Abstract
Plasma SARS-CoV-2 viral RNA (vRNA) levels are predictive of COVID-19 outcomes in hospitalized patients, but whether plasma vRNA reflects lower respiratory tract (LRT) vRNA levels is unclear. We compared plasma and LRT vRNA levels in serially collected samples from mechanically ventilated patients with COVID-19. LRT and plasma vRNA levels were strongly correlated at first sampling (n = 33, r = 0.83, P < 10−9) and then declined in parallel in available serial samples except in nonsurvivors who exhibited delayed vRNA clearance in LRT samples. Plasma vRNA measurement may offer a practical surrogate of LRT vRNA burden in critically ill patients, especially early after ICU admission.
Keywords: COVID-19, SARS-CoV-2, viremia, RNAemia
Lower respiratory tract (LRT) and plasma viral RNA (vRNA) levels are strongly correlated in mechanically ventilated COVID-19 patients. There is delayed LRT vRNA clearance in nonsurvivors. Plasma vRNA may offer a practical surrogate of LRT vRNA early in critical illness.
Plasma severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA (vRNA) levels (RNAemia) are significantly associated with coronavirus disease 2019 (COVID-19) severity indices and predict adverse clinical outcomes in hospitalized patients [1–6]. We and others have recently shown that RNAemia can indicate presence of virions in plasma (ie, true viremia) [1, 7], but how RNAemia relates to vRNA levels in the lower respiratory tract (LRT) has not been well defined. LRT infection by SARS-CoV-2 can lead to pneumonia and acute respiratory distress syndrome, which is the most common cause of COVID-19 mortality [8]. Viral particles are readily detectable by electron microscopy in mechanically ventilated patients with COVID-19, and vRNA levels in LRT secretions have been shown to be associated with COVID-19 outcome [9–11], but the relationship between LRT and plasma vRNA is not well defined. Levels of plasma vRNA could be an important biomarker of the extent of LRT infection. To explore this possibility, we compared plasma and LRT vRNA levels in simultaneously collected specimens, measured the temporal evolution of vRNA levels in each compartment, and examined for associations between vRNA levels and clinical outcomes.
METHODS
Study Cohort
From April 2020 through May 2021, we prospectively enrolled hospitalized patients with COVID-19 from 3 UPMC hospitals, in an observational cohort study. We included critically ill patients 18–90 years of age diagnosed with SARS-CoV-2 infection by a positive nasopharyngeal swab quantitative polymerase chain reaction (qPCR) test, who were intubated and mechanically ventilated for acute hypoxemic respiratory failure due to COVID-19 pneumonia.
All research protocols (protocols STUDY19050099 and STUDY20040036) were approved by University of Pittsburgh institutional review board and were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all research participants or their legally authorized representatives.
Clinical Data Extraction
We recorded baseline demographics, COVID-19 timelines (dates of symptom onset, SARS-CoV-2 infection diagnosis, intensive care unit [ICU] admission and intubation), severity indices at time of ICU admission (World Health Organization [WHO] 10-point ordinal scale and radiographic edema by the radiographic assessment of lung edema [RALE] score [12]), administered COVID-19–targeted therapies with plausible impact on vRNA levels (remdesivir, convalescent plasma, corticosteroids, and tocilizumab), 60-day survival, and time to liberation from mechanical ventilation (ie, successful extubation).
Experimental Analyses
We collected blood samples and endotracheal aspirates (ETA) on enrollment day (day 1, baseline), and then again on days 5 and 10 postenrollment while the subjects remained in the ICU. Blood samples were centrifuged for separation of plasma. ETA samples were inactivated by 4-fold dilution in DNA/RNA Shield (Zymo Research) under biosafety level 2+ conditions and then stored at −80°C.
We performed qRT-PCR for SARS-CoV-2 as previously described for plasma samples [1]. Briefly, we extracted total RNA from 0.5–1.0 mL of plasma or 150 µL inactivated ETA (diluted to 400 µL in phosphate buffered saline to reduce viscosity) using the MagMax Viral Pathogens Kit (Thermofisher), and performed 1-step quantitative RT-PCR of the SARS-CoV-2 N gene and human RNaseP gene, as previously described, using the following N-specific primers (forward: 5′-GTTTGGTGGACCCTCAGATT-3′, reverse: 5′-CGCAGTATTATTGGGTAAACCTTG-3′, Probe: 5′6-FAM-TAACCAGAATGGAGAACGCAGTGGG-3′BHQ1) [1].
