Abstract
Objectives
To advance the initiative of ending the global epidemic, long-lasting HIV protection is needed through sustained release of antiretroviral drugs for months to years. We investigated in macaques the safety and efficacy of biodegradable polycaprolactone implants releasing tenofovir alafenamide for HIV pre-exposure prophylaxis (PrEP).
Methods
Implants were administered subcutaneously in the arm using a contraceptive trocar. Efficacy against vaginal simian-HIV (SHIV) infection was investigated in six pigtailed macaques that received two tenofovir alafenamide implants (0.35 mg/day), one in each arm, for a total release rate of tenofovir alafenamide at 0.7 mg/day. Macaques were exposed to SHIV twice weekly for 6 weeks. Statistical analyses were used to compare outcome with eight untreated controls. Histological assessments were performed on skin biopsies collected near implantation sites.
Results
Median (range) tenofovir diphosphate level in PBMCs was 1519 (1068–1898) fmol/106 cells. All macaques with tenofovir alafenamide implants were protected against vaginal SHIV infection. In contrast, 7/8 controls were infected after a median of 4 SHIV exposures (P = 0.0047). Histological assessment of tissues near tenofovir alafenamide implant sites showed inflammation and necrosis in 5/6 animals, which were not evident by visual inspection.
Conclusions
We demonstrated complete protection against vaginal SHIV infection with two implants releasing a total of 0.7 mg of tenofovir alafenamide per day. We also identified tenofovir diphosphate concentrations in PBMCs associated with complete vaginal protection. Consistent with previous findings, we observed adverse local toxicity and necrosis near the tenofovir alafenamide implant site. Improved tenofovir alafenamide implants that are safe and maintain high efficacy have the potential to provide long-lasting protection against vaginal HIV infection.
Introduction
The HIV epidemic remains one of the highest global public health priorities.1 Although new HIV infections have steadily declined since the peak in 1997, an estimated 1.5 million became newly infected in 2020.2 Daily oral pre-exposure prophylaxis (PrEP) with the combination of emtricitabine and tenofovir disoproxil fumarate or tenofovir alafenamide has been highly effective in preventing HIV acquisition when taken as prescribed.3,4 However, inadequate adherence to the daily oral regimen reduces effectiveness and public health impact. As efforts to scale up PrEP worldwide accelerate, it is a high priority to identify novel PrEP modalities that accommodate the different needs among users. To this end, the pipeline for HIV prevention options is moving beyond daily to develop long-acting (LA) PrEP products that do not require frequent dosing and may overcome some of the adherence challenges associated with daily oral PrEP.
An injectable formulation of the integrase inhibitor cabotegravir was among the first systemic LA products to be developed for PrEP. LA cabotegravir is administered intramuscularly on a bimonthly schedule and showed high efficacy in preclinical macaque models against vaginal, rectal and penile virus acquisition.5–8 Recent results from the HPTN 083 and 084 trials showed that LA cabotegravir was safe and highly protective; its efficacy exceeded that of daily oral emtricitabine/tenofovir disoproxil fumarate, likely reflecting the adherence advantage of LA PrEP.9,10 These findings led to the recent FDA approval of LA cabotegravir (APRETUDE®) for PrEP use in at-risk adults to reduce the risk of sexually acquired HIV.11 However, these studies also raised important questions on how best to implement LA cabotegravir for PrEP and how to manage the long drug tail after treatment discontinuation to minimize risks of infection and possible selection of drug-resistant viruses.9 Another potential issue with LA cabotegravir is the inability to remove cabotegravir in the case of an adverse reaction.
Substantial efforts are currently ongoing to develop novel LA prevention products that have a longer duration of protection than LA cabotegravir. Subdermal implants are effective drug delivery platforms that have been extensively used as LA hormonal contraceptives to deliver levonorgestrel (Norplant, Jadelle) or etonogestrel (Implanon, Nexplanon) to prevent unintended pregnancies.12,13 Implant technologies designed to deliver antiretroviral (ARV) drugs for HIV PrEP are in different stages of preclinical and clinical development and include injectable subcutaneous hydrogels, silicone implants, osmotic pump systems, polyurethane tubes, refillable nanofluidic implants and biodegradable poly-ε-caprolactone (PCL) implants.14–20 Efforts with implants for HIV PrEP have focused on the delivery of different classes of ARVs, including tenofovir alafenamide, given its high potency (50% effective concentration in the low-nanomolar range), long intracellular half-life of the active metabolite tenofovir diphosphate, and low systemic tenofovir exposures.21 A silicone implant releasing tenofovir alafenamide (0.92 mg/day) resulted in high tenofovir diphosphate levels in PBMCs (∼500 fmol/106 cells) for up to 35 days in dogs and has now advanced to Phase I/II clinical trials (PACTR201809520959443).16,22 A refillable nanofluidic tenofovir alafenamide implant showed no adverse effects in macaques and maintained tenofovir diphosphate levels in PBMCs at about 300 fmol/106 cells for up to 4 months.17 A polyurethane reservoir implant sustained ∼50 fmol/106 cells in PBMCs for up to 14 weeks in macaques and exhibited local toxicity.22 The reasons for poor tolerability of certain tenofovir alafenamide delivery platforms remain unknown. Importantly, pharmacological benchmarks for high vaginal efficacy by tenofovir alafenamide-releasing implants remain poorly defined.
