Abstract
Background
The interplay among respiratory syncytial virus (RSV) loads, mucosal interferons (IFN), and disease severity in RSV-infected children is poorly understood.
Methods
Children <2 years of age with mild (outpatients) or severe (inpatients) RSV infection and healthy controls were enrolled, and nasopharyngeal samples obtained for RSV loads and innate cytokines quantification. Patients were stratified by age (0–6 and >6–24 months) and multivariable analyses performed to identify predictors of disease severity.
Results
In 2015–2019 we enrolled 219 RSV-infected children (78 outpatients; 141 inpatients) and 34 healthy controls. Type I, II, and III IFN concentrations were higher in children aged >6 versus 0–6 months and, like CXCL10, they were higher in outpatients than inpatients and correlated with RSV loads (P < .05). Higher IL6 concentrations increased the odds of hospitalization (odds ratio [OR], 2.30; 95% confidence interval [CI], 1.07–5.36) only in children >6 months, while higher IFN-λ2/3 concentrations had the opposite effect irrespective of age (OR, 0.38; 95% CI, .15–.86). Likewise, higher CXCL10 concentrations decreased the odds of hospitalization (OR, 0.21; 95% CI, .08–.48), oxygen administration (OR, 0.42; 95% CI, .21–.80),PICU admission (OR, 0.39; 95% CI, .20–.73), and prolonged hospitalization (OR, 0.57; 95% CI, .32–.98) irrespective of age.
Conclusions
Children with milder RSV infection and those aged >6 months had higher concentrations of mucosal IFNs, suggesting that maturation of mucosal IFN responses are associated with protection against severe RSV disease.
Keywords: RSV, cytokines, innate immune response, mucosal interferons, IFN-γ, CXCL10 (IP-10), disease severity, type III interferon
Higher mucosal interferon concentrations and RSV loads were associated with milder RSV disease in infants. Cytokine concentrations were also higher in children >6–24 vs 0–6 months, suggesting a maturational protective role of mucosal interferons on RSV disease severity.
Respiratory syncytial virus (RSV) accounts for 33 million cases of lower respiratory tract infection (LRTI), and more than 100 000 deaths annually in children <5 years of age [1]. Despite the high disease burden, therapy remains supportive and preventive strategies are exclusively approved for high-risk children [2]. The upper respiratory tract is the primary site for RSV infection. Upon infection, the respiratory mucosa maintains a balance between the interferon (IFN) antiviral response, that limits viral replication, and a proinflammatory response that can lead to enhance airway inflammation and worse clinical outcomes [3].
Studies of in vitro models and in children hospitalized with RSV LRTI have described associations between a number of innate immunity cytokines measured in the upper respiratory mucosa and disease severity with conflicting results [3–9]. Most of those studies did not comprehensively evaluate the role of mucosal type III IFNs, or the impact of age. Another important gap to help define the significance of mucosal cytokines in shaping RSV disease severity is to better define the mucosal cytokine profile of infants with mild RSV disease managed as outpatients, as most studies have been conducted in children hospitalized with severe disease. Understanding the interdependence between mucosal IFN responses, RSV loads, and the impact of age in children with mild RSV infection has important implications. On the one hand, the mucosal cytokine profile of mild disease could be used as a marker of ideal vaccine-elicited safe and protective responses, which is especially relevant to optimize the development of live attenuated vaccines designed to be administered intranasally [10]. On the other hand, from a practical perspective, this knowledge may aid with patient stratification in the clinical setting, and identification of the ideal therapeutic window for targeted therapeutic interventions.
The goal of this study was to define how the interplay between antiviral and proinflammatory mucosal cytokine responses, viral replication as defined by RSV loads, and age influences disease severity and clinical outcomes in young children with RSV infection.
METHODS
Study Design
This was a prospective, single-center, observational study that included a convenience sample of previously healthy children with RSV infection and healthy controls <2 years of age. Patients with RSV infection were enrolled at Nationwide Children’s Hospital (NCH) within a median (25%–75% interquartile range (IQR) time of 13 (5–19) hours of hospitalization, or as outpatients at NCH Primary Care Pediatric Offices, Emergency Department, and Urgent Care Clinics over 5 respiratory seasons. RSV diagnosis was established by rapid antigen testing or as part of a polymerase chain reaction (PCR) respiratory panel obtained per standard of care [8]. We also enrolled as a reference a cohort of asymptomatic, healthy, age-matched controls during well child visits or minor elective surgeries not involving the respiratory tract [11]. Exclusion criteria for RSV patients included gestational age <36 weeks, underlying medical conditions (ie, congenital heart disease, chronic lung disease, congenital or acquired immunodeficiency), concomitant bacterial infections, and systemic use of immunomodulatory drugs including systemic steroids within 2 weeks of presentation. We also excluded children with symptoms for more than 7 days, previous history of wheezing or hospitalization for RSV bronchiolitis, and RSV hospitalization for apnea or social reasons. Healthy controls were excluded if they met any of those criteria, or if they had a history of fever or respiratory symptoms within 2 weeks of enrollment (Supplementary Material).
The study was approved by the Institutional Review Board at NCH, classified as a level 1 risk clinical study—no greater than minimal risk (pursuant under 45 CFR 46.404; and 21 CFR 50.51). Informed consent was obtained from legal guardians before study participation, in compliance with Nationwide Children's Research Responsible Conduct Guidelines.
At enrollment we collected patients' demographic and clinical data using a questionnaire purposely designed for this study [8]. In parallel, we also collected a midturbinate swab for RSV quantification and typing by real-time PCR and for cytokine analysis. Disease severity was assessed using standardized clinical disease severity score (Ohio-RSV CDSS; Supplementary Table 1) that ranged from 0 (normal) to 15 (most severe disease). This CDSS has been validated in other studies [8, 11–14]. Among inpatients, we evaluated additional parameters of severity including administration and duration of supplemental oxygen, need and duration of noninvasive and invasive ventilatory support, admission to the pediatric intensive care unit (PICU), and duration of hospitalization.