Statistical Analyses
We performed log10-transformed vRNA level comparisons between sample types and clinical groups with nonparametric Wilcoxon tests. We examined plasma-ETA vRNA correlations with the Spearman rank method. We examined the dynamics of vRNA levels over time in linear regression models of plasma or ETA vRNA against days of sample acquisition from symptom onset, PCR diagnosis, ICU admission, and intubation. For the time-to-event outcomes of 60-day survival and time to liberation from invasive mechanical ventilation, we constructed Cox proportional hazards models with plasma or ETA vRNA as exposure, adjusted for age and time from symptom onset. We limited clinical outcome analyses to include only those samples collected within 6 days from ICU admission to account for immortal time bias (a period of follow-up during which, by design, death or the study outcome cannot occur). We analyzed temporal changes in vRNA levels using mixed linear regression models with random patient intercepts.
RESULTS
Comparisons of Plasma and ETA vRNA Levels
We included 54 subjects (median age 63 years, 63% men), who contributed a total of 96 plasma and 77 ETA samples (Supplementary Figures 1 and 2) and allowed for matched ETA-plasma comparisons of 33, 27, and 15 sample pairs on days 1, 5, and 10, respectively. Clinical characteristics in 60-day survivors (n = 28) vs nonsurvivors (n = 26) are shown in Supplementary Table 1.
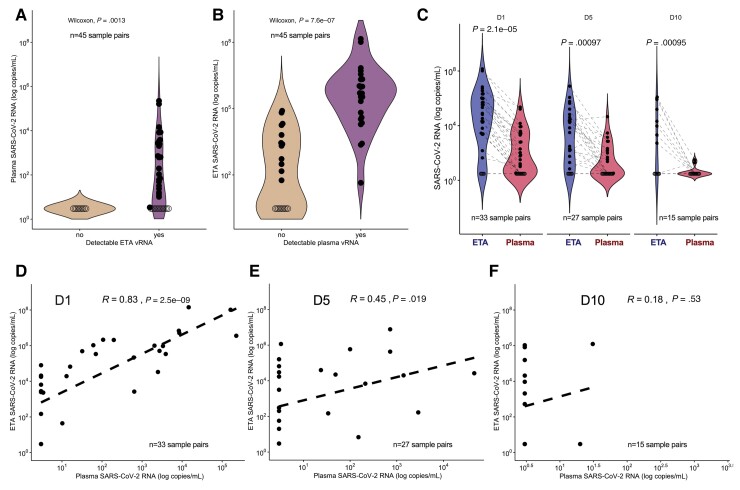
First, we compared the vRNA levels in the first available matched sample pair (ETA and plasma) postintubation from each subject (n = 45). vRNA was detectable in 24/45 (53%) plasma and 36/45 (80%) ETA samples (P value for difference in proportions = .007). The 9 samples with undetectable ETA vRNA also had undetectable plasma vRNA (Figure 1A). Conversely, subjects with undetectable plasma vRNA had significantly lower ETA vRNA levels compared to subjects with detectable plasma vRNA (P < .0001; Figure 1B).
Figure 1.
Plasma SARS-CoV-2 viral RNA levels reflect lower respiratory tract viral RNA levels. A, Plasma vRNA levels in the first available sample pair (n = 45) separated by nondetectable (left) or detectable (right) ETA vRNA. B, ETA vRNA levels in first available sample pair (n = 45) separated by nondetectable (left) or detectable (right) plasma vRNA. Comparisons in (A) and (B) were performed using Wilcoxon nonparametric test. Samples with undetectable vRNA levels are depicted with empty circles. C, ETA and plasma vRNA levels by day of sampling (days 1, 5, and 10 postenrollment). Each point represents a single sample and the dotted lines indicate paired ETA and plasma samples. Comparisons in (C) were done by Wilcoxon tests. D–F, Scatter plots of paired ETA and plasma SARS-CoV-2 RNA levels stratified by sampling day with displayed linear regression lines and Spearman rank test correlations, and P values: (D) D1, n = 33; (E) D5, n = 27; (F) D10, n = 15. Levels of vRNA were undetectable on D1 (ETA = 5/33, plasma = 12/33), D5 (ETA = 6/27, plasma = 15/27), and D10 (ETA = 7/15, plasma = 13/15). Abbreviations: D1, enrollment day 1; D5, postenrollment day 5; D10, postenrollment day 10; ETA, endotracheal aspirate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; vRNA, SARS-CoV-2 RNA.
