating more challenges towards that goal [7, 8]. Therefore, more efforts are urgently needed to maintain measles vaccination progress and further quantify the value of measles vaccination on childhood health.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Siyang Xia, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Cricket C Gullickson, Department of Ecology and Evolutionary Biology, Princeton University, Princeton, New Jersey, USA.
C Jessica E Metcalf, Department of Ecology and Evolutionary Biology, Princeton University, Princeton, New Jersey, USA; Princeton School of Public and International Affairs, Princeton University, Princeton, New Jersey, USA.
Bryan T Grenfell, Department of Ecology and Evolutionary Biology, Princeton University, Princeton, New Jersey, USA; Princeton School of Public and International Affairs, Princeton University, Princeton, New Jersey, USA.
Michael J Mina, Department of Pathology at Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Notes
Acknowledgments. We appreciate the valuable discussion and advice from Professor Marcia C. de Castro and Professor James M. Robins regarding the Brazilian data and statistical analyses. We also thank the Mina group and the Center for Communicable Disease Dynamics at Harvard T.H. Chan School of Public Health for helpful feedback.
Financial support. This work was supported by the National Institutes of Health Directors Early Independence Award (grant number DP5OD028145 to M. J. M.) and the Bill and Melinda Gates Foundation (grant number INV-016091 to C. J. E. M.).
References
- 1. Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Primer 2016; 2:16049. [DOI] [PubMed] [Google Scholar]
- 2. Patel MK. Progress toward regional measles elimination—worldwide, 2000–2018. MMWR Morb Mortal Wkly Rep 2019; 68:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paules CI, Marston HD, Fauci AS. Measles in 2019—going backward. N Engl J Med 2019; 380:2185–7. [DOI] [PubMed] [Google Scholar]
- 4. Dimala CA, Kadia BM, Nji MAM, Bechem NN. Factors associated with measles resurgence in the United States in the post-elimination era. Sci Rep 2021; 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner L, Dong E, Khan K, Sarkar S. Persistence of US measles risk due to vaccine hesitancy and outbreaks abroad. Lancet Infect Dis 2020; 20:1114–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sbarra AN, Rolfe S, Nguyen JQ, et al. Mapping routine measles vaccination in low- and middle-income countries. Nature 2021; 589:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durrheim DN, Andrus JK, Tabassum S, Bashour H, Githanga D, Pfaff G. A dangerous measles future looms beyond the COVID-19 pandemic. Nat Med 2021; 27:360–1. [DOI] [PubMed] [Google Scholar]
- 8. Roberts L. How COVID hurt the fight against other dangerous diseases. Nature 2021; 592:502–4. [DOI] [PubMed] [Google Scholar]
- 9. Mina MJ. Measles, immune suppression and vaccination: Direct and indirect nonspecific vaccine benefits. J Infect 2017; 74:S10–7. [DOI] [PubMed] [Google Scholar]
- 10. Shanks GD, Hu Z, Waller M, et al. Measles epidemics of variable lethality in the early 20th century. Am J Epidemiol 2014; 179:413–22. [DOI] [PubMed] [Google Scholar]
- 11. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189:S4–6. [DOI] [PubMed] [Google Scholar]
- 12. Galassi FM, Varotto E, Mussini C, Cossarizza A. Measles-induced immune amnesia likely recorded in the 18th century. J Clin Virol 2021; 141:104899. [DOI] [PubMed] [Google Scholar]
- 13. Griffin DE. Measles virus-induced suppression of immune responses: Measles virus-induced immunosuppression. Immunol Rev 2010; 236:176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Vries RD, McQuaid S, van Amerongen G, et al. Measles immune suppression: Lessons from the macaque model. PLoS Pathog. 2012; 8:e1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mina MJ, Kula T, Leng Y, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019; 366:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petrova VN, Sawatsky B, Han AX, et al. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol 2019; 4:eaay6125. [DOI] [PubMed] [Google Scholar]
- 17. Laksono BM, de Vries RD, Verburgh RJ, et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun 2018; 9:4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mina MJ, Metcalf CJE, de Swart RL, Osterhaus ADME, Grenfell BT. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 2015; 348:694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aaby P, Bukh J, Kronborg D, Lisse IM, da Silva MC. Delayed excess mortality after exposure to measles during the first six months of life. Am J Epidemiol 1990; 132:211–9. [DOI] [PubMed] [Google Scholar]