Sample Collection and Processing
Briefly, midturbinate nasal swab samples (FLOQswabs; Copan Diagnostics) were introduced into a nostril and rotated once to soak up secretions. The tip of the swab was then placed in 3 mL of viral transport media (M4-VTM; Thermo Fisher Scientific), transported on ice to the laboratory, vortexed for 30 seconds within 2–4 hours of sample collection, and aliquoted into 5 vials with approximately 500 µL in each. All vials were stored at −80°C until further processing in batches.
When ready for processing, samples were thawed in a laminar flow hood at room temperature for 20 minutes, and separate aliquots from the collected specimen were used for mucosal cytokine concentrations and RSV load quantitation. None of the samples included for cytokine analyses underwent more than 1 thawing cycle. For complete protocol, see Supplementary Material.
We decided to use midturbinate swabs instead of nasal wash samples to maximize enrollment and to improve retention. Before implementing midturbinate sampling, we performed validation studies as described below. In addition, nasopharyngeal or midturbinate nasal swab samples have been successfully used in studies assessing the host mucosal immune response of children and adults with RSV and other respiratory viral infections [15–17].
RSV Loads and Viral Coinfections
RSV loads were measured by quantitative real-time PCR targeting the N gene, as described [12]. In addition, to assess for viral coinfections all samples underwent testing using the FilmArray Respiratory Viral Panel (BioFire; BioMerieux) [8].
Cytokine Analysis
We measured the concentrations of 13 cytokines, in duplicates, using a bead-based multiplex assay (LEGENDplex Human Anti-Virus Response Panel; BioLegend) following manufacturer instructions and used a BD FACS flow cytometer and FACSDiva software for cytokine analyses. This assay allowed for the simultaneous quantification of type I ([IFN-α2], [IFNβ]), type II ([IFN-γ]), and type III ([IFN-λ1], [IFN-λ2/3]) interferons, interferon-γ–induced protein 10 (CXCL10 [IP-10]), interleukins ([IL-1β], IL-6, CXCL8 [IL-8], IL-10, IL-12), (TNF-α), and (GM-CSF). The lower limit of detection of the assay ranged from 0.7 to 12.8 pg/mL [18–21]. To assess the value of midturbinate samples for cytokine measurement, validation studies were performed with paired midturbinate swab and nasal wash samples in the first 55 children enrolled (RSV inpatients, n = 34; RSV outpatients, n = 16; healthy controls, n = 5). Overall, cytokine concentrations were higher in nasal wash than in midturbinate samples and were strongly correlated (Supplementary Table 2).
Statistical Analysis
Data are reported with frequencies and percentages for categorical variables and means (SD) or medians with 25%–75% IQR for continuous variables according to data distribution. Categorical data were analyzed by χ2 or Fisher exact test, and continuous variables by Mann-Whitney or Kruskal-Wallis tests with Benjamini-Hochberg tests to adjust for multiple comparisons. Nonlinear associations between cytokine concentrations and viral loads were analyzed using polynomial and restricted cubic spline regression, and linear associations using Pearson correlation coefficient. To account for differential batch assignment in the samples included in the study, we applied the censored likelihood multiple imputation method, as described [22]. Briefly, cytokine concentrations that were below the level of quantitation were imputed according to the batch and the subject's cohort (healthy controls, RSV inpatients and outpatients) using the lodi package in R environment (version 4.1.2). This technique allowed to standardize the cytokines' lower limit of detection across different batches, generating uniform and reliable results. In addition, sensitivity analyses using nonimputed data were performed.
To determine which factors were independently associated with worse clinical outcomes, defined as need for hospitalization, oxygen administration, PICU admission, and duration of hospitalization, we used multivariable logistic regression with Firth penalized likelihood, when warranted, to avoid small sample size bias. Covariates were included in the models if they were clinically relevant or had univariate P values of <.15, and were retained in the models if they had an adjusted P value of <.1, or if their inclusion had a substantial impact on models' goodness of fit based on Akaike information criterion [23]. These covariates included age (0–6 and >6–24 months), viral loads, viral coinfections, and cytokine concentrations that underwent log10 transformation. Analysis plan, including definitions of disease severity and age categories, were defined a priori. Analyses were conducted using R for statistical computing, and GraphPad Prism version 9 with a 2-sided P value <.05 considered statistically significant.