Stratified by enrollment day, ETA samples had significantly higher median number of vRNA copies compared to matched plasma samples per mL of specimen analyzed (day 1 ratio of ETA to plasma vRNA, 635; day 5, 31; day 10, 175; all P < .01; Figure 1C). In day 1 sample pairs (n = 33), ETA and plasma vRNA were strongly correlated (Spearman r = 0.83, P < 10−9), with attenuated correlation at day 5 (r = 0.45, P = .02, n = 27), and nonsignificant correlation by day 10 (n = 15), when most plasma samples had undetectable vRNA (Figure 1D–1F).
Plasma and Lower Respiratory Tract vRNA by Time From Symptom Onset
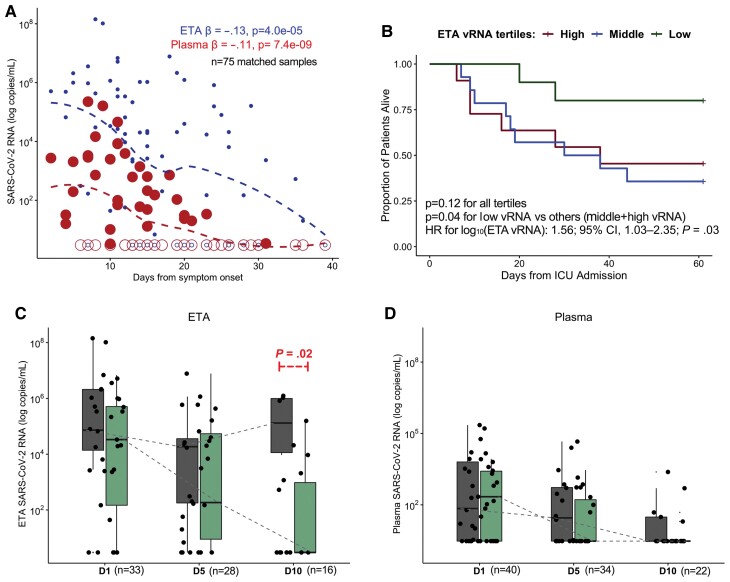
We then examined the impact of the recorded time elapsed from onset of SARS-CoV-2 clinical infection to sample acquisition on measured plasma and ETA vRNA levels, by building mixed linear regression models of vRNA against time (days) from symptom onset for all samples available. Both ETA and plasma vRNA levels significantly decreased over time (adjusted P < .001; Figure 2A), with plasma samples becoming undetectable earlier compared to ETA samples. We observed similar significant decrements in ETA and plasma vRNA levels over time when considering time periods from SARS-CoV-2 diagnosis, ICU admission, and intubation (data not shown).
Figure 2.