RTI International has developed a biodegradable implant fabricated through the hot-melt extrusion of PCL.23,24 This implant has a reservoir configuration that permits zero-order release of drugs for ∼12 months at tunable dosages based on wall thickness, surface area and PCL composition.20,25,26 In addition to their biodegradable properties, PCL implants are compatible with medical-grade trocars and can be removed in the case of an adverse reaction.24 We recently showed that implants releasing tenofovir alafenamide at 0.7 mg/day achieved high and sustained tenofovir diphosphate levels in PBMCs in rhesus macaques for up to 5 months.27 The study also documented adverse skin reactions around the tenofovir alafenamide implants that were mild or non-existent during the first 6 to 8 weeks and worsened between Months 2 and 4. Here we sought to investigate whether implants releasing 0.7 mg of tenofovir alafenamide per day could prevent vaginal infection with simian HIV (SHIV) in macaques in order to inform on correlates of protection by tenofovir alafenamide and guide the development of implants that are safe and effective. We performed SHIV challenges during the first 1–2 months after implantation when implants were deemed to be safe. The macaque challenge model employed has been extensively used to investigate the efficacy of PrEP regimens for women, including oral emtricitabine/tenofovir disoproxil fumarate and LA cabotegravir, and predicted their clinical efficacy.7,28,29 We identified correlates of high vaginal protection with sustained release of tenofovir alafenamide but further documented subclinical toxicity associated with short-term exposure to this tenofovir alafenamide implant.
Materials and methods
Ethics statement
All animal procedures were performed under anaesthesia and approved by the CDC Institutional Animal Care and Use Committee (IACUC). Housing and care of pigtailed macaques were done in accordance with the Guide for the Care and Use of Laboratory Animals.
Tenofovir alafenamide implant fabrication
Medical-grade PCL pellets loaded with sesame oil only (placebo implant) or a 2:1 mass ratio of tenofovir alafenamide base and sesame oil (tenofovir alafenamide implant) were fabricated as described in the Supplementary Materials and methods, available as Supplementary data at JAC Online.20,24,25,30 Tenofovir alafenamide implants were designed to release tenofovir alafenamide throughout the entire surface area of the implant at a release rate of 0.35 mg/day.
Efficacy of tenofovir alafenamide implants against vaginal SHIV infection
The efficacy of tenofovir alafenamide implants in preventing vaginal infection was investigated using an established macaque model of repeated vaginal exposures to SHIV162P3 as previously described.31 Eight female pigtailed macaques (Macaca nemestrina; 6 to 12 years old) were exposed to SHIV twice weekly for up to 6 weeks. Six of the animals received two implants (one in each arm) releasing tenofovir alafenamide at 0.35 mg/day for a total release of 0.70 mg of tenofovir alafenamide per day. The remaining two animals did not receive implants and were used as real-time controls. An additional six untreated animals that were previously challenged vaginally twice weekly with the same SHIV dose and by the same personnel were included as historical controls. Vaginal SHIV challenges in the tenofovir alafenamide-implant animals were initiated 1 week after implantation to allow sufficient time for tenofovir diphosphate to accumulate in PBMCs. Implants were removed 1 week after the last SHIV challenge (Week 8 post implantation) to measure the drug tail and infection status during the drug washout period. Animals were closely monitored for SHIV infection, as described in the Supplementary Materials and methods. Infection outcome was compared with the eight untreated controls (two real-time and six historical).7
Measurement of tenofovir diphosphate concentrations in PBMCs and vaginal and lymphoid tissue
During efficacy assessments, tenofovir diphosphate concentrations were measured in PBMCs of the six tenofovir alafenamide-treated animals during the SHIV challenge period and after implant removal. A more detailed pharmacokinetic (PK) analysis to measure drug distribution of tenofovir alafenamide implants was assessed in three additional SHIV-infected pigtailed macaques that received a single tenofovir alafenamide implant (0.35 mg/day) in the right arm and a placebo implant containing sesame oil only in the left arm. The SHIV-infected macaques were humanely sacrificed after 5 weeks (n = 1) or 12 weeks (n = 2) post implantation.
Intracellular tenofovir diphosphate in PBMCs and purified mononuclear cells from lymphoid and vaginal tissues collected at necropsy was measured by LC-MS-MS as described in the Supplementary Materials and methods. The lower limit of quantification (LLOQ) for tenofovir diphosphate was 100 fmol/sample.32
Determination of tenofovir alafenamide residual concentration and purity in extruded implants
Tenofovir alafenamide was extracted from the extruded implants as indicated in the Supplementary Materials and methods. Concentration and purity were measured using HPLC at Alera Labs, LLC (Durham, NC, USA).