RESULTS
Characteristics of Study Population
From February 2015 to April 2019, we enrolled a convenience sample of 219 previously healthy children <2 years of age with RSV infection and 34 healthy controls. Among children with RSV infection, 78 (36%) were diagnosed with mild disease in the outpatient setting, and 141 (64%) required hospitalization for RSV LRTI (inpatients). Median age was 6.0 (IQR, 3.4–10.4) months for outpatients, 3.1 (IQR, 1.5–7.9) months for inpatients, and 4.9 (IQR, 3.0–7.2) months for healthy controls. The demographic and clinical characteristics of the study cohorts are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics of RSV Patients and Healthy Controls
| Characteristic | Healthy Controls (n = 34) |
All RSV Patients (n = 219) |
RSV Outpatients (n = 78) |
RSV Inpatients (n = 141) |
P Valuea | P Valueb |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, mo | 4.9 (3.0–7.2) | 4.1 (1.8–8.6) | 6.0 (3.4–10.4) | 3.1 (1.5–7.9) | .38 | <.01 |
| Female, sex | 6 (17.6) | 107 (48.9) | 37 (47.4) | 70 (49.6) | <.01 | .86 |
| Race | .32 | <.01 | ||||
| White | 26 (76.5) | 144 (65.7) | 42 (53.8) | 102 (72.3) | ||
| Black | 3 (8.8) | 42 (19.2) | 26 (33.3) | 16 (11.3) | ||
| Other | 5 (14.7) | 33 (15.1) | 10 (12.8) | 23 (16.3) | ||
| Breastfedc | 18 (52.9) | 91 (41.5) | 41 (52.6) | 50 (35.5) | .26 | .02 |
| Delivery type, vaginal | 22 (64.7) | 157 (71.7) | 59 (75.6) | 98 (69.5) | .4 | .35 |
| Daycare attendance | 6 (17.6) | 76 (34.7) | 28 (35.9) | 48 (34.0) | .06 | .88 |
| Smoke exposure | 6 (17.6) | 60 (27.4) | 19 (24.0) | 42 (29.8) | .29 | .34 |
| Vaccines up to date | 31 (91.2) | 194 (88.6) | 69 (88.5) | 125 (88.6) | >.99 | >.99 |
| Family history of asthma | 10 (29.4) | 134 (61.2) | 44 (56.4) | 90 (63.8) | <.01 | .31 |
| Clinical parameters | ||||||
| Days of symptoms | … | 4 (3–5) | 4 (3–5) | 4 (3–5) | … | .15 |
| Ohio RSV CDSS | … | 5 (3–7) | 3 (2–4) | 8 (6–10) | … | <.001 |
| Steroids use | … | 35 (15.9) | 11 (14.1) | 24 (17.0) | … | .70 |
| Virology data | ||||||
| RSV type | .04 | |||||
| RSV-A | … | 113 (51.6) | 33 (42.3) | 80 (56.7) | … | |
| RSV-B | … | 106 (48.4) | 45 (57.7) | 61 (43.2) | … | |
| RSV load, log10 copies/mL | … | 7.7 (6.7–8.3) | 7.91 (±1.12) | 7.24 (±1.24) | … | <.001 |
| Viral coinfectionsd | 13 (52.0) | 66 (30.1) | 20 (25.6) | 46 (32.6) | .28 | .35 |
| Rhinovirus/enterovirus | 10 | 28 | 9 | 19 | … | |
| hCoVs, non-SARS-CoV-2 | 0 | 17 | 5 | 12 | … | |
| Adenovirus | 0 | 7 | 1 | 6 | … | |
| Parainfluenza virus | 0 | 2 | 1 | 1 | … | |
| Influenza virus | 0 | 2 | 0 | 2 | … | |
| hMPV | 0 | 1 | 1 | 0 | … | |
| Bocavirus | 0 | 1 | 1 | 0 | … | |
| >1 respiratory viruse | 3 | 8 | 2 | 6 | … |
Categorical data expressed as frequencies (%) and analyzed using Fisher or χ2 test. All continuous variables except RSV loads are expressed as medians (interquartile range) and analyzed using Mann-Whitney rank test. RSV loads expressed in means ± SD.
Abbreviations: CDSS, clinical disease severity score; hCoV, human coronaviruses; hMPV, human metapneumovirus; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Healthy controls versus all RSV patients.
RSV inpatients versus outpatients.
Breastfed at any time since birth.
Film array panel performed in all RSV patients, and 25 of 34 healthy controls (73%). No coinfections with Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae were identified.
For >1 respiratory virus combinations included rhinovirus/enterovirus and hCoV or adenovirus in 4 RSV patients and 2 controls, or hCoV and parainfluenza virus or adenovirus in 4 RSV patients and 1 control.
Median duration of symptoms at enrollment was 4 (IQR, 3–5) days for RSV outpatients and inpatients, and as expected the CDSS was higher in inpatients. Overall, rates of RSV A and RSV B infections were similar (51.6% vs 48.4%), with a slightly higher proportion of RSV B infections in outpatients than inpatients (58% vs 43%; P = .04). RSV loads were higher in outpatients (7.91 [SD 1.12] log10 copies/mL) than in inpatients (7.24 [SD 1.24] log10 copies/mL; P < .001).
Other respiratory viruses were identified in 30% of RSV patients and at similar rates in outpatients (26%) and inpatients (33%). Rhinoviruses followed by seasonal coronaviruses were the most commonly codetected viruses.
Mucosal Cytokine Concentrations in Children With RSV Infection and Healthy Controls
First, we analyzed whether cytokine concentrations differed between children with RSV infection and healthy controls. Of all cytokines evaluated, GM-CSF and IL-12 concentrations were below the limit of detection in most RSV patients and controls and were not included in further analyses.
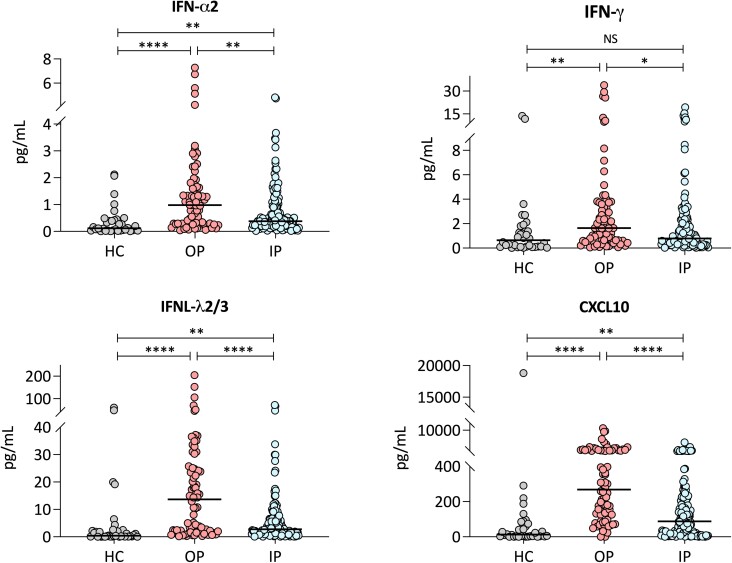
Concentrations of FN-α2, IFN-β, CXCL10, IFN-λ1, IFN-λ2/3, IL-1-β, TNF-α, IL-6, CXCL8, and IL-10 were significantly higher in RSV patients compared with controls after adjusting for multiple comparisons (Table 2). Further analyses according to disease severity showed that concentrations of type I (IFN-α2), type II (IFN-γ), type III (IFNλ1; IIFN-λ2/3) IFNs, and CXCL10 were significantly higher in outpatients with mild RSV infection than among inpatients with severe RSV disease (Figure 1).