Plasma and lower respiratory tract vRNA levels exhibit similar temporal decline although persistently elevated vRNA levels in lower respiratory tract samples are associated with increased mortality. A, Scatterplot of reported days from COVID-19 symptom onset and viral RNA (vRNA) levels (log10 transformed) in endotracheal aspirate (small circles) and plasma (large circles) samples. The β coefficients and corresponding P values of mixed linear regression models of log10-transformed vRNA levels with random patient intercepts and adjustment for time of sample acquisition from symptom onset are shown, with displayed dashed lines from locally weighted scatterplot smoothing. Samples with undetectable vRNA levels are depicted with empty circles. B, Kaplan-Meier curves of 60-day survival by ETA vRNA tertiles among patient samples obtained within the first 6 days of ICU admission: low vRNA tertile < 4844 (n = 10); middle 4482–490 922 (n = 14); high > 490 922 copies/mL (n = 11). C and D, Box and whisker plots showing ETA and plasma vRNA levels, stratified by sampling day and comparing nonsurvivors (dark gray) and survivors (light green). Boxes represent the interquartile ranges, horizontal lines represent the medians, and vertical lines extend from minimum to maximum values. Dashed lines connect the median values for each group (survivors vs nonsurvivors) at each sampling day to allow for visual appreciation of longitudinal trends in vRNA levels. Statistical comparisons for differences in trajectories were performed with mixed linear regression models with random patient intercepts and interaction terms for survivorship sampling day. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; D1, enrollment day 1; D5, postenrollment day 5; D10, postenrollment day 10; ETA, endotracheal aspirate; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; vRNA, SARS-CoV-2 RNA.
Associations of vRNA with Outcomes
Given variability in timing from ICU admission to sample acquisition (Supplementary Table 2), for clinical outcome analyses we only included samples obtained within the first 6 days of ICU admission to mitigate immortal time bias (Supplementary Figure 1; n = 47 plasma and n = 35 ETA unique subject samples). We found no significant association of either plasma or ETA vRNA levels with cross-sectional indices of COVID-19 severity (WHO ordinal scale or radiographic edema scores). Furthermore, we found no significant association for either plasma or ETA vRNA levels with receipt of the examined COVID-19 therapies prior to sample acquisition (data not shown).
ETA vRNA levels among samples obtained within 6 days of ICU admission (early levels) were significantly associated with 60-day survival in a Cox proportional hazards model adjusted for age and time (days) of sample acquisition from symptom onset (hazard ratio [HR] = 1.56; 95% confidence interval [CI], 1.03–2.35 for log10-transformed ETA vRNA levels; P = .03). By stratifying early ETA vRNA levels in tertiles and examining 60-day survival in Kaplan-Meier curve analysis, we found that patients in the low tertile (below 4842 copies/mL) had better survival compared to patients in the middle (between 4482 and 490 922 copies/mL) and high (above 490 922 copies/mL) tertiles combined (log-rank P = .04; Figure 2B). Similarly, higher early ETA vRNA levels were predictive of longer times to liberation from mechanical ventilation among 60-day survivors in an adjusted Cox proportional hazards model (HR for successful liberation per log10-transformed vRNA levels = 0.81; 95% CI, .65–1.00; P = .05). Early plasma vRNA levels were not significantly associated with worse 60-day survival (HR = 1.18; 95% CI, .81–1.71) or longer time to liberation from mechanical ventilation (HR = 0.82; 95% CI, .63–1.18).
We then examined the vRNA level trajectories of available longitudinal samples between 60-day survivors and nonsurvivors in mixed linear regression models with interaction terms for 60-day mortality and day of sampling (Figure 2C and 2D). We detected no significant difference in vRNA level decline for survivors and nonsurvivors (interaction term P values .06 and .43 for ETA and plasma samples, respectively). However, nonsurvivors had significantly higher ETA vRNA levels in day 10 samples (P = .02; Figure 2C), signifying delayed vRNA clearance in ETA samples compared to survivors. There was no significant difference in day 10 plasma samples between survivors and nonsurvivors, as by day 10 17/22 (78%) of samples had become undetectable for vRNA.
DISCUSSION
We show that SARS-CoV-2 vRNA levels in plasma and LRT secretions are strongly correlated in patients with severe COVID-19 early after ICU admission (Figure 1D). This finding supports plasma vRNA as an indicator of lung SARS-CoV-2 infection and suggests that plasma vRNA may be a useful biomarker for LRT viral burden early in the course of ICU care. A practical blood marker of LRT viral infection is desirable to improve sample accessibility and increase standardization of interpatient sample collection because there is greater variability in the methods used to collect LRT secretions.