- 20. Ashbaugh HR, Cherry JD, Hoff NA, et al. Association of previous measles infection with markers of acute infectious disease among 9- to 59-month-old children in the Democratic Republic of the Congo. J Pediatr Infect Dis Soc 2019; 8:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gadroen K, Dodd CN, Masclee GMC, et al. Impact and longevity of measles-associated immune suppression: a matched cohort study using data from the THIN general practice database in the U.K. BMJ Open 2018; 8:e021465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Behrens L, Cherry JD, Heininger U. The susceptibility to other infectious diseases following measles during a three year observation period in Switzerland. Pediatr Infect Dis J 2020; 39:478–82. [DOI] [PubMed] [Google Scholar]
- 23. van den Ent MMVX, Brown DW, Hoekstra EJ, Christie A, Cochi SL. Measles mortality reduction contributes substantially to reduction of all cause mortality among children less than five years of age, 1990–2008. J Infect Dis 2011; 204:S18–23. [DOI] [PubMed] [Google Scholar]
- 24. Aaby P, Bhuiya A, Nahar L, Knudsen K, de Francisco A, Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol. 2003; 32:106–15. [DOI] [PubMed] [Google Scholar]
- 25. Noori N, Rohani P. Quantifying the consequences of measles-induced immune modulation for whooping cough epidemiology. Philos Trans R Soc B Biol Sci 2019; 374:20180270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aaby P, Samb B, Simondon F, Seck AMC, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 1995; 311:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aaby P, Samb B, Andersen M, Simondon F. No long-term excess mortality after measles infection: A community study from Senegal. Am J Epidemiol 1996; 143:1035–41. [DOI] [PubMed] [Google Scholar]
- 28. Sato R, Haraguchi M. Effect of measles prevalence and vaccination coverage on other disease burden: evidence of measles immune amnesia in 46 African countries. Hum Vaccines Immunother 2021; 17:5361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akramuzzaman SM, Cutts FT, Wheeler JG, Hossain MJ. Increased childhood morbidity after measles is short-term in urban Bangladesh. Am J Epidemiol 2000; 151:723–35. [DOI] [PubMed] [Google Scholar]
- 30. Aaby P, Lisse IM, Mølbak K, Knudsen K, Whittle H. No persistent T lymphocyte immunosuppression or increased mortality after measles infection: a community study from Guinea-Bissau. Pediatr Infect Dis J 1996; 15:39–44. [DOI] [PubMed] [Google Scholar]
- 31. Gullickson C. The true burden of measles: The association between measles incidence and non-measles infectious mortality in children in Brazil, 1980–1995 [senior thesis]. Princeton University, 2015. http://arks.princeton.edu/ark:/88435/dsp019g54xk966. Accessed 12 October 2020. [Google Scholar]
- 32. Risi JB. Control of measles in Brazil. Clin Infect Dis 1983; 5:583–7. [DOI] [PubMed] [Google Scholar]
- 33. Prevots DR, Parise MS, Segatto TCV, et al. Interruption of measles transmission in Brazil, 2000–2001. J Infect Dis 2003; 187(Suppl 1):S111–20. [DOI] [PubMed] [Google Scholar]
- 34. Lindeløv JK. mcp: An R package for regression with multiple change points. OSF Preprints, 2020. https://osf.io/fzqxv/. Accessed 8 May 2021.
- 35. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/. Accessed 25 May 2021.
- 36. Beckford AP, Kaschula R, Stephen C. Factors associated with fatal cases of measles. Afr Med J 1985; 68:858–63. [PubMed] [Google Scholar]
- 37. Duke T, Mgone CS. Measles: Not just another viral exanthem. Lancet 2003; 361:763–73. [DOI] [PubMed] [Google Scholar]
- 38. Morales GB, Muñoz MA. Immune amnesia induced by measles and its effects on concurrent epidemics. J R Soc Interface 2021; 18:20210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plans-Rubió P. Vaccination coverage for routine vaccines and herd immunity levels against measles and pertussis in the world in 2019. Vaccines 2021; 9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel MK, Gacic-Dobo M, Strebel PM, et al. Progress toward regional measles elimination—worldwide, 2000–2015. Morb Mortal Wkly Rep 2016; 65:1228–33. [DOI] [PubMed] [Google Scholar]
- 41. Thompson KM. Evolution and use of dynamic transmission models for measles and rubella risk and policy analysis. Risk Anal 2016; 36:1383–403. [DOI] [PubMed] [Google Scholar]
- 42. Sim SY, Watts E, Constenla D, Brenzel L, Patenaude BN. Return on investment from immunization against 10 pathogens in 94 low- and middle-income countries, 2011–30: Study estimates return on investment from immunization programs against ten pathogens for ninety-four low- and middle-income countries from 2011 to 2030. Health Aff (Millwood) 2020; 39:1343–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.