Safety and tolerability of tenofovir alafenamide implants following efficacy assessments
Weekly wellness checks and examination of the implantation site were performed to assess the safety and tolerability of implants. Examination included monitoring animal weight, general health status (i.e. physical activity, social interactions, food consumption) and thorough inspection of the area surrounding the implants for visible skin reactions. Erythema and oedema were graded based on the Draize scale from 0 (no reaction) to 4 (severe reaction).33
Implants were removed 8 weeks after implantation. Briefly, a scalpel no. 15 blade was used to make a 2 cm incision in the skin near the implant. The skin over the incision was apposed with either surgical glue or an absorbable suture. Straight after implant removal, 3 mm surgical punch biopsies were collected immediately cranial to the displaced implant for histopathological examination, as described in the Supplementary Materials and methods. Sections were evaluated by a veterinary pathologist and semi-quantitatively scored for the type and intensity of inflammation, as well as the abundance of fibrosis and presence of necrosis. Inflammatory cells, including neutrophils, lymphocytes, plasma cells, macrophages, eosinophils and reactive fibroblasts within the dermis and subcutaneous tissues and fibroblast were evaluated. The distribution and severity of infiltration by each cell type was scored on a scale of 0 to 5, with 0 representing no inflammatory cells present and 5 representing extensive infiltration by the specific cell type.
Statistical analysis
Due to the small sample sizes, Fisher’s exact test was used to compare the number of animals protected in the treated group (n = 6) relative to untreated controls (n = 8). Survival analysis and efficacy were calculated as described in the Supplementary Materials and methods.
Results
Efficacy of tenofovir alafenamide implants in preventing vaginal SHIV transmission
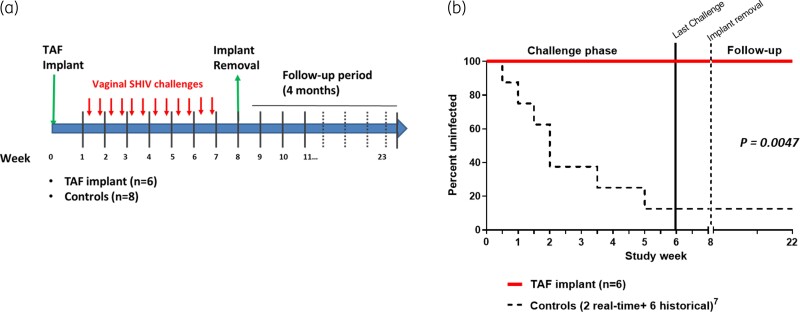
The study design of the vaginal SHIV challenge is shown in Figure 1a. Of the eight untreated controls, two were real-time controls and six were historical controls. The two real-time controls were infected at challenges 1 and 3. Overall, seven of the eight untreated animals became infected with SHIV after a median of 4 challenges (range 1–12). In contrast, all six macaques with tenofovir alafenamide implants remained uninfected after 12 vaginal challenges, resulting in an estimated efficacy of 100% (95% CI = undefined). The difference in survival distribution was statistically significant between the treated and untreated groups (P value = 0.0047, log-rank test) (Figure 1b).
Figure 1.
Protection of macaques against vaginal SHIV infection by tenofovir alafenamide (TAF) implants. (a) Study design. Six female pigtailed macaques received two TAF implants (placed in opposite arms), each releasing TAF at 0.35 mg/day (0.7 mg/day in total) 1 week prior to the challenge period. Animals were exposed vaginally to SHIV162P3 twice weekly for six consecutive weeks or a total of 12 challenges. Implants were removed 8 weeks post implantation. Infection outcome was compared with two real-time and six historical controls. (b) Survival analysis representing the cumulative percentage of uninfected macaques in the TAF-treated (n = 6) and untreated (n = 8) groups. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
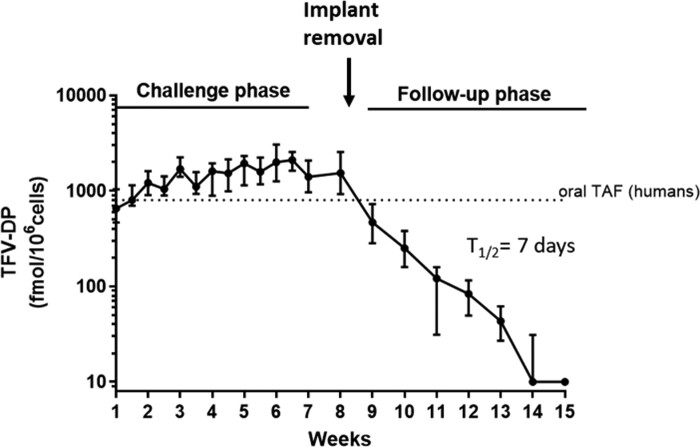
To define the concentrations of tenofovir diphosphate in PBMCs associated with protection, we monitored intracellular tenofovir diphosphate levels in PBMCs at the time of each virus exposure. Tenofovir diphosphate concentrations were high and sustained in all six animals with levels that ranged between 1068 and 1898 fmol/106 cells (median = 1519). After implant removal at Week 8, tenofovir diphosphate levels slowly declined over time and became undetectable 6 weeks after removal (Figure 2). The calculated terminal half-life of tenofovir diphosphate in PBMCs following implant removal was ∼7 days.