In the current study, we did not demonstrate a difference in clinical outcomes based on plasma vRNA even though we and several others have previously published that plasma vRNA is associated with clinical outcomes [1–6]. This divergent result is likely due to analyses restricted only to critically ill COVID-19 patients. Prior analyses showing associations of plasma vRNA with clinical outcome included subjects across a much wider spectrum of COVID-19 severity and patients earlier in their disease course [1–6]. We noted a wide range in the time interval between symptom onset and admission to the ICU in the current study (median = 7, range 2–31 days), indicating highly variable COVID-19 disease course. We show that by the time patients were admitted to the ICU and enrolled in our study, a higher proportion of plasma samples had become undetectable for vRNA compared to ETA samples (47% vs 20%, P = .007).
Consistent with prior evidence [13, 14], we showed that both LRT and plasma vRNA levels decrease over time during COVID-19 and that the 2 measures generally decline in parallel, providing further support for the use of plasma vRNA as a marker of the extent of LRT SARS-CoV-2 infection. However, we note higher levels and prolonged detection of LRT vRNA in nonsurvivors compared to survivors of ICU care (Figure 2C), highlighting the added value of monitoring LRT vRNA levels in estimating COVID-19 outcomes. Our findings also highlight the importance of adjusting for time from symptom onset in survival analyses, given the temporal variability of patient presentation and study enrollment during the clinical course of COVID-19.
The biological mechanisms that underlie the strong correlation between vRNA levels in the LRT and blood are unclear. One potential pathway for virion transit from lung to blood is disruption of the air-blood barrier due to inflammation and/or direct viral injury allowing spillover of virions from lung to bloodstream [15]. Regardless of the mechanism, transit of virions to the bloodstream may lead to extrapulmonary dissemination of infection; indeed, others have demonstrated extrapulmonary infectious virus [7].
Our analyses are limited by cohort size, inclusion of only ICU patients, and unavailability of matched ETA and plasma samples at all time points for logistical or clinical reasons (eg, some patients were intubated following enrollment). Nevertheless, the well-defined cohort of participants we studied allowed for direct, minimally invasive access to the LRT for vRNA quantification in ETA specimens, which are routinely obtained for clinical microbiology studies in COVID-19 subjects, as opposed to invasive bronchoscopic samples. We did not analyze upper respiratory tract vRNA levels because our focus was to examine LRT viral burden and its relationship with plasma vRNA levels in patients with severe disease, and also because viral shedding in severe COVID-19 is more prolonged in the lower compared to the upper respiratory tract [11]. Our findings also highlight the importance of analyzing the timing of measurement of vRNA biomarkers in relation to the clinical timeline of COVID-19, given the rapid and dynamic decline of vRNA levels in both the LRT and blood compartments.
In summary, plasma SARS-CoV-2 vRNA may serve as a useful biomarker of LRT infection in critically ill patients. Further research is necessary to confirm our findings in additional cohorts, using larger datasets to determine whether persistence of plasma viremia in critically ill patients is associated with worse clinical outcomes, and to investigate the biological mechanisms that underlie the relationship between lung and plasma viral burden.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Jana L Jacobs, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Asma Naqvi, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Faraaz A Shah, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Acute Lung Injury Center of Excellence, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Veteran’s Affairs Pittsburgh Healthcare System, Pittsburgh, Pennsylvania, USA.
Valerie F Boltz, HIV Dynamics and Replication Program, Center for Cancer Research, National Cancer Institute, Frederick, Maryland, USA.
Mary F Kearney, HIV Dynamics and Replication Program, Center for Cancer Research, National Cancer Institute, Frederick, Maryland, USA.
Bryan J McVerry, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Acute Lung Injury Center of Excellence, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Center for Medicine and the Microbiome, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Prabir Ray, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Caitlin Schaefer, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Acute Lung Injury Center of Excellence, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Meghan Fitzpatrick, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Barbara Methé, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Center for Medicine and the Microbiome, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Janet S Lee, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Acute Lung Injury Center of Excellence, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Alison Morris, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Center for Medicine and the Microbiome, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
John W Mellors, Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Georgios D Kitsios, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Acute Lung Injury Center of Excellence, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Center for Medicine and the Microbiome, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
William Bain, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Acute Lung Injury Center of Excellence, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Veteran’s Affairs Pittsburgh Healthcare System, Pittsburgh, Pennsylvania, USA.