Table 2.
Cytokine Concentrations in Children With RSV Infection and Healthy Controls
| Cytokine | Healthy Controls (n = 34) |
All RSV Patients (n = 219) |
RSV Outpatients (n = 78) |
RSV Inpatients (n = 141) |
P Valuea | P Valueb |
|---|---|---|---|---|---|---|
| IFN-α2 | 0.1 (0.03–0.43) | 0.5 (0.2–1.3) | 0.9 (0.3–1.7) | 0.4 (0.2–0.9) | <.001 | <.001* |
| IFN-β | 0.2 (0.03–1.1) | 1.5 (0.4–5.5) | 2.8 (0.6–6.9) | 1.1 (0.4–5.1) | <.001 | <.001 |
| CXCL10 | 12.9 (2.9–77.4) | 136.6 (36.4–328.0) | 268.6 (118.5–825.6) | 87.6 (15.4–221.2) | <.001 | <.001* |
| IFN-γ | 0.6 (0.1–1.5) | 0.9 (0.4–2.4) | 1.6 (0.6–3.5) | 0.8 (0.4–2.1) | .154 | .011* |
| IFN-λ1 | 2.2 (0.3–4.0) | 4.0 (1.6–10.6) | 5.2 (2.5–14.2) | 3.6 (1.3–9.2) | .022 | .004* |
| IFN-λ2/3 | 0.39 (0.08–2.3) | 3.5 (1.3–12.3) | 13.7 (2.3–24.9) | 2.8 (1.1–6.4) | <.001 | <.001* |
| IL-1β | 2.1 (0.3–4.1) | 11.6 (3.5–54.3) | 13.4 (5.9–49.9) | 10.4 (2.4–67.4) | <.001 | <.001 |
| TNF-α | 1.4 (0.1–3.9) | 4.4 (1.3–18.9) | 5.6 (2.1–18.6) | 3.5 (0.8–21.1) | <.001 | <.001 |
| IL-6 | 1.2 (0.2–2.3) | 15.4 (4.2–55.3) | 21.6 (5.3–53.7) | 13.8 (3.6–59.5) | <.001 | <.001 |
| CXCL8 | 414.1 (208.9–777.9) | 1471.0 (722.6–3011.0) | 1431 (669.5–3114) | 1484 (747.7–3101) | <.001 | <.001 |
| IL-10 | 0.4 (0.1–1.3) | 1.0 (0.4–2.6) | 1.5 (0.7–2.8) | 0.7 (0.3–2.2) | .008 | <.001* |
Cytokine concentrations (pg/mL) are expressed as medians (25%–75% interquartile range). The asterisk indicates significant differences between inpatients and outpatients by post hoc analyses using Dunn multiple comparison test. The Bonferroni correction was applied to adjust for multiple test comparisons within the 11 cytokines analyzed.
Abbreviations: IFN, interferon; IL, interleukin; RSV, respiratory syncytial virus; TNF, tumor necrosis factor.
P value for comparisons between healthy controls and all RSV patients by Mann-Whitney U test.
P value for comparison between healthy controls, RSV inpatients, and RSV outpatients by Kruskal-Wallis rank test.
Figure 1.
Mucosal cytokine concentrations in RSV patients and healthy controls. Concentrations of IFN-α2, IFN-γ, CXCL10, and IFN-λ2/3 in healthy controls (n = 34), RSV outpatients (n = 78), and RSV inpatients (n = 141). Y-axis represents cytokine concentrations in pg/mL and the X-axis the 3 study groups (healthy controls (HC), RSV outpatients (OP), and RSV inpatients (IP)). Analyses by Kruskal-Wallis and Dunn test for multiple test corrections. Number of asterisks indicate the strength of the P value: * < .01; ** < .001; **** < .0001. Abbreviations: NS, not significant; RSV, respiratory syncytial virus.
Cytokine Concentrations in Children With RSV Infection According to Age and Viral Loads
Next, we compared cytokine concentrations according to age. Within the RSV cohort, 140 (64%) infants were 0–6 months and 79 (36%) children >6–24 months of age. Of all cytokines analyzed, concentrations of IFN-α, IFN-γ, mucosal interferons (IFN-λ2/3), as well as TNF-α, IL-1β, CXCL8, and IL-10 were significantly higher in RSV infants >6 months of age compared with the 0–6 months age group (Supplementary Table 3).
Differences in concentrations of these cytokines in children younger than and older than 6 months also differed according to disease severity (Table 3). CXCL10 concentrations were consistently higher in RSV outpatients (mild disease) versus inpatients (severe disease) in both age groups. Similarly, IFN-λ2/3 concentrations were higher in RSV outpatients, both younger and older than 6 months, with differences that reached statistical significance only in the youngest age group. IL-10 concentrations were significantly higher in outpatients 0–6 months of age.
Table 3.