Figure 2.
Tenofovir diphosphate (TFV-DP) concentrations in PBMCs. TFV-DP levels were measured weekly during virus challenges and after implant removal at Week 8. Solid circles and bars represent medians and ranges, respectively. The horizontal dotted line denotes the concentrations of TFV-DP associated with steady-state levels with daily oral tenofovir alafenamide (TAF) in humans.
To investigate drug stability in implants, residual drug content was extracted from recovered implants and assessed for pure and degraded tenofovir alafenamide products. Residual pure tenofovir alafenamide remaining in the implants ranged from 96.2% to 97.9% (Table 1). Based on the pre-loaded yield and the recovery yield after 8 weeks, the calculated median in vivo release rate per implant was 0.38 mg/day, an elution rate that strongly correlated with the established in vitro release rate of 0.35 mg/day. After the implant removal, the incision healed quickly within a week.
Table 1.
Estimated stability and release rates of tenofovir alafenamide (TAF) calculated from explanted implants recovered from pigtailed macaques at Day 53
| Device ID | Original TAF loading (mg) | Experimental TAF recovered (mg) | TAF purity (%) | TAF released (mg) | Estimated release rate (mg/day) | Right (R)/left (L) ratio |
|---|---|---|---|---|---|---|
| A10024 L | 125.53 | 102 | 96.2 | 23.53 | 0.44 | 0.89 |
| A10024 R | 124.80 | 104 | 96.9 | 20.80 | 0.39 | |
| Z141191 L | 124.00 | 101.2 | 96.5 | 22.80 | 0.43 | 0.91 |
| Z141191 R | 124.73 | 103.8 | 97.1 | 20.93 | 0.39 | |
| Z08139 L | 127.26 | 109 | 96.9 | 18.26 | 0.34 | 0.53 |
| Z08139 R | 129.66 | 120 | 97.8 | 9.66 | 0.18 | |
| Z11328 L | 123.06 | 101 | 97.9 | 22.06 | 0.42 | 0.79 |
| Z11328 R | 126.46 | 109 | 97.5 | 17.46 | 0.33 | |
| Z13244 L | 127.13 | 105 | 96.4 | 22.13 | 0.42 | 0.86 |
| Z13244 R | 126.13 | 107 | 96.9 | 19.13 | 0.36 | |
| Z14009 L | 127.53 | 110 | 97.5 | 17.53 | 0.33 | 1.03 |
| Z14009 R | 126.06 | 108 | 97.5 | 18.06 | 0.34 |
TAF stability expressed as % purity of the excised implant devices. Purity determined by UV-UPLC.
Short-term safety and tolerability assessment of tenofovir alafenamide implant
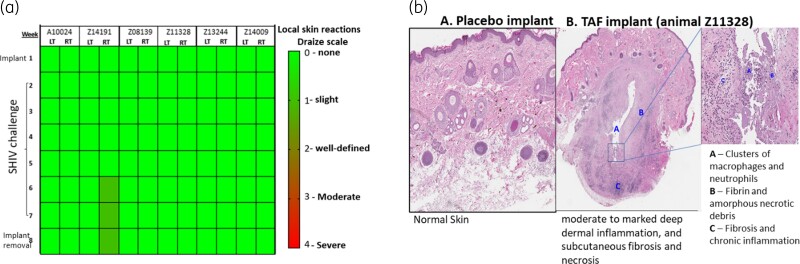
To assess short-term safety and tolerability of tenofovir alafenamide implants, we examined the area surrounding the tenofovir alafenamide implants during the 8 weeks of implantation. This assessment included the six macaques that received two tenofovir alafenamide implants for a cumulative analysis of 12 implantation sites (2 sites per animal). Using the Draize scale to assess local skin reactions, we found grade 1 erythema in one animal by Week 6, as indicated in the heat map (Figure 3a). The erythema quickly resolved following implant removal. All other tenofovir alafenamide implantation sites (n = 11) remained unremarkable during the 6 week treatment period.
Figure 3.