Notes
Acknowledgments. The authors wish to thank the patients and patients’ families that have enrolled in our research studies at the University of Pittsburgh. We also thank the physicians, nurses, respiratory therapists, and other staff at the UPMC Presbyterian, Shadyside and East Hospital units for assistance with coordination of patient enrollment and collection of patient samples. We thank Heather Gentry, Cathy Kessinger, and Cynthia Klamar for assistance with patient enrollment and processing research samples.
Disclaimer. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the National Institutes of Health (NIH) and the US Department of Veterans Affairs. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute or the NIH nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial support. This work was supported by a pilot COVID-19 award from the University of Pittsburgh Clinical and Translational Science Institute; the National Center for Advancing Translational Sciences; National Heart, Lung, and Blood Institute, NIH (grant numbers K23HL139987 to G. D. K. and P01HL114453 to B. J. M., J. S. L., and P. R.); National Cancer Institute, National Institutes of Health (grant numbers 75N91019D00024 to J. W. M. and 1OT2HL156812 intramural funds to M. F. K.); US Department of Veterans Affairs Biomedical Laboratory R&D Career Development Award (grant number IK2 BX004886 to W. B.); the University of Pittsburgh Vascular Medicine Institute; the Hemophilia Center of Western Pennsylvania; and the Institute for Transfusion Medicine (to W. B.).
References
- 1. Jacobs JL, Bain W, Naqvi A, et al. Severe acute respiratory syndrome coronavirus 2 viremia is associated with coronavirus disease 2019 severity and predicts clinical outcomes. Clin Infect Dis 2022; 74:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hagman K, Hedenstierna M, Gille-Johnson P, et al. SARS-CoV-2 RNA in serum as predictor of severe outcome in COVID-19: a retrospective cohort study. Clin Infect Dis 2021; 73:e2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hogan CA, Stevens BA, Sahoo MK, et al. High frequency of SARS-CoV-2 RNAemia and association with severe disease. Clin Infect Dis 2021; 72:e291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prebensen C, Hre PLM, Jonassen C, et al. SARS-CoV-2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID-19. Clin Infect Dis 2021; 73:e799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Veyer D, Kerneis S, Poulet G, et al. Highly sensitive quantification of plasma SARS-CoV-2 RNA sheds light on its potential clinical value. Clin Infect Dis 2021; 73: e2890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Cleemput J, van Snippenberg W, Lambrechts L, et al. Organ-specific genome diversity of replication-competent SARS-CoV-2. Nat Commun 2021; 12:6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ketcham SW, Bolig TC, Molling DJ, Sjoding MW, Flanders SA, Prescott HC. Causes and circumstances of death among patients hospitalized with COVID-19: a retrospective cohort study. Ann Am Thorac Soc 2021; 18:1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bain WG, Penaloza HF, Ladinsky MS, et al. Lower respiratory tract myeloid cells harbor SARS-Cov-2 and display an inflammatory phenotype. Chest 2021; 159:963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buetti N, Wicky PH, Le Hingrat Q, et al. SARS-CoV-2 detection in the lower respiratory tract of invasively ventilated ARDS patients. Crit Care 2020; 24:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen PZ, Bobrovitz N, Premji ZA, Koopmans M, Fisman DN, Gu FX. SARS-CoV-2 shedding dynamics across the respiratory tract, sex, and disease severity for adult and pediatric COVID-19. Elife 2021; 10:e70458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotok D, Yang L, Evankovich JW, et al. The evolution of radiographic edema in ARDS and its association with clinical outcomes: a prospective cohort study in adult patients. J Crit Care 2020; 56:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regan J, Flynn JP, Rosenthal A, et al. Viral load kinetics of severe acute respiratory syndrome coronavirus 2 in hospitalized individuals with coronavirus disease 2019. Open Forum Infect Dis 2021; 8:ofab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagman K, Hedensteirna M, Rudling J, et al. Duration of SARS-CoV-2 viremia and its correlation to mortality and inflammatory parameters in patients hospitalized for COVID-19: a cohort study. Diagn Microbiol Infect Dis 2022; 102:115595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020; 20:389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.