Cytokine Concentrations in Children With RSV Infection Stratified by Age and Severity
| Cytokine | Age 0–6 mo | Age >6 mo | ||||
|---|---|---|---|---|---|---|
| OP n = 41 |
IP n = 99 |
P Value | OP n = 37 |
IP n = 42 |
P Value | |
| IFN-α2 | 0.6 (0.3–1.6) | 0.3 (0.2–0.6) | .07 | 1.1 (0.3–1.7) | 0.9 (0.2–1.7) | >.99 |
| IFN-β | 3.4 (0.3–7.7) | 0.9 (0.3–3.8) | .76 | 2.4 (0.9–5.6) | 2.9 (0.7–6.4) | >.99 |
| CXCL0 | 278.1 (131.0–690.6) | 83.7 (11.4–223.1) | <.01 | 259 (91.1–1324.0) | 96.3 (20.9–175.3) | <.01 |
| IFN-γ | 1.2 (0.6–3.0) | 0.7 (0.3–1.4) | .15 | 1.8 (0.6–3.8) | 1.6 (0.6–3.5) | >.99 |
| IFN-λ1 | 4.0 (1.8–12.9) | 3.7 (1.4–8.9) | >.99 | 7.2 (2.9–21.6) | 3.8 (1.2–9.7) | .45 |
| IFN-λ2/3 | 10.4 (1.7–22.7) | 2.6 (1.0–5.9) | <.01 | 15.8 (3.1–31.9) | 3.2 (1.3–12.2) | .06 |
| IL-1β | 13.2 (4.8–32.9) | 6.1 (1.4–53.4) | >.99 | 13.6 (6.2–62.7) | 16.5 (7.2–105.7) | >.99 |
| TNF-α | 4.8 (1.6–12.4) | 2.1 (0.6–15.1) | .43 | 8.7 (2.7–35.2) | 9.0 (2.5–77.7) | >.99 |
| IL-6 | 27.7 (3.7–51.4) | 12.1 (3.1–50.8) | >.99 | 16.7 (5.3–55.7) | 22.6 (6.6–66.5) | >.99 |
| CXCL8 | 1212 (662.4–1964) | 1226 (613.3–2249) | >.99 | 2301 (671.6–3946) | 2060 (1233–4879) | >.99 |
| IL-10 | 1.7 (0.8–3.6) | 0.6 (0.3–1.6) | .02 | 1.5 (0.7–2.5) | 1.3 (0.4–4.6) | >.99 |
Continuous data are expressed as median (interquartile range) and analyzed using Mann-Whitney rank test. Values in bold indicate significant 2-sided P values. Bonferroni correction was applied to adjust for multiple test comparisons.
Abbreviations: IFN, interferon; IL, interleukin; IP, inpatient (severe RSV infection); OP, outpatient (mild RSV infection); RSV, respiratory syncytial virus; TNF, tumor necrosis factor.
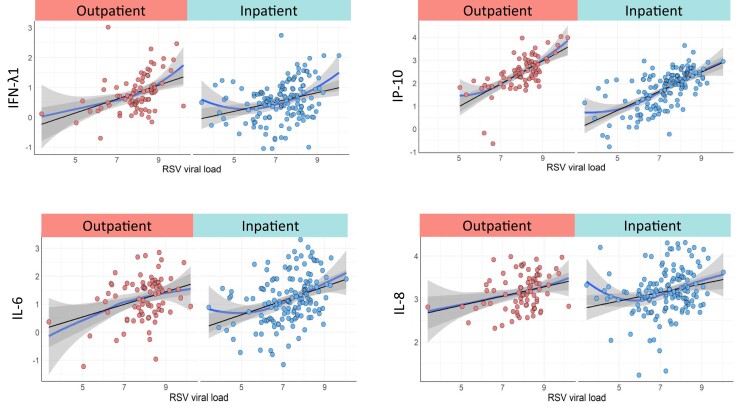
We also assessed the associations between RSV loads and cytokine concentrations. Using polynomial regression and smoothed scattered plot curves (LOESS curves) we found significant direct linear associations between RSV loads and CXCL10, IFN-λ1, IL-6, CXCL8, and IL-10 in RSV outpatients and inpatients (Figure 2 and Supplementary Table 4).
Figure 2.
Association between RSV loads and cytokine concentrations in RSV patients. LOESS curves for CXCL10(IP-10), IFN-λ1, IL-6, and CXCL8 in RSV outpatients (n = 78), and RSV inpatients (n = 141). The Y-axis represents cytokine concentrations in pg/mL and the X-axis RSV load in log10 copies/mL in the 2 study groups (RSV outpatients in red and RSV inpatients in blue). Analyses by polynomial and restricted cubic spline regression with the linear regression line included as a reference. Abbreviations: IFN, interferon; IL, interleukin; RSV, respiratory syncytial virus.
Adjusted Risk Factors for Severe RSV Disease
Lastly, we constructed different multivariable models to identify which cytokines were predictive of disease severity after adjusting for age, viral loads, and viral coinfections. Disease severity was defined by the need for hospitalization in all children with RSV infection, and among hospitalized children by the administration of supplemental oxygen, need for PICU, and prolonged hospital stay, which was defined as >62 hours (2.6 days) based on the median duration of hospitalization of the complete inpatient cohort.
To assess for the odds for hospitalization, children were stratified according to age as 0–6 months and >6–24 months (Table 4). Adjusted for the covariates mentioned above, higher concentrations of CXCL10 and IFN-λ2/3 were independently associated with reduced odds of hospitalization in children 0–6 months and >6 months of age. On the other hand, higher IL-6 concentrations were significantly associated with increased odds of hospitalization only in children >6 months of age. Among inpatients, models were also adjusted for age, viral loads, and viral coinfections, and constructed separately for each cytokine to avoid overfitting. Of all cytokines analyzed, only higher concentrations of CXCL10 were significantly associated with reduced odds of supplemental oxygen administration, reduced odds of PICU admission, and reduced odds of prolonged hospitalization (Table 5). Sensitivity analyses using nonimputed data showed similar results (Supplementary Tables 5 and 6).
Table 4.
Multivariable Analyses of Factors Associated With the Need for Hospitalization in Children With RSV Infection
| Factor | Age 0–6 mo | Age >6 mo | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| RSV load, log10 copies/mL | 1.01 (.63–1.58) | .98 | 0.67 (.33–1.24) | .22 |
| IFN-α2 | 0.43 (.16–1.13) | .09 | 0.77 (.26–2.20) | .63 |
| CXCL10 | 0.21 (.08–.48) | <.01 | 0.30 (.10–.82) | .02 |
| IFN-λ2/3 | 0.44 (.19–.95) | .04 | 0.38 (.15–.86) | .03 |
| IL-6 | 1.59 (.91–2.87) | .11 | 2.30 (1.07–5.36) | .04 |
Adjusted odds of hospitalization among patients in the study stratified by age. Bolded values indicate significant 2-sided P values (P < .05).