(a) Heatmap of local skin reactions at implant site. Local skin reactions observed during the 8 week study period were scored using a Draize scale: 0 (none) to 4 (severe). Visual assessments of the tenofovir alafenamide (TAF) implant site in the left (LT) and right (RT) arm are indicated for each animal. (b) Histological observations of implantation sites. Panels show a representative H&E stain of skin biopsies collected near the implantation site from an animal with a placebo or TAF implant. Left panel: skin biopsies surrounding the placebo PCL implants show normal and unremarkable tissue. Central and right panels: tissue surrounding the TAF implant show moderate to marked inflammation and subcutaneous fibrosis (A, B and C). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A semiquantitative histopathological assessment was also done by haematoxylin and eosin (H&E) staining of skin biopsies collected directly above the implant site in the treated group (Table 2). H&E staining revealed reactions indicative of a foreign-body response, with macrophages, multinucleated giant cells, mononuclear cell infiltrates and reactive fibrosis, which is common following subcutaneous administration of medical implants.34,35 In general, the inflammatory reactions appeared similar between the implant sites in both arms of the same animal, with intra-animal differences in inflammatory responses likely due to sampling. Notably, 7 of the 12 sites contained debris consistent with necrotic material (Table 2). There was moderate variation in the overall inflammatory response scores between different animals. The biopsies from one animal (A10024) showed minimal inflammatory response with only mild reactive fibroblasts, oedema and acute haemorrhage, while biopsies from two other animals (Z14191, Z11328) showed a more pronounced, mixed inflammatory response composed of aggregates of lymphocytes and plasma cells around vessels and adnexa, with mixed eosinophils and scattered macrophages and neutrophils. Oedema and prominent reactive fibroblasts were also present in the surrounding deep dermis and superficial subcutaneous tissue. The inflammation often centred on amorphous eosinophilic debris, interpreted to be fibrin and acellular necrotic material, surrounding the implant site. The total inflammatory response scores observed in the other three animals (Z08139, Z14009, Z13244) fell between the least and most severe responses described above (Table 2). Figure 3b shows the H&E staining from animal Z11328, illustrating moderate to marked deep dermal inflammation and subcutaneous fibroses and necrosis, while normal skin was observed in an animal from the terminal PK study that was implanted with a placebo implant. Overall, the histopathological evaluation indicated moderate to deep dermal necrosis and a median implant reactivity score of 15 (scale 0–45).36
Table 2.
H&E scoring
| Animal | Median (range) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A10024 | Z14191 | Z08139 | Z11328 | Z13244 | Z14009 | ||||||||
| Implantation arm | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT/LT |
| Lymphocytes | 0 | 0 | 3 | 3 | 2 | 2 | 1 | 3 | 1 | 3 | 4 | 1 | 2.0 (0–4) |
| Plasma cells | 0 | 0 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 3 | 2 | 1 | 2.0 (0–3) |
| Macrophages | 0 | 0 | 1 | 2 | 1 | 3 | 4 | 3 | 0 | 3 | 2 | 1 | 1.5 (0–4) |
| Neutrophils | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 0 | 1 | 2 | 0 | 1.0 (0–2) |
| Eosinophils | 0 | 0 | 1 | 1 | 0 | 1 | 4 | 4 | 0 | 1 | 1 | 0 | 1.0 (0–4) |
| Fibroblasts | 1 | 1 | 2 | 1 | 1 | 2 | 3 | 2 | 0 | 2 | 2 | 1 | 1.5 (0–3) |
| Acute haemorrhage | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0.5 (0–2) |
| Oedema | 1 | 1 | 3 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1.0 (0–3) |
| Fibrosis | 0 | 0 | 0 | 1 | 2 | 4 | 3 | 4 | 1 | 2 | 4 | 0 | 1.5 (0–4) |
| Acellular/necrotic material | − | − | + | + | − | + | + | + | − | + | + | − | |
Subcutaneous tissues collected near the implant site were scored (0 to 5 scale) for the presence of lymphocytes, plasma cells, histiocytes/macrophages, neutrophils, fibrosis, proliferating fibroblasts and acute haemorrhage. Presence of extravascular RBCs was only scored as acute haemorrhage if present in deeper tissue. Necrosis was documented based on the presence (+) or absence (−) of acellular and necrotic material.
Tenofovir diphosphate concentrations in vaginal and lymphoid tissue
To investigate the distribution of tenofovir diphosphate in vaginal tissues and in lymph nodes collected proximal or distal to the implantation site, we implanted three macaques with a single implant releasing tenofovir alafenamide at 0.35 mg/day in the right arm followed by tissue collection 5 or 12 weeks post implantation. Median (range) tenofovir diphosphate levels in PBMCs at the time of implant removal were 1104 (789–1608) fmol/106 cells. Median tenofovir diphosphate concentrations in axillary lymph nodes collected from the upper right side of the body and close to the implantation site were ∼10-fold higher than those detected in the axillary lymph nodes collected from the left side (3870 and 307.8 fmol/106 cells, respectively) although the sample size was too small to demonstrate statistical significance (Table 3). In contrast, median tenofovir diphosphate levels in inguinal lymph nodes collected from the lower right and left sides of the body were similar (499 and 393 fmol/106 cells, respectively). Notably, tenofovir diphosphate levels in vaginal lymphocytes (median = 50.7 fmol/106 cells) were ∼10-fold lower than those seen in the nearby inguinal lymph nodes (Table 3).
Table 3.
Tenofovir diphosphate (TFV-DP) concentrations in lymphocytes isolated from vaginal tissue, inguinal nodes and axillary nodes from three different macaques implanted with a single tenofovir alafenamide implant (0.35 mg/day) in the right arm
Discussion
Implants for HIV prevention that provide sustained drug release for months to years can be a desirable option for people who need or want long-term protection from HIV and are unable to adhere to daily oral PrEP. We used a validated macaque model of vaginal SHIV transmission to investigate the efficacy of a biodegradable PCL implant releasing tenofovir alafenamide (base) and identify correlates of protection by tenofovir alafenamide implants. We show that implants delivering 0.7 mg of tenofovir alafenamide per day result in high tenofovir diphosphate levels in PBMCs and provide complete protection against repeated vaginal SHIV exposures over 6 weeks or 12 challenges. However, despite a short exposure to the tenofovir alafenamide implants, we noted signs of local toxicity at the implantation site that were not evident through visual inspection, thus underscoring the need for improving the drug delivery system to more safely deliver tenofovir alafenamide at efficacious levels.
We have previously determined that an oral daily tenofovir alafenamide dose of 1.5 mg/kg in macaques, which represents about 15 mg in an average 10 kg animal, reproduces the drug exposure of the clinical 25 mg tenofovir alafenamide dose in humans and results in tenofovir diphosphate concentrations in PBMCs of about 350 fmol/106 cells.37 Here we show that the release of ∼0.7 mg of tenofovir alafenamide per day from implants, which is about 20-fold lower than the oral dose, results in higher tenofovir diphosphate concentrations. The mechanisms of the high dosing efficiency from implants cannot be entirely explained by the oral bioavailability of tenofovir alafenamide and may be also related to the implant formulation. A high dosing efficiency is highly desirable for implants because it can reduce the amount of drug needed while lengthening the duration of its effect.
Our exploratory analysis of drug distribution in lymph nodes and vaginal tissues revealed 10-fold higher tenofovir diphosphate levels in lymph nodes that were near the implantation site compared with the levels in the nodes in the opposite side of the implant location. These results are intriguing and need to be confirmed in a larger number of animals. If confirmed, the results suggest that tissues close to the implant location may be dosed more efficiently with tenofovir alafenamide and raise some considerations about the optimal body location for implants designed for HIV prevention. It will be important to expand this analysis to implants releasing other drugs and investigate whether an implant placed in a different location provides higher drug levels in neighbouring tissues. For instance, will a tenofovir alafenamide implant placed in the inner thigh result in higher tenofovir diphosphate levels in vaginal and rectal tissues and inguinal lymph nodes, and thus potentially increase efficacy against sexual HIV infection?
The observed focal necrosis in biopsies taken near the implantation sites was likely attributed to tenofovir alafenamide since placebo PCL implants formulated with sesame oil had no evidence of histopathological toxicity or local skin reactions and were found to be safe and well tolerated in macaques. Notably, the toxicities seen in the biopsy specimens were not evident by visual inspection as tenofovir alafenamide implantation sites remained unremarkable during the 8 week treatment period. Su et al.22 reported fibrosis, haemorrhagic abscesses and severe granulomatosis with non-degradable polyurethane (PU) tenofovir alafenamide implants, although in this instance some of the adverse histopathology was also noted with placebo implants. Pons-Faudoa et al.18 performed a limited toxicity evaluation of a refillable nanofluidic titanium tenofovir alafenamide implant and found mild tissue responses with little or no inflammatory cell infiltration. These observations raise questions about the source and mechanisms of toxicity associated with different tenofovir alafenamide implants and stress the need to further define implant characteristics that associate with better safety profiles. Possible attributes may include the implant material and configuration, including surface area for ARV release, use of tenofovir alafenamide hemifumarate or free base, and the degree of tenofovir alafenamide metabolite production at the implantation sites.24 The finding of delayed wound healing with high doses of tenofovir in primary epithelial cells and fibroblasts from the female reproductive tract is noteworthy and point to a possible role of tenofovir alafenamide or its metabolites in interfering with the wound healing process that must take place after incision and implant insertion subdermally.34,35,38
Our study also provides important preclinical information on the correlates of vaginal protection with tenofovir alafenamide in macaques. In a previous study using a clinically relevant dose of oral tenofovir alafenamide (1.5 mg/kg), we found 58% vaginal protection at tenofovir diphosphate concentrations in PBMCs of ∼350 fmol/106 cells.37 We document in this study 100% protection at concentrations of tenofovir diphosphate in PBMCs between 1100 and 1900 fmol/106 cells, suggesting that the benchmark for complete vaginal efficacy by tenofovir alafenamide is high. While dose titration can help define the precise benchmark for protection, more recent data from a high oral tenofovir alafenamide dose (27.4 mg/kg) study in macaques indicated that tenofovir diphosphate levels of ∼1300 fmol/106 PBMCs were associated with 93% efficacy.39 Collectively, these data all point to high tenofovir diphosphate concentrations in PBMCs needed for vaginal protection by tenofovir alafenamide. It is not known, however, if the same benchmark will also apply for rectal protection. Pons-Faudoa et al.18 found a 62% rectal efficacy with a nanofluidic tenofovir alafenamide implant that maintained tenofovir diphosphate levels at 390 fmol/106 PBMCs, although the efficacy estimate was not precise due to the small sample size. In an earlier study, we documented no rectal protection with a weekly oral tenofovir alafenamide dose of 13.7 mg/kg capable of sustaining tenofovir diphosphate levels between 2581 and 966 fmol/106 PBMCs over an entire week.40 These observations suggest that tenofovir diphosphate levels in PBMCs alone may not be a good surrogate for predicting rectal protection with tenofovir alafenamide.