Abbreviations: CI, confidence interval; IFN, interferon; IL, interleukin; OR, odds ratio; RSV, respiratory syncytial virus.
Table 5.
Multivariable Analyses of Factors Associated With Clinical Outcomes in Hospitalized Children With RSV Infection
| Factor | Supplemental O2 | PICU | Prolonged Hospitalization | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age >6 mo | 1.06 (.99–1.16) | .121 | 0.57 (.22–1.38) | .228 | 0.83 (.39–1.75) | .625 |
| RSV load, log10 copies/mL | 1.08 (.71–1.62) | .702 | 1.3 (.87–2.04) | .220 | 1.34 (.94–1.96) | .113 |
| Viral coinfections | 1.57 (.67–3.86) | .311 | 1.92 (.84–4.35) | .117 | 1.46 (.70–3.10) | .315 |
| CXCL10 | 0.42 (.21–.80) | .011 | 0.39 (.20–.73) | .004 | 0.57 (.32–.98) | .047 |
Prolonged hospitalization was defined as length of stay >62 hours based on median duration of hospitalization. Bolded values indicate significant 2-sided P values (P < .05).
Abbreviations: CI, confidence interval; OR, odds ratio; O2, oxygen; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus.
DISCUSSION
The role of mucosal type III IFNs in shaping disease severity in infants and young children with RSV infection has not been comprehensively evaluated. Nor has a careful assessment been made of the role of mucosal antiviral and inflammatory responses in children with mild RSV infection managed as outpatients, and whether those responses vary according to age. In this prospective study we showed that young children with mild RSV infection had significantly higher mucosal concentrations of type I, II, and more importantly of type III IFNs compared with children hospitalized with severe disease. In addition, we found that the concentrations of these cytokines were significantly higher in children >6 months of age and correlated with RSV loads. Of all cytokines measured, multivariable analyses showed that CXCL10 was consistently associated with improved clinical outcomes across age groups. Mucosal interferons (IFN-λ2/3) also played a protective role, being associated with decreased odds of hospitalization in younger and older infants.
The identification of biomarkers predictive of RSV disease severity has remained a topic of interest due to the high burden of disease and limited preventive and therapeutic strategies available. Because the primary site of RSV infection is the respiratory mucosa, there has been great interest in improving our understanding of the antiviral and proinflammatory mucosal immune response elicited by RSV [5–7, 24–29]. While studies have evaluated the role of various cytokines, including IFN-γ, IL1-β, IL-6, CXCL8, CXCL10, IL-10, or TNF-α, on RSV disease severity [30], the role of type III interferons, comprising IFN-λ1–4 [31, 32], is not completely defined.
Type III IFNs are relatively newer cytokines that play a fundamental role in mucosal immunity, promoting an antiviral state in mucosal barriers with less collateral damage than type I IFNs [33]. Studies in animal models described increased viral loads of RSV, influenza, human metapneumovirus (hMPV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-1) in mice lacking the interferon-λ receptor IL28Raα [34], while exogenous treatment with IFN-λ1 in primary human nasal epithelial cell cultures resulted in reduced RSV replication [31, 35]. Data in children with RSV infection is limited. A study conducted in infants hospitalized with RSV infection showed increased IFN-λ1 and IFN-λ3 mRNA expression in nasal wash samples compared with those with rhinovirus infection. In addition, higher IFN-λ1 mRNA expression was associated with tachypnea and a higher disease severity index only in infants with RSV infection [24]. In contrast, we found significantly higher concentrations of IFN-λ1 and IFN-λ2/3 in outpatients with mild RSV infection compared with inpatients with severe RSV disease. These differences may have been related to differences in study design, as the aforementioned study included exclusively children hospitalized with severe RSV infection, and lambda IFNs were measured by gene expression rather than at the protein level.
Two studies conducted in children <24 months of age with RSV bronchiolitis found that increased concentrations of IFN-γ and CXCL10 in nasal wash samples were associated with decreased risk of hospitalization [26, 27]. In agreement with those studies, we found that higher CXCL10 concentrations were protective in terms of hospitalization, but also associated with decreased odds of oxygen administration, need for PICU, and shorter duration of hospitalization. Secretion of CXCL10 is stimulated by type I, II, and III IFNs, and appears to promote dendritic cell maturation, T-cell stimulation, and IL-12 secretion that overall favors viral clearance of infected respiratory epithelial cells [36, 37]. The dynamic and functions of CXCL10 are nevertheless complex, as this cytokine can also promote a proinflammatory and profibrotic state [38]. Different studies have shown that high IL1-β, CXCL8, and TNF-α concentrations in nasal wash and bronchoalveolar lavage samples in children with RSV infection were associated with worse clinical outcomes [3, 25, 28, 29, 39–42]. In the present study, concentrations of IL1-β, TNF-α, CXCL8, and IL-6 were significantly higher in children with RSV infection compared with healthy controls; however, only in older children higher IL-6 concentrations were independently associated with increased risk of hospitalization.
We observed that RSV loads were significantly higher among outpatients with mild disease than in infants hospitalized with severe RSV infection. In addition, we found that RSV loads were directly correlated with CXCL10, IFN-λ1, IL-6, CXCL8, and IL-10 concentrations. These observations conflict with earlier studies that reported higher viral loads and an exaggerated immune response associated with severe RSV disease [9, 43, 44]. There has been, however, recent cumulative evidence suggesting that higher viral loads early in RSV infection coupled with a robust antiviral interferon response may lead to more favorable clinical outcomes in children with RSV infection [8, 12, 27, 29, 45]. Overall, these results suggest that clinical outcomes in RSV infection depend on the interplay between RSV replication and an adequate balance between the interferon and proinflammatory responses and that those responses are influenced by age.