In summary, we demonstrated that two biodegradable PCL implants releasing a total of 0.7 mg of tenofovir alafenamide per day resulted in high and sustained tenofovir diphosphate levels in PBMCs that was associated with full protection against vaginal SHIV infection, although the observed local toxicities emphasize the need for implant modifications to improve their safety. The identification of tenofovir diphosphate levels needed for full vaginal protection with tenofovir alafenamide also provides benchmark drug levels required for protection by other implant technologies that safely release tenofovir alafenamide.
Supplementary Material
Acknowledgements
We thank Drs Mrotz and Condrey for providing assistance during the implantation and removal of implants in macaques and Ms Virginia Butts for measuring drug levels. We also thank Croda (Edison, NJ, USA) for kindly providing the sesame oil excipient.
This research was made possible by the generous support of the American people through the U.S. President’s Emergency Plan for AIDS Relief (Cooperative Agreement number AID-OAA-A-14-00012). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, PEPFAR or the CDC.
Contributor Information
I Massud, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
A Krovi, RTI International, Research Triangle Park, NC, USA.
K Nishiura, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
S Ruone, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
L Li, RTI International, Research Triangle Park, NC, USA.
A Holder, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
J Gary, Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infection Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
P Mills, Comparative Medicine Branch, Division of Scientific Resources, National Center for Emerging and Zoonotic Infection Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
J Mitchell, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
G Khalil, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Y Pan, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
E Luecke, RTI International, Research Triangle Park, NC, USA.
G Gatto, RTI International, Research Triangle Park, NC, USA.
W Heneine, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
J G Garcίa-Lerma, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
L Johnson, RTI International, Research Triangle Park, NC, USA.
A van der Straten, Center for AIDS Prevention Studies (CAPS), Department of Medicine, University of California San Francisco, San Francisco, CA and ASTRA Consulting, Kensington, CA, USA.
C Dobard, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Funding
CDC intramural funds and Cooperative Agreement AID-OAA-A-14-00012 between RTI International and USAID.
Transparency declarations
J.G.G.-L. and W.H. are named in US Government patents on ‘Inhibition of HIV infection through chemoprophylaxis’. I.M., W.H. and J.G.G.-L. are named in a US Government patent on ‘HIV post-exposure prophylaxis’ and a patent application on ‘HIV pre-exposure prophylaxis’. The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official views of the CDC. All other authors: none to declare.
Supplementary data
Supplementary materials and methods are available as Supplementary data at JAC Online.
References
- 1. UNAIDS . UNAIDS data 2019. 2019.. https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data.
- 2. UNAIDS . Global HIV & AIDS statistics—2021 Fact sheet. 2021. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- 3. Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–34. [DOI] [PubMed] [Google Scholar]
- 5. Andrews CD, Spreen WR, Mohri H et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343: 1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrews CD, Yueh YL, Spreen WR et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 2015; 7: 270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Radzio J, Spreen W, Yueh YL et al. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Trans Med 2015; 7: 270ra5. [DOI] [PubMed] [Google Scholar]
- 8. Dobard C, Makarova N, Nishiura K et al. Long-acting cabotegravir protects macaques against repeated penile simian-human immunodeficiency virus exposures. J Infect Dis 2020; 222: 391–5. [DOI] [PubMed] [Google Scholar]
- 9. Marzinke MA, Grinsztejn B, Fogel JM et al. Characterization of HIV infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J Infect Dis 2021; 224: 1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landovitz RJ, Donnell D, Clement ME et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA Approves First Injectable Treatment for HIV Pre-Exposure Prevention 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention.
- 12. Winner B, Peipert JF, Zhao Q et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366: 1998–2007. [DOI] [PubMed] [Google Scholar]
- 13. Rocca ML, Palumbo AR, Visconti F et al. Safety and benefits of contraceptives implants: a systematic review. Pharmaceuticals (Basel) 2021; 14: 548–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romano JW, Baum MM, Demkovich ZR et al. Tenofovir alafenamide for HIV prevention: review of the Proceedings from the Gates Foundation Long-Acting TAF Product Development meeting. AIDS Res Hum Retroviruses 2021; 37: 409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kovarova M, Benhabbour SR, Massud I et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun 2018; 9: 4156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunawardana M, Remedios-Chan M, Miller CS et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother 2015; 59: 3913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chua CYX, Jain P, Ballerini A et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J Control Release 2018; 286: 315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pons-Faudoa FP, Sizovs A, Shelton KA et al. Preventive efficacy of a tenofovir alafenamide fumarate nanofluidic implant in SHIV-challenged nonhuman primates. Adv Ther (Weinh) 2021; 4: 2000163–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asai D, Kanamoto T, Takenaga M et al. In situ depot formation of anti-HIV fusion-inhibitor peptide in recombinant protein polymer hydrogel. Acta Biomater 2017; 64: 116–25. [DOI] [PubMed] [Google Scholar]
- 20. Li L, Gatto GJ, Brand RM et al. Long-acting biodegradable implant for sustained delivery of antiretroviral (ARV) and hormones. J Control Release 2021; 340: 188–99. [DOI] [PubMed] [Google Scholar]
- 21. Wassner C, Bradley N, Lee Y. A review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide. J Int Assoc Provid AIDS Care 2020; 19: 2325958220919231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su JT, Simpson SM, Sung S et al. A subcutaneous implant of tenofovir alafenamide fumarate causes local inflammation and tissue necrosis in rabbits and macaques. Antimicrob Agents Chemother 2020; 64: e01893-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlesinger E, Johengen D, Luecke E et al. A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharm Res 2016; 33: 1649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson LM, Krovi SA, Li L et al. Characterization of a reservoir-style implant for sustained release of tenofovir alafenamide (TAF) for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics 2019; 11: 315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Johnson LM, Krovi SA et al. Performance and stability of tenofovir alafenamide formulations within subcutaneous biodegradable implants for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics 2020; 12: 1057–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krovi SA, Johnson LM, Luecke E et al. Advances in long-acting injectables, implants, and vaginal rings for contraception and HIV prevention. Adv Drug Deliv Rev 2021; 176: 113849. [DOI] [PubMed] [Google Scholar]
- 27. Massud I, Krovi A, Ruone S et al. Pharmacokinetics and safety of long-acting tenofovir alafenamide implants in macaques for HIV prevention. Twenty-third International AIDS conference 2020. Abstract PEA0087. https://programme.aids2020.org/Abstract/Abstract/7107. [Google Scholar]
- 28. García-Lerma JG, Otten RA, Qari SH et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med 2008; 5: e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Massud I, Mitchell J, Babusis D et al. Chemoprophylaxis with oral emtricitabine and tenofovir alafenamide combination protects macaques from rectal SHIV infection. J Infect Dis 2016; 214: 1058–62. [DOI] [PubMed] [Google Scholar]
- 30. Li L, Areson C, van der Straten A et al. Effects of polymer blending on the performance of a subcutaneous biodegradable implant for HIV pre-exposure prophylaxis (PrEP). Int J Mol Sci 2021; 22: 6529–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One 2012; 7: e50632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuklenyik Z, Martin A, Pau CP et al. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J Chromatogr Sci 2009; 47: 365–72. [DOI] [PubMed] [Google Scholar]
- 33. Farage MA, Maibach HI, Andersen KE et al. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermatitis 2011; 65: 65–75. [DOI] [PubMed] [Google Scholar]
- 34. Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A 2017; 105: 927–40. [DOI] [PubMed] [Google Scholar]
- 35. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008; 20: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. International Organization for Standardization . Biological evaluation of medical devices, 3rd edition. 2016. [Google Scholar]
- 37. Massud I, Cong ME, Ruone S et al. Efficacy of oral tenofovir alafenamide/emtricitabine combination or single agent tenofovir alafenamide against vaginal SHIV infection in macaques. J Infect Dis 2019; 220: 1826–33. [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez-Garcia M, Patel MV, Shen Z et al. Tenofovir inhibits wound healing of epithelial cells and fibroblasts from the upper and lower human female reproductive tract. Sci Rep 2017; 8: 45725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Massud I, Nishiura K, Ruone S et al. Weekly oral tenofovir alafenamide protects macaques from vaginal SHIV infection. Conference on Retrovirus and Opportunistic Infections, March 2021. Abstract 714. https://www.natap.org/2021/CROI/croi_07.htm. [Google Scholar]
- 40. García-Lerma JG, Aung W, Cong ME et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 2011; 85: 6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.