Our study has limitations. We analyzed innate immunity cytokines in the nasal mucosa, which may not reflect the innate immune response occurring in the lower respiratory tract. However, we and others have shown in previous studies the value of upper respiratory tract samples as a surrogate of lower respiratory tract disease [3, 5, 26, 39, 40, 46]. In addition, the primary objective of our study was to understand the mucosal innate immune response at the initial site of infection and to potentially identify factors that may confer protection against severe RSV disease. Additionally, nasal mucosa sampling is a convenient, noninvasive procedure that will facilitate its implementation in large multicenter clinical trials. We did not perform sequential measurements of cytokine concentrations to assess the dynamics of mucosal innate immune responses. Nonetheless duration of symptoms at enrollment was comparable between children with mild and severe disease, and we limited study participation to children with up to 7 days of illness. In addition, a recent study that analyzed sequential samples in the first 7 days of symptoms of children hospitalized with RSV infection showed that the greatest differences in the concentrations of most mediators, particularly CXCL10 and IFN-γ, were observed in the first 7 days of illness [29]. As previously mentioned, children in the RSV inpatient cohort were younger than outpatients, and this may be an intrinsic limitation of studying RSV disease, given that younger age is a known risk factor for more severe illness. We performed age-matched analyses between inpatients and outpatients to confirm the consistency of our observations, and age was included as a covariate in all multivariable models.
In summary, we were able to consistently measure and define the protective role of mucosal type III interferons and other antiviral cytokines in a large cohort of infants with RSV infection. We found that those responses were influenced by age, correlated with viral loads, and demonstrated significant associations with clinical outcomes. This novel information will help with the development and evaluation of RSV live attenuated vaccines designed to be administered via the intranasal route [10]. In addition, these findings may aid patient stratification in the clinical setting and could facilitate the clinical evaluation of therapeutic interventions for RSV infections in children.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jeanette Taveras, Department of Pediatrics, Division of Infectious Diseases, Nationwide Children's Hospital and The Ohio State College of Medicine, Columbus, Ohio, USA.
Cristina Garcia-Maurino, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Melissa Moore-Clingenpeel, Biostatistics Core, Abigail Wexner Research Institute at Nationwide Children's Hospital, Columbus, Ohio, USA.
Zhaohui Xu, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Sara Mertz, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Fang Ye, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Phyl Chen, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Shira H Cohen, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Daniel Cohen, Department of Pediatrics, Division of Emergency Medicine at Nationwide Children's Hospital and The Ohio State University College of Medicine, Columbus, Ohio, USA.
Mark E Peeples, Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Octavio Ramilo, Department of Pediatrics, Division of Infectious Diseases, Nationwide Children's Hospital and The Ohio State College of Medicine, Columbus, Ohio, USA; Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Asuncion Mejias, Department of Pediatrics, Division of Infectious Diseases, Nationwide Children's Hospital and The Ohio State College of Medicine, Columbus, Ohio, USA; Center for Vaccines and Immunity, Abigail Wexner Research Institute at Nationwide Children's Hospital, The Ohio State College of Medicine, Columbus, Ohio, USA.
Notes
Acknowledgments . We thank all members of the clinical research team at Nationwide Children’s Hospital for their extraordinary efforts to help enrolling our patients, and especially our patients and their families for their participation in the study.
Disclaimer . Funding agencies had no input in data analyses and interpretation of the study findings.
Financial support. This work was supported by the National Institutes of Health (NIH; grant number AI112524 to A. M., O. R., and M. E. P.); Janssen and the generosity of the Rusch and Ireland family (to A. M. and O. R.). M. M. C. at The Abigail Wexner Research Institute at Nationwide Children’s Hospital is supported in part by the NIH (grant number UL1TR002733).
References
- 1. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399:2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taveras J, Ramilo O, Mejias A. Preventive strategies for respiratory syncytial virus infection in young infants. Neoreviews 2020; 21:e535–45. [DOI] [PubMed] [Google Scholar]
- 3. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30:481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glaser L, Coulter PJ, Shields M, Touzelet O, Power UF, Broadbent L. Airway epithelial derived cytokines and chemokines and their role in the immune response to respiratory syncytial virus infection. Pathogens 2019; 8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheeran P, Jafri H, Carubelli C, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J 1999; 18:115–22. [DOI] [PubMed] [Google Scholar]
- 6. Bennett BL, Garofalo RP, Cron SG, et al. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis 2007; 195:1532–40. [DOI] [PubMed] [Google Scholar]
- 7. Garcia C, Soriano-Fallas A, Lozano J, et al. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis J 2012; 31:86–9. [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Maurino C, Moore-Clingenpeel M, Thomas J, et al. Viral load dynamics and clinical disease severity in infants with respiratory syncytial virus infection. J Infect Dis 2019; 219:1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis 2015; 211:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karron RA, Atwell JE, McFarland EJ, et al. Live-attenuated vaccines prevent respiratory syncytial virus-associated illness in young children. Am J Respir Crit Care Med 2021; 203:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinonen S, Velazquez VM, Ye F, et al. Immune profiles provide insights into respiratory syncytial virus disease severity in young children. Sci Transl Med 2020; 12:eaaw0268. [DOI] [PubMed] [Google Scholar]
- 12. Brenes-Chacon H, Garcia-Maurino C, Moore-Clingenpeel M, et al. Age-dependent interactions among clinical characteristics, viral loads and disease severity in young children with respiratory syncytial virus infection. Pediatr Infect Dis J 2021; 40:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mejias A, Brenes-Chacon H, Garcia-Maurino C, Moore-Clingenpeel M, Ramilo O. Clinical disease severity scores and viral loads in children with RSV infection. Clin Infect Dis 2021; 72:e1160–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haddadin Z, Beveridge S, Fernandez K, et al. Respiratory syncytial virus disease severity in young children. Clin Infect Dis 2021; 73:e4384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu X, Lakerveld AJ, Imholz S, et al. Antibody and local cytokine response to respiratory syncytial virus infection in community-dwelling older adults. mSphere 2020; 5:e00577-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopez SMC, Shaikh N, Johnson M, Liu H, Martin JM, Williams JV. Viral coinfection and nasal cytokines in children with clinically diagnosed acute sinusitis. Front Pediatr 2022; 9:783665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel JA, Nair S, Revai K, Grady J, Chonmaitree T. Nasopharyngeal acute phase cytokines in viral upper respiratory infection: impact on acute otitis media in children. Pediatr Infect Dis J 2009; 28:1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehmann JS, Rughwani P, Kolenovic M, Ji S, Sun B. LEGENDplex: bead-assisted multiplex cytokine profiling by flow cytometry. Methods Enzymol 2019; 629:151–76. [DOI] [PubMed] [Google Scholar]
- 19. Zhao H, Dong N, Liu T, et al. Clinical significance of serum type III interferons in patients with gastric cancer. J Interferon Cytokine Res 2019; 39:155–63. [DOI] [PubMed] [Google Scholar]
- 20. d’Alessandro M, Bergantini L, Cameli P, et al. BAL and serum multiplex lipid profiling in idiopathic pulmonary fibrosis and fibrotic hypersensitivity pneumonitis. Life Sci 2020; 256:117995. [DOI] [PubMed] [Google Scholar]
- 21. Johnston AW, Routh JC, Purves JT, Wiener JS, Sinani A, Holl EK. Immune expression in children with vesicoureteral reflux: a pilot study. Urology 2021; 148:254–9. [DOI] [PubMed] [Google Scholar]
- 22. Boss J, Mukherjee B, Ferguson KK, et al. Estimating outcome-exposure associations when exposure biomarker detection limits vary across batches. Epidemiology 2019; 30:746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001; 57:120–5. [DOI] [PubMed] [Google Scholar]
- 24. Selvaggi C, Pierangeli A, Fabiani M, et al. Interferon lambda 1–3 expression in infants hospitalized for RSV or HRV associated bronchiolitis. J Infect 2014; 68:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNamara PS, Flanagan BF, Selby AM, Hart CA, Smyth RL. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur Respir J 2004; 23:106–12. [DOI] [PubMed] [Google Scholar]
- 26. Nicholson EG, Schlegel C, Garofalo RP, et al. Robust cytokine and chemokine response in nasopharyngeal secretions: association with decreased severity in children with physician diagnosed bronchiolitis. J Infect Dis 2016; 214:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piedra FA, Mei M, Avadhanula V, et al. The interdependencies of viral load, the innate immune response, and clinical outcome in children presenting to the emergency department with respiratory syncytial virus-associated bronchiolitis. PLoS One 2017; 12:e0172953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabarani CM, Bonville CA, Suryadevara M, et al. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J 2013; 32:e437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thwaites RS, Coates M, Ito K, et al. Reduced nasal viral load and IFN responses in infants with respiratory syncytial virus bronchiolitis and respiratory failure. Am J Respir Crit Care Med 2018; 198:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bont L, Heijnen CJ, Kavelaars A, et al. Local interferon-γ levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 2001; 184:355–8. [DOI] [PubMed] [Google Scholar]
- 31. Okabayashi T, Kojima T, Masaki T, et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res 2011; 160:360–6. [DOI] [PubMed] [Google Scholar]
- 32. Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. Plos Pathog 2008; 4:e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity 2019; 50:907–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mordstein M, Neugebauer E, Ditt V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol 2010; 84:5670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hillyer P, Mane VP, Chen A, et al. Respiratory syncytial virus infection induces a subset of types I and III interferons in human dendritic cells. Virology 2017; 504:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krathwohl MD, Anderson JL. Chemokine CXCL10 (IP-10) is sufficient to trigger an immune response to injected antigens in a mouse model. Vaccine 2006; 24:2987–93. [DOI] [PubMed] [Google Scholar]
- 37. Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon lambda-1 (IFN-λ/IL-29) induces ELR− CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-γ-independent manner. Genes Immun 2007; 8:177–80. [DOI] [PubMed] [Google Scholar]
- 38. Julian DR, Kazakoff MA, Patel A, Jaynes J, Willis MS, Yates CC. Chemokine-based therapeutics for the treatment of inflammatory and fibrotic convergent pathways in COVID-19. Curr Pathobiol Rep 2021; 9:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diaz PV, Valdivia G, Gaggero AA, et al. Pro-Inflammatory cytokines in nasopharyngeal aspirate from hospitalized children with respiratory syncytial virus infection with or without rhinovirus bronchiolitis, and use of the cytokines as predictors of illness severity. Medicine 2015; 94:e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hornsleth A, Klug B, Nir M, et al. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr Infect Dis J 1998; 17:1114–21. [DOI] [PubMed] [Google Scholar]
- 41. Vazquez Y, Gonzalez L, Noguera L, et al. Cytokines in the respiratory airway as biomarkers of severity and prognosis for respiratory syncytial virus infection: an update. Front Immunol 2019; 10:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu S, Hartert TV, Everard ML, et al. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr Pulmonol 2016; 51:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol 2010; 82:1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011; 204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mejias A, Brenes-Chacon H, Garcia-Mauriño C, Moore-Clingenpeel M, Ramilo O. Clinical disease severity scores and viral loads in children with respiratory syncytial virus infection. Clin Infect Dis 2021; 72:e1160–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thwaites RS, Ito K, Chingono JMS, et al. Nasosorption as a minimally invasive sampling procedure: mucosal viral load and inflammation in primary RSV bronchiolitis. J Infect Dis 2017; 215:1240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.